Abstract
Acute kidney injury represents a common complication in critically ill patients affected by septic shock and in many cases continuous renal replacement therapy (CRRT) may be required. In this scenario, antimicrobial dose optimization is highly challenging as the extracorporeal circuit may cause several pharmacokinetic alterations, which add up to volume of distribution and clearance variations resulting from sepsis. Variations in CRRT settings (i.e. modality of solute removal, type of filter material, blood flow rate and effluent flow rate), coupled with the presence of residual and/or recovering renal function, may cause dynamic variations in the clearance of hydrophilic antimicrobials. This means that dose reduction may not always be needed. Nowadays, the lack of pharmacokinetic data for novel antimicrobials during CRRT limits evidence-based dose recommendations for critically ill patients in this setting, thus making available evidence hardly applicable in real-world scenarios. This review aims to summarize the major determinants involved in antimicrobial clearance, and the available pharmacokinetic studies performed during CRRT involving novel antibiotics used for the management of multidrug-resistant Gram-positive and Gram-negative infections (namely ceftolozane–tazobactam, ceftazidime–avibactam, cefiderocol, imipenem–relebactam, meropenem–vaborbactam, ceftaroline, ceftobiprole, dalbavancin, and fosfomycin), providing a practical approach in guiding dose optimization in this special population.
Key Points
| Evidence assessing the pharmacokinetic behaviour of novel antibiotics used in the treatment of multidrug-resistant Gram-positive- and Gram-negative-related infections in patients undergoing continuous renal replacement therapy (CRRT) are limited. Most studies investigated ceftolozane–tazobactam pharmacokinetics, and no real-world evidence was found regarding the use of cefiderocol or imipenem–relebactam. |
| In most cases, a priori dose reduction of novel antibiotics in patients undergoing CRRT seems to be an inappropriate strategy rather than a real need. |
| Antimicrobial physicochemical/pharmacokinetic properties, CRRT settings, pathophysiological conditions, site of infection, and minimum inhibitory concentration of isolated pathogens should be carefully evaluated in dose adjustment decision making. |
| A paradigm shift from a ‘drug-centred’ approach to a ‘patient-centred’ approach could be useful and manageable, especially in settings where antibiotic therapeutic drug monitoring is unavailable. |
Introduction
Sepsis is the most common cause of acute kidney injury (AKI) in critically ill patients [1], requiring the initiation of continuous renal replacement therapy (CRRT) in approximately 70% of cases [2]. In this scenario, the mortality rate may exceed 60% [3]. Given that septic patients undergoing CRRT require prompt and optimized antimicrobial therapy, the choice of appropriate antibacterial dosing is highly challenging.
Several factors may affect the achievement of optimal pharmacokinetic/pharmacodynamic (PK/PD) targets in critically ill patients requiring CRRT, directly influencing antibiotic clearance (CL): physicochemical/PK properties of selected antibiotics, acute pathophysiological variations, CRRT settings, minimum inhibitory concentration (MIC) of isolated pathogens, and site of infection [4–8]. In this scenario, the ‘one dose fits all’ approach is completely unfeasible [8], potentially resulting in unnecessary dose reduction, as recently found in the SMARRT trial [9].
The widespread diffusion of multidrug-resistant (MDR) pathogens, both Gram-positive (e.g. methicillin-resistant Staphylococcus aureus [MRSA], vancomycin-resistant Enterococcus faecium [VRE]) and Gram-negative (e.g. carbapenemase-producing Enterobacteriaceae [CPE], MDR or extensively drug-resistant (XDR) Pseudomonas aeruginosa, Acinetobacter baumannii), represents a worrisome health concern [10]. In recent years, several novel antibiotics have been licensed for the management of MDR Gram-positive (i.e. dalbavancin, ceftaroline, ceftobiprole) [11] or Gram-negative (i.e. ceftolozane–tazobactam, ceftazidime–avibactam, meropenem–vaborbactam, imipenem–relebactam, cefiderocol) infections [12]. Additionally, some older agents (i.e. fosfomycin) showed promising results in this setting [13]. With the exception of dalbavancin, all of these novel agents share common physicochemical and PK properties, namely low molecular weight, pronounced hydrophilicity, limited volume of distribution (Vd) and protein binding, and predominant renal CL (Table 1), making them prone to relevant elimination via CRRT [4, 5].
Table 1.
Physicochemical and pharmacokinetic features of novel agents used for the management of multidrug-resistant Gram-positive and Gram-negative infections retrieved in healthy volunteers
| Antibiotic | Dose (mg) |
MW (Da) | Hydrosolubility (LogP) | Protein binding (%) | Vd (L/kg) | t½ (h) | Renal excretion (%) | AUC (mg*h/L) |
CL (L/h) |
Cmax (mg/L) | Cmin (mg/L) | Optimal PK/PD target |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefiderocol [45] | 2000 | 752 | 1.84 | 40 | 0.26 | 2.74 | 62 | 389.7 | 5.13 | 156 | NA | 100%fT> 4 × MIC |
| Ceftaroline [72] | 600 | 684 | − 0.79 | 20 | 0.29 | 2.17 | 88 | 62.7 | 8.45 | 27.3 | NA | 100%fT> 4 × MIC |
| Ceftazidime–avibactam [73] | 2000/500 | 547/265 | − 1.2/− 1.8 | 10/8 | 0.28/0.31 | 1.98/1.59 | 90/97 | 251.5/43.1 | 7.95/11.6 | 124.1/26.5 | 4.2/0.4 | 100%fT> 4 × MIC |
| Ceftolozane–tazobactam [74] | 1000/500 | 666/300 | − 1.2/− 1.8 | 20/22 | 0.19/0.40 | 3.1/1.1 | 95/80 | 230/29.8 | 4.3/16.6 | 72.8/17.0 | NA | 100%fT> 4 × MIC |
| Ceftobiprole [75] | 500 | 534 | v1.3 | 16 | 0.25 | 3.1 | 83 | 104 | 4.89 | 29.2 | NA | 100%fT> 4 × MIC |
| Dalbavancin [76] | 1000 | 1817 | 3.8 | 93 | 0.11 | 383 | 50 | 24,561 | 0.042 | 248.8 | NA | fAUC/MIC > 111.1 |
| Fosfomycin [77] | 8000 | 138 | − 0.86 | < 1 | 0.45 | 2.8 | 81 | 1056 | 7.8 | 370 | NA | 100%fT> 4 × MIC |
| Imipenem–relebactam [78] | 500/250 | 317/348 | − 0.19/− 2 | 20 | 0.23/0.23 | 1.0/1.6 | 70/90 | NA/30.0 | NA/8.3 | NA/17.9 | NA | 100%fT> 4 × MIC |
| Meropenem–vaborbactam [23] | 1000/1000 | 383/297 | − 0.69/1.02 | 2/23 | 0.28/0.25 | 1.30/1.65 | 70/95 | 87.1/99.4 | 12.5/11.1 | 27.5/27.8 | NA | 100%fT> 4 × MIC |
AUC area under the concentration-time curve, CL clearance, Cmax peak concentration, Cmin trough concentration, MIC minimum inhibitory concentration, MW molecular weight, NA not available, PK/PD pharmacokinetic/pharmacodynamic, t½ half-life, Vd volume of distribution
Despite growing use in critically ill patients, the lack of PK data during CRRT nowadays limits evidence-based antibiotic dosing recommendations for novel agents [14]. Furthermore, several studies reported CRRT as an independent predictor of clinical failure and the development of resistance to ceftolozane–tazobactam and ceftazidime–avibactam [15–17], and this could potentially be associated with antibiotic underexposure and failure to achieve optimal PK/PD targets. Consequently, implementation of a tailored approach in patients requiring CRRT and treated with novel agents represents an urgent clinical need. Addressing this need would allow the provision of adequate antimicrobial exposure that is mandatory for both improving clinical outcome and minimizing resistance development [18].
The aim of this review was to summarize relevant PK features of novel agents in critically septic patients requiring CRRT, performing a comparison with PK parameters retrieved in healthy volunteers. Additionally, we provide a critical reappraisal of their applicability in different clinical scenarios, to guide clinicians in the choice of the best dosage against MDR infections.
Methods
A literature search was conducted using PubMed/MEDLINE (search performed on 5 March 2021) in order to retrieve prospective or retrospective observational studies, population PK studies, or case series/reports investigating the PK behaviour of novel agents in critically septic patients requiring CRRT. The antibiotics cefiderocol, ceftaroline, ceftazidime–avibactam, ceftobiprole, ceftolozane–tazobactam, dalbavancin, fosfomycin, imipenem–relebactam, and meropenem–vaborbactam were included, and the following search string was specifically created: (‘ceftolozane’ OR ‘ceftolozane-tazobactam’ OR ‘ceftazidime-avibactam’ OR ‘avibactam’ OR ‘imipenem-relebactam’ OR ‘relebactam’ OR ‘cefiderocol’ OR ‘meropenem-vaborbactam’ OR ‘vaborbactam’ OR ‘fosfomycin’ OR ‘ceftaroline’ OR ‘ceftobiprole’ OR ‘dalbavancin’) AND (‘renal replacement therapy’ OR ‘continuous renal replacement therapy’ OR ‘hemofiltration’ OR ‘haemofiltration’ OR ‘hemodiafiltration’ OR ‘haemodiafiltration’ OR ‘hemodialysis’ OR ‘haemodialysis’ OR ‘crrt’ OR ‘rrt’ OR ‘cvvh’ OR ‘cvvhd’ OR ‘cvvhdf’ OR ‘continuous venovenous hemodialysis’ OR ‘continuous venovenous haemodialysis’ OR ‘continuous venovenous hemofiltration’ OR ‘continuous venovenous haemofiltration’ OR ‘continuous venovenous hemodiafiltration’ OR ‘continuous venovenous haemodiafiltration). Studies investigating selected agents in non-critically ill patients or ex vivo models, or lacking quantitative data on PK parameters, were excluded.
For each included study or case report, the following data were extracted: study design, demographic characteristics (age, sex, weight), antibiotic dose and modality of infusion, site of infection, isolated pathogen and relative MIC, CRRT modality (continuous venovenous haemofiltration [CVVH], continuous venovenous haemodialysis [CVVHD], or continuous venovenous haemodiafiltration [CVVHDF]), CRRT settings (type of filter, effluent flow rate, ultrafiltrate rate [Quf], dialysate rate [Qd], blood flow rate [Qb], pre/post-dilution, and net removal), residual diuresis, clinical outcome, and PK parameters (peak concentration [Cmax], trough concentration [Cmin], Vd, total CL, CRRT CL, half-life [t½], area under the concentration-time curve [AUC], and sieving coefficient [SC] or saturation coefficient [SA]). In studies where PK parameters were not fully provided, variables were calculated using the following equations: half-life was calculated as t½ = 0.693/kel, where kel is the elimination rate constant; CLtot was calculated as dose/AUC; and Vd was calculated as CLtot/kel.
Optimal antibiotic exposure was arbitrarily defined as the achievement of aggressive PK/PD targets, which were considered a fourfold 100% of time of the dosing interval in which the unbound concentration is maintained above the MIC (100%fT> 4 × MIC) for β-lactams [19] and fosfomycin [20], and an fAUC/MIC > 111.4 for dalbavancin [21]. PK/PD targets were calculated as the ratio between unbound trough concentration and the MIC for β-lactams and fosfomycin, respectively, and as the ratio between fAUC and the MIC for dalbavancin. In studies where unbound concentrations were not provided, free antibiotic levels were calculated according to the proportion of protein binding reported in healthy volunteers (Table 1). For β-lactamase inhibitors, optimal PK/PD targets corresponding to 100%fT > 4 mg/L for tazobactam and avibactam, respectively, and to AUC/MIC ≥ 24 for vaborbactam, were implemented according to preclinical models [22, 23]. The MIC value was set at the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoint when no pathogen was isolated.
A comparison between PK parameters retrieved in different CRRT modalities and in healthy volunteers was performed by calculating the percentage of the ratio between values retrieved in CRRT patients and healthy subjects for each specific PK parameter. When multiple studies assessed the PK behaviour of a given agent in CRRT patients, that agent having the larger sample size and/or the same dosing schedule tested in healthy volunteers was selected for the comparison.
Pharmacokinetics (PK) of Novel Antibiotics in Patients Undergoing Continuous Renal Replacement Therapy (CRRT)
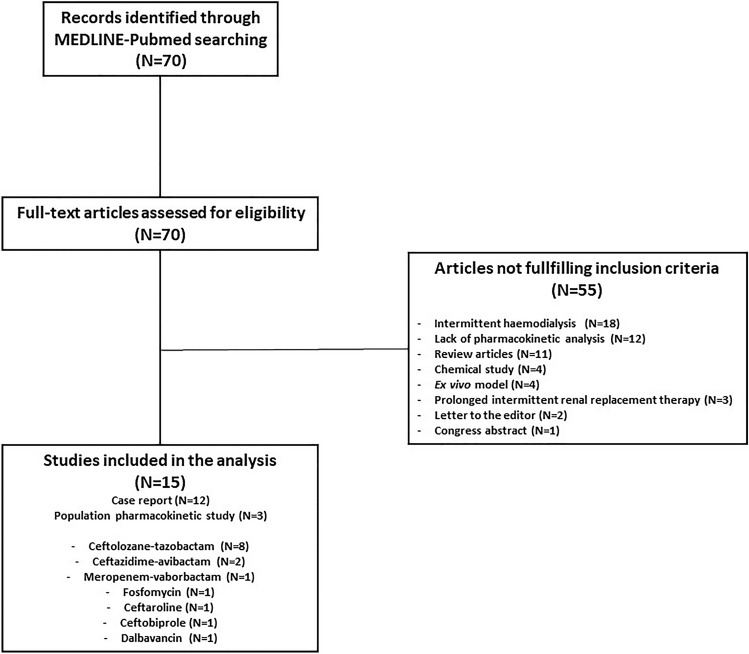
Overall, the search strategy identified 70 articles that were assessed for eligibility. Fifty-five of these articles did not fulfil the inclusion criteria, resulting in the inclusion of 15 original studies (3 population PK studies and 12 case reports) [24–38] assessing the PK behaviour of novel agents in critically septic patients requiring CRRT (Fig. 1). Details of the demographic/clinical characteristics and PK parameters retrieved in these studies are provided in Tables 2 and 3, respectively. Comparison of the PK parameter values between CRRT patients and healthy subjects is shown in Table 4.
Fig. 1.
Study selection process
Table 2.
Demographics/clinical features and CRRT settings collected from the included studies
| Novel agent (study reference) | Study design | No. of patients | Age, years/sex | Weight (kg) |
Dose | Site of infection | Pathogen/MIC | CRRT modality | Filter | Pre/post-dilution | Effluent flow rate (mL/h) | Blood flow rate (mL/h) |
Net removal (mL/h)—residual diuresis |
Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ceftolozane–tazobactam (Sime et al. [24]) |
PK population study | 6 |
55.8 ± 16.5 5M, 1F |
79.7 ± 17.9 |
1.5 g q8h (1-h infusion) |
4 BSI 3 HAP/VAP |
2 Pseudomonas aeruginosa 2 Stenotrophomonas maltophilia 2 Serratia marcescens 1 Klebsiella pneumoniae |
CVVHDF |
ST100/ST150 (surface area 0.9 m2/1.5 m2) |
NA |
Qd: 1250 ± 273.9 mL/ha Quf: 1277.8 ± 743.2 mL/ha |
150 ± 44.7 |
170 ± 173 NA |
NA |
|
Ceftolozane–tazobactam (Kuti et al. [28]) |
Case report | 1 | 75/M | 66 |
3 g q8h (1-h infusion) |
VAP |
Pseudomonas aeruginosa MIC: 0.75/4 mg/L |
CVVHDF | AN-69 high-flux M100 | NA |
Qd: 1000 Quf: 200 |
150 |
NA 76 mL/day |
Clinical cure |
|
Ceftolozane–tazobactam (Bremmer et al. [32]) |
Case report | 1 | 47/M | 82 |
3 g q8h (1-h infusion) |
Bacteraemic VAP and osteomyelitis |
Pseudomonas aeruginosa MIC: 2 mg/L |
CVVHDF | AN-69 high-flux M100 |
50% pre-filter 50% post-filter |
Qd: 1000 Quf: 750 |
200 |
NA 50 mL/day |
Clinical cure (death occurred for non-infective complications) |
|
Ceftolozane–tazobactam (Oliver et al. [27]) |
Case report | 1 | 61/M | 78.8 |
1.5 g q8h (EI 4 h) |
Osteomyelitis |
Pseudomonas aeruginosa MIC: 1.5 mg/L |
CVVH |
AN-69 high-flux M150 (surface area 1.5 m2) |
NA | 2000 | 250 | NA | Clinical cure |
|
Ceftolozane–tazobactam (Aguilar et al. [30]) |
Case report | 1 | 68/NA | NA |
3 g q8h (1-h infusion) |
cIAI | NA | CVVHD | Polysulphone membrane | NA |
Qd: 2000 Quf: 1000 |
100 |
NA Anuric |
Clinical cure |
|
Ceftolozane–tazobactam (Carbonell et al. [29]) |
Case report | 1 | 37/F | NA |
3 g q8h (3-h infusion) |
CR-BSI | Pseudomonas aeruginosa |
CVVHDF + MARS |
AN-69 high-flux M150 (surface area 1.5 m2) |
NA |
Qd: 1600 Quf: 500 |
180 | NA | Clinical failure |
|
Ceftolozane–tazobactam (Butragueño-Laiseca et al. [33]) |
Case report | 1 | 9 months | 5.8 | 30 mg/kg q8h | BSI |
Pseudomonas aeruginosa Escherichia coli ESBL MIC: 4 mg/L |
CVVHDF | NA | NA |
Qd: 250 Quf: 220 |
30 mL/min |
NA Anuric |
NA |
|
Ceftolozane–tazobactam (Mahmoud et al. [31]) |
Case report | 1 | 35/M | 187 | 3 g q8h CI | VAP |
Pseudomonas aeruginosa MIC: 2/4 mg/L |
CVVHDF | HF1400 polyarylethersulfone haemofilter | 100% post-filter |
Qd: 2000 Quf: 2000 |
250 |
25 Anuric |
NA |
|
Ceftazidime–avibactam (Wenzler et al. [35]) |
Case report | 1 | 53/F | NA |
1.25 g q8h (2-h infusion) |
Bacteraemic VAP |
Pseudomonas aeruginosa MIC: 6/4 mg/L |
CVVH |
Polyethersulfone membrane filter (surface area 1.6 m2) |
100% pre-filter | 2000 | 200 | NA | NA |
|
Ceftazidime–avibactam (Soukup et al. [34]) |
Case report | 1 | 50/M | 94 |
2.5 g q8h (2-h infusion) |
VAP |
Pseudomonas aeruginosa MIC 8 mg/L |
CVVHDF | M100 filter |
80% pre-filter 20% post-filter |
Qd: 1500 Quf: 1250 |
250 |
30–73 Anuria |
Clinical cure |
|
Meropenem–vaborbactam (Kufel et al. [36]) |
Case report | 1 | 60/M | 76 |
1 g/1 g q8h (3-h infusion) |
Joint infection |
Carbapenem- resistant Klebsiella pneumoniae MIC 0.094/8 mg/L |
CVVHD |
Polyethersulfone membrane filter (surface area 1.6 m2) |
NA | 3000 | 250 |
NA No residual diuresis |
Clinical failure |
|
Fosfomycin (Gattringer et al. [26]) |
PK study | 12 |
68 ± 8 10M, 2F |
78.7 ± 13.4 | 8 g q12h |
4 pneumonia 2 septic shock 2 endocarditis 2 cardiac failure 1 cIAI 1 aortic aneurysm |
2 Staphylococcus aureus 1 Streptococcus mitis 1 Pseudomonas aeruginosa |
CVVH |
Polyethylene membrane filter (surface area 1.2 m2) |
NA | 1966.7 ± 336 | 180 | NA | NA |
|
Ceftaroline (Kalaria et al. [25]) |
PK study | 4 |
52.3 ± 18.3 1M, 3F |
89.1 ± 19.8 |
300-600 mg q12h (1-h infusion) |
2 BSI 1 Endocarditis 1 HAP |
3 MRSA (MIC not provided) |
2 CVVHD 2 CVVDHF |
AN-69 high-flux M150 (surface area 1.5 m2) |
100% pre-filter | 3190 ± 510.3 |
250 (3 cases) 300 (1 case) |
125 ± 86.6 NA |
Clinical cure rate: 100% |
|
Ceftobiprole (Cojutti et al. [37]) |
Case report | 1 | 48/M | 92 |
250 mg q12h (2-h infusion) |
HCAP |
Empirical (MRSA EUCAST breakpoint 2 mg/L) |
CVVHDF | HF1400 polyarylethersulfone haemofilter |
75% pre-filter 25% post-filter |
Qd: 1500 Quf: 2000 |
150 | NA |
Clinical cure (death occurred for non-infective complications) |
|
Dalbavancin (Corona et al. [38]) |
Case report | 1 | 58/M |
BMI: 31.1 kg/m2 |
1500 mg single dose | NSTI |
Staphylococcus epidermidis MIC: 0.05 mg/L |
NA | NA | NA | NA | NA | NA | Clinical cure |
BMI body mass index, BSI bloodstream infection, cIAI complicated intra-abdominal infection, CI continuous infusion, CR-BSI catheter-related bloodstream infection, CVVH continuous venovenous haemofiltration, CVVHD continuous venovenous haemodialysis, CVVHDF continuous venovenous haemodiafiltration, CRRT continuous renal replacement therapy, EI extended infusion, ESBL extended spectrum β-lactamases, EUCAST European Committee on Antimicrobial Susceptibility Testing, F female, HCAP healthcare-associated pneumonia, HAP hospital-acquired pneumonia, M male, MARS methicillin-resistant Staphylococcus aureus, MIC minimum inhibitory concentration, MRSA methicillin-resistant Staphylococcus aureus, NA not available, NSTI necrotizing soft tissue infection, PK pharmacokinetic, Quf ultrafiltrate rate, Qd dialysate rate, q8h every 8 VAP ventilator-associated pneumonia
aData are expressed as mean ± standard deviation
Table 3.
Pharmacokinetic parameters of novel agents retrieved in patients undergoing continuous renal replacement therapy
| Novel agent (study reference) | Dose |
Cmax (mg/L) |
Cmin (mg/L) |
Vd (L) |
CLtot (L/h) |
CLCRRT (L/h) |
CLCRRT/total CL ratio |
t½ (h) |
AUC (mg × h/L) |
Sieving or saturation coefficient | PK/PD target attainment |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ceftolozane–tazobactam (Sime et al. 2019 [24]) |
1.5 g q8h (1-h infusion) |
LOZ: 54 (47–78) TAZ: 17.5 (16.4–20.2) |
LOZ: 28 (21-42) TAZ: 6.1 (5.5–6.7) |
LOZ: 73.4 ± 39.0 TAZ: 77.2 ± 32.3 |
LOZ: 3.52 ± 0.6 TAZ: 6.1 ± 0.8 |
LOZ: 2.92 ± 0.6 TAZ: 2.85 ± 0.6 |
LOZ: 83.0% TAZ: 46.7% |
LOZ: 14.5 TAZ: 8.8 |
LOZ: 284.1 TAZ: 82.0 |
LOZ: 0.94 ± 0.24 TAZ: 1.08 ± 0.3 |
3 g LD followed by 0.75 g q8h achieved a PK/PD target of 100%fT>MIC according to the CRRT setting of the study |
|
Ceftolozane–tazobactam (Kuti et al. [28]) |
3 g q8h (1-h infusion) |
LOZ: 127.2 TAZ: 40.6 |
LOZ: 43.3 LOZ: 6.7 (ELF) TAZ: 8.4 TAZ: 1.7 (ELF) |
LOZ: 17.9 TAZ: 19.2 |
LOZ: 2.6 TAZ: 4.9 |
NA | NA |
LOZ: 4.7 TAZ: 2.7 |
LOZ: 1153.8c TAZ: 612.2c |
NA |
LOZ: 100%fT>57.7×MICb TAZ: 100%T>4 mg/Lb LOZ (ELF): 100%T>8.93×MIC |
|
Ceftolozane–tazobactam (Bremmer et al.) [32] |
3 g q8h (1-h infusion) |
LOZ: 163.9 TAZ: 35.9 |
LOZ: 79.4 TAZ: 13.1 |
LOZ: 55.7c TAZ: 92.0c |
LOZ: 2.9 TAZ: 7.5 |
LOZ: 2.4 TAZ: 2.72 |
LOZ: 82.8% TAZ: 36.3% |
LOZ: 13.3 TAZ: 8.5 |
LOZ: 689 TAZ: 132.5 |
NA |
LOZ: 100%fT>39.7×MICb TAZ: 100%T>4 mg/Lb |
|
Ceftolozane–tazobactam (Oliver et al. [27]) |
1.5 g q8h (EI 4 h) |
LOZ: 38.57 TAZ: 10.94 |
LOZ: 31.63 TAZ: 7.81 |
LOZ: 153.0c TAZ: 273.9c |
LOZ: 3.52c TAZ: 6.76c |
NA | NA |
LOZ: 30.7 TAZ: 28.1 |
LOZ: 284.4 TAZ: 74.0 |
NA |
LOZ: 100%fT>21.1×MICb TAZ: 100%T>4 mg/Lb |
|
Ceftolozane–tazobactam (Aguilar et al. [30]) |
3 g q8h (1-h infusion) |
LOZ: 53.0 TAZ: 14.5 |
LOZ: 25.8 TAZ: 5.1 |
LOZ: 97.5 TAZ: 194.2 |
LOZ: 5.4 TAZ: 17.4 |
NA | NA |
LOZ: 12.6 TAZ: 7.8 |
LOZ: 373 TAZ: 57.6 |
NA |
LOZ: 100%fT>6.4×MICd TAZ: 100%T>4 mg/L |
|
Ceftolozane–tazobactam (Carbonell et al. [29]) |
3 g q8h (3-h infusion) |
LOZ: 112.7 | LOZ: 51.44 | LOZ: 24.0 | LOZ: 3.39 | NA | NA | LOZ: 5.3 | LOZ: 589 | NA | LOZ: 100%fT>12.86×MICd |
|
Ceftolozane–tazobactam (Butragueño-Laiseca et al. [33]) |
30 mg/kg q8h | LOZ: 75.8 | LOZ: 18.1 | LOZ: 1.91 | NA | LOZ: 0.39 | NA | LOZ: 3.51 | LOZ: 448.72 |
LOZ: 0.99–1.14 |
LOZ: 100%fT>4.53×MICb |
|
Ceftolozane–tazobactam (Mahmoud et al. [31]) |
3 g q8h CI |
LOZ (Css mean): 44.9 TAZ (Css mean): 18.9 |
NA |
LOZ: 5.6 TAZ: 6.6 |
LOZ: 4.15 TAZ: 4.27 |
LOZ: 74.1% TAZ: 64.7% |
NA |
LOZ: 359 TAZ: 151 |
LOZ: 0.88 ± 0.02 TAZ: 0.9 ± 0.02 |
LOZ: 100%fT>17.96×MIC TAZ: 100%T>4 mg/L |
|
|
Ceftazidime–avibactam (Wenzler et al. [35]) |
1.25 g q8h (2-h infusion) |
CAZ: 61.1 AVI: 14.5 |
CAZ: 32.0 AVI: 8.45 |
CAZ: 27.23 AVI: 30.81 |
CAZ: 2.87 AVI: 2.92 |
CAZ: 1.51 AVI: 1.52 |
CAZ: 57.1% AVI: 54.3% |
CAZ: 6.07 AVI: 6.78 |
CAZ: 347.9 AVI: 85.7 |
CAZ: 0.96 AVI: 0.93 |
CAZ: 100%T>5.33×MICb AVI: 100%T>4 mg/Lb |
|
Ceftazidime–avibactam (Soukup et al. [34]) |
2.5 g q8h (2-h infusion) |
CAZ: 152.39 AVI: 35.83 |
CAZ: 70 AVI: 17.2 |
CAZ: 11.51 AVI: 12.44 |
CAZ: 1.54 AVI: 1.45 |
NA | NA |
CAZ: 5.17 AVI: 5.92 |
CAZ: 1295.4 AVI: 343.4 |
NA |
CAZ: 100%T>8.75×MICb AVI: 100%T>4 mg/Lb |
|
Meropenem–vaborbactam (Kufel et al. [36]) |
1 g/1 g q8h (3-h infusion) |
MER: 35.0 VAB: 44.1 |
MER: 7.5 VAB: 17.2 |
MER: 50.28c VAB: 83.9c |
MER: 5.48c VAB: 3.44c |
NA | NA |
MER: 6.38 VAB: 16.81 |
MER: 182.42 VAB: 290.65 |
NA |
MER: 100%T>79.8×MICb VAB: AUC/MIC = 36.33b |
|
Fosfomycin (Gattringer et al. [26]) |
8 g q12h | 442.7 ± 124 | 103.1 ± 36.6 | 33.7 ± 12.7 | 6.4 ± 7.7 | 1.1 ± 0.2 | 76.7% ± 6.2% | 12.1 ± 5.2 | 2159.4 ± 609.8 | 0.7 ± 0.1 | 100%T>4×MIC achieved for MIC up to 16 mg/L |
|
Ceftaroline (Kalaria et al. [25]) |
300–600 mg q12h (1-h infusion) |
12.5 ± 2.4 | 2.86 ± 1.62 | 41.8 ± 16.1 | 6.68 ± 1.04 | 2.52 ± 0.60 | 35.3% ± 5.8% | 4.13 ± 1.59 | 58.3 ± 18.2 | 0.81 ± 0.1 | 100%T>MIC achieved in 75% of patients; none achieved an aggressive target of 100%T>4–5×MICb |
|
Ceftobiprole (Cojutti et al. [37]) |
250 mg q12h (2-h infusion) |
9.21 | 2.82 | 21.17 | 2.98 | NA | NA | 4.93c | 83.89c | NA | 100%T>1.41×MICb |
|
Dalbavancina (Corona et al. [38]) |
1500 mg single dose | 55.1 | NA | 27.2 | 0.334 | NA | NA | 56.8c | 4491 | NA | AUC/MIC = 89,820b |
Considering 20% protein binding for ceftolozane: 100%fT> 46.19 × MIC, 100%fT> 31.76 × MIC, 100%fT> 16.9 × MIC, and 100%fT> 3.62 × MIC []; considering 22% protein binding for tazobactam: 100%fT > 6.55 mg/L [], 100%fT > 6.01 mg/L [], 100%fT > 10.21 mg/L []. Considering 10% protein binding for ceftazidime: 100%fT> 4.79 × MIC [], and 100%fT> 7.88 × MIC []; considering 8% protein binding for avibactam: 100%fT > 7.77 mg/L [], and 15.8 mg/L []. Considering 2% protein binding for meropenem: 100%fT> 78.19 × MIC; considering 33% protein binding for vaborbactam: AUC/MIC = 24.34. Considering 20% protein binding for ceftaroline: the target of 100%fT> MIC was achieved in only 50% of cases. Considering 16% protein binding for ceftobiprole: 100%fT> 1.18 × MIC. Considering 93% protein binding for dalbavancin: fAUC/MIC = 6287 (optimal PK/PD target achieved)
AUC area under the concentration-time curve, AVI avibactam, CAZ ceftazidime, CI continuous infusion, CL clearance, CLCRRT continuous renal replacement therapy clearance, CLtot total clearance, Cmax peak concentration, Cmin trough concentration, CRRT continuous renal replacement therapy, Css mean mean concentration at steady state, EI extended infusion, ELF epithelial lining fluid, LD loading dose, LOZ ceftolozane, MER meropenem, MIC minimum inhibitory concentration, PK/PD pharmacokinetic/pharmacodynamic, qxh every x h, TAZ tazobactam, t½ half-life, VAB vaborbactam, Vd volume of distribution
aPK analysis was performed at day 8 after dalbavancin administration
bFree concentration was not calculated
cNot provided in original articles and calculated according to the formulae reported in the Methods section
dConsidering an MIC of 4 mg/L (clinical breakpoint) in the absence of isolates
Table 4.
Percentage difference in pharmacokinetic parameter values observed in patients undergoing continuous renal replacement therapy compared with healthy volunteers
| Antibiotic | Cmax (mg/L) | Vd (L/Kg) | t½ (h) | AUC (mg × h/L) | CL (L/h) |
|---|---|---|---|---|---|
| Ceftaroline | 45.8%a | 205.9% | 190.3% | 93.0%a | 79.1% |
| Ceftazidime–avibactam |
CAZ: 122.8% AVI: 133.2% |
CAZ: 58.7% AVI: 57.3% |
CAZ: 261.1% AVI: 372.3% |
CAZ: 515.1% AVI: 796.8% |
CAZ: 19.4% AVI: 12.5% |
| Ceftolozane–tazobactam |
LOZ: 74.2% TAZ: 103.0% |
LOZ: 551.9% TAZ: 275.7% |
LOZ: 467.7% TAZ: 800.0% |
LOZ: 123.5% TAZ: 275.2% |
LOZ: 81.9% TAZ: 36.7% |
| Ceftobiprole | 31.5%b | 121.0% | 159.0% | 80.7%b | 60.9% |
| Dalbavancin | 22.1%c | 353.2% | 14.8% | 18.3% | 795.2% |
| Fosfomycin | 177.9% | 107.0% | 432.1% | 204.5% | 82.1% |
| Meropenem–vaborbactam |
MER: 127.3% VAB: 158.6% |
MER: 256.5% VAB: 479.4% |
MER: 490.8% VAB: 1,018.8% |
MER: 209.4% VAB: 292.4% |
MER: 43.8% VAB: 31.0% |
AUC area under the concentration-time curve, AVI avibactam, CAZ ceftazidime, CL clearance, Cmax peak concentration, CRRT continuous renal replacement therapy, LOZ ceftolozane, MER meropenem, TAZ tazobactam, t½ half-life, VAB vaborbactam, Vd volume of distribution
aA different dosage was administered in CRRT patients (400 mg) compared with healthy volunteers (600 mg)
bA different dosage was administered in CRRT patients (250 mg) compared with healthy volunteers (500 mg)
cA different dosage was administered in CRRT patients (1500 mg) compared with healthy volunteers (1000 mg)
The total number of critically ill patients in whom some PK features of novel agents were assessed during CRRT was 34. Most studies investigated ceftolozane–tazobactam, whereas, to date, no real-world evidence related to the use of cefiderocol or imipenem–relebactam.
Ceftolozane–Tazobactam
One population PK study and seven case reports assessed the PK behaviour of ceftolozane–tazobactam in 13 different critically patients requiring CRRT, including one child [24, 27–33]. Different dosing schedules (1.5 g or 3 g every 8 h) were administered. Extended infusion (EI; in 3 or 4 h) and continuous infusion (CI) were adopted in two cases and one case, respectively. Pseudomonas aeruginosa represented the most frequently isolated pathogen (8 of 13 patients), and pneumonia and bloodstream infections (BSIs) were the most represented types of infection. CVVHDF was performed in 84.6% of patients. The effluent flow rate ranged from 1200 to 4000 mL/h, and in only four cases the flow rate was ≥ 3 L/h. All patients were anuric, with a residual diuresis of a maximum of 76 mL/day (Table 2).
PK parameters for both ceftolozane and tazobactam are shown in Table 3. Ceftolozane and tazobactam trough concentrations ranged from 18.1 to 79.4 mg/L and from 5.1 to 18.9 mg/L, respectively, while the ratio between CLCRRT and total CL ranged from 74.1 to 83.0% for ceftolozane and from 36.3 to 64.7% for tazobactam. SA was 0.88–0.99 for ceftolozane and 0.90–1.08 for tazobactam. Sime et al. [24] performed a population PK study in six critically ill patients treated with ceftolozane–tazobactam 1.5 g every 8 h in intermittent infusion regimens and undergoing CVVHDF, and found that a loading dose (LD) of 3 g followed by a 750 mg every 8 h CI could be adequate in achieving 100%T> MIC as the PK/PD target. However, it should be mentioned that the mean effluent flow rate was quite low (< 2.5 L/h) and none of the patients had residual diuresis. Notably, Aguilar et al. [30] reported that an intermittent infusion of ceftolozane–tazobactam 3 g every 8 h achieved an optimal PK/PD target (100%fT> 4 × MIC) in one patient undergoing CVVHD with an effluent flow rate of 3 L/h. Similarly, Mahmoud et al. [31] found that a high-dose CI of ceftolozane–tazobactam (3 g every 8 h) reached an optimal PK/PD target in a critically obese (187 kg) patient requiring CVVHDF with a high effluent flow rate (4 L/h). Prolonged infusion and/or high-dose (3 g every 8 h) ceftolozane–tazobactam achieved an aggressive PK/PD target in patients undergoing CVVH or CVVHDF with highly adsorptive membranes [27–29, 32]. However, it is worth noting that the effluent flow rate was below 2 L/h in all patients, meaning that a higher dose could be needed in patients undergoing CRRT characterized by highly adsorptive membranes and/or greater effluent flow rates (> 2.5–3 L/h).
The comparison of ceftolozane–tazobactam PK parameters between patients undergoing CRRT and healthy subjects (Table 4) showed a slight increase in AUC (1.23-fold) coupled with a negligible reduction in total CL (below 20%). Notably, a fivefold increase in ceftolozane Vd was found, possibly reflecting the remarkable PK alterations commonly reported in critically septic patients [39].
Ceftazidime–Avibactam
Only two case reports assessed the PK behaviour of ceftazidime–avibactam in critically ill patients requiring CRRT [34, 35]. Wenzler et al. [35] found that ceftazidime–avibactam 1.25 g every 8 h administered in 2-hourly infusions achieved an optimal PK/PD target in one patient affected by bacteraemic ventilator-associated pneumonia (VAP) due to XDR Pseudomonas aeruginosa and undergoing supportive treatment with CVVH. The ratio between continuous renal replacement therapy clearance (CLCRRT) and total CL was 57.1% for ceftazidime and 54.3% for avibactam. SC was 0.96 for ceftazidime and 0.93 for avibactam, and the effluent flow rate was set at only 2 L/h. Conversely, Soukup et al. [34] found that ceftazidime–avibactam full-dose (2.5 g every 8 h in 2-hourly infusions) achieved an aggressive PK/PD target in one anuric patient affected by Pseudomonas aeruginosa VAP undergoing CVVHDF. The effluent flow rate was set to 2750 mL/h, and trough ceftazidime and avibactam concentrations were 70 mg/L and 17.2 mg/L, respectively. Unfortunately, CLCRRT and SA measurements were not provided.
Notably, a significant increase in exposure was reported for both ceftazidime (up to fivefold) and avibactam (approximately eightfold) in a CRRT setting compared with healthy subjects. Total CL in CRRT patients was significantly reduced versus healthy subjects (below 20% for both ceftazidime and avibactam) (Table 4).
Meropenem–Vaborbactam
Only one case report assessed the PK behaviour of meropenem–vaborbactam in one anuric critically ill patient undergoing CVVHD during treatment of a joint infection due to carbapenemase-producing Klebsiella pneumoniae [36]. Qd was set to 3 L/h and a polyethersulfone membrane filter (surface area 1.6 m2) was used. The administration of a halved-dose (1 g/1 g every 8 h in 3-hourly EIs) allowed the achievement of an optimal PK/PD target for both meropenem and vaborbactam. Trough meropenem and vaborbactam concentrations were 7.5 mg/L and 17.2 mg/L, respectively. Unfortunately, CLCRRT and SA measurements were not provided. Interestingly, by means of an ex vivo model, Sime et al. [40] calculated the SC of meropenem–vaborbactam, resulting in an SC of 0.97–1.13 for meropenem and 0.64–0.78 for vaborbactam.
A significant AUC increase in meropenem (up to twofold) and vaborbactam (approximately threefold) was reported during CRRT compared with healthy subjects. Total CL in CRRT patients was significantly reduced (approximately 43% and 31% for meropenem and vaborbactam, respectively). Notably, Vd was increased by 2.5-fold for meropenem and 4.8-fold for vaborbactam, which could be due to the remarkable PK alterations commonly reported in critically septic patients [39] (Table 4).
Fosfomycin
A PK population study assessed the PK behaviour of fosfomycin among 12 critically ill patients undergoing CVVH [26]. Fosfomycin was administered at a dosage of 8 g every 12 h and pneumonia was the most frequent type of infection. Mean Quf was 1966.7 ± 336 mL/h, and a polyethylene membrane filter (surface area 1.2 m2) was used. The mean ratio between CLCRRT and total CL of fosfomycin was 76.7% ± 6.2%, resulting in an SC of 0.7 ± 0.1. Mean trough serum concentrations were 103.1 ± 36.6 mg/L, and all patients achieved an optimal PK/PD target (100%fT> 4 × MIC) for an MIC of up to 16 mg/L.
Compared with healthy volunteers, negligible alterations in both Vd (1.07-fold increase) and CL (below 20%) of fosfomycin were observed in critically ill patients undergoing CRRT compared with healthy subjects (Table 4).
According to some studies that support administration in prolonged or continuous infusions to maximize the PK/PD target [41, 42], fosfomycin at an EI/CI dosage of 16 g/day could be suggested for achieving the optimal PK/PD target in the presence of pathogens with higher MICs, especially in patients with residual diuresis or undergoing a higher effluent flow rate (> 2.5–3 L/h).
Ceftaroline
A population PK study assessed the PK behaviour of ceftaroline among four critically septic patients undergoing CRRT [25]. Ceftaroline was administered at a dosage of 300–600 mg every 12 h, and in three out of four patients, MRSA was isolated from blood cultures. CVVHD and CVVHDF were performed in two cases each. The mean effluent flow rate was 3190 ± 510.3 mL/h, and a highly adsorptive membrane (AN-69 high-flux M150 with a surface area of 1.5 m2) was adopted. The mean ratio between CLCRRT and total CL of ceftaroline was 35.3% ± 5.8%, resulting in an SC of 0.81 ± 0.1, and mean trough serum concentrations were 2.86 ± 1.62 mg/L. Considering the EUCAST clinical breakpoint for MRSA, no patients achieved the optimal PK/PD target (100%fT> 4 × MIC), and only half of the patients achieved the conservative PK/PD target of 100%fT> MIC.
A twofold increase in Vd coupled with a slight reduction in total CL (approximately 20%) of ceftaroline was observed in critically ill patients requiring CRRT compared with healthy volunteers (Table 4).
The authors recommended a ceftaroline dosing regimen of 400 mg every 12 in patients undergoing CRRT with an effluent flow rate of 3 L/h. However, it should be highlighted that in those patients with residual renal function, a full dose (600 mg every 12 h) coupled with prolonged infusion up to 12 h [43] could be needed to achieve more aggressive PK/PD targets.
Ceftobiprole
Only one case report assessed the PK behaviour of ceftobiprole in a critically ill patient affected by healthcare-associated pneumonia and undergoing CVVHDF [37]. No pathogen was isolated from both blood and respiratory cultures. The effluent flow rate was set to 3500 mL/h and a polyarylethersulfone membrane haemofilter was used. A conservative PK/PD target of 100%fT>MIC was achieved by administering ceftobiprole at a reduced dosage of 250 mg every 12 h (one-third of the full dosage). Trough ceftobiprole concentrations were 2.82 mg/L. Unfortunately, CLCRRT and SA were not assessed.
A 1.21-fold increase in Vd coupled with a moderate reduction in total CL (approximately 40%) of ceftobiprole was found among critically ill patients requiring CRRT compared with healthy volunteers (Table 4).
It should be noted that higher dosages (up to 500 mg every 12 h) administered by prolonged infusion (at least 2 h) could be needed in order to achieve an aggressive PK/PD target, especially in patients with residual renal function or when adopting an effluent flow rate > 3 L/h in the post-filter dilution mode.
Dalbavancin
Only one case report assessed the PK behaviour of dalbavancin in a critically ill patient requiring CRRT who was affected by necrotizing soft tissue infection due to Staphylococcus epidermidis [38]. Unfortunately, CRRT modality and settings were not provided. At day 7 after administration of a single 1500 mg dose, the PK/PD target was optimal (fAUC/MIC > 111.4).
Notably, a significantly lower t½ and total exposure (AUC) coupled with a remarkable increase in total CL (up to eightfold) of dalbavancin were estimated among the CRRT patients compared with healthy subjects (Table 4). This could be due to the underlying severe hypoalbuminaemia that affected this patient [38].
Imipenem–Relebactam
Currently, no real-world evidence regarding the administration of imipenem–relebactam during CRRT exists; however, imipenem–relebactam CL during CRRT was assessed in validated bovine models of continuous haemofiltration and continuous haemodialysis testing different ultrafiltrate and dialysate flow rates [44]. Both imipenem and relebactam were not removed by adsorption, readily crossing the haemodiafilter membrane in the two different modalities. Transmembrane CL of both imipenem and relebactam approximated the effluent rates [44].
Cefiderocol
Currently, no real-world evidence regarding the administration of cefiderocol during CRRT exists; however, cefiderocol is the first antibiotic that had dosing recommendations reported in the summary of product characteristics. Moreover, CRRT cefiderocol CL was predicted according to the CRRT CL reported for cefepime and by adjusting for the difference in unbound fraction [45]. This approach was implemented according to the similarity of the physicochemical and PK features shared by the two agents. The probability of target attainment was >90% against pathogens with an MIC ≤ 4 mg/L by considering a dose regimen of 1 g every 12 h (3-hourly infusions) during CVVH, and 1.5 g every 12 h (3-hourly infusions) during CVVHD and CVVHDF [45].
Discussion
We provided a comprehensive overview of the evidence regarding the PK features of novel agents targeted for MDR/XDR Gram-positive and Gram-negative pathogens in patients undergoing CRRT. Overall, evidence is limited to small population PK studies and a few case reports. Notably, wide variations in CRRT modalities and settings were found and this might limit the generalizability of these findings to different clinical scenarios.
Although it is well-known that the ‘one dose fits all’ approach may be hazardous in the critical care setting [46, 47], the implementation of CRRT in severely ill patients needs more attention in order to individualize antibiotic therapy. A ‘patient-centred’ approach is required and should consist of a 360° assessment of the critical septic patient undergoing CRRT for guidance in antibiotic dosage adjustment. Consequently, the development of dedicated guidelines for dosage adjustment based on a ‘drug approach’ could not fit the clinical needs. An a priori antimicrobial dosage reduction in patients undergoing CRRT should no more be considered appropriate, as reported in different studies [9, 48], and the need for full β-lactam doses is not an uncommon requirement. In this regard, high-doses of ceftolozane–tazobactam or ceftazidime–avibactam (3 g every 8 and 2.5 g every 8 h) in a prolonged infusion demonstrated achievement of the optimal PK/PD target during CRRT [29–31, 34]. The achievement of aggressive PK/PD targets (100%fT> 4 × MIC) is fundamental, for both improving clinical outcomes and limiting the development of resistance [18, 19]. In this regard, it should not be overlooked that the occurrence of breakthrough resistance to ceftazidime–avibactam during CRRT was reported [17].
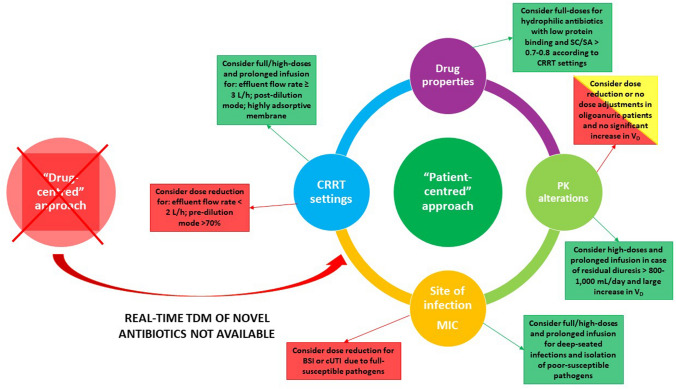
Four major determinants should guide antimicrobial dose adjustment during CRRT (Fig. 2): (1) physicochemical and PK features of novel agents; (2) CRRT modalities, settings and typology of filter; (3) PK alterations of critically ill patients (large increase in Vd and residual renal function); and (4) site of infection and MIC of the pathogen.
Fig. 2.
‘Patient-centred’ approach for dosing adjustment of novel antibiotics in critically ill patients during continuous renal replacement therapy. BSI bloodstream infection, cUTI complicated urinary tract infection, CRRT continuous renal replacement therapy, MIC minimum inhibitory concentration, PK pharmacokinetic. SA saturation coefficient, SC sieving coefficient, TDM therapeutic drug monitoring, Vd volume of distribution
Physicochemical and PK Features of Specific Antibiotics
Physicochemical and PK features represent a critical determinant involved in the extent of CRRT elimination of each novel agent. Molecular weight, hydrophilicity, electric charge, protein binding, and Vd of the selected antimicrobial should be considered in patients requiring CRRT [4, 49]. With the exception of dalbavancin, all of the novel agents developed for the management of MDR Gram-positive or Gram-negative infections are characterized by small molecular weight and size (ranging from 138 Da for fosfomycin to 966 Da for ceftolozane–tazobactam), high hydrophilicity (resulting in a LogP ranging from − 1.3 for ceftobiprole to 1.84 for cefiderocol), low protein binding (generally below 20%), and limited Vd (lower than 0.3 L/kg). These specific features make novel agents highly prone to remarkable CRRT elimination.
Considering that only the free fraction of a drug may be removed by CRRT and that the SC (i.e. the ratio of ultrafiltrate to serum antibiotic concentrations) correlates well with free antibiotic fraction in convective CRRT modalities (i.e. CVVH), novel antibiotics usually exhibit a high SC (> 0.7–0.8) [4, 50]. Similarly, also in diffusive CRRT modalities (i.e. CVVHD or CVVDHF), the SA correlates with the free moiety, although it depends more on the molecular weight as it usually decreases (leading to lowering in drug CL) for agents with a molecular weight > 1000 to 1500 Da [4, 50]. Among novel antibiotics, only dalbavancin showed these characteristics [51].
Furthermore, a close relationship between molecular weight and membrane characteristics may exist. In case of ‘low-flux’ membrane use, CL could be negligible for antimicrobials with a molecular weight > 1000 Da. Conversely, when ‘high-flux’ membranes are adopted, removal is also significant for antimicrobials with a molecular weight > 1000 Da in diffusive CRRT modalities [4, 52].
CRRT Modalities, Settings and Filter Types
Several CRRT factors, including CRRT modalities, settings and filter type, may significantly affect antibiotic CL [5]. The three main modalities of CRRT are represented by CVVH, CVVHD and CVVHDF, based on convective, diffusive, or mixed depurative techniques, respectively. While antibiotic CL during haemofiltration is directly proportional to both SC and ultrafiltration rate, during diffusive modalities CL estimation is more challenging due to the large variability of SA [4]. Indeed, significant variations may also occur for the SC of highly bound antimicrobials (e.g. dalbavancin or daptomycin), especially in critically ill patients affected by severe hypoalbuminaemia in whom the SC could not always reflect the theoretically unbound fraction of the antimicrobial agent [49]. Additionally, the type of dilution modality may significantly affect antimicrobial CL. In the post-dilution mode, the plasma directly crosses the membrane, and antimicrobial CL depends on the SC and ultrafiltration rate. Conversely, in the pre-dilution mode, the plasma is diluted by the addition of the replacement fluid before passing through the filter, and antimicrobial CL will be lower due to the dilution factor [5, 49]. Notably, when all other parameters are equal, the efficiency of antimicrobial removal is expected to be higher with CVVHDF compared with CVVH [49]. Roger et al. found a trend toward significant higher piperacillin total and CRRT CL, along with lower mean steady-state concentrations in patients undergoing CVVHDF compared with those undergoing CVVH [53].
As previously reported, the ultrafiltration rate (for convective modality) and the effluent flow rate (for diffusive/mixed modalities) are directly involved in the determination of antimicrobial CL [4, 5, 49]. The impact of high-intensity CRRT on antimicrobial dosing adjustment, especially for agents undergoing highly relevant CRRT removal, is much-debated [7, 54]. Different studies reported a linear relationship between effluent flow rate and total CL and/or CRRT CL for several antibiotics, namely meropenem, vancomycin, piperacillin–tazobactam, and ceftolozane–tazobactam [55–57]. Additionally, a significative relationship between effluent flow rate and CL was demonstrated in ex vivo models for ceftolozane–tazobactam, meropenem–vaborbactam, and dalbavancin [40, 58, 59]. Consequently, in patients undergoing high-intensity CRRT, altered dosing strategies of novel agents (full/high doses coupled with prolonged infusion) could be needed [14]. This is documented by different case reports involving ceftolozane–tazobactam [30, 31] or ceftazidime–avibactam [34], in which full doses or prolonged infusion were required to achieve optimal PK/PD targets when using an effluent flow rate >2.5–3 L/h. Conversely, administration of reduced doses of ceftaroline or ceftobiprole to patients requiring high-intensity CRRT failed in achieving the optimal PK/PD target [25, 37].
CRRT membrane types (e.g. polysulfone, polymethylmethacrylate and polyacrylonitrile membranes) have a relevant impact on antimicrobial CL according to the different adsorptive ability [5, 60]. Adsorptive capacity is high for AN69 surface-treated (ST) membrane, and negligible for polysulphone membrane [61, 62]. Even the surface area of the CRRT membrane has a relevant role in drug adsorption [5]. Ulldemolins et al. [63] found that in patients undergoing CVVHDF with 1.5 m2 AN69-ST membrane, the dose of piperacillin–tazobactam required to maintain a PK/PD target of 100%fT> MIC was double compared with those undergoing CVVHDF with a 0.9 m2 AN69-ST membrane. This example shows that membrane characteristics should also be taken into account when adjusting antimicrobial dose during CRRT, especially when highly adsorptive membranes with a large surface area are used (i.e. AN69-ST) [62].
PK Alterations of Critically Ill Patients
Regardless of specific CRRT settings and modalities, two main factors may directly affect the PK profile of antimicrobials in critically ill patients with severe AKI, namely an increase in extracellular fluids and residual renal function [64]. Considering that most of the novel agents are hydrophilic and have limited Vd (approximately < 15–20 L), the so-called ‘third spacing’ phenomenon commonly reported in septic shock may also strongly affect the achievement of adequate antibiotic concentration serum and tissue during CRRT [64, 65]. As reported in our analysis (Table 4), the Vd of several agents was significantly increased in CRRT patients, meaning that the magnitude of fluid load and/or the presence of a generalized oedematous state (potentially derived from the amount of fluid removal with CRRT) should be taken into account for proper adjustment of the LD.
Patients undergoing CRRT may sometimes also exhibit some degree of preserved residual renal function, defined by the absence of oliguria and a urine output ≥ 0.5 mL/kg/h according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria [66]. This may have an additive effect on CRRT CL. Some studies [67, 68] found a close relationship between residual renal function and the need for altered dosing strategies (higher doses or prolonged infusion) for achieving optimal PK/PD targets in CRRT patients treated with carbapenems. This need could theoretically be extended to all the other β-lactams with similar physicochemical and PK properties, including novel agents. Consequently, in critically ill patients undergoing CRRT directly, direct measurement of creatinine CL by means of 8-, 12-, or 24-h urine collection, rather than estimation by means of available formulas, is highly advisable [69] for accurate assessment of residual renal function. Unfortunately, data regarding residual renal function and/or net fluid removal during CRRT were poorly reported in studies investigating the PK of novel agents in CRRT patients (Table 2). Relevant residual diuresis was never reported, and high mean fluid removal ≥ 3000 mL/day during CRRT was only reported in the studies by Sime et al. [24] and Kalaria et al. [25].
Site of Infection
PK/PD issues directly associated with the specific infection should be considered for antibiotic dose adjustment in CRRT patients. Tissue penetration of a selected agent depends on its physicochemical properties and the site of infection. For example, higher doses of hydrophilic antimicrobials compared with lipophilic drugs are required for the management of VAP compared with BSI, considering the limited penetration rate into deep-seated infections [64]. Consequently, dose optimization of novel antibiotics during CRRT, by virtue of their hydrophilic properties, should closely consider this aspect.
Minimum Inhibitory Concentration of the Isolated Pathogens
Novel antibiotics are mainly used for targeted therapy of infections caused by MDR Gram-positive and Gram-negative pathogens, and the achievement of an optimal PK/PD target (100%fT> 4 × MIC) is imperative to maximize efficacy and minimize the development of new resistance [18]. It has been shown that bacterial isolates yielded in critically ill patients commonly exhibit MIC values two- to fourfold higher than those observed among those isolated from non-critical patients [65]. Consequently, novel agents could require the adoption of full/high-dose regimens administered by prolonged infusion, considering the clinical failure and resistance development reported with both ceftolozane–tazobactam and ceftazidime–avibactam among patients undergoing CRRT [15–17].
Administration of full-dose ceftolozane–tazobactam and/or ceftazidime–avibactam coupled with prolonged infusion led to optimal PK/PD target achievements in some cases during CVVHD or CVVHDF [30, 31, 34]. This strategy also granted adequate achievement of ceftolozane–tazobactam in the epithelial lining fluid [28]. Conversely, administration of reduced doses of ceftaroline or ceftobiprole during CVVHDF failed to achieve a Cmin/MIC ≥ 4 [25, 37]. In patients undergoing CVVH, all critical determinants involved in the extent of antibiotic CRRT elimination should be carefully assessed before considering the use of higher doses, given the fact that CL during CVVH could be lower compared with the other two CRRT modalities.
How Could Adequate Antibiotic Exposure be Granted? Therapeutic Drug Monitoring-Guided Strategy versus an Empirical Approach
Antimicrobial dose optimization in patients undergoing CRRT is challenging. An adaptive TDM strategy may be the most accurate approach to ensure the achievement of adequate antibiotic exposure in this scenario [64]. A TDM-guided strategy demonstrated that empirical β-lactams or vancomycin dosing failed in achieving optimal PK/PD targets during CRRT in up to 72% of cases [9, 70, 71].
Although TDM is invaluable in a CRRT scenario, it should be highlighted that extensive use of real-time TDM, particularly for β-lactams, is still limited. Furthermore, commercial kits for TDM are mainly available for traditional β-lactams, whereas methods for measuring novel antibiotics are still under construction. Optimization of antibiotic dosage during CRRT represents an important unmet clinical need in most hospitals as a TDM service is unavailable and only an empirical approach can be performed.
Overall, we believe that a paradigm shift from a ‘drug-centred’ approach to a ‘patient-centred’ approach should be considered in empirical dose adjustments during CRRT. This novel approach should consist of the development of an easy-to-apply ‘bedside algorithm’ evaluating the main variables affecting antibiotic CL during CRRT. The algorithm should be based on the physicochemical properties of the novel agent, the rate of residual renal function, the effluent flow rate, the site of infection, and the MIC of the isolated pathogens. This could allow a weighted choice in considering the need for an empirical antibiotic dosage increase or decrease according to the presence and extent of one or more of these determinants in each specific case. This approach may be directly tested in the different studies that we included in our analysis. In the case report by Mahmoud et al. [31], administration of full-dose ceftolozane–tazobactam by CI is supported by the application of high-intensity CVVHDF (effluent flow rate 4 L/h), the presence of deep-seated infection (VAP), and a borderline-susceptible Pseudomonas aeruginosa isolate (MIC 4 mg/L). Similarly, administration of full-dose ceftazidime–avibactam in the case report by Soukup et al. [34] is supported by the application of high-intensity CVVHDF (effluent flow rate 2750 mL/h), the presence of deep-seated infection (VAP), and borderline-susceptible Pseudomonas aeruginosa (MIC 8 mg/L). In these cases, the eventual presence of residual renal function would have also justified the need for a higher dosing regimen. The need for a ceftazidime–avibactam dosing reduction (1.25 g every 8 h) in the case report by Wenzler et al. [35] is supported by the application of low-intensity CVVH (Quf 2 L/h) with 100% pre-dilution mode in anuric patients affected by BSI. All these features justify a ceftazidime–avibactam dose reduction in a similar CRRT scenario. Conversely, in the PK study by Kalaria et al. [25], application of high-intensity CVVHD/CVVHDF (mean effluent flow rate > 3 L/h), presence of high adsorptive membrane exhibiting a large surface area, and high net fluid removal (ranging from 2 to 3 L/day) made ceftaroline dose reduction inappropriate, leading to failure in achieving the optimal PK/PD target in all four patients included.
This strategy strongly differs from the traditional ‘drug-centred’ view commonly found in the guidelines on dosage adjustment during CRRT [6], and could prospectively provide an alternative approach for dose adjustments, especially of novel antibiotics, which are affected by limited evidences in this setting.
Conclusion
Although evidence assessing the PK behaviour of novel antibiotics during CRRT are scanty, useful information directly applicable in clinical practice may be drawn. A priori dose reduction of these agents during CRRT seems to be an inappropriate strategy rather than a real need. Antimicrobial physicochemical/PK properties, CRRT settings, pathophysiological alterations, site of infection, and MIC of isolated pathogens should be carefully evaluated in dose-adjustment decision-making. A paradigm shift from a ‘drug-centred’ approach to a ‘patient-centred’ approach could be useful and manageable, especially in settings where antibiotic TDM is unavailable.
Declarations
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Conflicts of Interest/Competing Interests
Federico Pea participated in speakers’ bureaus for Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Pfzer and Sanofi Aventis, and in advisory boards for Angelini, Basilea Pharmaceutica, Correvio, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Novartis, Pfzer, Shionogi and Thermo-Fisher. Milo Gatti has no conflicts of interest to declare.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author Contributions
MG and FP made substantial contributions to the conception of the manuscript. MG was involved in drafting the manuscript, and FP made substantial contributions in revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
References
- 1.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 2.Hoste EAJ, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JMA, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–1030. doi: 10.1097/01.ASN.0000059863.48590.E9. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Pistolesi V, Morabito S, Di Mario F, Regolisti G, Cantarelli C, Fiaccadori E. A Guide to understanding antimicrobial drug dosing in critically ill patients on renal replacement therapy. Antimicrob Agents Chemother. 2019;63:e00583–e619. doi: 10.1128/AAC.00583-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Li X, Xia Y, Chu Y, Zhong H, Li J, et al. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front Pharmacol. 2020;11:786. doi: 10.3389/fphar.2020.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoff BM, Maker JH, Dager WE, Heintz BH. Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: an update. Ann Pharmacother. 2020;54:43–55. doi: 10.1177/1060028019865873. [DOI] [PubMed] [Google Scholar]
- 7.Jamal J-A, Mueller BA, Choi GYS, Lipman J, Roberts JA. How can we ensure effective antibiotic dosing in critically ill patients receiving different types of renal replacement therapy? Diagn Microbiol Infect Dis. 2015;82:92–103. doi: 10.1016/j.diagmicrobio.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Wong W-T, Choi G, Gomersall CD, Lipman J. To increase or decrease dosage of antimicrobials in septic patients during continuous renal replacement therapy: the eternal doubt. Curr Opin Pharmacol. 2015;24:68–78. doi: 10.1016/j.coph.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JA, Joynt G, Lee A, Choi G, Bellomo R, Kanji S, et al. The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: data from the multinational SMARRT Study. Clin Infect Dis. 2021;72:1369–1378. doi: 10.1093/cid/ciaa224. [DOI] [PubMed] [Google Scholar]
- 10.Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS. Antimicrobial resistance. JAMA. 2016;316:1193–1204. doi: 10.1001/jama.2016.11764. [DOI] [PubMed] [Google Scholar]
- 11.Koulenti D, Xu E, Song A, Sum Mok IY, Karageorgopoulos DE, Armaganidis A, et al. Emerging treatment options for infections by multidrug-resistant Gram-positive microorganisms. Microorganisms. 2020;8:191. doi: 10.3390/microorganisms8020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yahav D, Giske CG, Grāmatniece A, Abodakpi H, Tam VH, Leibovici L. New β-lactam-β-lactamase inhibitor combinations. Clin Microbiol Rev. 2020;34:e00115–e120. doi: 10.1128/CMR.00115-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31:e00079–e117. doi: 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatti M, Pea F. Pharmacokinetic/pharmacodynamic target attainment in critically ill renal patients on antimicrobial usage: focus on novel beta-lactams and beta lactams/beta-lactamase inhibitors. Expert Rev Clin Pharmacol. 2021 doi: 10.1080/17512433.2021.1901574. [DOI] [PubMed] [Google Scholar]
- 15.Bassetti M, Vena A, Giacobbe DR, Falcone M, Tiseo G, Giannella M, et al. Ceftolozane/tazobactam for treatment of severe ESBL-producing Enterobacterales infections: a multicenter nationwide clinical experience (CEFTABUSE II Study) Open Forum Infect Dis. 2020;7:ofaa139. doi: 10.1093/ofid/ofaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassetti M, Castaldo N, Cattelan A, Mussini C, Righi E, Tascini C, et al. Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: a multicentre nationwide clinical experience. Int J Antimicrob Agents. 2019;53:408–415. doi: 10.1016/j.ijantimicag.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2018;62:e02497–e2517. doi: 10.1128/AAC.02497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumi CD, Heffernan AJ, Lipman J, Roberts JA, Sime FB. What antibiotic exposures are required to suppress the emergence of resistance for Gram-negative bacteria? A systematic review. Clin Pharmacokinet. 2019;58:1407–1443. doi: 10.1007/s40262-019-00791-z. [DOI] [PubMed] [Google Scholar]
- 19.Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR) Crit Care. 2019;23:104. doi: 10.1186/s13054-019-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusanya OO, Bhavnani SM, Forrest A, et al. Exploration of the pharmacokinetic–pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother. 2015;59:7170–7177. doi: 10.1128/AAC.04955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cojutti PG, Rinaldi M, Zamparini E, Rossi N, Tedeschi S, Conti M, et al. Population pharmacokinetics of dalbavancin and dosing consideration for optimal treatment of adult patients with staphylococcal osteoarticular infections. Antimicrob Agents Chemother. 2021 doi: 10.1128/AAC.02260-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols WW, Newell P, Critchley IA, Riccobene T, Das S. Avibactam pharmacokinetic/pharmacodynamic targets. Antimicrob Agents Chemother. 2018;62:e02446–e2517. doi: 10.1128/AAC.02446-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novelli A, Del Giacomo P, Rossolini GM, Tumbarello M. Meropenem/vaborbactam: a next generation β-lactam β-lactamase inhibitor combination. Expert Rev Anti Infect Ther. 2020;18:643–655. doi: 10.1080/14787210.2020.1756775. [DOI] [PubMed] [Google Scholar]
- 24.Sime FB, Lassig-Smith M, Starr T, Stuart J, Pandey S, Parker SL, et al. A Population pharmacokinetic model-guided evaluation of ceftolozane-tazobactam dosing in critically ill patients undergoing continuous venovenous hemodiafiltration. Antimicrob Agents Chemother. 2019;64:e01655–e1719. doi: 10.1128/AAC.01655-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalaria S, Williford S, Guo D, Shu Y, Medlin C, Li M, et al. Optimizing ceftaroline dosing in critically ill patients undergoing continuous renal replacement therapy. Pharmacotherapy. 2021;41:205–211. doi: 10.1002/phar.2502. [DOI] [PubMed] [Google Scholar]
- 26.Gattringer R, Meyer B, Heinz G, Guttmann C, Zeitlinger M, Joukhadar C, et al. Single-dose pharmacokinetics of fosfomycin during continuous venovenous haemofiltration. J Antimicrob Chemother. 2006;58:367–371. doi: 10.1093/jac/dkl251. [DOI] [PubMed] [Google Scholar]
- 27.Oliver WD, Heil EL, Gonzales JP, Mehrotra S, Robinett K, Saleeb P, et al. Ceftolozane-tazobactam pharmacokinetics in a critically ill patient on continuous venovenous hemofiltration. Antimicrob Agents Chemother. 2016;60:1899–1901. doi: 10.1128/AAC.02608-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuti JL, Ghazi IM, Quintiliani R, Shore E, Nicolau DP. Treatment of multidrug-resistant Pseudomonas aeruginosa with ceftolozane/tazobactam in a critically ill patient receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents. 2016;48:342–343. doi: 10.1016/j.ijantimicag.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Carbonell N, Aguilar G, Ferriols R, Huerta R, Ferreres J, Calabuig M, et al. Ceftolozane pharmacokinetics in a septic critically ill patient under different extracorporeal replacement therapies. Antimicrob Agents Chemother. 2019;64:e01782–e1819. doi: 10.1128/AAC.01782-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguilar G, Ferriols R, Martínez-Castro S, Ezquer C, Pastor E, Carbonell JA, et al. Optimizing ceftolozane-tazobactam dosage in critically ill patients during continuous venovenous hemodiafiltration. Crit Care. 2019;23:145. doi: 10.1186/s13054-019-2434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoud A, Shah A, Nutley K, Nicolau DP, Sutherland C, Jain M, et al. Clinical pharmacokinetics of ceftolozane and tazobactam in an obese patient receiving continuous venovenous haemodiafiltration: a patient case and literature review. J Glob Antimicrob Resist. 2020;21:83–85. doi: 10.1016/j.jgar.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Bremmer DN, Nicolau DP, Burcham P, Chunduri A, Shidham G, Bauer KA. Ceftolozane/tazobactam pharmacokinetics in a critically ill adult receiving continuous renal replacement therapy. Pharmacotherapy. 2016;36:e30–e33. doi: 10.1002/phar.1744. [DOI] [PubMed] [Google Scholar]
- 33.Butragueño-Laiseca L, Troconiz IF, Grau S, Campillo N, García X, Padilla B, et al. Finding the dose for ceftolozane-tazobactam in critically ill children with and without acute kidney injury. Antibiot Basel Switz. 2020;9:887. doi: 10.3390/antibiotics9120887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soukup P, Faust AC, Edpuganti V, Putnam WC, McKinnell JA. Steady-state ceftazidime-avibactam serum concentrations and dosing recommendations in a critically ill patient being treated for Pseudomonas aeruginosa Pneumonia and undergoing continuous venovenous hemodiafiltration. Pharmacotherapy. 2019;39:1216–1222. doi: 10.1002/phar.2338. [DOI] [PubMed] [Google Scholar]
- 35.Wenzler E, Bunnell KL, Bleasdale SC, Benken S, Danziger LH, Rodvold KA. Pharmacokinetics and dialytic clearance of ceftazidime-avibactam in a critically ill patient on continuous venovenous hemofiltration. Antimicrob Agents Chemother. 2017;61:e00464–e517. doi: 10.1128/AAC.00464-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kufel WD, Eranki AP, Paolino KM, Call A, Miller CD, Mogle BT. In vivo pharmacokinetic analysis of meropenem/vaborbactam during continuous venovenous haemodialysis. J Antimicrob Chemother. 2019;74:2117–2118. doi: 10.1093/jac/dkz103. [DOI] [PubMed] [Google Scholar]
- 37.Cojutti PG, Merelli M, De Stefanis P, Fregonese C, Lucchese F, Bassetti M, et al. Disposition of ceftobiprole during continuous venous-venous hemodiafiltration (CVVHDF) in a single critically ill patient. Eur J Clin Pharmacol. 2018;74:1671–1672. doi: 10.1007/s00228-018-2535-0. [DOI] [PubMed] [Google Scholar]
- 38.Corona A, Agarossi A, Veronese A, Cattaneo D, D’Avolio A. Therapeutic drug monitoring of dalbavancin treatment in severe necrotizing fasciitis in 3 critically ill patients: a grand round. Ther Drug Monit. 2020;42:165–168. doi: 10.1097/FTD.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 39.Gonçalves-Pereira J, Póvoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care. 2011;15:R206. doi: 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sime FB, Pandey S, Karamujic N, Parker S, Alexander E, Loutit J, et al. Ex vivo characterization of effects of renal replacement therapy modalities and settings on pharmacokinetics of meropenem and vaborbactam. Antimicrob Agents Chemother. 2018;62:e01306–e1318. doi: 10.1128/AAC.01306-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonello RM, Di Bella S, Maraolo AE, Luzzati R. Fosfomycin in continuous or prolonged infusion for systemic bacterial infections: a systematic review of its dosing regimen proposal from in vitro, in vivo and clinical studies. Eur J Clin Microbiol Infect Dis. 2021;40:1117–1126. doi: 10.1007/s10096-021-04181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al Jalali V, Matzneller P, Wulkersdorfer B, Chou S, Bahmany S, Koch BCP, et al. Clinical pharmacokinetics of fosfomycin after continuous infusion compared with intermittent infusion: a randomized crossover study in healthy volunteers. Antimicrob Agents Chemother. 2020;65:e01375–e1420. doi: 10.1128/AAC.01375-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al Madfai F, Zaidi STR, Ming LC, Wanandy T, Patel RP. Physical and chemical stability of ceftaroline in an elastomeric infusion device. Eur J Hosp Pharm Sci Pract. 2018;25:e115–e119. doi: 10.1136/ejhpharm-2017-001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soo MY, Yessayan L, Dean M, Costello G, Katwaru R, Mueller B. P1959 Relebactam and imipenem clearance during ex vivo continuous renal replacement therapy. Abstract book 29TH ECCMID 13-16 April 2019 Amsterdam, Netherlands.
- 45.Katsube T, Echols R, Wajima T. Pharmacokinetic and pharmacodynamic profiles of cefiderocol, a novel siderophore cephalosporin. Clin Infect Dis. 2019;69:S552–S558. doi: 10.1093/cid/ciz828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatti M, Pea F. Should the clinical pharmacologist play a role in the multidisciplinary team managing severe necrotizing soft-tissue infections? Clin Pharmacokinet. 2021;60:403–407. doi: 10.1007/s40262-021-00986-3. [DOI] [PubMed] [Google Scholar]
- 47.Fujii M, Karumai T, Yamamoto R, Kobayashi E, Ogawa K, Tounai M, et al. Pharmacokinetic and pharmacodynamic considerations in antimicrobial therapy for sepsis. Expert Opin Drug Metab Toxicol. 2020;16:415–430. doi: 10.1080/17425255.2020.1750597. [DOI] [PubMed] [Google Scholar]
- 48.Seyler L, Cotton F, Taccone FS, De Backer D, Macours P, Vincent J-L, et al. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit Care. 2011;15:R137. doi: 10.1186/cc10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pea F, Viale P, Pavan F, Furlanut M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet. 2007;46:997–1038. doi: 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 50.Vincent HH, Vos MC, Akçahuseyin E, Goessens WH, van Duyl WA, Schalekamp MA. Drug clearance by continuous haemodiafiltration. Analysis of sieving coefficients and mass transfer coefficients of diffusion. Blood Purif. 1993;11:99–107. doi: 10.1159/000170103. [DOI] [PubMed] [Google Scholar]
- 51.Anderson VR, Keating GM. Dalbavancin. Drugs. 2008;68:639–648. doi: 10.2165/00003495-200868050-00006. [DOI] [PubMed] [Google Scholar]
- 52.Bugge JF. Influence of renal replacement therapy on pharmacokinetics in critically ill patients. Best Pract Res Clin Anaesthesiol. 2004;18:175–187. doi: 10.1016/j.bpa.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Roger C, Cotta MO, Muller L, Wallis SC, Lipman J, Lefrant J-Y, et al. Impact of renal replacement modalities on the clearance of piperacillin-tazobactam administered via continuous infusion in critically ill patients. Int J Antimicrob Agents. 2017;50:227–231. doi: 10.1016/j.ijantimicag.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Petersson J, Giske CG, Eliasson E. Poor correlation between meropenem and piperacillin plasma concentrations and delivered dose of continuous renal replacement therapy. Antimicrob Agents Chemother. 2021;65:e02029–e2120. doi: 10.1128/AAC.02029-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gatti M, Giannella M, Raschi E, Viale P, De Ponti F. Ceftolozane/tazobactam exposure in critically ill patients undergoing continuous renal replacement therapy: a PK/PD approach to tailor dosing. J Antimicrob Chemother. 2021;76:199–205. doi: 10.1093/jac/dkaa416. [DOI] [PubMed] [Google Scholar]
- 56.Jamal J-A, Udy AA, Lipman J, Roberts JA. The impact of variation in renal replacement therapy settings on piperacillin, meropenem, and vancomycin drug clearance in the critically ill: an analysis of published literature and dosing regimens*. Crit Care Med. 2014;42:1640–1650. doi: 10.1097/CCM.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 57.Roberts DM, Liu X, Roberts JA, Nair P, Cole L, Roberts MS, et al. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit Care. 2015;19:84. doi: 10.1186/s13054-015-0818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaijamorn W, Shaw AR, Lewis SJ, Mueller BA. Ex vivo ceftolozane/tazobactam clearance during continuous renal replacement therapy. Blood Purif. 2017;44:16–23. doi: 10.1159/000455897. [DOI] [PubMed] [Google Scholar]
- 59.Vilay AM, Shah KH, Churchwell MD, Patel JH, DePestel DD, Mueller BA. Modeled dalbavancin transmembrane clearance during intermittent and continuous renal replacement therapies. Blood Purif. 2010;30:37–43. doi: 10.1159/000316685. [DOI] [PubMed] [Google Scholar]
- 60.Michikoshi J, Matsumoto S, Miyawaki H, Morita M, Niu H, Seo K, et al. Evaluation of proteins and cells that adsorb to dialysis membranes used in continuous hemodiafiltration: comparison of AN69ST, polymethylmethacrylate, and polysulfone membranes. Blood Purif. 2019;48:358–367. doi: 10.1159/000501632. [DOI] [PubMed] [Google Scholar]
- 61.Honore PM, Mugisha A, Barreto Gutierrez L, Redant S, Kaefer K, Gallerani A, et al. Optimizing ceftolozane-tazobactam dosage during continuous renal replacement therapy: additional insights. Crit Care. 2019;23:406. doi: 10.1186/s13054-019-2692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aguilar G, Ferriols R, Martínez-Castro S, Ezquer C, Pastor E, Carbonell JA, et al. Optimizing ceftolozane-tazobactam dosage during continuous renal replacement therapy: some nuances. Crit Care. 2020;24:11. doi: 10.1186/s13054-019-2724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulldemolins M, Martín-Loeches I, Llauradó-Serra M, Fernández J, Vaquer S, Rodríguez A, et al. Piperacillin population pharmacokinetics in critically ill patients with multiple organ dysfunction syndrome receiving continuous venovenous haemodiafiltration: effect of type of dialysis membrane on dosing requirements. J Antimicrob Chemother. 2016;71:1651–1659. doi: 10.1093/jac/dkv503. [DOI] [PubMed] [Google Scholar]
- 64.Pea F, Viale P. Bench-to-bedside review: appropriate antibiotic therapy in severe sepsis and septic shock—does the dose matter? Crit Care. 2009;13:214. doi: 10.1186/cc7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 67.Ulldemolins M, Soy D, Llaurado-Serra M, Vaquer S, Castro P, Rodríguez AH, et al. Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother. 2015;59:5520–5528. doi: 10.1128/AAC.00712-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S, Xie F. Population pharmacokinetics and simulations of imipenem in critically ill patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents. 2019;53:98–105. doi: 10.1016/j.ijantimicag.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Udy AA, Morton FJA, Nguyen-Pham S, Jarrett P, Lassig-Smith M, Stuart J, et al. A comparison of CKD-EPI estimated glomerular filtration rate and measured creatinine clearance in recently admitted critically ill patients with normal plasma creatinine concentrations. BMC Nephrol. 2013;14:250. doi: 10.1186/1471-2369-14-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Economou CJP, Wong G, McWhinney B, Ungerer JPJ, Lipman J, Roberts JA. Impact of β-lactam antibiotic therapeutic drug monitoring on dose adjustments in critically ill patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents. 2017;49:589–594. doi: 10.1016/j.ijantimicag.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, et al. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523–1528. doi: 10.1097/CCM.0b013e318241e553. [DOI] [PubMed] [Google Scholar]
- 72.Merker A, Danziger LH, Rodvold KA, Glowacki RC. Pharmacokinetic and pharmacodynamic evaluation of ceftaroline fosamil. Expert Opin Drug Metab Toxicol. 2014;10:1741–1750. doi: 10.1517/17425255.2014.972932. [DOI] [PubMed] [Google Scholar]
- 73.Sy SKB, Zhuang L, Sy S, Derendorf H. Clinical pharmacokinetics and pharmacodynamics of ceftazidime-avibactam combination: a model-informed strategy for its clinical development. Clin Pharmacokinet. 2019;58:545–564. doi: 10.1007/s40262-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 74.Cho JC, Fiorenza MA, Estrada SJ. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination. Pharmacotherapy. 2015;35:701–715. doi: 10.1002/phar.1609. [DOI] [PubMed] [Google Scholar]
- 75.Torres A, Mouton JW, Pea F. Pharmacokinetics and dosing of ceftobiprole medocaril for the treatment of hospital- and community-acquired pneumonia in different patient populations. Clin Pharmacokinet. 2016;55:1507–1520. doi: 10.1007/s40262-016-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dash RP, Babu RJ, Srinivas NR. Review of the pharmacokinetics of dalbavancin, a recently approved lipoglycopeptide antibiotic. Infect Dis (Lond) 2017;49:483–492. doi: 10.1080/23744235.2017.1296968. [DOI] [PubMed] [Google Scholar]
- 77.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents. 2009;34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 78.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs. 2018;78:65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]