Abstract
We investigated the characteristics of regulatory T cells in adult‐onset Still’s disease (AOSD) with a focus on their plasticity, stability and relationship to disease severity. The proportion of circulating CD4+CD25+forkhead box protein 3 (FoxP3+) cells (Tregs) and intracellular expression of effector cytokines, including interferon (IFN)‐γ, interleukin (IL)‐17 and IL‐4, was analysed in 27 untreated patients with AOSD (acute AOSD), 11 of the 27 patients after remission and 16 healthy controls (HC) using flow cytometry. The suppressive ability of Tregs was also evaluated. Regression analyses of the results were performed. The proportion of Tregs was significantly lower in patients with acute AOSD than in the HC. The expression levels of IFN‐γ, IL‐17 and IL‐4 in Tregs were significantly increased in patients with acute AOSD. IFN‐γ and IL‐4 expression levels were inversely correlated with the proportion of Tregs and positively correlated with serum ferritin levels. Decreased expression of FoxP3 in CD4+CD25+ cells, which was correlated with increased expression of IL‐17, and impaired suppressive function were observed in Tregs in acute AOSD. However, these aberrant findings in Tregs, including the reduced circulating proportion and functional ability and altered intracellular expression levels of cytokines and FoxP3, were significantly improved after remission. In acute AOSD, Tregs show plastic changes, including effector cytokine production and reductions in their proportion and functional activity. IFN‐γ and IL‐4 expression levels in Tregs may be associated with disease severity. Also, down‐regulation of FoxP3 may be related to IL‐17 expression in Tregs. Importantly, the stability of Tregs can be restored in remission.
Keywords: adult‐onset Still’s disease, effector cytokines, helper T cell‐related cytokines, plasticity, regulatory T cells
This study investigated the plasticity and stability of regulatory T cells (Tregs) in adult‐onset Still’s disease. In the acute phase of disease, Tregs significantly showed plastic changes including overproduction of intracellular effector cytokines, as well as reductions in FoxP3 expression and functional ability. However, these aberrant findings in Tregs were significantly improved after remission.
INTRODUCTION
Adult‐onset Still’s disease (AOSD) is an autoinflammatory disease that is clinically characterized by spike fever, skin rash, sore throat and arthritis with elevated neutrophil counts and serum ferritin levels. Severe complications, such as macrophage activation syndrome (MAS), thrombotic thrombocytopenic purpura or other visceral impairments, sometimes develop [1, 2, 3]. In the disease mechanism of AOSD, activation of the innate immune system, including macrophages and neutrophils, is the hallmark of pathogenesis [3]. In the acute phase of AOSD, expression of proinflammatory cytokines is increased, and the relationship between serum proinflammatory cytokine levels and disease activity has been demonstrated [1, 2]. Among the proinflammatory cytokines in AOSD, interleukin (IL)‐18 was found to be a potential biomarker [4, 5, 6], and interferon (IFN)‐γ may be associated with the induction of macrophages and neutrophils during the development of AOSD [7, 8, 9]. Our recent study revealed the characteristics of the natural killer cells that produce IFN‐γ in AOSD [10]. Polarization of helper T (Th) type 1 cells, which also produce IFN‐γ, has also been demonstrated in AOSD [11], implicating acquired immune responses in the development of AOSD. Increased frequency of Th17 cells was found to be associated with disease activity in AOSD [12]. In the acquired immune system, regulatory T cells (Tregs), which express CD4, CD25 and the transcription factor forkhead box P3 (FoxP3), play a crucial role in mediating immune tolerance and regulating immune responses [13, 14]. To the best of our knowledge, only one study has examined the role of Tregs in AOSD, which showed a reduced frequency of CD4+CD25high cells [15]. However, it has been reported that the plasticity of Tregs, i.e. a shift to Th‐like cells by effector cytokines, may be promoted in the inflammatory environments of autoimmune disease [16, 17]. Accordingly, it is necessary to assess the plasticity and stability of Tregs in AOSD, a representative systemic inflammatory disease.
In this study, we investigated the characteristics of circulating Tregs, including their proportion, intracellular expression of effector cytokines related to Th cells and suppressive ability in AOSD. In addition, the relationships between Tregs and clinical findings were also evaluated.
MATERIAL AND METHODS
Patients
Twenty‐seven patients with AOSD who had not yet received immunosuppressive therapy (acute AOSD), with a mean age [standard deviation (SD)] of 53.5 (16.9) years, were enrolled into this study. All patients fulfilled the diagnostic criteria for AOSD proposed by Yamaguchi et al. [18]. For the control group, 16 age‐matched healthy controls (HC), with a mean age (SD) of 51.5 (11.6) years (seven men and nine women), were included for comparison. Whole blood samples were obtained from all study participants. The clinical findings of patients with acute AOSD (Table 1) were recorded when whole blood samples were provided. The comprehensive disease score (Pouchot score) [19] and the development of macrophage activation syndrome (MAS) [20, 21] were also included in the analysis. Eleven of the 27 patients whose Pouchot score was zero after remission were also evaluated as the remission AOSD group. Whole blood samples were collected at a mean (SD) of 33.1 (34.3) months after initiating immunosuppressive therapy. Of the 11 patients in the remission AOSD group, nine were on maintenance therapy, including prednisolone [mean (SD) = 4.4 (4.3) mg daily) (n = 8)], methotrexate (n = 3), cyclosporin (n = 4) and/or tocilizumab (n = 2). The local ethics committee of Shinshu University approved this study (approval no.: 601/4294). All participants provided written informed consent.
TABLE 1.
Epidemiological and clinical findings of patients with AOSD
| Acute AOSD | Remission AOSD | HC | p value* | ||
|---|---|---|---|---|---|
| Number | 27 | 11 | 16 | ||
| Mean age, years | 53.5 | ― | 51.5 | 0.889 | |
| Sex (M/F) | 7/20 | ― | 7/9 | 0.091 | |
| Physical findings, n (%) | |||||
| Spike fever (more than 38℃) | 27 (100) | ― | ― | ||
| Skin eruption | 25 (93) | ― | ― | ||
| Sore throat | 20 (74) | ― | ― | ||
| Lymphadenopathy | 14 (52) | ― | ― | ||
| Arthritis | 24 (89) | ― | ― | ||
| Myalgia | 9 (33) | ― | ― | ||
| Pleuritis | 5 (19) | ― | ― | ||
| Lung involvement | 3 (11) | ― | ― | ||
| Pericarditis | 1 (4) | ― | ― | ||
| Hepatomegaly | 10 (37) | ― | ― | ||
| Splenomegaly | 13 (48) | ― | ― | ||
| Abdominal pain | 2 (7) | ― | ― | ||
| Laboratory data, mean ± SD | p value ** | ||||
| White blood cells/µl | 17 205 ± 11352 | 7345 ± 1787 | ― | 0.001 | |
| Neutrophils/µl | 13 878 ± 10527 | 4988 ± 1547 | ― | <0.0001 | |
| Lymphocytes/µl | 971 ± 509 | 1706 ± 596 | ― | 0.001 | |
| C‐reactive protein, mg/dl | 11.23 ± 8.16 | 0.04 ± 0.05 | ― | <0.0001 | |
| ESR, mm/h | 66 ± 36 | 5.1 ± 2.9 | ― | 0.010 | |
| Ferritin, ng/ml | 9618 ± 9545 | 70 ± 48 | ― | <0.0001 | |
| AST, U/l | 82 ± 87 | 20 ± 6 | ― | <0.0001 | |
| ALT, U/l | 86 ± 103 | 20 ± 8 | ― | 0.001 | |
| LDH, U/l | 622 ± 435 | 215 ± 43 | ― | <0.0001 | |
| Activity evaluation | |||||
| Pouchot score, mean ± SD | 5.5 ± 1.7 | 0 | ― | ||
| Fulfilled MAS criteria, n (%) | 8 (30) | ― | ― |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; ESR, erythrocyte sedimentation rate; HC, healthy controls; MAS, macrophage activation syndrome; SD, standard deviation.
Comparisons between 27 acute adult‐onset Still’s disease (AOSD) patients and 16 HC using the Mann–Whitney U‐test for age and Fisher’s exact probability test for sex.
Comparisons between 27 acute and 16 remission AOSD patients using the Mann–Whitney U‐test. A p value less than 0.05 was considered statistically significant.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples, which were collected into ethylene diamine tetraacetic acid (EDTA)‐coated tubes by gradient centrifugation with Ficoll‐Hypaque PLUS (GE Healthcare, Pittsburgh, Pennsylvania, USA). Surface and intracellular markers in PBMCs were detected by flow cytometric analysis. The population of CD4+CD25+FoxP3+ cells was defined as Tregs. PBMCs were stimulated with 0.5 μg/ml ionomycin, 0.04 μg/ml phorbol myristate acetate (both from Sigma‐Aldrich, St Louis, Missouri, USA) and 2 μM monensin (BD Biosciences, San Diego, California, USA) at 37°C for 4 h. Stimulated PBMCs were stained with phycoerythrin/cyanin 7 (PE/Cy7)‐conjugated anti‐CD4 (BioLegend, San Diego, California, USA) and PC5‐conjugated anti‐CD25 (Beckman Coulter, Brea, California, USA) with or without PE‐conjugated anti‐CD152 [cytotoxic T lymphocyte antigen 4 (CTLA‐4] (Beckman Coulter) antibodies. For detecting the population of CD4+CD25+CD127− /lowCD45RA+FoxP3+ cells, stimulated PBMCs were alternatively stained with fluorescein isothiocyanate (FITC)‐conjugated anti‐CD4 (Beckman Coulter), allophycocyanin (APC)‐conjugated anti‐CD25 (BioLegend), PE/Cy7‐conjugated anti‐CD127 (BD Biosciences) and PE/Cy5‐conjugated anti‐CD45RA (BioLegend) antibodies. The stained PBMCs were permeabilized with Cytofix/Cytoperm (BD Biosciences) and then stained with FITC‐conjugated anti‐IFN‐γ (Beckman Coulter), PE‐conjugated anti‐IL‐17 (BD Biosciences), PE‐conjugated anti‐IL‐4 (Beckman Coulter), FITC‐conjugated transforming growth factor (TGF)‐β1 (BioLegend) or PE‐conjugated IL‐10 (BioLegend) antibodies, as well as PE‐conjugated (BD Biosciences), FITC‐conjugated or Pacific blue‐conjugated anti‐FoxP3 (both BioLegend). The permeabilized PBMCs, which were stained with anti‐CD4, anti‐CD25, anti‐CD127 and anti‐CD45RA antibodies, were alternatively stained with PE‐conjugated anti‐T‐bet, anti‐RAR‐related orphan receptor gamma (RORγt) or anti‐GATA binding protein 3 (GATA3) antibody (all from BD Biosciences), as well as Pacific blue‐conjugated anti‐FoxP3 antibody. To assess the suppressive ability of Tregs, suppression assays were performed and evaluated as previously described [22, 23]. The CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) was used to isolate conventional T (con‐T) cells (CD4+CD25− cells) and Tregs from unstimulated PBMCs. Allogenic con‐T cells labeled with carboxyfluorescein succinimidyl ester (CFSE, 2 μM; Invitrogen, Carlsbad, California, USA) and Tregs were co‐cultured with anti‐CD3/CD28 microbeads (at 1:1:1) at 37℃ for 4 days. The proliferation of con‐T cells was analysed by the CSFE dilution method, both with and without Tregs. Treated cells were acquired on a FACSCanto II flow cytometer (BD Bioscience), and the data were analysed using FlowJo software version 7.6.5 (Tree Star Inc., Ashland, Oregon, USA).
Enzyme‐linked immunosorbent assay (ELISA)
ELISA kits were used to measure serum levels of IL‐4, IL‐6, IL‐12, IL‐17, IL‐21, IFN‐γ (R&D Systems, Minneapolis, MN, USA) and IL‐18 (Medical and Biological Laboratories, Nagoya, Japan).
Statistical analysis
All data are presented as mean ± standard deviation (SD). The Mann–Whitney U‐test and Fisher’s exact probability test were used to compare two independent groups. Patient characteristics before and after treatment were compared by Wilcoxon’s signed‐rank test. The Kruskal–Wallis test was performed for comparisons between three independent groups and the Steel–Dwass test was used for multiple comparisons. A correlation coefficient test was performed to evaluate the significance of relationships. A p value less than 0.05 was defined statistically significant. All statistical analyses were performed using BellCurve for Excel (SSRI, Tokyo, Japan) and JMP (SAS Institute, Cary, North Carolina, USA).
RESULTS
Clinical characteristics of patients with AOSD
Of the 27 AOSD study patients, all (100%) had spike fever, 25 (93%) indicated skin eruption and eight (30%) fulfilled the criteria for MAS (Table 1). The mean Pouchot score was 5.5 ± 1.7 in the acute AOSD group. In the comparisons between three different clinical courses, which are classified into monocyclic, polycyclic and chronic pattern [1, 2], serum levels of C‐reactive protein at AOSD diagnosis were significantly higher in the polycyclic pattern than the chronic pattern (Supporting information, Table S1). Laboratory findings were significantly improved in the remission AOSD group and were within the normal ranges (Table 1).
Proportion of Tregs and intracellular expression of effector cytokines in acute AOSD
The proportion of circulating Tregs was significantly lower in the acute AOSD patients than in the HC (5.06 ± 3.92 versus 7.33 ± 3.47, p = 0.016) (Figure 1a). This value was significantly increased in the remission AOSD patients (8.90 ± 4.40, p = 0.004) (Figure 1b). Intracellular expression of IFN‐γ, IL‐17 and IL‐4 in Tregs was significantly higher in the acute AOSD patients than in the HC (p < 0.0001) (Figure 2a, Table 2). Expression levels of the aforementioned cytokines were significantly lower in the remission AOSD patients [median fluorescence index (MFI): p = 0.003, p = 0.003, p = 0.004, respectively (Figure 2b) (frequency: p = 0.004, p = 0.003, p = 0.003, respectively] (Table 2). Meanwhile, the proportion of circulating CD4+CD25+CD127−/lowCD45RA+FoxP3+ cells was also significantly lower in the acute AOSD patients than in the HC (1.50 ± 0.51 versus 7.13 ± 3.27, p = 0.0001), and was significantly increased after remission (9.20 ± 2.50, p = 0.027) (Supporting information, Figure S1). Intracellular expression of IFN‐γ, IL‐17, and IL‐4 in CD4+CD25+CD127−/lowCD45RA+FoxP3+ cells was also significantly higher in the acute AOSD patients than in the HC (p = 0.0006, p = 0.006, p = 0.0006, respectively), and their expression was significantly decreased after remission (p = 0.027) (Supporting information, Figure S2). In addition, intracellular expression of T‐bet, RORγt and GATA3, which are T helper type (Th)1, Th17 and Th2‐related transcription factors, respectively, in CD4+CD25+CD127−/lowCD45RA+FoxP3+ cells, were also significantly higher in the acute AOSD patients than in the HC (p = 0.0001, p = 0.0001, p = 0.0006, respectively), and their expression was significantly decreased after remission (p = 0.027) (Supporting information, Figure S3).
FIGURE 1.
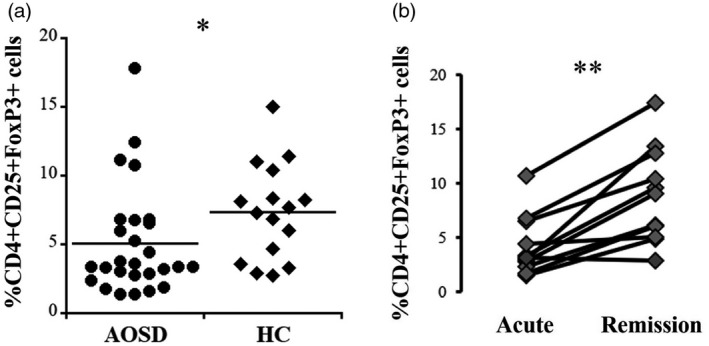
Proportions of circulating CD4+CD25+forkhead box protein 3 (FoxP3+) cells [regulatory T cells (Tregs)]. (a) Comparison of Tregs between patients with acute adult‐onset Still’s disease (AOSD) and healthy controls (HC). (b) Differences in the proportions of Tregs in 11 patients between the acute and remission phases of AOSD. The Mann–Whitney U‐test was used for comparison between the acute AOSD patients and HC. Wilcoxon’s signed‐rank test was used for comparison between acute and remission phases in 11 AOSD patients. *p < 0.05, **p < 0.005
FIGURE 2.
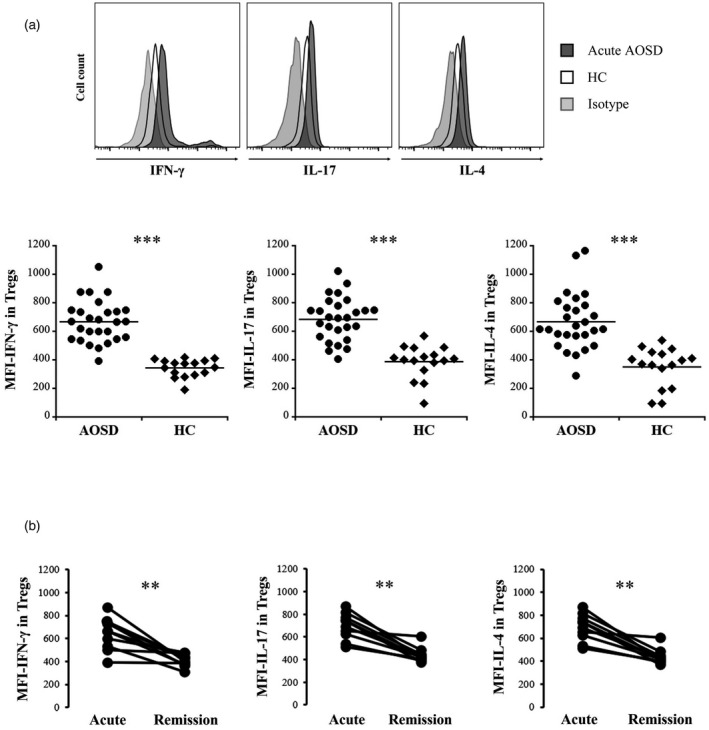
Intracellular expression levels of effector cytokines in CD4+CD25+forkhead box protein 3 (FoxP3+) cells [regulatory T cells (Tregs)]. (a): (Upper panels) Representative histograms of interferon (IFN)‐γ, interleukin (IL)‐17 and IL‐4 expression in Tregs (lower bar graphs). Comparisons of the median fluorescence index (MFI) of IFN‐γ, IL‐17 and IL‐4 in Tregs between patients with acute adult‐onset Still’s disease (AOSD) and HC. (b) Differences in the MFI of IFN‐γ, IL‐17 and IL‐4 in Tregs from 11 patients between acute and remission phases. The Mann–Whitney U‐test was used for comparisons between the acute AOSD patients and the HC. The Wilcoxon signed‐rank test was used for comparisons between acute and remission phases in 11 AOSD patients. **p < 0.005, ***p < 0.0001
TABLE 2.
Frequencies of intracellular cytokines in Tregs in patients with acute AOSD, remission AOSD and healthy controls
| Acute AOSD (n = 27) | Remission AOSD (n = 11) | HC (n = 16) | p value | ||
|---|---|---|---|---|---|
| */** | |||||
| In CD4+CD25+FoxP3+ cells | |||||
| %IFN‐γ | 24.18 ± 14.92 | 5.20 ± 9.61 | 4.33 ± 3.64 | 0.004/<0.0001 | |
| %IL‐17 | 23.58 ± 21.15 | 1.20 ± 1.36 | 1.84 ± 2.19 | 0.003/<0.0001 | |
| %IL‐4 | 26.97 ± 23.27 | 0.78 ± 1.41 | 2.04 ± 2.15 | 0.003/<0.0001 |
Data are presented as mean ± standard deviation (SD).
Abbreviations: FoxP3, forkhead box protein 3; HC, healthy controls; IFN‐γ, interferon‐γ; IL, interleukin; Tregs, regulatory T cells.
Comparisons between acute and remission phases in 11 acute adult‐onset Still’s disease (AOSD) patients using Wilcoxon’s signed‐rank test.
Comparisons between 27 acute AOSD patients and 16 HC using the Mann–Whitney U‐test. A p value less than 0.05 was considered statistically significant.
Serum levels of Th cell‐related cytokines and their relationships with Treg plasticity in AOSD
Serum levels of IL‐4, IL‐6, IL‐12, IL‐17, IL‐18, IL‐21 and IFN‐γ were significantly higher in the acute AOSD patients than in the HC (Table 3). Comparisons of the acute and remission AOSD patients showed no significant differences in serum levels of IL‐4, IL‐12 or IL‐17, despite significant reductions in the levels of the other evaluated cytokines. However, serum levels of these cytokines were not significantly correlated with the proportion of circulating Tregs or the intracellular expression levels of IFN‐γ, IL‐17 and IL‐4 in Tregs (data not shown).
TABLE 3.
Serum cytokine levels in patients with acute and remission AOSD and healthy controls
| Acute AOSD | Remission AOSD | HC | p value | |
|---|---|---|---|---|
| Cytokines | (n = 27) | (n = 11) | (n = 16) | */** |
| IL‐4 (pg/ml) | 13.1 ± 6.1 | 8.7 ± 2.1 | 8.5 ± 2.0 | 0.102/0.033 |
| IL‐6 (pg/ml) | 109.7 ± 139.4 | 11.7 ± 20.3 | 1.7 ± 0.6 | 0.005/<0.0001 |
| IL‐12 (pg/ml) | 1.5 ± 0.5 | 1.8 ± 1.0 | 0.7 ± 0.2 | 0.726/<0.0001 |
| IL‐17 (pg/ml) | 32.3 ± 17.6 | 23.3 ± 2.8 | 21.5 ± 4.1 | 0.368/0.025 |
| IL‐18 (pg/ml) | 2039.4 ± 779.6 | 351.6 ± 286.6 | 72.0 ± 11.1 | <0.0001/<0.0001 |
| IL‐21 (pg/ml) | 26.6 ± 41.2 | 8.4 ± 1.5 | 9.2 ± 1.8 | <0.0001/<0.0001 |
| IFN‐γ (pg/ml) | 75.3 ± 167.3 | 3.3 ± 2.0 | 2.3 ± 3.2 | <0.0001/<0.0001 |
Data are presented as the mean ± standard deviation (SD).
Abbreviations: HC, healthy controls; IL, interleukin; IFN‐γ, interferon‐γ.
Comparisons between acute and remission phases in 11 acute adult‐onset Still’s disease (AOSD) patients using Wilcoxon’s signed‐rank test.
Comparisons between 27 acute AOSD patients and 16 HC using the Mann–Whitney U‐test. A p value less than 0.05 was considered statistically significant.
Correlations among circulating Tregs, intracellular effector cytokine expression and disease severity in acute AOSD
Correlation analyses of circulating Tregs showed that the proportion of Tregs was inversely correlated with the MFI of IFN‐γ and IL‐4 in Tregs in the acute AOSD patients, but was not correlated with that of IL‐17 (Table 4). Frequencies of IFN‐γ, IL‐17 and IL‐4 were inversely correlated with the proportion of Tregs. Moreover, the MFI and frequencies of IFN‐γ and IL‐4 in Tregs were correlated with serum ferritin levels in the acute AOSD patients, whereas those of IL‐17 showed no correlation. There was no significant correlation between the proportion of Tregs and serum ferritin levels (data not shown).
TABLE 4.
Correlations between intracellular cytokine levels and the proportions of Tregs or serum ferritin in patients with acute AOSD
| Versus %CD4+CD25+FoxP3+ cells | Versus serum ferritin levels | ||||
|---|---|---|---|---|---|
| Coefficient | p value | Coefficient | p value | ||
| MFI in CD4+CD25+FoxP3+ cells | |||||
| MFI−IFN‐γ | −0.421 | 0.029 | 0.433 | 0.027 | |
| MFI−IL‐17 | −0.324 | 0.099 | 0.156 | 0.425 | |
| MFI−IL‐4 | −0.449 | 0.019 | 0.413 | 0.035 | |
| % Frequency in CD4+CD25+FoxP3+ cells | |||||
| % IFN‐γ | −0.499 | 0.008 | 0.390 | 0.047 | |
| % IL‐17 | −0.547 | 0.003 | 0.184 | 0.349 | |
| % IL‐4 | −0.680 | 0.0005 | 0.435 | 0.030 | |
A p value less than 0.05 was considered statistically significant.
Abbreviations: AOSD, acute adult‐onset Still’s disease; Coefficient, correlation coefficient; FoxP3, forkhead box protein 3; IL, interleukin; IFN‐γ, interferon‐γ; MFI, median fluorescence index.
Suppressive ability of Tregs in AOSD
To evaluate the suppressive ability of Tregs, the proliferation of con‐T target cells was evaluated with and without Tregs (Figure 3a,b). The proliferation of con‐T cells was significantly lower in the presence of Tregs from HC than in the absence of Tregs (p = 0.001). The proliferation of con‐T cells did not differ significantly in the presence of Tregs from acute AOSD patients or in the absence of Tregs (p = 0.193); however, proliferation was significantly higher in the presence of Tregs from acute AOSD patients than in the presence of Tregs from HC (p = 0.003), indicating abrogated suppressive function of Tregs in acute AOSD. The suppressive ability of Tregs from remission AOSD patients was significantly improved (p = 0.043) (Figure 3c). The expression levels of representative suppressive mediators, including TGF‐β1, IL‐10 and CTLA‐4 in Tregs from acute AOSD patients were not significantly different than those in Tregs from HC (p = 0.845, p = 0.220, p = 0.259, respectively) (Figure 4a). In contrast, the MFI and frequencies of FoxP3 in CD4+CD25+ cells were significantly lower in the acute AOSD patients than in the HC (p < 0.0001) (Figure 4b, Table 5) and were significantly increased after remission (p = 0.004, p = 0.013, respectively) (Figure 4c, Table 5). In CD4+CD25+CD127−/lowCD45RA+ cells, the MFI and frequency of FoxP3 were also significantly lower in the acute AOSD patients than in the HC (p = 0.0002, p = 0.0001, respectively), and were significantly increased after remission (p = 0.027) (Supporting information, Figure S4). The MFI of FoxP3 was inversely correlated with the MFI and frequency of IL‐17 in Tregs (Figure 4d), but was not correlated with those of either IFN‐γ or IL‐4 (data not shown).
FIGURE 3.
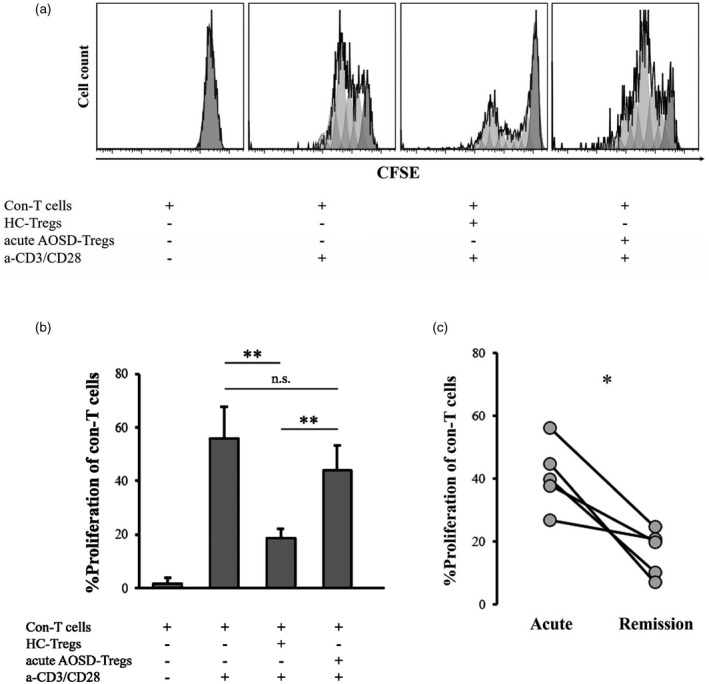
Suppression assay of regulatory T cells [regulatory T cells (Tregs)]. (a) Representative histograms showing the proliferation of conventional T cells (con‐T cells) with and without Tregs. (b) Comparisons of proliferative frequency of con‐T cells without Tregs (n = 9) and with Tregs from either acute adult‐onset Still’s disease (AOSD) patients (n = 8) or healthy controls (HC; n = 9). (c) Differences in the proliferative frequency of con‐T cells in the presence of Tregs from five patients collected in the acute and remission phases (n = 5). CFSE = carboxyfluorescein diacetate succinimidyl ester; HC‐Tregs = Tregs from HC; acute AOSD‐Tregs = Tregs from patients with acute AOSD; a‐CD3/CD28 = anti‐CD3/CD28 microbeads; NS = not significant. The Kruskal–Wallis and Steel–Dwass tests were used for comparisons of proliferative frequency of con‐T cells between three independent groups. Wilcoxon’s signed‐rank test was used for comparisons between acute and remission phases in five AOSD patients. *p < 0.05, **p < 0.005
FIGURE 4.
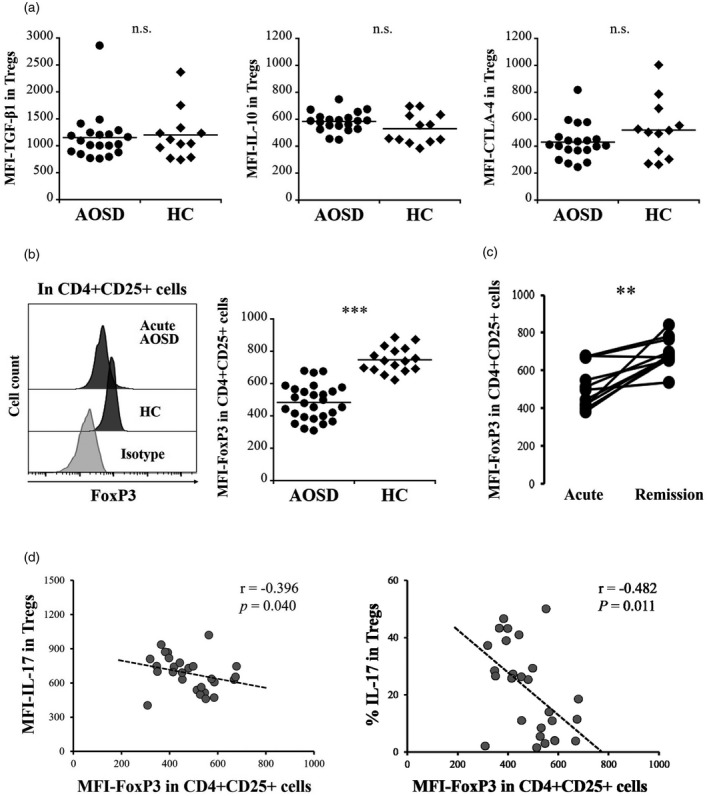
Expression of functional mediators in regulatory T cells (Tregs). (a) Comparisons of median fluorescence index (MFI) of transforming growth factor (TGF)‐β, interleukin (IL)‐10, and cytotoxic T lymphocyte antigen (CTLA)‐4 in CD4+CD25+forkhead box protein 3 (FoxP3+) cells collected from patients with acute acute adult‐onset Still’s disease (AOSD) and healthy controls (HC). (b) (Left panel) Representative histograms of FoxP3 expression in CD4+CD25+ cells from patients with AOSD and HC (right bar graph). Comparison of the MFI for FoxP3 in CD4+CD25+ cells from patients with acute AOSD and HC. (c) Differences in the MFI of FoxP3 in CD4+CD25+ cells from 11 patients with AOSD collected in the acute and remission phases. (d) Regression analyses of MFI and frequency of IL‐17 in MFI−FoxP3 in CD4+CD25+ cells from patients with acute AOSD. The Mann–Whitney U‐test was used for comparisons between the acute AOSD patients and the HC. Wilcoxon’s signed‐rank test was used for comparisons between acute and remission phases in 11 AOSD patients. **p < 0.005, ***p < 0.0001; NS = not significant
TABLE 5.
Frequency of FoxP3 in CD4+CD25+ cells in patients with acute AOSD, remission AOSD and healthy controls
| Acute AOSD | Remission AOSD | HC | p value | ||
|---|---|---|---|---|---|
| (n = 27) | (n = 11) | (n = 16) | */** | ||
| In total lymphocytes | |||||
| % CD4+CD25+ cells | 45.75 ± 15.48 | 43.40 ± 10.89 | 40.14 ± 18.02 | 0.374/0.218 | |
| In CD4+CD25+ cells | |||||
| % FoxP3 | 8.43 ± 7.83 | 18.73 ± 8.48 | 25.24 ± 9.62 | 0.013/<0.0001 |
Data are presented as the mean ± standard deviation (SD).
Abbreviations: HC, healthy controls; FoxP3, forkhead box protein 3.
Comparisons between acute and remission phases in 11 acute adult‐onset Still’s disease (AOSD) patients by using Wilcoxon’s signed‐rank test.
Comparisons between 27 acute AOSD patients and 16 HC using the Mann–Whitney U‐test. A p value less than 0.05 was considered statistically significant.
The relationship with clinical findings and clinical course
We investigated the relationship between the obtained results and clinical findings shown in Table 1. The expression of IL‐17 in Tregs was significantly higher in patients with sore throat than in those without (p = 0.015) (Supporting information, Figure S5). The expression of IL‐4 in Tregs was significantly higher in patients with pleuritis than in those without (p = 0.005). In accordance with previous investigation in which a cut‐off at 7.0 of the Pouchot score has an impact on the prognosis [24], we additionally compared the experimental results between patients showing more and less than this score, resulting in no significant differences (data not shown). Any experimental results had no significant differences between monocyclic, polycyclic and chronic pattern (data not shown).
DISCUSSION
In our first attempt at studying AOSD, we investigated the characteristics of circulating Tregs in AOSD, with a focus on their plasticity and stability. Previous investigations of Tregs in autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematous, systemic vasculitis and multiple sclerosis, inconsistently showed decreased and increased expression; however, defective Treg function was largely a consistent result [17, 22, 25, 26]. Our study demonstrated a decreased proportion of Tregs and dysfunction in acute AOSD patients as significant results. However, an increase in the proportion of Tregs and improved suppressive activity were both observed in remission AOSD patients, suggesting that the Treg disorder in AOSD may be reversible.
Intracellular expression of the effector cytokines IFN‐γ, IL‐17 and IL‐4 in the population of CD4+CD25+FoxP3+ cells was significantly higher in patients with acute AOSD. Tregs may include memory/effector phenotypes in the environment of chronic inflammation and/or in the process of immune‐aging [27, 28]. Meanwhile, subsets of CD127−/low and CD45RA+ are found to be phenotypical markers for certifying the homeostasis of Tregs in healthy individuals [29, 30]. The proportion of CD4+CD25+CD127−/lowCD45RA+FoxP3+ cells was significantly lower than that of CD4+CD25+FoxP3+ cells in the acute AOSD patients, suggesting that conversion to effector phenotypes might be shown even in the population expressing FoxP3. However, expression of IFN‐γ, IL‐17, IL‐4 and their transcription factors in CD4+CD25+CD127−/lowCD45RA+FoxP3+ cells was significantly higher in the acute AOSD patients than the HC, demonstrating that the phenotypical changes may be the pivotal features of Tregs in the acute phase of AOSD. The plasticity of Tregs includes Th‐like changes, characterized by the expression of effector cytokines in the conventional phenotype of Tregs that express FoxP3 [16, 17, 31, 32], ultimately resulting in an imbalance in immune tolerance due to insufficient intervention by Tregs [16, 33]. We have two hypotheses to explain the impaired homeostasis of Tregs in AOSD. First, the decrease in the proportion of circulating Tregs might be attributed to intracellular induction of effector cytokines. Notably, IFN‐γ and IL‐4 expression levels in Tregs were associated with the reduction in circulating Tregs. Although no significant correlation was found between the MFI of IL‐17 in Tregs and the proportion of Tregs in this study, plastic alteration of Tregs into Th17‐like cells has also been shown in some autoimmune diseases [34, 35]. Secondly, the increased intracellular expression of IFN‐γ, IL‐17 and IL‐4 might impair the suppressive function of Tregs. In fact, both proportion and functional capacity of Tregs recovered after remission with a significant reduction in intracellular cytokine expression. However, in this study there were no significant deficiencies in TGF‐β1, IL‐10 or CTLA‐4, which are mediators of the suppressive activity of Tregs [14, 25]. Our result demonstrated significantly decreased FoxP3 expression in CD4+CD25+ cells in patients with acute AOSD. Moreover, this was related to increased intracellular IL‐17 expression in Tregs. Conventional Tregs express FoxP3 as a pivotal regulator preventing extreme immune responses [36, 37]; Tregs down‐regulate FoxP3 expression in response to inflammatory signals [38, 39, 40]. It has been shown that intracellular induction of IL‐17 in Tregs leads to the down‐regulation of FoxP3 expression [39, 41]. Therefore, we assumed that the Tregs in patients with acute AOSD could be converted into not only Th‐like Tregs that retain FoxP3 expression but also Th phenotypes that lose FoxP3 expression, resulting in a decreased proportion of circulating Tregs. In addition, intracellular expression of IL‐17 might contribute to the loss of FoxP3 in Tregs, leading to impaired function. In contrast, it has also been reported that Th‐like Tregs which retain FoxP3 have less suppressive activity than conventional Tregs, although their suppressive capacity is not abolished [16, 31]. Under our experimental conditions, CD4+CD25+ cells, which were isolated using a commercially available magnetic isolation kit, were defined as Tregs and their suppressive activity was evaluated. Therefore, the isolated Tregs might include cell populations with down‐regulated FoxP3 levels and Th‐like FoxP3+ populations, suggesting that both populations have defective suppressive activity.
Some studies have shown elevated serum levels of IFN‐γ or IL‐17 as well as an increase in the proportion of circulating Th1 or Th17 cells in AOSD [8, 11, 12, 42]. Increased serum IL‐4 levels were also shown to be significantly correlated with disease activity [43, 44]. Accordingly, Th1, Th2 and Th17‐related cytokines may be strongly implicated in the pathogenesis of AOSD. Based on the results of previous investigations, plastic alteration of Tregs is promoted by exposure to Th cell‐related inflammatory cytokines [16, 17, 32, 45, 46, 47, 48]. However, our results showed that serum levels of these cytokines were not significantly correlated with the intracellular expression levels of IFN‐γ, IL‐17 or IL‐4 in Tregs. In contrast, IFN‐γ and IL‐4 expression levels in Tregs were associated with serum levels of ferritin, which is a biomarker commonly used for evaluating disease severity in AOSD [2, 3, 49, 50]. Hence, it was suggested that the plastic change of Tregs especially expressing IFN‐γ or IL‐4 may be significantly associated with disease severity in AOSD.
There are some limitations in this study. Our results statistically demonstrated low correlation coefficients in the regression analyses of intracellular cytokines in Tregs, suggesting that their correlations with FoxP3 expression, the proportion of Tregs and serum ferritin levels are very weak. Therefore, Th‐like changes in Tregs may have limited implications for the variation of Tregs and disease severity in AOSD. Also, it has been shown that ethnic and genetic variations may affect the immunity of AOSD [1, 2, 3] as well as the development of Tregs [51, 52], although a small number of Japanese patients were employed in this study.
In conclusion, in acute AOSD, circulating Tregs, which showed increases in the intracellular expression levels of IFN‐γ, IL‐17 and IL‐4, were significantly reduced in both proportion and suppressive activity. The expression levels of IFN‐γ and IL‐4 in Tregs might be implicated in a decreased proportion of Tregs and elevated serum levels of ferritin. In addition, decreased expression of FoxP3 in CD4+CD25+ cells was significantly shown in acute AOSD, and might be associated with IL‐17 expression in Tregs. These findings were significantly improved in patients with remission AOSD, suggesting that the Th‐like shift and functional impairment of Tregs are reversible. Disease activity may affect the stability of Tregs in AOSD. However, numerous immunopathogenic mechanisms are involved in the development of AOSD, and the immune response of Tregs can be affected by broad immune system interactions. Thus, further investigations on the immune signals affecting the plasticity of Tregs in AOSD are required.
CONFLICTS OF INTEREST
The authors declare that they have no financial or personal conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors designed this study, developed the structure and argument for this study. Y.‐S., T.‐I., D.‐K. and R.‐T. recruited blood samples and clinical data. Y.‐S. performed laboratory investigations and analyzed obtained data and prepared the draft of this manuscript. Y.‐S. and Y.‐S. contributed to revision of the manuscript. All authors revised and approved the final manuscript.
Supporting information
Fig S1‐S5
Table S1
ACKNOWLEDGEMENTS
This study was supported by the Shinshu Public Utility Foundation for the Promotion of Medical Science and a Health and Labour Sciences Research Grant on Rare and Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan. We thank all members of the Department of Medicine (Neurology and Rheumatology) at Shinshu University Hospital for treating the study patients.
Shimojima Y, Ichikawa T, Kishida D, Takamatsu R, Sekijima Y. Circulating regulatory T cells in adult‐onset Still’s disease: Focusing on their plasticity and stability. Clin Exp Immunol. 2021;206:184–195. 10.1111/cei.13648
DATA AVAILABILITY STATEMENT
The data for the analyses in this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still’s disease. J Autoimmun. 2018;93:24–36. [DOI] [PubMed] [Google Scholar]
- 2. Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult‐onset Still’s disease. Nat Rev Rheumatol. 2018;14:603–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerfaud‐Valentin M, Jamilloux Y, Iwaz J, Seve P. Adult‐onset Still’s disease. Autoimmun Rev. 2014;13:708–22. [DOI] [PubMed] [Google Scholar]
- 4. Girard C, Rech J, Brown M, Allali D, Roux‐Lombard P, Spertini F, et al. Elevated serum levels of free interleukin‐18 in adult‐onset Still’s disease. Rheumatology. 2016;55:2237–47. [DOI] [PubMed] [Google Scholar]
- 5. Priori R, Colafrancesco S, Alessandri C, Minniti A, Perricone C, Iaiani G, et al. Interleukin 18: a biomarker for differential diagnosis between adult‐onset Still’s disease and sepsis. J Rheumatol. 2014;41:1118–23. [DOI] [PubMed] [Google Scholar]
- 6. Chen DY, Lan JL, Lin FJ, Hsieh TY. Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset Still’s disease. J Rheumatol. 2004;31:2189–98. [PubMed] [Google Scholar]
- 7. Billiau A, Matthys P. Interferon‐gamma: a historical perspective. Cytokine Growth Factor Rev. 2009;20:97–113. [DOI] [PubMed] [Google Scholar]
- 8. Han JH, Suh CH, Jung JY, Ahn MH, Han MH, Kwon JE, et al. Elevated circulating levels of the interferon‐γ‐induced chemokines are associated with disease activity and cutaneous manifestations in adult‐onset Still’s disease. Sci Rep. 2017;7:46652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Komiya A, Matsui T, Nogi S, Iwata K, Futami H, Takaoka H, et al. Neutrophil CD64 is upregulated in patients with active adult‐onset Still’s disease. Scand J Rheumatol. 2012;41:156–8. [DOI] [PubMed] [Google Scholar]
- 10. Shimojima Y, Kishida D, Ueno KI, Ushiyama S, Ichikawa T, Sekijima Y. Characteristics of circulating natural killer cells and their interferon‐γ production in active adult‐onset Still disease. J Rheumatol. 2019;46:1268–76. [DOI] [PubMed] [Google Scholar]
- 11. Chen DY, Lan JL, Lin FJ, Hsieh TY, Wen MC. Predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult onset Still’s disease. Ann Rheum Dis. 2004;63:1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen DY, Chen YM, Lan JL, Lin CC, Chen HH, Hsieh CW. Potential role of Th17 cells in the pathogenesis of adult‐onset Still’s disease. Rheumatology. 2010;49:2305–12. [DOI] [PubMed] [Google Scholar]
- 13. Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Zheng SG. Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. [DOI] [PubMed] [Google Scholar]
- 15. Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. The associations of circulating CD4+CD25high regulatory T cells and TGF‐β with disease activity and clinical course in patients with adult‐onset Still’s disease. Connect Tissue Res. 2010;51:370–7. [DOI] [PubMed] [Google Scholar]
- 16. Qiu R, Zhou L, Ma Y, Zhou L, Liang T, Shi L, et al. Regulatory T cell plasticity and stability and autoimmune diseases. Clin Rev Allergy Immunol. 2020;58:52–70. [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Olsen N, Zheng SG. The progress and prospect of regulatory T cells in autoimmune diseases. J Autoimmun. 2020;111:102461. [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992;19:424–30. [PubMed] [Google Scholar]
- 19. Pouchot J, Sampalis JS, Beaudet F, Carette S, Decary F, Salusinsky‐Sternbach M, et al. Adult Still’s disease: manifestations, disease course, and outcome in 62 patients. Medicine. 1991;70:118–36. [PubMed] [Google Scholar]
- 20. Ahn SS, Yoo BW, Jung SM, Lee SW, Park YB, Song JJ. Application of the 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome in patients with adult‐onset Still disease. J Rheumatol. 2017;44:996–1003. [DOI] [PubMed] [Google Scholar]
- 21. Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A, et al. 2016 Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation collaborative initiative. Arthritis Rheumatol. 2016;68:566–76. [DOI] [PubMed] [Google Scholar]
- 22. Shimojima Y, Ishii W, Kishida D, Fukushima K, Ikeda SI. Imbalanced expression of dysfunctional regulatory T cells and T‐helper cells relates to immunopathogenesis in polyarteritis nodosa. Mod Rheumatol. 2017;27:102–9. [DOI] [PubMed] [Google Scholar]
- 23. Wen Z, Shimojima Y, Shirai T, Li Y, Ju J, Yang Z, et al. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J Clin Invest. 2016;126:1953–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruscitti P, Cipriani P, Masedu F, Iacono D, Ciccia F, Liakouli V, et al. Adult‐onset Still’s disease: evaluation of prognostic tools and validation of the systemic score by analysis of 100 cases from three centers. BMC Med. 2016;14:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–55. [DOI] [PubMed] [Google Scholar]
- 26. Morgan MD, Day CJ, Piper KP, Khan N, Harper L, Moss PA, et al. Patients with Wegener’s granulomatosis demonstrate a relative deficiency and functional impairment of T‐regulatory cells. Immunology. 2010;130:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jagger A, Shimojima Y, Goronzy JJ, Weyand CM. Regulatory T cells and the immune aging process: a mini‐review. Gerontology. 2014;60:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of Treg‐mediated T cell suppression. Front Immunol. 2012;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodríguez‐Perea AL, Arcia ED, Rueda CM, Velilla PA. Phenotypical characterization of regulatory T cells in humans and rodents. Clin Exp Immunol. 2016;185:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hori S. Lineage stability and phenotypic plasticity of Foxp3⁺ regulatory T cells. Immunol Rev. 2014;259:159–72. [DOI] [PubMed] [Google Scholar]
- 32. Brown CY, Sadlon T, Hope CM, Wong YY, Wong S, Liu N, et al. Molecular insights into regulatory T‐cell adaptation to self, environment, and host tissues: plasticity or loss of function in autoimmune disease. Front Immunol. 2020;11:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frangogiannis NG. Protean functions and phenotypic plasticity of regulatory T cells in chronic ischemic heart failure. Circulation. 2019;139:222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–77. [DOI] [PubMed] [Google Scholar]
- 35. Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh‐hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–8. [DOI] [PubMed] [Google Scholar]
- 36. Wan YY, Flavell RA. Regulatory T‐cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–70. [DOI] [PubMed] [Google Scholar]
- 37. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. [DOI] [PubMed] [Google Scholar]
- 38. Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–55. [DOI] [PubMed] [Google Scholar]
- 39. Yurchenko E, Shio MT, Huang TC, Da Silva MM, Szyf M, Levings MK, et al. Inflammation‐driven reprogramming of CD4+ Foxp3+ regulatory T cells into pathogenic Th1/Th17 T effectors is abrogated by mTOR inhibition in vivo . PLOS ONE. 2012;7:e35572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–97. [DOI] [PubMed] [Google Scholar]
- 41. Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoshino T, Ohta A, Yang D, Kawamoto M, Kikuchi M, Inoue Y, et al. Elevated serum interleukin 6, interferon‐gamma, and tumor necrosis factor‐alpha levels in patients with adult Still's disease. J Rheumatol. 1998;25:396–8. [PubMed] [Google Scholar]
- 43. Saiki O, Uda H, Nishimoto N, Miwa T, Mima T, Ogawara T, et al. Adult Still's disease reflects a Th2 rather than a Th1 cytokine profile. Clin Immunol. 2004;112:120–5. [DOI] [PubMed] [Google Scholar]
- 44. Fujii T, Nojima T, Yasuoka H, Satoh S, Nakamura K, Kuwana M, et al. Cytokine and immunogenetic profiles in Japanese patients with adult Still’s disease. Association with chronic articular disease. Rheumatology. 2001;40:1398–404. [DOI] [PubMed] [Google Scholar]
- 45. Dominguez‐Villar M, Baecher‐Allan CM, Hafler DA. Identification of T helper type 1‐like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2‐cell‐like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42:512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma‐associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17‐like cells. Nat Med. 2016;22:1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL‐17‐producing cells. Blood. 2008;112:2340–52. [DOI] [PubMed] [Google Scholar]
- 49. Lee SW, Park YB, Song JS, Lee SK. The mid‐range of the adjusted level of ferritin can predict the chronic course in patients with adult onset Still’s disease. J Rheumatol. 2009;36:156–62. [DOI] [PubMed] [Google Scholar]
- 50. Kong XD, Xu D, Zhang W, Zhao Y, Zeng X, Zhang F. Clinical features and prognosis in adult‐onset Still’s disease: a study of 104 cases. Clin Rheumatol. 2010;29:1015–9. [DOI] [PubMed] [Google Scholar]
- 51. Arvey A, van der Veeken J, Plitas G, Rich SS, Concannon P, Rudensky AY. Genetic and epigenetic variation in the lineage specification of regulatory T cells. eLife. 2015;4:e07571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huehn J, Beyer M. Epigenetic and transcriptional control of Foxp3+ regulatory T cells. Semin Immunol. 2015;27:10–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S5
Table S1
Data Availability Statement
The data for the analyses in this study are available from the corresponding author upon reasonable request.


