Abstract
Background:
Studies in lung transplantation have shown variable association between hospital volume and clinical outcomes. We aimed to identify the pattern of effect of hospital volume on individual survival after lung transplantation.
Methods:
We performed a retrospective analysis using the United Network for Organ Sharing national thoracic organ transplantation database. Adult patients who underwent lung transplantation between January 2013 and December 2017 were included. The association between mean annual center volume and 1-year overall survival was examined using restricted cubic splines in a random-effect multivariable Cox model. The volume threshold for optimal 1-year overall survival was subsequently approximated via the maximum likelihood approach using segmented linear splines in the same model.
Results:
The study included 10,007 patients at 71 transplant centers. Median annual center volume was 22.0 cases (IQR 10.6–38.0 cases). A center volume threshold was identified at 33 cases/year (95% CI 28–37 cases). Higher center volume up to 33 cases/year was associated with better 1-year survival (HR = 0.989, 95% CI 0.980 – 0.999 every additional case). Further increase in center volume above 33 cases/year showed no additional benefit (HR = 1.000, 95% CI 0.996 – 1.003 every additional case). Twenty-three centers (32.4%) reached the volume threshold of 33 cases/year.
Conclusions:
One-year survival after lung transplantation improved with increasing center volume up to 33 cases/year. Low volume centers below the 33 cases/year threshold had large variations in their outcomes and had a higher risk of performing poorly, though many of them maintained good performance.
Lung transplantation has been the definitive treatment for end-stage lung disease, and the annual number of lung transplants performed worldwide and in the United States has grown steadily over the past two decades1.2 The Centers for Medicare and Medicaid Services mandate an institutional volume of 10 cases annually to quality for funding3. Despite this mandate, annual procedural volume is highly variable4. Previous studies have attempted to characterize a volume-outcome relationship for lung transplantation and have suggested that higher transplantation volume may be associated with improved postoperative complications, rescue from complications, and survival4–6. These conclusions are frequently cited in arguments for in favor of regionalization and shifting lung transplantation to “high volume” centers.
However, existing volume-outcomes studies in lung transplantation have notable limitations in their methods used to define high volume. Most of these investigations have attempted to define volume using arbitrarily defined categories (i.e. tertiles, quartiles, etc.) rather than evaluating it as a continuous variable. Imposing artificial categorical boundaries on a continuous variable can lead to loss of information and can inflate the effect of volume on risk of postoperative complications or mortality when using odds ratios7–9. Previous studies that have examined transplantation as a continuous variable have assumed a linear relationship between transplantation and their outcome of interest, while the true relationship may not be linear10. Finally, with multiple definitions used for low and high volume in the literature, it is difficult to reach a consensus on what a high-volume transplantation center truly represents. Despite these methodological limitations, these studies serve as the backbone for arguments for regionalization of care in lung transplantation, which can have far-reaching consequences.
Hence, we proposed a study to characterize the relationship between lung transplantation procedural volume and post-transplant survival. We hypothesized that, after adjusting for other factors, a higher annual case volume would be associated with better 1-year survival. Additionally, we evaluated the relationship between center volume and 5-year overall survival using the same methodology.
Material and Methods
Design and Cohort Selection
We performed a retrospective analysis using the United Network for Organ Sharing (UNOS) national thoracic organ transplantation data of adult patients who underwent lung transplantation between January 2013 and December 2017. We assessed the relationship between annual transplant center volume and post-transplant 1-year overall survival and determined the volume threshold for optimal 1-year overall survival in lung transplantation. The individual patient was used as the unit of analysis, and each patient was assigned a variable representing the volume of the center at which they underwent transplantation. One-year survival, which is often used as a quality metric, was chosen as our primary endpoint to ensure a contemporaneous cohort with sufficient follow-up. Relationship between center volume and post-transplant 5-year overall survival was additionally evaluated using the same methodology.
We included all patients ≥ 18 years of age who underwent lung transplantation. Patients who underwent combined heart-lung or multiorgan transplantation, a redo lung transplantation, or had a previous organ transplantation of any type were excluded.
Center volume
Annual transplant center volume was estimated by the average number of transplants per active year during the study period. Centers that performed ≥ 1 transplantation during a year were considered active for that year.
Statistical analysis
Random-effect multivariable Cox modeling with restricted cubic splines (RCS) was initially used to estimate and visualize the functional relationship between center volume and post-transplant 1-year overall survival. Use of RCS allows for non-linear characterization of the relationship between volume and survival. Four knots at 5, 35, 65, and 95 percentiles were chosen to fit the RCS in the Cox model as recommended by Harrell11 and as evidenced by Akaike Information Criteria (AIC) score comparison with RCS using more knots. Variables in the Cox models were determined a priori based on literature review and clinical experience and included recipient age, gender, ethnicity, diagnosis grouping, lung allocation score, medical acuity, mean pulmonary artery pressure, creatinine, preoperative ventilator support, preoperative ECMO support, ischemic time, single lung transplant, donor age, donor non-heart beating status and donor PO2/FiO2. Center-specific variation was accounted for by incorporating a random-effect term of transplant centers into the model12. Only patients with complete covariable data were included in the Cox models.
Because a non-linear relationship between center volume and overall survival was observed on RCS, segmented linear splines (SLS) were used to approximate this functional relationship in the same model via a maximum likelihood approach with visual evidence from RCS, as described by Muggeo13. The resulting Cox model with SLS was examined by AIC and was compared with the RCS model for goodness of fit. While the RCS model smoothly estimates the relationship between center volume and post-transplant survival, regression coefficients of RCS terms in the model do not have any meaningful interpretation. The use of SLS allows center volume to be treated as a segmented continuous variable with hazard ratios corresponding to each segment. The effects of increased center volume within each volume segment can thus be quantified and evaluated.
Hazard ratios (HR) in the final Cox model with SLS were shown with 95% confidence interval (CI). All p-values were two-tailed and p-values <0.05 were considered statistically significant. All statistical analyses were performed using R version 3.5.3 (R Core Team, Vienna, Austria) and SPSS software version 25.0 (IBM, Armonk, NY, USA).
Results
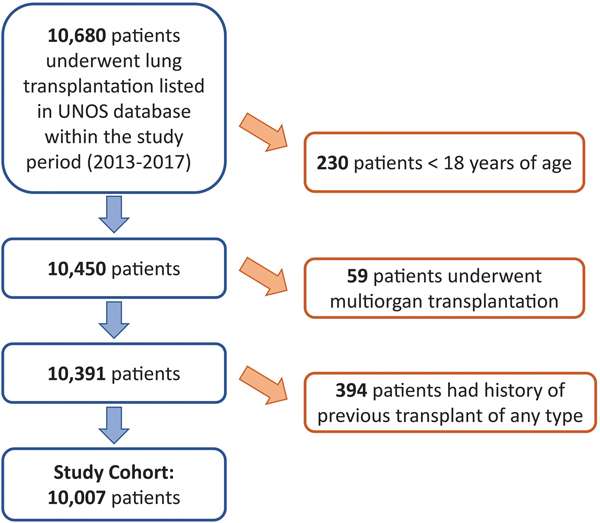
A total of 10,007 lung transplant recipients were included in this study (Figure 1), Median follow-up was 24.7 months (IQR 12.6 – 41.8 months). Baseline recipient and donor characteristics are shown in Table 1. During the study period, 71 transplant centers were active for at least one year and the median annual volume was 22.0 cases (IQR 10.6 – 38.0 cases). The highest annual volume was 102.2 cases and the lowest was 1 case.
Figure 1.
Consort Diagram of Study Cohort Selection.
Table 1:
Baseline characteristics (n = 10007).
| Variables | Valid Cases (% missing) | |
|---|---|---|
|
| ||
| Recipient age | 57.3 (±12.3) | 10007 (0%) |
| Male recipient | 5987 (59.8%) | 10007 (0%) |
| Recipient ethnicity | 10007 (0%) | |
| White | 8127 (81.2%) | |
| Black | 917 (9.2%) | |
| Others | 963 (9.6%) | |
| BMI | 25.5 (±4.5) | 9996 (0.1%) |
| LAS | 48.4 (±18.2) | 10007 (0%) |
| Diagnosis | 10007 (0%) | |
| Restrictive lung disease | 5752 (57.5%) | |
| Obstructive lung disease | 2805 (28.0%) | |
| Pulmonary vascular disease | 340 (3.4%) | |
| Cystic fibrosis | 1110 (11.1%) | |
| Medical acuity | 10007 (0%) | |
| Not hospitalized | 7797 (77.9%) | |
| Hospitalized but not in ICU | 980 (9.8%) | |
| In ICU | 1230 (12.3%) | |
| Mean pulmonary artery pressure | 27.5 (±10.8) | 9519 (4.9%) |
| Creatinine | 0.8 (±0.4) | 10007 (0%) |
| Preoperative ventilator support | 560 (5.6%) | 10007 (0%) |
| Preoperative ECMO support | 485 (4.8%) | 10007 (0%) |
| Ischemic time (hour) | 5.2 (±1.8) | 9961 (0.5%) |
| Single lung transplant | 2894 (28.9%) | 10006 (<0.1%) |
| Donor age | 35.1 (±14.0) | 10007 (0%) |
| Donor non-heart beating | 316 (3.2%) | 10007 (0%) |
| Donor PaO2/FiO2 | 436 (372–496) | 9954 (0.5%) |
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LAS, lung allocation score.
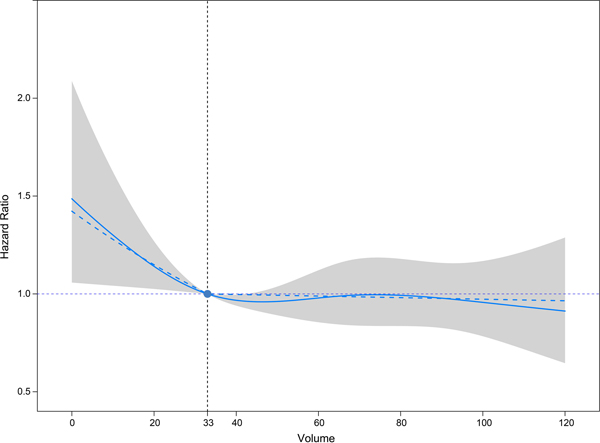
Final multivariable Cox models included 9423 patients for whom complete covariable data were available (5.8% missing, missing variables detailed in Table 1). RCS in the random-effect multivariable Cox model showed a non-linear relationship between center volume and post-transplant 1-year overall survival (Figure 2). Based on visual evidence from RCS, a maximum likelihood approach with SLS was used to approximate the functional relationship between center volume and 1-year survival in the same model. The threshold of annual lung transplant volume for optimal 1-year survival was identified at 33 cases (95% CI 28 – 37; Figure 2). AIC showed that the resulting model with SLS was non-inferior to the model with RCS (SLS 18914.84 vs. RCS 18915.84). Our model demonstrated that higher lung transplant center volume up to 33 cases per year was associated with better 1-year survival (HR = 0.989, 95% CI 0.980 – 0.999 every additional case). Additional increase in center volume over the 33 transplants per year threshold had no impact on 1-year survival (HR = 1.000, 95% CI 0.996 – 1.003 every additional case; Table 2). Additionally, higher recipient age (HR = 1.016, 95% CI 1.008 – 1.023), black ethnicity (HR = 1.270, 95% CI 1.040 – 1.552), higher LAS (HR = 1.006, 95% CI 1.001 – 1.012), pulmonary vascular disease diagnosis (HR = 1.424, 95% CI 1.026 – 1.977), preoperative ventilator support (HR = 1.469, 95% CI 1.088 – 1.985) and longer ischemic time (HR = 1.070, 95% CI 1.033 – 1.108) were associated with decreased 1-year survival.
Figure 2.
Volume Outcome Curve Demonstrating Relationship Between Annual Transplant Center Volume and Hazard of Post-Transplant Mortality Within One Year (n=9423). Solid curve represents restricted cubic splines; dashed curve represents segmented linear splines; shaded area represents the 95% confidence interval of restricted cubic splines.
Table 2:
Random-effect Cox model with segmented linear splines for 1-year survival after lung transplantation (n = 9423).
| Hazards ratio (95% CI) | P value | |
|---|---|---|
|
| ||
| Higher transplant volume (per case)* | ||
| When <33 cases | 0.989 (0.980 – 0.999) | 0.036 |
| When ≥33 cases | 1.000 (0.996 – 1.003) | 0.798 |
| Higher recipient age (per year) | 1.016 (1.008 – 1.023) | <0.001 |
| Male recipient | 1.105 (0.969 – 1.260) | 0.137 |
| Recipient ethnicity | ||
| White | Reference | |
| Black | 1.270 (1.040 – 1.552) | 0.019 |
| Others | 1.020 (0.823 – 1.265) | 0.856 |
| Higher LAS (per unit) | 1.006 (1.001 – 1.012) | 0.027 |
| Diagnosis grouping | ||
| Restrictive lung disease | Reference | |
| Obstructive lung disease | 1.013 (0.859 – 1.196) | 0.876 |
| Pulmonary vascular disease | 1.424 (1.026 – 1.977) | 0.035 |
| Cystic fibrosis | 0.996 (0.733 – 1.352) | 0.978 |
| Medical acuity | ||
| Not hospitalized | Reference | |
| Hospitalized but not in ICU | 0.958 (0.751 – 1.222) | 0.732 |
| In ICU | 1.012 (0.754 – 1.359) | 0.935 |
| Higher mean pulmonary artery pressure (per mmHg) | 1.006 (1.000 – 1.012) | 0.052 |
| Higher creatinine (per unit) | 1.078 (0.989 – 1.174) | 0.088 |
| Preoperative ventilator support | 1.469 (1.088 – 1.985) | 0.012 |
| Preoperative ECMO support | 1.119 (0.812 – 1.542) | 0.493 |
| Longer ischemic time (per hour) | 1.070 (1.033 – 1.108) | <0.001 |
| Single lung transplant | 1.067 (0.914 – 1.244) | 0.413 |
| Higher donor age (per year) | 1.003 (0.999 – 1.008) | 0.123 |
| Donor non-heart beating | 1.127 (0.819 – 1.551) | 0.463 |
| Higher donor PaO2/FiO2 (per 10 mmHg) | 0.998 (0.993 – 1.003) | 0.416 |
Two-segment linear splines.
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LAS, lung allocation score.
The relationship between center volume and post-transplant 5-year survival was analyzed using the same methodology, and a similar 2-segment relationship was observed with threshold identified at 35 cases per year (Supplemental Figure 1). Post-transplant 5-year survival improved with increasing center volume up to 35 cases per year (HR = 0.994, 95% CI 0.988 – 1.000 every additional case), and good survival was maintained beyond 35 cases per year (HR = 1.001, 95% CI 0.999 – 1.003 every additional case; Supplemental Table 1).
Twenty-three of 71 lung transplant centers (32.4%) in this study reached the volume threshold of 33 cases per year. Patients transplanted at high-volume centers (≥33 cases/year) were older (High-volume 57.4±12.8 vs. Low-volume 55.8±12.8, P<0.001; Table 3), had higher LAS (High-volume 49.1±18.7 vs. Low-volume 46.9±17.2, P<0.001), and more often required preoperative ventilator (High-volume 6.5% vs. Low-volume 3.7%, P<0.001) or ECMO support (High-volume 5.3% vs. Low-volume 3.9%, P=0.003). Diagnosis of restrictive lung disease was more frequently seen at high-volume centers (High-volume 59.3% vs. Low-volume 53.7%, P<0.001). Ischemic time was longer at high volume centers (High-volume 5.4±1.9 hours vs. Low-volume 5.0±1.7 hours, P<0.001) and non-heart beating donors were more frequently utilized at high volume centers (High-volume 3.7% vs. Low-volume 1.9%, P<0.001). On unadjusted comparison, incidence of reintubation (High-volume 1194/6722 [17.8%] vs. Low-volume 607/3229 [18.8%], P=0.209; missing 0.6%) and airway dehiscence (High-volume 97/6706 [1.4%] vs. Low-volume 45/3230 [1.4%], P=0.834; missing 0.7%) was similar between the high- and low-volume groups. Recipient and donor risk-adjusted one-year survival rate corresponding to each center with volume ≥ 5 cases/year is shown in Supplemental Figure 2.
Table 3:
Baseline characteristics comparison between high-volume (≥33 cases/year) and low-volume (<33 cases/year) centers (n = 10007).
| Variables | Low-volume (n=3258) | High-volume (n=6749) | P value |
|---|---|---|---|
|
| |||
| Recipient age | 55.8 (±12.8) | 57.4 (±12.8) | <0.001 |
| Male recipient | 1946/3258 (59.7%) | 4041/6749 (59.9%) | 0.889 |
| Recipient ethnicity | <0.001 | ||
| White | 2642/3258 (81.1%) | 5485/6749 (81.3%) | |
| Black | 369/3258 (11.3%) | 548/6749 (8.1%) | |
| Others | 247/3258 (7.6%) | 716/6749 (10.6%) | |
| BMI | 25.6 (±4.6) | 25.5 (±4.4) | 0.205 |
| LAS | 46.9 (±17.2) | 49.1 (±18.7) | <0.001 |
| Diagnosis | <0.001 | ||
| Restrictive lung disease | 1749/3258 (53.7%) | 4003/6749 (59.3%) | |
| Obstructive lung disease | 979/3258 (30.0%) | 1826/6749 (27.1%) | |
| Pulmonary vascular disease | 110/3258 (3.4%) | 230/6749 (3.4%) | |
| Cystic fibrosis | 420/3258 (12.9%) | 690/6749 (10.2%) | |
| Medical acuity | 0.158 | ||
| Not hospitalized | 2567/3258 (78.8%) | 5230/6749 (77.5%) | |
| Hospitalized but not in ICU | 320/3258 (9.8%) | 859/6749 (9.8%) | |
| In ICU | 371/3258 (11.4%) | 660/6749 (12.7%) | |
| Mean pulmonary artery pressure | 27.6 (±10.5) | 27.4 (±10.9) | 0.505 |
| Creatinine | 0.9 (±0.6) | 0.8 (±0.3) | 0.002 |
| Preoperative ventilator support | 120/3258 (3.7%) | 440/6749 (6.5%) | <0.001 |
| Preoperative ECMO support | 128/3258 (3.9%) | 357/6749 (5.3%) | 0.003 |
| Ischemic time (hour) | 5.0 (±1.7) | 5.4 (±1.9) | <0.001 |
| Single lung transplant | 1002/3257 (30.8%) | 1892/6749 (28.0%) | 0.005 |
| Donor age | 33.8 (±13.4) | 35.7 (±14.2) | <0.001 |
| Donor non-heart beating | 63/3258 (1.9%) | 253/6749 (3.7%) | <0.001 |
| Donor PaO2/FiO2 | 432 (368–492) | 438 (374–497) | 0.007 |
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LAS, lung allocation score.
Comment
Hospital procedural volume has long been promoted as an indicator of increased quality and superior outcomes for multiple complex surgical procedures. Prima facie, the “practice-makes-perfect” concept suggests that surgeons in higher volume facilities have accumulated more experience, leading to improved patient outcomes14. Conversely, a prevailing notion indicates that over time, leading facilities with good surgical outcome may garner increased patient volume based on reputation, thus achieving and maintaining higher surgical volume.
Given the complex nature of lung transplantation, previous studies have hypothesized that a similar volume-outcome relationship exists. Weiss and colleagues utilized UNOS transplant registry data to examine the relationship between hospital lung transplant volume and 30-day, one-year, and five-year survival6. They divided 10,496 patients into volume categories based on quartiles, with low volume hospitals performing 0–2.1 cases/year and high-volume hospitals performing 20–58.2 cases/year. They found that centers in the lowest volume quartile had an 89% increased risk of 30-day mortality compared to hospitals in the highest volume quartile. Additionally, low-volume hospitals had a 95% greater risk of 1-year mortality and 46% increased risk of 5-year cumulative mortality, when compared to high-volume hospitals. Kilic and colleagues utilized UNOS registry data to examine the relationship between lung transplant center volume and risk of postoperative complications5. They examined outcomes in 12,565 patients from 77 hospitals and divided volume by tertiles, with low-volume defined as an annual transplant volume of <21.8 transplants/year and high-volume defined as >34.2 transplants/year. They observed that low-volume centers had significantly elevated risk of developing certain postoperative complications, including a 79% increased risk of postoperative renal failure and a 27% increased risk postoperative infection. They also observed that when exclusively evaluating patients who developed postoperative complications, those transplanted at low-volume centers had a 52% greater risk of 90-day mortality compared to patients transplanted at high-volume centers. This suggested that high-volume centers may have a greater ability to “rescue” patients who developed postoperative complications, which translated into differences in survival15.
These studies provide preliminary evidence supporting a volume-outcome relationship in lung transplantation. However, arbitrary definitions of volume (i.e. tertiles, quartiles, or quintiles) in these studies represent methodological flaws that prevent an accurate understanding and quantification of the relationship of volume to post-transplantation survival7, 8. The categorical definitions may not represent the true inflection point(s) where a statistically significant change occurs. Alternative methods have been to define volume as a continuous variable. However, existing studies that have used this method have assumed a linear relationship when a non-linear relationship may be more accurate5, 6, 10.
Utilizing RCS to assess the relationship between continuous variables and an outcome of interest11,16, we demonstrated a non-linear relationship between hospital transplant volume and one-year transplant survival. Mortality risk decreased for every additional case up to 33 cases per year, with no additional benefit after 33 cases. Several factors may explain the volume-outcome relationship observed in lung transplantation. In addition to surgeon and pulmonologist experience, patients at higher-volume centers likely benefit from experienced multidisciplinary specialty teams proficient in the perioperative care of lung transplant patients5. These specialists include cardiothoracic anesthesiologists, nutritionists, psychologists, respiratory therapists, and many others. Anecdotal experience shows that with the input of these multidisciplinary teams, higher-volume centers use efficient pre-transplant evaluation algorithms and develop standardized care pathways for post-transplantation care, akin to enhanced recovery (ERAS) after major surgery17,18. Additionally, higher-volume centers have access to greater intensive care unit capacity and improved nurse to patient ratios, which are important in the immediate postoperative care of transplant recipients19. Finally, as suggested by Kilic, higher-volume centers may have greater experience and multidisciplinary expertise to “rescue” patients who develop postoperative complications5. However, not all lower volume centers had poorer performance. As shown in Supplemental Figure 2, many low volume centers had similar survival compared to high volume centers. The overall down-trending survival at lower center volume below the 33 cases/year threshold was associated with the higher likelihood of undergoing transplantation at a poor-performing center. On the other hand, good performance is more consistently observed above the volume threshold.
Given the prior evidence for a volume-outcome relationship, regionalization of lung transplantation to higher-volume centers has been deliberated. Proponents of regionalization have portrayed multiple benefits beyond improved patient outcomes and increased survival6. As high-volume centers have been shown to transplant older and potentially sicker patients, such centers can be an avenue for high-risk patients to gain access to transplantation, when they may not have been accepted to the waitlist at lower-volume hospitals. Additionally, high-volume centers may be more likely to use marginal donors, thus allowing increased organ utilization20,21. Finally, performing lung transplantation at higher-volume hospitals may be more cost-effective. Mooney and colleagues utilized Medicare claims data to compare cost of lung transplantation admission between low (<20 cases/year), intermediate (20–34 cases/year), and high-volume (≥35 cases/year) centers22. In a risk-adjusted analysis, they found compared to high-volume centers, low-volume centers were associated with an 11.7% higher cost of hospitalization. With fewer complications and lower in-hospital mortality, the authors concluded that higher-volume transplant centers offered high-quality and cost-effective care.
However, there are potential adverse consequences of implementing volume-based regionalization policies for lung transplantation. Regionalization can limit accessibility for patients who live far from a high-volume center. A 2016 UNOS study simulated the impact of closing the lowest-volume lung transplant centers and referring their patients to the highest-volume centers in the same region23. On adjusted analysis, with the closure of five centers per region, 240 lives would be saved per year. However, at the same time, 2999 patients would be referred away from the original center. Depending on region, travel distances for lung transplant patients range from 143 ±189 miles (California/Southwest region) to 325±420 miles (Pacific Northwest region)23. Given the limited number of lung transplant centers in each region, further regionalization of care could significantly increase travel distances and out of pocket expenditures for patients. In a recent national survey study investigating whether patients would be willing to travel to safer hospitals for complex cancer surgery, 74% of all respondents identified at least one barrier to travel, with the most common ones being the belief of insurance restriction, a higher cost of surgery, difficulty of following up, and the traffic. Interestingly, 94% of respondents with barriers would travel if some forms of facilitation, such as insurance coverage or transportation, were offered24. It needs to be noted that lower income, nonwhite race, and metropolitan residence were associated with reluctance to travel to a specialty hospital. The possibility of widening the disparity in access to care may be another critical barrier for regionalization of lung transplantation.
Successful regionalization has been reported in the field of thoracic surgery. Ely et al. analyzed the impact of regionalization of lung resection25 and esophagectomy26 in the Kaiser Permanente system in Northern California. This change was associated with shorter hospitalization and lower rate of complications for both operations. In 2007, a regionalization policy for lung cancer was implemented in Ontario, Canada. It was reported that in the years after policy implementation, length of hospital stay decreased more than expected from the baseline trend by 7% per year, while total travel distance by all patients to the hospital increased by 4% per year27. Although no significant reduction in operative mortality over and above the preexisting trend was observed in the overall cohort, significantly decreased mortality was seen in the ≥70 years age group, suggesting that patients with high risk profile likely benefit more from regionalization. Findings from these studies suggest that selection of high-risk patients, close collaboration between referring and regional centers, and the expansion of insurance coverage to provide travel and ancillary support to patients and caregivers will likely facilitate successful introduction of lung transplant regionalization. Additionally, given the large variation in center performance at lower volume centers, deliberate center evaluation is necessary to avoid referring patients away from low volume centers with good performance.
There are some limitations to our study that should be noted. First, with limitations inherent to retrospective analyses, our data demonstrated associations and not causality. While our multivariable modeling attempted to utilize all available and relevant covariates for risk-adjustment, unmeasured confounders could bias our analysis. We selected one-year post transplant survival as our outcome of interest to ensure a contemporaneous cohort with sufficient follow-up. Although we additionally evaluated the relationship between center volume and 5-year survival, this long-term survival analysis could be biased by the large number of patients censored due to our study period from 2013 to 2017 with maximal follow-up till the end of 2018. Moreover, there are other relevant outcomes including the risk of postoperative complications, number of readmissions, and chronic rejection. Our models cannot comment on volume thresholds for these outcomes. Lastly, the current study focuses on characterizing the overall volume-survival relationship. The comparisons between the categorized low- and high-volume groups in this study are only descriptive, and many centers with volume lower than the 33 cases/year had similar outcomes as high volume centers. Our study also has some important strengths. It utilizes a large repository of validated, prospectively collected data, employs a robust statistical technique to understand the volume-outcomes relationship, and offers evidence-based guidance for policymakers.
In summary, we identified a non-linear relationship between hospital lung transplant volume and one-year survival. Survival improved with increasing center volume up to 33 cases/year. Low volume centers below the 33 cases/year threshold had large variations in their outcomes and had a higher risk of performing poorly, though many of them maintained good performance.
Supplementary Material
References
- 1.Shigemura N. Lung transplantation and beyond: continued challenges in the wake of significant progress. J Thorac Dis 2019; 11(Suppl 3):S413–S416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant 2018; 37(10):1169–1183. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Medicare & Medicaid Services (CMS) HHS. Medicare program; hospital conditions of participation: requirements for approval and re-approval of transplant centers to perform organ transplants. Final rule. Fed Regist 2007; 72(61):15197–280. [PubMed] [Google Scholar]
- 4.Scarborough JE, Bennett KM, Davis RD, et al. Temporal trends in lung transplant center volume and outcomes in the United States. Transplantation 2010; 89(6):639–43. [DOI] [PubMed] [Google Scholar]
- 5.Kilic A, George TJ, Beaty CA, et al. The effect of center volume on the incidence of postoperative complications and their impact on survival after lung transplantation. J Thorac Cardiovasc Surg 2012; 144(6):1502–8; discussion 1508–9. [DOI] [PubMed] [Google Scholar]
- 6.Weiss ES, Allen JG, Meguid RA, et al. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg 2009; 88(4):1062–70. [DOI] [PubMed] [Google Scholar]
- 7.Shahian DM, Normand SL. The volume-outcome relationship: from Luft to Leapfrog. Ann Thorac Surg 2003; 75(3):1048–58. [DOI] [PubMed] [Google Scholar]
- 8.Kozower BD, Stukenborg GJ. Volume-Outcome Relationships in Thoracic Surgery. Thorac Surg Clin 2017; 27(3):251–256. [DOI] [PubMed] [Google Scholar]
- 9.Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology 1995; 6(4):450–4. [DOI] [PubMed] [Google Scholar]
- 10.Stukenborg G, Wagner D, Harrell F. Temporal Order and Nonlinearity in the Relationship Between Lung Cancer Resection Volume and In-Hospital Mortality. Health Serv Outcomes Res Method 2004; 5:59–73. [Google Scholar]
- 11.Harrell FE. Regression Modeling Strategies: with applications to linear models, logistic regression, and survival analysis. Springer-Verlag New York, Inc. New York, USA. 2010. [Google Scholar]
- 12.Therneau T, Grambsch P, Pankratz P. Penalized survival models and frailty. Journal of Computational and Graphical Statistics 2003; 12(1):156–171. [Google Scholar]
- 13.Muggeo VM. Estimating regression models with unknown break-points. Stat Med 2003; 22(19):3055–71. [DOI] [PubMed] [Google Scholar]
- 14.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979; 301(25):1364–9. [DOI] [PubMed] [Google Scholar]
- 15.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care 2011; 49(12):1076–81. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant. 2020; 55(4):675–680. [DOI] [PubMed] [Google Scholar]
- 17.Roussel MG, Gorham N, Wilson L, Mangi AA. Improving recovery time following heart transplantation: the role of the multidisciplinary health care team. J Multidiscip Healthc. 2013; 6:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cajita MI, Baumgartner E, Berben L, et al. Heart transplant centers with multidisciplinary team show a higher level of chronic illness management - Findings from the International BRIGHT Study. Heart Lung. 2017. 46(5):351–356. [DOI] [PubMed] [Google Scholar]
- 19.Kelly DM, Kutney-Lee A, McHugh MD, et al. Impact of critical care nursing on 30-day mortality of mechanically ventilated older adults. Crit Care Med 2014; 42(5):1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafranca JA, Spoon EQW, van de Wetering J, IJzermans JNM, Dor FJMF. Attitudes among transplant professionals regarding shifting paradigms in eligibility criteria for live kidney donation. PLoS One. 2017;12(7):e0181846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilic A, Weiss ES, Allen JG, George TJ, Yuh DD, Shah AS, Conte JV. Should orthotopic heart transplantation using marginal donors be limited to higher volume centers? Ann Thorac Surg. 2012; 94(3):695–702. [DOI] [PubMed] [Google Scholar]
- 22.Mooney JJ, Weill D, Boyd JH, et al. Effect of Transplant Center Volume on Cost and Readmissions in Medicare Lung Transplant Recipients. Ann Am Thorac Soc 2016; 13(7):1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magruder JT, Shah AS, Crawford TC, et al. Simulated Regionalization of Heart and Lung Transplantation in the United States. Am J Transplant. 2017; 17(2):485–495. [DOI] [PubMed] [Google Scholar]
- 24.Resio BJ, Chiu AS, Hoag JR, et al. Motivators, Barriers, and Facilitators to Traveling to the Safest Hospitals in the United States for Complex Cancer Surgery. JAMA Netw Open. 2018; 1(7):e184595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ely S, Jiang SF, Patel AR, Ashiku SK, Velotta JB. Regionalization of Lung Cancer Surgery Improves Outcomes in an Integrated Health Care System. Ann Thorac Surg. 2020; 110(1):276–283. [DOI] [PubMed] [Google Scholar]
- 26.Ely S, Alabaster A, Ashiku SK, Patel A, Velotta JB. Regionalization of thoracic surgery improves short-term cancer esophagectomy outcomes. J Thorac Dis. 2019; 11(5):1867–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bendzsak AM, Baxter NN, Darling GE, Austin PC, Urbach DR. Regionalization and Outcomes of Lung Cancer Surgery in Ontario, Canada. J Clin Oncol. 2017; 35(24):2772–2780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.