Abstract
Background
Nanocolloidal human serum albumin radiolabelled with 99mTc provides a diagnostic radiopharmaceutical for sentinel node lymphoscintigraphy. NanoHSA (Nanotop), a commercially available kit, enables the simple preparation of this radiopharmaceutical via reconstitution with pertechnetate eluted from a generator. Thin-layer chromatography is widely used for determining radiochemical purity in clinical nuclear medicine. Quality control methods recommended by the manufacturer were sometimes reported to yield variable results. Therefore, we proposed and evaluated three alternative thin-layer chromatography methods for the quality control of [99mTc]Tc-NanoHSA from a commercially available kit.
Results
The radiochemical purity of [99mTc]Tc-NanoHSA determined with all methods was reproducible and met the requirements of the SPC and the European Pharmacopoeia (≥ 95%). Our quality control using iTLC-SG chromatographic paper in methyl ethyl ketone mobile phase identified only free pertechnetate as impurity, resulting in > 99% RCP. The quality control using iTLC-SG in 85% methanol or iTLC-SA in 0.9% NaCl identified an additional small fraction of a hydrophilic impurity, resulting in 95–97% RCP. Glucose was identified as a potential 99mTc-carrying hydrophilic species contributing to hydrophilic impurities.
Conclusion
Our quality control of [99mTc]Tc-NanoHSA with non-polar mobile phase tended to underestimate the amount of hydrophilic impurities, although without compromising the final quality of the radiopharmaceutical. Alternative TLC methods using aqueous mobile phases enabled a more accurate determination of hydrophilic impurities.
Keywords: Nanocolloidal albumin, Lymphoscintigraphy, NanoHSA, Nanotop, 99mTc, Thin-layer chromatography, Radiochemical purity, Glucose, Sep-Pak®
Introduction
Human serum albumin (HSA) is the most abundant protein in the blood serum and is readily radiolabelled with 99mTc (technetium-99m). Its relatively short half-life (6.02 h) and low-energy gamma emission (141 keV) facilitate diagnostic applications of this radionuclide in a large number of small nuclear medicine centres involving minimal infrastructure. Affordable, commercially available radionuclide generators supply 99mTc as sodium pertechnetate [99mTc]NaTcO4, which is compatible with the requirements of Good Manufacturing Practices (GMP) and national pharmacopoeias. The pertechnetate solution eluted from a certified generator is then ready for HSA radiolabelling. The exact nature of how 99mTc binds to HSA is unknown, however the most likely mechanism is the binding of reduced 99mTc to sulfur atoms following the reduction of disulfide bonds of the protein by tin (II) added to the HSA kit (Vanbilloen et al. 1993).
Different physico-chemical forms of HSA labelled with 99mTc enable a range of versatile diagnostic applications (Grosser et al. 2016). Intravenous injection of the soluble [99mTc]Tc-HSA is used as a dynamic blood pool tracer, while slightly denatured, macroaggregated [99mTc]Tc-HSA particles sized 10–100 µm are used for lung perfusion studies. Smaller, 50–80 nm HSA nanoparticles labelled with 99mTc provide a diagnostic radiopharmaceutical agent for sentinel lymph nodes scintigraphy (Giammarile et al. 2013; Sadkin et al. 2020). Several commercially available kit formulations for preparing [99mTc]Tc-HSA nanocolloid have become available recently and are described in the literature (Nanotop®, Nanoalbumon®, Nanocoll®) (Persico et al. 2015; Gommans et al. 2009; Marenco et al. 2021). Since the radiopharmaceutical is prepared shortly prior to administration, a quality control (QC) must be performed immediately after radiolabelling in order to determine the radiochemical purity (RCP). Thin-layer chromatography (TLC) is the most widely used method for QC of radiopharmaceuticals prepared from commercially available kits. Free (unbound) pertechnetate [99mTc]TcO4− is the main impurity separated via TLC through a migration with the solvent front, because the nanocolloidal [99mTc]Tc-HSA and colloidal [99mTc]TcO2 remain at the origin of the chromatogram. It is essential that at least 95% of the 99mTc is bound to the nanocolloidal HSA before releasing the radiopharmaceutical for administration to patient; this amount must therefore be verified. The 95% RCP benchmark is used as a reference from individual pharmacopoeial monographs of several other 99mTc radiopharmaceuticals. The free pertechnetate [99mTc]TcO4− anion is similar to the iodide I− anion and is taken up by sodium/iodide symporter (NIS). This effect can cause an undesired uptake of [99mTc]TcO4− into the thyroid, but also into the salivary and gastric tissues, compromising image quality and delivering an unjustified radiation dose to the patient (Hingorani et al. 2010). The efficacy of radiolabelling of the nanocolloidal HSA depends on the size distribution of the particles, however, any excipients such as buffers, inorganic ions, or large amount of glucose present within the kit, as well as the quality of the generator eluate may all play a role in the speciation of 99mTc (Persico et al. 2015; Metaye et al. 2012; Waight et al. 2011).
Several publications covering nanocolloidal human serum albumin include different methods for quality control of the radiolabelled product. The summary of product characteristics (SPC) accompanying the ROTOP-NanoHSA kit recommends two TLC methods for determining the RCP of the final radiopharmaceutical. Method 1 is to use SG-60 aluminium plates as a stationary phase and acetone as a mobile phase. Method 2 is to use iTLC-SA paper as a stationary phase and methyl ethyl ketone as a mobile phase. Both methods enable the migration of free pertechnetate with the solvent front resulting in a retardation factor (Rf) of 0.9–1, while labelled [99mTc]Tc-NanoHSA remains at the origin, with Rf 0.-0.1. These methods are described for RCP determination in different monographs of the European Pharmacopoeia (Council of Europe 2019). The SPC of a similar product called Nanocoll® (GE Healthcare), recently discontinued, recommended 85% aqueous methanol as a mobile phase with iTLC-SA or Whatman No. 1 chromatographic paper, which enables the migration of free pertechnetate with the solvent front yielding Rf 0.9–1. As briefly reported elsewhere for [99mTc]Tc-Nanocoll, different TLC methods resulted in varied radiochemical purity for this radiopharmaceutical (Dumas et al. 2009). The SPC of Nanoalbumon® (Medi-Radiopharma Ltd, Erd, Hungary) stipulates 85% aqueous methanol mobile phase with iTLC-SG or Whatman No. 1 for determining the RCP (Marenco et al. 2021). The United States Pharmacopoeia includes a monograph on [99mTc]Tc-albumin colloid injection, giving a method for determining RCP using iTLC-SG with methyl ethyl ketone (United States Pharmacopoeia Convention. United States Pharmacopoeia (USP) 2019).
This study thus aimed at evaluating alternative QC methods for determining the RCP of nanocolloidal 99mTc-labelled HSA prepared from a commercially available kit: NanoHSA (ROTOP Pharmaka AG, Dresden, Germany). In addition to suitability verification, we employed three alternative compendial TLC methods to better separate small hydrophilic impurities. Solid-phase extraction (SPE) using a Sep-Pak® cartridge was used as a complementary method to separate [99mTc]Tc-NanoHSA from hydrophilic impurities, enabling their further analysis with TLC. To evaluate the potential of glucose as a hydrophilic 99mTc-carrying impurity, radiolabelling of glucose with [99mTc]TcO4− was tested and analysed with SPE and TLC methods. Finally, we evaluated a range of needles used for QC of radiopharmaceuticals in nuclear medicine departments in order to determine the volume of the spotted sample and its effect on TLC outcome.
Materials and methods
The drop size produced by syringe needles of different gauges was determined by weighing water drops (n = 5) deposited in a tall glass vial to minimise evaporation (balance XPE205, Mettler Toledo, Columbus, United States). To test the effect of drop size on TLC, 450 MBq of [99mTc]TcO4− in 1.5 mL eluate was partially reduced to [99mTc]TcO2 in the presence of 40 µg of Sn2+. After 10 min incubation at room temperature, samples of 2, 5, 10, 15 and 20 µL in triplicate (n = 3 technical replicates) were spotted onto 1.5 cm × 10 cm iTLC-SG chromatographic paper strips and developed in methyl ethyl ketone. The fraction of free [99mTc]TcO4− was determined using a miniGITA TLC scanner (Elysia-Raytest GmbH, Straubenhardt, Germany).The radiolabelling of nanocolloidal human serum albumin was carried out by reconstitution of a commercially available radiopharmaceutical kit: NanoHSA (ROTOP Pharmaka GmbH, Dresden, Germany), according to the instructions provided in the SPC (version 22,509). To verify the suitability of alternative compendial methods for quality control of [99mTc]Tc-NanoHSA, three individual radiolabellings were carried out in identical conditions on three consecutive days. Each NanoHSA kit (batch nr. EP 11 20 11 1 DE, authorisation nr. 905.79.00.00, expiry date 03.2022) was labelled with sodium pertechnetate [99mTc]NaTcO4 Ph. Eur. at maximum volume (4–5 mL) and maximum activity (4.2–5.5 GBq) eluted from a Pertector-15 generator (Polatom, Otwock, Poland, code MTcG-4, batch 086/20, 15 GBq on 26.10.2020 at 12 h 00). The radiochemical purity of [99mTc]Tc-NanoHSA was determined with thin-layer chromatography using three alternative methods:
Method A Stationary phase: 1.5 cm × 10 cm strip of iTLC-SG chromatographic paper, origin at 15 mm, solvent front 70 mm; mobile phase: methyl ethyl ketone.
Method B Stationary phase: iTLC-SG chromatographic paper, origin at 15 mm, solvent front 70 mm; mobile phase: methanol/water 85/15 v/v.
Method C Stationary phase: iTLC-SA chromatographic paper, origin at 15 mm, solvent front 60 mm; mobile phase: 0.9% aqueous NaCl.
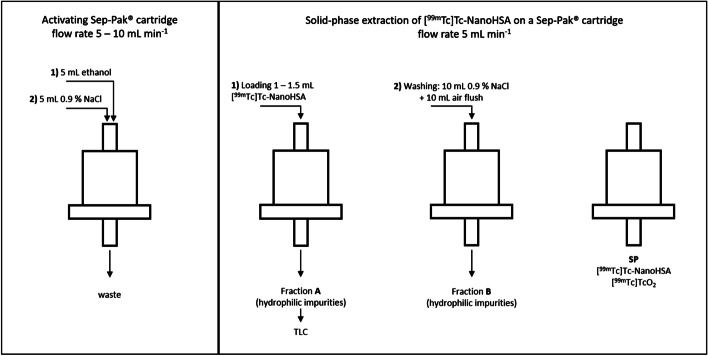
For each method, 10 µL of [99mTc]Tc-NanoHSA was deposited on a chromatographic strip immediately after radiolabelling was completed (t0, allowing 10 min incubation time according to the SPC), 30 min after radiolabelling (t1), and 60 min after radiolabelling (t2). The sample drop was not allowed to dry after spotting. Each iTLC was carried out in triplicate (n = 3 technical replicates) for three individual radiolabellings on consecutive days (n = 3 experimental replicates).The radiochemical separation of hydrophilic impurities in [99mTc]Tc-NanoHSA was carried out by solid-phase extraction using Sep-Pak® C18 reversed phase cartridge (Fig. 1). Sep-Pak® cartridge was activated with 5 mL ethanol followed by 5 mL 0.9% aqueous NaCl. Next, 1–1.5 mL [99mTc]Tc-NanoHSA was loaded onto the cartridge and fraction A was collected. The cartridge was washed with 10 mL 0.9% aqueous NaCl followed by 10 mL air flush providing fraction B. The radioactivity of the 99mTc was measured in fraction A, fraction B, and in the cartridge (SP) using a VDC-405 dose calibrator (Veenstra Instruments, Joure, The Netherlands). The radiochemical purity (%) of the [99mTc]Tc-NanoHSA was calculated as follows:
| 1 |
Fig. 1.
Solid-phase extraction of NanoHSA using Sep-Pak® cartridge. Colloidal [99mTc]TcO2 and [99mTc]Tc-NanoHSA were retained on Sep-Pak® cartridge enabling further TLC analysis of soluble hydrophilic impurities in fraction A
To identify other hydrophilic impurities in [99mTc]Tc-NanoHSA that were not retained on the Sep-Pak® cartridge, fraction A was analysed additionally using thin-layer chromatography. To determine the free [99mTc]TcO4−, 10 µL was spotted onto a TLC SG-60 strip on an aluminium support (origin: 15 mm, solvent front: 70 mm) and developed in methyl ethyl ketone. To determine other polar impurities, 10 µL was spotted onto a TLC SG-60 strip (origin: 15 mm, solvent front: 70 mm) and developed in 0.9% aqueous NaCl. To display glucose distribution in the analyte, a sample of fraction A from one NanoHSA radiolabelling was submitted to TLC using a SG-60 strip in methyl ethyl ketone and 0.9% NaCl mobile phases, and after chromatographic separation the strips were developed by briefly dipping in 2% aqueous KMnO4. The developed TLC strips were imaged in a UV–visible scanner (Camag, Muttenz, Switzerland).
To evaluate a potential contribution of glucose as a 99mTc-carrying impurity, radiolabelling of glucose was attempted with [99mTc]TcO4− in conditions similar to the radiolabelling of the NanoHSA kit. Briefly, 15 mg glucose in 0.9% aqueous NaCl in the presence of 40 µg (in one experiment – 200 µg) of Sn2+ was radiolabelled with 0.6–1.5 GBq of [99mTc]TcO4−. The final volume was adjusted with saline to 1.5–5 mL, and the experiment was reproduced in triplicate (n = 3 experimental replicates). To determine the free pertechnetate, TLC was carried out using SG-60 on an aluminium support as a stationary phase in acetone and in methyl ethyl ketone. To determine other hydrophilic 99mTc species, TLC was carried out with methanol/water 85/15 v/v mobile phase using SG-60 on aluminium and iTLC-SA stationary phase, and with 0.9% NaCl mobile phase using SG-60 on aluminium stationary phase. The distribution of glucose on the TLC strips was revealed by developing the strips in 2% KMnO4 and imaging in a UV–visible scanner (Camag, Muttenz, Switzerland). Additionally, after 10–20 min incubation at room temperature, radiolabelled glucose was loaded on a Sep-Pak® cartridge and fraction A was collected. The cartridge was washed with 10 mL 0.9% aqueous NaCl followed by 10 mL air flush providing fraction B. The radioactivity of 99mTc was measured in fraction A, fraction B, and in the cartridge (SP).
Results
Quality control of 99mTc-HSA nanocolloid
Effect of needle gauge on TLC performance
To determine the size of a drop deposited for thin-layer chromatography using a syringe, we tested a range of needles of different diameters routinely used for the QC of radiopharmaceuticals in clinical nuclear medicine. As expected, the drop size increased with a larger needle diameter, resulting in drops between 10 µL and 26 µL (Table 1).
Table 1.
Water drop size as a function of needle diameter. Water density assumed as 1 g mL−1
| 25G × 5/8″ 0.5 × 16 mm |
23G × 1 1/4″ 0.6 × 30 mm |
22G × 1 1/4″ 0.7 × 30 mm |
21G × 2″ 0.8 × 50 mm |
20G × 23/16″ 0.9 × 55 mm |
19G × 1 1/2″ 1.1 × 40 mm |
18G × 1 1/2″ 1.2 × 40 mm |
|
|---|---|---|---|---|---|---|---|
| m, g (n = 5) | 0.0100 | 0.0146 | 0.0156 | 0.0170 | 0.0171 | 0.0202 | 0.0264 |
| V, µL | 10 | 14.6 | 15.6 | 17 | 17.1 | 20.2 | 26.4 |
| SD, ± g (k = 2) | 0.0020 | 0.0043 | 0.0040 | 0.0023 | 0.0012 | 0.0021 | 0.0023 |
We then determined the fraction of free [99mTc]TcO4− as a function of volume spotted following a partial reduction with Sn2+. A range of samples from 2 µL to 20 µL spotted on iTLC-SG paper and developed in methyl ethyl ketone showed that radiochemical yield of free [99mTc]TcO4− appeared to decrease with a larger drop size (Table 2).
Table 2.
Fraction of free [99mTc]TcO4− determined with iTLC as a function of drop size
| Drop volume | 2 µL | 5 µL | 10 µL | 15 µL | 20 µL |
|---|---|---|---|---|---|
| % free [99mTc]TcO4− | 1.57 ± 0.20 | 0.60 ± 0.11 | 0.62 ± 0.15 | 0.29 ± 0.04 | 0.23 ± 0.03 |
Each experiment was carried out in triplicate (n = 3) using iTLC-SG strips developed in MEK mobile phase
Thin-layer chromatography
We tested three alternative compendial methods to differentiate radiochemical species in [99mTc]Tc-NanoHSA. iTLC-SG paper as stationary phase was used with MEK and 85% methanol mobile phases, and iTLC-SA paper with 0.9% NaCl. Our quality control results obtained with iTLC showed that the radiochemical purity of [99mTc]Tc-NanoHSA in all radiolabellings fulfilled the requirements set in the SPC (≥ 95%, Table 3). iTLC with methyl ethyl ketone as mobile phase showed consistent and reproducible quantitative radiolabelling at all time points from t0 to 60 min. Conversely, iTLC with aqueous mobile phases indicated lower RCP compared to MEK after 10 min incubation: 96.2 ± 0.3% in 85% methanol and 96.5 ± 0.3% in 0.9% NaCl, against 99.7 ± 0.2% in MEK. The RCP determined with 85% methanol and with saline mobile phases was similar and showed a slight increase between t0 (96.2 ± 0.3% and 96.5 ± 0.3% respectively) and 30 min incubation time (98.1 ± 0.4% and 98.0 ± 0.1% respectively).
Table 3.
Radiochemical purity of [99mTc]Tc-NanoHSA. RCP determined with iTLC and SPE for three individual radiolabellings (n = 3) carried out on three consecutive days
| MEK iTLC-SG |
85% Methanol / H2O iTLC-SG |
0.9% NaCl iTLC-SA |
Sep-Pak® | |
|---|---|---|---|---|
| t0 | 99.7 ± 0.2 | 96.2 ± 0.3 | 96.5 ± 0.3 | 95.7 ± 0.1# |
| + 30 min | 99.9 ± 0.1 | 98.1 ± 0.4 | 98 ± 0.1 | 97.6 ± 0.0* |
| + 60 min | 100 ± 0.1 | 98.6 ± 0.1 | 98.7 ± 0.0 | 98.3 ± 0.1* |
#n = 3 Sep-Pak® experimental replicates *n = 2 Sep-Pak® experimental replicates
Solid-phase extraction
We used solid-phase extraction on a Sep-Pak® C18 reversed phase cartridge to further separate and identify potential hydrophilic impurities from the [99mTc]Tc-NanoHSA. The RCP of the [99mTc]Tc-NanoHSA determined with a Sep-Pak® cartridge (Table 3) was in good agreement with the iTLC results obtained in aqueous mobile phases (85% methanol and saline), but not in MEK. We observed a similar trend towards an increase of RCP with an incubation time of over 30 min. Additional TLC analysis of the fraction A collected from the Sep-Pak® cartridge enabled a separation of hydrophilic impurities into several species. The TLC results for fraction A (Table 4) showed that the distribution of different 99mTc species varied in different experiments with a 20–50% standard deviation. Such variability is typical for non-specific impurities, which are rarely reproducible with a high degree of accuracy. According to TLC results obtained in MEK mobile phase (Table 4), about 50% of the fraction A contained free pertechnetate [99mTc]TcO4−, with the remaining 50% attributed to colloidal [99mTc]TcO2 and/or other polar hydrophilic impurities that did not migrate in MEK. However, TLC of the same sample in 0.9% NaCl mobile phase identified only 24–25% of colloidal [99mTc]TcO2 (Table 4). Collating these results suggests that approximately 25% of the fraction A, or 1–2% of the total radioactivity in [99mTc]Tc-NanoHSA, represent a different polar species carrying 99mTc, other than free pertechnetate.
Table 4.
Radiochemical speciation of hydrophilic impurities in [99mTc]Tc-NanoHSA
| Sep-Pak® fraction A | |||
|---|---|---|---|
| MEK | 0.9% NaCl | ||
| % [99mTc]TcO4− | % [99mTc]TcO2 | % polar impurities | |
| t0* | 49.4 ± 8.2 | 24.1 ± 8.4 | 75.9 ± 8.4 |
| + 30 min# | 53.4 ± 22.7 | 20.1 ± 7.2 | 79.9 ± 7.2 |
| + 60 min# | 48.0 ± 16.9 | 25.7 ± 12.4 | 74.3 ± 12.4 |
Determined in fraction A, containing hydrophilic impurities not retained on the Sep-Pak® cartridge, with TLC SG-60 stationary phase
*n = 3 experimental replicates
#n = 2 experimental replicates
Radiolabelling glucose with [99mTc]TcO4−
Thin-layer chromatography of glucose in the presence of [99mTc]TcO4− identified hydrophilic species carrying 99mTc capable of migrating with the solvent front in aqueous mobile phases (Table 5). Free pertechnetate fraction separated with acetone (16.0%) and MEK (25.7%) mobile phases was higher in the experiment with a high activity concentration of 99mTc (1.5 GBq in 2 mL), compared to 3.3% in acetone and 3.1% in MEK when glucose was radiolabelled with less 99mTc (0.06 GBq in 1.5 mL). Furthermore, the free [99mTc]TcO4− fraction appeared to decrease with incubation time from t0 to 1 h. The TLC in aqueous mobile phases identified a consistently higher fraction of mobile 99mTc-carrying species in both low activity concentration (36.1–39.0%) and high activity concentration (36.1–53.8%) radiolabellings. Again, the fraction of mobile 99mTc-carrying species decreased from 36.1–39.0% to 12.4–12.9% within 1 h incubation time. The radiolabelling of glucose attempted in the presence of 200 µg of Sn2+ resulted entirely in colloidal [99mTc]TcO2 immobilised at the origin on TLC strips.
Table 5.
Fraction (%) of mobile 99mTc species in radiolabelled glucose determined with TLC
| [1] | [2] | [1] | [2] | [1] | [2] | [1] | [2] | [1] | [2] | |
|---|---|---|---|---|---|---|---|---|---|---|
| GBq mL−1 99mTc | 0.75 | 0.04 | 0.75 | 0.04 | 0.75 | 0.04 | 0.75 | 0.04 | 0.75 | 0.04 |
| TLC conditions |
TLC SG-60 Acetone 30–35 min |
TLC SG-60 MEK 14–15 min |
TLC-SA 85% methanol 14–15 min |
TLC SG-60 85% methanol 30–32 min |
TLC SG-60 0.9% NaCl 14–15 min |
|||||
| Identification | Free [99mTc]TcO4− | Free [99mTc]TcO4− | Free [99mTc]TcO4− and 99mTc-glucose | Free [99mTc]TcO4− and 99mTc-glucose | Free [99mTc]TcO4− and 99mTc-glucose | |||||
| t0 | 16.0 | 3.3 | 25.7 | 3.1 | 53.8 | 39.0 | 36.1 | 36.1 | 48.1 | 36.5 |
| + 30 min | 1.6 | 2.2 | 19.3 | 17.2 | 19.1 | |||||
| + 60 min | 2.2 | 2.0 | 12.9 | 12.4 | 12.5 | |||||
Determined for two individual radiolabellings with different activity concentration of 99mTc (15 mg glucose, 40 µg Sn2+, 1.5–2 mL of 0.9% NaCl)
The distribution of glucose using different mobile phases on TLC plates developed in 2% KMnO4 showed that glucose remained at the origin in MEK (Fig. 2, strip 1), and in acetone (not shown). When TLC was carried out in 85% methanol or in saline, the glucose migrated with the solvent front (Fig. 2, strip 2 and 3).
Fig. 2.
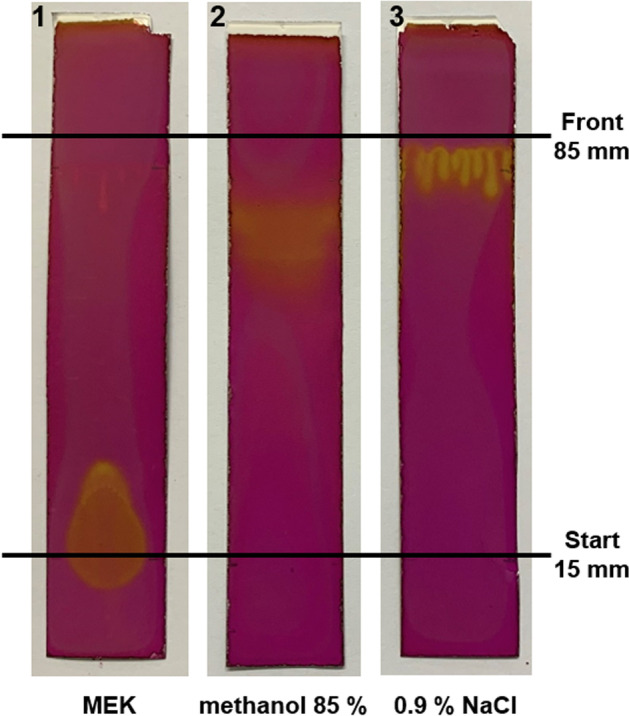
Distribution of glucose on TLC SG-60 strips. Glucose (yellow spot) migrated with the solvent front in 0.9% NaCl and in 85% methanol, and remained at the origin in MEK
The solid-phase extraction of glucose radiolabelled with [99mTc]TcO4− showed that glucose was not retained on the C18 Sep-Pak® cartridge. A nearly equal distribution of 99mTc radioactivity between fraction A collected upon loading and fraction B collected upon washing the cartridge speaks in favour of a weak non-specific retention of soluble 99mTc species (Table 6). Approximately 30–40% of the 99mTc was not released from the cartridge upon washing and was attributed to colloidal [99mTc]TcO2.
Table 6.
Distribution of [99mTc]Tc-glucose on Sep-Pak® cartridge
| [1] % 99mTc |
[2] % 99mTc |
[3] % 99mTc |
Identification | |
|---|---|---|---|---|
| Fraction A | 47.2 | 30.6 | 34.9 |
Free [99mTc]TcO4− and [99mTc]Tc-glucose |
| Fraction B | 21.7 | 29.1 | 28.5 | |
| A + B | 68.9 | 59.7 | 63.4 | |
| Residual 99mTc Sep-Pak® | 31.1 | 40.3 | 36.6 | Colloidal [99mTc]TcO2 |
Determined for three individual experiments (15 mg glucose, 40 µg Sn2+, 4 mL of 0.9% NaCl)
Discussion
Effect of needle gauge on TLC performance
The amount of a sample deposited on the chromatographic support for thin-layer chromatography is essential for the efficient separation of species present in a radiopharmaceutical. Different SPCs and pharmacopoeial monographs recommend spotting between 2 and 5 µL, and up to 10 µL per sample. Small nuclear medicine centres which employ radiopharmaceutical kits often do not have a laboratory equipped with pipettes that are checked and calibrated on a regular basis. Thus, quality control of radiopharmaceuticals is performed by withdrawing a small volume of radiolabelled product using a sterile syringe and needle and spotting a drop on an iTLC strip. The drop size distribution as a function of needle gauge summarised in Table 1 showed that the smallest 25G needle produced a 10 µL drop, which is the largest amount of sample recommended for iTLC by QC guidelines. In clinical nuclear medicine, needle sizes ranging from gauge 20G to 26G are used, and are routinely available to professionals carrying out the QC of [99mTc]Tc-NanoHSA. Needles 25G or 27G are recommended for subcutaneous injections for lymphoscintigraphy with radiotracers which include nanocolloidal HAS (Bluemel et al. 2015). In this study, only a 25G needle was shown to provide 10 µL, equivalent to the volume deposited with a pipette. This means that caution should be taken when interpreting iTLC results obtained with larger needles.The determination of free pertechnetate in partially reduced eluate as a function of volume spotted for iTLC showed a trend towards an apparent decline of the free pertechnetate fraction with increased sample volume: from 1.57 ± 0.20% when spotted with 2 µL and up to 0.23 ± 0.03% when spotted with 20 µL. These small numbers obtained with iTLC do not provide the most robust conclusions regarding the effect of sample volume on chromatographic separation and should be considered indicative results only. However, it must be noted that chromatograms of larger sample volumes – with 15 and 20 µL spots – had to be analysed with the TLC scanner once they had been left to decay by one half-life. When using the same settings as for the 2–10 µL spots immediately after developing the strips, the 15 and 20 µL spots saturated the radioactivity detector, thus compromising the quantification.
Thin-layer chromatography
According to the SPC, the methods recommended for determining the RCP of [99mTc]Tc-NanoHSA employ thin-layer chromatography with SG-60 on aluminium stationary phase in acetone as mobile phase, or iTLC-SA stationary phase in methyl ethyl ketone. For both methods 99mTc-labelled human serum albumin nanocolloid remains at the origin, while free unbound pertechnetate [99mTc]TcO4− migrates with the solvent front. These methods are widely recommended in the literature. However, for some time now, acetone has not been recommended for this purpose because it tends to overestimate the free [99mTc]TcO4− fraction due to its higher water content (Decristoforo and Zolle 2007). Methyl ethyl ketone is the ultimate mobile phase to enable an accurate separation of free pertechnetate with TLC (World Health Organisation 2019).
Therefore, we selected three alternative methods and verified their suitability for the QC of [99mTc]Tc-NanoHSA in a coherent, reproducible fashion with a minimal number of replicates. We chose iTLC-SG chromatographic paper for MEK mobile phase because this stationary phase is more widely used in practise compared to iTLC-SA, and it is easier to handle because it does not require thermal activation prior to use, unlike iTLC-SA. Additionally, we chose methanol/water 85/15 mobile phase for iTLC-SG because it enables the migration of small hydrophilic species along with the free pertechnetate [99mTc]TcO4−. Methanol may contribute to the chemical degradation of delicate biological molecules such as human serum albumin. Therefore, iTLC was also carried out in a 0.9% NaCl solution as mobile phase with iTLC-SA stationary phase (World Health Organisation. The International Pharmacopoeia. Technetium (99mTc) pentetate complex injection (Technetii (99mTc) pentetatis multiplex injectio); 2019.
The QC of the 99mTc-labelled NanoHSA using MEK showed quantitative RCP in all radiolabellings, with the free [99mTc]TcO4− fraction below 1% shortly after reconstitution of the kit. To determine the presence of other potential soluble impurities, more polar, aqueous mobile phases are necessary. iTLC-SG in 85% methanol showed quantitative radiolabelling of NanoHSA, however a slightly lower RCP after 10 min incubation (96.2 ± 0.3%). After 30 min incubation the RCP increased to over 98%. Although the initial result fulfilled the QC requirements of the SPC, it suggested the presence of a labile hydrophilic species carrying 99mTc that did not migrate in MEK. iTLC-SA in 0.9% NaCl showed remarkably similar results with methanol/water (Table 3). The RCP after 10 min incubation was 96.5 ± 0.3%, comparable to methanol/water (96.2 ± 0.3%) but slightly lower than with MEK (99.7 ± 0.2%). After 30 min incubation, the RCP increased to 98%, same as methanol/water. A comparison of the results of three iTLC methods revealed that MEK overestimated the RCP of [99mTc]Tc-NanoHSA, identifying only free pertechnetate impurity. Aqueous mobile phases identified approximately 1–2% of additional hydrophilic impurities, which consistently tended to decline within the timeframe of experiment (10–60 min).
Solid-phase extraction
Solid-phase extraction is used as an alternative to TLC for the quality control of some radiopharmaceuticals. Sep-Pak® cartridges with C18 hydrophobic reversed phase retain non-polar compounds and particles (Straub et al. 2018; Ramirez et al. 2000). Colloidal [99mTc]TcO2 is also retained on the Sep-Pak® and we hypothesised that larger colloidal particles (≤ 80 nm) of human serum albumin labelled with 99mTc could also be retained on the cartridge. The RCP of [99mTc]Tc-NanoHSA determined with SPE was similar to iTLC in methanol/water and in saline (Table 3), showing that Sep-Pak® makes it possible to separate the same hydrophilic impurities. Furthermore, this method may be used as a less expensive and rapid alternative for QC in the absence of a TLC scanner. However, the manual handling of SPE cartridges during this QC can result in a higher radiation dose to the operator and should be avoided. Furthermore, uncontrolled, manual elution may result in inaccurate and irreproducible results due to variations in the speed of elution.
Identification of a hydrophilic 99mTc impurity
Examining the results of the TLC and SPE of [99mTc]Tc-NanoHSA suggested the presence of a labile hydrophilic species carrying 99mTc. Although such species in no way impairs the final quality of the radiopharmaceutical, it is of interest to speculate on its nature. NanoHSA from ROTOP Pharmaka is a freeze-dried radiopharmaceutical kit containing 0.5 mg of colloidal particles of human serum albumin. According to the SPC, after reconstituting the kit, 95% of the particles sized ≤ 80 nm are radiolabelled with 99mTc. The excipients present in the kit include: tin chloride dihydrate as a reducing agent, glucose as a filling agent, an antifoaming agent called poloxamer 238, a sodium phosphate di-hydrate buffering agent, and a sodium phytate chelating agent for scavenging unbound 99mTc.Considering the chemical properties of each excipient, the hypothetical 99mTc-labelled species can be narrowed down to phytate or glucose. Phytic acid is a strong chelating molecule that forms stable complexes with multivalent transition metal ions (Vasca et al. 2002). Applications of phytate labelled with 99mTc have been reported in the literature, and a radiopharmaceutical kit (Phytacis®, IBA Molecular) is commercially available for diagnostic applications (Rao et al. 1990; Tavares et al. 2001; Takei et al. 2006). The chromatographic behaviour of [99mTc]Tc-phytate does not exhibit migration with solvent front in methanol/water mobile phase according to the QC method described in the SPC. Thus, [99mTc]Tc-phytate can be excluded as a hydrophilic impurity observed in this study.
Native non-functionalised glucose is only a very weak ligand for transition metals, and there are no stable complexes reported with 99mTc (Klufers and Kunte 2001; Bowen and Orvig 2008). Furthermore, 99mTc chemistry exhibits a strong preference towards N and S atoms when forming radiolabelled compounds (Alberto et al. 2020). Nevertheless, the imaging of glucose distribution on a TLC of the hydrophilic fraction A separated from [99mTc]Tc-NanoHSA using a Sep-Pak® cartridge showed that the glucose followed the distribution of 99mTc radioactivity in 0.9% NaCl and in 85% methanol mobile phases (Figs. 2 and 3). A large excess of glucose in the kit (15 mg) compared to nanocolloidal albumin (0.5 g) and 99mTc (1 GBq of 99mTc equivalent to 5 × 10–9 g) could enable the formation of a transient, elusive complex of [99mTc]Tc-glucose, sufficient to be picked up with TLC. This type of intermediate reaction supports our finding that the RCP of [99mTc]Tc-NanoHSA tended to increase between 10 and 60 min incubation time. In experiments with radiolabelling of glucose, the fraction of mobile 99mTc-carrying species decreased from 39 to 12% within 1 h incubation time (Table 5), which can be attributed to the formation of insoluble [99mTc]TcO2 colloid owing to dissociation of the labile [99mTc]Tc-glucose compound. Furthermore, varying distribution of radioactivity in the different fractions following the SPE of radiolabelled glucose (Table 6) showed an irreproducible behaviour, which was expected as a result of the weak, non-specific interaction of glucose with 99mTc. Another alternative explanation may be that a [99mTc]Tc-gluconate complex forms when a small fraction of the glucose in the kit becomes oxidised to gluconic acid.
Fig. 3.
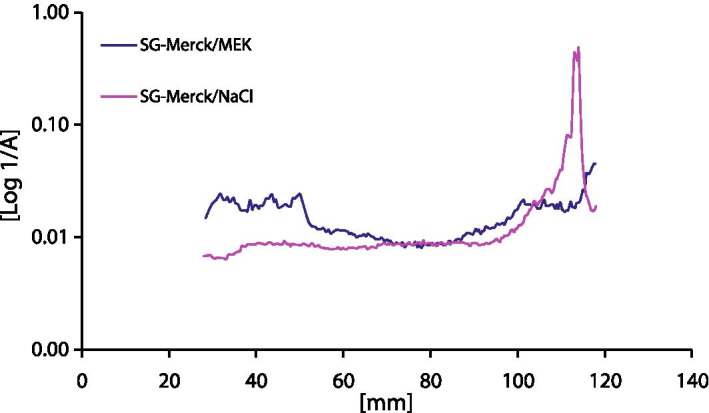
Distribution of glucose on a TLC of the hydrophilic fraction of [99mTc]Tc-NanoHSA. [99mTc]Tc-NanoHSA from one radiolabelling was retained on a Sep-Pak® cartridge and hydrophilic fraction A was submitted to TLC. Glucose migrated with the solvent front in 0.9% NaCl (purple trace) and mostly remained at the start in MEK (blue trace) mobile phase
Conclusions
The quality control of a commercially available NanoHSA kit labelled with 99mTc showed a reproducible RCP that fulfilled the criteria of the SPC, and the minimal 95% RCP requirement recommended by the European Pharmacopoeia for 99mTc-radiopharmaceuticals. A slight divergence was observed between polar and non-polar mobile phases used with iTLC. Thin-layer chromatography with polar aqueous mobile phases identified the presence of a small fraction of hydrophilic 99mTc-carrying species, potentially a weak labile complex of glucose, which contributes to overestimating the RCP in non-polar mobile phases. Solid-phase extraction using a Sep-Pak® cartridge yielded a RCP comparable to TLC with methanol/water and saline mobile phases and made it possible to separate hydrophilic impurities from the nanocolloidal [99mTc]Tc-HSA. A further TLC analysis of this SPE-separated hydrophilic fraction identified the presence of a hydrophilic impurity other than free pertechnetate. The chromatographic behaviour of glucose in the presence of [99mTc]TcO4− supported our hypothesis of a 99mTc-carrying glucose species as a potential hydrophilic impurity in the [99mTc]Tc-NanoHSA. Finally, nuclear medicine professionals running iTLC in a clinical setting should be aware of a potential misinterpretation of the RCP when using larger needles for spotting the samples.
Abbreviations
- GMP
Good manufacturing practices
- HSA
Human serum albumin
- iTLC
Instant thin-layer chromatography
- MEK
Methyl ethyl ketone
- NIS
Sodium/iodide symporter
- QC
Quality control
- Rf
Retardation factor
- RCP
Radiochemical purity
- SPC
Summary of product characteristics
- SPE
Solid-phase extraction
- TLC
Thin-layer chromatography
Authors' contributions
RC performed the experimental work, analysed the data and wrote the manuscript. ML performed the experimental work and revised the manuscript. CP performed the experimental work and revised the manuscript. MS supervised the work and revised the manuscript. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Availability of data and materials
Data can be obtained upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alberto R, Braband H, Nadeem Q. Bioorganometallic technetium and rhenium chemistry: fundamentals for applications. Chimia. 2020;74:953–959. doi: 10.2533/chimia.2020.953. [DOI] [PubMed] [Google Scholar]
- Bluemel C, Herrmann K, Giammarile F, Nieweg OE, Dubreuil J, Testori A, et al. EANM practice guidelines for lymphoscintigraphy and sentinel lymph node biopsy in melanoma. Eur J Nucl Med Mol Imaging. 2015;42:1750–1766. doi: 10.1007/s00259-015-3135-1. [DOI] [PubMed] [Google Scholar]
- Bowen ML, Orvig C. 99m-Technetium carbohydrate conjugates as potential agents in molecular imaging. Chem Commun. 2008 doi: 10.1039/b809365b. [DOI] [PubMed] [Google Scholar]
- Council of Europe. European Pharmacopoeia (Ph. Eur.). Technetium (99mTc) pentetate injection (Technetii (99mTc) pentetatis solution iniectabilis); 2019.
- Decristoforo C, Zolle I. Quality control methods of Tc-99m pharmaceuticals. In: Lever SZ, Lever JR, editors. Technetium-99m pharmaceuticals: preparation and quality control in nuclear medicine. 1. Berlin: Springer; 2007. pp. 132–137. [Google Scholar]
- Dumas C, Llamazares A, Koch P, Westera G. Labelling and quality control procedure of the 99mTc-human albumin nanocolloid kit. Eur J Nucl Med Mol Imaging. 2009;36:S281–S496. doi: 10.1007/s00259-009-1235-5. [DOI] [Google Scholar]
- Giammarile F, Alazraki N, Aarsvold JN, Audisio RA, Glass E, Grant SF, et al. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur J Nucl Med Mol Imaging. 2013;40:1932–1947. doi: 10.1007/s00259-013-2544-2. [DOI] [PubMed] [Google Scholar]
- Gommans GMM, Gommans E, van der Zant FM, Teule GJJ, van der Schors TG, de Waard JWD. Tc-99m Nanocoll: A radiopharmaceutical for sentinel node localisation in breast cancer-In vitro and in vivo results. Appl Radiat Isot. 2009;67:1550–1558. doi: 10.1016/j.apradiso.2009.02.091. [DOI] [PubMed] [Google Scholar]
- Grosser OS, Ruf J, Kupitz D, Pethe A, Ulrich G, Genseke P, et al. Pharmacokinetics of Tc-99m-MAA- and Tc-99m-HSA-microspheres used in preradioembolization dosimetry: influence on the liver-lung shunt. J Nucl Med. 2016;57:925–927. doi: 10.2967/jnumed.115.169987. [DOI] [PubMed] [Google Scholar]
- Hingorani M, Spitzweg C, Vassaux G, Newbold K, Melcher A, Pandha H, et al. The biology of the sodium iodide symporter and its potential for targeted gene delivery. Curr Cancer Drug Targets. 2010;10:242–267. doi: 10.2174/156800910791054194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klufers P, Kunte T. A transition metal complex of D-glucose. Angewandte Chemie-International Edition. 2001;40:4210–4212. doi: 10.1002/1521-3773(20011119)40:22<4210::AID-ANIE4210>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Marenco M, Canziani L, De Matteis G, Cavenaghi G, Aprile C, Lodola L. Chemical and physical characterisation of human serum albumin nanocolloids: kinetics, strength and specificity of bonds with 99mTc and 68Ga. Nanomaterials. 2021;11:1776. doi: 10.3390/nano11071776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metaye T, Rosenberg T, Guilhot J, Bouin-Pineau M-H, Perdrisot R. The presence of sodium nitrate in generator eluate decreases the radiochemical purity of 99mTc-sestamibi. J Nucl Med Technol. 2012;40:187–193. doi: 10.2967/jnmt.111.101246. [DOI] [PubMed] [Google Scholar]
- Persico MG, Lodola L, Buroni FE, Morandotti M, Pallavicini P, Aprile C. Tc-99m-human serum albumin nanocolloids: particle sizing and radioactivity distribution. J Labelled Compd Radiopharm. 2015;58:376–382. doi: 10.1002/jlcr.3317. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Arroyo T, Diaz-Alarcon JP, Garcia-Mendoza A, Muros MA, Del Valle MDM, et al. An alternative to the reference method for testing the radiochemical purity of Tc-99(m)-tetrofosmin. Nucl Med Commun. 2000;21:199–203. doi: 10.1097/00006231-200002000-00013. [DOI] [PubMed] [Google Scholar]
- Rao PN, Murthy SN, Muddukrishna SN, Devaru S, Radhakrishnan ER, Bhargava MK. Comparion of technetium-99m-phytate and technetium-99m-sulfur colloid in primary bone tumors. Eur J Nucl Med. 1990;16:847–853. doi: 10.1007/bf01280249. [DOI] [PubMed] [Google Scholar]
- Sadkin V, Skuridin V, Nesterov E, Stasyuk E, Rogov A, Varlamova N, et al. Tc-99m-labeled nanocolloid drugs: development methods. Sci Rep. 2020;10:9. doi: 10.1038/s41598-020-70991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub M, Leresche M, Pilloud C, Devynck F, Stritt N, Hesselmann R. A new two-strip TLC method for the quality control of technetium-99m mercaptoacetyl-triglycine (99mTc-MAG3) EJNMMI Radiopharm Chem. 2018;3:5. doi: 10.1186/s41181-018-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei H, Suemasu K, Kurosumi M, Ninomiya J, Horii Y, Inoue K, et al. Tc-99m-phytate is better than Tc-99m-human serum albumin as a radioactive tracer for sentinel lymph node biopsy in breast cancer. Surg Today. 2006;36:219–224. doi: 10.1007/s00595-005-3128-y. [DOI] [PubMed] [Google Scholar]
- Tavares MGM, Sapienza MT, Galeb NA, Belfort FA, Costa RR, Osorio C, et al. The use of Tc-99m-phytate for sentinel node mapping in melanoma, breast cancer and vulvar cancer: a study of 100 cases. Eur J Nucl Med. 2001;28:1597–1604. doi: 10.1007/s002590100625. [DOI] [PubMed] [Google Scholar]
- United States Pharmacopoeia Convention. United States Pharmacopoeia (USP). Technetium Tc-99m Albumin Colloid Injection; 2019.
- Vanbilloen HP, Verbeke KA, De Roo MJ, Verbruggen AM. Tc-99m labeled human serum albumin for ventriculography—a comparative evaluation of 6 labeling kits. Eur J Nucl Med. 1993;20:465–472. doi: 10.1007/bf00175158. [DOI] [PubMed] [Google Scholar]
- Vasca E, Materazzi S, Caruso T, Milano O, Fontanella C, Manfredi C. Complex formation between phytic acid and divalent metal ions: a solution equilibria and solid state investigation. Anal Bioanal Chem. 2002;374:173–178. doi: 10.1007/s00216-002-1469-6. [DOI] [PubMed] [Google Scholar]
- Waight LA, Cunnane CM, O'Brien LM, Millar AM. Effect of Tc-99 on the radiochemical purity of Tc-99m radiopharmaceuticals. Nucl Med Commun. 2011;32:752–756. doi: 10.1097/MNM.0b013e32834755df. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. The International Pharmacopoeia. Technetium ( 99m Tc) pentetate complex injection (Technetii ( 99m Tc) pentetatis multiplex injectio); 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained upon request.