Abstract
Purpose
The aim of this study was to show that bariatric surgery (BS) is more effective than medical therapy (MT) in Asian obese patients.
Methods
In this prospective, multicenter, nonrandomized, controlled trial, obese patients with body mass index of ≥35 kg/m2 or 30.0–34.9 kg/m2 with obesity-related comorbidities were assigned to undergo BS, such as laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass, or MT. Patients who underwent BS were evaluated 4, 12, 24, and 48 weeks after surgery, whereas patients who received MT were monitored at a hospital every 6 weeks for 1 year. At each visit, weight, waist and hip circumference, and blood pressure were measured, and patients underwent physical examination and laboratory testing. Health-related quality of life (HQOL) was investigated using Euro QOL-5 Dimension, Impact of Weight on Quality of Life questionnaire-Lite and Obesity-related Problems scale.
Results
The study included 264 patients from 13 institutions; of these, 64 underwent BS and 200 received MT. Of the patients who underwent BS, 6.3% experienced early complications. Relative weight changes from baseline to 48 weeks were significantly greater in the BS than in the MT group (26.9% vs. 2.1%, P < 0.001), as were the rates of remission of diabetes (47.8% vs. 16.7%, P = 0.014), hypertension (60.0% vs. 26.1%, P < 0.001), and dyslipidemia (63.2% vs. 22.0%, P < 0.001). HQOL was better in the BS than in the MT group at 48 weeks.
Conclusion
BS was safe and effective in Korean obese patients, with greater weight reduction, remission of comorbidities, and quality of life improvement than MT.
Keywords: Asia, Bariatric surgery, Metabolic diseases, Obesity
INTRODUCTION
The rapid increase in obesity rates worldwide and the greater awareness of obesity as a risk factor for chronic diseases have increased the need for obesity management, with many health policies including obesity as a medical condition. Similar findings have been observed in Asian countries. The prevalence of obesity is lower in Asia than in Western countries, but the prevalence of and mortality from obesity-related cardiovascular diseases are high, and the body mass index (BMI) cutoff for obesity is set lower than in the West [1,2]. Although there are some differences among countries, the percentage of obese individuals with BMI of 25–30 kg/m2 has decreased or been stagnant, whereas the percentage with BMI of >30 kg/m2 has increased continuously in Asian countries, including in Korea [3]. Similar trends have also been observed in Asian children and adolescents [4].
Bariatric surgery (BS) as a treatment for severe obesity was first performed in the West in the 1950s [5,6]. In Asia, however, the performance of BS began to increase in the late 2000s due to lack of indications and technical or cultural reasons [7]. Although BS in Korea was first performed in the early 2000s [8], few studies have assessed outcomes of BS in Korean as well as Asian patients. Moreover, previous studies have been limited to retrospective observational studies or studies of BS in patients with obesity and specific comorbidities such as diabetes. Although BS has been reported to ameliorate underlying conditions, such as diabetes, hypertension, and dyslipidemia, and to improve quality of life [9,10,11], these effects have not been thoroughly investigated in Asian patients.
Indications for BS in Western studies including a BMI of >35 kg/m2. Because BMI is generally lower in Asians than in Western obese individuals, studies of the effects of BMI on Asian individuals with BMI of 30–35 kg/m2 are necessary. This prospective clinical trial evaluated the efficacy and safety of BS in Korean obese individuals, with a BMI of >30 kg/m2, in comparison with medical therapy (MT).
METHODS
Patients and study design
This prospective multicenter clinical trial, performed at 13 university hospitals from August 2016 to April 2019, compared BS with MT in Korean patients with obesity. Obese patients were eligible if they had a BMI of ≥35 kg/m2 or a BMI of 30.0–34.9 kg/m2 with obesity-related comorbidities, such as diabetes, hypertension, or hyperlipidemia. Diabetes was defined as a fasting glucose concentration of ≥126 mg/dL, an HbA1c concentration of ≥6.5%, or treatment with antidiabetic medications. Hypertension was defined as a systolic blood pressure of ≥140 mmHg, a diastolic blood pressure of ≥90 mmHg, or treatment with antihypertensive agents. Dyslipidemia was defined as a total cholesterol concentration of ≥240 mg/dL, a triglyceride concentration of ≥200 mg/dL, a low-density lipoprotein cholesterol concentration of ≥160 mg/dL, a high-density lipoprotein cholesterol concentration of ≤40 mg/dL, or treatment with antihyperlipidemic medications. Patients were assigned to undergo BS or receive MT based on their personal preference. Patients in the BS group underwent Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) based on their personal medical condition, including the risk of gastric cancer, reflux esophagitis, and mental health status as determined by interviews with investigators. Patients in the MT group visited hospitals regularly and were treated mainly with medication (lorcaserin). Diet and exercise played an auxiliary role, and the methods were varied. MT group was treated by a doctor of Department of Family Medicine.
Patients who underwent BS were examined at 4, 12, 24, and 48 weeks after surgery, whereas those who received MT were monitored at a hospital every 6 weeks for 1 year. At each visit, weight, waist and hip circumference, and blood pressure were measured, and patients underwent physical examination and laboratory testing. Health-related quality of life (HQOL) was investigated using Euro QOL-5 Dimension (EQ-5D) 3-level, Impact of Weight on Quality of Life questionnaire (IWQoL)-Lite, and Obesity-related Problems (OP) scale.
This study was registered at ClinicalTrials.gov (NCT03100292) and approved by the Institutional Review Board of each center (approval number for the coordinating investigator: INHA 2016- 06-015). All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All subjects voluntarily agreed to participate in this study, and all provided written informed consent.
Clinical effectiveness
The primary clinical outcome was percent weight change from baseline 1 year after BS or MT. Secondary outcomes included changes in waist circumference and waist to hip ratio (WHR), and remission rate of obesity-related comorbidities such as hypertension, type 2 diabetes, and dyslipidemia, at 1 year. The remission of obesity-related comorbidities was defined as the normal value of all related laboratory assessments without medication.
The validated Korean versions of the EQ-5D (EQ-5D 3 level, EQ-5D visual analog scale [VAS]), IWQoL-Lite, and OP scale were used. EQ-5D 3 level had 5 dimensions, including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, with utility scores ranging from 0 to 1 (perfect health status) [12].
EQ-5D VAS was measured on a scale of 0 (worst imaginable state of health) to 100 (best imaginable state of health). IWQoL-Lite had 5 dimensions: physical function, self-esteem, sexual life, public distress, and work. Each question had 5 points from 0 to 5, these scores were converted to values of 0 to 100 (i.e., better health status) [13]. The OP scale, which measures psychological and social stresses related to obesity, consists of 8 body weight-related items that cause problems for obese individuals. These 8 items were private gatherings on one’s own, private gatherings at a friend or relative’s home, going to a restaurant, going to community activities, courses, etc., vacations away from home, trying on and buying clothes, bathing in public baths, and intimate relations [14]. Scores are converted to a scale of 0 to 100, with higher scores indicating greater psychological and social stress.
Data collection and statistical analysis
Sample size based on the primary endpoint (change in body weight) was fewer than 5 people per group using previous results [8] of 22.6% vs. 6.7% with 5% alpha error and 90% power. In addition, sample size based on the secondary endpoint of diabetes remission was 58 per group, using previous results of 18.4% vs. 0.9% with 5% alpha error and 90% power. In considering the number of patients treated for obesity, 64 patients were enrolled in the BS group and 200 in the MT group.
Data were recorded from electronic case report forms. This study included centralized monitoring and regular site monitoring every 2 months based on Data Validation Specification. Continuous variables were reported as mean and standard deviation and compared by 2-sided independent sample t-tests, and categorical variables were reported as frequency and percentage and compared by the chi-square tests. In addition, multivariable logistic regression was used to investigate the associations of BMI reduction to <25 kg/m2 and remission of comorbidities with obesity treatment method, after adjusting for covariates. The results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). A P-value of <0.05 was considered statistically significant. Most patients (93.7%) in the BS group, but only 49.0% in the MT group, were followed up for 1 year. Thus, missing data were analyzed using the Last Observation to Carried Forward (LOCF) method.
RESULTS
Clinical characteristics
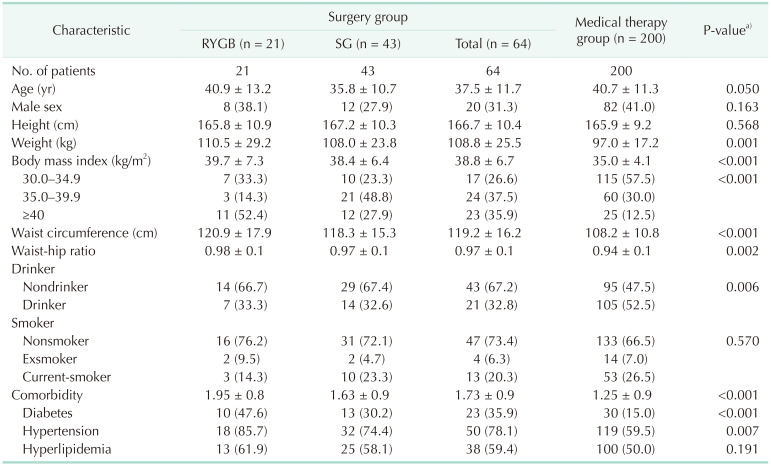
A total of 311 subjects, including 76 who underwent BS and 235 who received MT, provided written informed consent for study participation. Based on inclusion and exclusion criteria, 264 subjects, 64 who underwent BS and 200 who received MT, participated in this study (Supplementary Fig. 1). The baseline demographic and clinical characteristics of the 2 groups are presented in Table 1. Mean age (37.5 years vs. 40.7 years) and the percentage of men (31.1% vs. 41.0%) were lower, whereas mean body weight was significantly higher (108.8 kg vs. 97.0 kg), in the BS than in the MT group. In addition, BMI, WHR, number of comorbidities, and rates of diabetes and hypertension were significantly higher in the BS group.
Table 1. Baseline characteristics of the patients.
Values are presented as number only, mean ± standard deviation, or number (%).
RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
a)Surgery vs. medical therapy.
Clinical effectiveness and quality of life
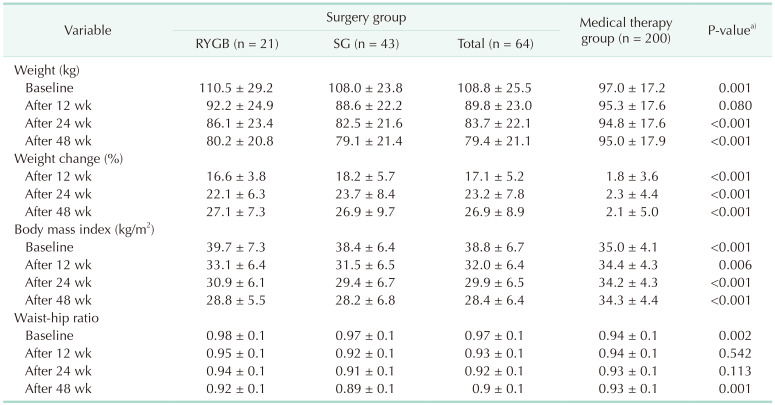
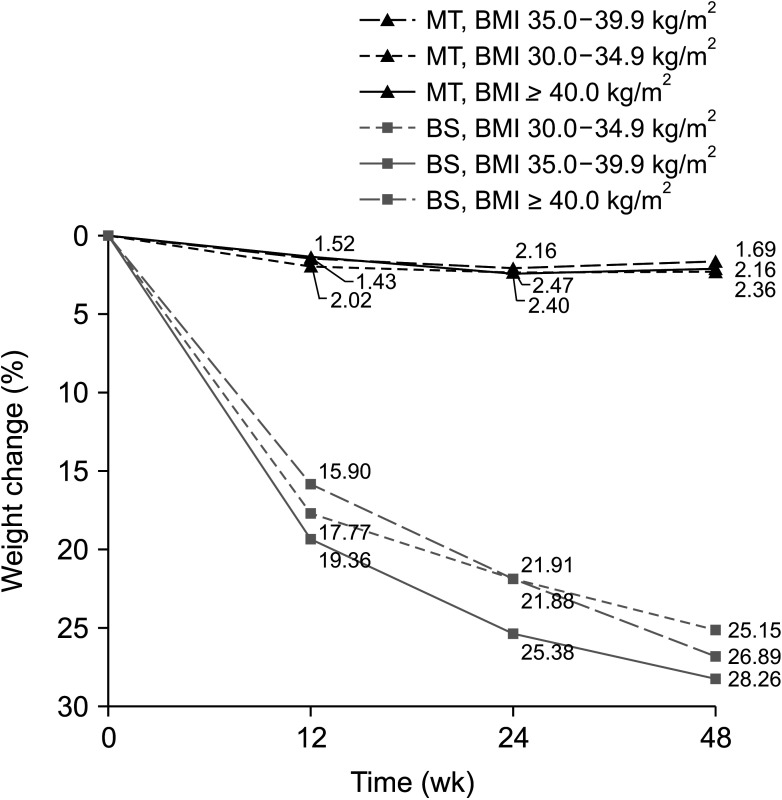
Table 2 shows clinical outcomes in the 2 groups of patients. Percent weight change from baseline to 48 weeks was significantly greater in the BS than in the MT group (26.9% vs. 2.1%, P < 0.001). The change of weight in the BS group was increased steadily over time, being 17.1%, 23.2%, and 26.9% at 12, 24, and 48 weeks, respectively. By contrast, there was little change in body weight over time in the MT group, being 1.8%, 2.3%, and 2.1% at 12, 24, and 48 weeks, respectively. Similar results were observed when BMI and WHR were compared in the BS and MT groups. The percent change in weight by BMI category at baseline (30.0–34.9 kg/m2, 35.0–39.9 kg/m2, and ≥40.0 kg/m2) did not differ significantly between the 2 groups (Fig. 1). HQOL scores are shown in Supplementary Fig. 2. At baseline, HQOL scores for all instruments were poorer in the BS than in the MT group. This difference, however, was reversed over time, with subjects in the BS group having better HQOL scores for all instruments after 48 weeks, regardless of poorer HQOL at baseline.
Table 2. Outcomes between surgery and conventional therapy group.
Values are presented as mean ± standard deviation.
RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
aSurgery vs. medical therapy.
Fig. 1. Percentages of weight change. MT, medical therapy group; BMI, body mass index; BS, bariatric surgery group.
Changes in comorbidities and laboratory assessments
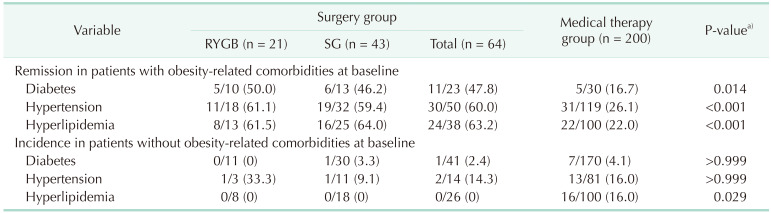
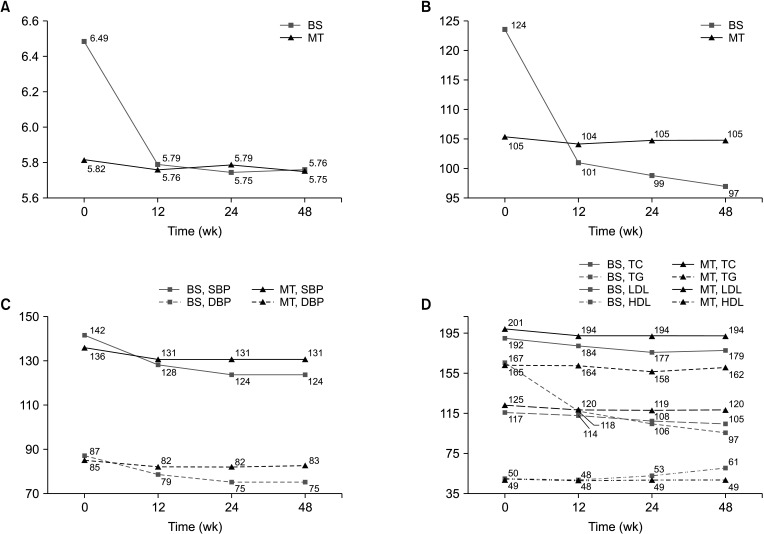
The remission and incidence of obesity-related comorbidities were presented in Table 3. Remission rates for diabetes (47.8% vs. 16.7%), hypertension (60.0% vs. 26.1%) and dyslipidemia (63.2% vs. 22.0%) were all significantly higher in the BS than in the MT group. The incidence of new-onset comorbidities after 48 weeks, among subjects who had no comorbidities at baseline, were more frequent in the MT than in the BS group. Fig. 2 shows changes in laboratory assessments in the 2 groups, regardless of medication administration. The subjects in the BS group showed improvements in all laboratory parameters, whereas subjects in the MT group showed almost no changes.
Table 3. Change of obesity-related comorbidities.
Values are presented number/total number (%).
RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
a)Surgery vs. medical therapy.
Fig. 2. Laboratory results. (A) HbA1c, (B) fasting blood sugar (FBS), (C) systolic (SBP) and diastolic blood pressure (DBP), and (D) total cholesterol (TC), TG, LDL, HDL. BS, bariatric surgery group; MT, medical therapy group.
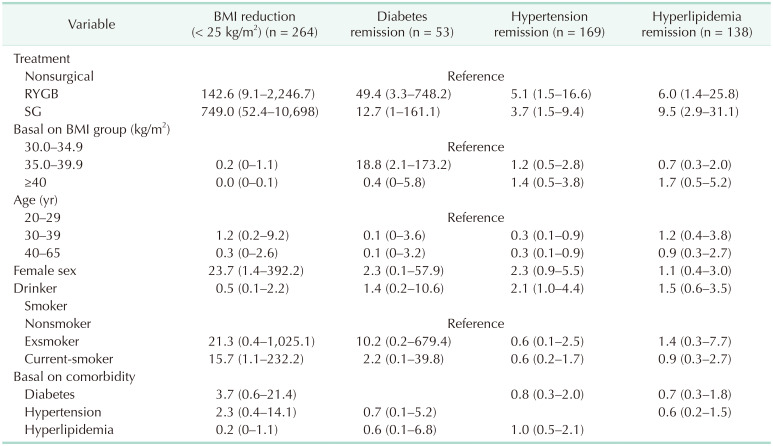
Factors associated with BMI reduction to <25 kg/m2 and remission of comorbidities
Table 4 shows the results of multivariable logistic regression analysis of the association between BMI reduction to <25 kg/m2 or remission of comorbidities with obesity treatment method. Although BS was the main factor associated with BMI reduction, baseline BMI had little effect on the decline in BMI. BS was also the main factor for remission of diabetes, hypertension, and dyslipidemia. Compared with the MT group, remission of diabetes was significantly greater in subjects who underwent RYGB (OR, 49.4; 95% CI, 3.3–748.2) and marginally greater in those who underwent SG (OR, 12.7; 95% CI, 1.0–161.1).
Table 4. Factors associated BMI reduction (< 25 kg/m2) and comorbidities remission after obesity treatment.
Values are presented as odds ratio (95% confidence interval).
BMI, body mass index; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
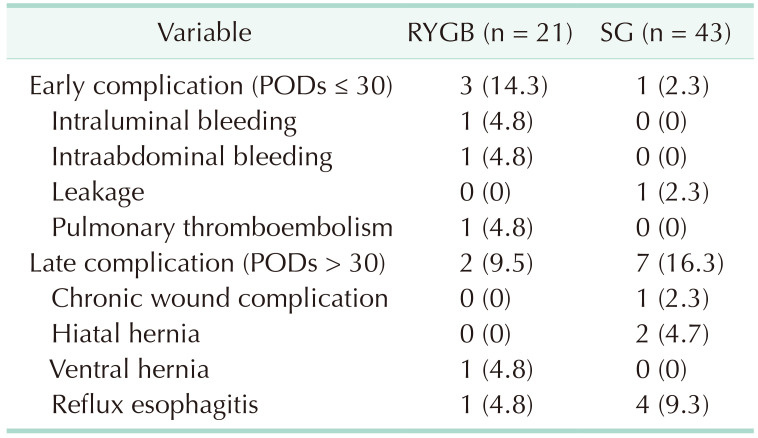
Postoperative complications
Postoperative complications in the BS group are shown in Table 5. Four patients (6.3%), including 3 who underwent RYGB and 1 who underwent SG, experienced early complications within 30 days, whereas 9 (14.1%), 2 who underwent RYGB and 7 who underwent SG, experienced late complications after 30 days.
Table 5. Postoperative complications in the surgery group.
Values are presented as number (%).
RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; POD, postoperation day.
DISCUSSION
To our knowledge, this study is the first prospective clinical trial conducted in Korea comparing the efficacy and safety of BS and MT in patients with BMI of >35.0 kg/m2 or 30.0–34.9 kg/m2 with obesity-related conditions. After 48 weeks, rates of body weight change and comorbidity improvements were greater in the BS than in the MT group, indicating that BS is effective in obese Korean individuals. Other studies have also shown that BS is effective in patients with obesity [15,16,17,18], with current obesity guidelines suggesting that surgical therapy is an effective treatment for severe obesity [19,20].
Most Western studies have been conducted on obese populations with a BMI of >35 kg/m2. The present study, however, included obese individuals with a BMI of 30–35 kg/m2, indicating that the therapeutic effect of BS on BMI is the same in both subpopulations. Similarly, BS has been shown to be effective in the treatment of Western patients with relatively lower BMI and with diabetes [11,21].
The remission of obesity-related comorbidities after BS correlates with weight loss. In the present study, the remission rates of diabetes, hypertension, and hyperlipidemia were 47.8%, 60.0%, and 63.2%, respectively. Remission rates of diabetes, hypertension, and hyperlipidemia after BS were 66.7%, 38.2%, and 60.4%, respectively, in a recent meta-analysis [9] and 78%, 69%, and 60%, respectively, in another study [22]. In addition, a study in Japan found that 3-year remission rates of diabetes, hypertension, and dyslipidemia after BS were 78%, 60%, and 65%, respectively [16]. Compared with these studies, remission rates in the BS group of the present study were rather low. By contrast, the remission rates of comorbidities in the MT group were higher than previously reported [23]. This may be due to this study being a prospective clinical trial in which patients visited clinics and received intensive medical care every 6 weeks, whereas previous studies were observational retrospective analyses.
Diabetic remission rates have been reported in several prospective randomized trials, as well as observational studies and meta-analyses. A prospective randomized clinical trial of patients in the United States with type 2 diabetes and BMI of 30–40 kg/m2 found that the diabetes remission rate 12 months after RYGB was 50% [21]. Moreover, rates of diabetes remission, defined as HbA1c of ≤6.0% without taking diabetic drugs, at 12 months after SG and RYGB were 27% and 42%, respectively [11], similar to the diabetes remission rate observed in the BS group of the present study.
The 20-year follow-up of the Swedish Obese Subjects (SOS) study suggested that the lowest weight loss 1–2 years after BS was slightly below baseline weight, followed by slight increases over time. In particular, weight loss rate 20 years after RYGB was >25%. A retrospective study in Korea confirmed that bodyweight loss was maintained for at least 5 years after surgery [24]. The SOS study also found that the remission rate of obesity-related comorbidities, such as diabetes, tended to decrease slightly 2 years after surgery [25]. In Korea, it is necessary to continuously monitor changes in obesity-related comorbidities obesity as well as weight loss after BS.
The present study also evaluated the incidence of comorbidities after obesity treatment. Although there were no significant between-group differences in rates of treatment-associated comorbidities, comorbidities occurred in both groups. For example, the incidence of comorbidities was reported to be 0%–4% in patients who underwent surgery and 5%–26% in those treated nonsurgically group [23]. In that study, the incidence rates of diabetes mellitus (2.4% [1 of 41] vs. 4.1% [7 of 170]), hypertension (14.3% [2 of 14] vs. 16.0% [13 of 81]), and hyperlipidemia (0% [0 of 26] vs. 16.0% [16 of 100]) were generally lower in the surgery than in the nonsurgery groups. However, the duration of follow-up in that study was insufficient for assessing the development of comorbidities after obesity treatment.
The present study investigated the quality of life before and after obesity treatment using 3 types of questionnaires; EQ-5D, which measures the general quality of life and IWQoL-Lite and OP scale, which measure obesity-specific quality of life. Surgery improved patient quality of life, confirming the results of a systematic review of quality of life after obesity surgery [10]. In contrast, the quality of life in the MT group showed little change, except for IWQoL-Lite. This trend was similar to that reported in a retrospective study of highly obese patients [26].
The incidence of early complications within 30 days after surgery was 6.3%, a rate lower than previously reported rates of 8.4%–17.2% [8,27,28]. However, the incidence of late complications, occurring more than 30 days after surgery was 14.1%, higher than previously reported rates of 2.5%–12.3% [8,29]. Two patients in the present study had hiatal hernias after SG, as shown by endoscopic examination.
This study had several limitations. First, allocation to the BS or MT group was not randomized. Randomized clinical trials comparing surgical and nonsurgical interventions are difficult to perform. For example, a recent randomized control trial comparing BS and MT in diabetic patients showed that rates of randomization after screening were low at 10.3%, with 10% of patients randomized to surgery disagreeing with the assigned treatment [21]. Thus, because of ethical issues as well as the time and costs associated with randomization to surgical and nonsurgical treatments, patients in the present study were not randomized. Second, the observation period was only 1 year, making it difficult to determine the long-term effects of BS. Third, the LOCF method was selected to impute missing values based on the missing completely at random (MCAR) hypothesis. Obesity, however, is expected to improve over time with treatment, making the LOCF method a conservative approach. Despite these limitations, this study was the first prospective clinical trial to evaluate the effectiveness and safety of BS in obese Koreans and to confirm the effect of BS according to baseline BMI.
In conclusion, this prospective clinical trial compared the efficacy and safety of BS with MT in Korean obese patients. The BS group showed significantly greater reductions in weight and remission of associated diseases than the MT group regardless, of the BMI category. In addition, improvements in patient quality of life were greater after BS than after MT.
Footnotes
Fund/Grant Support: This work was supported by grants from the Korean Health Technology R&D Project (HC15C1322), Ministry of Health & Welfare, Republic of Korea.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: YH, JHK, JWK, DJP, YJK, SMH.
- Formal Analysis: JWK, SA, JHL.
- Investigation: DJP, YSP, JHK, JWK, TKH, SUH.
- Methodology: YH, JHK, JWK, SUH, HJL, SWR, MWY.
- Project Administration: YH, JHK, DJP, JWK, SP.
- Writing — Original Draft: DJP, YSP, JWK, MWY.
- Writing — Review & Editing: All authors.
SUPPLEMENTARY MATERIALS
Supplementary Figs. 1 and 2 can be found via https://doi.org/10.4174/astr.2021.101.4.197.
CONSORT (Consolidated Standards of Reporting Trials) diagram. SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass.
Quality of life according to treatments. (A) Euro QOL-5 Dimension (EQ-5D) 3-level, (B) EQ-5D visual analog scale, (C) Impact of Weight on Quality of Life questionnaire-Lite, and (D) Obesity-related Problems scale. BS, bariatric surgery group; MT, medical therapy group; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; HQOL, health-related quality of life.
References
- 1.Deurenberg-Yap M, Yian TB, Kai CS, Deurenberg P, VAN Staveren WA. Manifestation of cardiovascular risk factors at low levels of body mass index and waist-to-hip ratio in Singaporean Chinese. Asia Pac J Clin Nutr. 1999;8:177–183. doi: 10.1046/j.1440-6047.1999.00091.x. [DOI] [PubMed] [Google Scholar]
- 2.Low S, Clin MC, Ma S, Heng D, Deurenberg-Yap M. Rationale for redefining obesity in Asians. Ann Acad Med Singap. 2009;38:66–69. [PubMed] [Google Scholar]
- 3.Shin HY, Kang HT. Recent trends in the prevalence of underweight, overweight, and obesity in Korean adults: the Korean National Health and Nutrition Examination Survey from 1998 to 2014. J Epidemiol. 2017;27:413–419. doi: 10.1016/j.je.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celio AC, Pories WJ. A history of bariatric surgery: the maturation of a medical discipline. Surg Clin North Am. 2016;96:655–667. doi: 10.1016/j.suc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Phillips BT, Shikora SA. The history of metabolic and bariatric surgery: development of standards for patient safety and efficacy. Metabolism. 2018;79:97–107. doi: 10.1016/j.metabol.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Umemura A, Lee WJ, Sasaki A, Wakabayashi G. History and current status of bariatric and metabolic surgeries in East Asia. Asian J Endosc Surg. 2015;8:268–274. doi: 10.1111/ases.12190. [DOI] [PubMed] [Google Scholar]
- 8.Heo YS, Park JM, Kim YJ, Kim SM, Park DJ, Lee SK, et al. Bariatric surgery versus conventional therapy in obese Korea patients: a multicenter retrospective cohort study. J Korean Surg Soc. 2012;83:335–342. doi: 10.4174/jkss.2012.83.6.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puzziferri N, Roshek TB, 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312:934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raaijmakers LC, Pouwels S, Thomassen SE, Nienhuijs SW. Quality of life and bariatric surgery: a systematic review of short-and long-term results and comparison with community norms. Eur J Clin Nutr. 2017;71:441–449. doi: 10.1038/ejcn.2016.198. [DOI] [PubMed] [Google Scholar]
- 11.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YK, Nam HS, Chuang LH, Kim KY, Yang HK, Kwon IS, et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health. 2009;12:1187–1193. doi: 10.1111/j.1524-4733.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 13.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan M, Karlsson J, Sjöström L, Backman L, Bengtsson C, Bouchard C, et al. Swedish obese subjects (SOS): an intervention study of obesity. Baseline evaluation of health and psychosocial functioning in the first 1743 subjects examined. Int J Obes Relat Metab Disord. 1993;17:503–512. [PubMed] [Google Scholar]
- 15.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and metaanalysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haruta H, Kasama K, Ohta M, Sasaki A, Yamamoto H, Miyazaki Y, et al. Long-term outcomes of bariatric and metabolic surgery in Japan: results of a multi-institutional survey. Obes Surg. 2017;27:754–762. doi: 10.1007/s11695-016-2361-3. [DOI] [PubMed] [Google Scholar]
- 17.Park CH, Nam SJ, Choi HS, Kim KO, Kim DH, Kim JW, et al. Comparative efficacy of bariatric surgery in the treatment of morbid obesity and diabetes mellitus: a systematic review and network metaanalysis. Obes Surg. 2019;29:2180–2190. doi: 10.1007/s11695-019-03831-6. [DOI] [PubMed] [Google Scholar]
- 18.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial: a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 19.Fried M, Hainer V, Basdevant A, Buchwald H, Deitel M, Finer N, et al. Interdisciplinary European guidelines on surgery of severe obesity. Obes Facts. 2008;1:52–59. doi: 10.1159/000113937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan DH, Kahan S. Guideline recommendations for obesity management. Med Clin North Am. 2018;102:49–63. doi: 10.1016/j.mcna.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149:707–715. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatoum IJ, Blackstone R, Hunter TD, Francis DM, Steinbuch M, Harris JL, et al. Clinical factors associated with remission of obesity-related comorbidities after bariatric surgery. JAMA Surg. 2016;151:130–137. doi: 10.1001/jamasurg.2015.3231. [DOI] [PubMed] [Google Scholar]
- 23.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JY, Heo Y, Kim YJ, Park JM, Kim SM, Park DJ, et al. Long-term effect of bariatric surgery versus conventional therapy in obese Korean patients: a multicenter retrospective cohort study. Ann Surg Treat Res. 2019;96:283–289. doi: 10.4174/astr.2019.96.6.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 26.Oh SH, Song HJ, Kwon JW, Park DJ, Lee YJ, Chun H, et al. The improvement of quality of life in patients treated with bariatric surgery in Korea. J Korean Surg Soc. 2013;84:131–139. doi: 10.4174/jkss.2013.84.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falk V, Twells L, Gregory D, Murphy R, Smith C, Boone D, et al. Laparoscopic sleeve gastrectomy at a new bariatric surger y centre in Canada: 30-day complication rates using the Clavien-Dindo c lassi f icat ion. Can J Surg. 2016;59:93–97. doi: 10.1503/cjs.016815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterli R, Borbély Y, Kern B, Gass M, Peters T, Thurnheer M, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258:690–695. doi: 10.1097/SLA.0b013e3182a67426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterli R, Wölnerhanssen BK, Vetter D, Nett P, Gass M, Borbély Y, et al. Laparoscopic sleeve gastrectomy versus Roux-Y-gastric bypass for morbid obesity-3-year outcomes of the prospective randomized Swiss Multicenter Bypass or Sleeve Study (SM-BOSS) Ann Surg. 2017;265:466–473. doi: 10.1097/SLA.0000000000001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT (Consolidated Standards of Reporting Trials) diagram. SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass.
Quality of life according to treatments. (A) Euro QOL-5 Dimension (EQ-5D) 3-level, (B) EQ-5D visual analog scale, (C) Impact of Weight on Quality of Life questionnaire-Lite, and (D) Obesity-related Problems scale. BS, bariatric surgery group; MT, medical therapy group; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; HQOL, health-related quality of life.