Abstract
In some types of human cancer, aldehyde dehydrogenases represent stemness markers and their expression is associated with advanced disease stages and poor prognosis. Although several biological functions are mediated by their product Retinoid acid, the molecular mechanism is tissue-dependent and only partially understood.
In this review, we summarize the current knowledge about the role of ALDH in solid tumours, especially ALDH1A1 and ALDH1A3 isoforms, regarding the molecular mechanism of their transcription and regulation, and their crosstalk with main molecular pathways resulting in the excessive proliferation, chemoresistance, stem cells properties and invasiveness. The recent knowledge of the regulatory effect of lnRNA on ALDH1A1 and ALDH1A3 is discussed too.
Keywords: Aldehyde dehydrogenase, Molecular regulation, Stem cells marker, Transcriptional control, Retinoic acid
Abbreviations: ALDH1A13, aldehyde dehydrogenase 1 isoforms A1, A3; AR, androgen receptor; atRA, all trans retinoic acid; BAX, Bcl-2-like protein 4, apoptosis regulator; Bcl-2, B-cell lymphoma 2, apoptosis regulator; BET, bromodomain and extraterminal protein family; BRD4, bromodomain-containing protein 4; C/EBPβ, CCAAT/enhancer-binding protein β; CD31, Platelet endothelial cell adhesion molecule 1; CD24, signal transducer 24, sialoglycoprotein; CD44, homing cell adhesion molecule; CDK2, Cyclin- dependent kinase 2; CHOP, C/EBP homologous protein; cMET (HGFR), Hepatocyte growth factor receptor; CSC, cancer stem cell; DANCR (KIAA0114), Differentiation antagonizing non-protein coding RNA; DDIT3, DNA damage inducible transcript 3; DHT, Dihydrotestosterone; EMT, epithelial-mesenchymal transition; ER, Estrogen receptor; ERK, Extracellular signal-regulated kinase; EZH2, Enhancer of zeste homolog 2; GADD153, Growth arrest and DNA damage 153; HGF, Hepatocyte growth factor; HNSCC, Head and neck squamous Cancer; HOX, Homeobox protein; HPCs, hematopoietic progenitors; HSP27, heat shock protein 27; IL-6, Interleukin 6; MDR, multidrug resistance; MEK, Mitogen-activated protein kinase; MMP, matrix metalloproteinase; mTOR, Mammalian target of rapamycin; MUC1-C, Mucin 1 glycoprotein, subunit C; NANOG, homeobox protein, transcriptional factor; NFκB, Nuclear factor kappa-light-chain-enhancer of activated B cells; NQO1, NAD(P)H dehydrogenase [quinone] 1; NRAD1 (LINC00284), Non-coding RNA in the aldehyde dehydrogenase 1A pathway; NRF2, Nuclear respiratory factor 1,; NSPc1, Polycomb 1 of the nervous system; PCAF, acetyltransferase P300/CBP-associated factor; PI3K, Phosphatidylinositol 3-kinase; PPARβ/δ, Peroxisome-proliferator-activated receptor β/δ; PTEN, Phosphatase and tensin homolog; RA, Retinoic acid; RAR, Retinoic acid receptor; RARE, RA responsive element; RARRES1, Retinoic acid receptor responder 1; RD16, retinol dehydrogenase 16; ROS, reactive oxygen species; RXR, Retinoid X receptor; S1P, Sphingosine-1-phosphate; SIRT2, Sirtuin 2; SMAD4, Mothers against decapentaplegic homolog 4; SNAI1, (Snail) zinc finger protein 1; SNAI2, (Slug) zinc finger protein 2; SOX2, SRY (sex determining region Y)-box 2; SRC, proto-oncogene tyrosine-protein kinase; STAT3, Signal transducer and activator of transcription 3; TCF4 (TCF7L2), Transcription factor 7-like 2; TGF- β, Transforming growth factor beta; TNBC, triple negative breast cancer; TLX-1, T-cell leukemia homeobox protein 1
Highlights
-
•
Aldehyde dehydrogenases are important stem cell markers in many human cancer types.
-
•
ALDH1A1 or ALDH1A3 activation participates in tumour progression, chemoresistance, stem-cell properties and invasiveness.
-
•
ALDH1A1 interacts with oncogenic pathways Notch, NRF, CXCR4, Polycomb, MDR, and HOX.
1. Characterization of ALDH
In human cells, 19 subtypes of aldehyde dehydrogenase (ALDH) - encoded genes have been identified on different chromosomes, with alternative splicing providing even more translational variability. ALDH proteins are divided into eleven protein families and four subfamilies that are localized in different cell compartments: in the cytoplasm, nucleus or mitochondria [1]. In addition to their enzymatic activity, they are necessary during embryogenesis and fetal development [2].
The ALDH family is characterized by engagement in a wide-range spectrum of biological processes essential for cell survival along with cell protection. It has a crucial role in the conversion of vitamin A to its active metabolite, retinoic acid (RA). ALDHs are also upregulated in mammals in response to both oxidative stress and lipid peroxidation [3]. They detoxicate potentially hazardous aldehydes [4], [5]. They have antioxidant and osmoregulatory functions and they are also engaged in drug metabolism and cell differentiation [4].
2. ALDH expression in neoplasia
Large study covering RNA sequencing data from The Cancer Genome Atlas (TCGA) and the analysis of expression of 19 ALDH isoforms linked this data with the patient's prognosis in the most frequent cancer types. Each ALDH isoform has a specific differential expression pattern, most of them have individual functional roles in cancer prognosis [6]. Higher ALDH activity was discovered in aggressive CSC cells and linked to their stemness properties. Moreover, ALDHs expression is specific for each tumour type, depending on organ and tissue [7]. Multiple ALDH isoforms can contribute to the ALDH activity detected by ALDEFLUOR™ method; ALDH1A1, 1A2, 1A3, 2, 7A1, 1B1, 3A1 and 8 [8].
ALDH1A1 and ALDH1A3 are largely studied in relation to CSCs [9] and we focus on these isoforms in this review. ALDH1A1 is localized on chromosome 9q21.13, with a length of 59,383 bp. The protein forms a homotetramer and is widely distributed in human adult brain, eyes, lungs, testes, liver and kidney. Increased ALDH1A1 expression mostly correlates with worse prognosis in patients and increased in vitro proliferation, colony/sphere formation, resistance and/or motility and in vivo tumorigenicity [10]. Human ALDH1A3 is located on chromosome 15 (15q26.3), with the length of 43,823 bp, and is involved in amino acids metabolism pathways [11]. ALDH1A3 expression is tissue- and cancer- specific and is usually associated with increased colony/sphere formation, tumorigenesis and angiogenic activity [11]. Both ALDH1A1 and 1A3 are required for the biosynthesis of retinoic acid and have an important role in the embryonic development of the eye & nose. Preferred substrate for both enzymes is retinal. ALDH1A3 has high activity with an all-trans retinal, and much lower in vitro activity with acetaldehyde. ALDH1A1 oxidizes a wider spectrum of aldehydes in vivo, but prefers 9-cis retinal [11]. Genetic homology of both proteins is 71% according to NCBI BLAST.
3. Role of ALDH in tumour progression, chemoresistance and stem-cell properties
3.1. ALDH can detoxify antineoplastic drugs
Oxidative stress damages DNA and proteins as long as it triggers lipid peroxidation of cellular phospholipids yielding over 200 species of reactive aldehydes. By metabolizing a wide range of aldehydes, ALDH can attenuate oxidative stress (Fig. 1A). Downregulation of ALDH activity has been shown to accumulate ROS in CSCs, leading to DNA damage and apoptosis [3]. Elevated levels of ALDH enzyme in many neoplastic cell lines as well as in patients led to the development of resistance to both chemotherapy and radiotherapy [12].
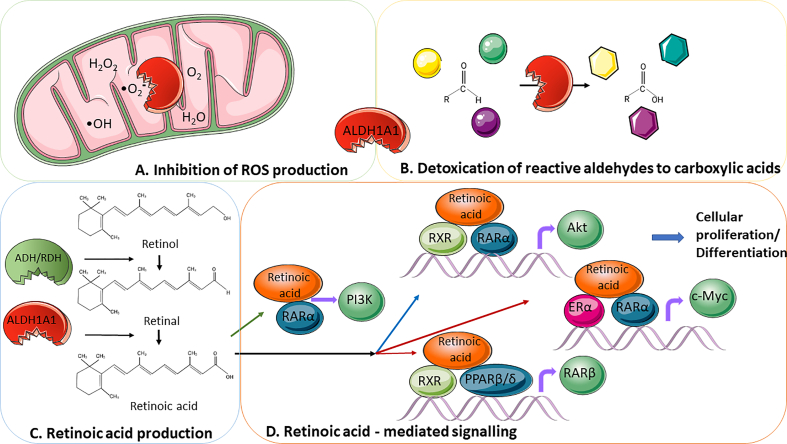
Fig. 1.
Biological processes affected by ALDH1A1 or ALDH1A3 function.
A. Inhibition of ROS production. Chemotherapeutics and radiation produce elevated levels of oxidative stress in cancer cells as part of their treatment effect; the “ROS scavenging” activity of ALDH could protect cancer cells against these therapeutic approaches by maintaining ROS at low levels. Attenuation of ALDH activity has been shown to accumulate ROS in CSCs, leading to DNA damage and apoptosis [3].
B. Detoxification of reactive aldehydes to carboxylic acids is a natural trait of both enzymes. Oxidative stress damages DNA and proteins as long as it triggers lipid peroxidation of cellular phospholipids yielding over 200 species of reactive aldehydes. ALDH1A1 and ALDH1A3 detoxify them and protect the cell, leading to the detoxification of commonly used anticancer treatment regimens [3].
C. Retinoid acid production. Alcohol dehydrogenases control the reversible conversion of retinol to retinal. Retinal is subsequently metabolized irreversibly to retinoic acid (RA) (all-trans, 9-cis, 13-cis) under control of ALDH1A1, ALDH1A2, ALDH1A3 and ALDH8A1 [4], [20]. RA acts as a signalling molecule to regulate gene transcription, leading to changes in cellular differentiation and proliferation [21].
D. Retinoid acid-mediated signalling. In the classic pathway (blue arrow) after entering the nucleus, RA is a ligand for heterodimer formed by nuclear retinoic acid receptor (RARα) and retinoid X receptor (RXRs), which anneals to DNA via a conserved DNA-binding domain at two RA responsive element sites (RARE and RXRE) [22]. These responsive elements are found in promoters of many retinoid-inducible genes, and transcriptional activation occurs upon binding the RAR/ RXR complex to them [23]. The RARs do not bind DNA alone, but RXR can bind DNA as a homodimer. In the absence of ligands, the RAR/ RXR complex still acts as a transcriptional repressor, thanks to bound corepressors, resulting in deacetylation of histones and thus repressing transcription of the target genes [24]. Conversely, after RA binding, conformational changes and subsequent binding to coactivators (SRC or histone acetylases) lead to histone acetylation and transcriptional activation [25]. In the non-classic pathway (red arrows), RA has multiple targets. It can bind to RXR and peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) heterodimers or RXR and ERα heterodimers and trigger the expression of its downstream target genes like AKT or c-MYC, respectively. RA can also bind with the RARα monomer (green arrow) outside of the nucleus and activate the PI3K/AKT pathway [9], [26]. The activation of the AKT, c-MYC or PI3K/AKT pathway leads to marked changes in cellular differentiation and proliferation, tumorigenesis, stemness and resistance to therapy [27].
Abbreviations: ER – estrogen receptor, PPARβ/δ - peroxisome-proliferator-activated receptor β/δ, RAR - retinoic acid receptor, RXR - retinoid X receptor, ROS - reactive oxygen species. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
ALDH1A1 and ALDH1A3 are most likely to provide drug and radiation resistance. ALDH1A1 upregulation was reported as the reason for chemoresistance to cyclophosphamide [13] and doxorubicine and paclitaxel [12] in breast cancer, to paclitaxel in lung cancer [14] and to cisplatin in cervical cancer [15]. Other examples of ALDH connection to resistance to temozolomide, irinotecan, paclitaxel, doxorubicin and epirubicin have been reviewed in [16]. A cross-resistance to 5-fluorouracil, oxaliplatin, cisplatin and irinotecan acquired by long-term passages in increasing 5-FU concentrations in colorectal cancer cells HT29 was associated with ALDH1A1 switch to ALDH1A3 isoform [17]. siRNA inhibition of ALDH1A1 isoforms sensitizes HT-29 cells to capecitabine and 5-fluorouracil [18]. Downregulation of ALDH1A1 by si-RNA increased the lung cancer cells sensitivity to cyclophosphamide [19].
3.2. ALDH participates in the regulation of tumour initiation and progression through the production of RA
ALDH plays an important role in the tumour progression via production of retinoic acid (RA) (all-trans, 9-cis, 13-cis) from retinal under control of ALDH1A1, ALDH1A2, ALDH1A3 or ALDH8A1 and this process is irreversible [4], [20] (Fig. 1C). One of the most important activities of intracellular retinoids is the ability to regulate gene transcription resulting in the changes of cellular differentiation and proliferation [21]. RA acts as a signalling molecule involved in two pathways, classic and non-classic, described in Fig. 1D.
Thus, RA enhances the expression of genes with RAREs in their promoters. The human genome contains over 14,000 RAREs; most of which are located in intragenic regions [28]. Initial studies concentrated on direct induction of genes containing RARE sequences; however, RA-mediated gene expression is more complex and regulated by other cellular processes, including the interaction of co-repressors and co-activators. Differential RA-regulated gene expression is cell-type specific and responsible for the diverse cellular effects induced by RA in different tissues [29].
Study of Marcato group in 2017 suggested a novel mechanism of RARE-independent RA-mediated gene regulation. They indicated that PPARβ/δ-directed transcription is not a major regulator of the pro- or anti-tumour effects of atRA. Instead, they claimed atRA-induced gene expression was dictated by expression of additional atRA-inducible transcription factors, specifically interferon regulatory factor 1 [30].
3.3. ALDH has functional role in stem cells
ALDH itself plays an important role in retinoid metabolism and in the removal of toxic aldehydes and ROS from CSCs, thereby affecting their essential properties, like stemness and cellular differentiation. RA influences the expression of CSCs markers such as Nestin and SOX2, which are important for the maintenance of stem cell properties. The atRA treatment of CSCs reduces the levels of some markers of these cells, including SOX2 and ABC transporters [31], [32]. Furthermore, retinoids are involved in many regulatory and signalling pathways in CSCs like Notch, Wingless (Wnt)/β-catenin or Sonic Hedgehog (in 4, 6) and affect the expression of Hox genes responsible for differentiation and self-renewal processes.
Due to its high concentration in CSCs, ALDH is an established marker used for CSC identification and purification [33]. However, the expression of certain ALDH isoforms can be used as a CSC marker only in tissue types that do not normally express high levels of ALDH. As an example, ALDH1A1 should be used as a CSC marker in breast, lung, colon and stomach epithelium (none or low level in their normal tissue); however not in liver and pancreas [20].
3.4. ALDH has impact on migratory ability, clonogenicity and metastatic potential
Migratory and invasive abilities are considered as the main in vitro characteristics of metastatic cells, whereas the capacity to form tumour spheres in vitro reflects tumorigenic capability in vivo. Tumour cells with high ALDEFLUOR™ activity displayed increased motility and invasive capability through a 3D basement membrane in breast and ovarian cancer, osteosarcoma, esophageal and prostate cancer. Moreover, ALDH+ cells originated from breast, ovarian, prostate, colon, esophageal and cervical cancer, brain tumours, head and neck squamous cell carcinoma and non-squamous cell lung cancer showed higher capacity to form tumour spheres (reviewed in [34]). These observations support the model of enhanced metastatic capacity of ALDH+ cells.
A number of pathways that are known to regulate CSCs, including Notch, Wnt, transforming growth factor-β (TGF-β), and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), are interconnected to ALDH1A1 or ALDH1A3 respectively, and also are capable of inducing epithelial-mesenchymal transition (EMT) [34]. The molecular interactions of these pathways with ALDH1A1 and ALDH1A3 will be discussed in next sections.
However, there is some controversial data about the link between ALDH and metastatic ability. For example, Croker and colleagues in 2009 reported that ALDHhighCD24-CD44+ cells were the only cancer cell subpopulation able to form metastases beyond the lungs in a pattern that mirrors the clinical behaviour of breast cancer [35]. Liu et al. in 2014 compared expression of EMT- and mesenchymal-epithelial transition (MET)-associated genes in CD24-CD44+ and ALDH+ tumour cells isolated from primary human breast cancers. While CD24-CD44+ cells exhibited EMT-associated genes, genes associated with the alternative epithelial-like state, such as cadherin, occludin, claudins and desmoplakin, were elevated in the ALDH+ population. Next, they examined the transition of breast CSCs between mesenchymal and epithelial states and found that ALDH participates during the epithelial and more proliferative phase of metastatic colonization in distant tissues. Interestingly, this suggests that ALDH may not be a key marker expressed during the intravascular and more mesenchymal phase of the metastatic cascade, but rather a marker and potential mechanistic promoter of a more proliferative/epithelial CSC phenotype via MET [36].
Also, Marcato et al. have reported that ALDH1A3 may have a dual role in breast cancer metastasis promotion. Expression of ALDH1A3 and RARRES1 (but not ALDH1A1) correlated with more aggressive triple-negative breast cancers. Patient tumours with high ALDH1A3 levels had significantly higher levels of RARRES1 and RARβ, while tumours with high ALDH1A1 showed higher levels of RARβ and CYP26A. Overexpression of ALDH1A3 (but not ALDH1A1) induced significant expression of RARE bearing genes RARβ and RARRES1. The effects of RA and ALDH1A3 were opposing in the breast cancer cell lines: tumour-promoting in MDA-MB-231 and MDA-MB-435 cells, but tumour-suppressive in MDA-MB-468 cells. It seems like ALDH1A3 induces RA signalling, but it does not determine the downstream effect of that RA signalling (promoting in MDA-MB-231 and MDA-MB-435 cells, and inhibiting in MDA-MB-468 cells). Downstream factors can determine whether ALDH1A3/RA promotes tumour growth or suppresses it. The DNA methylation inhibition experiments indicate that differential methylation contributes to divergent gene expression induced by ALDH1A3/RA in MDA-MB-468 and MDA-MB231 cells. Other transcription regulatory mechanisms (e.g. histone modifications) could contribute to differential expression of ALDH1A3/RA inducible genes in breast cancer [37].
4. Regulation of ALDH1A1 and ALDH1A3 expression
4.1. Transcriptional regulation of ALDH1A1 isoform
Functional analysis of ALDH1A1 promoter region revealed positive regulatory region (−91 to −53 bp) with a CCAAT box as the major cis-acting element that mediates basal ALDH1 promoter activity in human hepatome Hep3B cells expressing ALDH1 but not in erythroleukemic K562 cells or in fibroblast LTK-cells, which do not express ALDH1. The results suggested that cell type-specific factors regulate ALDH1 gene expression [38]. Next, Elizondo et al., in 2000 showed that RAR transactivates the ALDH1A1 promoter by binding to a RA response-like element (RARE) located at positions −91 to −75 bp. In addition, C/EBPβ has been shown to transactivate the ALDH1A1 promoter by recognizing and binding to the CCAAT region residing at 75 to 71 bp adjacent to the RARE [39].
To this day, several different transcriptional activation of ALDH1A1 have been identified (Fig. 2). It was proven that the Wnt pathway regulates ALDH1A1 through β-catenin and T-cell factor (TCF)-dependent transcription in breast cancer. Analysis of the ALDH1A1 promoter region displayed two main β-catenin/TCF binding sites 5′-A/T A/T CAAAG-3′ [41]. β-catenin can be triggered also by Wnt independent mechanism, downstream of AKT activity [42].
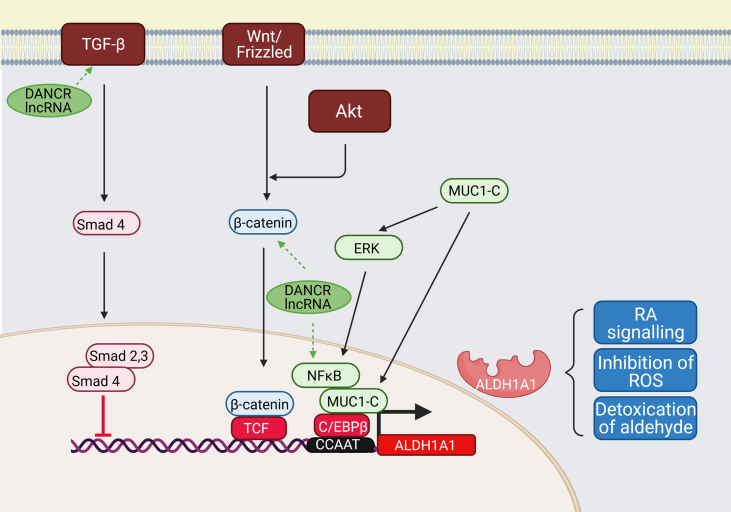
Fig. 2.
Transcriptional regulation of ALDH1A1.
ALDH1A1 promoter can be activated by several molecular pathways: 1) Wnt pathway regulating ALDH1A1 through β-catenin and T-cell factor (TCF)-dependent transcription; 2) Transforming growth factor β (TGF-β) - downregulating ALDH1A1 in Smad4-dependent manner; 3) Mucin 1-C (MUC1-C) inducing extracellular-signal-regulated kinase (ERK) signalling and phosphorylating CCAAT enhancer-binding protein (C/EBPβ), which leads to induction of ALDH1A1 expression.
TGF-β. Negative regulation of ALDH1A1 by TGF-β through Smad4 binding was shown in pancreatic adenocarcinoma cells. Suppression of Smad4 by specific si-RNA upregulated the expression of ALDH1A1, and overexpression of Smad4 downregulated it, leading to the decrease of the ALDH+ population and their tumour-initiating activity [40].
Wnt/ β- catenin. Suppression of β-catenin through specific siRNA resulted in a reduction of the ALDH+ cell population of prostate cancer progenitors. It sensitized it to radiation, proving that the Wnt pathway directly regulates ALDH1A1 through β-catenin/TCF dependent transcription [41]. Following study of ALDH+ cervical cancer cells revealed that ALDH expression could be triggered by β-catenin through a Wnt/Frizzled-independent mechanism, and this effect is downstream of AKT activity. Pharmacological inhibition of AKT reduced the active form of the β-catenin and inactive form of GSK3 in ALDH+ cells and reduced ALDH protein level and activity [42].
Mucin 1. In breast cancers, MUC1-C induced ERK-mediated phosphorylation and activation of the C/EBPβ transcription factor, forming the complex on the ALDH1A1 promoter and activating it. [43].
Long ncRNA. The effect of DANCR is described in Fig. 3.
Abbreviations: C/EBPβ - CCAAT/enhancer-binding protein β, ERK - extracellular signal-regulated kinase, GSK3 - glycogen synthase kinase-3, TCF - T-cell factor, TGF-β - Transforming growth factor β.
Transforming growth factor β (TGF-β) negatively downregulates ALDH1A1 in a Smad4-dependent manner. Overexpression of Smad4 inhibits it and reduces both the ALDHhigh population and the tumour-initiating activity of pancreatic cancer cells [40]. The inhibition of TGF-β signalling increased ALDH1high population in cholangiocarcinoma [44], breast cancer [45] and in uterine endometrioid cancer [46]. TGF-β signal stimulator Nodal had a tumour suppressor function in ALDHhigh cells via the activation of TGF- β pathway [46].
The oncogenic subunit of mucin 1 (MUC1-C), which is aberrantly overexpressed in many human breast cancers, induces ERK-mediated phosphorylation and activation of the C/EBPβ transcription factor in breast cancer cells. By forming a complex on the ALDH1A1 promoter, MUC1-C and C/EBPβ activate ALDH1A1 transcription [43].
In murine HPCs, TLX1/HOX11, an oncogenic transcription factor involved in human T-cell leukemia, transcriptionally regulates ALDH1A1 expression. ALDH1A1 gene was identified as a TLX1-responsive gene and transcriptional target, its overexpression perturbs murine haematopoiesis, favouring myeloid differentiation over lymphopoiesis [47].
4.2. Transcriptional regulation of ALDH1A3 isoform
ALDH1A3 expression level is tissue-specific. The abnormal expression of ALDH1A3 in tumours is regulated by several different signalling pathways, including signal transducer and activator of transcription 3 (STAT3), promoter methylation, KRAS oncogene, Androgen receptor, HGF/c-Met axis and by specific microRNAs [11] (summarized in Fig. 3).
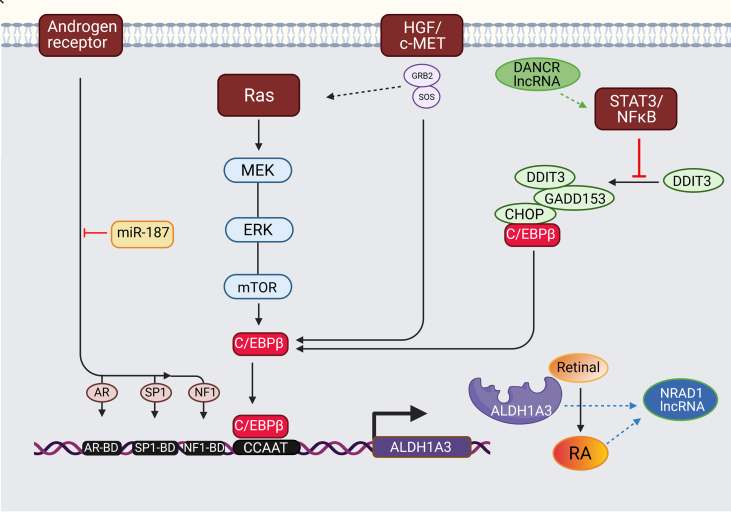
Fig. 3.
Transcriptional regulation of ALDH1A3.
The human ALDH1A3 gene promoter contains a CCAAT box (71 to 67 bp) activated by CCAAT/enhancer-binding protein beta (C/EBPβ). The binding of C/EBPβ to the promoter activates ALDH1A3 expression. ALDH1A3 impacts CSCs through the biosynthesis of retinoic acid (RA).
K-RAS. A key regulator of ALDH1A3 expression is the K-RAS oncogene. Signalling from K-RAS to C/EBPβ2 is likely through phosphorylation and subsequent activation of C/EBPβ2, allowing C/EBPβ2 to activate genes involved in proliferation and EMT transcriptionally. In a pancreatic cancer model using transgenic mice, mutant K-RAS upregulates ALDH1A3 expression via the MEK/ERK/mTOR pathways, a crucial regulator of cell proliferation, differentiation, and apoptosis [49].
STAT3. Aberrant activation of STAT3 and subsequent interactions with apoptosis-related or angiogenic factors can promote tumour progression. Activation of the STAT3 pathway significantly promoted ALDH1A3 expression [50]. Without the activity of STAT3-NFκB, C/EBPβ forms a complex with DDIT3/CHOP/GADD153. STAT3-NFκB activity represses the formation of the complex leading to C/EBPβ-dependent ALDH1A3 promoter activation [51]. The inhibition of STAT3-NFκB activity increases DDIT3 expression and DDIT3-C/EBPβ complex formation, which reduces the occupancy of the ALDH1A3 promoter by C/EBPβ and suppresses ALDH1A3 expression [9], [51].
Androgen Receptor (AR). ALDH1A3 expression is also directly regulated by AR signalling in androgen-dependent prostate cancer (PCa) cell line expressing ALDH1A3 only [52]. The UTR of the ALDH1A3 promoter contains potential DNA binding domains for AR and its transcription factors Sp1 and NF1 located upstream of the transcription initiation site. The addition of androgen DHT to PCa cells significantly increased the expression and the activity of ALDH1A3. At the same time, genetic and pharmacological inhibition of AR eliminated the ability of DHT to induce ALDH1A3 mRNA. DHT upregulated ALDH1A3 directly through the induction of AR transcriptional activity and not via indirect involvement of ERK and Src kinase pathways [52]. A consecutive study identified ALDH1A3 as a miR187 target in prostate cancer. ALDH1A3 expression was inversely correlated to miR-187 and significantly downregulated in PCa cell lines after transient transfection of miR-187 precursor [53].
HGF/c-MET. Genetic or pharmacological inhibition of c-MET in pancreatic cancer cells significantly decreased ALDH1A3 expression and activity. Activation of the HGF/c-MET pathway significantly promoted ALDH1A3 expression. However, the specific mechanism is unknown [54].
Long ncRNA. DANCR impacts ALDH1A3 by derepression of β-catenin, leading to an increase of the stemness traits of hepatocellular carcinoma [67] and elevation of activated TGF-β and IL-6, NFκB and STAT3 production in TNBC [68]. In the same cancer model, DANCR regulates the promoter binding of EZH2 and stimulates the expression of CSC markers, MDR and ALDH1 [64]. The expression of NRAD1 is regulated by ALDH1A3 and its product RA [62].
Abbreviations: AR - Androgen Receptor, C/EBPβ - CCAAT/enhancer binding protein β, CHOP - C/EBP homologous protein, DDIT3 - DNA damage inducible transcript 3, DHT - dihydrotestosterone, ERK - extracellular signal-regulated kinase, EZH2 - enhancer of zeste homolog 2, GADD153 - growth arrest and DNA damage 153, HGF - hepatocyte growth factor, MDR - multidrug resistance, MEK - mitogen-activated protein kinase, mTOR - mammalian target of rapamycin, RA - retinoic acid.
The human ALDH1A3 gene promoter contains a CCAAT box (71 to 67 bp) as well as ALDH1A1 promoter that could be similarly activated by C/EBPβ. C/EBPβ1 and C/EBPβ2 are both activators of transcription because they contain the N-terminal transactivation domain along with the C-terminal DNA binding/dimerization domain. C/EBPβ2 is a key regulator in the proliferation and plays a role in cell growth, survival and transformation. C/EBPβ1 is the isoform responsible for oncogene-induced senescence and is degraded by the proteasome during RAS transformation [48].
One of the key regulators of ALDH1A3 expression is K-RAS oncogene. The mutation and transformation of K-RAS is essential for the activation of transcription factor C/EBPβ and in transformed tumour cells leads to the anchorage independence, the ability to form colonies in soft agar and increased invasive potential [47]. ALDH1A3 transcription was associated with the mutated KRAS/MEK/mTOR signalling while ALDH1A1 was linked to PI3K/Akt /mTOR signature [11], [49], which are key regulatory cascades that control cell proliferation, differentiation and apoptosis.
The aberrant activation of STAT3 and subsequent interactions with apoptosis related or angiogenic factors can promote tumour progression. Transcriptional regulation of ALDH1A3 has showed the connection between ALDH1A3 and STAT3 (Fig. 3). Activation of the STAT3 pathway significantly promoted ALDH1A3 expression [50]. Without the activity of STAT3-NFκB, C/EBPβ forms a complex with DNA damage inducible transcript 3 (DDIT3), C/EBP homologous protein (CHOP) and growth arrest and DNA damage 153 (GADD153). STAT3-NFκB activity represses forming the complex leading to C/EBPβ-dependent ALDH1A3 promoter activation [51].
Study of Trasino et al. [51] showed that ALDH1A3 promoter contains potential AR binding sites and that ALDH1A3 expression is directly regulated by the Androgen receptor (AR) pathway in androgen-dependent prostate cancer cell. Addition of androgen dihydrotestosterone (DHT) upregulated ALDH1A3 directly through the induction of AR transcriptional activity and not by ERK and Src kinase pathway [52], [53].
The abnormal activation of the HGF/c-MET axis is associated with the progression of many tumours. Kim et al., showed a connection of HGF/c-MET signalling to the ALDH1A3 expression. Fibulin-3 inhibited c-MET activation and gene expression leading to negative regulation of cellular levels of ALDH1 isoforms, both ALDH1A1 and ALDH1A3. Inhibition of c-MET expression in AsPC-1 pancreatic cancer cells significantly decreased ALDH1A3 expression and its activity. However, the specific HGF/c-MET- dependent mechanism of ALDH1A3 regulation is unknown [54].
4.3. Regulation of ALDH1A1 by post-translational modification
The amount of ALDH produced in cells is regulated at several levels. Retinoids (most often atRA) affect the production of ALDH1A1 and ALDH1A3 through post-translational modifications at the protein level [19]. A549 lung cancer cells treatment with retinoids decreased both protein levels and enzyme activity of ALDH1 isoforms, but not the corresponding mRNAs. The accumulation of RA regulates ALDH levels through ubiquitin-proteasome system [19].
The most common regulatory mechanisms include acetylation or deacetylation of already finished proteins (Fig. 4A). ALDH1A1 is also subjected to an epigenetic regulation via BRD4 protein through regulation of its superenhancer [56] and the associated RNA in ovarian cancer cells treated with cisplatin in vitro and in vivo [57] (Fig. 4B).
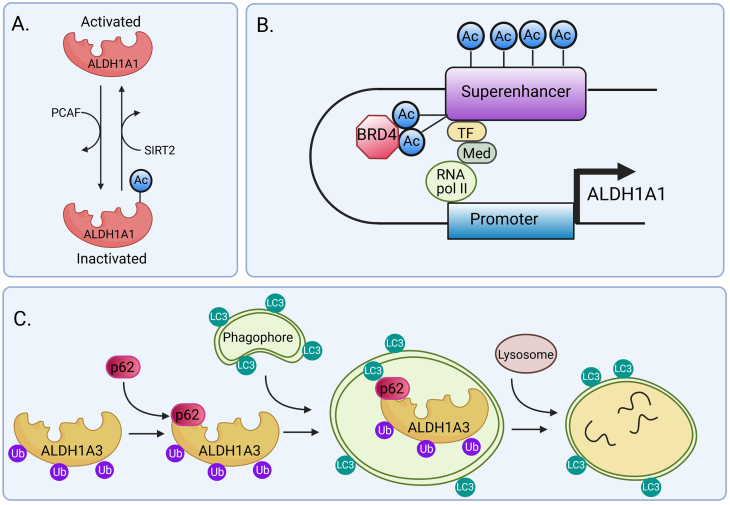
Fig. 4.
Epigenetic and post-transcriptional regulation of ALDH1A1 and ALDH1A3.
Regulation of ALDH1A1 activity by acetylation. ALDH1A1 activity is reduced by the acetylation of lysine 353, activated by acetyltransferase P300/CBP-associated factor (PCAF) and eliminated by deacetylase sirtuin 2 (SIRT2). ALDH1+ human breast cancer cells with low levels of ALDH1A1 acetylation display self-renewal characteristics. On the other hand, high levels of ALDH1A1 acetylation are associated with depletion of the stem cell population and decreased self-renewal [55].
Epigenetic regulation of ALDH1A1 via BRD4 protein. BRD4 belongs to the bromodomain and extraterminal family of proteins (BET). It recognizes acetylated lysine on histones through their bromodomains and controls transcription of the target gene, either directly or indirectly by enhancer elements. Genes hypersensitive to inhibition by BET proteins typically exhibit BRD4 occupancy at super-enhancer elements [56]. BRD4 binds to acetylated lysines (Ac) in super-enhancer and transcription factors (TF), bringing them together and mediating transcriptional co-activation and elongation via RNA polymerase II (RNA pol II) and mediator (Med). The enhancer and promoter interaction allows the formation of chromatin looping. Direct examination of chromatin looping between the super-enhancer and ALDH1A1 gene promoter in cells with or without treatment with the BET inhibitor revealed that the BET inhibitor abrogates the chromatin looping between the super-enhancer and the promoter of the ALDH1A1 gene. The data support BRD4 role in the transcriptional control of the ALDH1A1 gene through regulation of its super-enhancer and the associated RNA. The BET inhibitor suppresses the outgrowth of ovarian cancer cells treated with cisplatin in vitro and in vivo[9], [57].
Post-transcriptional regulation of ALDH1A3 by autophagy induced by high concentration-temozolomide treatment was reported in glioblastoma cell lines. Proximity ligation assay and co-immunoprecipitation showed direct interaction between ALDH1A3 and the autophagy adaptor protein p62. When autophagy is activated, LC3 protein is recruited to the autophagosome, and ALDH1A3 protein binds to autophagic substrate p62. ALDH1A3-p62 complex along with the attached ubiquitin cargo binds to the LC3 protein. Then, LC3 dissociates from the autolysosome while p62 and ALDH1A3 are degraded with ubiquitin [61].
Abbreviations: PCAF - acetyltransferase P300/CBP-associated factor, SIRT2 - sirtuin 2, BRD4 - Bromodomain-containing protein 4, Ac – acetylated lysine, BET - bromodomain and extraterminal protein family, TF - transcription factor, Med - mediator, LC3 - microtubule-associated protein 1A/1B-light chain 3, Ub - ubiquitin.
4.4. Regulation of ALDH1A3 by post-translation modification
Activity of ALDH1A3 promoter is regulated by methylation; it is hypermethylated in glioma, bladder cancer, lung cancer, colon cancer, breast cancer, stomach, bladder cancer, and prostate cancer. High levels of methylation of ALDH1A3 promoter results in a corresponding decrease in gene expression [58]. For example, 20% of glioblastoma (GBM) patients have a glioma CpG island methylation phenotype (G-CIMP1). The ALDH1A3 promoter is hypermethylated in 32.4% of G-CIMP negative GBM patients leading to a low level of ALDH1A3 protein production [59]. The DNA demethylation in AGS gastric cancer cells by methylation inhibitor 5-Aza-dC activated the ALDH1A3 gene, indicating that ALDH1A3 was silenced in AGS cells via promoter methylation [60].
Post-transcriptional regulation of ALDH1A3 by proteasomal degradation was reported in glioblastoma cell lines. ALDH1A3 was regulated by autophagy during high concentration- temozolomide treatment (described in Fig. 4C) [61].
5. Interaction of long non-coding RNA with ALDH1A1 and ALDH1A3
Recent evidence showed that two lncRNAs molecules – NRAD1 (previously LINC00284) and DANCR (differentiation antagonizing non-protein coding RNA, previously KIAA0114) were connected to ALDH1A1 or ALDH1A3 overexpression in CSC in breast cancer [62].
NRAD1 has been enriched in the ALDH+ CSC populations in breast cancer with worse patients' outcomes. Expression of NRAD1 is regulated by ALDH1A3 and its product retinoic acid (Fig. 3). It is localized in the nucleus; it regulates genes of differentiation and catabolic process. The pro-tumorigenic NRAD1 increases mammosphere formation and contributes to gene expression regulation by ALDH1A3 through chromatin interactions. Induction of NRAD1 is a novel mechanism through which ALDH1A3 regulates gene expression [63]. Si-NRAD1 or si–NFκB1 knockout led to the reduction in cell proliferation, migration, invasion, tube formation, angiogenesis, tumorigenic ability and promoted apoptosis in ovarian cancer by down-regulating MMP-2, MMP-9, Bcl-2, VEGF, and CD31 and up-regulating Bax [64].
Sha et al. [65] showed that DANCR was up-regulated in triple negative breast cancer (TNBC) patient tissues with worse TNM stages and overall survival. It contributes to tumour cell growth and metastasis by regulating the promoter binding of enhancer of zeste homolog 2 (EZH2) and stimulating the expressions of CSC markers, MDR and ALDH1 [65]. DANCR knockdown in breast cell lines reduced EMT, stemness, inflammation and production of stemness factors CD44, ABCG2, and ALDH1 [62]. In colorectal tissue samples and cell lines, DANCR knockdown significantly inhibits the proliferation, invasion and metastasis. DANCR promoted HSP27 expression and its mediation of proliferation/metastasis via miR-577 sponging [66] (Fig. 3).
Consistent with Sha et al. [65] and Brown et al.’ [62] findings, recent study showed that DANCR expression potently activated NFκB and STAT3 in TNBC. Their data suggest that elevation of TGF-β and IL-6 production is a mechanism by which DANCR promotes EMT and cancer stemness. Genetic attenuation of DANCR resulted in strong reduction of NFκB and STAT3 at protein level [68]. TGF-β and NFκB are an important part of pathways leading to transcriptional regulation of ADH1A1 (Fig. 2) and STAT3/NFκB also plays a role in transcriptional activation of ALDH1A3 (Fig. 3). It seems this might be one potential connection between DANCR and ALDH1.
Long ncRNA H19 belongs to lncRNA, which increases the intracellular ALDH activity in colorectal tumours. H19 activates Wnt- β-catenin pathway by sequestering miR-141, contributing to tumour development and chemoresistance in colorectal cancer tumours [69]. It promotes oncogenesis and drug resistance in colorectal, breast, and ovarian cancer cells. LncRNA H19 also interacts with SAHH to regulate the DNMT3B -dependent DNA methylation at different genetic loci [70].
6. Interaction of ALDH to main oncogenic pathways
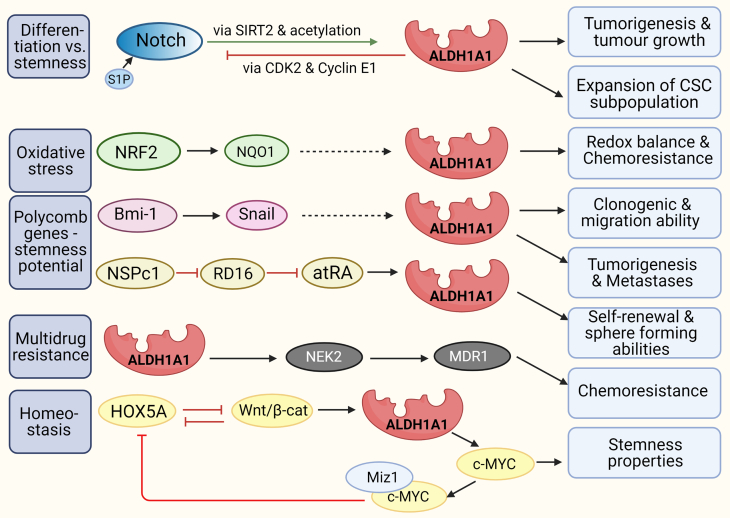
Functional studies demonstrated the correlation and association between several genes/ signalling pathways and ALDH1A1. Its interactions contribute to the excessive proliferation, tumorigenesis, chemoresistance, stemness, and invasiveness of cancer cells (Fig. 5).
Fig. 5.
Interactions of ALDH1A1 with other molecules.
Interaction with Notch. In the non-small lung cancer model, ALDH is regulated through the Notch pathway [74]. In breast cancer xenograft models, ALDH1A1 is controlled at a post-translational level by the Notch signalling pathway. Notch induces ALDH1A1 Lys-353 deacetylation and activation via SIRT2 induction and supporting ALDH1A1 to promote tumorigenesis and tumour growth [55]. Notch stimulation with sphingosine-1-phosphate (S1P) expands the breast cancer cells ALDH1+ subpopulation with CSC properties. Overexpression of sphingosine kinase 1 induces an increase in the levels of S1P and an expansion of the CSC subpopulation [72]. ALDH1A1 is involved in lung CSC activity via suppression of the Notch/Cyclin-dependent kinase 2 (CDK2)/CyclinE1 pathway. Upregulation of ALDH1A1 in lung cancer A549 cells significantly decreased NOTCH1, NOTCH2 and NOTCH3, CDK2, and CyclinE1 [75].
Interaction with NRF2. The levels of ALDH1A1, NRF2, and NQO1 (both controlling redox balance) are higher in ALDH+ HCT116 colorectal cells and doxorubicin-resistant ovarian cancer cells compared to ALDH-. The increase in ALDH1A1 and p62 was associated with NRF2 upregulation, indicating NRF2 signalling activation in ALDH+ cells [78].
Interaction with Polycomb genes Bmi-1 and NSPc1. Silencing of Bmi-1 expression in HNSCC- ALDH1+ cells resulted in the downregulation of Snail and ALDH1 expression. Bmi-1 modulates the tumorigenic traits of ALDH1+ cells by regulating Snail, but the mechanism is unknown [85] (dotted arrow). Polycomb 1 of the nervous system (NSPc1) in glioma CSCs causes the reduction of endogenous RA by inhibition of retinol dehydrogenase 16 (RD16). Suppression of NSPc1 expression reduced self-renewal and sphere-forming abilities in glioma CSCs and expression of Nestin and CD133 [32].
Interactions with MDR. RARα regulates MDR activity in human leukemic cells [86]. Retinol, retinyl acetate, and 13-cis-RA (but not atRA) reduce the activity of MDR1 by non-competitive inhibition and raising the membrane viscosity [87]. In the ovarian cancer model, the silencing of ALDH1A1 reduced the level of its downstream target NEK2. The downregulation of ABC transporters was detected due to ALDH1A1 or NEK2 silencing. They assume that ALDH1A1 can be involved in cisplatin resistance through the upregulation of NEK2 in ovarian cancer [88].
Interaction with HOX. There is reciprocal feedback between HOXA5 and Wnt signalling [90]. The Wnt pathway suppresses HOXA5 but activates ALDH1A1 transcription leading to c-MYC expression. C-MYC reacts with the Miz1 protein as a transcriptional repressor and prevents the transcription of HOXA5, thereby maintaining the properties of stem cells [91].
Abbreviations: atRA – all-trans retinoic acid, Bmi-1 - CDK2 – cyclin-dependent kinase 2, HNSCC - Head and Neck Squamous Cancer, HOX - Homeobox protein, MDR - multidrug resistance, MDR1, ABCG2, and MRP1 - receptors of MDR, drug transporters, Miz1 -Msx-interacting‑zinc finger, NRF2 - Nuclear respiratory factor 1, NQO1 - NAD(P)H dehydrogenase [quinone] 1, NSPc1 - Polycomb 1 of the nervous system, RD16 - retinol dehydrogenase 16, SIRT2 - sirtuin 2, S1P - sphingosine-1-phosphate.
6.1. Interaction with Notch
Notch pathway is a downstream target of retinoids, which induce cell differentiation and decrease proliferation. Constitutive activation of the Notch signalling pathway supported the differentiation of glioblastoma neurospheres in an RA-dependent process in a glioblastoma stem cell-like cellular model [70]. The data that atRA reduces the expression of some Notch receptors and ligands were reported in the breast cancer model [71], thereby regulating tumour cell invasiveness.
In breast cancer xenograft models, ALDH1A1 is regulated at a post-translational level by the NOTCH signalling pathway. Notch induces ALDH1A1 Lys-353 acetylation via induction of SIRT2 expression (Fig. 4A), promoting tumorigenesis and tumour growth. Replacement of endogenous ALDH1A1 with an acetylation mimetic mutant inhibited tumorigenesis and tumour growth. [55]. Notch stimulation with sphingosine-1-phosphate (S1P) expands the breast cancer cells ALDH1+ subpopulation with CSC properties. S1P binding to the S1P receptor 3 (S1PR3) stimulates ligand-independent NOTCH activation. Overexpression of sphingosine kinase 1 induces an increase in the levels of S1P and the CSC expansion [72]. Similar correlations between Notch signalling, ALDH activity, and CSCs have been observed in murine osteosarcoma [73].
In the non- small lung cancer model, ALDH is regulated through the Notch pathway since the chemical or genetic suppression of NOTCH3 results in decreased ALDH activity [74]. ALDH1A1 is involved in lung CSC activity via suppression of the Notch/Cyclin-dependent kinase 2 (CDK2)/CyclinE1 pathway. Upregulation of ALDH1A1 in lung cancer A549 cells significantly decreased expression of NOTCH1, NOTCH2 and NOTCH3, CDK2, and CyclinE1 and vice versa. ALDH1A1 expression maintained the stemness of the A549 cells [75].
6.2. Interaction with NRF2
NRF2 is involved in redox balance and, under oxidative stress, induces the transcription of cytoprotective genes that inhibit potentially carcinogenic reactive molecules [76]. On the other hand, high levels of NRF2 correlated with chemoresistance caused by enhanced induction of antioxidants, detoxifying enzymes, and drug efflux pumps [77].
In ALDH+ subpopulation isolated from colon cancer cell line HCT116, ALDH1A1, NRF2, and NQO1 expression were higher than in ALDH- cells. High levels of NRF2 and subsequent target genes were also detected in ALDH1A1+ subpopulation from doxorubicin-resistant ovarian cancer cells. The increase in ALDH1A1 and p62 was associated with NRF2 upregulation in the CSC-like ALDH+ cells [78]. Silencing of NRF2 in pancreatic cancer AsPC-1, COLO-357, and PANC-1 cells reduced the expression of ALDH1A1 and ALDH3A1 on mRNA and protein level and significantly enhanced the sensitivity of pancreatic cancer cells to 5-FU [79].
6.3. Interaction with CXCR4
CXCR4 is a chemokine receptor that belongs to seven transmembrane G protein-coupled cell surface receptors [80]. Its ligand, CXCL12, acts on CXCR4-expressing cells by inducing their migration by chemotaxis. Moreover, signalling of this ligand-receptor system results in multiple intracellular responses like cell survival, proliferation, and gene transcription [81]. CXCR4 transfers the signal via three potential pathways inducing ALDH activation: (i) PI3K/Akt/NFκB connected to ALDH1A1 transcription; (ii) Jak/STAT and (iii) SOS/Shc/Grb2 - Ras/Raf connected to ALDH1A3 transcription (in Chapter 4.1 and 4.2). All pathways result in the process of cell survival and proliferation [82].
Feng and colleagues explored the relationship between ALDH1A1, ALDH1A3, CXCR7, and CXCR4 on 58 human cancer cell lines. ALDH1A3 expression was negatively correlated with CXCR4 in analysed cell lines [83]. In three cell lines with attenuated ALDH1A3, colorectal HCT116 and SW480 cell lines together with A549 lung cells, they showed slower growth and less invasive capacity together with low CXCR4 expression. In contrast, CXCR4 expression did not change in SW620 and LOVO. The overexpression of CXCR4 could rescue cell growth suppression mediated by ALDH1A3 knockdown in SW480 and A549 cells but did not affect the growth of LOVO cells. These observations showed ALDH1A3 attenuation induced divergent CXCR4 regulation in different colon cancer cells. Treatment with atRA did not affect CXCR4 protein change, which suggested that other mechanisms rather than the RA pathway might be responsible for the regulation of CXCR4 caused by ALDH1A3 attenuation [83].
6.4. Interaction with polycomb proteins
Polycomb family of proteins represent essential players in maintaining the characteristics of stem cells and their deregulation is connected to CSCs and tumour transformation. Bmi-1 is a member of the Polycomb (PcG) family of transcriptional repressors that mediate gene silencing by regulating chromatin. It inhibits Myc-induced apoptosis by repressing the Cdkn2a locus. It also conceals the tumour suppressor PTEN and induces EMT [84]. Silencing of Bmi-1 expression in Head and Neck Squamous Cancer (HNSCC) - ALDH1+ cells resulted in Snail and ALDH1 downregulation, inhibition of the clonogenic and migration ability in vitro and reduction the number of lung metastases and tumour size in vivo. Bmi-1 modulates the tumorigenic properties in HNSCC-ALDH1+ or ALDH1− cells by regulating Snail, but the mechanism is unknown [85].
Polycomb 1 of the nervous system (NSPc1) regulates RA synthesis in glioma CSCs. It can inhibit the expression of retinol dehydrogenase 16 by binding to the promoter of its gene via RARE and thus reduce the level of endogenous atRA in cells. Suppression of NSPc1 expression reduces self-renewal and sphere formation in glioma CSCs and expression of Nestin and CD133. Reduction of NSPc1 leads to positive feedback during atRA formation and subsequent glioma stem cell differentiation [32].
6.5. Interactions with MDR
Multidrug resistance (MDR) -1 protein activity is one of the fundamental mechanisms responsible for multidrug resistance. RARα regulates MDR activity in human leukemic cells and leads to MDR [85].
However, the action of several retinoids is different. Retinol, retinyl acetate, and 13-cis-RA (not atRA) reduce the activity of MDR1, using the principle of non-competitive inhibition and the raising of the cytoplasmic membrane viscosity. Due to the reduced flexibility, ABC transporters cannot efficiently transport drugs from the cells [86]. A study in the ovarian cancer model reported that silencing of ALDH1A1 reduced its downstream target NEK2. The downregulation of ABC transporters (MDR1, ABCG2, and MRP1) was detected due to ALDH1A1 or NEK2 silencing. On the other hand, NEK2 overexpression leads to MDR1 upregulation. They assume that ALDH1A1 can be involved in cisplatin resistance through the upregulation of NEK2 in ovarian cancer [87].
6.6. Interaction with HOX
Hox genes encode a highly conserved family of transcription factors with homeodomain in their structure. They participate in the maintenance of the adult tissue homeostasis and also in the development of tumours [88]. Same as the homeotic genes, retinoids could control the expression of Hox genes. Langston et al. [89] identified RARE as part of the regulatory regions of Hox genes and demonstrated the possibility of influencing their transcription by RA. There is reciprocal feedback between HOXA5 and Wnt signalling. HOXA5 is suppressed by the Wnt pathway to maintain stemness and becomes active only outside the intestinal crypt, inhibiting Wnt signalling from enforcing differentiation. Wnt/ β-catenin signalling activates ALDH1A1 transcription leading to c-MYC expression. C-MYC reacts with the Miz1 protein as a transcriptional repressor and prevents the transcription of HOXA5, thereby maintaining the properties of stem cells. Tumour regression by HOXA5 induction can be triggered by retinoids [90]. Application of atRA to colon carcinoma CSCs results in decreased expression of HOXA4 and HOXA9, inhibition of proliferation, and an overall reduction in viability of affected cells [88].
7. Conclusion
Aldehyde dehydrogenases 1A1 and 1A3 are generally known as stem cell markers. Much lies hidden beneath this label: the ability to detoxify the drugs, antineoplastic regimen; the regulation of the tumour initiation and progression by triggering retinoid acid signalling; the impact on several pathways that are known to regulate stemness potential and EMT.
Numerous processes were studied in models of ALDEFLUOR-sorted ALDH+ cells with no specification of the correct isoform/s used. These studies detected the functional differences in ALDH+ vs. ALDH- cells, however molecular interactions require isoform-specific approach. This review summarizes the current state of knowledge of the partly elucidated network around ALDH1A1 and ALDH1A3 proteins and their adjacent signalling. The present data shows a large difference in transcriptional activation of both proteins depending on tissue and tumour type examined.
This review highlights the complex network of molecules which affect, regulate and influence ALDH1A1 and 1A3 isoforms resulting in cancer cells' resistance, tumorigenicity and motility.
Funding
This study was supported by the VEGA grant No. 2/0050/19, by Ministry of Health of the Slovak Republic under the contract 2019/60-BMCSAV-4 and by funding from the European Union's Horizon 2020 Research and Innovation Strategies to Programme under grant agreement No. 857381.
Authors' contributions
All authors contributed to this paper with conception of the study, literature review and analysis. MP prepared the figures. All authors read and approved the final version of the manuscript.
Declaration of Competing Interest
Authors declare no competing interest.
Acknowledgements
Special thanks belongs to Martin Benej, PhD for language editing.
Footnotes
Fig. 1 was created using PowerPoint cliparts (mitochondria & molecules) from Servier Medical Art de Servier (https://smart.servier.com) under the terms of licence of Creative Common Attributions 3.0 France. Fig. 2, Fig. 3, Fig. 4, Fig. 5 were created with a purchased version of BioRender.com.
References
- 1.Black W.J., Stagos D., Marchitti S.A., Nebert D.W., Tipton K.F., Bairoch A., Vasiliou V. Human aldehyde dehydrogenase genes: alternatively spliced transcriptional variants and their suggested nomenclature. Pharmacogenet. Genomics. 2009;19(11):893–902. doi: 10.1097/FPC.0b013e3283329023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haselbeck R.J., Hoffmann I., Duester G. Distinct functions for Aldh1 and Raldh2 in the control of ligand production for embryonic retinoid signaling pathways. Dev. Genet. 1999;25(4):353–364. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S., Brocker C., Koppaka V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013;56(89–101):2013. doi: 10.1016/j.freeradbiomed.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasiliou V., Pappa A., Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab. Rev. 2004;36(2):279–299. doi: 10.1081/dmr-120034001. [DOI] [PubMed] [Google Scholar]
- 5.Marchiti S.A., Brocker C., Stagos D., Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin. Metab. Toxicol. 2008;4(6):697–720. doi: 10.1517/17425255.4.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang P.M., Chen C.H., Yeh C.C., Lu H.J., Liu T.T., Chen M.H., Liu C.Y., Wu A., Yang M.H., Tai S.K., Mochly-Rosen D., Huang C.F. Transcriptome analysis and prognosis of ALDH isoforms in human cancer. Sci. Rep. 2018;8(1):2713. doi: 10.1038/s41598-018-21123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasiliou V., Thompson D.C., Smith C., Fujita M., Chen Y. Aldehyde dehydrogenases: from eye crystallins to metabolic disease and cancer stem cells. Chem. Biol. Interact. 2013;202(1–3):2–10. doi: 10.1016/j.cbi.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng H., Liu Y., Bian X. ALDH1A3 affects colon cancer in vitro proliferation and invasion depending on CXCR4 status. Br. J. Cancer. 2018;118:224–232. doi: 10.1038/bjc.2017.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassalli G. Aldehyde dehydrogenases: not just markers, but functional regulators of stem cells. Stem Cells Int. 2019;3904645 doi: 10.1155/2019/3904645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald T.L., McCubrey J.A. Pancreatic cancer stem cells: association with cell surface markers, prognosis, resistance, metastasis and treatment. Adv. Biol. Regulat. 2014;56:45–50. doi: 10.1016/j.jbior.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Duan J.J., Yu J.C., Xiu F.G., Shi W.B., Yu C. ALDH1A3, a metabolic target for cancer diagnosis and therapy. Int. J. Cancer. 2016;139(5):965–975. doi: 10.1002/ijc.30091. [DOI] [PubMed] [Google Scholar]
- 12.Croker A.K., Allan A.L. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44 human breast cancer cells. Breast Cancer Res. Treat. 2012;133(1):75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 13.Sladek N.E. Leukemic cell insensitivity to cyclophosphamide and other oxazaphosphorines mediated by aldehyde dehydrogenase[s] Cancer Treat. Res. 2002;112:161–175. doi: 10.1007/978-1-4615-1173-1_8. [DOI] [PubMed] [Google Scholar]
- 14.Ql Sun, Hf Sh.a., Yang Xh. Comparative proteomic analysis of paclitaxel sensitive A549 lung adenocarcinoma cell line and its resistant counterpart A549-taxol. J. Cancer Res. Clin. Oncol. 2011;137:521–532. doi: 10.1007/s00432-010-0913-9. [DOI] [PubMed] [Google Scholar]
- 15.Liu S.Y., Zheng P.S. High aldehyde dehydrogenase activity identifies cancer stem cells in human cervical cancer. Oncotarget. 2013;4(12):2462–2475. doi: 10.18632/oncotarget.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Januchowski R., Wojtowicz K., Zabel M. The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biomed. Pharmacother. 2013;67(7):669–680. doi: 10.1016/j.biopha.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Durinikova E., Kozovska Z., Poturnajova M., Plava J., Cierna Z., Babelova A., Bohovic R., Schmidtova S., Tomas M., Kucerova L., Matuskova M. ALDH1A3 upregulation and spontaneous metastasis formation is associated with acquired chemoresistance in colorectal cancer cells. BMC Cancer. 2018;18(1):848. doi: 10.1186/s12885-018-4758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozovska Z., Patsalias A., Bajzik V., Durinikova E., Demkova L., Jargasova S., Smolkova B., Plava J., Kucerova L., Matuskova M. ALDH1A inhibition sensitizes colon cancer cells to chemotherapy. BMC Cancer. 2018;18(1):656. doi: 10.1186/s12885-018-4572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreb J.S., Gabr A., Vartikar G.R., Gowda S., Zucali J.R., Mohuczy D. Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroperoxycyclophosphamide and acetaldehyde. J. Pharmacol. Exp. Ther. 2005;312(1):339–345. doi: 10.1124/jpet.104.072496. [DOI] [PubMed] [Google Scholar]
- 20.Toledo-Guzmán M.E., Hernández M.I., Gómez-Gallegos Á.A., Ortiz-Sánchez E. ALDH as a stem cell marker in solid tumors. Curr. Stem Cell Res. Therapy. 2019;14(5):375–388. doi: 10.2174/1574888X13666180810120012. [DOI] [PubMed] [Google Scholar]
- 21.Muzio G., Maggiora M., Paiuzzi E., Oraldi M., Canuto R.A. Aldehyde dehydrogenases and cell proliferation. Free Radic. Biol. Med. 2012;52(4):735–746. doi: 10.1016/j.freeradbiomed.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 22.di Masi A., Leboffe L., De Marinis E., Pagano F., Cicconi L., Rochette-Egly C., Lo-Coco F., Ascenzi P., Nervi C. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol. Asp. Med. 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Alique M., Lucio-Cazaña F.J., Moreno V., Xu Q., Konta T., Nakayama K., Furusu A., Sepulveda J.C., Kitamura M. Upregulation of cyclooxygenases by retinoic acid in rat mesangial cells. Pharmacology. 2007;79(1):57–64. doi: 10.1159/000097785. [DOI] [PubMed] [Google Scholar]
- 24.Loinder K., Söderström M. The nuclear receptor corepressor (N-CoR) modulates basal and activated transcription of genes controlled by retinoic acid. J. Steroid Biochem. Mol. Biol. 2003;84(1):15–21. doi: 10.1016/s0960-0760(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 25.Wallace B.D., Betts L., Talmage G., Pollet R.M., Holman N.S., Redinbo M.R. Structural and functional analysis of the human nuclear xenobiotic receptor PXR in complex with RXRa. J. Mol. Biol. 2013;425(14):2561–2577. doi: 10.1016/j.jmb.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S., Arcaroli J., Thompson D.C., Messersmith W., Vasiliou V. Acetaldehyde and retinaldehyde-metabolizing enzymes in colon and pancreatic cancers. Adv. Exp. Med. Biol. 2015;815:281–294. doi: 10.1007/978-3-319-09614-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark D.W. Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Ann. Transl. Med. 2016;4(24):518. doi: 10.21037/atm.2016.11.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalevée S. Genome-wide in silico identifcation of new conserved and functional retinoic acid receptor response elements. J. Biol. Chem. 2011;286:33322–33334. doi: 10.1074/jbc.M111.263681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bookout A.L. Anatomical profling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coyle K.M., Maxwell S., Thomas M.L. Profiling of the transcriptional response to all-trans retinoic acid in breast cancer cells reveals RARE-independent mechanisms of gene expression. Sci. Rep. 2017;7:16684. doi: 10.1038/s41598-017-16687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim Y.C., Kang H.J., Kim Y.S., Choi E.C. All-trans-retinoic acid inhibits growth of head and neck cancer stem cells by suppression of Wnt/ ß-catenin pathway. Eur. J. Cancer. 2012;48(17):3310–3318. doi: 10.1016/j.ejca.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Hu P.S., Xia Q.S., Wu F., Li D.K., Qi Y.J., Hu Y., Wei Z.Z., Li S.S., Tian N.Y., Wei Q.F. NSPc1 promotes cancer stem cell self-renewal by repressing the synthesis of all-trans retinoic acid via targeting RDH16 in malignant glioma. Oncogene. 2017;36(33):4706–4718. doi: 10.1038/onc.2017.34. [DOI] [PubMed] [Google Scholar]
- 33.van den Hoogen C., van der Horst G., Cheung H., Buijs J.T., Lippitt J.M., Guzmán-Ramírez N., Hamdy F.C., Eaton C.L., Thalmann G.N., Cecchini M.G., Pelger R.C., van der Pluijm G. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70(12):5163–5173. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Torres M., Allan A.L. Aldehyde dehydrogenase as a marker and functional mediator of metastasis in solid tumors. Clin. Experiment. Metastasis. 2016;33(1):97–113. doi: 10.1007/s10585-015-9755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Croker A.K., Goodale D., Chu J., Postenka C., Hedley B.D., Hess D.A., Allan A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 2009;13(8B):2236–2252. doi: 10.1111/j.1582-4934.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S., Cong Y., Wang D., Sun Y., Deng L., Liu Y., Martin-Trevino R., Shang L., McDermott S.P., Landis M.D. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2(1):78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcato P., Dean C.A., Liu R.Z., Coyle K.M., Bydoun M., Wallace M., Clements D., Turner C., Mathenge E.G., Gujar S.A., Giacomantonio C.A., Mackey J.R., Godbout R., Lee P.W. Aldehyde dehydrogenase 1A3 influences breast cancer progression via differential retinoic acid signaling. Mol. Oncol. 2015;9(1):17–31. doi: 10.1016/j.molonc.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagawa Y., Chen J.C., Hsu L.C., Yoshida A. The transcriptional regulation of human aldehyde dehydrogenase I gene. The structural and functional analysis of the promoter. J. Biol. Chem. 1995;270(29):17521–17527. doi: 10.1074/jbc.270.29.17521. [DOI] [PubMed] [Google Scholar]
- 39.Elizondo G., Corchero J., Sterneck E., Gonzalez F.J. Feedback inhibition of the retinaldehyde dehydrogenase gene ALDH1 by retinoic acid through retinoic acid receptor alpha and CCAAT/enhancer-binding protein beta. J. Biol. Chem. 2000;275(50):39747–39753. doi: 10.1074/jbc.M004987200. [DOI] [PubMed] [Google Scholar]
- 40.Hoshino Y., Nishida J., Katsuno Y., Koinuma D., Aoki T., Kokudo N., Miyazono K., Ehata S. Smad4 decreases the population of pancreatic cancer-initiating cells through transcriptional repression of ALDH1A1. Am. J. Pathol. 2015;185(5):1457–1470. doi: 10.1016/j.ajpath.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Cojoc M., Peitzsch C., Kurth I., Trautmann F., Kunz-Schughart L.A., Telegeev G.D., Stakhovsky E.A., Walker J.R., Simin K., Lyle S., Fuessel S., Erdmann K., Wirth M.P., Krause M., Baumann M., Dubrovska A. Aldehyde dehydrogenase is regulated by ß-Catenin/TCF and promotes radioresistance in prostate cancer progenitor cells. Cancer Res. 2015;75(7):1482–1494. doi: 10.1158/0008-5472.CAN-14-1924. [DOI] [PubMed] [Google Scholar]
- 42.Sarabia-Sánchez M.Á., Alvarado-Ortiz E., Toledo-Guzman M.E., García-Carrancá A., Ortiz-Sánchez E. ALDHHIGH population is regulated by the AKT/ß-catenin pathway in a cervical cancer model. Front. Oncol. 2020;10:1039. doi: 10.3389/fonc.2020.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alam M., Ahmad R., Rajabi H., Kharbanda A., Kufe D. MUC1-C oncoprotein activates ERK?C/EBPß signaling and induction of aldehyde dehydrogenase 1A1 in breast cancer cells. J. Biol. Chem. 2013;288(43):30892–30903. doi: 10.1074/jbc.M113.477158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shuang Z.Y., Wu W.C., Xu J., Lin G., Liu Y.C., Lao X.M., Zheng L., Li S. Transforming growth factor-ß1-induced epithelial-mesenchymal transition generates ALDH-positive cells with stem cell properties in cholangiocarcinoma. Cancer Lett. 2014;354(2):320–328. doi: 10.1016/j.canlet.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Bhola N.E., Balko J.M., Dugger T.C., Kuba M.G., Sánchez V., Sanders M., Stanford J., Cook R.S., Arteaga C.L. TGF-ß inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Invest. 2013;123(3):1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Jiang Y., Tian T., Hori Y., Wada N., Ikeda J., Morii E. Inhibitory effect of nodal on the expression of aldehyde dehydrogenase 1 in endometrioid adenocarcinoma of uterus. Biochem. Biophys. Res. Commun. 2013;440(4):731–736. doi: 10.1016/j.bbrc.2013.09.139. [DOI] [PubMed] [Google Scholar]
- 47.Rice K.L., Izon D.J., Ford J., Boodhoo A., Kees U.R., Greene W.K. Overexpression of stem cell associated ALDH1A1, a target of the leukemogenic transcription factor TLX1/HOX11, inhibits lymphopoiesis and promotes myelopoiesis in murine hematopoietic progenitors. Leuk. Res. 2008;32(6):873–883. doi: 10.1016/j.leukres.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Atwood A.A., Sealy L.J. C/EBPß's role in determining ras-induced senescence or transformation. Small GTPases. 2011;2(1):41–46. doi: 10.4161/sgtp.2.1.15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong B., Wu W., Cheng T., Schlitter A.M., Qian C., Bruns P., Jian Z., Jäger C., Regel I., Raulefs S., Behler N., Irmler M., Beckers J., Friess H., Erkan M., Siveke J.T., Tannapfel A., Hahn S.A., Theis F.J., Esposito I., Michalski C.W. A subset of metastatic pancreatic ductal adenocarcinomas depends quantitatively on oncogenic Kras/Mek/Erk-induced hyperactive mTOR signalling. Gut. 2016;65(4):647–657. doi: 10.1136/gutjnl-2014-307616. [DOI] [PubMed] [Google Scholar]
- 50.Shao C., Sullivan J.P., Girard L., Augustyn A., Yenerall P., Rodriguez-Canales J., Liu H., Behrens C., Shay J.W., Wistuba I.I., Minna J.D. Essential role of aldehyde dehydrogenase 1A3 for the maintenance of non-small cell lung cancer stem cells is associated with the STAT3 pathway. Clin. Cancer Res. 2014;20(15):4154–4166. doi: 10.1158/1078-0432.CCR-13-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canino C., Luo Y., Marcato P., Blandino G., Pass H.I., Cioce M. A STAT3-NFkB/DDIT3/CEBPß axis modulates ALDH1A3 expression in chemoresistant cell subpopulations. Oncotarget. 2015;6(14):12637–12653. doi: 10.18632/oncotarget.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trasino S.E., Harrison E.H., Wang T.T. Androgen regulation of aldehyde dehydrogenase 1A3 (ALDH1A3) in the androgen-responsive human prostate cancer cell line LNCaP. Exp. Biol. Med. 2007;232(6):762–771. [PubMed] [Google Scholar]
- 53.Casanova-Salas I., Masiá E., Armiñán A., Calatrava A., Mancarella C., Rubio-Briones J., Scotlandi K., Vicent M.J., López-Guerrero J.A. MiR-187 targets the androgen-regulated gene ALDH1A3 in prostate cancer. PloS one. 2015;10(5) doi: 10.1371/journal.pone.0125576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim I.G., Lee J.H., Kim S.Y., Kim J.Y., Cho E.W. Fibulin-3 negatively regulates ALDH1 via c-MET suppression and increases ?-radiation-induced sensitivity in some pancreatic cancer cell lines. Biochem. Biophys. Res. Commun. 2014;454(3):369–375. doi: 10.1016/j.bbrc.2014.10.084. [DOI] [PubMed] [Google Scholar]
- 55.Zhao D., Mo Y., Li M.T., Zou S.W., Cheng Z.L., Sun Y.P., Xiong Y., Guan K.L., Lei Q.Y. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J. Clin. Invest. 2014;124(12):5453–5465. doi: 10.1172/JCI76611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovén J., Hoke H.A., Lin C.Y., Lau A., Orlando D.A., Vakoc C.R., Bradner J.E., Lee T.I., Young R.A. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153(2):320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokoyama Y., Zhu H., Lee J.H., Kossenkov A.V., Wu S.Y., Wickramasinghe J.M., Yin X., Palozola K.C., Gardini A., Showe L.C., Zaret K.S., Liu Q., Speicher D., Conejo-Garcia J.R., Bradner J.E., Zhang Z., Sood A.K., Ordog T., Bitler B.G., Zhang R. BET inhibitors suppress ALDH activity by targeting ALDH1A1 super-enhancer in ovarian cancer. Cancer Res. 2016;76(21):6320–6330. doi: 10.1158/0008-5472.CAN-16-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y.J., Yoon H.Y., Kim J.S., Kang H.W., Min B.D., Kim S.K., Ha Y.S., Kim I.Y., Ryu K.H., Lee S.C., Kim W.J. HOXA9, ISL1 and ALDH1A3 methylation patterns as prognostic markers for nonmuscle invasive bladder cancer: array-based DNA methylation and expression profiling. Int. J. Cancer. 2013;133(5):1135–1142. doi: 10.1002/ijc.28121. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W., Yan W., You G., Bao Z., Wang Y., Liu Y., You Y., Jiang T. Genome-wide DNA methylation profiling identifies ALDH1A3 promoter methylation as a prognostic predictor in G-CIMP- primary glioblastoma. Cancer Lett. 2013;328(1):120–125. doi: 10.1016/j.canlet.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita S., Tsujino Y., Moriguchi K., Tatematsu M., Ushijima T. Chemical genomic screening for methylation-silenced genes in gastric cancer cell lines using 5-aza-2'-deoxycytidine treatment and oligonucleotide microarray. Cancer Sci. 2006;97(1):64–71. doi: 10.1111/j.1349-7006.2006.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu W., Schecker J., Würstle S., Schneider F., Schönfelder M., Schlegel J. Aldehyde dehydrogenase 1A3 (ALDH1A3) is regulated by autophagy in human glioblastoma cells. Cancer Lett. 2018;417:112–123. doi: 10.1016/j.canlet.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 62.Brown J.M., Wasson M.D., Marcato P. The missing lnc: the potential of targeting triple-negative breast cancer and cancer stem cells by inhibiting long non-coding RNAs. Cells. 2020;9(3):763. doi: 10.3390/cells9030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vidovic D., Huynh T.T., Konda P., Dean C., Cruickshank B.M., Sultan M., Coyle K.M., Gujar S., Marcato P. ALDH1A3-regulated long non-coding RNA NRAD1 is a potential novel target for triple-negative breast tumors and cancer stem cells. Cell Death Differ. 2020;27(1):363–378. doi: 10.1038/s41418-019-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruan Z., Zhao D. Long intergenic noncoding RNA LINC00284 knockdown reduces angiogenesis in ovarian cancer cells via up-regulation of MEST through NF-?B1. FASEB J. 2019;33(11):12047–12059. doi: 10.1096/fj.201900101RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sha S., Yuan D., Liu Y., Han B., Zhong N. Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol. Open. 2017;6(9):1310–1316. doi: 10.1242/bio.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y., Lu Z., Wang N., Feng J., Zhang J., Luan L., Zhao W., Zeng X. Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp. Mol. Med. 2018;50(5):1–17. doi: 10.1038/s12276-018-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan S.X., Wang J., Yang F., Tao Q.F., Zhang J., Wang L.L., Yang Y., Liu H., Wang Z.G., Xu Q.G., Fan J., Liu L., Sun S.H., Zhou W.P. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology (Baltimore, MD) 2016;63(2):499–511. doi: 10.1002/hep.27893. [DOI] [PubMed] [Google Scholar]
- 68.Zhang K.J., Tan X.L., Guo L. The long non-coding RNA DANCR regulates the inflammatory phenotype of breast cancer cells and promotes breast cancer progression via EZH2-dependent suppression of SOCS3 transcription. Mol. Oncol. 2020;14(2):309–328. doi: 10.1002/1878-0261.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren J., Ding L., Zhang D., Shi G., Xu Q., Shen S., Wang Y., Wang T., Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNAH19. Theranostics. 2018;8:3932–3948. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ying M., Wang S., Sang Y., Sun P., Lal B., Goodwin C.R., Guerrero-Cazares H., Quinones-Hinojosa A., Laterra J., Xia S. Regulation of glioblastoma stem cells by retinoic acid: role for notch pathway inhibition. Oncogene. 2011;30(31):3454–3467. doi: 10.1038/onc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zanetti A., Affatato R., Centritto F., Fratelli M., Kurosaki M., Barzago M.M., Bolis M., Terao M., Garattini E., Paroni G. All-trans-retinoic acid modulates the plasticity and inhibits the motility of breast cancer cells: role of notch1 and transforming growth factor(TGFß) J. Biol. Chem. 2015;290(29):17690–17709. doi: 10.1074/jbc.M115.638510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirata N., Yamada S., Shoda T., Kurihara M., Sekino Y., Kanda Y. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent notch activation. Nat. Commun. 2014;5:4806. doi: 10.1038/ncomms5806. [DOI] [PubMed] [Google Scholar]
- 73.Mu X., Isaac C., Greco N., Huard J., Weiss K. Notch signaling is associated with ALDH activity and an aggressive metastatic phenotype in murine osteosarcoma cells. Front. Oncol. 2013;3:143. doi: 10.3389/fonc.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sullivan J.P., Spinola M., Dodge M., Raso M.G., Behrens C., Gao B., Schuster K., Shao C., Larsen J.E., Sullivan L.A., Honorio S., Xie Y., Scaglioni P.P., DiMaio J.M., Gazdar A.F., Shay J.W., Wistuba I.I., Minna J.D. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70(23):9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z., Xiang Y., Xiang L., Xiao Y., Li F., Hao P. ALDH maintains the stemness of lung adenoma stem cells by suppressing the Notch/CDK2/CCNE pathway. PloS one. 2014;9(3) doi: 10.1371/journal.pone.0092669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tao S., Wang S., Moghaddam S.J., Ooi A., Chapman E., Wong P.K., Zhang D.D. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res. 2014;74(24):7430–7441. doi: 10.1158/0008-5472.CAN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu S., Lu H., Bai Y. Nrf2 in cancers: a double-edged sword. Cancer Med. 2019;8(5):2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim D., Choi B.H., Ryoo I.G., Kwak M.K. High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis. 2018;9(9):896. doi: 10.1038/s41419-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duong H.Q., You K.S., Oh S., Kwak S.J., Seong Y.S. Silencing of NRF2 reduces the expression of ALDH1A1 and ALDH3A1 and sensitizes to 5-FU in pancreatic cancer cells. Antioxidants (Basel, Switzerland) 2017;6(3):52. doi: 10.3390/antiox6030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacobson O., Weiss I.D., Szajek L.P., Niu G., Ma Y., Kiesewetter D.O., Peled A., Eden H.S., Farber J.M., Chen X. Improvement of CXCR4 tracer specificity for PET imaging. J. Control. Release. 2012;157(2):216–223. doi: 10.1016/j.jconrel.2011.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tahirovic Y.A., Pelly S., Jecs E., Miller E.J., Sharma S.K., Liotta D.C., Wilson L.J. Small molecule and peptide-based CXCR4 modulators as therapeutic agents. A patent review for the period from 2010 to 2018. Exp. Opin. Therapeutic Patents. 2020;30(2):87–101. doi: 10.1080/13543776.2020.1707186. [DOI] [PubMed] [Google Scholar]
- 82.Chatterjee S., Behnam Azad B., Nimmagadda S. The intricate role of CXCR4 in cancer. Adv. Cancer Res. 2014;124:31–82. doi: 10.1016/B978-0-12-411638-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng H., Liu Y., Bian X., Zhou F., Liu Y. ALDH1A3 affects colon cancer in vitro proliferation and invasion depending on CXCR4 status. Br. J. Cancer. 2018;118(2):224–232. doi: 10.1038/bjc.2017.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li D.W., Tang H.M., Fan J.W., Yan D.W., Zhou C.Z., Li S.X., Wang X.L., Peng Z.H. Expression level of Bmi-1 oncoprotein is associated with progression and prognosis in colon cancer. J. Cancer Res. Clin. Oncol. 2010;136(7):997–1006. doi: 10.1007/s00432-009-0745-7. [DOI] [PubMed] [Google Scholar]
- 85.Yu C.C., Lo W.L., Chen Y.W., Huang P.I., Hsu H.S., Tseng L.M., Hung S.C., Kao S.Y., Chang C.J., Chiou S.H. Bmi-1 regulates snail expression and promotes metastasis ability in head and neck squamous cancer-derived ALDH1 positive cells. J. Oncol. 2011;2011 doi: 10.1155/2011/609259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stromskaya T.P., Rybalkina E.Y., Zabotina T.N., Shishkin A.A., Stavrovskaya A.A. Influence of RARalpha gene on MDR1 expression and P-glycoprotein function in human leukemic cells. Cancer Cell Int. 2005;5:15. doi: 10.1186/1475-2867-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarapcsák S., Szalóki G., Telbisz Á., Gyöngy Z., Matúz K., Csosz É., Nagy P., Holb I.J., Rühl R., Nagy L., Szabó G., Goda K. Interactions of retinoids with the ABC transporters P-glycoprotein and breast cancer resistance protein. Sci. Rep. 2017;7:41376. doi: 10.1038/srep41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uddin M.H., Kim B., Cho U., Azmi A.S., Song Y.S. Association of ALDH1A1-NEK-2 axis in cisplatin resistance in ovarian cancer cells. Heliyon. 2020;6(11) doi: 10.1016/j.heliyon.2020.e05442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhatlekar S., Ertel A., Gonye G.E., Fields J.Z., Boman B.M. Gene expression signatures for HOXA4, HOXA9, and HOXD10 reveal alterations in transcriptional regulatory networks in colon cancer. J. Cell. Physiol. 2019;234(8):13042–13056. doi: 10.1002/jcp.27975. [DOI] [PubMed] [Google Scholar]
- 90.Langston A.W., Thompson J.R., Gudas L.J. Retinoic acid-responsive enhancers located 3' of the hox a and hox B homeobox gene clusters. Functional analysis. J. Biol. Chem. 1997;272(4):2167–2175. doi: 10.1074/jbc.272.4.2167. [DOI] [PubMed] [Google Scholar]
- 91.Ordóñez-Morán P., Dafflon C., Imajo M., Nishida E., Huelsken J. HOXA5 counteracts stem cell traits by inhibiting wnt signaling in colorectal cancer. Cancer cell. 2015;28(6):815–829. doi: 10.1016/j.ccell.2015.11.001. [DOI] [PubMed] [Google Scholar]