Abstract
Polygenic risk for schizophrenia has been associated with lower cognitive ability and age-related cognitive change in healthy individuals. Despite well-established neuropsychological sex differences in schizophrenia patients, genetic studies on sex differences in schizophrenia in relation to cognitive phenotypes are scarce. Here, we investigated whether the effect of a polygenic risk score (PRS) for schizophrenia on childhood, midlife, and late-life cognitive function in healthy individuals is modified by sex, and if PRS is linked to accelerated cognitive decline. Using a longitudinal data set from healthy individuals aged 25–100 years (N = 1459) spanning a 25-year period, we found that PRS was associated with lower cognitive ability (episodic memory, semantic memory, visuospatial ability), but not with accelerated cognitive decline. A significant interaction effect between sex and PRS was seen on cognitive task performance, and sex-stratified analyses showed that the effect of PRS was male-specific. In a sub-sample, we observed a male-specific effect of the PRS on school performance at age 12 (N = 496). Our findings of sex-specific effects of schizophrenia genetics on cognitive functioning across the lifespan indicate that the effects of underlying disease genetics on cognitive functioning is dependent on biological processes that differ between the sexes.
Subject terms: Learning and memory, Genomics
Introduction
Schizophrenia is a severe neuropsychiatric disorder that affects about 1% of the population [1, 2]. Cognitive deficits are considered a core feature of schizophrenia, affecting about 80% of the patients [1, 3]. The cognitive symptoms in schizophrenia involve working memory, episodic memory, reasoning and problem solving, speed of processing, and social cognition [3, 4]. Cognitive deficits in schizophrenia have been observed before the onset of positive symptoms, indicating that cognitive symptoms are not only related to secondary effects of the disease such as antipsychotic medication [5, 6]. Schizophrenia has also been suggested to be associated with accelerated cognitive aging [7]. To what extent this link may be due to disease-related factors, such as psychosis or taking antipsychotics, or underlying genetics is poorly understood. Evidence from studies reporting a link between poor school performance and risk of developing schizophrenia later in life [8, 9] supports the possibility that the cognitive deficits in schizophrenia patients may arise due to developmental causes already at young age [10]. However, it is not known if schizophrenia genetics is related to school performance in the general population.
Sex differences have long been observed in schizophrenia patients, with males being affected more frequently and more severely than females [11, 12]. Moreover, male patients have an earlier age at onset, worse negative symptoms, and worse treatment response to antipsychotics compared to female patients [13–16]. Several studies reported that male patients have more severe cognitive impairment than female patients [17–22], but other studies have found the opposite [23, 24] or no sex differences in cognitive deficits in schizophrenia [25]. Proposed explanations for sex differences in cognitive abilities in schizophrenia include sex hormone differences [26, 27] and sex differences in brain structure and volume [27, 28].
Genetics is known to play a crucial role in the development of schizophrenia, with an estimated heritability of ~80% [1, 2]. The schizophrenia genome-wide association study (GWAS) performed by the Psychiatric Genomics Consortium (PGC) [29] has identified a large number of genetic risk variants, typically single nucleotide polymorphisms (SNPs), that individually have weak effects on the phenotype [30]. To capture the polygenic nature of schizophrenia, a polygenic risk score (PRS) can be calculated to examine the impact of cumulative genetic risk for schizophrenia on related phenotypes [31, 32]. Studying PRS in unrelated healthy individuals has the advantage that genetic effects can be separated from secondary disease-related factors such as medication. A genetic overlap between schizophrenia and cognitive functioning in healthy individuals has been identified [33–35] and schizophrenia PRS have been linked to decreased cognitive ability across different cognitive domains in both schizophrenia patients [34, 36] and healthy individuals [37, 38]. However, sex differences in relation to cognitive phenotypes were typically not reported in past genetic studies of schizophrenia [34, 36–39], but a recent study suggests male-specific effects of schizophrenia PRS on memory in healthy older adults [40].
In the present study, we investigated sex-specific effects of schizophrenia PRS on cognitive performance in healthy individuals. We used longitudinal cognitive data from a large sample of healthy adults (25–100 years) to investigate genetic effects on both cognitive level and slope (episodic memory, semantic memory, visuospatial ability). In a sub-sample, we examined the impact of PRS on school grade data at age 12 as a proxy for childhood cognition.
Methods
Participants
Data in the present study come from the longitudinal population-based Betula Prospective Cohort Study on memory, health and aging, conducted in Umeå, Sweden [41, 42], including measurements of cognitive functions from six test waves (T1–T6) 5 years apart, with a total follow-up period of 25 years. Exclusion criteria were dementia and known neurologic or psychiatric disease. From three cohorts (S1, S3, and S6), a total of 1746 individuals had been successfully genotyped. We excluded individuals that had developed dementia (N = 287), resulting in 1459 individuals (with an average of 3.6 time-points, ranging from 1 to 6) included in the current study (678 males, 781 females) aged between 25 and 100 years (see Supplementary Table 1 for age distribution by time from inclusion). Dementia diagnosis was done by a geropsychiatrist based on the DSM-IV criteria [43] as previously described elsewhere [41, 44, 45]. All participants had European ancestry. The research was approved by the regional ethical review board at Umeå University (EPN), and all participants gave written informed consent.
Genotyping and construction of polygenic risk scores
Two genotyping datasets were used in the present study. For the first dataset (comprising 1481 individuals, 56% females), most of the DNA extraction for SNP genotyping was done at VIB-U Antwerp Center for Molecular Neurology in Belgium, and a minor part of DNA samples was extracted at Genome-wide genotyping LGC Genomics Ltd, UK. All DNA samples were genotyped using two types of Illumina arrays: The Infinium Exome Array and the Infinium Human OmniExpress-12v1_H Array. This was done at the Genotyping Platform of the Broad Institute of MIT and Harvard, USA, between 2012 and 2014. Imputation and standard quality control (QC) of the raw genotypes were done using the 1000 Genomes data and the imputation pipeline RICOPILI [46] using IMPUTE2 [47] used by the psychiatric genomic consortia (PGC). For the second genotyping dataset (comprising 361 individuals, 53% females), the DNA extraction for SNP genotyping was done at the Institute of Human Genetics, University of Bonn, Germany. All DNA samples were genotyped using two types of Illumina arrays: Illumina Omni Express and Omni 1S Bead chip kits. Imputation of the raw genotypes was done according to the ENIGMA2 protocol of the ENIGMA Consortium (http://enigma.ini.usc.edu/) to the 1000 Genomes reference panel [48] using minimac (version 2013.7.17) [49]. For both genotyping datasets, post-imputation QC was performed based on genotype call rate < 10%, minor allele frequency (MAF) < 1%, SNP missingness < 5%, and imputation info < 0.8. After removal of SNPs with ambiguous strand alignment and strand mismatch between the two datasets, we merged these datasets using PLINK [50] (version 1.9) resulting in one dataset including 1746 individuals (N = 96 in both datasets). Thereafter, we performed principal component analysis (PCA) for genetic ancestry on the merged dataset (shown in Supplementary Figure 1), and calculated the schizophrenia PRS in PLINK [50] based on the summary statistics of the PGC2 schizophrenia GWAS [29] including 35,476 cases and 46,839 controls, after excluding the current sample as well as SNPs within the extended major histocompatibility complex (MHC) region (25–34 Mb on chromosome 6 on the hg19 assembly) due to the high linkage disequilibrium (LD) of this region. First, LD clumping was performed according to parameters used by the PGC2 [29]: discarding SNPs within 500 kb of, and in r2 ≥ 0.1 with another more significant SNP, and excluding SNPs with MAF < 10%, after removal of SNPs with genotype call rate < 1% and MAF < 1%. PRS were based on SNPs located on autosomal chromosomes and calculated for each individual by summing the alleles of the clumped SNPs (N = 75,992) weighted by the natural log of the odds ratio from the PGC2 GWAS results [29]. The explained variance in case-control discrimination has been shown to increase with increasing PRS p-value threshold, reaching a plateau at p < 0.05 [29, 51]. However, the p-value threshold explaining the largest variance in case-control discrimination may not be the same as that for the amount of variance explained in endophenotypes. Moreover, it has been shown that the p-value threshold of 1 has the highest empirical power in traits with high polygenicity [52], and both schizophrenia [2] and cognitive performance [53] show highly polygenic inheritance. Therefore, PRS were constructed at 10 p-value thresholds (0.0001, 0.001, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 1), and the p-value threshold that explained the largest amount of variance in the cognitive composite cross-sectionally, using Nagelkerke’s pseudo-R2, was used in our main analyses. Additional PRS p-value thresholds were reported for comparisons and transparency, as the optimal p-value threshold may differ depending on the outcome variable. In addition, we calculated a polygenic score (PGS) on cognitive performance in the same way as the schizophrenia PRS using summary statistics from a large multicenter GWAS on cognitive performance measured across at least three cognitive domains including 257,841 individuals [53], and we used the PGS including all clumped SNPs (N = 75,639) from the GWAS summary statistics (p-value threshold ≤1). To complement the traditional clumping and thresholding (C + T) method for PRS calculation, we also calculated a schizophrenia PRS using the principal component approach (PCA-PRS), following Coombes et al. [52]. This method uses PCA to gather the maximum variation from PRSs calculated across the 10 p-value thresholds in a single principal component and avoids multiple testing without suffering loss of power [52].
Cognitive tests
The analyses were based on a cognition composite score that was calculated as the sum of z-transformed tests of visuospatial ability, episodic memory and semantic memory, as well as on the three cognitive domains separately. For episodic memory, a composite score was calculated as the sum of z-transformed tests of episodic memory (two tests of free oral recall of verb-noun sentences, two tests of category-cued recall of nouns from the sentence recall, and one test of free recall of presented nouns). For semantic memory, a composite score was calculated as the sum of z-transformed tests of semantic knowledge/verbal fluency (three tests of verbal generation of as many words as possible during 1 min: words that begin with A; words that begin with M, exactly 5 letters; professions that begin with B). Visuospatial ability was measured with the block design task from the Wechsler Adult Intelligence Scale (WAIS-R) [54]. For details about cognitive tasks see Nilsson et al. [41].
School performance
School grade data at approximately age 12 (sixth grade) was available for a subset of 496 Betula participants (247 males and 249 females) from the cohorts S1 and S3. The analyses were based on a school grade composite score that was calculated as the sum of z-transformed school grades from six school subjects: mathematics, Swedish, history, biology, geography, and scripture knowledge (Christianity) as well as on the six grades separately. For details see Pudas et al. [55].
Statistical analyses
To examine the association of PRS with level and change in cognitive measures over 25 years, we performed linear mixed-effect models that were fitted in R using the lme4 function available through the lme4 package. P-values were estimated based on the Satterthwaite approximations implemented in the lme4Test package. The models included the PRS as covariate of interest and the following covariates of no interest: age, age2, sex, and the first 5 principal components for genetic ancestry (to control for population stratification). Time from inclusion (years) was used as time-scale to represent slope, and interaction with time was allowed for all covariates. The models also included random subject-specific intercepts. In addition, we ran the same models adding a polygenic score (PGS) for cognitive performance. Model comparisons were done with a likelihood-ratio-test. To test for interaction effects, an interaction term for age and PRS (PRS*age) as well as for sex and PRS (PRS*sex) was included in the longitudinal regression models. Cross-sectional analyses of school performance at age 12 were done using the lm-function in R including the PRS as well as birth month (to account for differences in cognitive maturity), sex, and the first 5 principal components. Both the longitudinal and the cross-sectional analyses were performed for all individuals as well as sex-stratified. The variance in cognitive task performance explained by PRS was calculated as Nagelkerke’s pseudo-R2, by comparing the full model (PRS plus covariates) to the reduced model (covariates only), which was done for cross-sectional models using the lm-function in R. These models were based on each individual’s first cognitive test occasion, and included sample (cohort) as additional covariate. Levene’s test was used to assess the equality of variance in baseline cognitive test performance and schizophrenia PRS in males and females. Welch two-sample t-tests were performed to investigate if males and females differ according to their cognitive test performance at baseline as well as their PRS. A logistic regression analysis was performed to test if intake of antipsychotics is associated with PRS. All statistical analyses were performed in R version 4.0.3.
Results
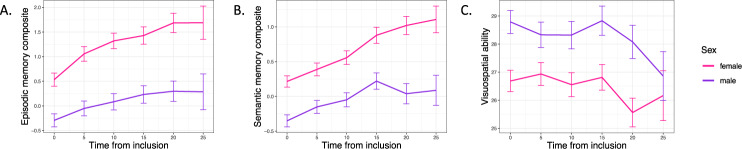
Descriptive statistics for each cognitive test and test occasion (T1–T6) are shown in Supplementary Table 2 separately for males and females as well as for all individuals. The effect of schizophrenia PRS (calculated with 10 different p-value thresholds) on cognition composite at baseline can be found in Supplementary Table 3, showing that the variance in cognitive test performance explained by schizophrenia PRS is largest for the PRS calculated with a p-value threshold ≤1, which was used for subsequent analyses. Using the cognition composite, Levene’s test showed that males and females had equal variance in their cognitive test performance (f = 2.659, p-value = 0.103). The distribution of cognitive data in males and females is shown in Supplementary Figure 2. Consistent with reported cognitive sexual dimorphisms [56], Welch two-sample t-tests showed that males performed better on the visuospatial task (t = −3.7691, df = 1423.3, p-value = 0.0002), whereas females performed better on the episodic memory (t = 4.3799, df = 1443.7, p-value = 1.273e−05) and semantic memory tasks (t = 4.7805, df = 1427.9, p-value = 1.929e−06), shown in Fig. 1. Moreover, Welch two-sample t-test showed that males and females did not differ significantly regarding their schizophrenia PRS (t = 1.888, df = 1419.8, p-value = 0.059, males having a slightly higher PRS). Levene’s test showed that males and females had equal variance in their PRS (f = 0.012, p-value = 0.9173).
Fig. 1. Cognitive test performance for each cognitive domain across 25 years separately for males and females.
A Episodic memory. B Semantic memory. C Visuospatial ability. Error bars show standard error.
Effect of schizophrenia genetics on cognitive test performance
Overall, schizophrenia PRS was associated with a lower cognition composite (t-value = −2.224, p-value = 0.026), but not with cognitive change over time (t-value = 0.188, p-value = 0.851). Results subdivided by three cognitive domains showed that PRS was negatively associated with episodic memory and semantic memory, but not with visuospatial ability (Table 1). For any cognitive domain, no significant effects of PRS on cognitive change over time could be observed (Table 1). Complementary analyses of PRS using more stringent p-value thresholds again showed no effects on visuospatial ability, nor on cognitive slope in any examined domain (Supplementary Table 4).
Table 1.
Effect of schizophrenia PRS (p-value threshold ≤1) on intercept and slope of individual cognitive tests, including all six test occasions (T1–T6), separately for males and females as well as for all individuals.
| N | Estimate (95% CI) | t-value | p-value | |||
|---|---|---|---|---|---|---|
| Episodic memory composite | PRS | All | 1454 | −0.154 (−0.298 to −0.010) | −2.083 | 0.0374* |
| Time*PRS | −0.002 (−0.009 to 0.005) | −0.526 | 0.5987 | |||
| PRS | Females | 778 | −0.015 (−0.215 to 0.186) | −0.142 | 0.8869 | |
| Time*PRS | −0.003 (−0.013 to 0.008) | −0.510 | 0.6104 | |||
| PRS | Males | 676 | −0.303 (−0.507 to −0.095) | −2.854 | 0.0044* | |
| Time*PRS | 0.006 (−0.011 to 0.009) | −0.171 | 0.8644 | |||
| Semantic memory composite | PRS | All | 1459 | −0.122 (−0.226 to −0.017) | −2.276 | 0.0230* |
| Time*PRS | 0.003 (0.002 to 0.008) | 1.064 | 0.2873 | |||
| PRS | Females | 781 | −0.056 (−0.199 to 0.087) | −0.767 | 0.4433 | |
| Time*PRS | 0.004 (−0.003 to 0.011) | 1.042 | 0.2977 | |||
| PRS | Males | 678 | −0.191 (−0.343 to −0.038) | −2.441 | 0.0149* | |
| Time*PRS | 0.002 (−0.006 to 0.009) | 0.492 | 0.6227 | |||
| Visuospatial ability | PRS | All | 1457 | −0.023 (−0.063 to 0.020) | −1.017 | 0.3091 |
| Time*PRS | −0.001 (−0.002 to 0.001) | −0.481 | 0.6306 | |||
| PRS | Females | 780 | 0.027 (−0.003 to 0.084) | 0.905 | 0.3657 | |
| Time*PRS | −0.001 (−0.0003 to 0.002) | −0.493 | 0.6219 | |||
| PRS | Males | 677 | −0.076 (−0.133 to −0.012) | −2.324 | 0.0204* | |
| Time*PRS | 0.001 (−0.003 to 0.002) | −0.101 | 0.9193 |
Bold: p < 0.02 (corrected for multiple testing across three tests). Linear mixed-effect models included sex (when applicable), age, age2, and the first 5 genetic principal components for genetic ancestry as covariates of no interest.
CI confidence interval, PRS polygenic risk score.
*p < 0.05 (uncorrected).
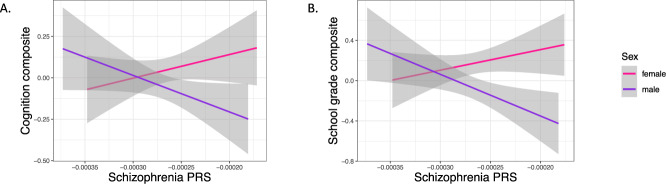
Next, we tested for interaction effects of PRS with age or sex on cognitive test performance cross-sectionally using the cognition composite within linear models. There was no significant interaction between PRS and age (t-value = 1.789, p-value = 0.074), but a significant interaction effect between PRS and sex could be observed (t-value = −2.262, p-value = 0.024). There was a significant interaction effect between PRS and sex for all 10 PRS calculated with different p-value thresholds for the cognition composite (Supplementary Table 5). Sex-stratified analyses showed that the association between PRS and lower cognitive performance across all cognitive domains was male-specific with no trend seen in females (Table 1, Fig. 2A) and again, no longitudinal effect of PRS on cognitive performance could be observed in either sex (Table 1). As a sensitivity analysis, we also included a polygenic score for cognitive performance (Cog-PGS) in the models to see if the effects of schizophrenia genetics are independent of genetic variants with a known effect on cognition, based on GWAS on cognitive ability in healthy individuals [53]. As expected, the Cog-PGS was strongly predictive of cognitive ability (Supplementary Table 6), but no interaction effects with sex were seen on the cognition composite score (t-value = −0.628, p-value = 0.530). Including all individuals, the negative association between the schizophrenia PRS and cognitive performance was no longer significant when also including the Cog-PGS in the same model. Model comparisons showed that models including the Cog-PGS alone did not differ significantly regarding fit to data from the models including both the Cog-PGS and schizophrenia PRS (episodic memory: χ2 = 3.888 (2), p = 0.143; semantic memory: χ2 = 3.272 (2), p = 0.195; visuospatial ability: χ2 = 0.643 (2), p = 0.725). However, sex-stratified analyses showed that the negative association between schizophrenia PRS and cognitive performance (episodic memory and semantic memory) was still significant in males when including the Cog-PGS in the models (Supplementary Table 6). In another sensitivity analysis, we ran the models including years of education as additional covariate showing that education was strongly predictive for cognitive performance, but the relationship between schizophrenia PRS and cognitive performance was independent of participants’ education (shown for all individuals as well as sex-stratified in Supplementary Table 7). Data on self-reported drug use was available for T3–T6. Logistic regression analysis showed that intake of antipsychotics (N = 14) was associated with higher PRS (OR = 3.204, p-value = 3.65e−06). To test if the effect of schizophrenia genetics on cognitive performance is independent of antipsychotic use, we ran the models excluding individuals taking one or more antipsychotics (N = 14). For all three cognitive domains, the significance level of the PRS as well as the interaction effect with sex did not change.
Fig. 2. Association between schizophrenia PRS and baseline cognitive test performance cross-sectionally in males and females.
A PRS associated with a cognition composite score that was calculated as the sum of z-transformed tests of visuospatial ability, episodic memory, and semantic memory (males: t-value = −3.397, p-value = 0.0007); females: t-value = 0.612, p-value = 0.5404). B PRS associated with a school grade composite score that was calculated as the sum of z-transformed school grades from six school subjects: mathematics, Swedish, history, biology, geography, and scripture knowledge (Christianity) (males: t-value = −2.707, p-value = 0.0073; females: t-value = 0.296, p-value = 0.7673). Gray areas indicate standard error. PRS p-value threshold ≤1.
Effect of schizophrenia genetics on school performance
We tested for association between schizophrenia PRS and school performance at age 12 (N = 496), including sex interactions, with school grades used as a proxy for childhood cognitive ability. There was a trend for an association between PRS and lower school grade composite across six school subjects (t-value = −1.908, p-value = 0.0570). Importantly, there was a significant interaction between PRS and sex (t-value = −2.962, p-value = 0.0032). Sex-stratified analyses showed the same patterns as for cognition in mid- and old age; a significant negative association in males (t-value = −3.235, p-value = 0.0014), with no trend in females (t-value = 0.305, p-value = 0.7604) (Fig. 2B). Results subdivided by each school subject separately showed the same patterns with no trend for females in any school subject (Table 2).
Table 2.
Effect of schizophrenia PRS (p-value threshold ≤1) on each school grade at age 12, separately for males and females as well as for all individuals.
| N | Estimate (95% CI) | t-value | p-value | ||
|---|---|---|---|---|---|
| Mathematics | All | 496 | −0.082 (−0.170 to 0.006) | −1.837 | 0.0668 |
| Females | 249 | −0.012 (−0.132 to 0.1081) | −0.195 | 0.8457 | |
| Males | 247 | −0.170 (−0.299 to −0.041) | −2.603 | 0.0098* | |
| Swedish | All | 496 | −0.033 (−0.119 to 0.054) | −0.748 | 0.4549 |
| Females | 249 | 0.030 (−0.091 to 0.152) | 0.491 | 0.6240 | |
| Males | 247 | −0.111 (−0.234 to 0.013) | −1.767 | 0.0785 | |
| History | All | 496 | −0.100 (−0.189 to −0.011) | −2.208 | 0.0278* |
| Females | 249 | −0.018 (−0.142 to 0.106) | −0.281 | 0.7788 | |
| Males | 247 | −0.195 (−0.324 to −0.066) | −2.969 | 0.0033* | |
| Biology | All | 496 | −0.077 (−0.167 to 0.013) | −1.683 | 0.0931 |
| Females | 249 | 0.042 (−0.081 to 0.165) | 0.676 | 0.4999 | |
| Males | 247 | −0.214 (−0.344 to −0.084) | −3.236 | 0.0014* | |
| Geography | All | 496 | −0.050 (−0.139 to 0.039) | −1.110 | 0.2678 |
| Females | 249 | 0.081 (−0.039 to 0.202) | 1.331 | 0.1845 | |
| Males | 247 | −0.202 (−0.332 to −0.072) | −3.061 | 0.0025* | |
| Scripture knowledge (Christianity) | All | 496 | −0.096 (−0.181 to −0.012) | −2.239 | 0.0256* |
| Females | 249 | −0.028 (−0.145 to 0.089) | −0.474 | 0.6357 | |
| Males | 247 | −0.186 (−0.308 to −0.064) | −3.005 | 0.0029* |
Bold: p < 0.008 (corrected for multiple testing across six subjects). Linear regression models included sex (when applicable), birth month, and the first 5 genetic principal components for genetic ancestry as covariates of no interest.
CI confidence interval, PRS polygenic risk score.
*p < 0.05 (uncorrected).
Complementary analyses using PCA-PRS
There was a significant interaction with sex (PCA-PRS*sex) both for the cognition composite (t-value = −2.842, p-value = 0.005) and the school grade composite (t-value = −2.627, p-value = 0.009). Similar male-specific negative associations as for the schizophrenia PRS and cognition could also be observed for the PCA-PRS, as shown for all individuals as well as sex-stratified in Supplementary Table 8 (cognitive performance) and Supplementary Table 9 (school performance).
Discussion
Using a large sample of healthy individuals, we found a robust interaction effect between schizophrenia PRS and sex on cognitive ability. A male-specific effect of the PRS was seen on school grades at age 12 across 6 subjects and on cognitive test performance across three domains, episodic memory, semantic memory, visuospatial ability in mid- to old age. Using a longitudinal data set spanning a 25-year period, we also showed that PRS was not associated with accelerated cognitive decline.
Sex differences can be described as sex-specific effects (presence in one sex only) or sex-dependent (quantitative differences between the sexes) [57]. In the present study, the association between the PRS and worse cognitive performance in mid- and old age, as well as worse school performance was seen in males only with no trend effects in females, indicating sex-specific effects of schizophrenia genetics on cognitive ability in healthy individuals across the entire lifespan. Although it is possible that there may be a smaller effect on cognitive ability in females that we could not detect in our study, there was no consistent direction of statistical effects for the different cognitive tests in females, which is suggestive of no effect even at trend level in this group.
Although it has been proposed that the impact of sex should be tested in genetic studies of schizophrenia given the significant differences in disease manifestation [57–59], to our knowledge, sex differences in the effect of schizophrenia PRS on cognition has only been considered in one previous study, where sex-stratified analyses showed that the effect of schizophrenia PRS on verbal memory and semantic fluency in older adults reached significance only in males [40]. Here, we were able to demonstrate a significant sex*PRS interaction thus demonstrating that there are statistically reliable differences in the effect of PRS between the sexes, and showed that this effect was not seen for a genetic score for general cognitive ability, but instead specific to schizophrenia genetics. In line with reported cognitive sexual dimorphisms [56], we show that males performed better on the visuospatial task, whereas females performed better on the memory tasks. Thus, our observations of a male-specific effect of schizophrenia genetics on both memory tasks and visuospatial ability indicate that this effect is independent of normal cognitive sexual dimorphisms. Finally, we show that male-specificity not only relates to cognitive functioning in adulthood and old age, but also to childhood cognitive ability.
A schizophrenia GWAS stratified by sex did not reveal any genome-wide significant sex-specific associations [60], but a genome-wide genotype-by-sex interaction analysis of risk for schizophrenia found genome-wide significant SNP-by-sex interactions [58]. However, no consistent sex differences in SNP-based heritability have been identified in schizophrenia [59]. Taken together, our results suggest that the effect of schizophrenia genetics on a certain disease endophenotype, cognitive ability, is sex-specific. As the effect of schizophrenia PRS on cognition might differ between healthy individuals and schizophrenia patients [61], our results warrant replication in patient cohorts to examine if sex differences are related to underlying disease genetics on specific symptoms and disease manifestations. Further, it will be relevant to not only replicate the observed sex-specific PRS effect for episodic memory, semantic memory, and visuospatial ability as investigated here, but also to explore its generality in other cognitive domains. Finally, to investigate if the male-specific effects on cognition are driven by specific biological pathways of schizophrenia, future studies investigating pathway-specific PRS would be of importance.
Using a long follow-up time, we did not observe any association between schizophrenia PRS and accelerated cognitive decline. These results are in contrast to a previous publication by McIntosh et al. [62] where schizophrenia PRS was associated with change in cognitive ability from childhood to old age in healthy individuals [62]. In a previous study of older adults, only a modest decline in overall cognitive performance associated with schizophrenia PRS could be observed [63], and in another two longitudinal studies of older adults [40, 64], no such association was found. In our sample, there was a trend for a negative interaction between age and PRS on cognition (p = 0.07), indicating that the effect of PRS on cognitive task performance may be somewhat stronger in young individuals. The cognitive deficits in schizophrenia patients may arise at younger age due to developmental causes, as also our school grade data suggest. Indeed, it has been shown that children who later developed schizophrenia had deficits in tests of processing speed, attention, visuospatial ability, and working memory when they entered school at age 7, and were further impaired in attention and working memory as they got older compared to children that did not develop schizophrenia [65]. This was further confirmed by our finding of worse school performance at age 12 in relation to schizophrenia PRS. However, it has also been reported that schizophrenia PRS has no effect on the association between low school performance and developing schizophrenia [66], which could be due to sex differences that were not considered in that study.
Pharmacological treatment options mainly include antipsychotics that primarily treat the positive symptoms [67], and besides non-pharmacological treatment options, there are currently no available medications that efficiently treat the cognitive symptoms in schizophrenia [68]. As sex differences observed in cognitive performance in schizophrenia may be due to differences in sex hormone levels, estradiol and other sex hormones have been suggested for the treatment of cognitive symptoms in schizophrenia [69, 70]. The selective estrogen receptor modulator raloxifene in combination with antipsychotics has shown beneficial effects on cognition in both women and men with schizophrenia [69–71]. However, the genetic mechanisms of sex differences related to cognitive processing in schizophrenia require further investigation. It should be tested if our results translate to schizophrenia patients, which may enable the development of sex-specific pharmocological treatments for schizophrenia and a greater understanding of the biology of cognitive impairments in the disease.
In conclusion, our results strongly suggest male-specific effects of schizophrenia genetics on episodic memory, semantic memory, and visuospatial ability in healthy individuals. Our findings indicate that the effect of underlying schizophrenia genetics on cognition is dependent on biological processes that differ between the sexes. These results open up for replication in patients and further investigations of interactions between schizophrenia genetics and biological processes with known sex differences, which could provide valuable insight into disease biology, and potentially lead to the development of novel sex-specific pharmacological treatment options.
Supplementary information
Author contributions
EK performed the data analyses and drafted the manuscript. LN and AL advised on data analysis, statistical methods, and interpretation of results. SP and RA provided critical data and advised on data analysis. KK was responsible for the study concept and design. LN is the principal investigator of the Betula study, SP is responsible for the Betula school grade data, and RA is responsible for the Biobank in the Betula study. All authors critically revised the manuscript.
Funding
This work was supported by a grant to KK from the Swedish Research Council (Grant no. 2017-03011). The retrieval of school grades was supported by a grant from the Royal Swedish Academy of Sciences (AS2015-0004) to SP. Open access funding provided by Umea University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01649-4.
References
- 1.Tsuang M, Lyons M, Faaone S. Heterogeneity of schizophrenia: conceptual models and analytic strategies. Br J Psychiatry. 1990;156:17–26. doi: 10.1192/bjp.156.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait. Arch Gen Psychiatry. 2003;60:1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 3.Young JW, Geyer MA, Diego S. Developing treatments for cognitive deficits in schizophrenia: the challenge of translation. J Psychopharmacol. 2015;29:178–96. doi: 10.1177/0269881114555252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J, Ragland J, Carter C. Memory and cognition in schizophrenia. Mol Psychiatry. 2019;24:633–42. doi: 10.1038/s41380-018-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Khandaker GM, Barnett JH, White IR, Jones PB. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res. 2011;132:220–7. doi: 10.1016/j.schres.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajji TK, Miranda D, Mulsant BH. Cognition, function, and disability in patients with schizophrenia: a review of longitudinal studies. Can J Psychiatry. 2014;59:13–17. doi: 10.1177/070674371405900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacCabe JH, Lambe MP, Cnattingius S, Torrång A, Björk C, Sham PC, et al. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med. 2008;38:1133–40. doi: 10.1017/S0033291707002048. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, Ohlsson H, Mezuk B, Sundquist K, Sundquist J. A Swedish national prospective and co-relative study of school achievement at age 16, and risk for schizophrenia, other nonaffective psychosis, and bipolar illness. Schizophr Bull. 2016;42:77–86. doi: 10.1093/schbul/sbv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones P, Murray R, Rodgers B, Marmot M. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–402. doi: 10.1016/S0140-6736(94)90569-X. [DOI] [PubMed] [Google Scholar]
- 11.Kraepelin E. Dementia praecox and paraphrenia. Robert E. Krieger Publishing Co.; 1971.
- 12.Jongsma HE, Turner C, Kirkbride JB, Jones PB. International incidence of psychotic disorders, 2002–17: a systematic review and meta-analysis. Lancet Public Heal. 2019;4:e229–e244. doi: 10.1016/S2468-2667(19)30056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Häfner H, Maurer K, Löffler W, Riecher-Rössler A. The influence of age and sex on the onset of early course of schizophrenia. Br J Psychiatry. 1993;162:80–86. doi: 10.1192/bjp.162.1.80. [DOI] [PubMed] [Google Scholar]
- 14.Moriarty PJ, Lieber D, Bennett A, White L, Parrella M, Harvey PD, et al. Gender differences in poor outcome patients with lifelong schizophrenia. Schizophr Bull. 2001;27:103–13. doi: 10.1093/oxfordjournals.schbul.a006850. [DOI] [PubMed] [Google Scholar]
- 15.Rubin L, Haas G, Keshavan M, Sweeney J, Maki P. Sex difference in cognitive response to antipsychotic treatment in first episode schizophrenia. Neuropsychopharmacology. 2008;33:290–7. doi: 10.1038/sj.npp.1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castle DJ, Wessely S, Murray RM. Sex and schizophrenia: effects of diagnostic stringency, and associations with premorbid variables. Br J Psychiatry. 1993;162:658–64. doi: 10.1192/bjp.162.5.658. [DOI] [PubMed] [Google Scholar]
- 17.Seidman LJ, Goldstein JM, Goodman JM, Koren D, Turner WM, Faraone SV, et al. Sex differences in olfactory identification and Wisconsin card sorting performance in schizophrenia: Relationship to attention and verbal ability. Biol Psychiatry. 1997;42:104–15. doi: 10.1016/S0006-3223(96)00300-9. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, et al. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry. 1998;155:1358–64. doi: 10.1176/ajp.155.10.1358. [DOI] [PubMed] [Google Scholar]
- 19.Sota TL, Heinrichs RW. Sex differences in verbal memory in schizophrenia patients treated with ‘typical’ neuroleptics. Schizophr Res. 2003;62:175–82. doi: 10.1016/S0920-9964(02)00373-0. [DOI] [PubMed] [Google Scholar]
- 20.Vaskinn A, Sundet K, Simonsen C, Hellvin T, Melle I, Andreassen OA. Sex differences in neuropsychological performance and social functioning in schizophrenia and bipolar disorder. Neuropsychology. 2011;25:499–510. doi: 10.1037/a0022677. [DOI] [PubMed] [Google Scholar]
- 21.Han M, Huang XF, Chen DC, Xiu MH, Hui L, Liu H, et al. Gender differences in cognitive function of patients with chronic schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:358–63. doi: 10.1016/j.pnpbp.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XY, Chen DC, Xiu MH, Yang FD, Haile CN, Kosten TA, et al. Gender differences in never-medicated first-episode schizophrenia and medicated chronic schizophrenia patients. J Clin Psychiatry. 2012;73:1025–33. doi: 10.4088/JCP.11m07422. [DOI] [PubMed] [Google Scholar]
- 23.Lewine RR, Walker EF, Shurett R, Caudle J, Haden C. Sex differences in neuropsychological functioning among schizophrenic patients. Am J Psychiatry. 1996;153:1178–84. doi: 10.1176/ajp.153.9.1178. [DOI] [PubMed] [Google Scholar]
- 24.Roesch-Ely D, Hornberger E, Weiland S, Hornstein C, Parzer P, Thomas C, et al. Do sex differences affect prefrontal cortex associated cognition in schizophrenia? Schizophr Res. 2009;107:255–61. doi: 10.1016/j.schres.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg TE, Gold JM, Torrey EF, Weinberger DR. Lack of sex differences in the neuropsychological performance of patients with schizophrenia. Am J Psychiatry. 1995;152:883–8. doi: 10.1176/ajp.152.6.883. [DOI] [PubMed] [Google Scholar]
- 26.Sweeney JA, Maki PM. Effects of sex, menstrual cycle phase, and endogenous Hormones on Cognition in Schizophrenia. Schizophr Res. 2016;166:269–75. doi: 10.1016/j.schres.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendrek A, Mancini-Marïe A. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev. 2016;67:57–78. doi: 10.1016/j.neubiorev.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–45. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Ripke S, Neale BM, Corvin A, Walters JT, Farh KH, Holmans PA, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Schizophrenia C, Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, et al. Common polygenic variation contributes to risk of schizophremia that overlaps with bipolar disease. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi SW, Mak TSH, O’Reilly PF. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc. 2020;15:2759–72. doi: 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smeland OB, Frei O, Kauppi K, Hill WD, Li W, Wang Y, et al. Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal-numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry. 2017;74:1065–75. doi: 10.1001/jamapsychiatry.2017.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hubbard L, Tansey KE, Rai D, Jones P, Ripke S, Chambert KD, et al. Evidence of common genetic overlap between schizophrenia and cognition. Schizophr Bull. 2016;42:832–42. doi: 10.1093/schbul/sbv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch E, Rosenthal B, Lundquist A, Chen C-H, Kauppi K. Interactome overlap between schizophrenia and cognition. Schizophr Res. 2020;222:167–74. doi: 10.1016/j.schres.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Wang SH, Hsiao PC, Yeh LL, Liu CM, Liu CC, Hwang TJ, et al. Polygenic risk for schizophrenia and neurocognitive performance in patients with schizophrenia. Genes, Brain Behav. 2018;17:49–55. doi: 10.1111/gbb.12401. [DOI] [PubMed] [Google Scholar]
- 37.Mallet J, Le Strat Y, Dubertret C, Gorwood P. Polygenic risk scores shed light on the relationship between schizophrenia and cognitive functioning: review and meta-analysis. J Clin Med. 2020;9:341. doi: 10.3390/jcm9020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Germine L, Robinson EB, Smoller JW, Calkins ME, Moore TM, Hakonarson H, et al. Association between polygenic risk for schizophrenia, neurocognition and social cognition across development. Transl Psychiatry. 2016;6:e924. doi: 10.1038/tp.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dezhina Z, Ranlund S, Kyriakopoulos M, Williams SCR, Dima D. A systematic review of associations between functional MRI activity and polygenic risk for schizophrenia and bipolar disorder. Brain Imaging Behav. 2019;13:862–77. doi: 10.1007/s11682-018-9879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kępińska AP, MacCabe JH, Cadar D, Steptoe A, Murray RM, Ajnakina O. Schizophrenia polygenic risk predicts general cognitive deficit but not cognitive decline in healthy older adults. Transl Psychiatry. 2020;10:1–9. doi: 10.1038/s41398-020-01114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson LG, Adolfsson R, Bäckman L, de Frias CM, Molander B, Nyberg L. Betula: a prospective cohort study on memory, health and aging. Aging, Neuropsychol Cogn. 2004;11:134–48. doi: 10.1080/13825580490511026. [DOI] [Google Scholar]
- 42.Nyberg L, Boraxbekk CJ, Sörman DE, Hansson P, Herlitz A, Kauppi K, et al. Biological and environmental predictors of heterogeneity in neurocognitive ageing: evidence from Betula and other longitudinal studies. Ageing Res Rev. 2020;64:1–23. doi: 10.1016/j.arr.2020.101184. [DOI] [PubMed] [Google Scholar]
- 43.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington; 2000.
- 44.Rönnlund M, Sundström A, Adolfsson R, Nilsson LG. Subjective memory impairment in older adults predicts future dementia independent of baseline memory performance: evidence from the Betula prospective cohort study. Alzheimer’s Dement. 2015;11:1385–92. doi: 10.1016/j.jalz.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Kauppi K, Rönnlund M, Nordin Adolfsson A, Pudas S, Adolfsson R. Effects of polygenic risk for Alzheimer’s disease on rate of cognitive decline in normal aging. Transl Psychiatry. 2020;10:1–8. doi: 10.1038/s41398-020-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam M, Awasthi S, Watson HJ, Goldstein J, Panagiotaropoulou G, Trubetskoy V, et al. Genome analysis RICOPILI: Rapid Imputation for COnsortias PIpeLIne. Bioinformatics. 2020;36:930–3. doi: 10.1093/bioinformatics/btz633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2016;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2013;44:955–9. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calafato MS, Thygesen JH, Ranlund S, Zartaloudi E, Cahn W, Crespo-Facorro B, et al. Use of schizophrenia and bipolar disorder polygenic risk scores to identify psychotic disorders. Br J Psychiatry. 2018;213:535–41. doi: 10.1192/bjp.2018.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coombes BJ, Ploner A, Bergen SE, Biernacka JM. A principal component approach to improve association testing with polygenic risk scores. Genet Epidemiol. 2020;44:676–86. doi: 10.1002/gepi.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–21. doi: 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rönnlund M, Nilsson LG. Adult life-span patterns in WAIS-R Block Design performance: cross-sectional versus longitudinal age gradients and relations to demographic factors. Intelligence. 2006;34:63–78. doi: 10.1016/j.intell.2005.06.004. [DOI] [Google Scholar]
- 55.Pudas S, Rönnlund M, Gutchess A. School performance and educational attainment as early-life predictors of age-related memory decline: protective influences in later-born cohorts. J Gerontol - Ser B. 2019;74:1356–65. doi: 10.1093/geronb/gby137. [DOI] [PubMed] [Google Scholar]
- 56.Hyde JS. Sex and cognition: gender and cognitive functions. Curr Opin Neurobiol. 2016;38:53–6. doi: 10.1016/j.conb.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein JM, Cherkerzian S, Tsuang MT, Petryshen TL. Sex differences in the genetic risk for schizophrenia: history of the evidence for sex-specific and sex-dependent effects. Am J Med Genet Part B Neuropsychiatr Genet. 2013;162:698–710. doi: 10.1002/ajmg.b.32159. [DOI] [PubMed] [Google Scholar]
- 58.Blokland G, Grove J, Chen CY, Cotsapas C, Tobet S, Handa R, et al. Sex-dependent shared and non-shared genetic architecture across mood and psychotic disorders. Biol Psychiatry. 2021;21:S0006–3223. doi: 10.1016/j.biopsych.2021.02.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin J, Khramtsova EA, Goleva SB, Blokland G, Traglia M, Walters RK, et al. Examining sex-differentiated genetic effects across neuropsychiatric and behavioral traits. Biol Psychiatry. 2021;89:1127–37. doi: 10.1016/j.biopsych.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergen SE, O'Dushlaine CT, Lee PH, Fanous AH, Ruderfer DM, Ripke S, et al. Genetic modifiers and subtypes in schizophrenia: investigations of age at onset, severity, sex and family history. Schizophr Res. 2014;154:48–53. doi: 10.1016/j.schres.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richards AL, Pardiñas AF, Frizzati A, Tansey KE, Lynham AJ, Holmans P, et al. The relationship between polygenic risk scores and cognition in Schizophrenia. Schizophr Bull. 2020;46:336–44. doi: 10.1093/schbul/sbz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McIntosh AM, Gow A, Luciano M, Davies G, Liewald DC, Harris SE, et al. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol Psychiatry. 2013;73:938–43. doi: 10.1016/j.biopsych.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Ritchie SJ, Hill WD, Marioni RE, Davies G, Hagenaars SP, Harris SE, et al. Polygenic predictors of age-related decline in cognitive ability. Mol Psychiatry. 2019;25:2584–98. doi: 10.1038/s41380-019-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liebers DT, Pirooznia M, Seiffudin F, Musliner KL, Zandi PP, Goes FS. Polygenic risk of schizophrenia and cognition in a population-based survey of older adults. Schizophr Bull. 2016;42:984–91. doi: 10.1093/schbul/sbw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–9. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sørensen HJ, Debost JC, Agerbo E, Benros ME, McGrath JJ, Mortensen PB, et al. Polygenic risk scores, school achievement, and risk for schizophrenia: a Danish population-based study. Biol Psychiatry. 2018;84:684–91. doi: 10.1016/j.biopsych.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Rampino A, Marakhovskaia A, Soares-Silva T, Torretta S, Veneziani F, Beaulieu JM. Antipsychotic drug responsiveness and dopamine receptor signaling; old players and new prospects. Front Psychiatry. 2019;10:1–13. doi: 10.3389/fpsyt.2018.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riaz N, Wolden SL, Gelblum DY, Eric J. Developing treatments for cognitive deficits in schizophrenia: the challenge of translation. J Psychopharmacol. 2016;118:6072–8. doi: 10.1177/0269881114555252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gogos A, Ney LJ, Seymour N, Van Rheenen TE, Felmingham KL. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: are gonadal hormones the link? Br J Pharmacol. 2019;176:4119–35. doi: 10.1111/bph.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGregor C, Riordan A, Thornton J. Estrogens and the cognitive symptoms of schizophrenia: possible neuroprotective mechanisms. Front Neuroendocrinol. 2017;47:19–33. doi: 10.1016/j.yfrne.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Weickert TW, Weinberg D, Lenroot R, Catts SV, Wells R, Vercammen A, et al. Adjunctive raloxifene treatment improves attention and memory in men and women with schizophrenia. Mol Psychiatry. 2015;20:685–94. doi: 10.1038/mp.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.