Abstract
Background
The approval of monoclonal antibodies for prevention of migraine has revolutionized treatment for patients. Oral preventatives are still considered first line treatments as head-to-head trials comparing them with antibodies are lacking.
Methods
The main purpose of this study was to provide a comparative overview of the efficacy of three commonly prescribed migraine preventative medication classes. For this systematic review and meta-analysis, we searched the databases CENTRAL, EMBASE, and MEDLINE until 20 March 2020. We included RCTs reporting the 50% response rates for topiramate, Botulinum Toxin Type A and monoclonal antibodies against CGRP(r). Studies were excluded if response rates were not reported, treatment allocation was unclear, or if study quality was insufficient. Primary outcome measure were the 50% response rates. The pooled odds ratios with 95% confidence intervals were calculated with the random effects model. The study was registered at PROSPERO (CRD42020222880).
Findings
We identified 6552 reports. Thirty-two were eligible for our review. Studies assessing monoclonal antibodies included 13,302 patients and yielded pooled odds ratios for the 50% response rate of 2.30 (CI: 2.11–2.50). Topiramate had an overall effect estimate of 2.70 (CI: 1.97–3.69) with 1989 included patients and Botulinum Toxin Type A achieved 1.28 (CI: 0.98–1. 67) with 2472 patients included.
Interpretation
Topiramate, botulinum toxin type A and monoclonal antibodies showed higher odds ratios in achieving a 50% response rate compared to placebo. Topiramate numerically demonstrated the greatest effect size but also the highest drop-out rate.
Keywords: Migraine disorders, prevention, treatment, efficacy, responder rate, outcome
Introduction
Migraine is a frequent and highly debilitating disorder ranking second regarding years lived with disability (1). Recognizing the high prevalence of more than 12% and the fact that it peaks in the most productive years of life has sparked an increasing interest in medical and pharmaceutical research for this disease (2–4). For many patients, the abortive treatment of the acute attack with simple analgesics or triptans is insufficient to taper the burden of the disease, as these medications are ineffective in at least 30% of attacks, may be poorly tolerated, and may even worsen the migraine if overused. Besides ineffective acute medication, physicians’ decision to start preventive therapy is based on attack frequency, headache days, severity of attacks, and impact on the patient’s quality of life. Thus, more than a third of the patients qualify for prophylactic treatment (5–9).
The desired outcome of preventative treatments for migraine is a reduction in monthly migraine days (MMD) or migraine frequency, as well as the reduction of the severity of migraine attacks. These outcomes have first been observed with pharmacological compounds that have been initially developed for other indications than migraine. The adherence to these oral preventive anti-migraine treatments is still somewhat disappointing: They are ineffective in 40–50% of patients and this, together with poor tolerance due to side effects, explains why over 60% of chronic migraine sufferers abandon them after 2 months (10). There is thus a real need for better-performing and especially better-tolerated treatments (11). Neurologists, headache specialists and patients eagerly awaited the approval of human monoclonal antibodies (mABs) targeting calcitonin gene-related peptide (CGRP) or its receptor (CGRPr). They were developed specifically for migraine prevention and demonstrated good efficacy and low adverse reaction rates in various clinical trials (12). However, major drawbacks in the therapy with mABs in migraine prevention are the lack of long-term data and the costs that render them a second- or third-line option in many countries to date. Therefore, it is necessary to evaluate the risk- and cost-benefit ratio for each individual patient before initiating therapy. The efficacy of pharmacological agents is vital information needed to assess this ratio. A common measurement to assess efficacy is the response rates. The 50% response rate depicts the percentage of patients with a reduction of mean monthly migraines of at least 50% compared to baseline. In this systematic review and meta-analysis, we evaluated the efficacy as expressed with the 50% response rate for topiramate (TPM), botulinum toxin type A (BoNTA), and CGRP pathway monoclonal antibodies (mABs).
Methods
The aim of this review was to provide a comparison between monoclonal antibodies targeting the CGRP pathway and the already established therapeutic means, TPM and BoNTA, using a meta-analysis. TPM and BoNTA were selected for this review as they are usually administered to a collective of patients that qualifies for treatment with monoclonal antibodies.
Literature search
This review was conducted in adherence with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement (13). We performed a systematic search of the Cochrane Controlled Trials Register (CENTRAL), EMBASE, and MEDLINE. The search strategy was established to include published clinical trials assessing the efficacy of preventative treatments for migraines. Language was restricted to English or German, and reference lists of retrieved studies or other meta-analyses were searched manually. In order not to omit any relevant data, the pharmacological agents were not restricted to mABs, TPM and BoNTA. Relevant studies were searched through 20 May 2020. Our search strategy comprised four concepts defining disorder, application, intervention, and outcome. We used free text terms as well as controlled vocabulary terms (MeSH).
The detailed search strategy and keywords are included in the supplemental material.
Study selection
Two researchers screened the titles and abstracts of the candidate studies individually for inclusion and exclusion criteria. Duplicate studies were excluded from data extraction. For study selection flow, see Figure 1.
Figure 1.
Flow diagram of study selection process. The database search yielded more than 6000 results. Of these, 2812 duplicates were identified. All drug regimens have been considered for initial full-text review to include studies using mABs, TPM or BoNTA as comparators.
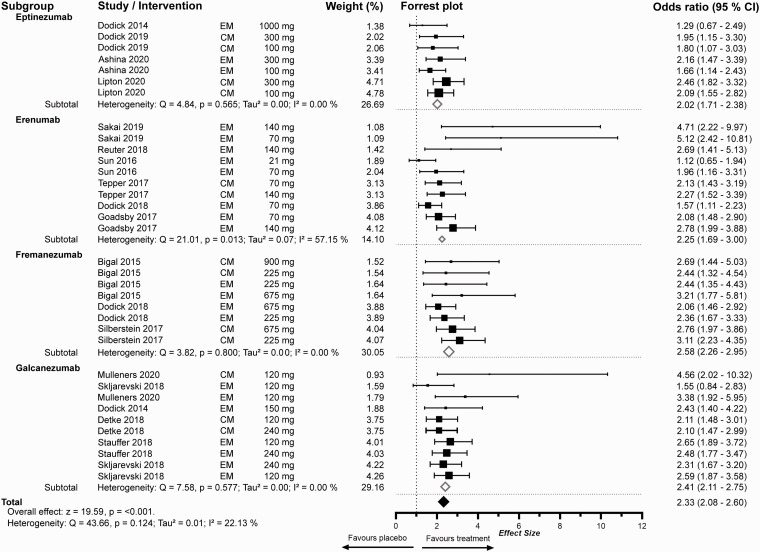
Figure 2.
Meta-analysis of 50% response rates of mABs for the prevention of migraine. The random effects model depicts the odds-ratios (OR) of individual studies and dosing regimens regarding our primary endpoint of 50% response rate compared to placebo. Studies are ordered by weight. Weighting was performed with the inverse variance method. Bigger boxes indicate higher weighting. The model’s confidence level was defined as 95%. Subgroup analysis was performed for the respective monoclonal antibody. Total effect size was calculated with consideration of the weighting of the respective subgroups in comparison to the total effect size presented in Figure 5 with weighting applied regarding to episodic or chronic migraine. The x-axis is presented as standard decimal plot. The study by Mulleners et al. 202039 evaluated Galcanezumab for chronic and episodic migraine.
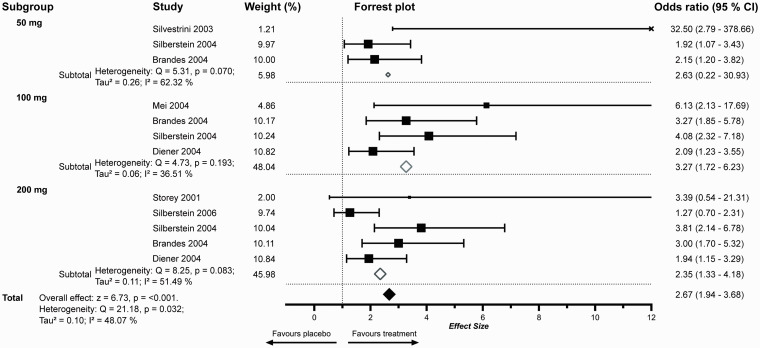
Figure 3.
Meta-Analysis of 50% response rates of TPM separated by dosing regimens. The x-axis is presented as standard decimal plot. Box sizes indicate weighting. OR outside of the selected interval is marked with (x). The study by Silvestrini et al. 200349 was the sole study evaluating TPM for chronic migraine that fulfilled our inclusion criteria. The sample size was comparatively low (n = 28), additionally only one subject in the placebo group reached a 50% response. TPM in the recommended doses of 100 or 200 mg was superior to placebo regarding the 50% response rate.
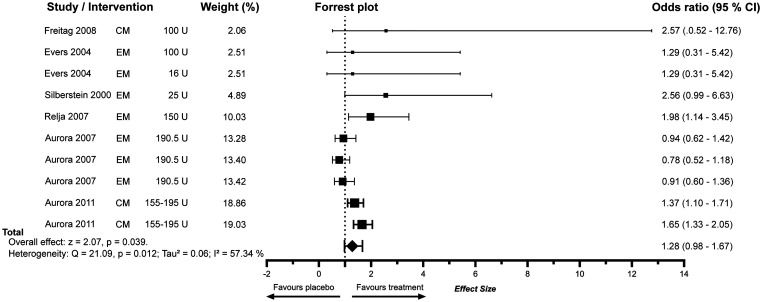
Figure 4.
Meta-analysis of 50% response rates of BoNTA. Studies are in ascending order of respective weight. Box sizes indicate weighting. The x-axis is presented as standard decimal plot.
For eligibility, trials had to be placebo-controlled and randomized. The defined diseases studied were episodic and chronic migraine according to the criteria valid at the time of conducting the trial (e.g. ICHD criteria) (14,15). The eligible pharmacologic interventions were TPM, BoNTA and mABs targeting CGRP for preventative migraine treatment. For this review, we included all available dosing regimens, regardless of the formal approval for the respective pharmacological agent. Studies were required to report response rates (the proportion of subjects reporting a reduction in migraine attack frequency or mean migraine days of 50%, 75% and/or 100%) and the number of subjects in each intervention group. As secondary outcome variables, we collected reduction in migraine days or headache days. Outcome variables reported as least square means were included and denoted as such.
We included placebo-controlled RCTs of sufficient quality reporting the 50% response rates for mABs against CGRP(r), TPM, and BoNTA. Studies were excluded if response rates were not reported, treatment allocation was unclear, or if study quality was insufficient. Therefore, we applied the JADAD score, the most widely used tool to assess the methodological quality of a clinical trial. JADAD allocates a score between zero (low quality) and five (high quality) to trials; we excluded studies with a JADAD score below 3 (16). Furthermore, we excluded studies assessing other headaches than migraine or migraine variants (i.e. menstrual migraine, status migrainosus, etc). Trials regarding any other species than human were not included.
Data extraction
Two investigators extracted the data independently. Information extracted comprised full title, authors, publication date, study population, interventions and duration of intervention, baseline data, outcomes, and potential source of bias. Risk of bias of all included studies was assessed using the Cochrane collaboration tool (17). Both individual patient-level data and summary estimates were extracted where available. The extracted data was entered in a purpose-built database and consolidated for relevant analyses by the lead investigator. For the primary outcome measurement, we compared the 50% response rates (RR50) of included studies. We chose the RR50 since a 50% response to any preventative migraine medication is used to assess efficacy in clinical practice and required as outcome by regulatory agencies.
Statistical analysis
Duplicates were identified and removed using the citation manager Mendeley® (Mendeley Ltd.). Furthermore, studies reporting the same dataset were identified manually. If the dataset comprised the whole information needed for our analyses, we selected the earlier report. If data were distributed between multiple reports, a master case was created encompassing all necessary data. Aggregated study data were transferred to Excel and meta-analyses were performed using MetaEssentials and GraphPad Prism 8 (GraphPad Software, San Diego, US) (18). The primary outcome was defined as the 50% response rate, comprising reductions in mean monthly migraine days (90.0%), mean monthly headache days (5.0%), and mean monthly headache hours (5.0%). Odds ratios for 50% response rates were selected as effect measures. We chose to use odds ratios as the effect measure because it is a conservative, robust measure, therefore minimizing discrepancies in study designs (inclusion criteria, length of treatment, length of observation, etc). Heterogeneity across studies was assessed using the I2 statistics (19). Odds ratios for achieving 50% response rates were pooled across studies using random effects meta-analyses (20). Because of considerable heterogeneity (I2 > 50%) in the different analyses, summarized ORs from the random effects model were chosen and reported as final results. Effect sizes were graphically displayed using forest plots.
Studies were weighted by calculating the inverse variance of their effect estimates. We determined the baseline migraine days (where available) as a moderator variable to perform a meta-regression analysis. Funnel plots that allow visual interpretation regarding publication bias were generated. In case of suspected publication bias, missing study data were imputed into these plots to facilitate the interpretation of whether a publication bias was likely to exist.
For mABs, we performed a subgroup analysis of the different available agents and further divided the results in studies evaluating episodic or chronic migraine. For TPM and BoNTA, subgroups were defined for episodic and chronic migraine only.
Role of the funding source
There was no funding source for this study.
Results
The database and trial registry search yielded 6552 results (2943 MedLine, 438 Cochrane Library, 2946 Embase). After removing duplicates, letters, case reports and studies where full text could not be retrieved, 429 studies were identified as eligible for full review. By restricting the results to studies on mABs, TPM and BoNTA, we obtained 131 studies. Of these, 32 randomized, placebo-controlled trials reporting responder rates finally fulfilled our inclusion criteria. Both studies investigating episodic and chronic migraine were included. Study characteristics are summarized in Tables 1–3. Main reasons for exclusion of retrieved studies was failure to report response rates, investigation of excluded treatments or a JADAD score below 3. One study of mABs assessing patients with episodic and chronic migraine had to be excluded from the analysis as the 50% response rate was not reported individually for these groups. Another study with a mixed episodic and chronic migraine population reported data separately and was assigned to the respective subgroups.
Table 1.
Overview of included studies assessing monoclonal antibodies for migraine prevention. All studies were blinded and used placebo as comparator. For the dropout rate, we considered overall dropouts (including the placebo group) to capture study design related withdrawals. Unless otherwise noted, drop-out rate resembles withdrawal for any reason. Studies marked with “*” report subjects terminating due to treatment-emergent adverse events. Unless stated otherwise, adverse-event rates are the percentage of subjects in the treatment group reporting adverse events.
|
Monoclonal antibodies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Drug (doses) | Study design (allocation) | Sample size | Population | Primary endpoint | Duration (DBTP) | Baseline frequency | Concurrent prevention (%) | AE-Rate (%) | Dropouts (%) | Age, years (SD or range) |
| Ashina 2020 (23) | Eptinezumab (30, 100, 300) | Parallel(1:1:1:1) | 888 | EM | Efficacy | 12 weeks | 8.6 MD | 0 | 59.7 | 3.5 | 39.8 (±11.4) |
| Dodick 2014 (24) | Eptinezumab (1000) | Parallel (1:1) | 163 | EM | Safety | One Dose | 8.6 MD | 0 | 57.0 | 4.3* | 38.8 (±10.2) |
| Dodick 2019 (25) | Eptinezumab (10, 30, 100, 300) | Parallel (1:1:1:1:1) | 616 | CM | Efficacy | 12 weeks | 16.5 MD | 34.9 | 56.0 | 2.0 | 36.6 (±12.1) |
| Lipton 2020 (26) | Eptinezumab (100, 300) | Parallel (1:1:1) | 1072 | CM | Efficacy | 12 weeks | 16.1 MD | 44.7 | 47.8 | 1.0 | 40.5 (±11.2) |
| Dodick 2018 (27) | Erenumab (70) | Parallel+OLE (1:1) | 577 | EM | Efficacy (75-% Risk Reduction) | 12 weeks (28 weeks OLE) | 8.3 MD | 6.1 | 48.4 | 1.1 | 42.0 (±11.5) |
| Goadsby 2017 (28) | Erenumab (70, 140) | Parallel (1:1:1) | 955 | EM | Efficacy | 28 weeks | 8.3 MD | 2.8 | 56.4 | 2.3 | 40.9 (±11.2) |
| Reuter 2018 (29) | Erenumab (140) | Parallel (1:1) | 246 | EM | Efficacy | 12 weeks | 9.3 MD | 0 | 54.5 | 2.4* | 44.4 (±10.6) |
| Sakai 2019 (30) | Erenumab (28, 70, 140) | Parallel (2:1:2:2) | 475 | EM | Efficacy | 12 weeks | 7.8 MD | 9.2 | 66.8 | 2.5 | 44.3 (n.a) |
| Sun 2016 (31) | Erenumab (7, 21, 70) | Parallel (3:2:2:2) | 483 | EM | Efficacy | 12 weeks | 8.7 MD | 0 | 51.7 | 3.6* | 41.1 (±10.8) |
| Tepper 2017 (32) | Erenumab (140, 70) | Parallel (3:2:2) | 667 | CM | Efficacy | 12 weeks | 18.0 MD | 0 | 45.5 | 3.5* | 42.1 (±11.2) |
| Bigal 2015 (33) | Fremanezumab(675/225, 900) | Parallel (1:1:1) | 264 | CM | Efficacy | 12 weeks | 161.9 HH | 39.7 | 50.5 | 1.1 | 40.7 (±12.0) |
| Bigal 2015 (34) | Fremanezumab (675, 225) | Parallel (1:1:1) | 295 | EM | Efficacy | 12 weeks | 11.4 MD | 29.3 | 52.5 | 9.1* | 41.2 (±12.2) |
| Dodick 2018 (35) | Fremanezumab (225, 675) | Parallel (1:1:1) | 875 | EM | Efficacy | 12 weeks | 9.1 MD | 20.8 | 62.4 | 1.7 | 41.8 (±12.0) |
| Ferrari 2019† (36) | Fremanezumab (675, 225) | Parallel (1:1:1) | 838 | EM + CM | Efficacy | 12 weeks (12 weeks OLE) | 14.2 MD | 0 | 50.0 | 1.0 | 46.2 (±11.1) |
| Silberstein 2017 (37) | Fremanezumab (675, 675/225) | Parallel (1:1:1) | 1130 | CM | Efficacy | 12 weeks | 13.1 HD | 21.0 | 70.5 | 1.7 | 41.3 (±12.1) |
| Detke 2018 (38) | Galcanezumab (240/120, 240) | Parallel+OLE (2:1:1) | 1113 | CM | Efficacy | 12 weeks (36 weeks OLE) | 19.4 MD | 15.0 | 57.5 | 0.9 | 40.8 (±12.1) |
| Mulleners 2020 (39) | Galcanezumab (120) | Parallel (1:1) | 462 | EM + CM | Efficacy | 12 weeks | 13.2 MD | 0 | 51.0 | 2.4* | 45.8 (±11.8) |
| Skljarevski 2018 (40) | Galcanezumab (240, 120) | Parallel (1:1:2) | 915 | EM | Efficacy | 24 weeks | 9.1 MD | 0 | 68.3 | 13.4* | 41.7 (±11.1) |
| Skljarevski 2018 (41) | Galcanezumab (5, 50, 120, 300) | Parallel (2:1:1:1:1) | 410 | EM | Efficacy | 12 weeks | 6.7 MD | 0 | 51.3 | 8.5* | 40.0 (±12.0) |
| Stauffer 2018 (42) | Galcanezumab (240,120) | Parallel (2:1:1) | 858 | EM | Efficacy | 24 weeks | 9.1 MD | 0 | 66.6 | 17.8* | 40.4 (±11.6) |
| Total | 13,302 | 11.18 | 56.2 | 5.1 | 41.5 (±2.2) | ||||||
†Excluded from final analysis because 50% response rate was not reported individually for the episodic or chronic migraine group.
DBTP: double-blind treatment phase; OLE: open-label extension; MD: mean migraine days; HD: mean headache days; HH: mean headache hours; CM: chronic migraine; EM: episodic migraine. Baseline frequency is given in numbers and the respective primary outcome measure of the studies.
Table 2.
Overview of included studies assessing topiramate for migraine prevention. All studies were blinded and used placebo as comparator. For the dropout rate we considered overall dropouts (including placebo group) to capture study design-related withdrawals. Unless otherwise noted, dropout rate resembles withdrawal for any reason. Studies marked with “*” report subjects terminating due to treatment-emergent adverse events. Unless stated otherwise, adverse event rates are the percentage of subjects in the treatment group reporting adverse events.
|
Topiramate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Drug (doses) | Study design (allocation) | Sample size | Population | Primary endpoint | Duration (DBTP) | Baseline frequency | Concurrent prevention (%) | AE rate (%) | Dropouts (%) | Age, years (SD or range) |
| Brandes 2004 (43) | TPM (50, 100, 200) | Parallel (1:1:1:1) | 483 | EM | Efficacy | 18 weeks | 5.5 MF | 0 | n.a† | 44.1* | 38.9 (±12.3) |
| Diener 2004 (44) | TPM (100, 200, PRO) ‡ | Parallel (1:1:1:1) | 575 | EM | Efficacy | 18 weeks | 5.1 MF | 0 | 36.0§ | 62.6* | 40.9 (±10.9) |
| Mei 2004 (45) | TPM (100) | Parallel (1:1) | 115 | EM | Efficacy | 12 weeks | 5.5 MF | 0 | 30.4 | 16.5 | 39.2 (±11.5) |
| Mei 2006¶(46) | TPM (100) | Parallel (2:3) | 50 | CM | Efficacy | 12 weeks | 23.9 HD | 0 | n.a | 30.0 | 45.9 (±8.7) |
| Silberstein 2004 (47) | TPM (50, 100, 200) | Parallel(1:1:1:1) | 487 | EM | Efficacy | 18 weeks | 5.5 MF | 0 | n.a | 43.5* | 40.4 (±11.4) |
| Silberstein 2006 (48) | TPM (200) | Parallel (2:1) | 211 | EM | Efficacy | 12 weeks | 4.9 MF | 0 | 90.0 | 26.5* | 40.5 (±11.1) |
| Silvestrini 2003 (49) | TPM (50) | Parallel (1:1) | 28 | CM | Efficacy | 8 weeks | 20.0 HF | 0 | 21.4 | 3.6 | 43.5 (34–58) |
| Storey 2001 (50) | TPM (200) | Parallel (1:1) | 40 | EM | Efficacy | 20 weeks | 4.8 MF | 52.5 | n.a† | 12.5 | 38.2 (19–62) |
| Total | 1949 | 6.56 | 44.5 | 29.9 | 40.9 (±2.4) | ||||||
†No overall AE rate reported. This study reported adverse events that were commonly associated with topiramate and occurred in 10% or more of the patients. Most common AE was paraesthesia.
‡Propranolol was used as active comparator in addition to placebo.
§This study reported treatment-limiting adverse invents. No overall AE rate reported.
¶Excluded from final analysis as the population consisted of migraine patients with medication overuse headache.
DBTP: double-blind treatment phase; OLE: open-label extension; MD: mean migraine days; HD: mean headache days; MF: mean migraine frequency; HF: mean headache frequency; HH: mean headache hours; CM: chronic migraine; EM: episodic migraine; PRO: propranolol; n.a.: not available/not reported.Baseline frequency is given in numbers and the respective primary outcome measure of the studies.
Table 3.
Overview of included studies assessing botulinum toxin type A for migraine prevention. All studies were blinded and used placebo as comparator. For the dropout rate, we considered overall dropouts (including placebo group) to capture study design-related withdrawals. Unless otherwise noted, dropout rate resembles withdrawal for any reason. Studies marked with “*” report subjects terminating due to treatment-emergent adverse events. Unless stated otherwise, adverse-event rates are the percentage of subjects in the treatment group reporting adverse events.
|
Botulinum Toxin Type A | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Drug (doses) | Study design (Allocation) | Sample size | Population | Primary endpoint | Duration (DBTP) | Baseline frequency | Concurrent prevention (%) | AE rate (%) | Dropouts (%) | Age, years (SD or range) |
| Aurora 2007 (51) | BoNTA (110–260U) | Parallel (1:1) † | 369 | EM | Efficacy | 26 weeks | 6.5 MF | 38.2 | 60.4 | 1.9 | 21.6 (±11.6) |
| Aurora 2011 (52) | BoNTA (155–195U) | Parallel (1:1) +OLE | 1384 | CM | Efficacy | 24 weeks (32 weeks OLE) | 19.9 HD | 0 | 62.4 | 2.6 | 41.3 (±10.6) |
| Evers 2004 (53) | BoNTA (16U, 100U) | Parallel (1:1:1) | 60 | EM | Efficacy | 12 weeks | 3.8 MF | 30.0 | 36.7 | 0 | 38.0 (±11.0) |
| Freitag 2008 (54) | BoNTA (100U) | Parallel (1:1) | 41 | CM | Efficacy | 16 weeks | 14.2 MD | n.a | n.a | 12.2 | 42.2 (19–64) |
| Relja 2007 (55) | BoNTA (225U, 150U, 75U) | Parallel (1:1:1:1) † | 495 | EM | Efficacy | 38 weeks | 4.1 MF | 0 | 77.2 | 2.8 | 43.2 (±10.3) |
| Silberstein 2000 (56) | BoNTA (25U, 75U) | Parallel (1:1:1) | 123 | EM | Efficacy | 12 weeks | 5.0 MF | n.a | 50.0‡ | 0.8* | 44.0 (22–63) |
| Total | 2472 | 17.1 | 57.3 | 3.4 | 38.4 (±7.7) | ||||||
†Patients were stratified into placebo responders and placebo non-responders.
‡No overall AE rate reported. Adverse events were reported in 50% in the 75U group and 24% in the vehicle (placebo) group.
DBTP: double-blind treatment phase; OLE: open-label extension; MD: mean migraine days; HD: mean headache days; MF: mean migraine frequency; HF: mean headache frequency; HH: mean headache hours; CM: chronic migraine; EM: episodic migraine; PRO: propranolol. Baseline frequency is given in numbers and the respective primary outcome measure of the studies.
Mean patient age across all included studies varied between 21.6 to 46.2. Total summarized study population was 17,763 participants with 74.9% investigated in trials evaluating mABs, 11.2% in TPM trials and 13.9% in BoNTA studies. The mean percentages of participants using concomitant preventative medication during the respective studies varied from 0.0% to 52.5%. Studies allowing concomitant preventive migraine medication did not consistently report drug, dosing or treatment duration but required the patients to be on a stable regime prior to and during the study. Studies not allowing concomitant prophylaxis required an adequate wash-out period prior to participation. The mean overall adverse-event rate and drop-out rate ranged from 21.4% to 90% and 0.0% to 62.6% respectively. The corresponding rates for the respective treatment arms are presented in Tables 1–3.
The primary outcome measures in mABs studies comprised reduction in mean monthly migraine days (90.0%), mean monthly headache days (5.0%), and mean monthly headache hours (5.0%). TPM studies assessed mean monthly migraine frequency (71.43%), mean monthly headache frequency (14.29%) and mean monthly headache days (14.29%). The most common primary outcome measurements in BoNTA studies were mean monthly migraine frequency (66.67%), mean monthly headache frequency (16.67%) and mean monthly headache days (16.67%). All included studies applied a parallel-arm study design with placebo as control. Date of publication ranged from 2014 to 2020 for mABs, 2003 to 2006 for TPM and 2000 to 2011 for BoNTA.
Efficacy
Each included study reached their primary endpoint and showed superiority compared to placebo regarding efficacy.
Monoclonal antibodies
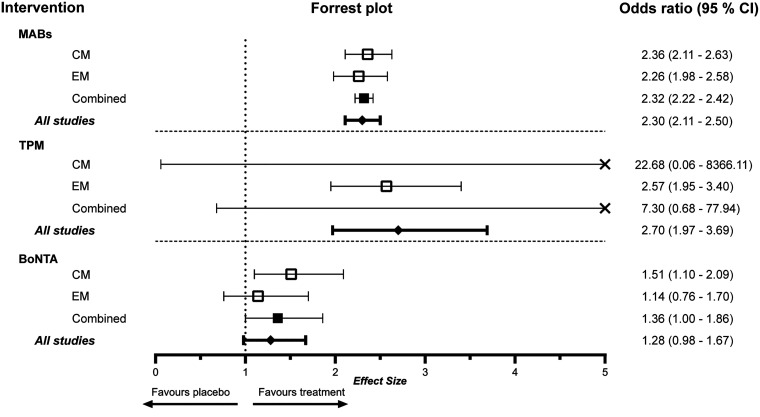
All included studies investigating mABs for the prevention of episodic migraine had higher OR for achieving 50% response compared to placebo. Three studies with antibodies for the prevention of episodic migraine demonstrated a lower bound of the confidence interval below the threshold of 1 and hence were statistically not significant. All studies assessing mABs for chronic migraine showed significant higher efficacy regarding RR50 compared to placebo. The combined effect size regarding the 50% response rate for mABs in the preventative treatment of episodic and chronic migraine was 2.26 (CI 1.98–2.58) and 2.34 (CI 2.13–2.56) respectively.
In the subgroup analysis of the four available monoclonal antibodies for the prevention of episodic migraine, all drugs showed a higher OR in achieving 50% response rate compared to placebo. Eptinezumab demonstrated the lowest effect size, with only two studies fulfilling our inclusion criteria, while erenumab, fremanezumab and galcanezumab demonstrated similar effect sizes. For the prevention of chronic migraine, all antibodies yielded comparable ORs and were at least twice as effective as placebo (Figure 2).
Topiramate
Topiramate for the prevention of episodic migraine was evaluated in doses ranging from 50 mg to 200 mg per day. Two studies of 200 mg TPM did not show a significant positive effect in achieving 50% response compared to placebo. The combined effect size for all TPM studies was 2.70 (CI: 1.97–3.69). Only one study evaluating TPM for chronic migraine met our inclusion criteria. When separated by episodic or chronic migraine, TPM showed ORs of 2.57 (CI: 1.95–3.40) and 22.68 (CI: 0.06–8366.11) respectively. The different dosing regimens of topiramate were analysed separately and showed an OR of 3.27 (CI: 1.72–6.23) for 100 mg, 2.35 (CI: 1.33–4.18) for 200 mg and 2.63 (CI: 0.22–30.93) for 50 mg (Figure 3).
Botulinum toxin type A
Studies assessing the efficacy of BoNTA were more heterogeneous. The dosing ranged from 16 Units to 225 Units of BoNTA. The injection sites and respective units across them were different in each study. The response regarding RR50 was similar in four of the studies for BoNTA and placebo, yet overall BoNTA was more favourable. Overall, the combined effect size was 1.36 (CI 1.00–1.86) for BoNTA when weighting was performed with consideration to episodic and chronic migraine. Analysing all BoNTA studies together, the combined effect size was 1.28 (CI: 0.98–1.67). In accordance with the approval for the use of BoNTA in migraine prevention, BoNTA for chronic migraine showed positive results with an OR of 1.51 (CI 1.10–2.09). For episodic migraine, the OR of BoNTA achieving RR50 was not significant (OR 1.14; CI 0.76–1.70) (Figure 4).
Study heterogeneity
Study heterogeneity (I2) was 22.1% for mABs, 48.1% for TPM and 57.3% for BoNTA when not analysed separately for episodic and chronic migraine. To rule out migraine frequency to be a predictor of response to treatment, we performed a regression analysis for each treatment regime. After dividing data into studies assessing episodic or chronic migraine, we found no significant association of effect size with baseline migraine/headache days or migraine/headache frequency (R2: 6.42% TPM; 35.36% BoNTA; 3.48% mABs).
Risk of bias
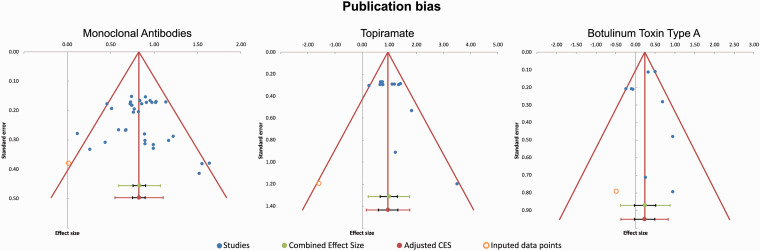
To test for publication bias an Egger’s regression test was performed. The p-values for testing 0 intercept revealed low significant results for topiramate (p = 0.042) and no significant results for BoNTA (p = 0.414) or mABs (p = 0.528 chronic migraine; p = 0.383 episodic migraine). See funnel plots for visualization (Figure 5).
Figure 5.
Funnel-plot of studies addressing mABs, TPM and BoNTA for the prevention of migraine.
(a) Results for mABs. There were fewer studies with higher standard error showing little or no benefit regarding 50% response rate leading to slight asymmetry in the funnel plot. Overall, published studies were consistently positive. (b) Results for TPM. No studies were published with a negative outcome regarding the 50% response rate. Data is skewed by the study of Silvestrini et al. 200349 with a Logs Odds Ratio of 3.48. This study showed an unexpected low placebo response. (c) Results for BoNTA. There were fewer studies published with a negative outcome regarding the 50% response rate compared to placebo. Studies with a lower standard error were published equally independent of outcome.
Discussion
Our evaluation confirmed the efficacy of mABs and TPM in the prevention of episodic and chronic migraine, as well as the efficacy of BoNTA in the prevention of chronic migraine. Utilizing the 50% response rate as a clinically relevant effect marker and authority required benchmark, enables decision-making. The RR50 has been assessed more consistently in recent studies, which led to a higher inclusion of studies evaluating mABs. Clinical trials assessing beta-blockers or antidepressants for the prevention of migraine scarcely report 50% response rates and were therefore not considered for this review.
Figure 6.
Subgroup-analysis of mABs, TPM and BoNTA for the prevention of episodic and chronic migraine. X-axis is depicted as standard decimal plot. Box size indicates weighting. All available antibodies showed overall superiority regarding the 50% response rate compared to placebo. The overall effect size of TPM for the prevention of migraine compared to placebo was the highest in our analysis. Robust data exist for TPM in the prevention of episodic migraine. When weighting studies according to episodic and chronic migraine the combined effect size was not significantly superior compared to placebo. This effect is caused by the abundance of large, randomized controlled studies evaluating TPM for chronic migraine and thus yielding a broad CI. Corresponding to international and national recommendations, BoNTA showed superiority in the prevention of chronic migraine regarding 50% response rate. Data to support its use in the prevention of episodic migraine is scarce and not consistent. The combined effect size of all studies when weighting regarding episodic or chronic migraine still results in a favourable outcome for BoNTA of an OR of 1.36 (CI: 1.00–1.86).
Direct comparisons of mABs, TPM and BoNTA have not been published to date, which is partially due to the difficulty of blinding in such a study. A comparison of studies in a meta-analysis has several limitations, such as different study durations or definitions of adverse events. Furthermore, the available studies also tend to use different outcome measures such as headache or migraine days or attack frequency. The RR50 can be used as a surrogate comparator between studies, as it is an artificial variable independent of the underlying efficacy measurement (i.e. frequencies). The utilization of odds ratios furthermore reduces variations emerging due to variable placebo rates.
In the prevention of episodic migraine, two antibody studies, Dodick 2014 and Skljarevski 2018 showed an exceptionally high placebo response, rendering their comparison inconclusive. Dodick et al. suggest the high placebo response might be due to the intravenous mode of administration of eptinezumab. The dose-finding study of erenumab by Sun 2016 also yielded no significant effect for the subtherapeutic dose of 21 mg. For the prevention of chronic migraine, all included mAB studies were favourable compared to placebo. We could find no significant difference between the respective antibodies regarding the RR50. As most of these studies were carried out in a similar fashion and comparable populations, the main source of uncertainty as expressed with broad confidence intervals is most likely a difference in sample size. No relevant publication bias was detected.
The studies by Silvestrini 2003 and Storey 2001 had the lowest sample size and were weighted ≤ 2.0% for calculating the combined effect size of all TPM studies. The distinctively broad CI for TPM in chronic migraine is mainly due to the low sample size in studies investigating TPM for chronic migraine. We recommend interpreting this finding with caution. The study by Silberstein 2006 evaluating the efficacy of TPM 200 mg/d compared to placebo was inconclusive. The authors report no significant superiority of TPM in the reduction of monthly migraine frequency when analysed in a per-protocol ANCOVA (analysis of co-variance). In a post-hoc intention to treat analysis, however, topiramate was significantly more effective than placebo.
Both TPM 100 mg and 200 mg per day were shown to be effective in achieving 50% reduction. Only one study on TPM for the prevention of chronic migraine fulfilled our inclusion criteria. This study had a small sample size of 28 patients and used a low dose of 50 mg per day. Thus, the results need to be interpreted with caution but TPM can be assumed to be effective in chronic migraine.
For BoNTA, only two studies demonstrated significant efficacy in achieving a 50% response rate. Relja 2007 evaluated BoNTA for the prevention of episodic migraine. Although not reaching the primary endpoint, the BoNTA group had a higher 50% response rate. Aurora 2011 introduced the PREEMPT protocol, which is the recommended use of BoNTA for the treatment of chronic migraine. The methods employed varied considerably within the included BoNTA studies. An interpretation of this data must therefore be made with caution (Figure 6).
The rate of concomitant preventive medication was lowest in the TPM group. This is not surprising, as in clinical practice patients only rarely receive concurrent daily oral medications for the prevention of migraines. Topiramate studies had reported a significant lower mean adverse event rate compared to mABs (p ≤ 0.001) or BoNTA (p = 0.005). Interestingly, mean dropout rate was also significantly higher in topiramate (mABs: p ≤ 0.001; BoNTA: p = 0.005). A potential explanation for these lower mean adverse event rates in TPM is a variable definition of adverse events, as several studies reported only treatment-emergent adverse events rather than overall adverse events. Although the CONSORT statement has been introduced as early as 1996, the approach to performing studies for the prevention of migraines has changed over the years (21). The introduction of electronic devices to record data as well as the inclusion of outcome variables not directly assessing efficacy is a more recent development. Due to the chronological dispersion of the included studies, a moderate heterogeneity of reporting in study design and outcomes must be assumed. This variance might be an explanation for relatively high reported adverse events in studies of mABs compared to TPM.
The effect size of reported response rates was highest for TPM and lowest for BoNTA. In a clinically practical view, one must assume that the new targeted therapies for migraine prevention are not more effective but considerably better tolerated than oral preventatives, at least in a placebo-controlled study setting. Although newer therapies have already been proven safe in long-term safety studies, TPM and BoNTA have been utilized for 2 decades enabling observation of seldom adverse reactions. Thus, long-term tolerability and late-onset effects of mABs still need evaluation. A factor in favour of mABs and BoNTA is the frequency of administration; while available oral preventative medications are administered daily, mABs require injection only monthly or quarterly, like BoNTA, accounting for a rather good treatment adherence.
Study heterogeneity was low for mABs but moderate for TPM and BoNTA, and not attributed to baseline headache or migraine frequency alone or publication bias. For better comparability, adherence to standard protocols of performing and reporting clinical trials is desirable. For a reliable direct comparison of preventative treatments in migraine head-to-head studies need to be conducted, such as the unpublished HER-MES study (22).
Conclusion
New targeted therapies are considered a milestone for the treatment of migraines. A positive mean treatment effect can be easily measured by a 50% response rate. In this review, we compared three treatment regimens that are commonly used in clinical practice – monoclonal CGRP antibodies, topiramate and botulinum toxin type A. In general, all treatments show higher ORs in achieving a 50% response compared to placebo. Topiramate demonstrated the greatest effect size but also the highest drop-out rate. This might indicate that new therapies are not more effective but more tolerable. A comparison of different treatment regimes, particularly from different decades, must be carried out with caution and requires head-to-head studies in the future.
Clinical implications
Efficacy, expressed as 50% response rate, is highest in topiramate.
The lower dropout rate in monoclonal antibodies against CGRP indicates better tolerability.
Patients tolerating adequate doses of oral preventative treatments might achieve a greater reduction in migraine headaches.
Acknowledgements
We are thankful to the University and State Library of Innsbruck (Universitäts- und Landesbibliothek Innsbruck) for their assistance in the conduction of our literature search.
Footnotes
Declaration of conflicting interests: FF reports personal fees from Novartis AG, personal fees from Eli Lilly and Company, personal fees from Teva Pharmaceuticals, outside the submitted work; FF is recipient of a scientific grant from the Austrian Academy of Sciences at the Department for Neurology, Medical University of Innsbruck. GB reports research support and/or consulting fees from Allergan, Grünenthal, Lilly, Menarini, Novartis, Pfizer, Reckitt Benckiser and Teva.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Florian Frank https://orcid.org/0000-0002-7236-5557
Contributors
FF planned the study and developed the search algorithm, and also carried out article review and data extraction as well as statistical analysis and drafted and wrote the manuscript. HU planned and carried out statistical analysis and revised figures and the manuscript. VS carried out article review and data extraction and revised the manuscript. GB planned the study and wrote the manuscript and supervised the data extraction process and resolved disagreements at the study selection level.
Glossary
CENTRAL: Cochrane Controlled Trials Register
RCT: randomized controlled trial
CI: confidence interval
MMD: mean monthly migraine daysm
AB(s): monoclonal antibody(ies)
CGRP: calcitonin gene-related peptide
CGRPr: calcitonin gene-related peptide receptor
TPM: topiramate
BoNTA: botulinum toxin type A
MeSH: medical subject headings
RR50: 50% response rate
FDA: Food and Drug Administration
EMA: European Medicines Agency
ICHD: International Classification of Headache Disorders
OR: odds ratio
ANCOVA: analysis of co-variance
CONSORT: Consolidated Standards of Reporting Trials
Search Algorithm
(((((((((((((migraine[Text Word]) OR episodic migraine[Text Word]) OR chronic migraine[Text Word]) OR migraine with aura[Text Word]) OR migraine without aura[Text Word]) OR migrain*[Text Word]) OR cephalgi*[Text Word]) OR migraine*[Text Word]) OR cephalalgi*[Text Word]) OR migraine disorders[MeSH Terms])) AND ((((((((((((((((((((((((((((((preventive therapy[Text Word]) OR prophylaxis[Text Word]) OR treatment[Text Word]) OR therapies[Title/Abstract]) OR prevention[Text Word]) OR prophylactic[Text Word]) OR preventive treatment[Text Word]) OR preventative treatment[Text Word]) OR pharmacologic[Title/Abstract]) OR prevent*[Text Word]) OR drug*[Text Word]) OR preventative*[Title/Abstract]) OR therapy[Title/Abstract]) OR effective[Title/Abstract]) OR frequency*[Title/Abstract]) OR reduction[Title/Abstract]) OR reduces[Title/Abstract]) OR reduced[Title/Abstract]) OR attack*[Title/Abstract]) OR month*[Text Word]) OR therapeutic[Title/Abstract]) OR effectively[Title/Abstract]) OR effect[Title/Abstract]) OR (Prevention and Control[MeSH Terms])) OR Drug Therapy[MeSH Terms]) OR Treatment Outcome[MeSH Terms]) OR Therapeutic Use[MeSH Subheading]) OR monthly[Text Word]) OR outcome*[Text Word]))) AND ((((((((((((((((((((((((((((((((((((((((((((beta-blocker[Text Word]) OR topiramate[Text Word]) OR anticonvu*[Text Word]) OR antiepilep*[Text Word]) OR topira*[Text Word]) OR valproate[Text Word]) OR valpro*[Text Word]) OR candesartan[Text Word]) OR candesart*[Text Word]) OR onabotulinum[Text Word]) OR botox[Text Word]) OR onabotulinum*[Text Word]) OR erenumab[Text Word]) OR CGRP[Text Word]) OR anti-CGRP[Text Word]) OR Calcitonin Gene-Related*[Text Word]) OR gepants[Text Word]) OR *gepant[Text Word]) OR Valproic Acid[MeSH Terms]) OR Botulinum Toxins, Type A[MeSH Terms]) OR Amitriptyline[MeSH Terms]) OR fremanezumab[Supplementary Concept]) OR TEV-48125[Text Word]) OR galcanezumab[Supplementary Concept]) OR eptinezumab[Supplementary Concept]) OR telcagepant[Supplementary Concept]) OR Propranolol[MeSH Terms]) OR Metoprolol[MeSH Terms]) OR Nebivolol[MeSH Terms]) OR Adrenergic Beta-Antagonists[MeSH Terms]) OR Bisoprolol[MeSH Terms]) OR Flunarizine[MeSH Terms]) OR Verapamil[MeSH Terms]) OR Anticonvulsants[MeSH Terms]) OR Calcitonin Gene-Related Peptide[MeSH Terms]) OR receptors, calcitonin gene related peptide[MeSH Terms]) OR atogepant[Text Word]) OR rimegepant[Text Word]) OR pituitary adenylate cyclase activating polypeptide[MeSH Terms]) OR PACAP*[Text Word]) OR AMG334[Text Word]) OR PAC1[Text Word]) OR LBR-101[Text Word]))) AND ((((((((((((((((((((((((((((((((randomized controlled trial[Text Word]) OR controlled clinical trial[Text Word]) OR randomized controlled*[Text Word]) OR random allocation[Title/Abstract]) OR double-blind method[MeSH Terms]) OR single-blind method[MeSH Terms]) OR clinical trial[Text Word]) OR clinical trials[Text Word]) OR prospective studies[MeSH Terms]) OR control*[Text Word]) OR prospectiv*[Text Word]) OR Placebos[MeSH Terms]) OR placebo*[Text Word]) OR random*[Text Word]) OR blind*[Text Word]) OR evaluation studies as topic[MeSH Terms]) OR Comparative Study[Publication Type]) OR trial*[Text Word]) OR controll*[Text Word]) OR compare*[Text Word]) OR Clinical Trials as Topic[MeSH Terms]) OR *blind*[Text Word]) OR Multicenter Study[Publication Type]) OR Clinical Studies as Topic[MeSH Terms]) OR cross-over studies[MeSH Terms]) OR clinical trial[Publication Type]) OR drug evaluation[MeSH Terms]) OR preliminary data[MeSH Terms]) OR pivot*[Text Word]) OR Review Literature as Topic[MeSH Terms]) OR Systematic Review[Publication Type]) OR Review[Publication Type])
ORDER By MOST RECENT!!!
MEDLINE – SEARCH
| Concept 1 | Search # | Concept 2 | Search # | Concept 3 | Search # | Concept 4 | |
|---|---|---|---|---|---|---|---|
| #1 | migraine[Text Word] | #11 | preventive therapy[Text Word] | #40 | beta-blocker[Text Word] | #85 | randomized controlled trial[Text Word] |
| #2 | episodic migraine[Text Word] | #12 | prophylaxis[Text Word] | #41 | topiramate[Text Word] | #86 | controlled clinical trial[Text Word] |
| #3 | chronic migraine[Text Word] | #13 | treatment[Text Word] | #42 | anticonvu*[Text Word] | #87 | randomized controlled*[Text Word] |
| #4 | migraine with aura[Text Word] | #14 | therapies[Title/Abstract] | #43 | antiepilep*[Text Word] | #88 | random allocation[Title/Abstract] |
| #5 | migraine without aura[Text Word] | #15 | prevention[Text Word] | #44 | topira*[Text Word] | #89 | Double-Blind Method[MeSH Terms] |
| #6 | migrain*[Text Word] | #16 | prophylactic[Text Word] | #45 | valproate[Text Word] | #90 | Single-Blind Method[MeSH Terms] |
| #7 | cephalgi*[Text Word] | #17 | preventive treatment[Text Word] | #46 | valpro*[Text Word] | #91 | clinical trial[Text Word] |
| #8 | migraine*[Text Word] | #18 | pharmacologic[Title/Abstract] | #47 | candesartan[Text Word] | #92 | clinical trials[Text Word] |
| #9 | cephalalgi*[Text Word] | #19 | prevent*[Text Word] | #48 | candesart*[Text Word] | #93 | Prospective Studies[MeSH Terms] |
| #10 | Migraine Disorders [Mesh Terms] | #20 | drug*[Text Word] | #49 | onabotulinum[Text Word] | #94 | control*[Text Word] |
| #21 | preventative*[Title/Abstract] | #50 | botox[Text Word] | #95 | prospectiv*[Text Word] | ||
| #22 | therapy[Title/Abstract] | #51 | onabotulinum*[Text Word] | #96 | Placebos[MeSH Terms] | ||
| #23 | effective[Title/Abstract] | #52 | erenumab[Text Word] | #97 | placebo*[Text Word] | ||
| #24 | frequency*[Title/Abstract] | #53 | CGRP[Text Word] | #98 | random*[Text Word] | ||
| #25 | reduction[Title/Abstract] | #54 | anti-CGRP[Text Word] | #99 | blind*[Text Word] | ||
| #26 | reduces[Title/Abstract] | #55 | Calcitonin Gene-Related*[Text Word] | #100 | Evaluation Studies as Topic[MeSH Terms] | ||
| #27 | reduced[Title/Abstract] | #56 | gepants[Text Word] | #101 | Comparative Study[Publication Type] | ||
| #28 | attack*[Title/Abstract] | #57 | *gepant[Text Word] | #102 | trial*[Text Word] | ||
| #29 | month*[Text Word] | #58 | Valproic Acid[MeSH Terms] | #103 | controll*[Text Word] | ||
| #30 | therapeutic[Title/Abstract] | #59 | Botulinum Toxins, Type A[MeSH Terms] | #104 | efficacy*[Text Word] | ||
| #31 | effectively[Title/Abstract] | #60 | Amitriptyline[MeSH Terms] | #105 | compare*[Text Word] | ||
| #32 | effect[Title/Abstract] | #61 | fremanezumab[Supplementary Concept] | #106 | Clinical Trials as Topic[MeSH Terms] | ||
| #33 | Prevention and Control[MeSH Terms] | #62 | TEV-48125[Text Word] | #107 | *blind*[Text Word] | ||
| #34 | Drug Therapy[MeSH Terms] | #63 | galcanezumab[Supplementary Concept] | #108 | Multicenter Study[Publication Type] | ||
| #35 | Treatment Outcome [MeSH Terms] | #64 | eptinezumab[Supplementary Concept] | #109 | Clinical Studies as Topic[MeSH Terms] | ||
| #36 | Therapeutic Use [MeSH Subheading] | #65 | telcagepant[Supplementary Concept] | #110 | Cross-Over Studies[MeSH Terms] | ||
| #37 | monthly[Text Word] | #66 | Propranolol[MeSH Terms] | #111 | Clinical Trial[Publication Type] | ||
| #38 | outcome*[Text Word] | #67 | Metoprolol[MeSH Terms] | #112 | Drug Evaluation[MeSH Terms] | ||
| #39 | preventative treatment[Text Word] | #68 | Nebivolol[MeSH Terms] | #113 | Preliminary Data[MeSH Terms] | ||
| #69 | Adrenergic Beta-Antagonists[MeSH Terms] | #114 | pivot*[Text Word] | ||||
| #70 | Bisoprolol[MeSH Terms] | #115 | Review Literature as Topic[MeSH Terms] | ||||
| #71 | Flunarizine[MeSH Terms] | #116 | Systematic Review[Publication Type] | ||||
| #72 | Verapamil[MeSH Terms] | #117 | Review[Publication Type] | ||||
| #73 | Anticonvulsants[MeSH Terms] | ||||||
| #74 | Calcitonin Gene-Related Peptide[MeSH Terms] | ||||||
| #75 | Receptors, Calcitonin Gene-Related Peptide[MeSH Terms] | ||||||
| #76 | atogepant[Text Word] | ||||||
| #77 | rimegepant[Text Word] | ||||||
| #78 | Pituitary Adenylate Cyclase-Activating Polypeptide[MeSH Terms] | ||||||
| #79 | PACAP*[Text Word] | ||||||
| #80 | AMG334[Text Word] | ||||||
| #81 | PAC1[Text Word] | ||||||
| #82 | filorexant[Text Word] | ||||||
| #83 | orexin[Text Word] | ||||||
| #84 | LBR-101[Text Word] |
References
- 1.Steiner TJ, Stovner LJ, Jensen R, et al. Migraine remains second among the world’s causes of disability, and first among young women: Findings from GBD2019. J Headache Pain 2020; 21: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipton RB, Stewart WF. Prevalence and impact of migraine. Neurol Clin 1997; 15: 1–13. [DOI] [PubMed] [Google Scholar]
- 3.Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: Updated statistics from government health surveillance studies. Headache 2015; 55: 21–34. [DOI] [PubMed] [Google Scholar]
- 4.Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav 2018; 8: e00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipton RB, Stewart WF. The epidemiology of migraine. Eur Neurol 1994; 34: 6–11. [DOI] [PubMed] [Google Scholar]
- 6.Lipton RB, Scher AI, Steiner TJ, et al. Patterns of health care utilization for migraine in England and in the United States. Neurology 2003; 60: 441–448. [DOI] [PubMed] [Google Scholar]
- 7.Zebenholzer K, Andree C, Lechner A, et al. Prevalence, management and burden of episodic and chronic headaches – a cross-sectional multicentre study in eight Austrian headache centres. J Headache Pain 2015; 16: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: A summary and comparison with other recent clinical practice guidelines. Headache 2012; 52: 930–945. [DOI] [PubMed] [Google Scholar]
- 9.Sacco S, Bendtsen L, Ashina M, et al. European Headache Federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain 2019; 20: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 2015; 35: 478–488. [DOI] [PubMed] [Google Scholar]
- 11.Schoenen J, Manise M, Nonis R, et al. Monoclonal antibodies blocking CGRP transmission: An update on their added value in migraine prevention. Rev Neurol 2020; 176: 788–803. [DOI] [PubMed] [Google Scholar]
- 12.Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies – Successful translation from bench to clinic. Nat Rev Neurol 2018; 14: 338–350. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 15.Friedman AP, Finley KH, Graham JR, et al. Ad Hoc Committee on Classification of Headache. Classification of Headache. JAMA 1962; 179(9): 717–718. [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res Synth Methods 2017; 8: 537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 21.Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: The CONSORT statement. JAMA 1996; 276: 637–639. [DOI] [PubMed] [Google Scholar]
- 22.U.S. National Library of Medicine, ClinicalTrials.gov. Head-to-head study of erenumab against topiramate in patients with episodic and chronic migraine (HER-MES), https://clinicaltrials.gov/ct2/show/NCT03828539 (2020, accessed 19 February 2021).
- 23.Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia 2020; 40: 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014; 13: 1100–1107. [DOI] [PubMed] [Google Scholar]
- 25.Dodick DW, Lipton RB, Silberstein S, et al. Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial. Cephalalgia 2019; 39: 1075–1085. [DOI] [PubMed] [Google Scholar]
- 26.Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 2020; 94: e1365–e1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodick DW, Ashina M, Brandes JL, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018; 38: 1026–1037. [DOI] [PubMed] [Google Scholar]
- 28.Goadsby PJ, Uwe R, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017; 377: 2123–2132. [DOI] [PubMed] [Google Scholar]
- 29.Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: A randomised, double-blind, placebo-controlled, phase 3b study. Lancet 2018; 392: 2280–2287. [DOI] [PubMed] [Google Scholar]
- 30.Sakai F, Takeshima T, Tatsuoka Y, et al. A randomized Phase 2 study of erenumab for the prevention of episodic migraine in Japanese adults. Headache 2019; 59: 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 382–390. [DOI] [PubMed] [Google Scholar]
- 32.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017; 16: 425–434. [DOI] [PubMed] [Google Scholar]
- 33.Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 34.Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 35.Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: A randomized clinical trial. JAMA 2018; 319: 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): A randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 2019; 394: 1030–1040. [DOI] [PubMed] [Google Scholar]
- 37.Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med 2017; 377: 2113–2122. [DOI] [PubMed] [Google Scholar]
- 38.Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology 2018; 91: e2211–e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulleners WM, Kim BK, Láinez MJA, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): A multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 2020; 19: 814–825. [DOI] [PubMed] [Google Scholar]
- 40.Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 2018; 38: 1442–1454. [DOI] [PubMed] [Google Scholar]
- 41.Skljarevski V, Oakes TM, Zhang Q, et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention a randomized clinical trial. JAMA Neurol 2018; 85259: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE-1 randomized clinical trial. JAMA Neurol 2018; 75: 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: A randomized controlled trial. JAMA 2004; 291: 965–973. [DOI] [PubMed] [Google Scholar]
- 44.Diener HC, Tfelt-Hansen P, Dahlöf C, et al. Topiramate in migraine prophylaxis: Results from a placebo-controlled trial with propranolol as an active control. J Neurol 2004; 251: 943–950. [DOI] [PubMed] [Google Scholar]
- 45.Mei D, Capuano A, Vollono C, et al. Topiramate in migraine prophylaxis: A randomised double-blind versus placebo study. Neurol Sci 2004; 25: 245–250. [DOI] [PubMed] [Google Scholar]
- 46.Mei D, Ferraro D, Zelano G, et al. Topiramate and triptans revert chronic migraine with medication overuse to episodic migraine. Clin Neuropharmacol 2006; 29: 269–275. [DOI] [PubMed] [Google Scholar]
- 47.Silberstein SD. Topiramate in migraine prevention: Evidence-based medicine from clinical trials. Neurol Sci 2004; 25: 244–245. [DOI] [PubMed] [Google Scholar]
- 48.Silberstein SD, Hulihan J, Karim MR, et al. Efficacy and tolerability of topiramate 200 mg/d in the prevention of migraine with/without aura in adults: 12-week pilot study. Clin Ther 2006; 28: 1002–1011. [DOI] [PubMed] [Google Scholar]
- 49.Silvestrini M, Bartolini M, Coccia M, et al. Topiramate in the treatment of chronic migraine. Cephalalgia 2003; 23: 820–824. [DOI] [PubMed] [Google Scholar]
- 50.Storey JR, Calder CS, Hart DE, et al. Topiramate in migraine prevention: A double-blind, placebo-controlled study. Headache 2001; 41: 968–975. [DOI] [PubMed] [Google Scholar]
- 51.Aurora SK, Gawel M, Brandes JL, et al. Botulinum toxin type A prophylactic treatment of episodic migraine: A randomized, double-blind, placebo-controlled exploratory study. Headache 2007; 47: 486–499. [DOI] [PubMed] [Google Scholar]
- 52.Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache 2011; 51: 1358–1373. [DOI] [PubMed] [Google Scholar]
- 53.Evers S, Vollmer-Haase J, Schwaag S, et al. Botulinum toxin A in the prophylactic treatment of migraine – a randomized, double-blind, placebo-controlled study. Cephalalgia 2004; 24: 838–843. [DOI] [PubMed] [Google Scholar]
- 54.Freitag FG, Diamond S, Diamond M, et al . Botulinum toxin type A in the treatment of chronic migraine without medication overuse. Headache 2008; 48: 201–209. [DOI] [PubMed] [Google Scholar]
- 55.Relja M, Poole AC, Schoenen J, et al. A multicentre, double-blind, randomized, placebo-controlled, parallel group study of multiple treatments of botulinum toxin type A (BoNTA) for the prophylaxis of episodic migraine headaches. Cephalalgia 2007; 27: 492–503. [DOI] [PubMed] [Google Scholar]
- 56.Silberstein S, Mathew N, Saper J, et al. Botulinum toxin type A as a migraine preventive treatment. Headache 2000; 40: 445–450. [DOI] [PubMed] [Google Scholar]