Abstract
Purpose: To investigate the expression of various angiogenesis and inflammation mediators in the vitreous fluid of eyes with proliferative diabetic retinopathy (PDR).
Methods: A total of 38 eyes with PDR and 37 control eyes were included. Vitreous fluid was collected during vitrectomy. Vitreous levels of colony stimulating factor-1 receptor (CSF-1R), syndecan-1, placental growth factor (PIGF), and angiopoietin-like protein 4 (ANGPTL-4) were measured by multiplex immunoassay. Vitreous levels of vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), and intercellular adhesion molecule-1 (ICAM-1) were measured by cytometric beads array. Levels of these mediators were compared between the PDR and control eyes. Correlations between levels of different mediators and between these mediators and kidney function metrics in the PDR group were also analyzed.
Results: Vitreous levels of syndecan-1, PIGF, ANGPTL-4, VEGF, and IL-8 were significantly higher in the PDR group compared to the control group (all p < 0.05). Levels of VEGF were significantly correlated with levels of syndecan-1, PIGF, and ANGPTL-4 (r = 0.370 to 0.497, all p < 0.05). Significant positive correlations were detected between levels of any two of the following mediators including syndecan-1, PIGF, ANGPTL-4, and IL-8 (r = 0.370 to 0.906, all p < 0.05). Apart from VEGF, levels of these mediators were positively correlated with serum creatinine and blood urea nitrogen (r = 0.328 to 0.638, all p < 0.05), and negatively correlated with fasting blood glucose and estimated glomerular filtration rate (r = −0.325 to −0.603, all p < 0.05).
Conclusions: Correlations between different angiogenesis and inflammation mediators were observed in eyes with PDR, suggesting cross-talks of different angiogenesis and inflammation pathways in the pathogenesis of PDR. The levels of angiogenesis and inflammation in PDR are correlated with kidney damage, indicating possible common pathways in diabetic retinopathy and nephropathy.
Keywords: diabetic retinopathy, proliferative diabetic retinopathy, angiogenesis, inflammation, vitreous fluid
Introduction
Diabetic retinopathy (DR) is the most common cause of vision loss in diabetes mellitus (DM). The overall prevalence of any DR in the world is 34.6% and prevalence of vision-threatening DR is estimated to be 10.2% (1). The number of people affected by moderate to severe vision impairment due to DR is estimated to be 2.6 million (2). The prevalence of DR increases with increasing DM duration, hemoglobin A1c, and blood pressure (1). Proliferative diabetic retinopathy (PDR) is the most vision-threatening type of DR and its prevalence is estimated to be 6.96% worldwide (1). PDR patients are at high risks of severe vision loss due to complications such as tractional retinal detachment and vitreous hemorrhage, and exploration of the mechanisms of PDR is critical for the treatment and prevention of the disease.
Angiogenesis and inflammation are two important mechanisms for the development of DR, including PDR (3). It has been shown that vascular endothelial growth factor (VEGF) plays a critical role in the development of the disease and anti-VEGF therapy has been commonly used in patients with PDR (4). Other angiogenesis and inflammation mediators such as placental growth factor (PIGF), angiopoietin-like protein 4 (ANGPTL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), and intercellular adhesion molecule-1 (ICAM-1) are also suggested to be involved in the pathogenesis of PDR (5–8). Colony stimulating factor-1 (CSF-1) and its receptor (CSF-1R) have also been found to be involved in the development of PDR and retinal inflammation (9, 10). And expression of syndecan-1 has been found to be elevated in vitreous of eyes with PDR (11, 12). Treatment targeting these mediators may be potential replacement therapy for anti-VEGF treatment, which is limited by short duration of action and heterogeneity of efficacy.
In the present study, we aim to investigate the expression of various angiogenesis and inflammation mediators mentioned above in the vitreous fluid of eyes with PDR.
Materials and Methods
Subjects
In this prospective study, 38 patients (38 eyes) with PDR who underwent vitrectomy for vitreous hemorrhage, proliferative epiretinal membrane, or tractional retinal detachment were recruited form the Department of Ophthalmology at Guangdong Provincial People's Hospital (GPPH) between August 2017 to August 2020. A total of 37 non-DM patients (37 eyes) who underwent vitrectomy for idiopathic pre-retinal membranes (IPM), idiopathic macular holes (IMH), or rhegmatogenous retinal detachment (RRD) were included as the control group. The exclusion criteria were: (1) coexistence of other ocular conditions associated with inflammation (such as age-related macular degeneration, glaucoma, uveitis etc.), (2) history of ocular surgery or trauma, (3) previous anti-VEGF treatment, (4) history of severe systemic inflammatory diseases, primary kidney diseases, or any other kidney diseases that are cause other than DM secondarily. The study was approved by the Research Ethics Committee of the Guangdong Provincial People's Hospital (Number:2016232A), and it was in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients before recruitment.
All patients underwent complete ocular examinations and measurements of blood pressure, fasting blood glucose (FBG), glycated hemoglobin (HbA1c), serum creatinine (sCr), blood urea nitrogen (BUN), and estimated glomerular filtration rate (eGFR) before surgery. The eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (13). Vitreous fluids were collected during pars plana vitrectomy using the 23-gauge trocar and cannula system (Alcon Laboratories, Inc. Fort Worth, Tex. the USA). About 0.2–0.4 ml of vitreous humor was aspirated into a sterile syringe before intraocular infusion. The vitreous samples were centrifuged immediately at 2,500 rpm at 4°C for 10 min. The supernatants were aspirated and subsequently stored at −80°C.
Vitreous levels of colony stimulating factor-1 receptor (CSF-1R), syndecan-1, placental growth factor (PIGF), and angiopoietin-like protein 4 (ANGPTL-4) were measured by multiplex immunoassay (Luminex Human Magnetic Assay, R&D Systems, Minneapolis, MN). Vitreous levels of vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), and intercellular adhesion molecule-1 (ICAM-1) were measured by cytometric beads array BD Bioscience, San Jose, CA, USA. All samples were measured once. The minimum and maximum detection values were 9.77–2,500 pg/ml for IL-6, IL-8, MCP-1, TNF-α, and VEGF, 35.32–25,750 pg/ml for 2.92–710 pg/ml for PIGF, 526.43–383,770 pg/ml for ANGPTL-4, 136.49–99,500 pg/ml for CSF-1R, 38.94–28,390 pg/ml for MCP-1, and 38.94–28,390 pg/ml for syndecan-1. The R2 was higher than 0.99 in all curves. When values were outside the detection limit of the kit, the detection threshold was calculated based on the standard curve (7, 14).
Statistical Analysis
Statistical analysis was performed using SPSS software package version 26 (SPSS. Inc, Chicago, IL, USA). Qualitative variables were presented as numbers and percentages. Quantitative variables were presented as median (lower quartile, upper quartile). Mann-Whitney U-test was applied to compare the basic characteristics, blood test parameters and levels of the angiogenesis and inflammation mediators between the PDR group and the control group. Spearman's correlation test was used to analyze the associations between different mediators which were significantly different between the two groups, or between the mediators and clinical characteristics/blood test parameters. For all the tests, p < 0.05 was considered statistically significant.
Results
Basic Characteristics
A total of 37 eyes with PDR and 38 control eyes were included. Basic characteristics of the two groups are shown in Table 1. There were significant differences in FBG, HbA1c, sCr, BUN, and eGFR between the two groups (all p < 0.001).
Table 1.
Basic characteristics of the subjects.
| Characteristics | PDR (n = 38) | Non-DM (n = 37) | p-value |
|---|---|---|---|
| Age, y | 57.00 (49.00, 69.00) | 59.00 (51.00, 64.75) | 0.480 |
| Male/female | 9/29 | 16/21 | 0.072 |
| Duration of DM, years | 8.30 (5.00, 10.00) | N/A | N/A |
| Duration of DR, months | 12.00 (5.25, 16.13) | N/A | N/A |
| SBP, mmHg | 128.00 (120.25, 144.00) | 127.00 (116.25,138.50) | 0.347 |
| DBP, mmHg | 83.00 (68.00, 89.25) | 78.00 (73.25, 86.75) | 0.718 |
| FBG, mmol/l | 10.10 (7.90, 13.27) | 6.15 (5.33, 7.08) | <0.001* |
| HbA1c, % | 7.70 (7.58, 7.90) | 5.95 (5.50, 6.10) | <0.001* |
| sCr, μmol/l | 169.40 (85.15, 297.90) | 76.68 (60.08, 86.01) | <0.001* |
| BUN, mmol/l | 9.96 (7.38, 16.42) | 5.20 (4.38, 6.06) | <0.001* |
| eGFR, ml/min/1.73 m2 | 34.83 (18.16, 69.16) | 93.10 (79.27, 100.24) | <0.001* |
PDR, proliferative diabetic retinopathy; DM, diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; sCr, serum creatinine; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate.
Statistically significant (p-value < 0.05).
Vitreous Levels of the Angiogenesis and Inflammation Mediators
Vitreous levels of the angiogenesis and inflammation mediators are shown in Table 2. Vitreous levels of syndecan-1 (p = 0.004), PIGF (p < 0.001), ANGPTL-4 (p < 0.001), VEGF (p = 0.001), and IL-8 (p = 0.038) were significantly higher in the PDR group compared to the control group. No significant differences were found in vitreous levels of CSF-1R (p = 0.695), IL-6 (p = 0.719), MCP-1 (p = 0.611), TNF-α (p = 0.354), and ICAM-1 (p = 0.080).
Table 2.
Vitreous levels of the angiogenesis and inflammation mediators.
| Mediators | PDR (n = 38) | Non-DM (n = 37) | p-value |
|---|---|---|---|
| Syndecan-1 (pg/ml) | 196.75 (106.86, 446.55) | 108.26 (68.78,171.45) | 0.004* |
| CSF-1R (pg/ml) | 24352.98 (13319.23, 58648.69) | 39665.10 (13459.57, 51903.36) | 0.695 |
| PIGF (pg/ml) | 35.60 (3.49, 283.08) | 0.81 (0.37, 1.29) | <0.001* |
| ANGPTL-4 (pg/ml) | 112378.13 (31458.05, 387799.98) | 26890.31 (8022.31, 54353.15) | <0.001* |
| VEGF (pg/ml) | 22.14 (5.40, 204.52) | 3.15 (2.38, 4.15) | 0.001* |
| IL-6 (pg/ml) | 45.32 (11.31, 360.45) | 31.84 (13.12, 207.56) | 0.719 |
| IL-8 (pg/ml) | 50.71 (19.84, 256.68) | 23.69 (10.70, 49.14) | 0.038* |
| MCP-1 (pg/ml) | 1107.18 (610.26, 2084.73) | 1315.25 (590.25, 2400.45) | 0.611 |
| TNF-α (pg/ml) | 8.81 (6.68, 46.28) | 7.92 (6.79, 9.83) | 0.354 |
| ICAM-1 (pg/ml) | 1209.64 (318.50, 2075.00) | 572.37 (224.68, 1215.11) | 0.080 |
PDR, proliferative diabetic retinopathy; DM, diabetes mellitus.
Statistically significant (p value < 0.05).
Correlations Between Vitreous Levels of Different Angiogenesis and Inflammation Mediators
Among the angiogenesis and inflammation mediators significantly different between the two groups, correlation coefficients between vitreous levels of any two mediators are shown in Table 3. Significant correlations were detected between levels of syndecan-1 and levels of PIGF (r = 0.813), ANGPTL-4 (r = 0.859), VEGF (r = 0.471), and IL-8 (r = 0.826), between levels of PIGF and levels of ANGPTL-4 (r = 0.906), VEGF (r = 0.497), and IL-8 (r = 0.879), and between levels of ANGPTL-4 and levels of VEGF (r = 0.370) and IL-8 (r = 0.902) (all p < 0.05). No significant correlation was found between levels of VEGF and levels of IL-8 (p = 0.078).
Table 3.
Correlations between vitreous levels of different angiogenesis and inflammation mediators in eyes with PDR.
| Mediators | Syndecan-1 (pg/ml) | PIGF (pg/ml) | ANGPTL-4 (pg/ml) | VEGF (pg/ml) | IL-8 (pg/ml) |
|---|---|---|---|---|---|
| r value | r value | r value | r value | r value | |
| (p-value) | (p-value) | (p-value) | (p-value) | (p-value) | |
| Syndecan-1 (pg/ml) | 0.813 (<0.001*) |
0.859 (<0.001*) |
0.471 (0.003*) |
0.826 (<0.001*) |
|
| PIGF (pg/ml) | 0.906 (<0.001*) |
0.497 (0.001*) |
0.879 (<0.001*) |
||
| ANGPTL-4 (pg/ml) | 0.370 (0.022*) |
0.902 (<0.001*) |
|||
| VEGF (pg/ml) | 0.289 (0.078) |
Statistically significant (p value < 0.05).
Correlations Between Vitreous Levels of the Angiogenesis and Inflammation Mediators and Kidney Function Metrics
Among the mediators significantly different between the two groups, correlation coefficients between vitreous levels of the mediators and systemic factors are shown in Table 4. Significant positive correlations were found between sCr and levels of syndecan-1, PIGF, ANGPTL-4, and IL-8 (r = 0.328 to 0.572, all p < 0.05). Similar correlations were detected between BUN and levels of syndecan-1, PIGF, ANGPTL-4, and IL-8 (r = 0.447 to 0.638, all p < 0.05). The eGFR and FBG were found to be significantly negatively correlated with levels of syndecan-1, PIGF, ANGPTL-4, and IL-8 (r = −0.325 to −0.603, all p < 0.05).
Table 4.
Correlations between vitreous levels of the angiogenesis and inflammation mediators and systemic factors.
| Systemic factors | FBG, mmol/l | HbA1c, % | sCr, μmol/l | BUN, mmol/l | eGFR, ml/min/1.73 m2 |
|---|---|---|---|---|---|
| r value | r value | r value | r value | r value | |
| (p-value) | (p-value) | (p-value) | (p-value) | (p-value) | |
| Syndecan-1 (pg/ml) | −0.325 (0.047*) |
−0.054 (0.746) |
0.572 (<0.001*) |
0.629 (<0.001*) |
−0.603 (<0.001*) |
| PIGF (pg/ml) | −0.350 (0.031*) |
−0.231 (0.163) |
0.338 (0.038*) |
0.447 (0.005*) |
−0.356 (0.028*) |
| ANGPTL4 (pg/ml) | −0.362 (0.026*) |
−0.279 (0.101) |
0.428 (0.007*) |
0.638 (<0.001*) |
−0.453 (0.004*) |
| VEGF (pg/ml) | −0.168 (0.315) |
−0.268 (0.104) |
0.000 (0.998) |
0.184 (0.270) |
−0.019 (0.910) |
| IL-8 (pg/ml) | −0.403 (0.012*) |
−0.226 (0.173) |
0.328 (0.044*) |
0.509 (0.001*) |
−0.365 (0.024*) |
FBG, fasting blood glucose; HbA1c, glycated hemoglobin; sCr, serum creatinine; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate.
Statistically significant (p value < 0.05).
Discussion
Angiogenesis and inflammation are the key feature of PDR and also thought to be critical mechanisms for PDR initiation and progression (3, 15). In this study, we showed higher vitreous levels of angiogenesis and inflammation mediators including syndecan-1, PIGF, ANGPTL-4, VEGF and IL-8 in eyes with PDR. We also detected significant positive correlations between vitreous levels of these mediators. Apart from VEGF, levels of these mediators were significantly correlated with FBG, sCr, BUN, and eGFR. Our results indicate that interactions between different angiogenesis and inflammation mediators are involved in the pathogenesis of PDR.
Results in our study showed that the vitreous levels of angiogenesis and inflammation mediators including syndecan-1, PIGF, ANGPTL-4, VEGF and IL-8 in the PDR group were significantly higher than in control group. Under high glucose (HG) and hypoxia conditions, increased hypoxia-inducible factors (HIFs) in the retina activated the transcription of multiple genes encoding angiogenesis mediators, including ANGPTL-4, VEGF, and PIGF (5, 16, 17). Elevated vitreous level of syndecan-1 was also detected in eyes of PDR in previous studies (11, 12). The reason for the increase of syndecan-1 might be due to activation of the heparinase/syndecan-1 axis or matrix metallopeptidases (MMPs)/syndecan-1 axis in retinal microvascular endothelial cells induced by HG, hypoxia, or inflammation (11, 12). On the other hand, HIFs can also lead to the up-regulation of chemokines, including IL-8, which can promote the infiltration of inflammatory cells (18). However, increased vitreous levels of several inflammation mediators noted in previous studies, including TNF-α, MCP-1, CSF-1R, ICAM-1 and IL-6, were not observed in our study. Similar negative findings were also noticed in some other studies (19, 20). The discrepancy might be due to different PDR phenotypes in different studies (21–23). Taken together, the roles of the angiogenesis and inflammation mediators in PDR are complicated, and the vitreous levels of these mediators may be affected by the phenotypes of PDR.
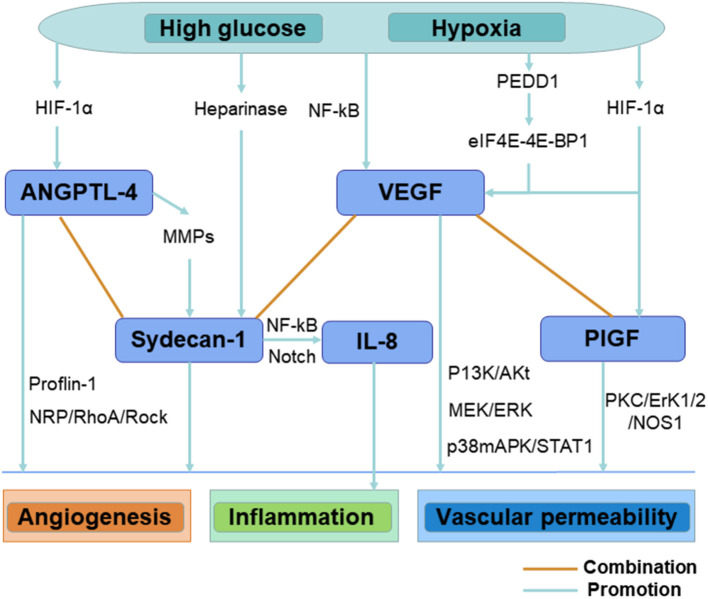
Correlation analysis revealed the complex relationships between different angiogenesis and inflammation mediators. Results in our study showed that the correlations between any two of following mediators including syndecan-1, PIGF, ANGPTL-4, and IL-8 were nearly two times stronger than those between VEGF and other mediators. These results suggested a critical role of the regulation of non-VEGF pathways in the pathogenesis of PDR. Emerging evidences suggest that ANGPTL4 may be a candidate target in DME and PDR treatment (5, 24). ANGPTL4 is up-regulated in hypoxic retinal Müller cells and plays an upstream role of VEGF, and it may accelerate the progression of PDR by increasing retinal angiogenesis and promoting vascular permeability (25, 26). A previous study demonstrated that inhibition of ANGPTL-4 was more efficient than inhibition of VEGF alone in reducing the angiogenesis effect in PDR, indicating pathways in addition to VEGF (25). What is more, ANGPTL-4 can induce the expression of MMPs, enhancing the shedding of syndecan-1 from the cell surface, and syndecan-1 is one of the ANGPTL-4 receptors and mediates ANGPTL-4-induced intracellular signaling (24, 27). Previous studies also reported that syndecan-1 could up-regulate the expressions of inflammation mediators such as IL-8 in chronic inflammation, leading to leukocyte chemotaxis and initiation of inflammatory response (28, 29). Therefore, the ANGPTL-4/MMP/syndecan-1/IL-8 axis may contribute to PDR progression (Figure 1). However, the interactions between different angiogenesis and inflammation mediators in PDR have not been completely elucidated, and future investigations are needed to explore these complicated mechanisms. Targeting both VEGF and non-VEGF angiogenesis pathways may be necessary for effective treatment or prevention of PDR. These findings suggest the value of non-VEGF angiogenesis pathways as the potential therapeutic targets of PDR.
Figure 1.
Illustration shows the interactions between different angiogenesis and inflammation mediators in eyes with PDR.
We also revealed that levels of the angiogenesis and inflammation mediators were significantly correlated with indicators of kidney damage including sCr, BUN, and eGFR. These results suggested that kidney damage tended to be more severe in patients with higher levels of angiogenesis and inflammation in the eye. There are substantial evidences supporting the association between DR and diabetic nephropathy (DN) (30–35): (1) both DR and DN are characterized by diabetic microangiopathy; (2) there are common risk factors for the occurrence and progression of DR and DN; (3) the severity of DR is correlated with the degree of kidney damage; (4) multiple pathways have been considered to be involved in the development and progression of both DR and DN. On the one hand, elevated sCr and BUN and reduced eGFR in our study reflected microvascular damage of the kidney in the PDR patients. On the other hand, levels of the vitreous mediators were indicators of angiogenesis and inflammation in the retina of PDR. In our study, apart from VEGF, these angiogenesis and inflammation mediators were significantly correlated with kidney damage, consistent with previous studies showing significant correlations between serum levels of these mediators and renal functions (36–38), suggesting that non-VEGF pathways might be involved in the pathogenesis of both DR and DN. Increased serum levels of ANGPTL-4, syndecan-1, PIGF, and IL-8 were observed in diabetes patients with complications (26, 39, 40). It was shown that ANGPTL-4 expression was positively correlated with the amount of 24-h urine protein, sCr and the kidney weight index (41). It was also demonstrated that ANGPTL-4 was over-expressed in DN rat and associated with HG-induced cell proliferation, inflammatory response, and extracellular matrix accumulation in glomerular mesangial cells, mediating damage to the glomerular filtration barrier (41, 42). Besides, syndecan-1 shedding may also play a role in the development of DN (40). These evidences support that DR and DN might share a common pathogenesis and clinical course, indicating that therapy targeting non-VEGF pathways may be beneficial to hinder the progression of both DR and DN.
In addition, results in our study showed that angiogenesis and inflammation mediators were negatively correlated with FBG. These results might be due to the increased intensity of glycemic control in PDR patients.
There are several limitations of this study. Firstly, we did not detect the levels of mediators in NPDR patients or DM patients without DR, so we were unable to investigate the relationship between the vitreous levels of the angiogenesis and inflammation mediators with the severity of DR. We also did not detect the serum levels of these mediators. Therefore, we could not analyze the correlations between serum levels of these mediators and renal functions. Secondly, heterogeneity of the control group might change the profile of the angiogenesis and inflammation mediators in the vitreous fluid, masking the true effects of certain mediators in PDR. Thirdly, since our study was based on the human level, the exact mechanisms of the correlations between these mediators and cross-talks of angiogenic and inflammatory pathways in the pathogenesis of PDR require further investigation in animal experiments. Finally, population-based randomized studies with larger samples and basic research are needed to verify our results, as well as explore the regulation mechanisms of the angiogenesis and inflammation mediators in PDR.
Conclusions
The present study revealed elevated vitreous levels of various angiogenesis and inflammation mediators and significant correlations between these mediators in eyes of PDR, providing clues to understand the link between angiogenesis and inflammation in PDR. Moreover, significant correlations between the vitreous levels of these mediators and kidney damage indicated a potential relationship between retinopathy and nephropathy in PDR patients.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data used during the current study are available from the corresponding authors on reasonable request. Requests to access these datasets should be directed to Yijun Hu, huyijun2014@163.com.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of the Guangdong Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YH, HY, GW, and BL: conception and design. BL, QW, ZD, and YF: data collection and collation. GW and ZD: laboratory analysis. GW, BL, QW, and CT: data analysis and interpretation. YH and GW: manuscript writing. YH and HY: data interpretation and final review of the manuscript. All authors revised and approved the submitted manuscript.
Funding
This work was supported by Grant 81870663 and 82171075 from the National Natural Science Foundation of China (HY), Grant KJ012019087 of the Outstanding Young Talent Trainee Program of Guangdong Provincial People's Hospital (HY), Grant KJ012019457 from the GDPH Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangdong Province (HY), Grant Y012018145 from the Talent Introduction Fund of Guangdong Provincial People's Hospital (HY), Grant A2021378 from the Medical Scientific Research Foundation of Guangdong Province, China (YH), Grant 2018SK50106 from the Technology Innovation Guidance Program of Hunan Province (YH), Grant AM1909D2 and AR1909D2 from the Science Research Foundation of Aier Eye Hospital Group (YH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. (2017) 5:e1221–34. 10.1016/s2214-109x(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 3.Nawaz IM, Rezzola S, Cancarini A, Russo A, Costagliola C, Semeraro F, et al. Human vitreous in proliferative diabetic retinopathy: characterization and translational implications. Prog Retin Eye Res. (2019) 72:100756. 10.1016/j.preteyeres.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Cheung N, Wong IY, Wong TY. Ocular anti-VEGF therapy for diabetic retinopathy: overview of clinical efficacy and evolving applications. Diabetes Care. (2014) 37:900–5. 10.2337/dc13-1990 [DOI] [PubMed] [Google Scholar]
- 5.Babapoor-Farrokhran S, Jee K, Puchner B, Hassan SJ, Xin X, Rodrigues M, et al. Angiopoietin-like 4 is a potent angiogenic factor and a novel therapeutic target for patients with proliferative diabetic retinopathy. Proc Natl Acad Sci USA. (2015) 112:E3030–9. 10.1073/pnas.1423765112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Wu J, Wu C, Bian AL, Geng S, Dai RP. Comparison of aqueous humor levels of PlGF and VEGF in proliferative diabetic retinopathy before and after intravitreal conbercept injection. Diabetes Res Clin Pract. (2020) 162:108083. 10.1016/j.diabres.2020.108083 [DOI] [PubMed] [Google Scholar]
- 7.Mallmann F, Canani LH. Intravitreal neurodegenerative and inflammatory mediators in proliferative diabetic retinopathy. Arq Bras Oftalmol. (2019) 82:275–82. 10.5935/0004-2749.20190055 [DOI] [PubMed] [Google Scholar]
- 8.McAuley AK, Sanfilippo PG, Hewitt AW, Liang H, Lamoureux E, Wang JJ, et al. Vitreous biomarkers in diabetic retinopathy: a systematic review and meta-analysis. J Diabetes Compl. (2014) 28:419–25. 10.1016/j.jdiacomp.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 9.Yoshida S, Kobayashi Y, Nakama T, Zhou Y, Ishikawa K, Arita R, et al. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: implications for M2 macrophage-involving fibrovascular membrane formation. Br J Ophthalmol. (2015) 99:629–34. 10.1136/bjophthalmol-2014-305860 [DOI] [PubMed] [Google Scholar]
- 10.Kokona D, Ebneter A, Escher P, Zinkernagel MS. Colony-stimulating factor 1 receptor inhibition prevents disruption of the blood-retina barrier during chronic inflammation. J Neuroinflammation. (2018) 15:340. 10.1186/s12974-018-1373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu El-Asrar AM, Alam K, Nawaz MI, Mohammad G, Van den Eynde K, Siddiquei MM, et al. Upregulated expression of heparanase in the vitreous of patients with proliferative diabetic retinopathy originates from activated endothelial cells and leukocytes. Invest Ophthalmol Vis Sci. (2015) 56:8239–47. 10.1167/iovs.15-18025 [DOI] [PubMed] [Google Scholar]
- 12.Abu El-Asrar AM, Nawaz MI, De Hertogh G, Alam K, Siddiquei MM, Van den Eynde K, et al. S100A4 is upregulated in proliferative diabetic retinopathy and correlates with markers of angiogenesis and fibrogenesis. Mol Vis. (2014) 20:1209–24. [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curnow SJ, Falciani F, Durrani OM, Cheung CM, Ross EJ, Wloka K, et al. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. (2005) 46:4251–9. 10.1167/iovs.05-0444 [DOI] [PubMed] [Google Scholar]
- 15.Abu El-Asrar AM, Nawaz MI, Ahmad A, De Zutter A, Siddiquei MM, Blanter M, et al. Evaluation of proteoforms of the transmembrane chemokines CXCL16 and CX3CL1, their receptors, and their processing metalloproteinases ADAM10 and ADAM17 in proliferative diabetic retinopathy. Front Immunol. (2020) 11:601639. 10.3389/fimmu.2020.601639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurihara T, Westenskow PD, Friedlander M. Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signaling in the retina. Adv Exp Med Biol. (2014) 801:275–81. 10.1007/978-1-4614-3209-8_35 [DOI] [PubMed] [Google Scholar]
- 17.Jiao W, Ji JF, Xu W, Bu W, Zheng Y, Ma A, et al. Distinct downstream signaling and the roles of VEGF and PlGF in high glucose-mediated injuries of human retinal endothelial cells in culture. Sci Rep. (2019) 9:15339. 10.1038/s41598-019-51603-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. (2011) 30:343–58. 10.1016/j.preteyeres.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loporchio DF, Tam EK, Cho J, Chung J, Jun GR, Xia W, et al. Cytokine levels in human vitreous in proliferative diabetic retinopathy. Cells. (2021) 10:1069. 10.3390/cells10051069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. (2011) 55:256–63. 10.1007/s10384-011-0004-8 [DOI] [PubMed] [Google Scholar]
- 21.Katagiri M, Shoji J, Inada N, Kato S, Kitano S, Uchigata Y. Evaluation of vitreous levels of advanced glycation end products and angiogenic factors as biomarkers for severity of diabetic retinopathy. Int Ophthalmol. (2018) 38:607–15. 10.1007/s10792-017-0499-1 [DOI] [PubMed] [Google Scholar]
- 22.Urbančič M, Petrovič D, Živin AM, Korošec P, FleŽar M, Petrovič MG. Correlations between vitreous cytokine levels and inflammatory cells in fibrovascular membranes of patients with proliferative diabetic retinopathy. Mol Vis. (2020) 26:472–82. [PMC free article] [PubMed] [Google Scholar]
- 23.El-Asrar AM, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S, et al. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. (2011) 17:1829–38. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Cheng Y, Su G. A review of the multifunctionality of angiopoietin-like 4 in eye disease. Biosci Rep. (2018) 38:BSR20180557. 10.1042/bsr20180557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Q, Lu P, Chen W, Lu L, Zheng Z. ANGPTL-4 induces diabetic retinal inflammation by activating Profilin-1. Exp Eye Res. (2018) 166:140–50. 10.1016/j.exer.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 26.Lu Q, Zou W, Chen B, Zou C, Zhao M, Zheng Z. ANGPTL-4 correlates with vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. (2016) 254:1281–8. 10.1007/s00417-015-3187-8 [DOI] [PubMed] [Google Scholar]
- 27.Kirsch N, Chang LS, Koch S, Glinka A, Dolde C, Colozza G, et al. Angiopoietin-like 4 is a Wnt signaling antagonist that promotes LRP6 turnover. Dev Cell. (2017) 43:71–82.e6. 10.1016/j.devcel.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Wang Z, Liu J, Zhang S, Fei J, Li J, et al. Cell surface-anchored syndecan-1 ameliorates intestinal inflammation and neutrophil transmigration in ulcerative colitis. J Cell Mol Med. (2017) 21:13–25. 10.1111/jcmm.12934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim SA, Gadalla R, El-Ghonaimy EA, Samir O, Mohamed HT, Hassan H, et al. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol Cancer. (2017) 16:57. 10.1186/s12943-017-0621-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh YT, Tsai MJ, Tu ST, Hsieh MC. Association of abnormal renal profiles and proliferative diabetic retinopathy and diabetic macular edema in an Asian population with type 2 diabetes. JAMA Ophthalmol. (2018) 136:68–74. 10.1001/jamaophthalmol.2017.5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia. (2013) 56:457–66. 10.1007/s00125-012-2796-6 [DOI] [PubMed] [Google Scholar]
- 32.Liu B, Hu Y, Wu Q, Zeng Y, Xiao Y, Zeng X, et al. Qualitative and quantitative analysis of B-cell-produced antibodies in vitreous humor of Type 2 diabetic patients with diabetic retinopathy. J Diabetes Res. (2020) 2020:4631290. 10.1155/2020/4631290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Ren H, Zhang J, Cao Y, Wang Y, Meng D, et al. Diabetic retinopathy, classified using the lesion-aware deep learning system, predicts diabetic end-stage renal disease in chinese patients. Endocr Pract. (2020) 26:429–43. 10.4158/ep-2019-0512 [DOI] [PubMed] [Google Scholar]
- 34.Phipps JA, Feener EP. The kallikrein-kinin system in diabetic retinopathy: lessons for the kidney. Kidney Int. (2008) 73:1114–9. 10.1038/ki.2008.9 [DOI] [PubMed] [Google Scholar]
- 35.Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus - A comprehensive review. J Diabetes Compl. (2020) 34:107613. 10.1016/j.jdiacomp.2020.107613 [DOI] [PubMed] [Google Scholar]
- 36.Colombo M, McGurnaghan SJ, Blackbourn LAK, Dalton RN, Dunger D, Bell S, et al. Comparison of serum and urinary biomarker panels with albumin/creatinine ratio in the prediction of renal function decline in type 1 diabetes. Diabetologia. (2020) 63:788–98. 10.1007/s00125-019-05081-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanova Y, Laikov A, Markelova M, Khadiullina R, Makseev A, Hasanova M, et al. Proteomic analysis of human serum from patients with chronic kidney disease. Biomolecules. (2020) 10:257 10.3390/biom10020257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saboia Z, Meneses GC, Martins AMC, Daher EF, Silva Junior GB. Association between syndecan-1 and renal function in adolescents with excess weight: evidence of subclinical kidney disease and endothelial dysfunction. Braz J Med Biol Res. (2018) 51:e7174. 10.1590/1414-431x20177174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med. (2020) 30:030502. 10.11613/bm.2020.030502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolseth IB, Reine TM, Parker K, Sudworth A, Witczak BJ, Jenssen TG, et al. Increased levels of inflammatory mediators and proinflammatory monocytes in patients with type I diabetes mellitus and nephropathy. J Diabetes Compl. (2017) 31:245–52. 10.1016/j.jdiacomp.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 41.Xue L, Feng X, Wang C, Zhang X, Sun W, Yu K. Benazepril hydrochloride improves diabetic nephropathy and decreases proteinuria by decreasing ANGPTL-4 expression. BMC Nephrol. (2017) 18:307. 10.1186/s12882-017-0724-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin L, Zhang R, Yang S, Chen F, Shi J. Knockdown of ANGPTL-4 inhibits inflammatory response and extracellular matrix accumulation in glomerular mesangial cells cultured under high glucose condition. Artif Cells Nanomed Biotechnol. (2019) 47:3368–73. 10.1080/21691401.2019.1649274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data used during the current study are available from the corresponding authors on reasonable request. Requests to access these datasets should be directed to Yijun Hu, huyijun2014@163.com.