Abstract
Finely tuned decidualization of endometrial stromal fibroblasts into decidual cells is crucial for successful implantation and a healthy pregnancy. Both insulin and androgens are known to modulate decidualization, however, their complex effect on this process has not been fully elucidated. As hyperinsulinemia and hyperandrogenism are associated in clinical conditions, we aimed to investigate the interaction between insulin and androgens on decidualization. Primary human endometrial stromal cells were decidualized in vitro in the presence of insulin and/or androgens (dihydrotestosterone (DHT), testosterone). Gene or protein expressions of decidualization markers were measured, and cells size characteristics were determined. Migration of decidualizing endometrial stromal cells and invasion of HTR‐8/SVneo trophoblast spheroids were assessed. We found that insulin and androgens in combination enhanced the upregulation of several decidualization markers including prolactin, tissue factor, tissue inhibitor of matrix metalloproteinase 3 and connexin‐43, and also interacted in modulating cell size characteristics resulting in enlarged decidualizing cells. However, insulin and DHT together restricted the migration of decidualizing cells and invasion of trophoblast spheroids. Our findings suggest that insulin and androgens interact to potentiate the process of decidualization. On the other hand, inhibited cell migration and trophoblast invasion might negatively impact the function of decidualizing endometrial stromal cells.
Keywords: androgens, decidualization, endometrium, insulin, interaction
1. INTRODUCTION
Decidualization denotes the morphological and biochemical differentiation of endometrial stromal cells in the secretory phase of the menstrual cycle. During decidualization, spindle‐shaped fibroblast‐like endometrial stromal cells enlarge and acquire epithelioid characteristics, which is associated with enlarged nucleus, increased number of nucleoli, accumulation of glycogen and lipid droplets, dilatation of the rough endoplasmic reticulum and the Golgi systems and increased number of gap junctions between the neighbouring cells. 1 , 2 This transformation is of importance for embryo implantation and the formation of a functional feto‐maternal interface as it controls trophoblast proliferation, migration and invasion. 3 A variety of factors are secreted from decidualized cells and have been proposed to serve as putative markers of decidualization, including prolactin (PRL), insulin‐like growth factor binding protein 1 (IGFBP1), tissue factor (TF), tissue inhibitor of matrix metalloproteinase 3 (TIMP3), prokineticin 1 (PROK1) and the main gap junction protein connexin‐43 (CX43). 4 Impaired decidualization is considered to be associated with reproductive disorders, including decreased implantation, recurrent miscarriage and placenta‐related disorders. 5
The role of insulin in decidualization has been investigated in several studies, however, its effect on decidualization markers has shown varying results. Thus, it has been demonstrated that insulin downregulates IGFBP1 in decidualizing human endometrial stromal cells, 6 , 7 , 8 whereas PRL production is stimulated. 9 We have furthermore reported that insulin downregulates a number of decidualization markers via the transcriptional inactivation of Forkhead box protein 1 (Foxo1), a crucial transcription factor in decidualization, 8 whereas PROK1 is highly enhanced by insulin in decidualizing human endometrial stromal cells. 10 However, it was recently shown that adequate insulin signalling via insulin receptor substrate‐2 supports the decidualization process. 7
Androgens may also play a role for decidualization. 11 , 12 , 13 In the endometrium, the androgen receptor (AR) expression is confined to the stroma and it fluctuates during the menstrual cycle with a gradual decrease from the early proliferative to the mid‐secretory phase. 14 Furthermore, endometrial expression levels of enzymes that play a role in the biosynthesis and conversion of androgens are higher in the secretory phase of the menstrual cycle, suggesting a role of locally synthesized androgens in decidualization. 14 , 15 It was recently demonstrated that androgens (testosterone and dihydrotestosterone (DHT)) enhance the expression of decidualization markers such as PRL and IGFBP1 and promote the morphological and ultrastructural changes associated with the decidualization process. 1 , 15 , 16
Hyperinsulinemia and hyperandrogenism are common clinical conditions in polycystic ovary syndrome (PCOS) and obesity. These conditions are related to implantation failure, increased risk of miscarriage and adverse pregnancy outcomes. 17 , 18 , 19 , 20 However, the mechanisms by which hyperinsulinemia and/or hyperandrogenism weaken the chance for a successful implantation and an uncomplicated pregnancy are still not elucidated. The purpose of the present study was to investigate the in vitro interaction between insulin and androgens on the decidualization process, focusing on the functional changes in decidualizing endometrial stromal cells.
2. MATERIALS AND METHODS
2.1. Subjects
Regularly cycling, healthy volunteers (n = 9) underwent collection of endometrial biopsy under local anaesthesia using a suction curette (Pipet Curet, CooperSurgical) in the proliferative phase of the menstrual cycle at cycle day 5–9. All women were between 18 and 35 years and had a body mass index between 19 and 28. Exclusion criteria were hormonal medication within 3 months prior to biopsy sampling, smoking, endocrine disorder, current chronic disease or continuous medication. They gave their written informed consent and the Regional Ethical Committee in Stockholm approved the study (DNR 2018/2199‐31).
2.2. Isolation of human endometrial stromal cells and culture conditions
Isolation of human endometrial stromal cells was carried out as described previously. 8 Endometrial stromal cells were seeded to 6‐well Costar plates (Sigma‐Aldrich) at a density of 105/well and cultured in DMEM/F12‐Glutamax medium (Thermo Fischer Scientific) supplemented with 10% heat‐inactivated foetal bovine serum (HI‐FBS; Sigma‐Aldrich) and 0.2% penicillin‐streptomycin (Sigma‐Aldrich) until 80%–90% confluency. In vitro decidualization of endometrial stromal cells was induced in phenol red‐free DMEM/F12 (Thermo Fischer Scientific) supplemented with 2% charcoal‐stripped FBS (Sigma‐Aldrich) 0.2% penicillin‐streptomycin (Sigma‐Aldrich) using 1 μM medroxyprogesterone‐17‐acetate (MPA; Sigma‐Aldrich) and 0.5 mM dibutyryl‐cAMP (db‐cAMP; Sigma‐Aldrich) in the presence or absence of 100 nM insulin (Sigma‐Aldrich), 1 μM DHT (Sigma‐Aldrich) or 1 μM testosterone (Sigma‐Aldrich), or the combination of insulin and DHT or insulin and testosterone for six days. The culture media was renewed after 3 days.
In the experiments of flow cytometry, wound‐healing assay and spheroid co‐culture invasion assay, we chose to treat the cells with insulin and/or DHT, but not with testosterone, since DHT has the strongest affinity to the androgen receptor 21 and does not convert to other hormones.
2.3. RNA isolation, cDNA synthesis and RT‐PCR
Total RNA was extracted using Quick‐RNA Miniprep Kit (Zymo Research) and subjected to cDNA synthesis using SuperScript VILO cDNA Synthesis Kit (Thermo Fischer Scientific). Gene expression levels of PRL and IGFBP1 were determined with TaqMan method. TF and TIMP3 were measured using the SybrGreen method. Ribosomal protein L13A (RPL13A) was used as a housekeeping gene to normalize gene expression levels. Gene expression levels were analysed with the ΔΔCt method. The employed TaqMan assays (Thermo Fischer Scientific) and oligonucleotides (Sigma‐Aldrich) are listed in Tables S1 and S2. All determinations were performed in triplicate.
2.4. Flow cytometry
Flow cytometry was used to determine protein expression of CX43. Cell cultures were harvested with 3 ml AutoMACS Rinsing solution (Miltenyi Biotec, Germany) at 37°C and pelleted with centrifugation at 400 g for 7 min. Cells were resuspended in 180 μl Cytofix/Cytoperm (BD Biosciences), transferred to V shaped 96‐well plate (Sigma‐Aldrich) and incubated for 20 min on ice. After centrifugation at 450 g for 6 min, cells were washed/permeabilized twice using 180 μl Perm/Wash buffer (BD Biosciences), and after centrifugation, cells were resuspended in 40 μl Perm/Wash buffer. Five microlitre Fc block (BD Biosciences) was applied for 15 min at room temperature in order to block non‐specific binding of potential Fc receptors. Cells were then incubated with APC‐conjugated CX43 or isotype control antibody (R&D Systems) for 1 h at 4°C in dark, washed twice using 180 μl Perm/Wash buffer and resuspended in 130 μl fixation buffer (BioLegend) prior to flow cytometric analysis. The fluorescence of the cells was measured using a NovoCyte flow cytometer (ACEA Biosciences, Inc.), and data were analysed with the FlowJo software (TreeStar).
We also determined forward scatter (FSC—reflecting cell volume), side scatter (SSC—reflecting the granularity or the internal complexity of the cells) and pulse‐width (reflecting cell diameter) parameters in order to approximate cell size.
2.5. Cell size determination using light microscopy
After 5 days of decidualization, cells were detached from the culture dishes using TrypLE Express (Thermo Fischer Scientific) and microphotographs were taken with 100x magnification using a Leica DFC420 C digital camera on a Nikon Eclipse TS 100 inverted microscope. At least 100 cells were analysed in each condition of each healthy donor. Photographs were analysed using ImageJ software.
2.6. Wound‐healing assay
A wound‐healing assay was used to study the migratory potential of decidualized cells after treatment with insulin, DHT or the combination of both. Endometrial stromal cells were seeded in 24‐well Costar plates (Sigma‐Aldrich) and cultured in DMEM/F12‐Glutamax supplemented with 10% HI‐FBS and 0.2% penicillin‐streptomycin until confluency. The cells were decidualized as stated above in the presence or absence of 100 nM insulin, 1 μM DHT or their combination for 6 days. The cells were scratched with a 200 μl pipette tip and washed twice with PBS. 700 μl phenol red‐free DMEM/F12 media supplemented with 2% charcoal‐stripped foetal bovine serum and 0.2% penicillin‐streptomycin was added to each well. No decidualization agents, insulin or DHT were added during the 24 h of the wound‐healing assay. The migration of the cells was followed by an IncuCyte S3 Live‐Cell Analysis System (Sartorius) using a 4x objective (whole well, phase contrast imaging) for 24 h. Photographs were analysed using ImageJ software. The experiments were performed with cells from five healthy volunteers. Each experiment was performed in duplicate.
2.7. Formation of trophoblast spheroids and co‐culture invasion assay
We applied a co‐culture invasion assay to investigate the invasion of decidualized cells by HTR‐8/SVneo spheroids in the presence of insulin, DHT or their combination. Spheroids consisting of 3*103 HTR‐8/SVneo, a first trimester derived immortalized trophoblast cell line, were formed as described previously. 10 The co‐culture experiments were performed in 24‐well Costar plates. Primary endometrial stromal cells were seeded at a density of 105/well and cultured until 90% confluency. Cells were either decidualized in the presence or absence of 100 nM insulin, 1 μM DHT or their combination as described above for 6 days or left untreated. Then, media was changed to 700 μl phenol red‐free DMEM/F12 supplemented with 2% charcoal‐stripped FBS and 0.2% penicillin‐streptomycin. One HTR‐8/SVneo spheroid was carefully transferred to each well onto the confluent stromal/decidual cells using 1 ml pipette tip previously cut with sterile blade in order to widen it. No decidualization agents, insulin or DHT were added during the 16 h of the co‐culture invasion assay. The invasion of HTR‐8/SVneo spheroids was followed by an IncuCyte S3 Live‐Cell Analysis System using a 4x objective (whole well, phase contrast imaging). The invasion areas of spheroids were measured using ImageJ. The experiments were performed with cells from five healthy volunteers.
2.8. Statistical analysis
Statistical analysis was performed with GraphPad Prism 9.0. Two‐way ANOVA for repeated measurements was used with the within group factors insulin (yes/no), DHT (yes/no) or testosterone (yes/no) and the interaction insulin*DHT or insulin*testosterone. Considering the limited sample size and the high risk of type II error (false‐negative results), we performed simple main effect tests if interactions corresponded to p < 0.15. All the data were log‐transformed because of skewness. p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Gene expression levels of decidualization markers in response to insulin, androgens and combined treatment
In vitro decidualization increased all studied markers (Figure S1A–D).
3.2. PRL
The interaction between insulin and DHT was significant, and the post hoc test showed higher PRL expression by insulin in combination with DHT than for insulin (p = 0.003) or DHT alone (p = 0.002; Figure 1 and Table 1). Furthermore, insulin increased PRL expression compared with no treatment (p = 0.038). There was no interaction between insulin and testosterone, but a significant main effect for both insulin and testosterone (p = 0.030 and p = 0.000, respectively (Figure 1 and Table 1).
FIGURE 1.
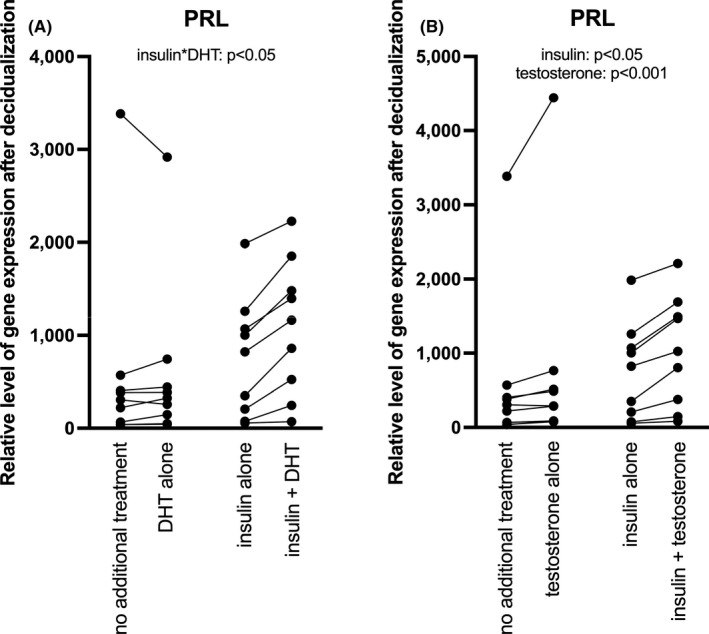
(A) Relative levels of PRL gene expression after 6 days in response to insulin and/or DHT in in vitro decidualized (treated with MPA and db‐cAMP) human endometrial stromal cells of healthy volunteers. Interaction effect between insulin and DHT: p < 0.05. (B) Relative levels of PRL gene expression after 6 days in response to insulin and/or testosterone in in vitro decidualized (treated with MPA and db‐cAMP) human endometrial stromal cells of healthy volunteers. Main effect of insulin: p < 0.05; main effect of testosterone: p < 0.001
TABLE 1.
Decidualization markers in in vitro decidualized endometrial stromal cells in response to insulin, DHT or testosterone and insulin+DHT or insulin+testosterone
| Gene | Decidual | Insulin | Androgen | Insulin+Androgen | Interaction and main effect | Post‐hoc effects | |
|---|---|---|---|---|---|---|---|
| DHT | PRL | 306 (39–3386) | 823 (56–1986) | 326 (44–2917) | 1164 (71–2228) | 1(*) | a(*), b(**), d(**) |
| IGFBP1 | 13,436 (191–277,298) | 13,805 (28–303,133) | 20,800 (373–220,117) | 19,510 (161–547,130) | |||
| TF | 2.22 (0.62–39.04) | 5.82 (1.37–248.00) | 3.01 (0.92–71.60) | 4.60 (1.35–220.50) | 1(*) | a(***), b(**), c(**) | |
| TIMP3 | 2.41 (1.33–9.63) | 16.74 (2.38–69.46) | 4.08 (2.13–19.62) | 36.47 (3.39–121.5) | A(**), B(*) | ||
| CX43 | 1.8x105 (1.4x105‐3.2x105) | 2.5x105 (1.7x105‐3.8x105) | 1.7x105 (1.2x105‐3.0x105) | 2.5x105 (1.7x105‐4.0x105) | 1(*) | a(**), b(**) | |
| Testosterone | PRL | 306 (39–3386) | 823 (56–1986) | 289 (79–4444) | 1025 (82–2209) | A(*), B(***) | |
| IGFBP1 | 13,436 (191–277,298) | 13,805 (28–303,133) | 14,889 (262–238,187) | 21,879 (128–462,294) | B(**) | ||
| TF | 2.22 (0.62–39.04) | 5.82 (1.37–248.00) | 2.56 (0.85–82.37) | 7.10 (1.48–307.50) | A(****), B(**) | ||
| TIMP3 | 2.41 (1.33–9.63) | 16.74 (2.38–69.46) | 4.83 (2.16–9.57) | 19.47 (3.75–65.77) | A(**), B(*) |
Data are median and ranges (min‐max). Values of PRL, IGFBP1, TF and TIMP3 are relative gene expression levels compared with those in stromal cells. Values of CX43 are protein expression levels (AU (arbitrary unit) of mean fluorescence intensity). 1 = Interaction between insulin and androgen, A = Main effect of insulin, B = Main effect of androgen. Post hoc test: a = insulin vs. decidual, b = insulin+androgen vs. androgen, c = androgen vs. decidual, d = insulin+androgen vs. insulin. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
3.3. IGFBP1
There was no interaction or significant main effects of insulin or DHT on IGFBP1 gene expression (Table 1 and Figure S2). Furthermore, there was no significant interaction between insulin and testosterone. However, testosterone increased IGFBP1 (p = 0.005) but not insulin (Table 1 and Figure S2).
3.4. TF
There was an interaction between insulin and DHT, and the post hoc test showed higher TF expression by the combined insulin and DHT treatment compared with DHT alone (p = 0.005). Furthermore, both insulin and DHT increased TF expression compared with no treatment (p = 0.000 and p = 0.004, respectively; Table 1 and Figure S2). There was no interaction between insulin and testosterone, but both insulin and testosterone increased TF (p = 0.000 and p = 0.006, respectively; Table 1 and Figure S2).
3.5. TIMP3
There was no significant interaction between insulin and DHT, however, both increased the gene expression of TIMP3 independently of each other (p = 0.001 and p = 0.016, respectively; Table 1 and Figure S2). The effect of testosterone exposure was similar to that of DHT treatment (Table 1 and Figure S2).
3.6. Protein expression levels of the gap junction protein CX43 in response to insulin, DHT and their combined treatment
In vitro decidualization did not significantly affect CX43 protein expression (not shown). There was an interaction between insulin and DHT, and the post hoc test showed a significantly higher protein expression of CX43 when cells were treated with insulin and DHT in combination compared with DHT alone (p = 0.002). Insulin alone, also increased CX43 compared with no treatment (p = 0.007; Table 1 and Figure S3).
3.7. Cell size determination
The interaction between insulin and DHT was significant for most parameters of cell characteristics (Table 2). Insulin in combination with DHT increased FSC‐A (reflecting cell volume) compared with insulin alone (p = 0.001), but not insulin or DHT alone (Table 2 and Figure S4). Furthermore, the combined treatment increased SSC‐A (reflecting cell complexity) compared with DHT alone (p = 0.000), and insulin alone increased SSC‐A compared with no treatment (p = 0.005; Table 2 and Figure S4). Insulin in combination with DHT also increased pulse‐width (reflecting cell diameter) compared with insulin and DHT alone (p = 0.0058 and p = 0.0067, respectively; Table 2 and Figure S4). Similarly, insulin in combination with DHT increased cell size compared with insulin and DHT alone (p = 0.002 and p = 0.020, respectively; Table 2 and Figure S4).
TABLE 2.
Cell characteristics of in vitro decidualized endometrial stromal cells in response to insulin, DHT and insulin+DHT
| Measure | Decidual | Insulin | DHT | Insulin+DHT | Interaction and main effect | Post hoc effects |
|---|---|---|---|---|---|---|
| FSC‐A | 6.0 x 105 (2.6 x 105−7.5 x 105) | 5.6 × 105 (2.9 × 105−8.0 × 105) | 5.4 × 105 (2.3 × 105−8.3 × 105) | 6.0 × 105 (3.4 × 105−8.6 × 105) | 1(*) | d(**) |
| SSC‐A | 8.7 × 105 (4.6 × 105−1.4 × 106) | 1.2 × 106 (7.9 × 105−1.6 × 106) | 8.3 × 105 (4.0 × 105−1.4 × 106) | 1.3 × 106 (7.3 × 105−1.7 × 106) | 1(*) | a(**), b(***) |
| Pulse width | 113.0 (89.0–149.0) | 121.0 (101.0–144.0) | 114.0 (87.0–149.0) | 121.0 (107.0–152.0) | 1( p =0.058) | b(**), d(**) |
| Cell size | 2468 (1817–2833) | 3184 (2492–3388) | 2257 (1794–2586) | 3456 (2719–3652) | 1(*) | b(*), d(**) |
Data are median and ranges (min‐max). The units of FSC‐A, SSC‐A and pulse width are AU (arbitrary unit) of mean fluorescence intensity. The units of cell size are pixel2. 1 = Interaction between insulin and DHT. Post hoc test: a = insulin vs. decidual, b = insulin+DHT vs. DHT, c = DHT vs. decidual, d = insulin+DHT vs. insulin. *p < 0.05, **p < 0.01 and ***p < 0.001.
3.8. Wound‐healing assay
In vitro decidualization did not significantly affect the migration of the cells as compared to undifferentiated stromal cells (not shown). Insulin and DHT interacted in cell migration of decidualizing cells, and post hoc test showed that the combined treatment inhibited cell migration in comparison with insulin or DHT alone (p = 0.045 and p = 0.005, respectively; Figure 2A–I and Table S3).
FIGURE 2.
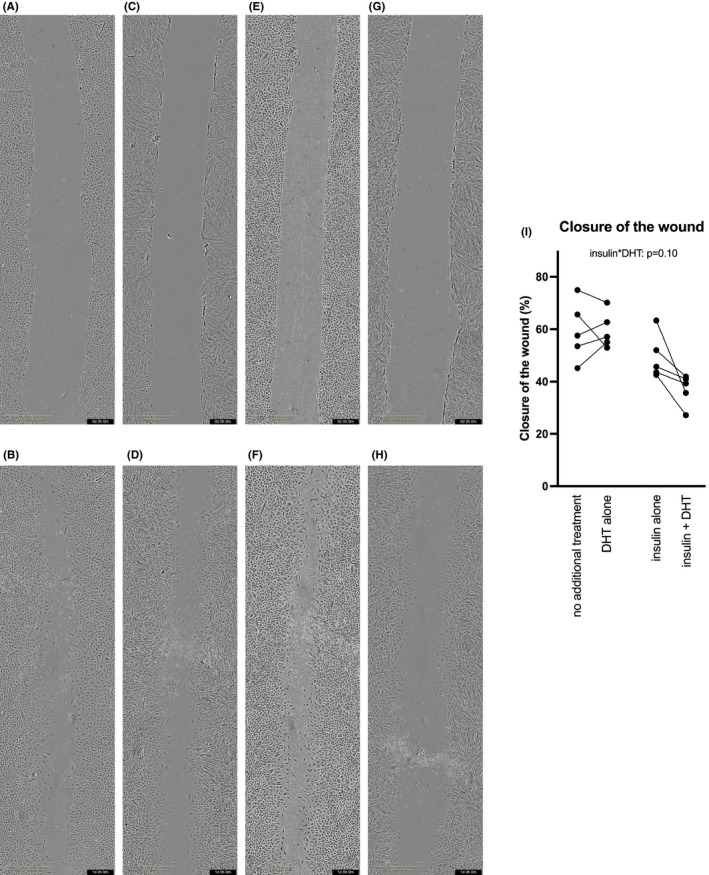
(A–H) Representative microphotographs (40x magnification) of wound‐healing assay of stromal cells decidualized (treated with MPA and db‐cAMP) for 6 days after wounding at 0h (A) and 24h (B), with insulin treatment at 0h (C) and 24h (D), with DHT treatment at 0h (E) and 24h (F) and with insulin and DHT treatment at 0h (G) and 24h (H) were taken with IncuCyte S3 Live‐Cell Analysis System. (I) Closure of the wound using in vitro decidualized (treated with MPA and db‐cAMP) human endometrial stromal cells of healthy volunteers in response to insulin and/or DHT after 6 days, at 24 h. Interaction effect between insulin and DHT: p = 0.10
3.9. Co‐culture invasion assay
In vitro decidualization did not significantly affect the invasion of the HTR‐8/SVneo spheroids when compared to that of undifferentiated stromal cells (not shown). Insulin and DHT interacted in invasion of trophoblast spheroids of decidualizing cells, and post hoc test demonstrated that the combined treatment with insulin and DHT inhibited the outgrowth of HTR‐8/SVneo trophoblast spheroids at 12 h compared with both insulin and DHT alone (p = 0.019 and p = 0.010, respectively). The effect of the combined treatment of insulin and DHT was similar at 16 h. Furthermore, at 16 h, insulin significantly restricted spheroid outgrowth (p = 0.001; Figure 3A–N, Videos [Link], [Link], [Link], [Link] and Table S3).
FIGURE 3.

(A‐L) Representative microphotographs (40x magnification) of co‐culture invasion assay using HTR‐8/SVneo spheroids and stromal cells decidualized (treated with MPA and db‐cAMP) for 6 days at 0h (A), 12h (B) and 16h (C), with insulin treatment at 0h (D), 12h (E) and 16h (F), with DHT treatment at 0h (G), 12h (H) and 16h (I), and with insulin and DHT treatment at 0h (J), 12h (K) and 16h (L) were taken with IncuCyte S3 Live‐Cell Analysis System. (M) Co‐culture invasion assay using HTR‐8/SVneo spheroids on in vitro decidualized (treated with MPA and db‐cAMP) human endometrial stromal cells of healthy volunteers in response to insulin and/or DHT after 6 days, at 12h. Interaction effect between insulin and DHT: p = 0.11. (N) Co‐culture invasion assay using HTR‐8/SVneo spheroids on in vitro decidualized (treated with MPA and db‐cAMP) human endometrial stromal cells of healthy volunteers in response to insulin and/or DHT after 6 days, at 16h. Main effect of insulin: p < 0.01
4. DISCUSSION
This is the first study demonstrating evidence of interaction between insulin and androgens in the regulation of decidualizing endometrial stromal cells. The upregulation of several decidualization markers and the increase of decidual cell size were enhanced by insulin, and also by androgens to a lesser extent, whereas the combination of insulin and DHT seemed to potentiate this effect even further. However, insulin and particularly the combined treatment of insulin and DHT inhibited migration of decidualizing cells and invasion of trophoblast spheroids.
Our results concerning the separate effects of insulin and androgens on the decidualization markers PRL and IGFBP1 are largely consistent with earlier data. Thus, it was previously shown that both intracrine and exogenous androgens enhance the production of PRL and IGFBP1 along with the morphological transformation into decidualizing endometrial cells. 1 , 15 , 16 , 22 On the other hand, previous studies have demonstrated that insulin enhances PRL and PROK1 productions, whereas IGFBP1 is downregulated in decidualizing endometrial stromal cells. 6 , 7 , 8 , 9 , 10
Here, we found that both insulin and androgens enhanced PRL, and testosterone also increased IGFBP1, whereas insulin had no significant effect on IGFBP1. In addition, we have shown that the decidualization markers TF and TIMP3 were upregulated by insulin and androgens. In these experiments, we tested both DHT and testosterone, however, since there were no major differences in the actions of these two androgens on decidualization markers, we chose to use only DHT in the following experiments. This was because DHT has a 2‐fold higher affinity to the androgen receptor than testosterone. 21 Furthermore, testosterone can exert diverse effects due to its conversion to both oestrogen and DHT in decidualizing endometrial stromal cells, via the action of aromatase and 5α‐reductase type 1, respectively. 22
The main findings in our study are the interactions between insulin and androgens in the regulation of decidualization, showing a potentiated upregulation of gene expression of PRL and TF and of protein expression of the gap junction protein CX43 by insulin and DHT in combination. There were also significant interactions in the modulation of cell size characteristics, with the greatest increase in decidualizing endometrial cell size by the combination of insulin and DHT. The mechanisms of these interactions are not known. However, hypothetically it may involve the phosphatidylinositol 3‐kinase (PI3K)‐Akt (protein kinase B) signalling pathway. In peripheral tissues, insulin and IGF‐I signalling involves activation of the PI3K‐Akt pathway. Several modes of interaction between Akt and androgen signalling via AR have been suggested, however, predominantly in prostate cancer, including direct phosphorylation and inhibition of AR by Akt, regulation by Akt via the wnt/GSK3B/ß‐catenin pathway, crosstalk of AR and Akt involving NF‐ kB and suppression of AR by Foxo1. 23 , 24 , 25 It remains to be determined whether insulin and androgens interact in decidualizing endometrial stromal cells via an action of PI3K/Akt signalling and which downstream pathways are involved in this process.
Successful invasion of the embryonic trophoblasts requires sufficient motility of maternal endometrial stromal cells. 26 , 27 , 28 , 29 , 30 It has been demonstrated that stromal cells migrate away from the implanting blastocyst, but also move around and encapsulate the early embryo, thereby facilitating the invasion of trophoblasts into the decidual bed. 26 , 29 , 31 However, there needs to be a delicate balance between mechanisms that promote trophoblast invasion and those that restrict it. Inadequate implantation is considered to increase the risk of early miscarriage and placental insufficiency, whereas excessive trophoblast invasion might lead to placenta accrete. 32
Several putative factors are involved in the regulation of endometrial stromal cell migration and trophoblast invasion including hormones, growth factors, chemokines and inflammatory mediators. 33 Wongwananuruk and collaborators studied the role of androgen signalling and demonstrated that DHT alone had no significant effect on trophoblast invasion in a co‐culture system using HTR‐8/SVneo trophoblast cells and decidualizing endometrial stromal cells. 31 We found that combined treatment with insulin and DHT inhibited migration of decidualizing stromal cells, whereas there was no significant effect of insulin or DHT alone. Furthermore, the combined treatment, as well as insulin alone, restricted the invasion of trophoblast spheroids.
The decreased migration and trophoblast invasion by the combined treatment could at least partly be explained by the increased expression of gap junction protein CX43. However, even though we washed the decidualizing stromal cells several times when performing the wound‐healing and co‐culture assays, it cannot be excluded that the effect of decidual secretion of other factors, such as PROK1 might have impacted the migration and invasion. We have previously demonstrated that PROK1, a crucial negative regulator of trophoblast invasion, 10 , 34 , 35 also decreases the migration of endometrial stromal cells 10 and is enhanced by the combined treatment with insulin and androgens. 36 Taken together, our results show that interaction of insulin and androgens causes decreased endometrial stromal cell migration along with increased expression of CX43, and inhibited trophoblast invasion in vitro, that suggest dysregulation of the decidualization process.
There are certain limitations with our study. We used extravillous trophoblast cell line HTR‐8/SVneo instead of primary cells and the number of endometrial samples are limited in the study. However, we consider the use of primary endometrial stromal cells instead of cell lines as a strength of the study.
In conclusion, our results demonstrate that insulin and androgens interact in the decidualization process, leading to enlarged decidualizing stromal cells and enhanced upregulation of several putative decidualization markers. However, at the same time the combined treatment of insulin and androgens caused inhibition of endometrial stromal cell migration and trophoblast invasion in vitro. We suggest that these results may indicate a dysregulation of the normally finely tuned decidualization. The results may have implications for the understanding of implantation failure, miscarriage and placenta‐related pregnancy complications in conditions of hyperinsulinemia and hyperandrogenism, such as PCOS and obesity.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Angelica Lindén Hirschberg: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (equal); Project administration (lead); Resources (lead); Supervision (lead); Validation (lead); Writing – original draft (lead); Writing – review and editing (equal). Ivika Jakson: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – review and editing (equal). Carlota Graells Brugalla: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing – review and editing (equal). Daniel Salamon: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – review and editing (equal). Dorina Ujvari: Conceptualization (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Project administration (lead); Resources (lead); Supervision (lead); Validation (lead); Visualization (lead); Writing – original draft (lead); Writing – review and editing (equal).
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Table S2
Table S3
Video S1
Video S2
Video S3
Video S4
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Lotta Blomberg, Berit Legerstam and Siv Rödin Andersson at the Women's Health Clinical Research Unit for the technical assistance. This work was supported by grants from Swedish Research Council (2017‐02051), Stockholm County Council (2019‐0248) and Åke Wiberg foundation (M18‐0114).
Hirschberg AL, Jakson I, Graells Brugalla C, Salamon D, Ujvari D. Interaction between insulin and androgen signalling in decidualization, cell migration and trophoblast invasion in vitro . J Cell Mol Med. 2021;25:9523–9532. 10.1111/jcmm.16892
DATA AVAILABILITY STATEMENT
The data that support the findings are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kajihara T, Tanaka K, Oguro T, et al. Androgens modulate the morphological characteristics of human endometrial stromal cells decidualized in vitro. Reprod Sci. 2014;21(3):372‐380. 10.1177/1933719113497280 [DOI] [PubMed] [Google Scholar]
- 2. Pan‐Castillo B, Gazze SA, Thomas S, et al. Morphophysical dynamics of human endometrial cells during decidualization. Nanomedicine. 2018;14(7):2235‐2245. 10.1016/j.nano.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 3. Kajihara T, Jones M, Fusi L, et al. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol. 2006;20(10):2444‐2455. 10.1210/me.2006-0118 [DOI] [PubMed] [Google Scholar]
- 4. Brar AK, Handwerger S, Kessler CA, Aronow BJ. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics. 2001;7(2):135‐148. 10.1152/physiolgenomics.00061.2001 [DOI] [PubMed] [Google Scholar]
- 5. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851‐905. 10.1210/er.2014-1045 [DOI] [PubMed] [Google Scholar]
- 6. Lathi RB, Hess AP, Tulac S, Nayak NR, Conti M, Giudice LC. Dose‐dependent insulin regulation of insulin‐like growth factor binding protein‐1 in human endometrial stromal cells is mediated by distinct signaling pathways. J Clin Endocrinol Metab. 2005;90(3):1599‐1606. 10.1210/jc.2004-1676 [DOI] [PubMed] [Google Scholar]
- 7. Neff AM, Yu J, Taylor RN, Bagchi IC, Bagchi MK. Insulin Signaling Via Progesterone‐Regulated Insulin Receptor Substrate 2 is Critical for Human Uterine Decidualization. Endocrinology. 2020;161(1):bqz021. 10.1210/endocr/bqz021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ujvari D, Jakson I, Babayeva S, et al. Dysregulation of in vitro decidualization of human endometrial stromal cells by insulin via transcriptional inhibition of forkhead box protein O1. PLoS ONE. 2017;12(1):e0171004. 10.1371/journal.pone.0171004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thrailkill KM, Golander A, Underwood LE, Richards RG, Handwerger S. Insulin stimulates the synthesis and release of prolactin from human decidual cells. Endocrinology. 1989;124(6):3010‐3014. 10.1210/endo-124-6-3010 [DOI] [PubMed] [Google Scholar]
- 10. Ujvari D, Jakson I, Oldmark C, et al. Prokineticin 1 is up‐regulated by insulin in decidualizing human endometrial stromal cells. J Cell Mol Med. 2018;22(1):163‐172. 10.1111/jcmm.13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cloke B, Huhtinen K, Fusi L, et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149(9):4462‐4474. 10.1210/en.2008-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kajihara T, Tochigi H, Prechapanich J, et al. Androgen signaling in decidualizing human endometrial stromal cells enhances resistance to oxidative stress. Fertil Steril. 2012;97(1):185‐191. 10.1016/j.fertnstert.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 13. Gibson DA, Simitsidellis I, Saunders PT. Regulation of androgen action during establishment of pregnancy. J Mol Endocrinol. 2016;57(1):R35‐47. 10.1530/JME-16-0027 [DOI] [PubMed] [Google Scholar]
- 14. Cloke B, Christian M. The role of androgens and the androgen receptor in cycling endometrium. Mol Cell Endocrinol. 2012;358(2):166‐175. 10.1016/j.mce.2011.06.031 [DOI] [PubMed] [Google Scholar]
- 15. Gibson DA, Simitsidellis I, Cousins FL, Critchley HO, Saunders PT. Intracrine androgens enhance decidualization and modulate expression of human endometrial receptivity genes. Sci Rep. 2016;6(1):19970. 10.1038/srep19970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Narukawa S, Kanzaki H, Inoue T, et al. Androgens induce prolactin production by human endometrial stromal cells in vitro. J Clin Endocrinol Metab. 1994;78(1):165‐168. 10.1210/jcem.78.1.8288699 [DOI] [PubMed] [Google Scholar]
- 17. Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet Gynecol. 1999;180(1 Pt 1):60‐63. 10.1016/s0002-9378(99)70150-x [DOI] [PubMed] [Google Scholar]
- 18. Solomon CG, Seely EW. Brief review: hypertension in pregnancy: a manifestation of the insulin resistance syndrome? Hypertension. 2001;37(2):232‐239. 10.1161/01.hyp.37.2.232 [DOI] [PubMed] [Google Scholar]
- 19. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8(1):8–41. 10.1186/1741-7015-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolf M, Sandler L, Munoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: a prospective study. J Clin Endocrinol Metab. 2002;87(4):1563‐1568. 10.1210/jcem.87.4.8405 [DOI] [PubMed] [Google Scholar]
- 21. Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126(2):1165‐1172. 10.1210/endo-126-2-1165 [DOI] [PubMed] [Google Scholar]
- 22. Gibson DA, Simitsidellis I, Kelepouri O, Critchley HOD, Saunders PTK. Dehydroepiandrosterone enhances decidualization in women of advanced reproductive age. Fertil Steril. 2018;109(4):728–734.e2. 10.1016/j.fertnstert.2017.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Kreisberg JI, Ghosh PM. Cross‐talk between the androgen receptor and the phosphatidylinositol 3‐kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7(6):591‐604. 10.2174/156800907781662248 [DOI] [PubMed] [Google Scholar]
- 24. Yanase T, Fan W. Modification of androgen receptor function by IGF‐1 signaling implications in the mechanism of refractory prostate carcinoma. Vitam Horm. 2009;80:649‐666. 10.1016/S0083-6729(08)00623-7 [DOI] [PubMed] [Google Scholar]
- 25. Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross‐talk in prostate cancer cells. Endocr Relat Cancer. 2008;15(4):841‐849. 10.1677/ERC-08-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast‐derived signals. Hum Reprod. 2010;25(4):862‐873. 10.1093/humrep/dep468 [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez M, Neufeld J, Reimann K, et al. Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin‐binding epidermal growth factor‐like growth factor and interleukin‐1 beta. Mol Hum Reprod. 2011;17(7):421‐433. 10.1093/molehr/gar015 [DOI] [PubMed] [Google Scholar]
- 28. Grewal S, Carver J, Ridley AJ, Mardon HJ. Human endometrial stromal cell rho GTPases have opposing roles in regulating focal adhesion turnover and embryo invasion in vitro. Biol Reprod. 2010;83(1):75‐82. 10.1095/biolreprod.109.080630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grewal S, Carver JG, Ridley AJ, Mardon HJ. Implantation of the human embryo requires Rac1‐dependent endometrial stromal cell migration. Proc Natl Acad Sci USA. 2008;105(42):16189‐16194. 10.1073/pnas.0806219105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teklenburg G, Salker M, Molokhia M, et al. Natural Selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS ONE. 2010;5(4):e10258. 10.1371/journal.pone.0010258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wongwananuruk T, Sato T, Kajihara T, et al. Endometrial androgen signaling and decidualization regulate trophoblast expansion and invasion in co‐culture: A time‐lapse study. Placenta. 2016;47:56‐62. 10.1016/j.placenta.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 32. Jauniaux E, Burton GJ. Pathophysiology of Placenta Accreta Spectrum Disorders: A Review of Current Findings. Clin Obstet Gynecol. 2018;61(4):743‐754. 10.1097/GRF.0000000000000392 [DOI] [PubMed] [Google Scholar]
- 33. Weimar CH, Macklon NS, Post Uiterweer ED, Brosens JJ, Gellersen B. The motile and invasive capacity of human endometrial stromal cells: implications for normal and impaired reproductive function. Hum Reprod Update. 2013;19(5):542‐557. 10.1093/humupd/dmt025 [DOI] [PubMed] [Google Scholar]
- 34. Alfaidy N, Hoffmann P, Boufettal H, et al. The multiple roles of EG‐VEGF/PROK1 in normal and pathological placental angiogenesis. Biomed Res Int. 2014;2014:1–10. 10.1155/2014/451906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffmann P, Saoudi Y, Benharouga M, et al. Role of EG‐VEGF in human placentation: Physiological and pathological implications. J Cell Mol Med. 2009;13(8B):2224‐2235. 10.1111/j.1582-4934.2008.00554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ujvari D, Graells Brugalla C, Hirschberg AL. Dihydrotestosterone potentiates insulin to up‐regulate prokineticin‐1 in decidualizing human endometrial stromal cells. J Cell Mol Med. 2020;24(5):3242‐3245. 10.1111/jcmm.14923 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Table S2
Table S3
Video S1
Video S2
Video S3
Video S4
Supplementary Material
Data Availability Statement
The data that support the findings are available from the corresponding author upon reasonable request.


