Abstract
Introduction:
Anti-oxidant, antispasmodic, anti-inflammatory, and analgesic effects have been reported for Apium graveolens Linn. ) Celery( seeds and its active component luteolin. So, this study was carried out to investigate the protective effects of hexane (AGHE) and methanol (AGME) extracts of A. graveolens seeds and luteolin on acetic acid-induced colitis in rats.
Methods:
Three doses of AGHE (100, 200, and 400 mg/kg), AGME (200, 400, and 800 mg/kg), and luteolin (5, 10, and 20 mg/kg) were administered orally (p.o.) to separate groups of male Wistar rats, 2 h before ulcer induction (acetic acid 4%) and continued once daily for 4 days. Prednisolone (4 mg/kg) and mesalazine (100 mg/kg) were used as reference and vehicle (2 mL/kg) as control groups. Colon biopsies were taken for weighting, macroscopic and histopathologic evaluation, and measuring myeloperoxidase (MPO) activity.
Results:
Our findings showed that AGHE (200 and 400 mg/kg), AGME (400 and 800 mg/kg), and luteolin (10 and 20 mg/kg) were effective to reduce colonic ulcer score, area, and index as well as total colitis index, and MPO activity significantly in comparison with controls. Since the lowest doses of extracts and luteolin were not significantly effective to diminish evaluated parameters of colitis, it is concluded that the ameliorative effect was dose related.
Conclusion:
It is also concluded that both extracts and luteolin, as an important ingredient of celery extract, were effective in the amelioration of colitis in rats, but further clinical and detailed mechanistic experiments are required to introduce these natural agents for colitis treatment or prevention in human.
Keywords: Acetic acid, animal model, Apium graveolense, colitis, luteolin
Introduction
Apium graveolens Linn (A. graveolens) belongs to Apiaceae family and known as celery. Celery is a biannual plant with a height of about 100 cm, a strong aroma, and fleshy solid stem. The fruit (seeds) of this plant is oval with 1.5–2 mm wideness. It is wingless, brown, and with black lines.[1] This plant grows wildly in salt-lined areas, swamps, saltwater, beaches, and salt springs. Celery is a native medicinal plant to Europe and the wild type could be found in countries such as Algeria, Abyssinia, Caucasus, Iran, India, and United States.[2] It is widely used in food, pharmaceutical, and ornamental industries.[2] Celery seeds have antifungal, antioxidant, antispasmodic, anti-inflammatory, and analgesic effects.[3,4] According to Atta and Alkofahi study, a dose-dependent anti-inflammatory and anti-nociceptive effect have been seen for celery seeds ethanol extract on acetic acid-induced writhing and hot-plate analgesic tests as well as ear edema in mice.[5] In addition, Brancovic et al. and Al-Howiriny et al. reported the antispasmodic and anti-ulcerative effects of celery extract both ex vivo and in vivo, respectively.[6,7] In Iranian traditional medicine, celery seeds are used to treat rheumatoid arthritis, gout, and renal diseases as an anti-inflammatory and diuretic agent.[8] Celery seeds have also been experimentally used to reduce pain and inflammation in oral area, ear, and for headache.[5,9] High contents of essential oil in celery seeds are likely responsible for its potent antispasmodic effects as the seeds contain 2–3% essential oil, which mainly consists of limonene (60%) and selinene (10%).2 About 15 flavonoid glycosides have been isolated in celery seeds for which glucopyranoside, luteolin 7-O-apiosylglucoside, chry soerizl7-O-ß-D-glucopyranoside, chrysoeriol7-O-apisoglucoside, apigenin7-O-ß-D-glucopyranoside, naringenin7-O-ß-D-glucopyranoside, quercetin, and apiin are most abundant.[5,10,11,12] Among these compounds, luteolin has a significant anti-inflammatory effect according to literature.[13,14]
Furthermore, inflammatory bowel disease (IBD) is a chronic and multifactorial gastrointestinal (GI) inflammatory condition, which is categorized into ulcerative colitis and Crohn's disease.[15] While etiology of IBD is still unknown and multifactorial, immune system dysfunction, changes in GI flora, oxidative stress, and intestinal mucosal damages are among the most suspicious reasons are presented in IBD development and aggravation.[16,17] Current medications include sulfasalazine, mesalazine, 5-amino salicylic acid (5-ASA) derivatives, corticosteroids and immunosuppressive agents, which have limited efficacy but major adverse reactions and toxicity like allergic reactions, bone marrow suppression, osteoporosis, liver toxicity, and pulmonary fibrosis.[18,19] The lack of specific and curative treatments with limited toxicity represents a growing need to develop safe and effective alternatives for IBD including herbal medicines.[16] So in the current study, the effect of A. graveolens two different extracts including hexane (AGHE) and methanol (AGME) extracts were examined on acetic acid-induced colitis and compared with its active and available constituent; luteolin.
Methods
Plant material and extract preparation
Celery seeds were purchased from Pakan Bazr Co. (Isfahan, Iran) in June 2019. The plant was identified by Dr. Seyed Mostafa Ghannadian; a pharmacognosist at the Pharmacognosy Department of Isfahan School of Pharmacy and Pharmaceutical Sciences. Herbarium voucher No1704 was deposited in the Pharmacognosy Department of the Isfahan School of Pharmacy. For preparation of extracts, 500 g dry powder of celery seeds was transferred into a filter paper extraction thimble and inserted into Soxhlet apparatus. Hexane (2000 mL) was poured into the Soxhlet flask and set the heater at 80°C. After 20 reflux cycles (about 6 h), the extract was let to be evaporated in a rotary evaporator under reduced pressure until there was no water left in it and kept in a freezer. Remaining powder of hexane extraction was dried and the previous steps were repeated with 1500 ml methanol in the soxhlet apparatus. Finally, methanol extract was collected and kept in the freezer.[7,20]
Measurement of yield value
Fresh weight of each harvest was measured when the final extract was obtained and the total yield was calculated regarding to primary crude material.[21]
Measurement of total flavonoids content
The aluminum chloride colorimetric method was used for the determination of the total flavonoid content of the sample.[22] Luteolin was used to make the standard calibration curve. Stock luteolin solution was prepared by dissolving 4.0 mg luteolin in 10 mL methanol, and then the standard solutions of luteolin were made by serial dilutions in methanol (4–500 μg/mL). An amount of 0.6 mL diluted standard luteolin solutions or extracts were separately mixed with 0.6 mL of 2% aluminum chloride and the solutions were incubated for 60 min at room temperature. The absorbance of the reaction mixtures was measured against blank at 405 nm wavelength by a Shimadzu UV-Vis spectrophotometer (Tokyo, Japan). The concentration of total flavonoid content in the test samples was calculated from the calibration plot (Y = 0.0714 × ‒0.0157, R2 = 0.999) and expressed as mg luteolin equivalent (LE)/100 mg of dried plant material. All the determinations were carried out in triplicate.[23]
Drugs and chemicals
Prednisolone and mesalazine were purchased from Iran Hormone Co. (Tehran, Iran). Luteolin, Orto-dianizidin dihydrocholoride (ODD), hexa-decyl trimethyl ammonium bromide (HTAB) and folin ciocalteu reagent were obtained from Sigma Company (St. Louis, MO, USA). Aluminum chloride hexahydrate, methanol, n-hexane, formaldehyde, and glacial acetic acid were purchased from Merck Company (Darmstadt, Germany). Normal saline was purchased from Shahid-ghazi Co. (Tabriz, Iran).
Animals
Seventy-eight male Wistar rats (200–240 g) were purchased from animal house of Isfahan School of Pharmacy and allowed to be acclimated with laboratory environment for 1 week before the start of the intervention. The rats were housed in plexiglass standard cages under uniform and controlled conditions of temperature (20–22°C), humidity (50–70%), and 12/12 h light/dark photoperiods. Rats were fed with chow pellets and tap water was freely accessible. The animal experiments were done according to the national guidelines provided by Ethics and Research Committee of Isfahan University of Medical Sciences, Isfahan, Iran, and the code of IR.MUI.RESEARCH.REC.1397.152 was allocated to this research project.
Animal Grouping
Animals were randomly divided into 13 groups with 6 rats in each as follows:
1: Normal (Sham) group: Normal rats without ulcer induction received vehicle (2 mL/kg tween 80 (0.1%) in normal saline).
2: Control group: Rats with induced colitis received vehicle (2 mL/kg).
3, 4, and 5: Hexane extract-treated groups; rats with induced colitis received three increasing doses of AGHE (100, 200, and 400 mg/kg)
6, 7, and 8: Methanol extract-treated groups; rats with induced colitis received three increasing doses of AGME (200, 400, and 800 mg/kg)
9, 10, and 11: Luteolin-treated groups; rats with induced colitis received three increasing doses of luteolin (5, 10, and 20 mg/kg)
12 and 13: Reference groups: rats with induced colitis received prednisolone (4 mg/kg) or mesalazine (100 mg/kg).
All the treatments were made orally (p.o.) at 2 h before colitis induction and continued for 4 days thereafter on a daily basis. Finally, the animals were euthanized by carbon-dioxide inhalation, 24 h after the last dose (a 5-day treatment).[24]
Experimental Protocol
All drugs or plant extracts were freshly prepared as suspensions or solutions. For inducing acute colitis, first, rats were fasted for 24 h with free access to tap water and observed to ensure their health before induction. Acute colitis was induced by 2 mL acetic acid (4%) via intra-colonic administration.[24] The rats were lightly anesthetized with halothane while a soft and flexible catheter (2 mm inner diameter and 8 cm in length) was inserted to the anus up to 8 cm and acetic acid was gently injected. Before taking the catheter out, the rats were maintained in a head-down position for 30 s in order to prevent solution spreading out. In Sham group, vehicle (2 mL/kg) was instilled. Colon biopsies were taken for macroscopic scoring of injured tissue, histopathologic examination and measuring myeloperoxidase (MPO) activity.
Evaluation of Colon Macroscopic Damage
Rats were sacrificed under overdose carbon dioxide inhalation at day 5. The abdomen was opened and distal colon, 8 cm in length, and 2 cm proximal to the anus, was excised and incised longitudinally and washed with normal saline. Wet colon weight was measured. Then, tissue was fixed on a light and transparent sheet and its changes were determined for each group. Photos of colon segments were taken by a Sony camera and then transferred to a personal computer and analyzed by Fiji-win 32 software for measuring the ulcerated areas (UA).[25] Ulcer severity (US) was evaluated through the following scores: 0 - no ulcer, 1 - inflammation, edema, thickness, and superficial erosions, 2 - hemorrhagic spots, bleeding, and deep ulcer, 3. necrosis and/or perforation. Ulcer index (UI) was determined by summing the ulcer score and the ulcer area for each colon (UI = US + UA). For further assessments, tissue samples were cut into two equal parts longitudinally, a part was stored immediately at freezer (‒70°C) till biochemical analysis (MPO determination) and the other part was stored in 10% formalin for pathological evaluation.[26]
Evaluation of colon histological damage
Fixed colon tissue was dehydrated, cleared, impregnated with paraffin, blocked, processed, sectioned in 4 μm thick slices, and stained with haemotoxylin and eosin (H&E). Inflammation severity and extent and crypt damage were evaluated on H&E-stained according to a validated scoring system described by Cooper et al.[27] and Dieleman et al.[28] after some modifications. Total colitis index as the sum of the three following sub-scores (inflammation severity, inflammation extent, and crypt damage) was finally measured for each specimen. Pathological evaluation and scoring were performed using a Zeiss microscope equipped with a Sony color video camera for digital imaging.
Evaluation of colonic MPO activity
MPO activity as a marker of polymorph-nuclear leukocyte accumulation was measured using a previously described method after setting up at this laboratory.[29,30] Colon tissues were removed from the freezer and after melting, each tissue sample (0.1 g) chopped in 5 ml of potassium phosphate buffer (pH 6) containing 0.5% w/v HTAB and transferred to a homogenizing tube and homogenized for 45 s 3 times at 1-min interval. Next, the homogenate mixture was sonicated in an ice bath for 10 s and then subjected to a sequence of freezing and thawing and sonicated again for 10 s more. After that, the suspensions were centrifuged at 4000 rpm for 15 min. The MPO activity was measured at 450 nm by UV-Vis spectrophotometrically: for this 0.1 mL of the solution was mixed with 2.9 ml of potassium buffer (pH 6) containing 0.167 mg/mL ODD and 0.005% hydrogen peroxide. MPO activity was defined as quantity of enzyme degrading 1 μm of peroxide per min at 25°C and was reported as units (U) per 100 mg weight of wet colon.
Statistical analysis
Statistical analysis was done using SPSS 22.0 statistical software. Differences among groups were examined using parametric one-way analysis of variance (ANOVA) with Turkey's HSD as post hoc test. Non-parametric data were analyzed using Kruskal–Wallis followed by Mann-Whitney U test. Data are expressed as mean ± S.E.M. or median (range). The minimal level of significance was considered at P < 0.05.
Results
Yield values and total flavonoids contents
Yield values for AGHE and AGME were obtained 16.2% and 8.4%, respectively. Moreover, dry material was assessed for both extracts after trice repeat and 91.2% and 83.0% were obtained for AGHE and AGME, respectively.
Total flavonoid was assessed according to a colorimetric method described above and standardized against luteolin (4, 10, 100, and 500 mcg/mL) as an important flavonoid of A. graveolense. Finally, total flavonoids as luteolin equivalent (LE/100 mg) was measured 0.17 ± 0.02 mg and 1.06 ± 0.08 mg for dried AGHE and AGME, respectively.
Macroscopic evaluation
As shown in Table 1 and Figure 1, macroscopic features of colitis revealed that in control group, maximum ulcer area, severity, and index as well as weight/length ratio, which are indicative of highest level of damage were obtained. Sham group showed no change which suggests that handling-related stress and rectal insertion of vehicle had no interference with experimental results. Treatment with prednisolone and mesalazine as reference drugs reduced the ulcer score (P < 0.01), ulcer area (P < 0.001), ulcer index (P < 0.001), and the weight/length of colon (P < 0.001) significantly [Table 1]. Treatment with higher doses of extracts; AGHE (400 mg/kg), AGME (400 and 800 mg/kg) and luteolin (10 and 20 mg/kg) significantly (at least P < 0.05) reduced all macroscopic assessed parameters representing a dose-related effect [Table 1].
Table 1.
Effects of A. graveolens seeds hexane extract (AGHE), methanol extract (AGME), and luteolin on macroscopic parameters of colitis induced by acetic acid in rats
| Groups/Dose (mg/kg) | Ulcer Area (cm2) | Ulcer Score (0-3) | Ulcer Index (0-10) | W/L Ratio (mg/cm2) |
|---|---|---|---|---|
| Sham | 0.00±0.00*** | 0.0 (0-0)** | 0.00±0.00*** | 74.33±5.09*** |
| Control | 5.94±0.32 | 3.0 (2-3) | 8.94±0.44 | 155.04±4.30 |
| AGHE100 | 4.02±1.24 | 2.0 (1-3) | 6.02±1.60 | 135.67±10.33 |
| AGHE200 | 2.49±0.82* | 1.5 (0-2)** | 3.99±1.09* | 116.91±10.58* |
| AGHE400 | 1.95±0.82** | 1.0 (0-2)* | 2.95±1.26** | 111.01±8.23** |
| AGME200 | 3.00±0.45 | 1.5 (1-2) | 4.50±0.63 | 124.75±9.14 |
| AGME400 | 2.08±0.25** | 1.0 (1-2)** | 3.08±0.41** | 110.53±8.65** |
| AGME800 | 1.16±0.55*** | 0.5 (0-2)** | 1.66±0.88*** | 104.53±8.43*** |
| Luteolin 5 | 3.46±0.83 | 1.5 (1-3) | 4.96±1.23 | 132.30±6.65 |
| Luteolin 10 | 1.76±0.76** | 1.0 (0-3)* | 2.76±1.23** | 113.53±9.38** |
| Luteolin 20 | 1.00±0.38*** | 1.0 (0-1)** | 2±0.59*** | 104.52±4.73*** |
| Prednisolone4 | 0.48±0.50*** | 0.5 (0-1)** | 0.98±0.27*** | 82.96±1.98*** |
| Mesalazine100 | 0.58±0.45*** | 1.0 (0-1)** | 1.58±0.24*** | 90.01±2.40*** |
Sham: normal rats received vehicle (2 mL/kg/day). W/L: weight/length ratio. Data are expressed as mean±S.E.M. or median (range) for scoring parameter, (n=6). *P<0.05, **P<0.01, ***P<0.001, indicate significant difference versus control group
Figure 1.
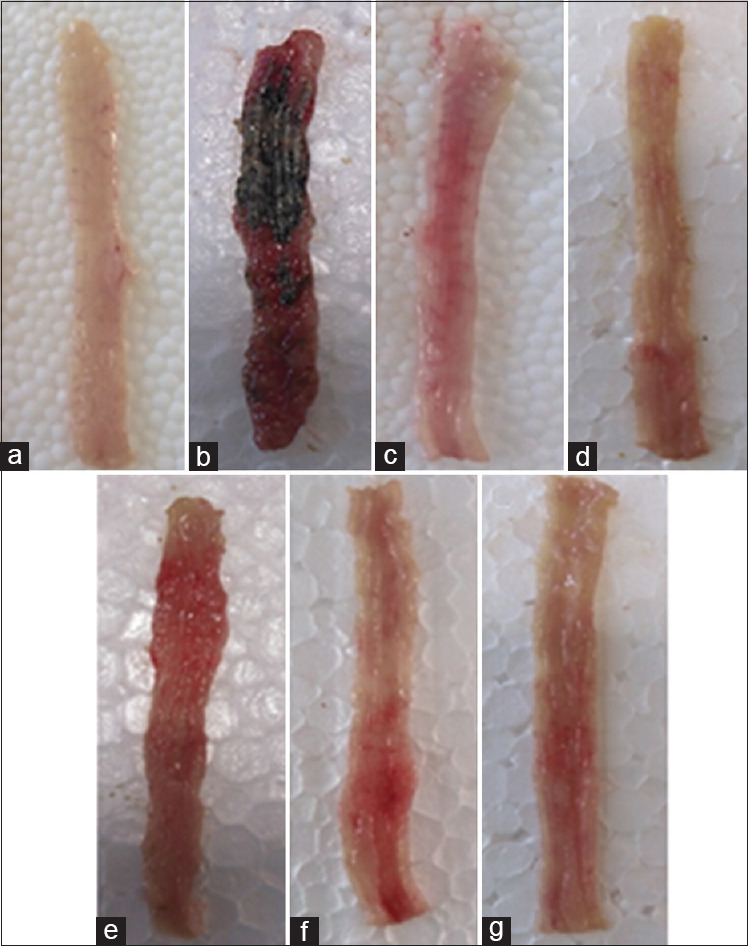
Photos of colon tissues, 5 days after colitis induction in rats. (a) Normal colon treated with vehicle (2 mL/kg). (b) Control colitis treated with vehicle (2 mL/kg). (c) Colitis treated with prednisolone (4 mg/kg). (d) Colitis treated with mesalazine (100 mg/kg). (e) Colitis treated with AGHE (400 mg/kg). (f) Colitis treated with AGME (800 mg/kg). (g) Colitis treated with luteolin (20 mg/kg)
Pathological presentation
In Sham group, no pathologic and/or histological damage was observed, whereas in control group, which colitis was induced by acetic acid instillation and treated with vehicle, clear destruction of epithelium, hemorrhage, edema, inflammatory cellular infiltration, crypt damage, and ulceration at mucus and sub-mucosal layers were found [Table 2 and Figure 2]. Treatment with prednisolone and mesalazine showed significant (at least P < 0.01) reduction in inflammation severity and inflammation extent, crypt damage and total colitis index. Oral administration of higher doses of AGHE (400 mg/kg), AGME (400 and 800 mg/kg) and luteolin (10 and 20 mg/kg) were similarly effective to reduce histopathologic scores (at least P < 0.05) [Table 2]. Lower doses of AGHE (100 and 200 mg/kg), AGME (200 mg/kg) and luteolin (5 mg/kg) couldn't significantly reduce pathological parameters (P > 0.05) indicating a dose-related effect too [Table 2].
Table 2.
Effect of A. graveolens seeds hexane extract (AGHE), methanol extract (AGME), and luteolin on microscopic parameters of colitis induced by acetic acid in rats
| Groups/Dose (mg/kg) | Inflammation Severity (0-3) | Inflammation Extent (0-3) | Crypt Damage (0-4) | Total Colitis Index (0-10) |
|---|---|---|---|---|
| Sham | 0.0 (0-0)** | 0.0 (0-0)** | 0.0 (0-0)** | 0.0 (0-0)** |
| Control | 3.0 (2-3) | 3.0 (2-3) | 4.0 (3-4) | 9.5 (8-10) |
| AGHE100 | 2.0 (1-3) | 2.0 (1-3) | 3.0 (2-4) | 7.0 (4-10) |
| AGHE200 | 2.0 (0-3) | 2.0 (1-2) | 2.0 (2-4)* | 5.5 (3-9) |
| AGHE400 | 1.0 (1-2)** | 1.0 (0-2)** | 2.0 (1-2)** | 4.0 (2-6)** |
| AGME200 | 2.0 (0-3) | 2.0 (1-3) | 3.0 (1-4) | 7.5 (2-10) |
| AGME400 | 2.0 (1-2)* | 2.0 (1-2)** | 2.0 (1-3)* | 5.5 (4-7)** |
| AGME800 | 1.0 (0-2)** | 1.0 (0-2)** | 1.0 (1-2)** | 2.5 (2-5)** |
| Luteolin 5 | 2.0 (1-3) | 2.0 (1-3) | 3.0 (2-4) | 6.5 (5-9) |
| Luteolin 10 | 2.0 (1-2)* | 1.5 (1-2)** | 2.0 (1-2)* | 5.0 (3-6)** |
| Luteolin 20 | 1.0 (0-1)** | 1.0 (0-2)** | 1.0 (1-2)** | 2.5 (2-5)** |
| Prednisolone4 | 0.5 (0-1)*** | 0.5 (0-1)*** | 1.0 (0-2)*** | 2.0 (1-3)*** |
| Mesalazine100 | 1.0 (0-1)** | 0.5 (0-2)*** | 1.0 (0-1)** | 2.0 (1-4)*** |
Sham: normal rats received vehicle (2 mL/kg). Data are expressed as median (range) for scoring parameter, (n=6). *P<0.05, **P<0.01, ***P<0.001, indicate significant difference versus control group
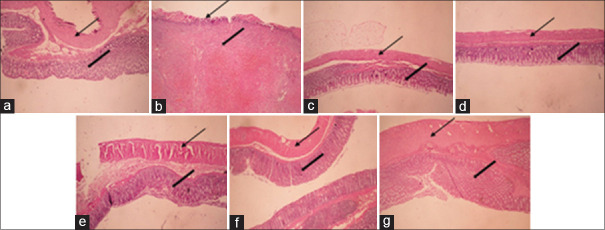
Figure 2.
Microscopic presentation of acetic acid-induced colitis in rats. (a) Normal colon treated with vehicle (2 mL/kg). (b) Control colitis treated with vehicle (2 mL/kg). (c) Colitis treated with prednisolone (4 mg/kg). (d) Colitis treated with mesalazine (100 mg/kg). (e) Colitis treated with AGHE (400 mg/kg). (f) Colitis treated with AGME (800 mg/kg). (g) Colitis treated with luteolin (20 mg/kg). H and E staining with ×40 magnification
MPO activity measurement
Treatment with two higher doses of AGHE (200 and 400 mg/kg), AGME (400 and 800 mg/kg) and luteolin (10 and 20 mg/kg) could reduce MPO activity (at least P < 0.05) similar to prednisolone and mesalazine (P < 0.001), whereas the lowest dose of AGHE (100 mg/kg), AGME (200 mg/kg) and luteolin (5 mg/kg) had no significant effect (P > 0.05) [Figure 3].
Figure 3.
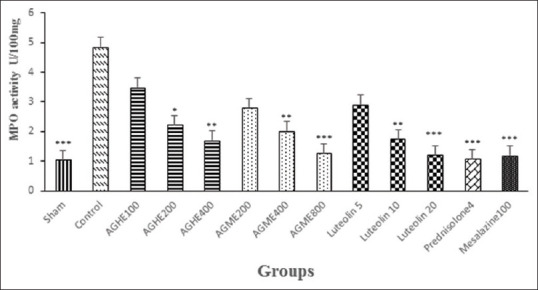
Myeloperoxidase (MPO) activity in colonic tissue of rats with colitis. Treatments were made with vehicle (2 mL/kg), GAHE (100, 200, and 400 mg/kg), GAME (200, 400, and 800 mg/kg) and Luteolin (5, 10, and 20 mg/kg). Prednisolone (4 mg/kg) and mesalazine (100 mg/kg) were used as reference agents. Data are expressed as mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 indicate significant difference versus control group
Discussion
Animal model of IBD is important for exploring disease mechanisms and evaluation of new therapeutic agents.[31] Acetic acid model of experimental colitis is a rapid, reproducible and cost-effective model with reasonable similarity to pathological and clinical features of human ulcerative colitis for screening of drugs with anti-colitis activity.[32]
In this study, prednisolone and mesalazine, which possess a different mechanism of actions, were used as reference drugs to clarify the efficacy of A. graveolens seeds extracts (AGHE and AGME) and their likely mechanism of action. Luteolin, on the other hand, is one of the main flavonoid glycosides in A. graveolens that has anti-inflammatory effects and its anti-colitis activity has not been evaluated yet.[9,13] So, in this study, the effect of AGHE and AGME on acetic acid-induced colitis was examined and compared with one of its active constituents, luteolin.
The results revealed that AGHE and AGME as well as luteolin were effective to reduce all macroscopic parameters of colitis and even weight of colons when larger doses of drugs were applied. Similar findings were obtained with MPO activity and pathological features like total colitis index in comparison with the untreated control group. This is in agreement with this idea that likely a dose-dependent activity is engaged. Albeit the results of AGME and luteolin were somewhat better than AGHE especially when greater doses are applied, but it is evident that by considering the dose, the most potent agent examined was luteolin which is an active and important ingredient of celery seeds.[14] Moreover, by referring to flavonoid contents of AGHE and AGME, it is concluded that luteolin had just a partial role in anti-colitis activity of both extracts and other ingredients rendered significant cooperation too. Luteolin and most of other flavonoids can reduce reactive oxygen species while result in increased superoxide activity, which in turn causes protection against oxidative damages.[33] We know that increased tumor necrosis factor Alfa (TNF-α) and interleukin-1β (IL-1 β) are evident and indicative for IBD disease condition.[34] It has been reported that luteolin inhibited both the synthesis and secretion of inflammatory cytokines such as TNF- α and IL-1 β from human mast cells (HMC-1) in a dose-dependent manner.[13] More activities might explain the beneficial effects of luteolin in current study could be suppression of histamine release, vascular permeability and edema, leukocyte infiltration and strengthening of mucosal layer and cyto-protection which have proven previously.[5,14,35] Phytochemical analysis suggested that the anti-inflammatory and anti-nociceptive effects of the hexane extract of celery (AGHE) might be due to their content of phthalids and coumarins.[4] There are many reports that polyphenols such as coumarins and phthalids may act like non-steroidal-anti-inflammatory drugs (NSAIDs) in acute inflammatory models.[3] Caffeic acid, p-coumaric acid, ferulic acid, apigenin, luteolin, tannin, saponin, and kaempferol are likely among other principal agents that might have an involvement in anti-colitis effect of celery seeds extracts in the recent study.[4]
It is notable that the 5-day treatment applied in the current study made an opportunity for delayed protective mechanisms such as scavenging of oxido-radicals and repairing mucosal layers to be appeared.[36] Since the examined drugs were administered orally, it is concluded that active ingredients of both extracts and luteolin had enough bioavailability in GI tract and/or could reach in a significant amount to distal colon to exert their beneficial anti-inflammatory effects.[3] To verify this hypothesis, a non-oral route of administration could be suggested in future studies. Antioxidant activity of A. graveolens seeds has been demonstrated in several studies. According to Kooti and Daraei[4] study, among the various extracts were assessed, methanol extract of celery seeds had the highest antioxidant activity; however, in recent study, the hexane extract was superior to methanolic one likely because, the methanol extract was processed on hexane extract residue. Celery seeds extract contains phenolic acids and flavonoids for which anti-inflammatory effects have been documented.[37,38] It has revealed that celery seeds contain apiin and apigenin as major flavonoids, which are known for anti-inflammatory properties.[11,39] In addition, seeds of celery are rich in vitamins A and C. It is suggested that powerful antioxidant property of celery seeds is associated with compounds such as apigenin, apiin, and vitamins A and C.[9] Apiin showed a significant inhibitory activity on nitrite production in vitro.[39] Apigenin is an antioxidant and suppresses the generation of hydrogen peroxide and anti-immunoglobin E-induced histamine release while its anti-colitis activity has been recently demonstrated by Sadraei et al.[26] We know that flavonoids inhibit cyclooxygenase-2 (COX-2) activity which in turn diminishes synthesis of prostaglandin E2 and plays a key role in inflammation and its associated diseases suppression.[38,40]
Conclusion
In conclusion, our results suggest that A. graveolens seeds extracts (AGME and AGME) are effective in IBD murine model and luteolin as one of its important components has a minor but significant role in this activity. More detailed studies including toxicological and clinical trials are required to distinguish the exact mechanism of A. graveolens seeds action and its suitability in IBD therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Syed-Sufiyan F, Rajeev KS. Review on the pharmacognostical and pharmacological characterization of Apium graveolens Linn. Indo Glob J Pharm Sci. 2012;2:36–42. [Google Scholar]
- 2.Kooti W, Ali-Akbari S, Asadi-Samani M, Ghadery H, Ashtary-Larky D. A review on medicinal plant of Apium graveolens. Adv Herb Med. 2014;1:48–59. [Google Scholar]
- 3.Ramezani M, Nasri S, Yassa N. Antinociceptive and anti-inflammatory effects of isolated fractions from Apium graveolens seeds in mice. Pharm Biol. 2009;47:740–3. [Google Scholar]
- 4.Kooti W, Daraei N. A review of the antioxidant activity of celery (Apium graveolens L) J Evid Bas Compl Alt Med. 2017;1:1–6. doi: 10.1177/2156587217717415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117–24. doi: 10.1016/s0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 6.Brankovic S, Gocmanac-Ignjatovic M, Kostic M, Veljkovic M, Miladinovic B, Milutinovic M, et al. Spasmolytic activity of the aqueous and ethanol celery leaves (Apium graveolens) extracts on the contraction of isolated rat ileum. Acta Med Medianae. 2015;54:11–6. [Google Scholar]
- 7.Al-Howiriny T, Alsheikh A, Alqasoumi S, Al-Yahya M, ElTahir K, Rafatullah S. Gastric antiulcer, antisecretory and cytoprotective properties of celery (Apium graveolens) in rats. Pharm Biol. 2010;48:786–93. doi: 10.3109/13880200903280026. [DOI] [PubMed] [Google Scholar]
- 8.Zargari A. 5th ed. Tehran: Tehran University Press; 1990. Medicinal Plants; pp. 476–82. [Google Scholar]
- 9.Evans WC. 15th ed. London: Bailliere Tindall; 1996. Trease and Evans Pharmacognosy; pp. 301–7. [Google Scholar]
- 10.Zhou K, Zhao F, Liu Z, Zhuang Y, Chen L, Qiu F. Triterpenoids and flavonoids from celery (Apium graveolens) J Nat Prod. 2009;72:1563–7. doi: 10.1021/np900117v. [DOI] [PubMed] [Google Scholar]
- 11.Lin LZ, Lu S, Harnly JM. Detection and quantification of glycosylated flavonoid malonates in celery, Chinese celery, and celery seed by LC-DAD-ESI/MS. J Agric Food Chem. 2007;55:1321–6. doi: 10.1021/jf0624796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar Sh, Pandey AK. Chemistry and biological activities of flavonoids: An overview. Sci World J. 2013;55:1321–6. doi: 10.1155/2013/162750. doi: 10.1155/2013/162750. Lin LZ, Lu S, Harnly JM. Detection and quantification of glycosylated flavonoid malonates in celery, Chinese celery, and celery seed by LC-DAD-ESI/MS. J Agric Food Chem 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabavi SF, Braidy N, Gortzi O, Sobarzo-Sanchez E, Daglia M, Skalicka-Wozniak K, et al. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res Bull. 2015;119:1–11. doi: 10.1016/j.brainresbull.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Jeon I, Soo Kim H, Ju Kang H, Seo Lee H, Jeong S, Soo Kim J. Anti-Inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) Leaves. Molecules. 2014;19:6941–51. doi: 10.3390/molecules19066941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 16.Sellin JH, Pasricha PJ. Pharmacotherapy of inflammatory bowel disease. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw Hill Companies; 2006. pp. 1037–56. [Google Scholar]
- 17.Sartor RB. Pathogenesis and immune mechanism of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5–11. [PubMed] [Google Scholar]
- 18.Robert W. Novel and future medical management of inflammatory bowel disease. Surg Clin North Am. 2007;87:727–47. doi: 10.1016/j.suc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 19.McQuaid KR. Drugs used in the treatment of gastrointestinal disease. Basic and Clinical Pharmacology. In: Katzung BG, editor. 10th ed. New York: McGraw Hill Companies; 2007. pp. 1009–40. [Google Scholar]
- 20.Minaiyan M, Zolfaghari B, Kamal A. Effect of hydroalcoholic and buthanolic extract of Cucumis sativus seeds on blood glucose level of normal and streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2011;14:436–42. [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra S, Khan Sh, Avula B, Lata H, Yang MH, ElSohly MA, et al. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid Based Complement Alternat Med 2014. 2014:253875. doi: 10.1155/2014/253875. doi: 10.1155/2014/253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinova D, Ribarova F, Atanassova M. Total phenolic and total flavonoids in Bulgarian fruits and vegetables. J Univ Cheml Technol Metal. 2005;40:255–60. [Google Scholar]
- 23.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary color metric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 24.Mascolo N, Izzo A, Autore G, Maiello F, Dicarlo G, Capsso F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J Pharmacol Exp Ther. 1995;272:469–75. [PubMed] [Google Scholar]
- 25.Mahdavi NS, Talebi A, Minaiyan M. Ameliorative effect of galantamine on acetic acid-induced colitis in rats. Res Pharm Sci. 2019;14:391–9. doi: 10.4103/1735-5362.268199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadraei H, Asghari G, Khanabadi M, Minaiyan M. Anti-inflammatory effect of apigenin and hydroalcoholic extract of Dracocephalum kotschyi on acetic acid-induced colitis in rats. Res Pharm Sci. 2017;12:322–9. doi: 10.4103/1735-5362.212050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper HS, Murthy S, Shah R, Sedergan D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis.Laboratory investigation. J Technic Meth Pathol. 1993;69:238–49. [PubMed] [Google Scholar]
- 28.Dieleman L, Palmen M, Akol H, Bloemena E, Pena A, Meuwissen S. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immuno. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motavallian-Naeini A, Minaiyan M, Rabbani M, Mahzuni P. Anti-inflammatory effect of ondansetron through 5-HT3 receptors on TNBS-induced colitis in rat. EXCLI J. 2012;11:30–44. [PMC free article] [PubMed] [Google Scholar]
- 30.Zabihi M, Hajhashemi V, Talebi A, Minaiyan M. Evaluation of central and peripheral effects of doxepin on acetic acid-induced colitis in rat and the involved mechanisms. EXCLI J. 2017;16:414–25. doi: 10.17179/excli2016-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cominelli F, Arseneau KO, Rodriguez-Palacios A, Pizarro T. Uncovering pathogenic mechanisms of inflammatory bowel disease using mouse models of Crohn's disease–like ileitis: What is the right model? Cell Mol Gastroenterol Hepatol. 2017;4:19–32. doi: 10.1016/j.jcmgh.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minaiyan M, Hajhashemi V, Rabbani M, Fattahian E, Mahzouni P. Beneficial effects of maprotiline in a murine model of colitis in normal and reserpinised depressed rats. Int Sch Res Notices. 2014 doi: 10.1155/2014/359841. doi: 10.1155/2014/359841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanmugapriya R, Ushadevi T. In vitro antibacterial and antioxidant activities of Apium graveolens L.seed extracts. Int J Drug Dev Res. 2014;6:165–70. [Google Scholar]
- 34.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325:928–37. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 35.Ziyan L, Yongmei Z, Nan Z, Ning T, Baolin L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 2007;73:221–6. doi: 10.1055/s-2007-967122. [DOI] [PubMed] [Google Scholar]
- 36.Minaiyan M, Sajjadi SE, Naderi N, Taheri D. Anti-Inflammatory effect of Kelussia odoratissima Mozaff.hydroalcoholic extract on acetic acid- induced acute colitis in rats. J Reports Pharm Sci. 2014;3:28–35. [Google Scholar]
- 37.Silvan AM, Abad MJ, Bermejo P, Villar A. Effect of compounds extracted from Santolina oblongifolia on TBX (2) release in human platelets. Inflammopharmacology. 1998;6:255–63. doi: 10.1007/s10787-998-0024-2. [DOI] [PubMed] [Google Scholar]
- 38.O'Leary KA, Pascual-Treasa SD, Needs PW, Bao YP, O'Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutation. 2004;551:245–54. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Mencherini T, Cau A, Bianco G, Della Loggia R, Aquino RP, Autore G. An extract of Apium graveolens var.dulce leaves: Structure of the major constituent, apiin, and its anti- inflammatory properties. J Pharm Pharmacol. 2007;59:891–7. doi: 10.1211/jpp.59.6.0016. [DOI] [PubMed] [Google Scholar]
- 40.Sultana S, Ahmed S, Jahangir T, Sharma S. Inhibitory effect of celery seeds extract on chemically induced hepatocarcinogenesis: Modulation of cell proliferation, metabolism and altered hepatic foci development. Cancer Lett. 2005;221:11–20. doi: 10.1016/j.canlet.2004.07.030. [DOI] [PubMed] [Google Scholar]