Abstract
Propylene epoxidation with O2 to propylene oxide is a very valuable reaction but remains as a long-standing challenge due to unavailable efficient catalysts with high selectivity. Herein, we successfully explore 27 nm-sized cubic Cu2O nanocrystals enclosed with {100} faces and {110} edges as a highly selective catalyst for propylene epoxidation with O2, which acquires propylene oxide selectivity of more than 80% at 90–110 °C. Propylene epoxidation with weakly-adsorbed O2 species at the {110} edge sites exhibits a low barrier and is the dominant reaction occurring at low reaction temperatures, leading to the high propylene oxide selectivity. Such a weakly-adsorbed O2 species is not stable at high reaction temperatures, and the surface lattice oxygen species becomes the active oxygen species to participate in propylene epoxidation to propylene oxide and propylene partial oxidation to acrolein at the {110} edge sites and propylene combustion to CO2 at the {100} face sites, which all exhibit high barriers and result in decreased propylene oxide selectivity.
Subject terms: Catalytic mechanisms, Heterogeneous catalysis, Materials for energy and catalysis
Direct propylene epoxidation with O2 to form propylene oxide (PO) selectively is a challenge. Here, the authors explore 27 nm-sized cubic Cu2O nanocrystals as thermocatalysts for this reaction which facilitate PO production with high selectivity, >80%, at moderate temperatures of 90–110 °C.
Introduction
Propylene oxide (PO) is a platform chemical for numerous commodity chemicals1, such as polyols and glycol ethers. The current industrial production of PO from propylene involves uses of chlorohydrin or H2O2 and is cost-ineffective and environment-unfriendly2,3. Among various alternative technologies4–7, propylene epoxidation with O2 to PO is considered most economic and environment-friendly. However, it is meanwhile one of the most challenging catalytic reactions due to unavailable efficient catalysts with high selectivity8–12. Cu-based catalysts have been widely studied as a promising catalyst13–18, but the reported PO selectivity is not satisfying.
Fundamental understanding of active sites for heterogeneous catalytic reactions is an efficient approach to explore novel catalysts. Successful examples have been only a few and they are all based on density functional theory (DFT) calculations19–23. Herein, we report a successful exploration of fine cubic Cu2O nanocrystals (NCs) enclosed with {100} faces and {110} edges as a highly selective catalyst for propylene epoxidation with O2 to PO, guided by an experimental fundamental understanding of the active site. We previously used large rhombic dodecahedral NCs (denoted as d-Cu2O) enclosed with Cu2O{110} facets to identify the Cu2O{110} facets as the active facet for propylene epoxidation with O217, in which, however, reaction temperatures above 150 °C were adopted due to the low density of the active site on the used large d-Cu2O NCs, favoring the combustion reaction and limiting the acquired PO selectivity. Later large d-Cu2O NCs with the Cl− dopant were reported to exhibit enhanced activity in catalyzing propylene epoxidation with O2 and consequently high PO selectivity at low temperatures9. Thus, a reasonable strategy to explore highly selective catalysts for propylene epoxidation with O2 to PO is to synthesize uniform fine d-Cu2O NCs with high densities of Cu2O{110} active site, which, unfortunately, has not been realized. Meanwhile, Cu2O cubes (denoted as c-Cu2O) are enclosed with the {100} faces and {110} edges. We found that the densities of {110} edges are high on Cu2O cubes (denoted as c-Cu2O) finer than 100 nm and the {110} edge sites on these fine c-Cu2O NCs, rather than the {100} face sites, are the dominant active site catalyzing the CO oxidation reaction24. Intrigued by these findings, we have investigated propylene oxidation with O2 over c-Cu2O NCs with different sizes and report herein that fine c-Cu2O NCs with an average size of 27 nm selectively catalyze propylene epoxidation with O2 to PO at temperatures below 110 °C with the Cu2O{110} edge sites as the active site. Interestingly, the reaction mechanism for PO production at the Cu2O{110} active site was found to switch from weakly adsorbed O2-participating Langmuir–Hinshelwood (LH) mechanism at low temperatures to surface lattice oxygen-participating Mars-van Krevelen (MvK) mechanism at high temperatures.
Results
Synthesis and structural characterizations catalysts
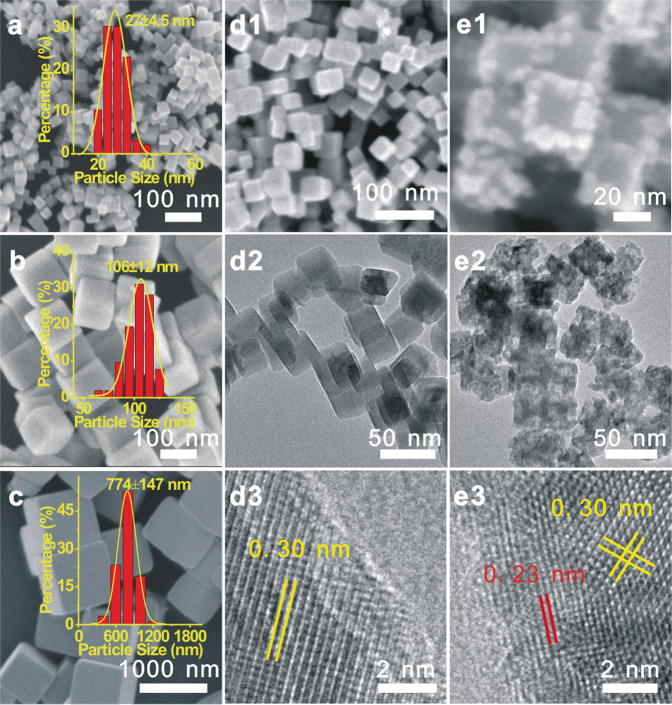
Following previously established procedures17,25–27, uniform surfactant-free c-Cu2O NCs with sizes of 27 ± 4.5, 106 ± 12, and 774 ± 147 nm were synthesized (Fig. 1a–c and Supplementary Fig. 1) and denoted as c-Cu2O-27, c-Cu2O-106, and c-Cu2O-774, respectively. BET-specific surface areas of c-Cu2O-27, c-Cu2O-106, and c-Cu2O-774 are 25.5, 11.2, and 1.5 m2 g−1, respectively. XPS spectra (Supplementary Fig. 2) show existences of only adventitious carbon and carbonates on the surfaces of various c-Cu2O NCs. c-Cu2O NCs are enclosed with O-terminated Cu2O{100} faces and (Cu(I), O)-terminated Cu2O{110} edges (Supplementary Fig. 3)24,28. Based on the size distributions of various c-Cu2O NCs, densities of Cu(110) edge sites and their fractions related to total surface Cu sites were estimated to be 6.44 × 1018/gCu2O and 1.61% on c-Cu2O-27, 4.08 × 1017/gCu2O, and 0.42% on c-Cu2O-106, and 7.84 × 1015/gCu2O and 0.06% on c-Cu2O-774 (Supplementary Table 1), respectively. Surface sites of various Cu2O NCs were probed by CO adsorption at 123 K with in-situ DRIFTS (Supplementary Fig. 4). Vibrational features of adsorbed CO were barely observed for c-Cu2O-774 NCs, but a vibrational feature at 2109 cm−1 arising from CO adsorbed at the Cu(I) site29 emerged for c-Cu2O-106 NCs and grew greatly for c-Cu2O-27 NCs. The Cu(I) sites for CO adsorption on c-Cu2O NCs exist on the (Cu(I), O)-terminated Cu2O{110} edges but not on the O-terminated Cu2O{100} faces. Therefore, the density of Cu2O{110} edges is too low on large c-Cu2O-774 NCs to be probed by CO adsorption measured with DRIFTS, but becomes high enough on fine c-Cu2O-106 and c-Cu2O-27 NCs.
Fig. 1. Microscopic characterization of Cu2O NCs.
SEM images and particle size distributions of a c-Cu2O-27, b c-Cu2O-106, and c c-Cu2O-774. SEM, TEM, and HRTEM images of c-Cu2O-27 after evaluated in C3H6 oxidation with O2 at 90 (d1–d3) and 150 °C (e1–e3). Lattice fringes of 0.30 and 0.23 nm correspond to the spacing of the Cu2O{110} (JCPDS card no. 78-2076) and CuO{111} (JCPDS card no. 89-5899) crystal planes, respectively.
Catalytic performance in C3H6 oxidation with O2
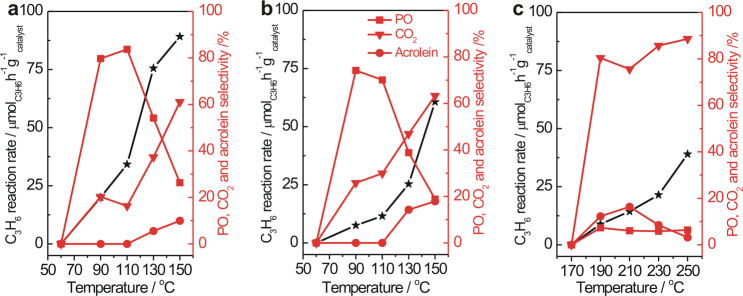
Various c-Cu2O NCs exhibit size-dependent catalytic performance in propylene oxidation with O2. As shown in Fig. 2 and Supplementary Fig. 5, c-Cu2O-774 NCs became active at 190 °C and dominantly catalyzed propylene combustion to produce CO2 with CO2 selectivity above 80%. c-Cu2O-27 and c-Cu2O-106 NCs were much more active than c-Cu2O-774 NCs, being catalytically active at 90 °C. Meanwhile, at comparable C3H6 conversions, c-Cu2O-27 and c-Cu2O-106 NCs exhibited much higher PO selectivities than c-Cu2O-774 NCs. Strikingly, c-Cu2O-27 and c-Cu2O-106 NCs selectively catalyzed the propylene epoxidation with PO selectivity respectively of above 80 and 70% between 90 and 110 °C, but barely catalyzed propylene partial oxidation to acrolein. As the temperature increased above 110 °C, the CO2 selectivity increased rapidly at the expense of PO selectivity, and the acrolein production emerged and grew.
Fig. 2. Catalytic performance in C3H6 oxidation with O2.
C3H6 reaction rate (black) and propylene oxide (PO), acrolein, and CO2 selectivities (red) of the C3H6 oxidation with O2 catalyzed by a c-Cu2O-27, b c-Cu2O-106, and c c-Cu2O-774 NCs. Reaction condition: 200 mg catalyst, 8% C3H6, and 4% O2 balanced with Ar at a flow rate of 50 mL min−1.
The catalytic performance of c-Cu2O-774 NCs is contributed by the Cu2O{100} face sites that selectively catalyze the propylene combustion reaction17, while the very different catalytic performances of c-Cu2O-27 and c-Cu2O-106 NCs arise from both the Cu2O{110} edge sites of enough high densities and the Cu2O{100} face sites. The Cu2O{110} facets were identified to selectively catalyze the propylene epoxidation reaction17. Therefore, the Cu2O{110} edge sites of c-Cu2O-27 and c-Cu2O-106 NCs are the dominant surface sites catalyzing propylene oxidation between 90 and 110 °C, giving high PO selectivity, while the contribution from the Cu2O{100} face sites increases with the reaction temperature, leading to increased CO2 selectivity at the expense of PO selectivity. These results, on one hand, demonstrate that the Cu2O{110} edge sites on c-Cu2O-27 and c-Cu2O-106 NCs are more active than the Cu2O{100} face sites, on the other hand, demonstrates that the Cu2O{110} site is active in selectively catalyzing the propylene epoxidation with O2 at low temperatures. As far as we know, PO selectivity above 80% in propylene oxidation with O2 catalyzed by c-Cu2O-27 NCs are much higher than all previously reported Cu-based catalysts except the recently-reported Cl–doped d-Cu2O NCs9. It is noteworthy that the catalytic selectivity of Cu2O{110} edge sites of c-Cu2O-27 and c-Cu2O-106 NCs in catalyzing propylene oxidation with O2 between 90 and 110 °C differs very much from that of Cu2O{110} face sites of large d-Cu2O NCs (d-Cu2O-439) which became active only above 150 °C (Supplementary Fig. 6)17. In addition to the significantly higher PO selectivity over c-Cu2O-27 and c-Cu2O-106 NCs than over d-Cu2O-439 NCs, acrolein was barely produced for c-Cu2O-27 and c-Cu2O-106 NCs but was a major product for d-Cu2O-439 NCs. Thus the catalytic behavior of Cu2O{110} sites in propylene oxidation with O2 sensitively depends on the reaction temperature.
Structures of spent Cu2O NCs after catalytic performance evaluations at different temperatures were characterized. Both microscopic (Fig. 1d, e and Supplementary Fig. 7) and spectroscopic (Supplementary Fig. 8) characterization results show that the structures of spent c-Cu2O-27 and c-Cu2O-106 NCs at 90 °C and spent d-Cu2O-439 at 210 °C are similar to their starting structures, whereas the surfaces of spent c-Cu2O-27 and c-Cu2O-106 NCs at 150 °C and spent c-Cu2O-774 NCs at 210 °C get oxidized, which can be associated with the selective CO2 production, a highly exothermic reaction. Surface oxidation is more extensive on finer c-Cu2O-27 NCs than on c-Cu2O-106 NCs. CuO ad-particles on spent c-Cu2O-27 and c-Cu2O-106 NCs resulting from surface oxidation were observed to locate preferentially at the edges, supporting that the Cu2O{110} edge sites are where the catalytic reactions dominantly occur. In-situ NAP-XPS spectra of c-Cu2O-27 NCs under 0.6 mbar C3H6 + 0.3 mbar O2 (Supplementary Fig. 9) do not show obvious surface oxidation at temperatures up to 150 °C. The discrepancy on surface oxidation of c-Cu2O-27 NCs at 150 °C under catalytic reaction and NAP-XPS measurement conditions can be attributed to the existing pressure gap.
Probed by CO adsorption, the oxidation of Cu2O{110} edges of c-Cu2O-27 and c-Cu2O-106 NCs at 130 and 150 °C also reduces the available surface Cu(I) sites (Supplementary Fig. 10). We found that C3H6 conversion rates of c-Cu2O-27 and c-Cu2O-106 NCs were proportional to the vibrational peak intensities of CO adsorbed at the surface Cu(I) sites at 90 and 130 °C but not at 150 °C (Supplementary Fig. 11). Therefore, the catalytic performances of c-Cu2O-27 and c-Cu2O-106 NCs up to 130 °C are dominantly contributed by the Cu2O{110} edges with the Cu(I) sites, and the observed decrease of PO selectivity and increase of CO2 selectivity at 130 °C should be due to the more extensive over-oxidation of PO. At 150 °C, although less active than the Cu2O{110} edge sites, the Cu2O{100} face sites of c-Cu2O-27 and c-Cu2O-106 NCs also contribute to the catalytic performance, enhancing the overall CO2 production and selectivity.
Stability of c-Cu2O-27 NCs at 90 °C was further evaluated. C3H6 conversion kept decreasing with the reaction time, while the PO selectivity gradually increased to almost 100% (Supplementary Fig. 12a). XPS spectra show that the surface of spent c-Cu2O-27 NCs is not oxidized (Supplementary Fig. 12b), while C–H species with the C 1s binding energy at 285.4 eV12 emerges (Supplementary Fig. 12c). Meanwhile, vibration features of carbonate species (1338 and 1537 cm−1)29, C–O–C (1046 cm−1)30, and C–H (~2928 cm−1) groups were observed on the spent catalyst (Supplementary Fig. 12d). These observations indicate that oligomers likely form and accumulate to block the active surface sites on c-Cu2O-27 NCs during the catalytic reaction.
Reaction mechanism of C3H6 oxidation with O2
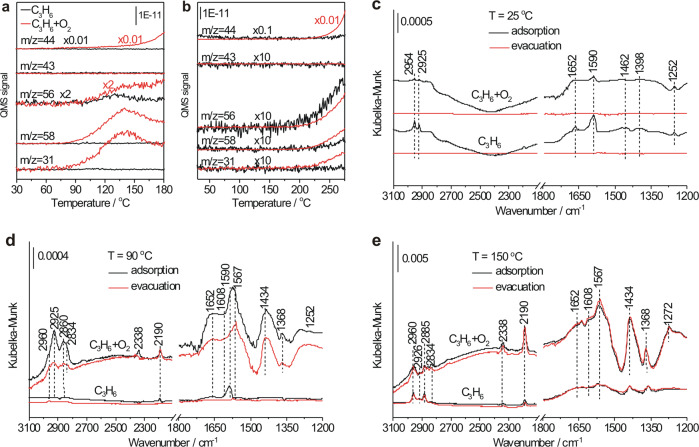
Figure 3a, b compare C3H6 and C3H6 + O2 temperature-programmed reaction spectra (TPRS) over c-Cu2O-27 and d-Cu2O-439 NCs. Over c-Cu2O-27 NCs (Fig. 3a), PO (m/z = 58 and 31) and CO2 (m/z = 44) productions did not appear in the C3H6-TPRS profile but appeared at ∼80 °C with similar traces in the C3H6 + O2-TPRS profile. Similar acrolein (m/z = 56) production traces appeared at ∼100 °C in both C3H6-TPRS and C3H6 + O2-TPRS profiles, and the acrolein production decreased with the temperature increasing in the C3H6-TPRS profile but increased in the C3H6 + O2-TPRS profile. Over d-Cu2O-439 NCs (Fig. 3b), acrolein, PO, and CO2 productions were observed above 200 °C to display similar traces in the C3H6-TPRS and C3H6 + O2-TPRS profiles with more productions in the presence of O2. Thus, no matter at low temperatures over c-Cu2O-27 NCs or at high temperatures over d-Cu2O-439 NCs, the acrolein production by C3H6 with O2 follows the surface lattice oxygen-participated MvK mechanism, consistent with the previous results31. The observed decrease of acrolein with the temperature in the C3H6-TPRS profile over c-Cu2O-27 NCs likely arises from the insufficient supply of surface lattice oxygen species at the Cu2O{110} edges. The PO production by C3H6 with O2 at high temperatures over d-Cu2O-439 NCs also follows the MvK mechanism, whereas the PO production at low temperatures over c-Cu2O-27 NCs does not, instead, it should follow a LH mechanism involving surface reactions between co-adsorbed propylene and oxygen species. Therefore, propylene epoxidation with O2 at the Cu2O{110} active site follows the LH mechanism at low temperatures and the MvK mechanism at high temperatures.
Fig. 3. Reaction mechanism.
C3H6-TPRS (8% C3H6 in Ar) and C3H6 + O2-TPRS (8% C3H6 + 4% O2 in Ar) of a c-Cu2O-27 and b d-Cu2O-439 NCs. DRIFTS spectra of C3H6 (PC3H6 = 50 Pa) and C3H6 + O2 (PC3H6 = 50 Pa, PO2 = 25 Pa) adsorption on c-Cu2O-27 NCs at c 25, d 90, and e 150 °C.
In-situ DRIFTS measurements of C3H6 and C3H6 + O2 adsorption on c-Cu2O-27 NCs at different temperatures were carried out to explore the temperature-dependent reaction mechanisms of propylene epoxidation with O2 (Fig. 3c–e). Assignments of observed vibrational features are summarized in Supplementary Table 2. In addition to gaseous C3H6 (2954 and 1652 cm−1), molecularly-adsorbed C3H6 species at the Cu(I) site of Cu2O{110} edges (C3H6(a)Cu) (2925 and 1590 cm−1) and bridgingly at Cu(I) and O sites of Cu2O{110} edges (C3H6(a)Cu,O) or bridgingly at O sites of Cu2O{100} face sites (C3H6(a)O,O) (2925 and 1462 cm−1) were observed upon both C3H6 and C3H6 + O2 adsorption at 25 °C, and all vibrational features disappeared upon evacuation, indicating reversible C3H6 adsorption and absence of C3H6 + O2 reaction at 25 °C. At 90 °C, C3H6 adsorption dominantly formed C3H6(a)Cu species, suggesting that C3H6(a)Cu should be more stable than C3H6(a)Cu,O and C3H6(a)O,O, whereas C3H6 + O2 adsorption formed not only reversibly-adsorbed C3H6 species desorbing upon subsequent evacuation but also surface intermediates remaining upon subsequent evacuation, including HCOO(a) (2960, 2885, 1567 and 1368 cm−1), allyl adsorbed at the O site (C3H5(a)O) (2834 and 1608 cm−1), CO2(a) (2338 and 2190 cm−1), adsorbed acrolein (C3H4O(a)) (1652 cm−1), and allyl adsorbed at the Cu site (C3H5(a)Cu) (1434 cm−1). HCOO(a) and CO2(a) species are the surface intermediates for the CO2 production, while C3H5(a) and C3H4O(a) species belong to the surface intermediates for the acrolein production. Previous DFT calculation results suggested that the presence of O2 promoted C3H6 dehydrogenation reactions to form adsorbed allyl and acrolein species on Cu2O surfaces32,33. Therefore, C3H6 + O2 adsorption at 90 °C involves surface reactions between co-adsorbed C3H6(a) and oxygen species following the LH mechanism, consistent with the above catalytic performance and TPRS results. Interestingly, few acrolein is produced although C3H5(a) and C3H4O(a) intermediates are formed, whereas PO is the dominant product but few relevant surface intermediates can be identified. This suggests that the desorption of C3H4O(a) to produce gaseous acrolein should exhibit a large barrier and barely occur at 90 °C, whereas surface reactions producing PO can occur. As the temperature increased to 150 °C, the vibrational features of all observed surface intermediates significantly grew, consistent with the enhanced C3H6 conversion. Meanwhile, C3H6 + O2 adsorption gave the same vibrational bands as C3H6 adsorption but with stronger intensities, supporting the above TPRS result that Cu2O{110}-catalyzed propylene oxidation with O2 at high temperatures follows the MvK mechanism. Acrolein production was observed, demonstrating the occurrence of C3H4O(a) desorption.
DFT calculations of C3H6 oxidation with O2
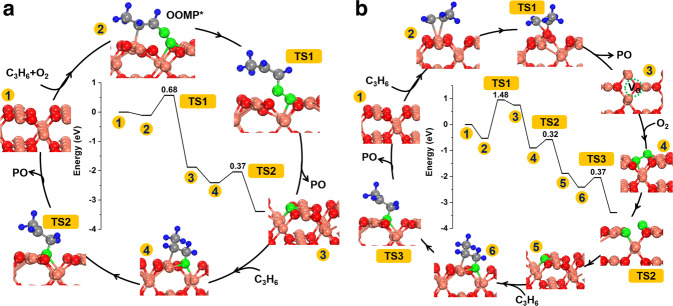
DFT calculations were performed to understand the mechanisms of propylene oxidation at the Cu2O{110} active site (Supplementary Table 3). C3H6(a)Cu and C3H6(a)Cu,O were calculated to exhibit adsorption energy of −0.53 and −0.33 eV and C=C stretch vibrational frequency of 1571 and 1444 cm−1, respectively (Supplementary Fig. 13), agreeing with the experimental results. O2 weakly adsorbs with an adsorption energy of −0.25 eV (Supplementary Fig. 14), and its dissociation into two oxygen adatoms exhibits an enthalpy of −0.12 eV but a barrier of 1.16 eV. It can thus be expected that O2 dissociation on the perfect Cu2O{110} surface is unlikely at low temperatures. Propylene epoxidation with O2 at the Cu2O{110} active site was found to occur via either a LH mechanism or a MvK mechanism (Fig. 4). The LH mechanism initiates via co-adsorption of C3H6 and O2 to form an oxametallacycle (OOMP) intermediate (Cu–O–O–CH2–CH(CH3)–Cu) with a reaction energy of −0.34 eV, which is stronger by 0.26 eV than the formation of Cu–O–O–(CH3)–CH–CH2–Cu intermediate. Then the OOMP intermediate dissociates to produce a PO molecule and an atomic O with a barrier of 0.68 eV. Finally, the resulting atomic O readily reacts with C3H6(a)Cu to produce another PO molecule with a barrier of 0.37 eV to close the catalytic cycle of propylene epoxidation with O2. Similar mechanisms of C3H6 epoxidation with molecularly-adsorbed O2 species on IB group metal surfaces were proposed by DFT calculations34. The MvK mechanism initiates via the reaction of C3H6(a)Cu with surface lattice O to produce a PO molecule and a surface oxygen vacancy (VO) with a barrier of 1.48 eV. Then O2 readily dissociates at the VO site to fill it and produce an atomic O with a barrier of 0.32 eV. Finally, the resulting atomic O readily reacts with C3H6(a)Cu to produce another PO molecule with a barrier of 0.37 eV to close the catalytic cycle of propylene epoxidation with O2.
Fig. 4. DFT calculations.
Energy profile and catalytic cycle along with the optimized structures of intermediates and transition states for propylene epoxidation on Cu2O(110) via a LH and b MvK mechanisms. The pink, red, green, gray, and blue spheres represent Cu, O in Cu2O, O in O2, C, and H atoms, respectively.
Propylene partial oxidation to acrolein at the Cu2O{110} active site following the Mvk mechanism was also calculated (Supplementary Fig. 15). It initiates by an abstract of an α-H atom of C3H6(a)Cu to produce C3H5(a)Cu or C3H5(a)O with a barrier of 0.75 eV. The C3H5(a)O is more stable than the C3H5(a)Cu species by 0.90 eV, resulting in a larger barrier of the C3H5(a)O-to-C3H4O(a) reaction (1.77 eV) than of the C3H5(a)Cu-to-C3H4O(a) reaction (1.05 eV). Then the resulting C3H4O(a) species desorbs to produce an acrolein molecule and create a surface oxygen vacancy with a barrier of 0.96 eV. The H-abstraction reactions of C3H6(a)Cu to produce C3H4O(a) were previously calculated to be promoted by co-adsorbed O2 species, but the C3H4O(a) desorption still exhibited a barrier of 0.99 eV32. Meanwhile, the C=C bond breaking of C3H6(a)O,O on the Cu2O{100} surface by surface lattice oxygen to eventually produce CO2 was previously calculated with a barrier of 1.05 eV17.
The above DFT calculation results suggest that the largest barrier is 0.68 eV among elementary surface reactions of LH mechanism for Cu2O{110}-catalyzed propylene epoxidation, but is 0.99–1.77 eV of MvK mechanism for Cu2O{110}-catalyzed propylene epoxidation, LH and MvK mechanisms for Cu2O{110}-catalyzed propylene partial oxidation to acrolein, and MvK mechanism for Cu2O{100}-catalyzed propylene combustion. Meanwhile, due to the very small adsorption energy but very large dissociation barrier of O2(a), O2(a) is the dominant adsorbed oxygen species on the stoichiometric Cu2O{110} site and can only form at low temperatures. Thus, at low temperatures at which O2(a) forms, Cu2O{110}-catalyzed propylene epoxidation following the LH mechanism occurs and other reactions with large barriers barely, leading to the high PO selectivity; however, at high temperatures at which O2(a) is few, Cu2O{110}-catalyzed propylene epoxidation following the LH mechanism barely occurs although with low barriers, and Cu2O{110}-catalyzed propylene epoxidation and propylene partial oxidation to acrolein and Cu2O{100}-catalyzed propylene combustion, all following the MvK mechanism, occur to produce PO, acrolein and CO2, respectively. These DFT calculation results agree well with the experimental observations of temperature-dependent catalytic selectivity of our fine c-Cu2O NCs in propylene oxidation with O2. Thus, the reactivity and temperature-dependent coverages of various surface species at different surface sites of Cu2O NCs are responsible for the observed apparent catalytic activity and selectivity in propylene oxidation with O2.
Discussion
In summary, based on the fundamental understanding of the active site of Cu2O catalysts for propylene epoxidation with O2, we successfully explore finely-sized cubic Cu2O NCs with a high density of active Cu2O{110} edge sites as the highly selective catalyst to catalyze propylene epoxidation with O2. Over the c-Cu2O-27 NCs catalyst, Cu2O{110}-catalyzed propylene epoxidation with weakly adsorbed O2(a) species as the active oxygen species exhibits a low barrier and is the dominant reaction occurring at low temperatures, selectively producing PO with a selectivity of above 80%, whereas Cu2O{110}-catalyzed propylene partial oxidation and propylene epoxidation and Cu2O{100}-catalyzed propylene combustion, all with surface lattice oxygen as the dominant active oxygen species and exhibiting large barriers, occur at high reaction temperatures, producing acrolein, PO and CO2, respectively. These results demonstrate the effectiveness of fundamental understanding in guiding the exploration of efficient catalysts for challenging heterogeneous catalytic reactions.
Methods
Chemicals and materials
All chemical reagents with the analytical grade were purchased from Sinopharm Chemical Reagent Co. C3H6 (99.95%), O2 (99.999%), CO (99.99%), and Ar (99.999%) were purchased from Nanjing Shangyuan Industrial Factory. All chemicals were used as received.
Synthesis of c-Cu2O-27 and c-Cu2O-106 NCs
c-Cu2O-27 and c-Cu2O-106 NCs were synthesized according to the method reported by Chang et al. 26. To synthesize c-Cu2O-27 NCs, 1 mL CuSO4 aqueous solution (1.2 mol L−1) was rapidly injected into 400 mL deionized water at 25 °C. After stirring for 5 min, 1 mL NaOH aqueous solution (4.8 mol L−1) was poured into the solution. After stirring for another 5 min, 1 mL ascorbic acid aqueous solution (1.2 mol L−1) was injected. Then the solution was kept for another 30 min, and the resulting precipitate was collected by centrifugation, decanting, and washing with distilled water and absolute ethanol, and finally dried in vacuum at RT for 12 h. c-Cu2O-106 NCs were synthesized similarly, except that 0.26 g sodium citrate was added to the initial 400 mL deionized water at 25 °C.
Synthesis of c-Cu2O-774 NCs
c-Cu2O-774 NCs were synthesized according to the following typical procedure25: 5.0 mL NaOH aqueous solution (2.0 mol L−1) was added dropwise into 50 mL CuCl2 aqueous solution (0.01 mol L−1) at 60 °C. After adequately stirring for 0.5 h, 5.0 mL ascorbic acid aqueous solution (0.6 mol L−1) was added dropwise into the solution. The mixed solution was adequately stirred at 60 °C for 5 h. The resulting precipitate was collected by centrifugation, decanting, and washing with distilled water and absolute ethanol, and finally dried in vacuum at RT for 12 h.
Synthesis of d-Cu2O-439 NCs
d-Cu2O-439 NCs capped with oleic acid (OA) (denoted as d-Cu2O-439-OA) were synthesized following Liang et al.’s procedure27. Typically, under vigorous stirring, 4 mL OA was mixed with 20 mL of absolute ethanol, and slowly added to 40 mL CuSO4 aqueous solution (0.025 mol L−1). The mixture was heated to 100 °C for 0.5 h. Then 10 mL NaOH aqueous solution (0.8 mol L−1) was added. After stirring for another 5 min, 30 mL d-(+)-glucose aqueous solution (0.63 mol L−1) was quickly added. The obtained mixture was stirred at 100 °C for another 1 h, and its color changed from black to green, and finally to brick red. The resulting precipitate was collected by centrifugation, decanting, and washing with distilled water and absolute ethanol, and finally dried in vacuum at RT for 12 h.
Capping ligands on as-synthesized d-Cu2O-439-OA NCs were removed following Hua et al.’s procedure17. Typically, 150 mg d-Cu2O-439-OA NCs were placed in a U-shaped quartz microreactor and purged in the stream of C3H6 + O2 + Ar gas mixture (C3H6: O2: Ar = 2: 1: 22) with a flow rate of 50 mL min−1 at RT for 0.5 h, and then heated to 215 °C at a heating rate of 5 °C min−1 and kept for another 0.5 h. Then the sample was cooled down to room temperature to acquire d-Cu2O-439 NCs.
In-situ C3H6 and C3H6 + O2 DRIFTS
Diffuse reflectance infrared spectroscopy (DRIFTS) measurements of chemisorption processes were performed on a Nicolet 6700 FTIR spectrometer equipped with an in-situ DRIFTS reaction cell (Harrick Scientific Products, INC) and a MCT/A detector. 50 mg catalyst was loaded onto the sample stage of the reaction cell. Prior to the experiments, the sample was heated at the desired temperatures at pressures better than 0.1 Pa, and the spectrum was measured and used as the background spectrum, then the adsorbed gas was admitted into the reaction cell to desirable pressures through a leak valve, and the DRIFTS spectra were recorded after the chemisorption processes reached a steady state.
In-situ C3H6 + O2 NAPXPS
Near-ambient pressure X-ray photoelectron spectroscopy (NAPXPS) measurements were carried out at BL02B01 of Shanghai Synchrotron Radiation Facility35. The bending magnet beamline delivered a soft X-ray with photon flux around 1 × 1011 photons s−1, energy resolution of E/∆E = 3700 and beam spot size of ~200 µm × 75 µm on the sample. XPS spectra were calibrated using Au 4f7/2 binding energy at 84.0 eV. During the NAPXPS experiments, 0.6 mbar C3H6 and 0.3 mbar O2 were introduced into the chamber, and the c-Cu2O NCs were heated and stabilized at desirable temperatures for 0.5 h, and then the NAPXPS spectra were measured.
Structural characterizations
Power X-ray diffraction (XRD) patterns were conducted on a Philips X’Pert PROS diffractometer using a nickel-filtered Cu Kα (wavelength: 0.15418 nm) radiation source with the operation voltage and operation current being 50 mA and 40 kV, respectively. X-ray photoelectron spectroscopy (XPS) was carried out on an ESCALAB 250 high-performance electron spectrometer using monochromatized Al Kα (hν = 1486.7 eV) as the excitation source. The likely charging of samples was corrected by setting the binding energy of the adventitious carbon (C 1s) to 284.8 eV. Scanning electron microscope (SEM) images were obtained on a JEOL JSM-6700 field emission scanning electron microscope. Transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) were obtained on a JEM-2100F high-resolution transmission electron microscope.
C3H6-temperature-programmed reaction spectra (C3H6-TPRS) and C3H6 + O2 TPRS were measured in a quartz tube microreactor equipped with an axial quartz sheathed thermocouple and connected to an online mass spectrometer (HIDEN QIC-20). In the C3H6-TPRS experiments, 50 mg catalyst was pretreated in Ar with a flow rate of 30 mL min−1 at 200 °C for 0.5 h and then cooled to 30 °C, then the gas stream was switched to 8% C3H6 in Ar with a flow rate of 50 mL min−1 and the catalyst was heated at a heating rate of 5 °C min−1. In the C3H6 + O2 TPRS experiments, 50 mg catalyst was pretreated in Ar with a flow rate of 30 mL min−1 at 200 °C for 0.5 h and then cooled to 30 °C, then the gas stream was switched to 8% C3H6 + 4% O2 in Ar with a flow rate of 50 mL min−1 and the catalyst was heated to the desired temperature at a heating rate of 5 °C min−1.
In-situ CO adsorption after catalytic reactions at different temperatures was performed on a Nicolet 6700 FTIR spectrometer equipped with an in-situ low-temperature and vacuum DRIFTS reaction cell (Harrick Scientific Products, Inc.) in order to enhance the chemisorption with minimum interference of gas-phase molecules. The DRIFTS spectra were measured with 256 scans and a resolution of 4 cm−1 using a MCT/A detector. 50 mg catalyst was loaded on the sample stage of the reaction cell. Prior to adsorption experiments, the sample was evacuated at 293 K for 1 h at a base pressure of 0.1 Pa and then cooled to 123 K, whose spectrum was taken as the background spectra. Then CO was admitted into the reaction cell to the desirable pressures via a leak valve, and the DRIFTS spectrum was recorded after the chemisorption reached the steady state.
Catalytic performance evaluation
Catalytic performance of Cu2O nanocrystals in propylene oxidation with O2 without any pretreatments was evaluated in a quartz tube microreactor equipped with an axial quartz sheathed thermocouple. 200 mg catalyst was used and heated to the desired reaction temperatures at a rate of 2 °C min−1 in a reaction gas mixture (C3H6: O2: Ar = 2: 1: 22, flow rate: 50 mL min−1). After the catalytic reaction reached a steady state, the composition of outlet gas was analyzed using an online Shimazu GC-2014 gas chromatograph equipped with two flame ionization detectors (FIDs) and one thermal conductivity detector (TCD). One FID was attached to a Stabilwax-DA capillary column (0.53 mm × 60 m) to detect propylene and oxygenates (acetaldehyde, PO, acetone, propionaldehyde, acrolein, acetic acid, and isopropanol) to a detection limit of 1 ppm, and the TCD was attached to a Porapak Q (3 mm × 3 m) and C13x compact column (3 mm × 3 m, Shimazu) to detect O2. A CH4 conversion oven was connected to the end of the TCD to convert trace CO2 to CH4, whose concentration was detected by the other FID. All the lines and valves between the exit of the reactor and the gas chromatographs were heated to 80 °C to prevent condensation of the products. The activity and selectivity of the catalytic reaction were calculated as the following, in which Xi represents conversion, Si selectivity, mi mass, and ni moles of substance i, represents the flow rate of C3H6:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
DFT calculations
DFT calculations were performed by Vienna ab initio Simulation Package (VASP)36,37. The exchange–correlation interaction was described by the Bayesian error estimation functional with van der Waals correlation (BEEF–vdW)38. The Kohn–Sham equations were solved by a plane wave basis set with a kinetic energy cutoff of 400 eV. A Cu2O(110) surface with (2 × 2) unit cell was modeled by a slab model including four-layer O and seven-layer Cu atoms. To prevent the artificial interaction between the repeated slabs along z-direction, 15 Å vacuum was introduced with correction of the dipole moment. The (2 × 2 × 1) k-point mesh was used to sample the Brillouin zone. During the optimization, the bottom two-layer O and four-layer Cu atoms were fixed in their bulk positions, while the remained atoms and adsorbates were relaxed until the residual forces were less than 0.02 eV Å−1. DFT + U correction was used with U-J = 6 eV for Cu 3d-orbitals39. Adsorption energies were calculated by Eads = Ead/sub – Ead – Esub, where Ead/sub, Ead, and Esub were the total energies of the optimized adsorbate/substrate system, the adsorbate in the gas phase, and the clean substrate, respectively. Transition states of the elementary steps were located by the climbing-image nudged elastic band (CI-NEB) method40.
Supplementary information
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (91945301, 21525313, 91745202), the Chinese Academy of Sciences, and the Changjiang Scholars Program of the Ministry of Education of China. The DFT calculations were performed on the supercomputing center of Wuhan University. The NAP-XPS measurements were carried out at the beamline 02B01 of the Shanghai Synchrotron Radiation Facility supported by the National Natural Science Foundation of China under contract no. 11227902.
Source data
Author contributions
W.X carried out the experiments. X.-K.G. carried out the DFT calculations. Z.Z., P.C., Y.Z., Z.Y., D.L., H.Z., and Z.L. assisted with the experiments. W.H. designed and supervised the project. All authors analyzed the data. W.H., W.X., and X.-K.G. prepared the manuscript and other authors commented on the manuscript.
Data availability
The data supporting the findings of the study are available within the paper and its Supplementary Information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Petar Djinović and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wei Xiong, Xiang-Kui Gu.
Change history
11/2/2021
A Correction to this paper has been published: 10.1038/s41467-021-26712-y
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-26257-0.
References
- 1.Myers, R. L. The 100 Most Important Chemical Compounds (Greenwood Press, London, 2007).
- 2.Nijhuis TA, Makkee M, Moulijn JA, Weckhuysen BM. The production of propene oxide: Catalytic processes and recent developments. Ind. Eng. Chem. Res. 2006;45:3447–3459. doi: 10.1021/ie0513090. [DOI] [Google Scholar]
- 3.Russo V, Tesser R, Santacesaria E, Di Serio M. Chemical and technical aspects of propene oxide production via hydrogen peroxide (HPPO Process) Ind. Eng. Chem. Res. 2013;52:1168–1178. doi: 10.1021/ie3023862. [DOI] [Google Scholar]
- 4.Leow WR, et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science. 2020;368:1228–1233. doi: 10.1126/science.aaz8459. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Haruta M. Gas-phase propene epoxidation over coinage metal catalysts. Res. Chem. Intermed. 2011;38:1–24. doi: 10.1007/s11164-011-0424-6. [DOI] [Google Scholar]
- 6.Khatib SJ, Oyama ST. Direct oxidation of propylene to propylene oxide with molecular oxygen: a review. Catal. Rev. Sci. Eng. 2015;57:306–344. doi: 10.1080/01614940.2015.1041849. [DOI] [Google Scholar]
- 7.Teržan J, Huš M, Likozar B, Djinović P. Propylene epoxidation using molecular oxygen over copper- and silver-based catalysts: a review. ACS Catal. 2020;10:13415–13436. doi: 10.1021/acscatal.0c03340. [DOI] [Google Scholar]
- 8.Lei Y, et al. Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science. 2010;328:224–228. doi: 10.1126/science.1185200. [DOI] [PubMed] [Google Scholar]
- 9.Zhan C, et al. Critical roles of doping Cl on Cu2O nanocrystals for direct epoxidation of propylene by molecular oxygen. J. Am. Chem. Soc. 2020;142:14134–14141. doi: 10.1021/jacs.0c03882. [DOI] [PubMed] [Google Scholar]
- 10.Marimuthu A, Zhang J, Linic S. Tuning selectivity in propylene epoxidation by plasmon mediated photo-switching of Cu oxidation state. Science. 2013;339:1590–1593. doi: 10.1126/science.1231631. [DOI] [PubMed] [Google Scholar]
- 11.He J, Zhai Q, Zhang Q, Deng W, Wang Y. Active site and reaction mechanism for the epoxidation of propylene by oxygen over CuOx/SiO2 catalysts with and without Cs+ modification. J. Catal. 2013;299:53–66. doi: 10.1016/j.jcat.2012.11.032. [DOI] [Google Scholar]
- 12.Yang X, et al. Direct epoxidation of propylene over stabilized Cu+ surface sites on titanium-modified Cu2O. Angew. Chem. Int. Ed. 2015;54:11946–11951. doi: 10.1002/anie.201504538. [DOI] [PubMed] [Google Scholar]
- 13.Zhu W, Zhang Q, Wang Y. Cu(I)-catalyzed epoxidation of propylene by molecular oxygen. J. Phys. Chem. C. 2008;112:7731–7734. doi: 10.1021/jp800927y. [DOI] [Google Scholar]
- 14.Vaughan O, Kyriakou G, Macleod N, Tikhov M, Lambert R. Copper as a selective catalyst for the epoxidation of propene. J. Catal. 2005;236:401–404. doi: 10.1016/j.jcat.2005.10.019. [DOI] [Google Scholar]
- 15.Wang Y, Chu H, Zhu W, Zhang Q. Copper-based efficient catalysts for propylene epoxidation by molecular oxygen. Catal. Today. 2008;131:496–504. doi: 10.1016/j.cattod.2007.10.022. [DOI] [Google Scholar]
- 16.Su W, Wang S, Ying P, Feng Z, Li C. A molecular insight into propylene epoxidation on Cu/SiO2 catalysts using O2 as oxidant. J. Catal. 2009;268:165–174. doi: 10.1016/j.jcat.2009.09.017. [DOI] [Google Scholar]
- 17.Hua Q, et al. Crystal-plane-controlled selectivity of Cu2O catalysts in propylene oxidation with molecular oxygen. Angew. Chem. Int. Ed. 2014;53:4856–4861. doi: 10.1002/anie.201402374. [DOI] [PubMed] [Google Scholar]
- 18.Torres D, Lopez N, Illas F, Lambert RM. Why copper is intrinsically more selective than silver in alkene epoxidation: ethylene oxidation on Cu(111) versus Ag(111) J. Am. Chem. Soc. 2005;127:10774–10775. doi: 10.1021/ja043227t. [DOI] [PubMed] [Google Scholar]
- 19.Besenbacher F, et al. Design of a surface alloy catalyst for steam reforming. Science. 1998;279:1913–1915. doi: 10.1126/science.279.5358.1913. [DOI] [PubMed] [Google Scholar]
- 20.Nørskov JK, Bligaard T, Rossmeisl J, Christensen CH. Towards the computational design of solid catalysts. Nat. Chem. 2009;1:37–46. doi: 10.1038/nchem.121. [DOI] [PubMed] [Google Scholar]
- 21.Seh ZW, et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science. 2017;355:eaad4998. doi: 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- 22.Studt F, et al. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 2014;6:320–324. doi: 10.1038/nchem.1873. [DOI] [PubMed] [Google Scholar]
- 23.Honkala K, et al. Ammonia synthesis from first-principles calculations. Science. 2005;307:555–558. doi: 10.1126/science.1106435. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, et al. Site-resolved Cu2O catalysis in the oxidation of CO. Angew. Chem. Int. Ed. 2019;58:4276–4280. doi: 10.1002/anie.201814258. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D-F, et al. Delicate control of crystallographic facet-oriented Cu2O nanocrystals and the correlated adsorption ability. J. Mater. Chem. 2009;19:5220–5225. doi: 10.1039/b816349a. [DOI] [Google Scholar]
- 26.Chang IC, et al. Large-scale synthesis of uniform Cu2O nanocubes with tunable sizes by in-situ nucleation. CrystEngComm. 2013;15:2363–2366. doi: 10.1039/c3ce26932a. [DOI] [Google Scholar]
- 27.Liang X, Gao L, Yang S, Sun J. Facile synthesis and shape evolution of single-crystal cuprous oxide. Adv. Mater. 2009;21:2068–2071. doi: 10.1002/adma.200802783. [DOI] [Google Scholar]
- 28.Huang W. Oxide nanocrystal model catalysts. Acc. Chem. Res. 2016;49:520–527. doi: 10.1021/acs.accounts.5b00537. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, et al. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J. Phys. Chem. C. 2016;120:21472–21485. doi: 10.1021/acs.jpcc.6b06158. [DOI] [Google Scholar]
- 30.Yang PF, Li JY, Li TD. Characterization on propylene oxide polymerization with in situ FT-IR. Adv. Mater. Res. 2010;160–162:60–64. doi: 10.4028/www.scientific.net/AMR.160-162.60. [DOI] [Google Scholar]
- 31.Reitz JB, Solomon EI. Propylene oxidation on copper oxide surfaces: electronic and geometric contributions to reactivity and selectivity. J. Am. Chem. Soc. 1998;120:11467–11478. doi: 10.1021/ja981579s. [DOI] [Google Scholar]
- 32.Xiao T-T, Li R-S, Wang G-C. A DFT study and microkinetic simulation in propylene oxidation on the “29” CuxO/Cu(111) surface. J. Phys. Chem. C. 2020;124:6611–6623. doi: 10.1021/acs.jpcc.9b11347. [DOI] [Google Scholar]
- 33.Song YY, Wang GC. Theoretical study of propylene epoxidation over Cu2O(111) surface: activity of O2–, O–, and O2– species. J. Phys. Chem. C. 2018;122:21500–21513. doi: 10.1021/acs.jpcc.8b07044. [DOI] [Google Scholar]
- 34.Dai Y, et al. Significant enhancement of the selectivity of propylene epoxidation for propylene oxide: a molecular oxygen mechanism. Phys. Chem. Chem. Phys. 2017;19:25129–25139. doi: 10.1039/C7CP02892J. [DOI] [PubMed] [Google Scholar]
- 35.Cai J, et al. An APXPS endstation for gas–solid and liquid–solid interface studies at SSRF. Nucl. Sci. Tech. 2019;30:81. doi: 10.1007/s41365-019-0608-0. [DOI] [Google Scholar]
- 36.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 37.Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996;6:15–50. doi: 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 38.Wellendorff J, et al. Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys. Rev. B. 2012;85:235149. doi: 10.1103/PhysRevB.85.235149. [DOI] [Google Scholar]
- 39.Isseroff LY, Carter EA. Importance of reference Hamiltonians containing exact exchange for accurate one-shot GW calculations of Cu2O. Phys. Rev. B. 2012;85:235142. doi: 10.1103/PhysRevB.85.235142. [DOI] [Google Scholar]
- 40.Henkelman G, Uberuaga BP, Jonsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000;113:9901–9904. doi: 10.1063/1.1329672. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of the study are available within the paper and its Supplementary Information. Source data are provided with this paper.