Abstract
Excess pain following visceral provocation has been suggested as a marker for chronic pelvic pain risk in women. However, few noninvasive tests have been validated that could be performed readily on youth in early risk windows. Therefore, we evaluated the validity and reliability of a noninvasive bladder pain test in 124 healthy premenarchal females (median age 11, [IQR 11-12]), as previously studied in adult women. We explored whether psychosocial, sensory factors, and quantitative sensory test results were associated with provoked bladder pain and assessed the relation of bladder pain with prior abdominal pain history. Compared with findings in young adult females (age 21 [20-28]), results were similar except that adolescents had more pain at first sensation to void (p=0.005) and lower maximum tolerance volume (p<0.001). Anxiety, depression, somatic symptoms, and pain catastrophizing predicted provoked bladder pain (p’s < 0.05). Bladder pain inversely correlated with pressure pain thresholds (r=−.25, p <0.05), but not with cold pressor pain or conditioned pain modulation effectiveness. Bladder pain was also associated with frequency of abdominal pain symptoms (r=0.25, p=0.039). We found strong retest reliability for bladder pain at standard levels of sensory urgency in 21 adolescents who attended repeat visits at 6-12 months (intraclass correlations = 0.88-0.90). Noninvasive bladder pain testing appears reproducible in adolescent females and may predict abdominal pain symptomatology. Confirmation of our findings and further investigation of the bladder test across menarche will help establish how visceral sensitivity contributes to the early trajectory of pelvic pain risk.
1. Introduction
Visceral chronic pelvic pain (CPP) disorders (e.g., endometriosis-associated pelvic pain, irritable bowel syndrome [IBS], and bladder pain syndrome [BPS]) cause substantial morbidity and cost upwards of 50 billion U.S. dollars in lost wages and health care expenditures annually. [7,34,51,76] Current treatments for CPP are often ineffective, making early identification of at-risk, asymptomatic individuals a critical research priority.[11] Excess pain induced by experimentally-controlled organ provocation could be a promising marker for CPP risk, [36,50,67] but future risk has been largely evaluated using symptom questionnaires.
In adults, prospective symptom questionnaires suggest illness behavior and somatic sensitivity predict new-onset IBS risk. [29,41] Warren and colleagues have found that new-onset BPS patients commonly report prodromal symptoms of urogenital dysfunction. [1,2] For an effective prevention strategy, such questionnaires ideally would identify vulnerable individuals prior to the onset of full-blown symptoms. However, existing studies have generally recruited individuals already exhibiting some degree of pain or distress. Self-reported dysmenorrhea intensity is readily ascertainable even in adolescence, and is a consistent and significant risk factor for premenopausal CPP.[2,60,70,77] Menstrual pain intensity alone lacks specificity for use to predict future pain risk, as moderate dysmenorrhea is highly prevalent, affecting 40-90% of women.[52] Given these limitations, there is clearly a need for additive approaches to pain-risk detection that complement symptom report with measures such as quantitative sensory testing (QST).[16,75]
Enhanced pain in response to intraluminal organ provocation, such as rectal distension, is a well-established experimental marker for IBS [36], but a less invasive test is needed for broader studies. To facilitate early chronic-pain risk detection in adults, we have validated a noninvasive bladder distension task using oral water loading.[60] Notably, women with CPP, regardless of BPS status, report more pain after bladder distension than healthy controls.[63] Separately, we have found some dysmenorrhea sufferers, without chronic pain, harbor silent bladder pain identifiable with this method.[22] These otherwise healthy young women with combined menstrual and bladder pain intriguingly also have psychosocial and QST profiles skewed towards results commonly observed in chronic pain.[23] Provoked bladder pain is also more closely related to pelvic pain symptom severity, compared to other QST results, in CPP and asymptomatic women.[23,63]
In this study, we have implemented the bladder test in pre-menarchal females. Using the initial data from an ongoing, longitudinal study, we establish the initial validity and retest reliability of provoked bladder pain in adolescents. We compare these results with our historical adult findings to assess for developmental differences. Because psychological factors and somatic symptom burden may predict experimental pain sensitivity, we test their hypothesized associations with provoked bladder pain response. We assess both sensory thresholds and descending modulation with QST, because visceral pain states may exhibit a pronociceptive profile with enhanced ascending nociceptive input or impaired descending modulation. [17,40,46] We also assess how anthropometric and developmental factors influence provoked bladder pain, given that pubertal maturation stage has been shown to influence pain sensitivity. [6,21,54] Finally, we test the hypothesis that provoked bladder pain is positively associated with the frequency of spontaneous abdominal pain symptoms.
2. Methods
2.1. Overview
The parent project for this analysis is the prospective observational study, Early Menstrual Pain Impact on Multisensory Hypersensitivity (EMPATHY), which seeks to characterize the trajectory of bodily symptom complaints and menstrual pain at the menarchal transition. NorthShore University HealthSystem’s Institutional Review Board approved this study. Participants were recruited through a letter from the adolescent’s pediatrician and advertisements throughout the community. Research assistants screened parents/guardians (hereafter referred to parents) of potential participants by telephone to determine study eligibility. Eligible participants were invited for a baseline study visit at the clinic where adolescents signed an assent form, and parents signed a consent form. The results presented here are from baseline data collected between January 22, 2019 and February 27, 2020.
2.2. Participants
Eligible participants were anatomically female adolescents age 9 to 14 years, enrolled before the onset of menses. In response to community advertisements and direct mailing from pediatricians to 1032 families, 170 parents contacted us for further information, and 162 participants were identified as potentially eligible. At the time of this data analysis, 124 participants had completed a baseline study visit. Exclusionary criteria for the study included: a) significant medical issues (those likely to limit availability for the study) or developmental issues that would limit the ability to complete assessments and interpret pain, b) participation in very intense physical activity programs such as competitive gymnastics or long-distance running and a BMI < 10% to avoid enrolling girls at high risk for prolonged amenorrhea, c) inability to read or comprehend the informed consent written in English, d) history of chronic pain (clinical pain > 3 months in duration), e) use of hormonal suppression, f) regular use of prescription analgesics, g) significant allergy to ultrasound or electrode gel, and h) other mental health or medical issue reported by the parent at phone screening that would interfere with being able to safely complete study procedures.
2.3. Baseline study visit questionnaires
Parents completed basic demographic and medical history questionnaires including age, ethnicity, and race, with assistance by their parent as needed. All data were entered into REDCap.[20] The complete results of parental history questionnaires will be reported elsewhere; other than the demographic information from the parents the data reported here comes from questionnaires and experimental tests completed by the adolescents.
After measurement of height, weight, and blood pressure, adolescents completed multiple questionnaires, including components of the Patient Reported Outcomes Measurement Information System (PROMIS) Pediatric Profile to assess psychosocial health. We used PROMIS Pediatric Profile measures for anxiety, depression, peer relationships, and pain interference. Each construct is measured with eight 5-point Likert scale items, reflecting self-impressions over the past 7 days. In the current sample, we calculated a Cronbach’s α of 0.91 for both the anxiety and depression scale. The PROMIS profile also contains a single item on the Physical Function measure assessing overall average pain over the past 7 days (on a numeric 0-10 numeric rating scale). The PROMIS Pediatric Profile domains have demonstrated construct validity and responsiveness to change in a recent pediatric chronic pain population study.[26]
We assessed somatic complaints with the Children’s Somatic Symptoms Inventory (CSSI). The CSSI assesses non-specific bodily symptoms (both painful and nonpainful) over the prior two weeks. In the revised and validated CSSI-24, 24 items are scored on a 5-point scale, ranging from “Never” (0) to “Always.” Prior studies have confirmed good internal consistency (Cronbach’s α=0.88), test-retest reliability (3 months r=0.5), and concurrent validity with disability and mood measures.[66] We obtained a similar Cronbach’s α (= 0.88) in this cohort. To assess adolescents’ psychological response to painful events, we included the 13-item Pain Catastrophizing Scale (α = 0.89).[44] Bowel symptoms were assessed using the Rome Questionnaire of Pediatric Gastrointestinal Symptoms.[45] Pubertal development was assessed using the Tanner Stage Developmental Questionnaire, a validated self-report questionnaire that asks adolescents to self-report their current developmental stage using model pictures of breasts and genitals across normal development (α = 0.75).[35,49]
2.4. Experimental Bladder Pain Test
Bladder sensitivity was assessed using a noninvasive filling task we previously validated in adult females to identify subclinical intermediate phenotypes for BPS.[22,59,60,63] Before the study visit, parents were instructed to make sure adolescents were well hydrated and there were no restrictions on how much adolescents could drink prior to testing. To avoid diuresis, adolescents were instructed to avoid caffeine 6 hours before the testing. Adolescents were asked to void at the beginning of this test. Although results are not reported in this paper, electromyography, electrocardiography, and respiratory rate monitors were also placed for autonomic assessment during the task. Adolescents were then given verbal directions for the test and asked to drink as much water as possible in 5 minutes (maximum of 20 oz). After the initial void and water loading, they were asked to indicate when they reached three levels of bladder urgency conventionally defined as first sensation, first urge, and maximum tolerance using the terms green, yellow and red zone, respectively. Green zone was defined to adolescents as “You are aware there is some urine in your bladder.” Yellow zone was defined as “You could go to the bathroom to pee, but you don’t have to rush off to do this.” Red zone was defined as “You would leave in the middle of your favorite movie to go to the bathroom even if you were missing the best part.” Staff members confirmed adolescents’ understanding of the task with a short multiple-choice quiz about performing the task. To ensure consistency, trained staff performed testing with a written script. After the initial void and at each level of bladder urgency, measurements of urgency (0- no urgency to 100 – worst urgency imaginable) and bladder pain (0 - no pain to 100 - worst pain imaginable) were obtained using a 0-100 visual analogue scale (VAS). At each cystometric threshold the associated bladder volume was measured using either a 3D ultrasound abdominal transducer (GE Voluson 750, Wauwatosa, WI) or a bladder scanner (Verathon Bladder Scan Prime, North Creek Parkway Bothell, WA). In addition to these thresholds, participants were also asked to rate their bladder pain and urgency every 15 minutes.
These provocation parameters appear safe and align with formal bladder testing standards in pediatric populations and pain-sensitive populations.[53] If maximum tolerance was not reached after 45 and after 60 minutes, they were asked to drink more water (a maximum of 10 oz at each time point). At 75 minutes, this testing was terminated regardless of urgency to minimize the visit's total length; the volume at this point was recorded as the proxy for maximum tolerance. In pilot testing approximately 80% of youth reach maximum tolerance by this point. All participants typically achieved the primary outcome variables at first urge within 75 minutes. The adolescent bladder test was similar to the adult version, except that adults were given additional 45 minutes to accommodate their larger bladder volume. Upon termination of bladder testing, adolescents urinated into a graded toilet hat in a bathroom. Urine was collected to validate ultrasound measurements and for further biospecimen analyses. We calculated intraclass correlation coefficients (ICCs, two-way mixed effects, absolute agreement, single rater/measurement) on all participants’ maximal volumes to voided volumes. There was a strong relationship between maximal volumes to voided volumes (ICC of 0.96 [95% CI: 0.91 - 0.97]) suggesting our ultrasonographic measurements were highly reliable.
2.5. Pressure Pain Threshold (PPT) Testing
Next, adolescents underwent pressure pain threshold testing following validated protocols from our published studies [61,62], using custom software to operate a commercially available digital pressure algometer with a 1 cm2 rubber tip (Wagner Instruments, Greenwich, CT). Before this test segment, we confirmed adolescents had no baseline pain at their left knee and left shoulder by asking them to rate their pain at these sites on a 0-10 numerical rating scale (NRS).
A practice test measurement was done on their right knee to confirm they understood instructions. They were instructed to squeeze a handheld trigger that recorded the instantaneous pressure when the provoked pain reached a 1 on a 0-10 NRS. To confirm they understood that this was the threshold at which they first felt pain, adolescents were asked to report their pain level using the NRS immediately after pressing the button for each site. If they reported a score >1, the instructions and the test were repeated until they demonstrated understanding.
We tested PPTs at two sites corresponding to the American College of Rheumatology fibromyalgia tender point sites: the left trapezius and the left medial knee fat pad.[73] Staff conducted an initial trial at each site, took a two-minute break, and then conducted a second trial at each site followed by another two-minute break. The algometer was used to conduct the external site measurements using a ramp rate of 4 Newtons (N)/s. The examiner terminated trials at 70 N if the adolescent did not reach the pressure pain threshold before then. There was high reliability in PPTs across repetitions and sites (α=0.86). Staff conducting PPT testing performed reliability testing in practice sessions to achieve consistent results (<10% variance in thresholds).
2.6. Conditioned Pain Modulation (CPM):
We employed a common strategy for assessing descending modulation of pain, CPM, by repeating PPT testing in the presence of a heterotopic stimulus (in this case, cold water). Multiple studies have suggested that pain perception is enhanced by impaired descending modulation, which CPM can measure.[74] Although impaired CPM in adolescents with functional abdominal pain or irritable bowel syndrome has been reported, [37,46] it remains unknown whether alterations in CPM are associated with visceral sensitivity. The test stimulus was mechanical pressure on the left medial knee fat pad and trapezius, with baseline pain thresholds determined from the initial tests above. Following both sets of pressure tasks, adolescents were given a two-minute break, and then the conditioning stimulus was applied by instructing them to insert their right hand, up to their wrist, into a bucket of circulating water (flow rate = 7.4 L/min) maintained at 8 ± 1° C. After 10s of immersion, they were asked to rate their hand pain on a 0-10 NRS. After 20s of immersion, they rated their hand pain again and then withdrew their hand. Immediately after, the pressure pain stimulus was re-applied to the knee and then the shoulder. Adolescents were asked to rate pain at each site again when they reached the provoked pain threshold.[33] CPM was measured by the standardized differences in pain threshold (post CPM PPT minus average of baseline PPTs). To measure the CPM effect, we analyzed change in PPTs after cold water for knee and shoulder separately, consistent with guidelines suggesting testing CPM at multiple sites.[74]
Prior studies have shown that participant expectancy of CPM is correlated with CPM effect. [8,13,14,32] To account for potential expectancy effects as described by others, and as a positive control, we looked at the relationship between CPM expectancy and CPM effect.
Prior to the actual cold water task, adolescents were asked to predict what the impact of the immersion would have on their test stimulus (PPT) response, similar to a prior study.[13] The predicted response, on a five-point Likert scale ranged from “It will decrease the pressure pain a lot” down to “It will increase the pressure pain a lot.”
2.7. Post-Menarchal Visits
3-9 months after the onset of first menses, adolescents were scheduled for a follow-up visit. All study procedures as described above were repeated: Questionnaires, Noninvasive Bladder Test, Pressure Pain Threshold Testing, and Conditioned Pain Modulation. To prevent potential confounding due to menstrual pain, this visit was scheduled on a day where participants were not menstruating. The data from this visit is only used here for the retest reliability analyses.
2.8. Data Analyses:
The overarching EMPATHY study seeks to enroll 375 adolescent/parent dyads to achieve the primary aims of identifying distinct statistically-derived trajectory patterns for initial menstrual distress and to determine if heightened menstrual pain predicts development of multisensory hypersensitivity. However, the sub-analyses in this paper focus separately on assessing the reliability of the bladder test itself, assessing its validity by comparing the results to those seen in adult women, identifying potential predictors of the bladder pain response, and testing one hypothesis related to test performance, described below.
To verify the reproducibility of the bladder test in adolescent females we calculated intraclass correlation coefficients (ICCs, two-way mixed effects, absolute agreement, single rater/measurement) on all parameters in all participants who had returned for a post-menarchal visit (n = 13). In this cohort, their median repeat test was 169 (interquartile range 154 – 211) days later. To maximize the sample size for this reliability estimate, we added repeated measurements (one year apart approximately) from a pilot study in eight healthy adolescents (median age 12.5 years [12-13]). In this pilot data set studied between July 2017 and July 2018, one adolescent was post-menarchal at the bladder test, and three more were post-menarchal at the repeat test. In place of power analysis to establish the sample size's adequacy, we have included 95% confidence intervals for the calculations.[39] Typical guidelines suggest values between 0.5 - 0.75 indicate moderate reliability, from 0.75 - 0.9 indicate good reliability, and > 0.90 indicate excellent reliability.[30]
To compare the bladder test results to adult findings, we conducted a pilot analysis comparing this data set to our prior published data.[59] Because our prior cohort recruited a highly enriched sample of adult participants with both dysmenorrhea and bladder sensitivity, the full raw data set would not be an appropriate comparison. Therefore, for this comparison, we analyzed a subset of the full adult dataset that would correspond to the approximate incidence in the community of both pain free controls (50% ), moderate/severe dysmenorrhea (~40%), and moderate/severe dysmenorrhea with bladder sensitivity (~10%). In a sensitivity analysis, we also compared the adolescents to the adult healthy control group.[60,70] Mann-Whitney tests were used to compare the adolescent and adult results for bladder pain, urgency, and volume at first sensation, first urge, and maximum tolerance. To make a conservative correction for multiple comparisons across all parameters and thresholds, the Benjamini-Hochberg procedure was used to limit the false discovery rate to 5%.[3] Adjusted P values (padj) are provided for significant results.
We also characterized potential influences on bladder sensitivity that have been identified as relevant for other experimental pain measures. We specifically focused on pain at first urge, which we identified as the optimal threshold to discriminate groups on relative bladder pain sensitivity in our prior adult studies [22,23]. The relationship between pain at first urge and other factors associated with the biopsychosocial pain model was analyzed for PROMIS anxiety and depression, somatic sensitivity (CSSI), and pain catastrophizing (PCS) scores. We also examined the relationship of bladder pain sensitivity to developmental (Tanner stage) and pain processing profiles (average body PPT, cold pressor pain, conditioned pain modulation). Because there was a comparable magnitude of PPT change after cold water (α=0.81), similar to Goodin et al., we combined results at the two sites for a single CPM effect measure.[15] Spearman’s correlation coefficient was calculated to evaluate the relationship between these factors. Applying the Benjamini-Hochberg procedure limited type I error as described above. Kendall’s Tau was used to examine the correlation between CPM and the adolescent’s expected CPM results.
We hypothesized that even in chronic pain-free adolescents, the bladder pain test would discriminate those with more days of self-reported abdominal pain from those with fewer days. Specifically, we analyzed the correlation between a single item from the Rome IV pediatric bowel questionnaire, "How many days in the past month did you have pain in the area around or below the belly button” and first urge pain.
Statistics were analyzed in STATA (version 13.1 College Station, TX) and graphed in R version 3.6.3.[48] To accommodate the lack of normal distributions in several of the variables (confirmed by Shapiro-Wilks test), nonparametric tests were used. Results are presented as medians (interquartile range).
3. Results
The median age of the 124 recruited adolescents at baseline was 11 years (11 – 12), and they reported a median breast and genital Tanner stage of 2 (2 – 3). For the purposes of the longitudinal validation analyses, 13 of these core study participants had reached menarche and had participated in a second visit; their data was combined with the 8 participants who had completed two visits in the prior pilot study.
The cohort included 25.8% minority and 5.6% Hispanic adolescents. Parents were of similar race and ethnicity (20.2% minority, 5.6% Hispanic). Overall, adolescents were slightly below population averages on levels of reported anxiety and depressive symptoms (PROMIS average score is set at 50, Table 1). On a 0-10 NRS, most reported relatively little pain over the past week (1 [0 - 3]) and few somatic symptoms on the CSSI (13 [8 - 20]).
Table 1:
Demographic and symptom profiles, premenarchal adolescent females.
| Factor | n | median [IQR] (%) |
|---|---|---|
| Age | 124 | 11 [11 - 12] |
| Tanner Stage | ||
| Breast Development | 123 | 2 [2 - 3] |
| Genital Hair | 123 | 2 [2 - 3] |
| Race | ||
| Asian | 21 | (16.9%) |
| Black | 9 | (7.3%) |
| Other | 2 | (1.6%) |
| White | 92 | (74.2%) |
| Hispanic ethnicity | 7 | (5.6%) |
| Self-Report Mood and Symptom Scales | ||
| PROMIS Anxiety* | 124 | 47 [43 - 55] |
| PROMIS Depression* | 124 | 46 [40 - 52] |
| CSSI | 124 | 13 [8 - 20] |
| Pain Catastrophizing Scale | 124 | 28 [23 -35] |
| Pain (0-10 NRS) past week | 124 | 1 [0 - 3] |
Complete data (n=124) was obtained on all participants except for one participant who felt uncomfortable filling out the Tanner Stage questionnaire.
T-scores; IQR = interquartile range; PROMIS = Patient Reported Outcomes Measurement Information System, CSSI = Children’s Somatic Symptoms Inventory, NRS = numeric rating scale.
3.1. Longitudinal validation of the bladder test
To establish the long-term stability of the provoked bladder test, we analyzed repeat test data available from 21 adolescents (Table 2). There were strong retest reliability between pain ratings at the initial and repeat visits at all thresholds (Table 2, ICCs = 0.88 - 0.90). Similar results were obtained for urgency ratings (ICCs = 0.79 - 0.96). Bladder volume measurements were not as stable (ICCs = 0.62 - 0.68); however, bladder scanner measurements were consistent with voided volume (ICC = 0.97 [95% CI 0.95 - 0.98]). Thus, although some changes in volume thresholds may occur over time, the relative pain and urgency at each threshold appear consistent.
Table 2:
Retest reliability for bladder testing in female adolescents.
| Pain | Urgency | Volume | |
|---|---|---|---|
| First Sensation | .89 (.73 - .96) | .79 (.31 -.85) | .68 (.20 - .87) |
| First Urge | .90 (.75 - .96) | .96 (.99 - .98) | .62 (.05 - .82) |
| Maximum Tolerance | .88 (.69 - .95) | .95 (.87 - .98) | .66 (.16 - .87) |
Results shown indicate intraclass correlation coefficients with 95% confidence intervals.
3.2. Adolescents’ pain report during bladder filling is similar to that in young adult women at first urge and maximum tolerance, but is greater at first sensation to void
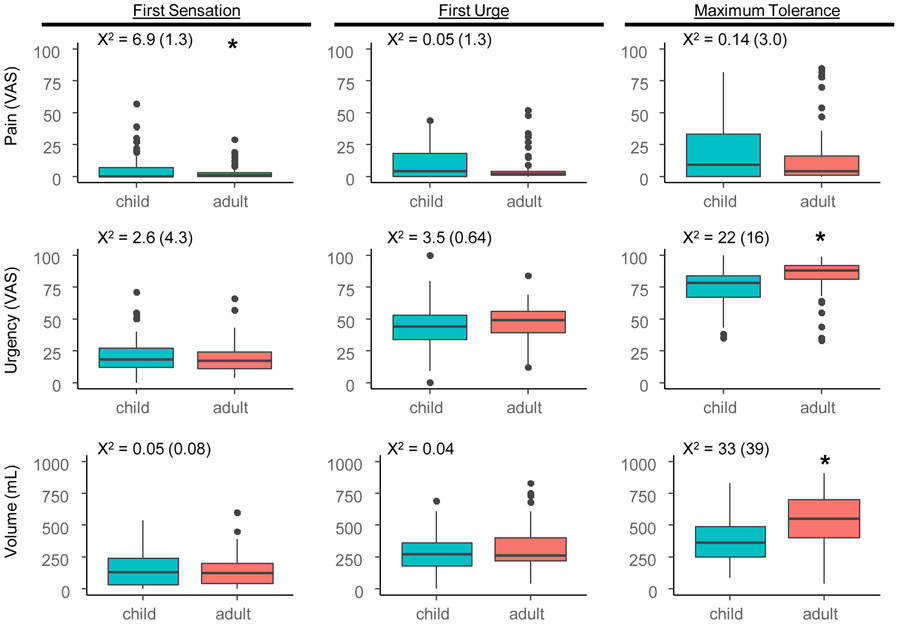
We obtained bladder sensitivity measurements in all adolescents (Figure 1) and compared them to a previously studied cohort of young adult females.[23] The average residual bladder volume post-voiding was low (1 [0 - 5] mL). At first sensation (17 [6 32] minutes), the adolescents reported some urgency (18 [12 - 26]) and no pain (0 [0 - 7]), at an median volume of 124 (28 – 236) mL. At first urge (40 [31 51] minutes), self-reported urgency increased (44 [34 - 52]) at a median volume of 272 (178 – 356) mL in adolescents. Although most reported negligible pain at first urge (4 [0 - 17]), 10% of them reported pain intensity of 32 or higher. At maximum tolerance (52 [44 63] minutes), urgency (77 [66 - 84]) and pain report (9 [0 - 32]) peaked with a median volume of 358 (250 – 484) mL.
Figure 1: Comparison between provoked bladder pain test between adolescent and adult females.
Box and whiskers plots indicate median (thick line), 25-75th confidence intervals (boxed interval), range (line) and outliers (circles). X2 values Kruskal Wallis tests are shown above each panel for differences between child and adult data including women with dysmenorrhea and pain free controls. Values in parentheses are X2 values for differences between child and adult data including only pain free controls. Asterisks designate significant differences (p < 0.05) with Kruskal Wallis tests between child and adult data.
Overall, assessed volume, pain, and urgency in adolescents were comparable to adults. All adolescents reached the threshold for first urge and 81% of adolescents (101/124) reached maximum tolerance within 75 minutes. However, intensity of pain in adolescents at first sensation was greater than in adults (p = 0.005). Although the difference was significant, there was only a 1-point median difference on a 0-100 scale (adolescents 0 [0 7] vs. adults 1 [0 4]). Adults reported higher maximum tolerance urgency ratings (p < 0.001) and volumes than adolescents (p < 0.001); their protocol did allow for up to 120 minutes of filling. These differences were also significant after correction for multiple comparisons (padj's < 0.05). In a sensitivity analysis, we also compared the adolescent cohort to the adult healthy control group. The statistical significance of differences between the adolescent cohort and adult healthy controls was similar to the mixed cohort above (Figure 1), except that the difference in pain at first sensation was not significant (p = 0.196).
With regard to tolerability, after voiding, all adolescents indicated their pain returned to baseline (0 [0 - 0]). After completing bladder testing and other tasks, most reported they had a positive experience (118/124), and that they would be willing to come back the following year for a similar experience (117/124).
3.3. Pressure pain thresholds (PPTs) and conditioned pain modulation (CPM) in adolescents
We also conducted QST to evaluate potential biological mechanisms related to bladder sensitivity (Table 3). Increases in PPTs were observed at both the knee and shoulder site (Table 3). Average body PPTs increased from 15.1 N (11.0 - 22.1) to 17.0 N (12.7 - 23.8) after cold water immersion (p <0.0001). Consistent with prior studies, there was a significant correlation between the expectation that cold water immersion will reduce pain thresholds and an actual reduction in pain threshold (τb = 0.31 , p < .0001).
Table 3:
Quantitative sensory testing results in adolescents.
| Average | Knee | Shoulder | |
|---|---|---|---|
| Before Cold Pressor | |||
| PPT (N) | 15.1 (11.0, 22.1) | 12.3 (8.4,17.2) | 18.2 (12.6, 26.4) |
| During Cold Pressor | |||
| Pain at 10s (NRS) | 5.0 (3.0, 7.0) | ||
| Pain at 20s (NRS) | 7.0 (5.0, 8.0) | ||
| After Cold Pressor | |||
| PPT (N) | 17.0 (12.7, 23.8) | 13.8 (9.8, 19.8) | 19.8 (14.4, 30.4) |
| CPM effect size | |||
| CPM (ΔN) | 2.3 (−0.5, 5.6) | 2.0 (−1.6, 5.4) | 2.7(−0.8, 7.4) |
| CPM (%baseline) | 13.3 (−3.6, 39.4) | 12.7 (−10.8, 42.9) | 15.2 (−5.6, 43.4) |
| Z score | 5.3 | 3.8 | 4.9 |
| p value | <0.0001 | <0.0001 | <0.0001 |
Results indicate median (interquartile range). CPM was computed as relative difference (ΔN) as well as relative change (%baseline). Results are shown for the average knee and shoulder PPTs as well. Z-score and p-values indicates Wilcoxon matched-pairs signed-ranks test comparing PPTs before and after cold pressor. NRS = numeric rating scale, PPT = pain pressure threshold, N = Newton, CPM = Conditioned Pain Modulation.
We next examined how strongly related provoked bladder pain was to other QST parameters (Table 3). Bladder pain was inversely correlated with PPTs (r = −0.25, padj = 0.030, but not associated with CPM or cold water immersion pain (Table 4). There were also no significant relationships between other QST results and other factors (anxiety, depression, somatic sensitivity and pain catastrophizing), except that PPTs were positively correlated with Tanner stage (r = 0.25, padj = 0.030).
Table 4:
Relationship of pain sensitivity, psychosocial, and developmental factors and bladder pain at first urge.
| Bladder Pain | PPT | Cold Pressor | CPM | Tanner Stage |
Anxiety | Depression | CSSI | |
|---|---|---|---|---|---|---|---|---|
| PPT | −.25 | |||||||
| Cold Pressor Pain | .03 | −.17 | ||||||
| CPM | .08 | −.02 | −.17 | |||||
| Tanner Stage | −.11 | .25 | −.04 | .18 | ||||
| Anxiety | .33 | −.17 | .06 | −.01 | −.17 | |||
| Depression | .26 | −.01 | −.03 | .19 | .01 | .57 | ||
| CSSI | .31 | −.15 | .12 | .00 | .02 | .63 | .53 | |
| PCS | .25 | −.07 | .17 | .16 | .16 | .46 | .35 | .47 |
Bold indicates results were significant after Benjamini-Hochberg false-discovery rate corrections for multiple comparisons (p<0.05). PPT = pressure pain threshold, CPM = conditioned pain modulation, CSSI = Children's Somatic Symptoms Inventory, PCS = pain catastrophizing scale.
3.4. Bladder pain sensitivity is associated with anxiety and somatic symptoms
Based on prior studies of rectal distension tasks, we hypothesized that bladder pain sensitivity would be associated with other psychological factors that may contribute to perceptual bias in chronic pain states.[18,71,72]. Indeed, bladder pain at first urge was positively associated with anxiety (Table 4, r = 0.33), depression (r = 0.26), somatic sensitivity as measured by the CSSI (r = 0.31), and PCS (r = 0.25). All these parameters were significant (pad’j’s < 0.05) even after correction for multiple comparisons. Notably, provoked bladder pain was not correlated (p's > 0.2) to Tanner stage or any other anthropomorphic factors (weight, height, blood pressure— data not shown).
3.5. The association between provoked bladder pain vs QST and prior self-reported lower abdominal pain
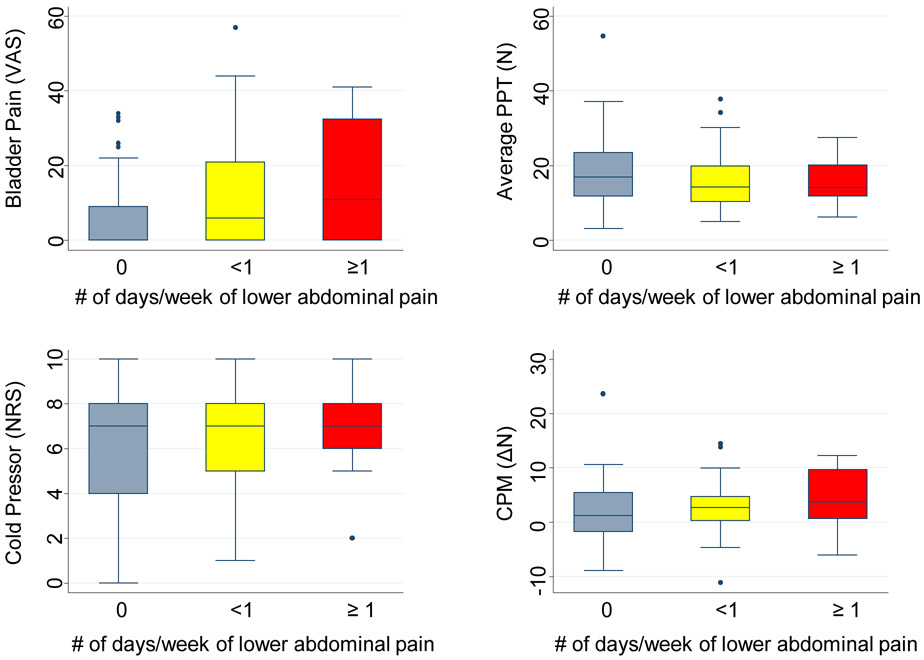
We next assessed the hypothesis that bladder pain would be related to self-reported frequency of spontaneous lower abdominal pain. Specifically, we analyzed a single item from the Rome IV pediatric bowel questionnaire, "How many days in the past month did you have pain in the area around or below the belly button.” Whereas 50% of adolescents did not report any recent abdominal pain, others reported pain on 1 day (28, 22.6%), 2 days (9, 7.3%), 3 days (9, 7.3%), 4 days (4, 3.2%), or 5 days or more (12, 9.7%). There was a significant correlation between the number of days of lower abdominal pain and provoked bladder pain (r = 0.23, padj = 0.030; Figure 2). To verify this, we also examined the relationship between bladder pain and the PROMIS pediatric physical function single-item overall pain rating (0-10 NRS). Bladder pain positively correlated with pain over the past week (r = 0.25, padj =0.039). We also examined the relationship between other QST parameters and the Rome IV self-reported frequency of spontaneous lower abdominal pain (Figure 2). Although CPM magnitude was correlated to lower abdominal pain frequency (r = 0.21, padj = 0.03), PPTs (r = 0.12, p = 0.2) and cold pressor pain (r = −0.13, p = 0.158) were not.
Figure 2: Associations between lower abdominal pain frequency and QST results.
Box and whiskers plots indicate median (thick line), 25-75th confidence intervals (boxed interval), range (line) and outliers (circles). Sample sizes for each category are: 0 days per week (n= 63) , <1 day per week (n = 45), ≥1 day per week: (n = 16). PPT = pain pressure threshold, CPM = conditioned pain modulation, VAS = visual analog scale, N = Newtons, NRS = numerical rating scale.
4. Discussion
This study supports the validity of a noninvasive bladder pain assessment in perimenarchal adolescents, a first step towards characterizing the evolution of pelvic visceral pain at the pubertal transition. The test performs similarly to results we obtained in young adult women and is consistent with prior studies that show a modestly lower volume at which adolescents reach maximum cystometric capacity.[69] Notably, the upper quartile of adolescents reported a pain threshold of 17 on a rating scale of 0-100 at first urge—which is above the threshold of 15 we established in adults as a potential marker of silent bladder hypersensitivity [22]. Given the presence of this phenotype and the high temporal stability in our pilot assessment (ICC 0.88-0.90), we will continue to follow this cohort into the postmenarchal years to understand the course of early bladder pain sensation. Even in this study of adolescents without chronic pain, provoked bladder pain correlated significantly with the frequency of self-reported, spontaneous abdominal pain in the past 3 months. Study results also corroborate prior published findings of the magnitude of somatic QST findings for pressure stimulation, cold water exposure, and conditioned pain modulation, suggesting that these adolescents comprehended the basic premise of such sensory testing.[46,47,56]
These results resemble those from other studies of experimental visceral pain provocation in youth. In symptomatic functional abdominal pain patients, Anderson et al. found that greater severity of clinical abdominal pain is predicted by higher ratings of stomach unpleasantness provoked by water loading.[1] We extend that literature by showing a modest linear relationship in adolescents between the number of days of spontaneous abdominal pain and reported level of provoked bladder pain (r = 0.23). A small number of studies employing invasive paradigms such as anal manometry have likewise found evidence of visceral hypersensitivity in children suffering from functional abdominal pain.[10,65] Faure and colleagues similarly found that 8 adolescent Crohn’s disease sufferers, histologically in remission, but with continued abdominal pain, had an enhanced pain response to rectal distension suggesting a prominent role for dysregulated sensory circuits.[5,12] Our protocol’s noninvasive nature lends itself to “pre-symptomatic” application across other visceral pain conditions in youth. Such studies are needed to standardize assessment of chronic visceral pain risk. In our prior study of young, chronic-pain free adult women, having a heightened bladder provocation response was linked to having other abnormal sensory responses, resembling what is observed in chronic pelvic pain[23] This altered bladder sensitivity may serve as a proxy for formal neuroimaging-identified neural changes linked to altered visceral organ threat assessment. More recent work from Deutsch and colleagues [9] and Kleinhans and others [28], using a similar bladder distension protocol in adults, showed that heightened responses predicted distinct differences in connectivity and regional cerebral blood flow in known pain perceptual sites (insula, cingulate), sensory/motor sites, and the limbic system (hippocampus, amygdala).
We found that levels of anxiety, depression, pain catastrophizing, and somatic sensitivity (historically labeled somatization) are all modestly associated with an adolescent’s bladder pain response. However, correlation coefficients between these factors were lower (.16 −.22) in adults compared to the adolescents here (.26 −.33), suggesting that visceral pain experience in youth may be more susceptible to these influences. We suggest accounting for these factors to minimize confounding in future comparisons of bladder pain sensitivity between groups. The literature is inconsistent in determining how psychological factors influence visceral pain testing results in youth. Several studies have failed to show a relationship between depression or anxiety and visceral hypersensitivity, using multiple methods including water-loading, rectal barostat, or esophageal catheters.[1,10,67] Very little of that data is drawn from healthy youth. Conceivably, the varying findings may reflect differences in the clinical burden of pain in prior studies, the exact modality being testing, and the amount of psychosocial dysfunction in healthy vs. symptomatic youth.
We are incidentally able to corroborate some key published findings of the magnitude of somatic QST findings for pressure stimulation, cold water exposure, and CPM in healthy youth. These confirmatory findings suggest that our adolescent participants comprehended the basic premise of such sensory testing. Average age differences of almost five years might explain the modestly lower average pressure pain thresholds we found compared to those reported from the large scale Tromso study, which recruited older Norwegian youth.[56] Our adolescents rated cold water unpleasantness similar to that described by youth in a 2009 normative study, even though that group employed a multi-stage protocol, and slightly warmer water.[57] Differences in CPM protocols across studies often make it difficult to compare results directly. Nevertheless, the magnitude (proportionally) of descending modulation in this cohort was similar to others.[37,46,47,56,58]
Although we identified a correlation between the bladder test, anxiety, depression and pain catastrophizing, there was not a significant correlation between other forms of QST and psychological factors. Similarly, in adults without chronic pain correlations between psychological factors and the bladder test were significant, but correlations between the other QST procedures and psychological factors were not.[23] However, in a large scale study of Norwegian youth (ages 15-19) with and without chronic pain, there was a significant correlation between psychological distress and QST. [56] Conversely, in several smaller studies, QST parameters, and psychological measures (chiefly anxiety and depression) do not generally predict experimental responses. [27,46,58] We hypothesize that correlations between QST and psychological factors observed in the Norwegian study and other studies in adults may be due to group differences in psychological factors, because patients with chronic pain also have worse psychological distress. [17,31] In the future, multivariate models of large data sets could be used to test this hypothesis.
The QST findings in relation to the provoked bladder pain results may provide some insight into the emergence of bladder hypersensitivity. The lack of a relationship between cold pressor testing and CPM suggest limited involvement of the brainstem descending modulatory pathways that are typically associated with abnormal sensitivity on these tests. [19,24] However, there are many other potential mechanisms that might be responsible for altered visceral pain sensitivity, such as aberrant connectivity in the central executive or default mode networks. [64] Additional research using the repeated assessments we have planned will likely continue to clarify the key underlying neurological mechanisms, as such sensitivity continues to evolve.
We have previously demonstrated that menstrual pain in adult women positively correlates with magnitude of pain from this bladder task [22,60], but it remains unknown whether heightened bladder pain precedes or follows changes in menstrual pain. Additionally, because menarche is a crucial time point where the prevalence of mood disorders diverges upwardly for girls [4], further use of this task can clarify the direction of influence of psychological states and pain sensitivity with trajectory models.[38] Even recent analyses of well-known, large studies such as the Avon Longitudinal Study of Parents and Children and the National Longitudinal Study of Adolescent to Adult Health, have not attempted to look at repeated measures of these constructs.[42,43]
The strengths of this study include the extension of a naturalistic visceral distension test to an adolescent cohort. Adapted from a water loading test originally described to assess gut sensitivity, our bladder-specific design allows us to reach a clinically meaningful maximum distension.[67] By studying premenarchal females, we also can limit potential confounding from prior cross-organ sensitization from dysmenorrhea experiences. Although menstrual period pain episodically distresses at least half of reproductive-age women and is a strong risk factor associated with non-menstrual pelvic pain, most prior pain studies fail to account for this.[70] The inclusion of multimodal QST also allows contrast with several parallel QST studies. We also provide an assessment of the impact of psychosocial factors on such visceral sensitivity.
Our paradigm has some limitations, including a predictable stimulus slope and inability to control the rate of distension. The usage of diuretics and/or graded distraction could potentially be employed in further studies when a more unpredictable paradigm is needed to study high-risk youth, who might be primed to have bladder awareness. We continue to actively recruit a broader demographic range to better generalize results; it is particularly important to study African-Americans, who may experience greater overall pelvic visceral pain burden given their higher prevalence of uterine pain and leiomyoma.[25,55]
In summary, this is a first attempt to validate a noninvasive provoked bladder pain test in adolescent females. The high level of acceptability reported by our participants and its ease of use may facilitate longitudinal research on the trajectory of visceral sensitivity. More work is needed to understand how this task performs in those with existing pain conditions or bladder dysfunction. The association of provoked bladder pain to psychological factors supports the need to account for potential confounding during such bladder testing. Further data from this cohort, over time, will allow us to understand the impact of cross-organ sensitization after menarche from menstrual pain on bladder sensitivity, and how other nonvisceral pain sensitivity measures evolve similarly postmenarche. A better understanding of such constructs is essential for identifying at-risk youth well before the onset of clinically bothersome symptoms to develop evidence-based pain prevention strategies.
Acknowledgments
The authors thank Dr. Gerald Gebhart for help with experimental design, advice, and editorial assistance, Margaret Schroer, Gabriela Ashenafi, and Ellen Garrison for conducting the study visits, and the NorthShore University HealthSystem Department of Pediatrics for supporting recruiting of their patients. The present study was funded by NICHD R01HD096332. Dr. Tu reports personal fees from AbbVie, personal fees from Wolters Kluwer, personal fees from UroShape, outside the submitted work. No other conflicts noted for other authors. The conducted research was not preregistered with an analysis plan.
References
- [1].Anderson JL, Acra S, Bruehl S, Walker LS. Relation between clinical symptoms and experimental visceral hypersensitivity in pediatric patients with functional abdominal pain. J Pediatr Gastroenterol Nutr 2008;47:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG 2008;115:1382–91. [DOI] [PubMed] [Google Scholar]
- [3].Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: series B (Methodological) 1995;57:289–300. [Google Scholar]
- [4].Born L, Shea A, Steiner M. The roots of depression in adolescent girls: is menarche the key? Curr Psychiatry Rep 2002;4:449–460. [DOI] [PubMed] [Google Scholar]
- [5].Castilloux J, Noble A, Faure C. Is visceral hypersensitivity correlated with symptom severity in children with functional gastrointestinal disorders? J Pediatr Gastroenterol Nutr 2008;46:272–278. [DOI] [PubMed] [Google Scholar]
- [6].Chumpitazi BP, Weidler EM, Czyzewski DI, Self MM, Heitkemper M, Shulman RJ. Childhood Irritable Bowel Syndrome Characteristics Are Related to Both Sex and Pubertal Development. J Pediatr 2017;180:141–147.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clemens JQ, Markossian T, Calhoun EA. Comparison of economic impact of chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis/painful bladder syndrome. Urology 2009;73:743–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cormier S, Piché M, Rainville P. Expectations modulate heterotopic noxious counter-stimulation analgesia. J Pain 2013;14:114–125. [DOI] [PubMed] [Google Scholar]
- [9].Deutsch G, Deshpande H, Frölich MA, Lai HH, Ness TJ. Bladder Distension Increases Blood Flow in Pain Related Brain Structures in Subjects with Interstitial Cystitis. J Urol 2016;196:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Di Lorenzo C, Youssef NN, Sigurdsson L, Scharff L, Griffiths J, Wald A. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr 2001;139:838–843. [DOI] [PubMed] [Google Scholar]
- [11].Fall M, Baranowski AP, Elneil S, Engeler D, Hughes J, Messelink EJ, Oberpenning F, de C Williams AC. EAU guidelines on chronic pelvic pain. Eur Urol 2010;57:35–48. [DOI] [PubMed] [Google Scholar]
- [12].Faure C, Wieckowska A. Somatic referral of visceral sensations and rectal sensory threshold for pain in children with functional gastrointestinal disorders. J Pediatr 2007;150:66–71. [DOI] [PubMed] [Google Scholar]
- [13].France CR, Burns JW, Gupta RK, Buvanendran A, Chont M, Schuster E, Orlowska D, Bruehl S. Expectancy Effects on Conditioned Pain Modulation Are Not Influenced by Naloxone or Morphine. Ann Behav Med 2016;50:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia – When the spine echoes what the brain expects. Pain 2007;130:137–143. [DOI] [PubMed] [Google Scholar]
- [15].Goodin BR, Kronfli T, King CD, Glover TL, Sibille K, Fillingim RB. Testing the relation between dispositional optimism and conditioned pain modulation: does ethnicity matter? J Behav Med 2013;36:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain 2011;12:T61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grinberg K, Granot M, Lowenstein L, Abramov L, Weissman-Fogel I. A common pronociceptive pain modulation profile typifying subgroups of chronic pelvic pain syndromes is interrelated with enhanced clinical pain. Pain 2017;158:1021–1029. [DOI] [PubMed] [Google Scholar]
- [18].Grinsvall C, Törnblom H, Tack J, Van Oudenhove L, Simrén M. Psychological factors selectively upregulate rectal pain perception in hypersensitive patients with irritable bowel syndrome. Neurogastroenterol Motil 2015;27:1772–1782. [DOI] [PubMed] [Google Scholar]
- [19].Harper DE, Ichesco E, Schrepf A, Hampson JP, Clauw DJ, Schmidt-Wilcke T, Harris RE, Harte SE. Resting Functional Connectivity of the Periaqueductal Gray Is Associated With Normal Inhibition and Pathological Facilitation in Conditioned Pain Modulation. J Pain 2018;19:635.e1–635.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hebert JJ, Leboeuf-Yde C, Franz C, Lardon A, Hestbæk L, Manson N, Wedderkopp N. Pubertal development and growth are prospectively associated with spinal pain in young people (CHAMPS study-DK). Eur Spine J 2019;28:1565–1571. [DOI] [PubMed] [Google Scholar]
- [22].Hellman KM, Datta A, Steiner ND, Kane Morlock JN, Garrison EF, Clauw DJ, Tu FF. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol 2018;219:84.e1–84.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hellman KM, Roth GE, Dillane KE, Garrison EF, Oladosu FA, Clauw DJ, Tu FF. Dysmenorrhea subtypes exhibit differential quantitative sensory assessment profiles. Pain 2020;161:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hendriks-Balk MC, Megdiche F, Pezzi L, Reynaud O, Da Costa S, Bueti D, Van De Ville D, Wuerzner G. Brainstem Correlates of a Cold Pressor Test Measured by Ultra-High Field fMRI. Front Neurosci 2020;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol 1996;87:55–8. [DOI] [PubMed] [Google Scholar]
- [26].Kashikar-Zuck S, Carle A, Barnett K, Goldschneider KR, Sherry DD, Mara CA, Cunningham N, Farrell J, Tress J, DeWitt EM. Longitudinal evaluation of patient-reported outcomes measurement information systems measures in pediatric chronic pain. Pain 2016;157:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL 3rd. Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain 2009;143:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kleinhans NM, Yang CC, Strachan ED, Buchwald DS, Maravilla KR. Alterations in Connectivity on Functional Magnetic Resonance Imaging with Provocation of Lower Urinary Tract Symptoms: A MAPP Research Network Feasibility Study of Urological Chronic Pelvic Pain Syndromes. J Urol 2016;195:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Klem F, Wadhwa A, Prokop LJ, Sundt WJ, Farrugia G, Camilleri M, Singh S, Grover M. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology 2017;152:1042–1054.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016;15:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].de Kruijf M, Peters MJ, C Jacobs L, Tiemeier H, Nijsten T, Hofman A, Uitterlinden AG, Huygen FJPM, van Meurs JBJ. Determinants for Quantitative Sensory Testing and the Association with Chronic Musculoskeletal Pain in the General Elderly Population. Pain Pract 2016;16:831–841. [DOI] [PubMed] [Google Scholar]
- [32].Larivière M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain 2007;23:506–510. [DOI] [PubMed] [Google Scholar]
- [33].Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, Solomon DH, Karlson EW. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum 2013;65:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Longstreth GF, Wilson A, Knight K, Wong J, Chiou C-F, Barghout V, Frech F, Ofman JJ. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol 2003;98:600–607. [DOI] [PubMed] [Google Scholar]
- [35].Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995;109:40–52. [DOI] [PubMed] [Google Scholar]
- [37].Morris MC, Walker LS, Bruehl S, Stone AL, Mielock AS, Rao U. Impaired conditioned pain modulation in youth with functional abdominal pain. Pain 2016;157:2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mulvaney S, Lambert EW, Garber J, Walker LS. Trajectories of symptoms and impairment for pediatric patients with functional abdominal pain: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry 2006;45:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 2007;82:591–605. [DOI] [PubMed] [Google Scholar]
- [40].Ness TJ, Lloyd LK, Fillingim RB. An endogenous pain control system is altered in subjects with interstitial cystitis. J Urol 2014;191:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nicholl BI, Halder SL, Macfarlane GJ, Thompson DG, O’Brien S, Musleh M, McBeth J. Psychosocial risk markers for new onset irritable bowel syndrome--results of a large prospective population-based study. Pain 2008;137:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Noel M, Groenewald CB, Beals-Erickson SE, Gebert JT, Palermo TM. Chronic pain in adolescence and internalizing mental health disorders: a nationally representative study. Pain 2016;157:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Norris T, Deere K, Tobias JH, Crawley E. Chronic Fatigue Syndrome and Chronic Widespread Pain in Adolescence: Population Birth Cohort Study. J Pain 2017;18:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 1997;20:589–605. [DOI] [PubMed] [Google Scholar]
- [45].Palsson OS, Whitehead WE, van Tilburg MAL, Chang L, Chey W, Crowell MD, Keefer L, Lembo AJ, Parkman HP, Rao SS, Sperber A, Spiegel B, Tack J, Vanner S, Walker LS, Whorwell P, Yang Y. Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology 2016;150:1481–91. [DOI] [PubMed] [Google Scholar]
- [46].Pas R, Rheel E, Van Oosterwijck S, Leysen L, Van De Vijver E, Nijs J, Ickmans K, Meeus M. Endogenous pain modulation in children with functional abdominal pain disorders. Pain 2019;160:1883–1890. [DOI] [PubMed] [Google Scholar]
- [47].Payne LA, Seidman LC, Sim M-S, Rapkin AJ, Naliboff BD, Zeltzer LK. Experimental evaluation of central pain processes in young women with primary dysmenorrhea. Pain 2019;160:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria., 2020. p. Available: https://www.R-project.org/. [Google Scholar]
- [49].Rasmussen AR, Wohlfahrt-Veje C, Tefre de Renzy-Martin K, Hagen CP, Tinggaard J, Mouritsen A, Mieritz MG, Main KM. Validity of self-assessment of pubertal maturation. Pediatrics 2015;135:86–93. [DOI] [PubMed] [Google Scholar]
- [50].Reddy H, Staahl C, Arendt-Nielsen L, Gregersen H, Drewes AM, Funch-Jensen P. Sensory and biomechanical properties of the esophagus in non-erosive reflux disease. Scand J Gastroenterol 2007;42:432–440. [DOI] [PubMed] [Google Scholar]
- [51].Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology 2002;122:1500–1511. [DOI] [PubMed] [Google Scholar]
- [52].Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol 2019;220:569.e1–569.e7. [DOI] [PubMed] [Google Scholar]
- [53].Schurman JV, Friesen CA, Andre L, Welchert E, Lavenbarg T, Danda CE, Cocjin JT, Hyman PE. Diagnostic utility of the water load test in children with chronic abdominal pain. J Pediatr Gastroenterol Nutr 2007;44:51–57. [DOI] [PubMed] [Google Scholar]
- [54].Sperotto F, Brachi S, Vittadello F, Zulian F. Musculoskeletal pain in schoolchildren across puberty: a 3-year follow-up study. Pediatr Rheumatol Online J 2015;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124:1501–1512. [DOI] [PubMed] [Google Scholar]
- [56].Tham SW, Palermo TM, Holley AL, Zhou C, Stubhaug A, Furberg A-S, Nielsen CS. A population-based study of quantitative sensory testing in adolescents with and without chronic pain. Pain 2016;157:2807–2815. [DOI] [PubMed] [Google Scholar]
- [57].Trapanotto M, Pozziani G, Perissinotto E, Barbieri S, Zacchello F, Benini F. The cold pressor test for the pediatric population: refinement of procedures, development of norms, and study of psychological variables. J Pediatr Psychol 2009;34:749–759. [DOI] [PubMed] [Google Scholar]
- [58].Tsao JCI, Seidman LC, Evans S, Lung KC, Zeltzer LK, Naliboff BD. Conditioned pain modulation in children and adolescents: effects of sex and age. J Pain 2013;14:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tu FF, Datta A, Atashroo D, Senapati S, Roth G, Clauw DJ, Hellman KM. Clinical profile of comorbid dysmenorrhea and bladder sensitivity: a cross-sectional analysis. Am J Obstet Gynecol 2020;222:594.e1–594.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A noninvasive bladder sensory test supports a role for dysmenorrhea increasing bladder noxious mechanosensitivity. Clin J Pain 2013;29:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tu FF, Fitzgerald CM, Kuiken T, Farrell T, Harden RN. Comparative measurement of pelvic floor pain sensitivity in chronic pelvic pain. Obstet Gynecol 2007;110:1244–1248. [DOI] [PubMed] [Google Scholar]
- [62].Tu FF, Fitzgerald CM, Kuiken T, Farrell T, Harden RN. Vaginal pressure-pain thresholds: initial validation and reliability assessment in healthy women. Clin J Pain 2008;24:45–50. [DOI] [PubMed] [Google Scholar]
- [63].Tu FF, Kane JN, Hellman KM. Noninvasive experimental bladder pain assessment in painful bladder syndrome. BJOG 2017;124:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tu Y, Zhang B, Cao J, Wilson G, Zhang Z, Kong J. Identifying inter-individual differences in pain threshold using brain connectome: a test-retest reproducible study. Neuroimage 2019;202:116049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Van Ginkel R, Voskuijl WP, Benninga MA, Taminiau JA, Boeckxstaens GE. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology 2001;120:31–38. [DOI] [PubMed] [Google Scholar]
- [66].Walker LS, Beck JE, Garber J, Lambert W. Children’s Somatization Inventory: psychometric properties of the revised form (CSI-24). J Pediatr Psychol 2009;34:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani T, Greene JW, Mertz H, Naliboff BD. Validation of a symptom provocation test for laboratory studies of abdominal pain and discomfort in children and adolescents. J Pediatr Psychol 2006;31:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Warren JW, Wesselmann U, Greenberg P, Clauw DJ. Urinary symptoms as a prodrome of bladder pain syndrome/interstitial cystitis. Urology 2014;83:1035–1040. [DOI] [PubMed] [Google Scholar]
- [69].Wen JG, Tong EC. Cystometry in infants and children with no apparent voiding symptoms. Br J Urol 1998;81:468–473. [DOI] [PubMed] [Google Scholar]
- [70].Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am J Obstet Gynecol 2013;209:422e.1–422.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Whitehead WE, Crowell MD, Davidoff AL, Palsson OS, Schuster MM. Pain from rectal distension in women with irritable bowel syndrome: relationship to sexual abuse. Dig Dis Sci 1997;42:796–804. [DOI] [PubMed] [Google Scholar]
- [72].Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology 1998;115:1263–71. [DOI] [PubMed] [Google Scholar]
- [73].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- [74].Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen KB, Stubhaug A, Treede RD, Wilder-Smith OHG. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19:805–806. [DOI] [PubMed] [Google Scholar]
- [75].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best L-A, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22–28. [DOI] [PubMed] [Google Scholar]
- [76].Zhao SZ, Wong JM, Davis MB, Gersh GE, Johnson KE. The cost of inpatient endometriosis treatment: an analysis based on the Healthcare Cost and Utilization Project Nationwide Inpatient Sample. Am J Manag Care 1998;4:1127–1134. [PubMed] [Google Scholar]
- [77].Zondervan KT, Yudkin PL, Vessey MP, Jenkinson CP, Dawes MG, Barlow DH, Kennedy SH. The community prevalence of chronic pelvic pain in women and associated illness behaviour. Br J Gen Pract 2001;51:541–547. [PMC free article] [PubMed] [Google Scholar]