Abstract
Brain α2-containing GABAA receptors play a critical role in the modulation of anxiety- and fear-like behavior. However, it is unknown whether these receptors also play a role in modulating resilience to chronic stress, and in which brain areas and cell types such an effect would be mediated. We evaluated the role of α2-containing GABAA receptors following chronic social defeat stress using male mice deficient in the α2 subunit globally or conditionally in dopamine D1- or D2-receptor-expressing neurons, e.g., within the nucleus accumbens (NAc). In addition, we examined the effect of the lack of the α2 subunit on intermediates of the glutathione synthesis pathway. We found that α2-containing GABAA receptors on D2-receptor-positive but not on D1-receptor-positive neurons promote resiliency to chronic social defeat stress, as reflected in social interaction tests. The pro-resiliency effects of α2-containing GABAA receptors on D2-receptor-positive neurons do not appear to be directly related to alterations in anxiety-like behavior, as reflected in the elevated plus-maze, light–dark box, and novel open field tests. Increases in indices of oxidative stress—reflected by increases in cystathionine levels and reductions in GSH/GSSG ratios—were found in the NAc and prefrontal cortex but not in the hippocampus of mice lacking α2-containing GABAA receptors. We conclude that α2-containing GABAA receptors within specific brain areas and cell populations promote stress resiliency independently of direct effects on anxiety-like behaviors. A potential mechanism contributing to this increased resiliency is the protection that α2-containing GABAA receptors provide against oxidative stress in NAc and the prefrontal cortex.
Subject terms: Ion channels in the nervous system, Behavioural genetics
Introduction
Stress is an important factor in the development of neuropsychiatric disorders such as major depressive disorder, generalized anxiety disorder, and post-traumatic stress disorder (PTSD). While most individuals display high resilience to stress and do not develop stress-related disorders, clinical studies have demonstrated that both exposures to stress earlier in life and genetic factors correlate with a heightened vulnerability to developing mood and anxiety disorders [1–5]. A stress sensitization hypothesis has been proposed whereby these genetic and environmental factors lower the threshold for the onset of depression following exposure to stressful episodes [6, 7]. Stress sensitization has also been observed in preclinical studies, where pre-exposure to stress enhances susceptibility to subsequent stressors, leading to depressive- and anxiety-like behaviors as well as cellular and molecular changes such as enhanced oxidative stress, which are particularly prevalent in depression [3, 8–10]. Understanding how gene × environment interactions enhance susceptibility to developing depression is critical to understanding the etiology of the disease.
Accumulating evidence suggests a role for the inhibitory neurotransmitter γ-aminobutyric acid (GABA) and its type-A receptor (GABAAR) in stress and depression. Clinical studies have identified decreased GABA levels in the cortex of patients with depression, while increases in GABA levels have been implicated in the therapeutic effects of standard antidepressants (ADs) [11–14]. GABAAR properties are defined by the assembly of five subunits, divided into subclasses based on sequence homology; α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3 [15]. Alterations in GABAAR subunit expression are also observed in the cortex of depressed patients following a suicide, as well as in mice exposed to stress [16]. In particular, decreases in γ2 subunit levels are observed in brain regions including the cortex and amygdala in mice exposed to maternal separation [17], and α2 subunit levels are decreased in the cortex and hippocampus of rats that show anxiety-like phenotypes following chronic exposure to restraint stress [18].
The role of individual GABAAR subunits in depression has been examined using genetically modified mice. Global heterozygous knockdown of the GABAAR γ2 subunit leads to hyperactivation of the HPA axis as well as depressive- and anxiety-like behaviors [19, 20]. Furthermore, we previously demonstrated that global knockdown of the GABAAR α2 subunit also leads to depressive-like (despair) and anxiety-like behavior [21, 22]. Surprisingly, we observed that chronic fluoxetine treatment has prodepressant- and anxiogenic-like effects in mice lacking α2-containing GABAARs [23]. Similar prodepressant-like effects of fluoxetine treatment have been observed in mice raised in stressful conditions [24]. We hypothesize that the knockdown of α2-containing GABAARs contributes to an enhanced reaction to the acute stress associated with the behavioral tasks utilized and to the prodepressant-like effects of fluoxetine. Clinical studies also support this hypothesis, as patients that expressed polymorphisms in the α2 subunit gene (GABRA2) and reported experiencing childhood trauma were at a greater risk for developing stress-related illness in adulthood [25]. The current studies were designed to further examine the potential impact of α2-containing GABAARs on susceptibility to partial social isolation, moderate or severe chronic stress, with “partial social isolation” referring to the housing conditions of control mice in the chronic social defeat paradigm, “moderate stress” referring to 5 days of chronic social defeat and “severe stress” referring to 10 days of chronic social defeat. Utilizing this spectrum of chronic stress, we examined the influence of α2-containing GABAARs on stress-induced changes in sociability and on a bias for threat cues in an ambiguous cue discrimination paradigm. To begin to identify specific subregions involved, baseline differences in markers of oxidative stress were measured in regions of the brain that have been implicated in depression and also have high Gabra2 expression (nucleus accumbens [NAc], medial prefrontal cortex [mPFC], and hippocampus). Furthermore, a conditional knockdown approach was utilized to identify a molecularly defined subpopulation of NAc neurons suppressing stress susceptibility via α2-containing GABAARs.
Materials and methods
Mice
All experiments were performed with male mice and were approved by the McLean Hospital Institutional Animal Care and Use Committee and in accordance with the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals (Eighth edition, 2011). Mice were bred on the C57BL/6J background (origin: Jackson Laboratory, Bar Harbor, ME). Male global α2 knockout (Gabra2tm2.2Uru) (wild type: α2+/+ and homozygous knockout: α2−/−, mice), as well as conditional D1- (α2FF and α2FF/D1-Cre) and D2-α2 knockout mice (α2FF and α2FF/D2-Cre), were biopsied for genotyping and ear-tagged at 3 weeks of age. The D1-Cre and D2-Cre mice were generated by the GENSAT project [26] and obtained from Dr. Nathaniel Heintz, and generation of the α2 floxed mice (Gabra2tm2.1Uru) has been reported previously [27]. Separate α2FF control groups were generated for the two conditional knockout strains. Food and water were provided ad libitum throughout the course of the experiments. Subjects were 6–12 weeks of age at the time of behavioral testing. Since stronger despair-like behavior was previously reported in α2−/− as compared to α2+/− and α2+/+ mice [21] and there were multiple variables being considered (defeat and genotype), only α2−/− and α2+/+ were examined in studies utilizing global α2 knockout mice. Mice were group-housed before experiments unless described otherwise. Lights were on from 0700 to 1900. Experiments were performed during the light period of the light cycle.
Behavioral experiments
Social defeat
The 10-day chronic social defeat stress (CSDS) paradigm was adapted from published work [28–30]. Male CD-1 retired breeders (obtained from Charles River Laboratories) were screened for aggressive behavior prior to the start of defeats. In the 5-day sub-CSDS studies, aggressive CD-1 mice were selected that had not been used in other experiments within 2 weeks. Target (Defeat) mice were introduced to the home cage of an aggressive CD-1 mouse, physically defeated for 10 min, and then separated by a perforated divider allowing for sensory contact over the next 24 h. The CD-1 and target mice had access to food and water within their respective cage side over the 24-h period. The defeat stress was repeated for 10 (CSDS) or 5 (sub-CSDS) days, with the defeated mouse being exposed to a novel aggressive CD-1 mouse each day. Control mice were handled daily and housed in a similarly sized cage with a conspecific on the opposite side of a perforated divider.
Following CSDS or subCSDS, all control and defeat mice were single housed for 24 h prior to the assessment of stress-induced social avoidance behavior using the social interaction test (SIT). The SIT chamber is a white polycarbonate box with a clear bottom, measuring 42 cm × 42 cm. An interaction zone (IZ) measuring 14 cm × 24 cm is defined on one of the sides of the chamber, while 9 cm × 9 cm squares are defined in the two opposite corners. A chrome cylindrical wire cage (11 cm tall with a bottom diameter of 10.5 cm and bars spaced 1 cm apart) is placed at the center of the IZ. Experimental mice were habituated to the testing chamber with an empty wire cage under red light conditions for 15 minutes for 2 days. On the day of the SIT, experimental mice were habituated to the testing room under the same lighting conditions for 1 h prior to testing, and then exposed to the SIT chamber for two 2.5-min trials; in Trial 1 an empty wire cage was placed in the IZ, in Trial 2 a novel aggressive CD-1 was placed under a different wire cage in the same location. Time spent in the IZ, the average distance from the IZ, time spent in the opposite corners, and average distance traveled during the two trials were tracked using Ethovision (Ethovision XT, Noldus Information Technology, Netherlands).
Open field
The open field chamber consists of a 42 cm L × 42 cm W × 31 cm H clear plexiglass arena. For the novel open field, the chamber was evenly lit to 100 lux. To assess anxiety-like behavior and locomotor activity in the novel open field, mice were placed into one corner of the testing chamber and allowed to explore freely for 30 minutes. Percentage time spent in the center (20 cm × 20 cm) and total distance traveled (cm) were analyzed using Ethovision. For the familiar open field, mice were retested in the same arena 1 day later under red light conditions. Locomotor activity over the course of the 30-min trial was assessed using the Ethovision XT system and analyzed as total distance traveled (cm).
Three-chamber SIT
The testing apparatus is a rectangular polycarbonate box that is separated into three chambers, each measuring 20 cm L × 40.5 cm W × 22.5 cm H. The two center chamber walls have removable partitions in the middle, which when removed allows mice to move freely between the three chambers. Two chrome cylindrical wire cages (11 cm H, bottom diameter 10.5 cm, with bars spaced 1 cm apart) were placed in the back-outer corners of the two side chambers. A weighted cup was placed on the top of the cages to prevent the experimental mice from climbing on the top of the wire cages. Mice were habituated to the 40-lux testing room for 30 min before testing. Following room habituation, mice were habituated to the testing chamber for 10 min. For the habituation trial, experimental mice were introduced to the center chamber with both partitions raised and time spent in the three chambers (with empty wire cages placed in the two back corners of the side chambers) was recorded using Ethovision. Following the chamber habituation trial, the experimental mouse was confined to the center chamber while a novel C57BL/6J control mouse was placed under one wire cage and a similar-sized novel object (black paperclip) was placed under the other wire cage. The side chamber location of the object and control mouse was varied per experimental trial. When clear side chamber preferences were observed during the habituation trial, the novel mouse location was counterbalanced and placed under the cage in the non-preferred side chamber. For the experimental trial, the partitions were raised again, and the experimental mouse explored the three chambers for an additional 10 min. Time spent in the three chambers and average distance from the two cups was recorded using Ethovision.
Ambiguous cue discrimination
The ambiguous cue discrimination protocol was adapted from published work [31]. The experiment was composed of 5 sessions each lasting 1600 s and separated by 24 h: pre-exposure (PE), 3 training days (T1–T3), and fear memory recall (FMR). Each day, mice were placed into fear conditioning chambers (Med Associates Inc., St. Albans, VT, USA) with an olfactory cue (peppermint extract on PE, T1–3 and 0.05% benzaldehyde on FMR) in the waste tray. After an initial 300 s habituation period, mice were exposed to 10 auditory cues of either 7000 Hz intermittent tone (72 dB, 5 s on and 5 s off for a total of 30 s) or white noise (65 dB, 30 s). During the training days, each cue was paired with a mild foot shock (0.3 mA, 0.5 s) on either 100% (CS+) or 20% (CS20) of total cue presentations. Mice received a total of 6 shocks per day on T1–T3, with no shocks given on PE or FMR days. On FMR, mice were placed into a different fear conditioning chamber with an insert covering the floor and a new olfactory cue. Auditory cue-CS type pairing was counterbalanced across groups and the position of the CS20 shock was randomized. Between each group, the chambers and waste trays were cleaned with water. For subCSDS-Ambiguous Cue, mice were defeated for 5 days as described above, single housed, and then tested in the ambiguous cue discrimination paradigm. Context-evoked freezing was measured by calculating the percentage of time spent freezing during the initial 300 s of the PE and FMR trials (% Fz). Cue-evoked freezing responses were measured by calculating the difference between the percentage of time spent freezing during and before the 30 s of tone presentation on FMR (% Fz diff).
Forced swim test (FST)
The FST was performed as previously described [23]. Despair-like behavior was manually scored by an observer blinded to genotype using JWatcher [32], and latency to first immobility and time spent immobile over the course of the entire 6-minute trial were assessed. Immobility was defined as lack of movement, or only those motions necessary to remain afloat.
Light–dark box (LDB)
The LDB test was carried out as described previously [33], with some minor modifications. The LDB testing apparatus is composed of one open brightly lit (250 lux) chamber (28 cm L × 28 cm W × 31 cm H) and one covered dark chamber (14 cm L × 14 cm W × 31 cm H) connected by an opening (5 cm × 5 cm). The testing room was lit to 70 lux, and D1- and D2-α2KO mice, as well as their respective α2FF cage mates, were habituated to the test room 1 h before testing. At the start of the 6-min trial, the experimental mouse was placed within the dark chamber and allowed to freely explore the two chambers. Time spent in the open chamber was recorded using Ethovision XT, and percent time in light ((time in the clear chamber)/ 6 min × 100) was calculated as a measure of anxiety-like behavior.
RNA extraction, reverse transcription, and quantitative polymerase chain reaction (PCR)
Following rapid decapitation and removal of the brain, bilateral 1 mm punches of the NAc were taken from 1 mm thick coronal sections and then immediately flash frozen on dry ice. RNA was isolated using the Qiagen miRNeasy micro kit (Qiagen, Germany). RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, California). Quantitative PCR was performed using Taqman gene expression assays (Applied Biosystems, Foster City, CA) for Gabra2 (Mm00433435_m1) and β-actin (Mm00607939_s1). All samples were run in triplicate and normalized to a calibrator sample (from the hippocampus) across RT-PCR plates. Relative changes in gene expression were quantified using the ΔΔCt method and expressed as percent change for α2FF/D1-Cre and α2FF/D2-Cre mice compared to their respective littermate control α2FF.
High-performance liquid chromatography (HPLC)
NAc, mPFC, and hippocampus were dissected in ice-cold PBS (pH 5) following rapid decapitation from global α2+/+ and α2−/− mice at 7–10 weeks of age. Tissue samples were immediately placed in microcentrifuge tubes and flash frozen with ice-cold isopentane. Frozen tissue was weighed and sonicated (approximately 1:10 w-v) in ice-cold 0.03 N perchloric acid solution. Protein and cellular debris were cleared by centrifugation at 20,000 × g at 4 °C for 45 min. Pellets were resuspended in 100 μl 0.1 N NaOH and protein content was measured for normalization using a Micro BCATM Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Tissue homogenates, cleared of protein and debris (10 μl) were injected onto an Eclipse XDB-C8 (3 × 150 mm, 3.5 μm) reverse-phase C8 column (Agilent, Santa Clara, CA) using a refrigerated autosampler (System Gold 508, Beckman Coulter Brea, CA) and measured with a CouloChem III running a BDD analytical cell model 5040 (ThermoFisher) electrochemical detector at an operating potential of 1500 mV. A dual mobile phase gradient elution was used to resolve analytes, consisting of mobile phase containing sodium phosphate (Sigma) 25 mM and 1-octanesulfonic acid (Sigma) 2.1 mM, adjusted to pH 2.65 with phosphoric acid (Sigma), with the second mobile phase (B) containing 50% acetonitrile (Sigma). The system was run at a flow rate of 1 mL/min at ambient temperature with the following gradients: 0 to 8 min 0% B, 8–20 min, gradient to 30% B. The system equilibrated at 0% B from 25 to 36 min. Peak area analysis (Beckman Coulter 32-Karat software v.8.0) was based on standard curves generated for each compound. Samples were normalized against protein content.
Statistical analyses
Statistical analysis was performed using SigmaPlot versions 11 and 14 (Systat Inc., San Jose, CA) and with GraphPad Prism (San Diego, CA). Statistical significance was evaluated using a 95% confidence interval. The overall variance was tested using a two-way analysis of variance (ANOVA) (CSDS, sub-CSDS, open field tests following CSDS, three-chamber SIT, and ambiguous cue context-evoked freezing following sub-CSDS, one-way ANOVA (forced swim test, LDB, novel open field), mixed-design three-way ANOVA (ambiguous cue discrimination) and Kruskal–Wallis one-way ANOVA based on ranks (familiar open field). When significant interactions or main effects were detected, differences between groups were resolved using the Holm–Sidak post hoc test. HPLC experiments were analyzed with a two-way ANOVA followed by Tukey’s multiple comparison test. Student’s t test (qRT-PCR experiments) and Mann Whitney U test (baseline ambiguous cue discrimination) were used to assessing variance. Outliers were identified using Grubb’s test. Data are graphically expressed as means ± the standard error of the mean.
Results
Mice globally lacking α2-containing GABAA receptors exhibit enhanced social avoidance to sub-CSDS but not to CSDS
To examine the influence of α2-containing GABAARs in chronic stress, we assessed stress-induced changes in sociability after “severe stress”, i.e., following 10 days of CSDS using the one-chamber SIT. Sociability was reduced in defeated α2+/+ and α2−/− mice. Defeated α2+/+ mice spent less time in the IZ (Fig. S1A) and had a greater average distance to the IZ (Fig. S1C) in the presence of a novel aggressive CD-1 (Trial 2) as compared to control α2+/+ mice. Surprisingly, control α2−/− mice appeared to spend less time in the IZ and averaged a greater distance to the IZ in Trial 2 although neither measure was statistically significant (p = 0.06 and 0 = 0.061 in Fig. S1A and S1C). When time in the opposite corners was assessed, only defeated α2−/− mice spent more time in the opposite corners in Trial 2 as compared to controls (Fig. S1D). No differences were detected in any measurement in Trial 1 (Fig. S1A, C, D). When time in the IZs and corners were compared between the two trials as ratios (Trial 2/Trial 1), both defeated α2+/+ and α2−/− mice had lower IZ ratios (Fig. S1B) and higher corner ratios (Fig. S1E) as compared to control mice. Control and defeated α2−/− mice moved less during the SIT as compared to control and defeated α2+/+ mice (Fig. S1F).
To examine stress-induced changes in mobility and anxiety, mice were tested in the novel and familiar open field following CSDS. In the novel open field test, which quantifies locomotor activity and anxiety-like behavior, both control and defeated α2−/− mice spent significantly less time in the center (Fig. S2A) and moved less (Fig. S2B) as compared to α2+/+ mice. These findings are not surprising, given that α2-containing GABAARs have been implicated in the modulation of anxiety [22, 34–36]. In the familiar open field, which quantifies locomotor activity but not anxiety-like behavior, no differences in mobility were detected (Fig. S2C) suggesting that the changes in mobility observed in the SIT can likely be attributed to anxiety-related to the stress rather than a change in general locomotor behavior. While these findings do not negate the results reported in the other measurements of the SIT, they are important to consider when interpreting differences between genotypes.
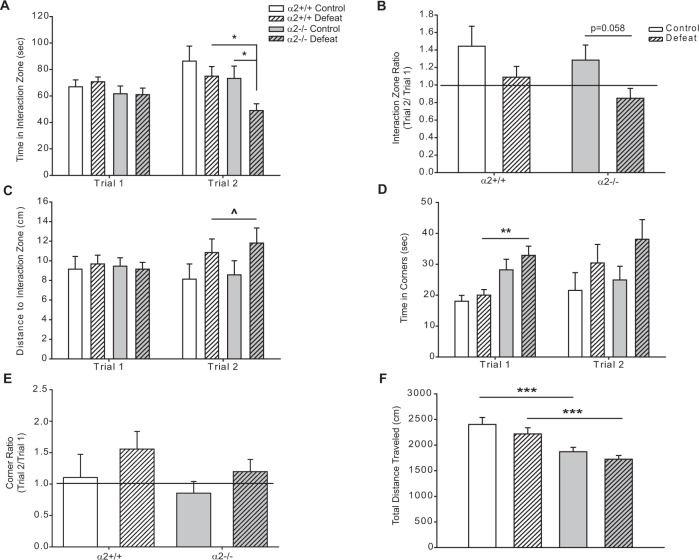
While no differences in CSDS-induced sociability were detected between α2+/+ and α2−/− defeat mice, enhanced despair-like behavior had previously been reported in α2−/− compared to α2+/+ mice [21]. It is possible that CSDS may have produced a ceiling effect, masking genotype differences in stress-induced sociability. The observed trend towards reduced sociability in control α2−/− mice (Fig. S1A, C), where 10 days of the control condition which involved partial social isolation with no physical contact with the cage-mate mouse suggested that a more moderate form of social stress might allow for the detection of genotype differences. Accordingly, we developed a “moderate stress” protocol with only five days of social defeat (sub-CSDS), and changes in sociability were assessed through the SIT. Following moderate stress, sociability was reduced in α2−/− but not α2+/+ mice. Defeated α2−/− mice spent less time in the IZ as compared to control α2−/− and defeated α2+/+ mice in Trial 2 (Fig. 1). Thus, α2−/− mice seem to be vulnerable to a moderate stressor that does not affect α2+/+ mice. This moderate stress did elicit an increase in the time in corners in trial 1 in α2−/− mice (Fig. 1D), although the distance to the IZ which also takes into account time spent in the corners was unaltered (Fig. 1C). However, the moderate stressor did not elicit other behavioral changes in Trial 2 in either α2+/+ or α2−/− mice (Fig. 1B–E), in contrast to the severe stress (Fig. S1). As previously observed under the severe stress condition, α2−/− mice subjected to moderate stress exhibited less locomotor activity over the course of the SIT as compared to α2+/+ mice (Fig. 1F). The behavior of the α2−/− mice after moderate stress is unlikely to be due to a general impairment, as both α2+/+ and α2−/− mice exhibited strong sociability in a three-chamber SIT (Fig. S3).
Fig. 1. Global knockdown of α2-GABAARs increases susceptibility to 5 days of social defeat stress (“moderate stress”).
A Five days of defeat stress reduces time spent in the interaction zone in α2−/− mice in Trial 2 (Two-way ANOVA, main effect of defeat F1,61 = 4.89, p < 0.05; main effect of genotype F1,61 = 5.843, p < 0.05, defeat × genotype interaction F1,61 = 0.633, p > 0.05) but not in Trial 1 (two-way ANOVA, the main effect of defeat F1,60 = 0.00581, p > 0.05, main effect of genotype F1,60 = 3.561, p > 0.05, defeat × genotype interaction F1,60 = 0.692, p > 0.05). B Ratios of time in the interaction zone (Trial 2/Trial 1) are reduced in α2−/− mice (trend p = 0.058) following defeats (two-way ANOVA, main effect of defeat F1,60 = 4.274, p = 0.043, main effect of genotype F1,60 = 1.234, p > 0.05, defeat × genotype interaction F1,60 = 0.344, p > 0.05). Line denotes the ratio of 1, animals with interaction zone ratios ≥ 1 are considered sociable. C Five days of defeat stress increases the average distance from the interaction zone in Trial 2, although no group differences were detected through post hoc analysis (two-way ANOVA, the main effect of defeat F1,64 = 4.515, p < 0.05, the main effect of genotype F1,64 = 0.259, p > 0.05, defeat × genotype interaction F1,64 = 0.0163, p > 0.05). The average distance from the interaction zone did not vary in Trial 1 (two-way ANOVA, the main effect of defeat F1,63 = 0.0429, p > 0.05, the main effect of genotype F1,63 = 0.00106, p > 0.05, defeat × genotype interaction F1,63 = 0.318, p > 0.05). D Five days of defeat stress does not alter time spent in the opposite corners in Trial 2 (two-way ANOVA, main effect of defeat F1,61 = 3.564, p > 0.05, main effect of genotype F1,61 = 0.853, p > 0.05, defeat × genotype interaction F1,59 = 0.00628, p > 0.05) but defeat α2−/− mice spent more time in the opposite corner in Trial 1 (two-way ANOVA, main effect of defeat F1,59 = 0.747, p > 0.05, main effect of genotype F1,59 = 12.473, p < 0.001, defeat × genotype interaction F1,59 = 0.00628, p > 0.05). E No differences in corner ratios were detected following 5 days of defeat stress (two-way ANOVA, main effect of defeat F1,58 = 3.219, p > 0.05, main effect of genotype F1,58 = 0.795, p > 0.05, defeat × genotype interaction F1,58 = 0.168 p > 0.05). Line denotes the ratio of 1, animals with corner ratios ≤ 1 are considered sociable. F Total distance traveled during the SIT is decreased with α2-GABAAR deletion (two-way ANOVA, main effect of genotype F1,61 = 25.957, p < 0.001, main effect of defeat F1,61 = 2.714, p > 0.05, defeat × genotype interaction F1,61 = 0.0368, p > 0.05). Control α2+/+ n = 12, defeat α2+/+ n = 18, control α2−/− n = 15, defeat α2−/− n = 21. *^p < 0.05, **p < 0.01, ***p < 0.001.
Mice globally lacking α2-containing GABAA receptors exhibit an enhanced bias for threat cues
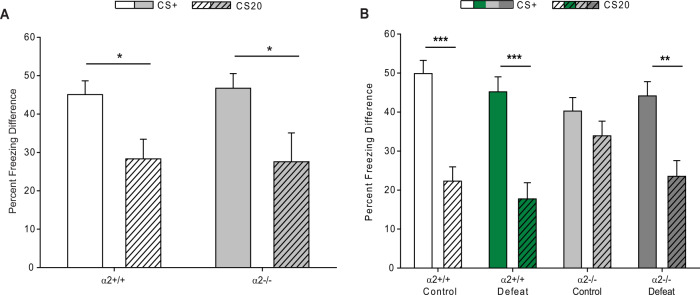
The impact of stress and α2-GABAARs on depression-related behaviors was further characterized by examining bias for threat cues (negativity bias is a cognitive symptom of depression in humans) through an ambiguous cue discrimination protocol. At baseline (no defeat), both α2+/+ and α2−/− mice froze significantly more in response to the CS+ than the CS20 tone (Fig. 2A), indicating their ability to discriminate between the two conditions. In addition, no differences in context-evoked freezing were detected between α2+/+ and α2−/− mice (Fig. S4A). After moderate stress (sub-CSDS), both control and defeat α2+/+ mice as well as defeat α2−/− mice could discriminate between the CS+ and the CS20 tones (Fig. 2B). Surprisingly, α2−/− mice that have undergone five days of the control condition of the chronic social defeat protocol which involved partial social isolation with no physical contact with the cage-mate mouse (“mild stress”) demonstrated heightened freezing behavior to the CS20, freezing for a similar percentage of time to the CS20 and CS+ conditions (Fig. 2B). Differences in context-evoked freezing were detected before and after ambiguous cue training. On the pre-exposure training day, defeat α2−/− mice froze significantly more. Exposure to moderate stress and global knockdown of Gabra2 enhanced context-evoked freezing on the FMR day, although group differences could not be resolved (Fig. S4B).
Fig. 2. Global knockdown of αα2-GABAARs results in bias for threat cues.
A At baseline both α2+/+ (Mann–Whitney Rank Sum test p < 0.05, n = 5) and α2−/− (Mann–Whitney Rank Sum test p < 0.05, n = 5) mice freeze more in response to the CS+ tone than the CS20 tones. B Following 5 days of social defeat stress (or partial social isolation or “mild stress” for the control mice), control α2+/+ (p < 0.001), defeat α2+/+ (p < 0.001), and defeat α2−/− mice (p < 0.01) freeze more in response to the CS+ tone than the CS20 tone. Control α2−/− mice freeze for a similar amount of time to the CS20 and CS+ tones, exhibiting heightened freezing in response to the CS20 tone (p > 0.05) (three-way ANOVA, mixed-effects model: main effect of trial type (CS20, CS+) F1,151 = 64.96, p < 0.0001, main effect of genotype F1,151 = 0.3719, p > 0.05, main effect of the social environment (control, social defeat stress) F1,151 = 2.026, p > 0.05, trial-type × genotype interaction F1,151 = 7.608, p < 0.01, trial-type × social environment interaction F1,151 = 1.933, p > 0.05; genotype × social defeat interaction F1,151 = 0.06083, p > 0.05, trial-type × genotype × social environment interaction F1,151 = 2.006, p > 0.05). Control α2+/+ n = 8, defeat α2+/+ n = 8, control α2−/− n = 7, defeat α2−/− n = 8. *p < 0.05, **p < 0.01, ***p < 0.001.
Markers of oxidative stress are elevated at baseline in mice globally lacking α2-containing GABAA receptors
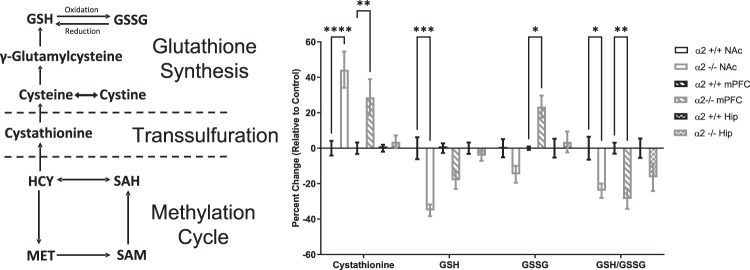
Accumulating evidence implicates heightened inflammatory processes in the development of neuropsychiatric disorders such as anxiety, depression, bipolar disorder, and schizophrenia [37]. Imbalances between reactive oxygen species and protective antioxidants contribute to a state of oxidative stress, contributing to cellular damage and ultimately cell death [38]. Decreases in antioxidant levels, in particular glutathione, are reported in the cortex of depressed patients as well as rodents exposed to stress [38–40]. Furthermore, rodent studies demonstrated that oxidative stress may enhance susceptibility to stress-induced depression-like behavior as mice lacking the redox-sensitive transcription factor Nrf2 exhibit high levels of oxidative stress and depressive-like behavior following exposure to mild forms of stress, which is prevented with antioxidant treatment [8]. In addition, knockdown of Nrf2 was sufficient to induce elevated oxidative stress and a vulnerability to stress-induced depressive-like behavior [8]. These studies provide evidence that alterations in redox-related genes can enhance susceptibility to environmental stress. As we observed that global knockdown of α2-GABAARs enhances susceptibility to environmental stress, we examined the possibility that reductions in α2-GABAAR function might be sufficient to induce oxidative stress. Utilizing changes in components of the glutathione synthesis pathway as readout, levels of oxidative stress were measured in the NAc, mPFC, and hippocampus of α2+/+ and α2−/− mice. When in its reduced state (GSH), glutathione can donate an electron to help neutralize free radicals, in turn becoming reactive and forming glutathione disulfide (GSSG) [41]. When the oxidant/antioxidant balance is tipped in times of oxidative stress, there is a greater demand for glutathione, GSH is depleted, and levels of GSSG are elevated. Region-specific increases in oxidative stress were detected in α2−/− mice (Fig. 3). Levels of GSH were decreased in the NAc and the ratio of GSH/GSSG was decreased in the NAc and mPFC, while cystathionine (a precursor for glutathione) was increased in the NAc and mPFC in α2−/− mice as compared to α2+/+ mice. GSSG levels were also increased in the NAc of α2−/− mice as compared to α2+/+ mice. No differences were detected in the hippocampus.
Fig. 3. Global knockdown of α2-GABAARs produces region-specific changes in glutathione production.
Left panel. Schematic of the methylation cycle, transsulfuration, and glutathione synthesis. Right panel. Baseline differences in the reduced (GSH) and oxidized (GSSG) forms of glutathione, the GSH/GSSG ratio, and cystathionine (an intermediate in the production of cysteine, a precursor to glutathione) were measured in the NAc, the mPFC, and the hippocampus of α2+/+ and α−/− mice. Region-specific increases in oxidative stress were detected in α2−/− mice as compared to α2+/+ mice (two-way ANOVA: main effect of metabolite F3,140 = 26.62, p < 0.0001, main effect of sample F5,140 = 1.371, p = 0.2389, metabolite × sample interaction, F15,140 = 9.514, p < 0001). Cystathionine was increased in the NAc (p < 0.0001) and the mPFC (p < 0.01), GSH was decreased in the NAc (p < 0.001), GSSG was increased in the mPFC (p < 0.05), and GSH/GSSG ratios were decreased in the NAc (p < 0.05) and the mPFC (p < 0.01) of α2−/− mice. α2+/+ n = 4, α2−/− n = 4. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. The absolute values of the metabolites in μmol/mg protein in the α2+/+ brains are the following. Cystathionine: NAc 0.0051; mPFC 0.0042; Hip 0.0046. GSH: NAc 18.253; mPFC 17.538, Hip 19.693. GSSG NAc 0.118; mPFC 0.1055; Hip 0.138. The GSH/GSSG ratio is NAc 154.6864; mPFC 166.237; Hip 142.7029.
Molecular and behavioral characterization of conditional α2-GABAAR knockdown in D1+ and D2+ cells
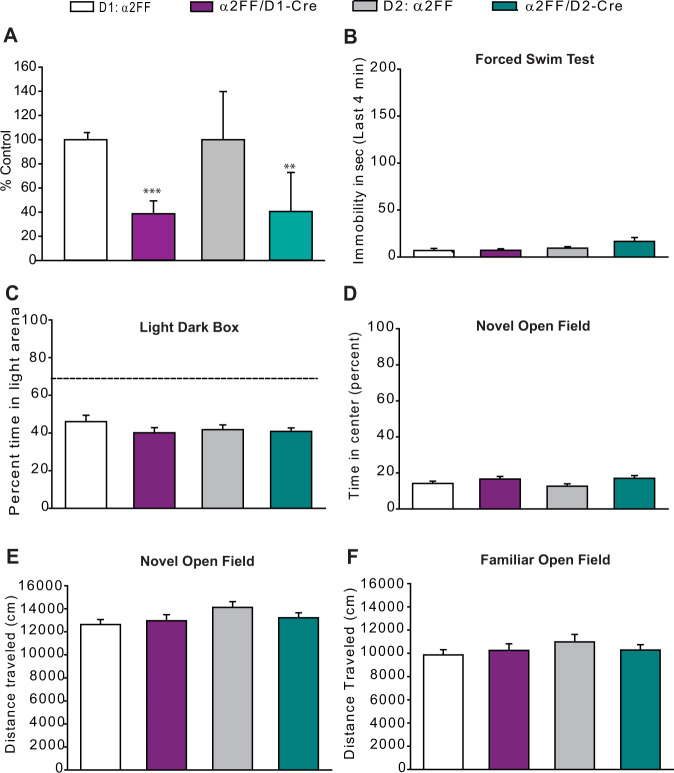
The NAc has been implicated in stress-induced decreases in sociability [9, 28]. Signaling through two major cell types in the region [dopamine 1 receptor-positive (D1+) and dopamine 2 receptor-positive (D2+) medium spiny neurons (MSNs)] has been shown to differentially alter stress susceptibility, with optogenetic activation of D1+ MSNs promoting resilience to CSDS, while repeated optogenetic activation of D2+ MSNs promotes susceptibility to mild social stress [42]. α2-GABAARs are the main α subtype found in the NAc [43], and we observed elevated markers of oxidative stress specifically in the NAc in mice lacking α2-GABAARs. Taken together, these findings highlight α2-GABAARs in the NAc as potential mediators of stress sensitivity. However, it is not known whether selective alterations in Gabra2 expression in D1+ or D2+ neurons could influence susceptibility to stress and depressive-like behavior. We hypothesized that selective decreases in α2-GABAAR expression in D1+ or D2+ neurons would disinhibit these cell types and mimic the effects of D1+ and D2+ optogenetic activation in the NAc on stress-susceptibility. Using qRT-PCR, significant decreases of the α2 subunit mRNA were noted in conditional D1+ knockdown (α2FF/D1-Cre) mice and in conditional D2+ knockdown mice (α2FF/D2-Cre) (Fig. 4A).
Fig. 4. Molecular and behavioral characterization of D1+ and D2+ conditional knockdown of α2-GABAARs.
A Decreases in Gabra2 expression are detected in the NAc of α2FF/D1-Cre (n = 5) compared to littermate control α2FF mice (n = 4) and α2FF/D2-Cre mice (n = 6) compared to littermate control α2FF mice (n = 5) (t test, α2FF vs. α2FF/D1-Cre, ***p < 0.001; α2FF vs. α2FF/D2-Cre, **p < 0.01). B In the forced swim test α2FF/D1-Cre, α2FF/D2-Cre, and their respective littermate α2FF controls spend little time immobile in the last 4 min of the test (one-way ANOVA, F1,58 = 1.824, p > 0.05; D1 α2FF n = 14, α2FF/D1-Cre n = 14, D2 α2FF n = 17, α2FF/D2-Cre n = 17). C In the light–dark box test, α2FF/D1-Cre, α2FF/D2-Cre, and their respective littermate α2FF controls spend a similar percentage of time in the lit chamber (one-way ANOVA, F1,58 = 1.134, p > 0.05; D1 α2FF n = 14, α2FF/D1-Cre n = 14, D2 α2FF n = 18, α2FF/D2-Cre n = 16). The dashed line represents the percentage time in the light chamber that would be expected if the distribution of time between the light and dark chambers were random (67%, chance level). D, E In the novel open field, α2FF/D1-Cre, α2FF/D2-Cre, and their respective littermate α2FF controls spend a similar percentage of time in the center portion of the chamber (one-way ANOVA, F1,44 = 2.204, p > 0.05) and move a similar distance (one-way ANOVA, F1,44 = 1.535, p > 0.05). D1 α2FF n = 11, α2FF/D1-Cre n = 13, D2 α2FF n = 10, α2FF/D2-Cre n = 14). F In the familiar open field, α2FF/D1-Cre, α2FF/D2-Cre, and their respective littermate α2FF controls travel a similar total distance (Kruskal–Wallis one-way ANOVA on Ranks H = 1.504, p > 0.05; D1 α2FF n = 14, α2FF/D1-Cre n = 15, D2 α2FF n = 15, α2FF/D2-Cre n = 17).
As global knockdown of α2-GABAARs produces despair- and anxiety-like behavior [21, 22], similar behaviors were assessed in α2FF/D1-Cre and α2FF/D2-Cre mice. No baseline differences in despair-like behavior (FST, Fig. 4B), anxiety-like behavior (LDB, Fig. 4C; novel open field, Fig. 4D, E), sociability (3-chamber SIT, Fig. S5A–C), or locomotor activity (familiar open field, Fig. 4F) were detected between any of the genotypes.
Mice lacking α2-containing GABAA receptors in D2+ but not D1+ cells exhibit enhanced susceptibility to moderate stress
The influence of conditional knockdown of α2-GABAARs in D1+ and D2+ cells on susceptibility to stress-induced social avoidance was examined following moderate stress (sub-CSDS). Conditional knockdown of α2 in D1+ cells did not alter sociability following moderate stress. No effects of defeat or genotype were observed in time spent in the IZ (Fig. S6A, B), distance to the IZ (Fig. S6C), or corners (Fig. S6D, E). Control α2FF and control α2FF/D1-Cre mice moved less during the SIT as compared to defeat α2FF mice (Fig. S6F). However, as these changes in locomotor behavior are not defeat- or genotype-specific, the changes in locomotion do not confound the other behavioral measurements.
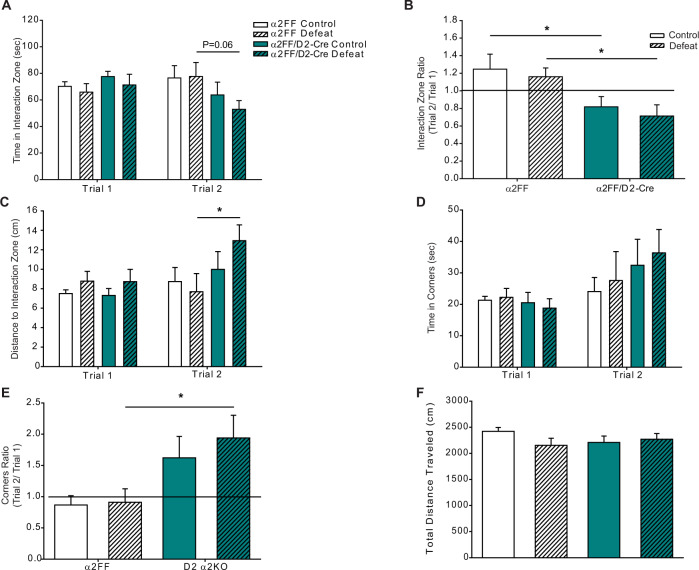
In contrast, conditional knockdown of α2 in D2+ cells reduced sociability following sub-CSDS. Defeated α2FF/D2-Cre mice had a greater average distance from the IZ in trial 2 (Fig. 5C) as compared to defeat α2FF mice. No differences were detected in time spent in the corners in Trial 2 (Fig. 5D), or in any of the measurements in Trial 1 (Fig. 5A, C, D). When IZ and corner ratios were compared, both control and defeat α2FF/D2-Cre mice had lower IZ ratios (Fig. 5B) and defeat α2FF/D2-Cre mice also had higher corner ratios (Fig. 5E). No defeat or genotype effects on total distance traveled were detected in the SIT (Fig. 5F).
Fig. 5. Conditional knockdown of α2-GABAARs in D2+ cells enhances susceptibility to stress-induced social avoidance.
A Mice lacking α2-GABAARs in D2+ cells display a trend to less time in the interaction zone in Trial 2 (two-way ANOVA, the main effect of genotype F1,45 = 4.204, p < 0.05; the main effect of defeat F1,45 = 0.288, p > 0.05, genotype × defeat interaction F1,45 = 0.423, p > 0.05; defeat α2FF/D2-Cre vs. defeat α2FF, p = 0.06) but not in Trial 1 (two-way ANOVA, the main effect of defeat F1,44 = 0.721, p > 0.05, the main effect of genotype F1,44 = 1.04, p > 0.05, genotype × defeat interaction F1,44 = 0.0256, p > 0.05). B Interaction zone ratios are decreased in mice lacking α2-GABAARs in D2+ cells (two-way ANOVA, the main effect of genotype F1,44 = 11.187, p < 0.01, main effect of defeat F1,44 = 0.527, p > 0.05, interaction defeat × genotype F1,44 = 0.00473, p > 0.05). Line denotes the ratio of 1, animals with interaction zone ratios ≥ 1 are considered sociable. C Following 5 days of defeat stress, α2FF/D2-Cre average a greater distance from the interaction zone in Trial 2 (two-way ANOVA, main effect of defeat F1,45 = 0.114, p > 0.05, main effect of genotype F1,45 = 4.129, p < 0.05, defeat × genotype interaction F1,45 = 2.220, p > 0.05) but not in Trial 1 (two-way ANOVA, main effect of defeat F1,45 = 0.623, p > 0.05, main effect of genotype F1,45 = 0.463, p > 0.05, defeat × genotype interaction F1,45 = 0.111, p > 0.05). D Time spent in corners does not differ between animals in either trial (Trial 1: two-way ANOVA, the main effect of defeat F1,42 = 0.0439, p > 0.05, main effect of genotype F1,42 = 1.565, p > 0.05, defeat × genotype interaction F1,42 = 0.0744, p > 0.05; Trial 2: two-way ANOVA, main effect of defeat F1,44 = 0.06, p > 0.05, main effect of genotype F1,44 = 1.152, p > 0.05, defeat × genotype interaction F1,44 = 0.712, p > 0.05). E Corner ratios are increased in defeated α2FF/D2-Cre mice (two-way ANOVA, main effect of genotype F1,43 = 9.694, p < 0.01, main effect of defeat F1,43 = 0.394, p > 0.05, defeat × genotype interaction F1,43 = 0.229, p > 0.05). Line denotes the ratio of 1, animals with corner ratios ≤ 1 are considered sociable. F No differences in total distance traveled were detected in the SIT (two-way ANOVA, main effect of defeat F1,44 = 0.996, p > 0.05, main effect of genotype F1,44 = 0.220, p > 0.05, defeat × genotype interaction F1,44 = 2.526, p > 0.05). Control α2FF n = 12, defeat α2FF n = 12, control α2FF/D2-Cre n = 12, defeat α2FF/D2-Cre n = 13. *p < 0.05.
Discussion
We investigated whether a reduction in the expression of α2-containing GABAA receptors may increase stress vulnerability by exposing the animals to three levels of stress: (1) mild stress: the control animals in the social defeat tests, where the mice are subjected to partial social isolation (i.e., two mice are housed in the same cage with a perforated divider, allowing no physical contact and co-nesting); (2) moderate stress: sub-CSDS, where defeat sessions are repeated for 5 days, (3) severe stress: full 10-day version of the CSDS. We found that α2−/− mice showed reduced social behavior following moderate stress, which did not affect the social behavior of α2+/+ mice. In addition, mild stress caused a putative bias for threat cues in α2−/−, but not α2+/+ control mice. Through control experiments, we ruled out the possibility that the changes in the behavior of the α2−/− mice were due to nonspecific changes in locomotion and an overall reduction in sociability, strengthening the conclusion that α2−/− mice are more susceptible to effects of mild and moderate stress than controls. In the case of severe stress, α2−/− and α2+/+ mice showed a similar reduction in social interaction, possibly suggesting an overall ceiling effect in the reactivity to stress.
We next examined whether the increased stress vulnerability observed in α2−/− mice may be due to changes in oxidative stress, as a reduction in antioxidant levels has been observed in depressed patients [3, 10, 44] and a relationship between stress vulnerability and oxidative stress has been demonstrated in animal models [8]. We found changes in oxidant/antioxidant balance indicative of oxidative stress in the NAc and the mPFC (but not the hippocampus) of α2−/− mice compared to α2+/+ controls. These findings are in line with a report that the prefrontal cortex was more sensitive to oxidative stress than the hippocampus following acute restraint or chronic isolation stress in rats [45]. Others have reported increased oxidative stress in the hippocampus in mice following chronic mild stress [8], suggesting that the hippocampus may be involved in non-social forms of stress.
Based on increased oxidative stress in the NAc of α2−/− mice, the well-established role of the NAc in the reduction of sociability induced by social stress [28, 46], and the abundant expression of α2-containing GABAA receptors in the NAc [47], we decided to focus on this brain region as a possible mediator of the behavioral and stress-vulnerability changes observed in α2−/− mice.
To explore the specific GABAergic circuitry mediating stress susceptibility we employed a combination of molecular and conditional knockdown strategies. Our studies suggest that α2-containing GABAAR-preferring compounds could have antioxidant effects. In support for this, pre-treatment with a single injection of diazepam (a positive modulator of α1-, α2-, α3-, and α5-containing GABAARs) was shown to prevent acute stress-induced increases in oxidative stress within the striatum [48]. Our findings suggest that α2-containing GABAARs may be mediating this effect.
Having identified the NAc as a potential stress-vulnerable region following Gabra2 knockdown, we wanted to further characterize the potential role of α2-containing GABAARs within the NAc in stress susceptibility, specifically the role of α2-containing GABAARs in D1+ and D2+ MSNs. Knockdown of α2-containing GABAARs in a cell type-specific manner is predicted to increase the activity of this cell type. Indeed, we observed that conditional knockdown of α2-containing GABAARs in D2+, but not D1+ cells, promotes susceptibility to moderate stress. A potential limitation to our approach is that D1+ and D2+ cells are also found outside of the NAc (e.g., in the dorsal striatum and cerebral cortex, see http://mouse.brain-map.org), although the NAc is where dopamine receptor 1, dopamine receptor 2, and Gabra2 expression have the greatest overlap (see also https://mouse.brain-map.org/) [43]. Our findings are supported by a previous report showing that optogenetic activation of D1+ and D2+ MSNs has opposing effects on defeat stress susceptibility. Enhancing activity in D1+ MSNs resulted in resilient behavioral outcomes while inhibiting activity in these cells induced depression-like outcomes after CSDS; activation or inhibition of D2+ MSNs had no effect [42]. Interestingly, however, while repeated activation of D1+ MSNs in stress naïve mice promoted resilience to CSDS-induced social avoidance, repeated activation of D2+ MSNs in stress naïve mice induced social avoidance following a single day of social defeat stress [42]. In contrast to these previous studies, the experiments reported here provide evidence for a specific endogenous molecular target in a defined neuronal cell type and a GABAergic signal as a molecular pathway modulating the reaction to social defeat stress.
When we used qRT-PCR to determine knockdown of the α2 mRNA in the NAc in D1-Cre/α2FF mice and in D2-Cre/α2FF mice, we found that α2 mRNA levels were decreased in both mutant lines. However, distinguishing α2-containing GABAARs on these two cell types at the protein level would be inherently difficult due to the diffuse expression of α2-containing GABAARs on processes. Potentially, dynamic processes affecting surface/synaptic expression of the α2 subunit in each cell type may be different. While it might be surprising that a relatively moderate decrease in Gabra2 expression in the NAc of D2-Cre/α2FF mice is able to influence stress-induced social avoidance behavior, selective knockdown of another GABAAR subtype (α5) in a single cell population within the central amygdala (where Gabra5 expression is low) [43] was shown to be sufficient to increase anxiety-like behavior [49]. The previous reports, as well as our findings, support the idea that a small population of GABAA receptors in a critical location in a behavioral circuitry can have disproportionately large effects on behavior [15].
It is important to consider the potential confound that anxiety has in studies utilizing stress. Comparisons of CSDS-induced changes in sociability in two different strains of mice suggest that Balb/c mice (an innately anxious mouse strain) are more susceptible to social defeat stress than C57BL/6 mice [50]. α2-containing GABAARs are necessary for the anxiolytic-like effects of diazepam [34, 51]. Furthermore, previous research reported anxiety-like behavior in global α2−/− mice [22]. In the current studies, we also observed anxiety-like behavior in the novel open field as well as decreased locomotor activity in the SIT in α2−/− mice that we attributed to increased anxiety-like behavior in these mice. In addition, the increased context-evoked freezing observed in control and defeat α2−/− mice, as well as defeated α2+/+ mice in the ambiguous cue test, may be attributed to heightened anxiety/fear-like behavior. In humans, depression and anxiety are frequently co-morbid, and it is likely that anxiety-like behavior may contribute to the enhanced reactivity to stress we observe in global α2 knockout mice. As we observed anxiety- and depressive-like behavior in the global α2−/− mice it is possible that the increased anxiety in these mice is responsible for the increased sensitivity to moderate stress. However, our conditional D1+ and D2+ knockdown studies suggest that the stress-induced changes in sociability that we observe cannot be explained by changes in anxiety-like behavior alone as conditional D2-α2 knockdown alone does not alter baseline anxiety-like behavior but does increase stress-induced social avoidance behavior. Our results demonstrate that α2-containing GABAARs in D2+ neurons modulate susceptibility to sub-CDSS in a manner independent of anxiety-like behavior.
It is unknown at present whether the α2 knockdown in the D1+ and D2+ MSNs results in a simple loss of the corresponding GABAA receptors and thus inhibitory synaptic inputs or whether there is a compensatory upregulation of other GABAA receptors α subunits which might then have different subcellular localizations. It should also be noted that other GABAA receptor subtypes have been reported to modulate the response to chronic stress. Both positive and negative allosteric modulators of the α5-containing GABAA receptors have been shown to promote resilience to chronic stress [52–54]. Moreover, it has been shown that a knockout of extrasynaptic δ-containing GABAA receptors results in activation of the HPA axis [55]. It thus appears that resilience to chronic stress is modulated by more than one GABAA receptor subtype.
Our studies indicate that changes in α2-containing GABAAR signaling reduce the threshold for behavioral and oxidative stress susceptibility. In support of the kindling hypothesis of stress and depression, our findings suggest that decreases in Gabra2 expression early in life may serve as a “first hit” or a genetic predisposition to an enhanced reactivity to subsequent stressors later in life. D2+ cells within the NAc may be particularly vulnerable to changes in Gabra2 expression. Identifying the precise α2-mediated circuitry involved in stress susceptibility and resilience would help understand how an impairment in GABAergic signaling contributes to the development of stress-based disorders such as depression and PTSD. Furthermore, our studies suggest that α2-containing GABAARs are promising targets for the development of novel ADs and may potentially contribute to the AD effects of the neurosteroid SAGE-217, a positive allosteric GABAA receptor modulator, in major depression [56].
Supplementary information
Acknowledgements
We thank Dr. Maltesh Kambali (University of Illinois at Urbana-Champaign, Urbana, IL) for help with statistical analysis.
Funding
This study was supported by award numbers R01MH095905 (UR), 5P50MH094271 (TKH), R01ES029097 (TKH), R01MH063266 (WAC), K01MH107787 (EE), a NARSAD Distinguished Investigator grant (#25623) as a P&S Fund Investigator (UR), an Explorer Award (#311334) from the Simons Foundation Autism Research Initiative (UR), a grant (# 2017-08-31) from the Whitehall Foundation (UR), a Rappaport Mental Health Research Scholar Award (RSB), and a McLean Presidential Fellowship Award (RSB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.
Author contributions
Substantial contributions to the conception or design of the work: RSB, WAC, TKH, and UR. Acquisition, analysis, and interpretation of data for the work: RSB, CC, NWH, NBH, RK, RJD, JWM, EE, WAC, TKH, and UR. All authors contributed to drafting the work or revising it critically for important intellectual content, provided final approval of the version to be published, and agree to be accountable for all aspects o the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
Within the last 2 years, WAC has served as a consultant for Psy Therapeutics, TKH as Scientific Advisor to BrainEver and as Co-Director, Child & Brain Development, Canadian Institute for Advanced Research (CIFAR), and UR as a consultant to Concert Pharmaceuticals. UR is also a Scientific Advisor for Damona Pharmaceuticals. RSB is currently employed by Vertex Pharmaceuticals. WAC is the current Editor-in-Chief of Neuropsychopharmacology.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/30/2017
The name of the author William A. Carlezon was changed to William A. Carlezon Jr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01144-w.
References
- 1.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–86. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 3.Glover V. Maternal stress or anxiety in pregnancy and emotional development of the child. Br J Psychiatry. 1997;171:105–6. doi: 10.1192/bjp.171.2.105. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. J Am Med Assoc. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 5.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 6.Harkness KL, Bruce AE, Lumley MN. The role of childhood abuse and neglect in the sensitization to stressful life events in adolescent depression. J Abnorm Psychol. 2006;115:730–41. doi: 10.1037/0021-843X.115.4.730. [DOI] [PubMed] [Google Scholar]
- 7.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 8.Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal JH, Cresto N, et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry. 2017;22:1701–13. doi: 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 11.Honig A, Bartlett JR, Bouras N, Bridges PK. Amino acid levels in depression: a preliminary investigation. J Psychiatr Res. 1988;22:159–64. doi: 10.1016/0022-3956(88)90001-5. [DOI] [PubMed] [Google Scholar]
- 12.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 13.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–5. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 15.Engin E, Benham RS, Rudolph U. An emerging circuit pharmacology of GABAA receptors. Trends Pharmacol Sci. 2018;39:710–32. doi: 10.1016/j.tips.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PloS ONE. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–29. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 18.Wislowska-Stanek A, Lehner M, Skorzewska A, Krzascik P, Maciejak P, Szyndler J, et al. Changes in the brain expression of alpha-2 subunits of the GABA-A receptor after chronic restraint stress in low- and high-anxiety rats. Behav Brain Res. 2013;253:337–45. doi: 10.1016/j.bbr.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, et al. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–54. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010;68:512–20. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollenweider I, Smith KS, Keist R, Rudolph U. Antidepressant-like properties of α2-containing GABAA receptors. Behav Brain Res. 2011;217:77–80. doi: 10.1016/j.bbr.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koester C, Rudolph U, Haenggi T, Papilloud A, Fritschy JM, Crestani F. Dissecting the role of diazepam-sensitive gamma-aminobutyric acid type A receptors in defensive behavioral reactivity to mild threat. Pharmacol Biochem Behav. 2013;103:541–9. doi: 10.1016/j.pbb.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Benham RS, Hewage NB, Suckow RF, Engin E, Rudolph U. Prodepressant- and anxiogenic-like effects of serotonin-selective, but not noradrenaline-selective, antidepressant agents in mice lacking α2-containing GABAA receptors. Behav Brain Res. 2017;332:172–9. doi: 10.1016/j.bbr.2017.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alboni S, van Dijk RM, Poggini S, Milior G, Perrotta M, Drenth T, et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol Psychiatry. 2015;22:552–61. doi: 10.1038/mp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson EC, Agrawal A, Pergadia ML, Lynskey MT, Todorov AA, Wang JC, et al. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Mol Psychiatry. 2009;14:234–5. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerfen CR, Paletzki R, Heintz N, GENSAT BAC. Cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–83. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, et al. Presynaptic α2-GABAA receptors in primary afferent depolarization and spinal pain control. J Neurosci. 2011;31:8134–42. doi: 10.1523/JNEUROSCI.6328-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 29.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76:550–8. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin HM, Shrestha Muna S, Bagalkot TR, Cui Y, Yadav BK, Chung YC. The effects of social defeat on behavior and dopaminergic markers in mice. Neuroscience. 2015;288:167–77. doi: 10.1016/j.neuroscience.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 31.McHugh SB, Barkus C, Lima J, Glover LR, Sharp T, Bannerman DM. SERT and uncertainty: serotonin transporter expression influences information processing biases for ambiguous aversive cues in mice. Genes Brain Behav. 2015;14:330–6. doi: 10.1111/gbb.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumstein D, Daniel J, Evans C. JWatcher Software. 2012. http://www.jwatcher.ucla.edu. Retrieved March 2, 2021.
- 33.Behlke LM, Foster RA, Liu J, Benke D, Benham RS, Nathanson AJ, et al. A pharmacogenetic ‘Restriction-of-Function’ approach reveals evidence for anxiolytic-like actions mediated by α5-containing GABAA receptors in mice. Neuropsychopharmacology. 2016;41:2492–501. doi: 10.1038/npp.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 35.Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN. Both α2 and α3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur J Neurosci. 2006;23:2495–504. doi: 10.1111/j.1460-9568.2006.04775.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith KS, Engin E, Meloni EG, Rudolph U. Benzodiazepine-induced anxiolysis and reduction of conditioned fear are mediated by distinct GABAA receptor subtypes in mice. Neuropharmacology. 2012;63:250–8. doi: 10.1016/j.neuropharm.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller AH, Haroon E, Felger JC. Therapeutic Implications of brain-Immune Interactions: treatment in translation. Neuropsychopharmacology. 2017;42:334–59. doi: 10.1038/npp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halliwell B, Gutteridge J. Free radicals in biology and medicine. 3rd edition, O Clarendon, editor. Oxford Science Publications; 2000.
- 39.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–30. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 40.Pandya CD, Howell KR, Pillai A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:214–23. doi: 10.1016/j.pnpbp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–53. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 2015;77:212–22. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 44.Liu T, Zhong S, Liao X, Chen J, He T, Lai S, et al. A meta-analysis of oxidative stress markers in depression. PloS ONE. 2015;10:e0138904. doi: 10.1371/journal.pone.0138904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zlatkovic J, Todorovic N, Boskovic M, Pajovic SB, Demajo M, Filipovic D. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol Cell Biochem. 2014;393:43–57. doi: 10.1007/s11010-014-2045-z. [DOI] [PubMed] [Google Scholar]
- 46.Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hortnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience. 2013;236:345–72. doi: 10.1016/j.neuroscience.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendez-Cuesta LA, Marquez-Valadez B, Perez-De La Cruz V, Escobar-Briones C, Galvan-Arzate S, Alvarez-Ruiz Y, et al. Diazepam blocks striatal lipid peroxidation and improves stereotyped activity in a rat model of acute stress. Basic Clin Pharmacol Toxicol. 2011;109:350–6. doi: 10.1111/j.1742-7843.2011.00738.x. [DOI] [PubMed] [Google Scholar]
- 49.Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, et al. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci. 2015;18:1493–500. doi: 10.1038/nn.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savignac HM, Finger BC, Pizzo RC, O’Leary OF, Dinan TG, Cryan JF. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience. 2011;192:524–36. doi: 10.1016/j.neuroscience.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 51.Engin E, Smith KS, Gao Y, Nagy D, Foster RA, Tsvetkov E, et al. Modulation of anxiety and fear via distinct intrahippocampal circuits. eLife. 2016;5:e14120. doi: 10.7554/eLife.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piantadosi SC, French BJ, Poe MM, Timic T, Markovic BD, Pabba M, et al. Sex-dependent anti-stress effect of an α5 subunit containing GABAA receptor positive allosteric modulator. Front Pharmaol. 2016;7:446. doi: 10.3389/fphar.2016.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prevot TD, Li G, Vidojevic A, Misquitta KA, Fee C, Santrac A, et al. Novel benzodiazepine-like ligands with various anxiolytic, antidepressant, or pro-cognitive profiles. Mol Neuropsychiatry. 2019;5:84–97. doi: 10.1159/000496086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM. Rapid antidepressant action and restoration of excitatory synaptic growth after chronic stress by negative modulators of α5-containing GABAA receptors. Neuropsychopharmacology. 2015;40:2499–509. doi: 10.1038/npp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee V, Sarkar J, Maguire J. Loss of Gabrd in CRH neuros blunts the corticosterone response to stress and diminishes stress-related behaviors. Psychoneuroendocrinology. 2014;41:75–88. doi: 10.1016/j.psyneuen.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. 2019;381:903–11. doi: 10.1056/NEJMoa1815981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.