Supplemental Digital Content is available in the text.
Keywords: administration, inhalation drug, clinical trial, phase 1, drug safety
BACKGROUND:
Ischemia-reperfusion injury is common in critically ill patients, and directed therapies are lacking. Inhaled hydrogen gas diminishes ischemia-reperfusion injury in models of shock, stroke, and cardiac arrest. The purpose of this study was to investigate the safety of inhaled hydrogen gas at doses required for a clinical efficacy study.
DESIGN:
Prospective, single-arm study.
SETTING:
Tertiary care hospital.
PATIENTS/SUBJECTS:
Eight healthy adult participants.
INTERVENTIONS:
Subjects underwent hospitalized exposure to 2.4% hydrogen gas in medical air via high-flow nasal cannula (15 L/min) for 24 (n = 2), 48 (n = 2), or 72 (n = 4) hours.
MEASUREMENTS AND MAIN RESULTS:
Endpoints included vital signs, patient- and nurse-reported signs and symptoms (stratified according to clinical significance), pulmonary function testing, 12-lead electrocardiogram, mini-mental state examinations, neurologic examination, and serologic testing prior to and following exposure. All adverse events were verified by two clinicians external to the study team and an external Data and Safety Monitoring Board. All eight participants (18–30 yr; 50% female; 62% non-Caucasian) completed the study without early termination. No clinically significant adverse events occurred in any patient. Compared with baseline measures, there were no clinically significant changes over time in vital signs, pulmonary function testing results, Mini-Mental State Examination scores, neurologic examination findings, electrocardiogram measurements, or serologic tests for hematologic (except for clinically insignificant increases in hematocrit and platelet counts), renal, hepatic, pancreatic, or cardiac injury associated with hydrogen gas inhalation.
CONCLUSIONS:
Inhalation of 2.4% hydrogen gas does not appear to cause clinically significant adverse effects in healthy adults. Although these data suggest that inhaled hydrogen gas may be well tolerated, future studies need to be powered to further evaluate safety. These data will be foundational to future interventional studies of inhaled hydrogen gas in injury states, including following cardiac arrest.
Ischemia-reperfusion injury (IRI) results in end-organ injury in a number of clinical scenarios, including myocardial infarction, stroke, and cardiac arrest, leading to significant morbidity in surviving patients (1). Care paradigms for these illnesses focus on timely restoration of optimal perfusion and the prevention of secondary injury; therapies that target IRI itself are generally lacking. One notable exception is targeted temperature management, an approach that has not demonstrated a consistent therapeutic advantage in randomized controlled trials in older children and adults (2). The need for targeted therapies addressing IRI is significant.
Recently, it has been discovered that hydrogen gas (i.e., molecular dihydrogen [H2]) has therapeutic benefits by selectively reducing the hydroxyl radical in vivo (3, 4), a mediator that results from excess oxygen-free radical formation during reperfusion injury and directly damages DNA and lipid membranes. H2 administration has been shown to decrease nuclear factor of activated T cells–activated calcium signaling (central to apoptosis), activate the NF-E2 p45-related factor 2 pathway (up-regulates production of protective proteins, such as glutathione and catalase), and down-regulate proinflammatory cytokines (e.g., interleukin-1, tumor necrosis factor-a) (5, 6). There are numerous preclinical studies demonstrating that peri-injury H2 inhalation results in clinically important improvements in animal models of cardiac arrest (7–12), cardiopulmonary bypass (13), stroke (3, 14), hypoxic-ischemic encephalopathy (15), and sepsis (16, 17).
To date, a rigorous clinical study of the safety of H2 is lacking. Previously, our group found that mice exposed continuously to 2.4% hydrogen in air for 72 hours experienced no clinically significant changes in neurologic or pulmonary function compared with controls exposed to medical air (18). Further, there have been numerous reports of clinical H2 exposure in early phase clinical trials, including in cardiac arrest (19), stroke (20), coronary reintervention (21), colorectal cancer (22), and lung cancer (23). Although reports of adverse events among these studies are rare, the H2 dosing and duration of H2 administration vary widely among them, often limited to several hours per day. Further, because these patients were otherwise ill, the identification of H2-related findings may have been confounded by disease-related findings. Finally, although each of these studies was well conducted, none mention good clinical practice rigor nor were they intended to be screening studies for adverse events. The purpose of this study was to rigorously screen for adverse effects (AEs) associated with H2 exposure in healthy subjects at the dose and duration that we intend to use for a future efficacy study.
METHODS
Study Design
The study was performed under an investigator-initiated Investigational New Drug (IND) application (IND 146967), was approved by the Institutional Review Board (IRB) of Boston Children’s Hospital (IRB-P00031196), was registered on ClinicalTrials.gov (NCT04046211), and was performed according to Good Clinical Practice guidelines. The study was monitored by an independent Data and Safety Monitoring Board (DSMB). Eligible subjects were 18–35 years old and otherwise healthy; subjects with a history of chronic or recent illness, including coronavirus disease 2019 (COVID-19) or respiratory disorders such as asthma, chronic obstructive pulmonary disease, prior acute lung injury/acute respiratory distress syndrome, inflammatory disorders, known heritable disorders, nasal septal or sinus disease, history of tobacco use, recent blood transfusions, or the regular use of prescription medications (excepting contraceptives), were excluded. Subjects were recruited using an advertisement at a local university and on ClinicalTrials.gov. Financial compensation was provided for participation. All respondents were screened via e-mail for inclusion and exclusion criteria. Participants were then randomly selected (but with a targeted 50/50 gender distribution) for phone screening. Assenting participants then underwent an in-person physical examination, testing for pregnancy and COVID-19, and an in-person, written informed consent. Consenting eligible subjects then proceeded to study participation during an inpatient admission.
Study Protocol
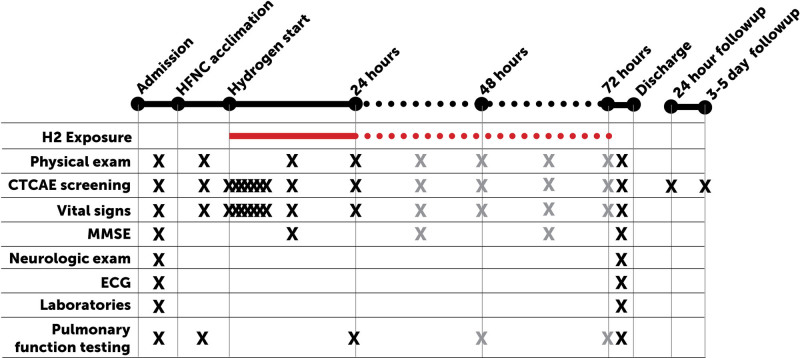
At the start of the inpatient admission, a complete physical examination, neurologic examination, pulmonary function testing, electrocardiogram (ECG), and baseline serologic testing were completed (Fig. 1). Thereafter, subjects underwent a 4-hour acclimation period to the high-flow nasal cannula (HFNC; 15 L/min, 21% oxygen, no hydrogen) to distinguish any symptoms arising from the HFNC itself. Participants were then assigned to either 24 (n = 2), 48 (n = 2), or 72 hours (n = 4) (sequential dose escalating design with 50/50 within-group sex assignment) of exposure to inhaled 2.4% hydrogen via HFNC (15 L/min, in 21% oxygen, balance nitrogen) during an inpatient stay. Gas mixtures were premixed using a Good Manufacturing Process and certified (part number Z03NI76T15A0000, Airgas Specialty Gases, Plumsteadville, PA), regulated via medical air flowmeter (part number FMAA07442FH, AmVex, Gurnee, IL), and administered with heat and humidification (part number MR850JHU, Fisher & Paykel Healthcare, Irvine, CA). Proper placement of the HFNC within the nares was observed at least hourly by a staff nurse. At this flow rate, we expected alveoli to be saturated with the inhaled gas (i.e. 2.4% H2) given that subjects were at rest and generally exhibited closed mouth breathing (24).
Figure 1.
Schematic of study treatment and testing. Upon hospital admission, subjects underwent a physical examination, mini-mental state examination (MMSE), a separate, detailed neurologic examination, 12-lead electrocardiogram (ECG), pulmonary function testing, and baseline laboratories. Subjects were then acclimated to high-flow nasal cannula (HFNC) for 4 hr, after which they were exposed to hydrogen gas (H2) for up to 72 hr. Subjects were regularly screened for signs and symptoms, which were graded according to the Common Terminology of Clinical Adverse Events (CTCAEs). Following exposure, measurements were repeated prior to discharge. A follow-up phone call took place at 24 hr and 3–5 d following discharge.
During the exposure period, subjects were observed for several endpoints as described subsequently. Broadly, our choice of endpoints was intended to represent a comprehensive screening for possible symptoms of H2 administration. Since there have been no consistent reports of adverse findings in clinical exposures, we began with a comprehensive screening tool frequently used to codify adverse events: National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAEs), Version 5.0. We also screened specifically for symptoms that might be expected from an inhaled gas (i.e., respiratory findings, such as wheezing or bronchospasm based on spirometry) or one with known clinical neurologic effects (i.e., neurologic examination). Given that we had previously described a decrease in locomotor activity following H2 exposure (albeit an isolated finding among a large battery of neurologic endpoints), we also performed a detailed neurologic examination to interrogate this finding in humans. Given that H2 exhibits rapid plasma transport and elimination within hours, the timing of the following endpoints was more frequent early in the exposure period and decreased over time. Following exposure, subjects underwent the same testing as at baseline. Follow-up phone interviews were conducted 1 day and 3–5 days after H2 exposure.
Adverse Event Screening
Adverse symptoms and signs were collected by the bedside nurse and separately by study team members at predefined intervals (i.e., during each vital sign measurements, as well as during the 1-d and 3–5-d follow-up phone calls). Any AEs were graded by the study team according to the CTCAE. A physical examination was performed by the bedside nurse at least every 12 hours and by a physician on the study team at least every 24 hours, including a respiratory, cardiovascular, and neurologic assessment. A mini-mental state examination (MMSE) was conducted at baseline and every 24 hours by a member of the study team. A comprehensive neurologic examination (including deep tendon reflexes, strength, coordination, fine motor skills, rapid alternating movements, and short-term memory) was separately performed by an attending neurologist once prior to and once following H2 exposure (prior to discharge). AE severity assignments were separately reviewed by both a physician and nurse removed from the study team, and all AEs were reported to the DSMB. All grade II and higher AEs and clinically significant grade I AEs (e.g., those which required treatment) were reported.
Pulmonary Function Testing
Pulmonary function testing was conducted every 24 hours using a calibrated bedside spirometer (Micro I spirometer, Vyaire Medical). Percent predicted forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and peak expiratory flow rate (PEFR) were recorded for each of three blows at each time point, and the blow with the highest FEV1 was chosen as representative of each time point.
Twelve-Lead ECG
A 12-lead ECG was performed prior to and following the H2 exposure period. Standard intervals were compared over time. All ECGs were interpreted by a board-certified cardiologist and abnormalities reported as adverse events.
Serological Testing
A predefined battery of laboratory testing was analyzed prior to and within 2 hours following the completion of H2 exposure. All testing was performed in the hospital’s core laboratory, including a complete blood count, chemistry panel 10, liver function tests, amylase and lipase levels, coagulation panel, and cardiac troponin.
Statistical Analysis
Patient characteristics and clinical measurements were summarized using mean and sd, median and interquartile range, and frequency and percentage. Serial measures of vital signs, MMSE, and pulmonary function testing were compared with baseline measurements (when relevant, the baseline was taken while the patient was breathing medical air without hydrogen added via HFNC) using a mixed effects analysis of variance model (random subject, fixed time points) with a compound symmetry covariance structure; these analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC). Comparisons of laboratory measurements and ECG findings pre versus post exposure were carried out using Wilcoxon matched-pairs signed rank testing. These analyses were performed in (and all graphs created in) GraphPad Prism 9.1 (GraphPad Software, San Diego, CA). A p value of less than 0.05 was defined as statistically significant for all tests. Normal values for each laboratory are displayed on each figure below for reference; values shown for adult females (LabCorp reference values).
RESULTS
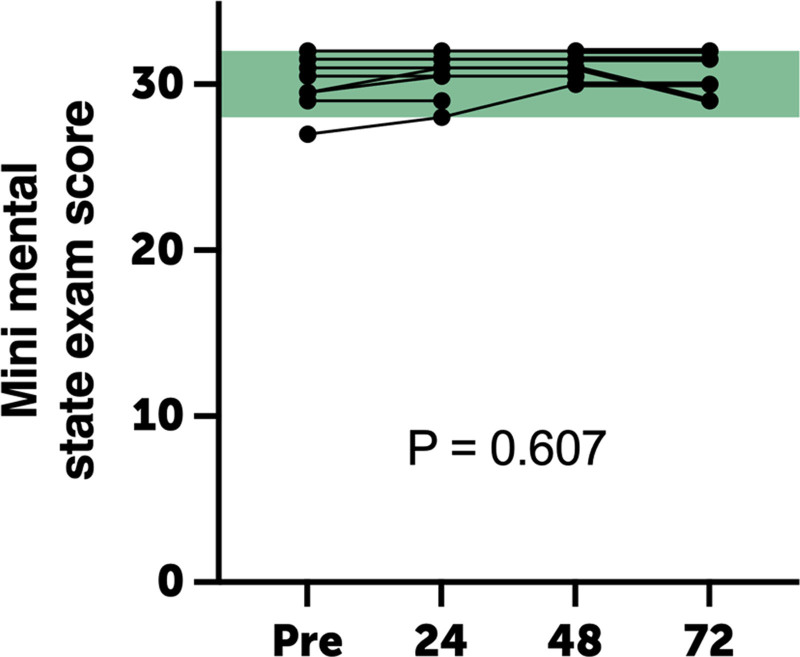
Of the nine subjects screened, eight met all eligibility criteria and provided written informed consent. All participants completed the study protocol as described without early termination (Table 1). The study cohort was 20.8 ± 4.1 years old, and 50% were male. One subject was observed to have a cannula displacement for less than 1 hour during sleep, and the exposure period was extended by an additional hour. No environmental hazard events occurred during the study. No clinically significant symptoms or adverse events occurred in any patient. Specifically, there were no complaints of respiratory distress, chest tightness, and findings of wheezing or tachypnea. There were also no clinically significant changes noted in neurologic examination (pre vs post exposure) nor in MMSE score over time (p = 0.607) (Fig. 2). There were no complaints of headache, malaise, fatigue, or other constitutional symptoms during or following H2 exposure through the follow-up periods.
TABLE 1.
Demographics of Study Participants
| Characteristics | n (%) | |
|---|---|---|
| Total Enrolled | ||
| Sex | Male | 4 (50) |
| Female | 4 (50) | |
| Ethnicity | Hispanic or Latino | 0 (0) |
| Not Hispanic or Latino | 8 (100) | |
| Unknown or not reported | 0 (0) | |
| Race | White | 3 (38) |
| Black/African American | 2 (25) | |
| Asian | 1 (13) | |
| Native American/Alaskan Native | 0 (0) | |
| Native Hawaiian/other Pacific Islander | 0 (0) | |
| Multiracial | 2 (25) | |
| Other | 0 (0) | |
| Unknown | 0 (0) | |
| Descriptor | Value | |
| Weight (kg) | Mean | 73.9 |
| Median | 76.7 | |
| sd | 11.0 | |
| Minimum | 56.7 | |
| Maximum | 86.5 | |
| Age at enrollment (yr) | Mean | 22.1 |
| Median | 20.8 | |
| sd | 4.1 | |
| Minimum | 18.5 | |
| Maximum | 30.7 | |
Figure 2.
Mini-mental state examination scores during hydrogen gas exposure did not differ from baseline values. Points are individual replicates; green shading represents reference range.
Vital Signs and ECG
Compared with baseline findings (HFNC breathing), there were no significant changes in systolic or diastolic blood pressure, respiratory rate, or oxygen saturation over time (Supplemental Fig. 1, http://links.lww.com/CCX/A804). There was a statistically significant but clinically insignificant decrease in heart rate over time (p < 0.05). There was no evidence of ectopic rhythm or conduction abnormality in any patient on telemetry or on 12-lead ECG (Supplemental Fig. 2, http://links.lww.com/CCX/A805).
Spirometry
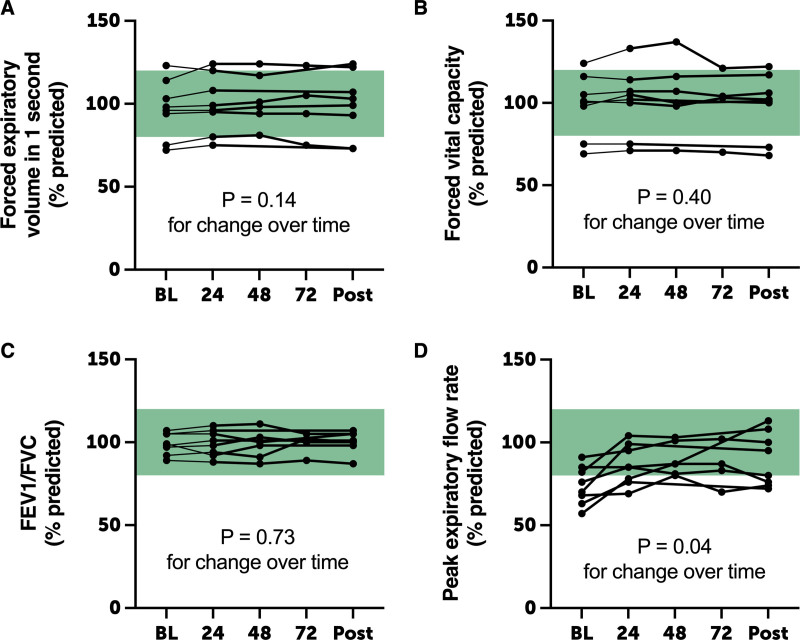
Compared with HFNC breathing, there were no changes over time in percent predicted FEV1, FVC, or FEV1/FVC ratio (Fig. 3). There was a statistically significant but clinically insignificant increase in PEFR over time during and following H2 breathing (p = 0.038).
Figure 3.
Pulmonary function testing. Compared with baseline (BL) measurements, there were no significant changes in percent predicted forced expiratory volume in 1 s (FEV1, A), forced vital capacity (FVC, B), or FEV1/FVC ratio (C) during and following hydrogen gas administration. There was a clinically insignificant increase in peak expiratory flow rate over time, perhaps related to improving technique over time. Points are individual replicates; green shading represents reference range.
Laboratory Findings
Compared with baseline findings, there were no significant changes in WBC count. There were statistically significant but clinically insignificant pre- versus postexposure increases in hemoglobin (mean increase, 1.3 g/dL [95% CI, 0.8–1.7 g/dL]), hematocrit (mean increase, 4.0% [2.4–5.6%]), and platelet count (mean increase, 22 cells/µL [4–41 cells/µL]) (Supplemental Fig. 3 A–D, http://links.lww.com/CCX/A806). Compared with baseline findings, there were no significant changes in serum chemistry profile (Supplemental Fig. 3 E–N, http://links.lww.com/CCX/A806). There was a decrease in serum chloride by 2.0 mmol/L (0.27–3.7 mmol/L) (p = 0.0391). Similarly, there were no significant changes in hepatic or pancreatic enzymes, coagulation profile, or cardiac troponin (Supplemental Fig. 3 O–AA, http://links.lww.com/CCX/A806).
DISCUSSION
We have shown that the administration of 2.4% H2 via HFNC appears to be safe and well tolerated, without clinically significant AEs in healthy participants. Subjects did not describe any odor or sensation, nor any respiratory signs or symptoms. There were no clinically detectable changes in neurologic function, including attention, memory, fine motor skills, and coordination associated with H2 inhalation. This was reassuring given our prior (likely artifactual) finding of diminished locomotor activity (one of many subsets of a battery of tests) in hydrogen-exposed mice (18). There was also no evidence that prolonged exposure to hydrogen in healthy subjects causes any clinically significant organ injury as evidenced by serologic testing. There was no evidence of clinically significant leukodepression. It is likely that the increases we found in hemoglobin, hematocrit, and platelet concentrations following H2 exposure were related to a mild dehydration in the hospitalized subjects; it is also possible that H2 stimulated bone marrow to increase production across cell catheters or decreased erythrocyte and platelet destruction, although these seem less likely. The statistically significant decrease in heart rate over time (always within the clinically normal range) may have been related to mild, transient anxiety early on in the study, particularly since there were no signs of arrhythmia on telemetry and no hemodynamic compromise. Similarly, the statistically significant improvement in PEFR was most likely related to improvements in spirometry technique over time, rather than a true H2 effect. Given that there were no meaningful changes in other spirometric endpoints, it is unlikely that this reflects a true H2 effect. The strength of this work was study rigor, including redundancy in examining for important endpoints (e.g., respiratory and neurologic symptoms), layers of quality control and endpoint adjudication, direct observation of hydrogen administration, and good clinical practice. This gives us confidence that the lack of positive findings in this study reflects a reassuring safety screening study.
These results are consistent with prior reports of hydrogen exposure in adult patients in illness, although dosing regimens in published studies vary. Perhaps the most rigorous study to date found that hematologic, liver, kidney, pancreas, cardiac enzymes, and electrolyte profiles did not significantly change in stroke patients breathing 3% H2 via nonrebreathing face mask for 1 hour bid for 7 days (20). Another study described no environmental safety hazards, no renal injury, and no constitutional symptoms (specifically dizziness, rash, constipation, or cystitis) in a small number of patients receiving periprocedural 1.3% H2 via face mask during percutaneous coronary reintervention (21). Similarly, another pilot study described no environmental hazards and no major attributable AEs following 18 hours of continuous delivery of 2% H2 via mechanical ventilator in a small number of postcardiac arrest patients (19).
We note the following limitations to our study. Given the low number of subjects in this safety, our study was limited to the identification of frequent AEs and was underpowered to detect findings that may be less common. Further, we intentionally enrolled healthy subjects for this initial study; the AE profile of H2 in illness may differ. Relatedly, the neurologic findings were requisitely measured using a different battery of tests than were used in the prior mouse study (since there is no direct correlate). As such, the lack of positive neurologic findings cannot be completely reassuring. Second, although we ensured H2 exposure by direct observation of cannula placement and of gas flow, we did not quantify serum H2 concentrations, as there is no Good Laboratory Practice-validated instrument to do so. However, we administered H2 at a flow rate (15 L/min) at which we expected alveoli to be saturated with the inhaled gas with minimal air entrainment given that subjects were at rest and generally exhibited closed mouth breathing (24). Third, this was a single-arm study in which the study team was not blinded to treatment allocation. However, most of the endpoints were objective, and subjective endpoints (e.g., neurologic findings) were confirmed by more than one observer.
CONCLUSIONS
Inhalation of 2.4% H2 appears to be well tolerated with no clinically significant AEs. Compared with baseline measures, there were no clinically significant changes in vital signs, neurologic examination, pulmonary function testing, or ECG changes, nor in any laboratory variables associated with up to 72 hours of H2 inhalation. Although these data suggest that inhaled H2 may be well tolerated, future studies need to be powered to further evaluate safety. These data should enable future studies of inhaled H2 in injury states.
ACKNOWLEDGMENTS
We thank Brenda Barton, Sandra Mariotti, Aanchal Gupta, Meg Fitzgerald, Gary Heyman, Peter Betit, Vassilios Bezzerides, the nurses of the Experimental Therapeutics Unit at Boston Children’s Hospital, and the members of the external Data and Safety Monitoring Board for assistance in completing this study.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Cole and Sperotto contributed equally to this work.
Drs. Cole, Sperotto, and Kheir designed the trial. Drs. Carlisle and Kheir designed and implemented the gas delivery device. Drs. Cole, Sperotto, DiNardo, Rivkin, and Kheir measured and adjudicated endpoints, Dr. Cole performed data entry. Drs. Sperotto and Sleeper performed statistical analyses. Drs. Cole and Kheir wrote the article. All authors reviewed and edited the article.
Supported, in part, by the Pappendick Family Therapeutic Acceleration Award. Further, this work was conducted with support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541), and financial contributions from Harvard University and its affiliated academic healthcare centers.
The authors have disclosed that they do not have any conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
REFERENCES
- 1.Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med. 2013; 368:1019–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moler FW, Silverstein FS, Holubkov R, et al. ; THAPCA Trial Investigators. Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med. 2017; 376:318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007; 13:688–694 [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Yu Q, Liu Y, et al. Hydrogen gas alleviates oxygen toxicity by reducing hydroxyl radical levels in PC12 cells. PLoS One. 2017; 12:e0173645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iuchi K, Imoto A, Kamimura N, et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep. 2016; 6:18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichihara M, Sobue S, Ito M, et al. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - Comprehensive review of 321 original articles. Med Gas Res. 2015; 5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashida K, Sano M, Kamimura N, et al. H(2) gas improves functional outcome after cardiac arrest to an extent comparable to therapeutic hypothermia in a rat model. J Am Heart Assoc. 2012; 1:e003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagatani K, Wada K, Takeuchi S, et al. Effect of hydrogen gas on the survival rate of mice following global cerebral ischemia. Shock. 2012; 37:645–652 [DOI] [PubMed] [Google Scholar]
- 9.Hayashida K, Sano M, Kamimura N, et al. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation. 2014; 130:2173–2180 [DOI] [PubMed] [Google Scholar]
- 10.Huo TT, Zeng Y, Liu XN, et al. Hydrogen-rich saline improves survival and neurological outcome after cardiac arrest and cardiopulmonary resuscitation in rats. Anesth Analg. 2014; 119:368–380 [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Jia L, Chen B, et al. Hydrogen inhalation is superior to mild hypothermia in improving cardiac function and neurological outcome in an asphyxial cardiac arrest model of rats. Shock. 2016; 46:312–318 [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Chen B, Dai C, et al. Hydrogen inhalation is superior to mild hypothermia for improving neurological outcome and survival in a cardiac arrest model of spontaneously hypertensive rat. Shock. 2018; 50:689–695 [DOI] [PubMed] [Google Scholar]
- 13.Cole AR, Perry DA, Raza A, et al. Perioperatively inhaled hydrogen gas diminishes neurologic injury following experimental circulatory arrest in swine. JACC Basic Transl Sci. 2019; 4:176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang JL, Liu WW, Manaenko A, et al. Hydrogen inhibits microglial activation and regulates microglial phenotype in a mouse middle cerebral artery occlusion model. Med Gas Res. 2019; 9:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Htun Y, Nakamura S, Nakao Y, et al. Hydrogen ventilation combined with mild hypothermia improves short-term neurological outcomes in a 5-day neonatal hypoxia-ischaemia piglet model. Sci Rep. 2019; 9:4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Yang Y, Bian Y, et al. Hydrogen gas protects against intestinal injury in wild type but not NRF2 knockout mice with severe sepsis by regulating HO-1 and HMGB1 release. Shock. 2017; 48:364–370 [DOI] [PubMed] [Google Scholar]
- 17.Xie K, Yu Y, Pei Y, et al. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010; 34:90–97 [DOI] [PubMed] [Google Scholar]
- 18.Cole AR, Raza A, Ahmed H, et al. Safety of inhaled hydrogen gas in healthy mice. Med Gas Res. 2019; 9:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura T, Hayashida K, Sano M, et al. Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome - First-in-human pilot study. Circ J. 2016; 80:1870–1873 [DOI] [PubMed] [Google Scholar]
- 20.Ono H, Nishijima Y, Ohta S, et al. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis. 2017; 26:2587–2594 [DOI] [PubMed] [Google Scholar]
- 21.Katsumata Y, Sano F, Abe T, et al. The effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infarction - First pilot study in humans. Circ J. 2017; 81:940–947 [DOI] [PubMed] [Google Scholar]
- 22.Akagi J, Baba H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol Rep. 2019; 41:301–311 [DOI] [PubMed] [Google Scholar]
- 23.Chen JB, Kong XF, Mu F, et al. Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer. Med Gas Res. 2020; 10:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward JJ. High-flow oxygen administration by nasal cannula for adult and perinatal patients. Respir Care. 2013; 58:98–122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.