Abstract
Objective:
To evaluate the association of CT/CT angiography (CTA) findings and clinical characteristics with subsequent vasospasm in patients with aneurysmal subarachnoid hemorrhage (aSAH).
Methods-:
Consecutive presentation CTA head exams in patients with aSAH between January 2005 and June 2015 were retrospectively evaluated for intracranial arterial calcification, undulation and non-calcified stenosis. Additional variables including modified Fisher Scale (mFS), Glasgow Coma Scale (GCS) and neurological exam status were reviewed. Associations of CTA findings with the incidence of angiographic vasospasm were assessed with multivariate logistic regression models using the least absolute shrinkage and selection operator machine-learning algorithm. Model performance was summarized using c-index with bootstrap optimism-adjustment.
Results
Intracranial arterial calcification, seen in 51.7% of 195 total patients, was protective against vasospasm (OR-0.6; 95% CI-0.52–0.67; p = 0.009), while arterial undulation (24%) was associated with subsequent vasospasm (OR-2.6; 95% CI-1.3–5.1; p = 0.007). Non-calcified intracranial arterial stenosis (5%) was associated with subsequent vasospasm, (OR-4.7; 95% CI-1.0–22.8; p = 0.054). Least absolute shrinkage and selection operator selected all three CTA findings as predictors in a multivariate model for vasospasm in addition to clinical factors, which demonstrated superior predictive performance (c-index-0.74; 95% CI-0.69–0.82) compared to a model based on mFS and clinical factors only (c-index-0.66; 95% CI-0.57–0.75; p = 0.010 for the difference).
Conclusion:
Presentation CTA findings combined with clinical factors may better predict the development of vasospasm in patients with aSAH compared to current prognostic models alone.
Advances in knowledge:
The combination of initial CT/CTA and clinical findings better predict development of vasospasm after aSAH. This can lead to better markers for use in future clinical trials to develop vasospasm preventative treatments and potentially provide better targets for early aggressive treatment.
Introduction
Intracranial vasospasm is defined as delayed luminal narrowing of the cerebral arteries after the onset of aneurysmal subarachnoid hemorrhage (aSAH).1 Angiographic vasospasm is seen in up to 70% of patients with aSAH, of which 20–30% exhibit clinical manifestations that include delayed cerebral ischemia (DCI), and up to half develop cerebral infarction.2,3 Angiographic vasospasm typically develops 7–10 days post-aneurysm rupture,1 which has led to investigation into predictive algorithms, to prevent the frequent poor outcomes from aSAH.
Multiple prognostic models are used to stratify risk of vasospasm in patients with aSAH, including characterization of intracranial hemorrhage, transcranial Doppler ultrasound features and lower scores on the Glasgow coma scale. Although no single criteria is an agreed-upon gold-standard, the modified Fisher scale (mFS) is a commonly used prognostic tool based on the volume and distribution of intracranial hemorrhage on non-contrast head CT. Revision to the original Fisher scale in 2006 added emphasis to the location of intracranial hemorrhage, better accounting for the increased development of vasospasm associated with cisternal and intraventricular hemorrhage.4 While this scale is widely used, the mFS does not take into account the volume of intraventricular or intraparenchymal hematoma, which may also impact vasospasm risk.5,6
Non-contrast head CT and CTA are standard imaging to identify the presence and location of a ruptured aneurysm, with accuracy comparable to digital subtraction angiography (DSA).7 The purpose of this study is to assess the role of findings on initial head CT/CTA performed on patients presenting with aSAH, in combination with clinical characteristics and mFS, for predicting subsequent angiographic vasospasm.
Methods and materials
Study population
This was a retrospective observational cohort study approved by our institutional ethics review board with patient informed consent waived. Consecutive patients presenting to a comprehensive stroke center between January 2005 and June 2015 with clinical suspicion of aSAH were reviewed. Inclusion criteria were: (1) CT and CTA head performed at presentation (within 24 h of symptom onset) and prior to aneurysm treatment and previous and/or long-term follow-up angiographic imaging for baseline comparison for luminal anatomy. (2) Evidence of aSAH on imaging or CSF analysis secondary to a ruptured intracranial aneurysm present on imaging. (3) Subsequent surgical or endovascular intervention of the ruptured aneurysm within 48 h of symptom onset. Exclusion criteria were: (1) CTA images not available for review or markedly limited/non-diagnostic image quality, (2) unavailable or insufficient medical records to evaluate clinical variables. All patients received standard clinical management.
An electronic medical record review was performed for each patient to determine the time of symptom onset and to document patient demographics; smoking status; longitudinal GCS; illicit stimulant use (methamphetamine or cocaine); cardiovascular risk factors and history of prior stroke, coronary artery disease, hypertension, hyperlipidemia, obesity, and diabetes mellitus. GCS was recorded at presentation and throughout the hospital stay and used to determine DCI. DCI was defined as new focal neurological impairment or a decrease of 2 points or greater of the GCS score lasting at least 1 h, not occurring immediately after aneurysm occlusion and without other identifiable causes for deterioration or evidence of infarct on imaging.8
Imaging parameters and analysis
Patients underwent non-contrast CT and CTA head examinations on a 64-slice multidetector CT scanner (Siemens Somatom Definition AS+; Siemens Healthineers, Erlangen, Germany) covering from the level of the upper cervical spine to the skull vertex. After non-contrast head CT acquisition, a 100 ml Omnipaque 350 contrast bolus was injected followed by a 30 ml saline flush through an 18-gauge cannula placed in the antecubital vein. SmartPrep was used for bolus timing with monitoring at the aortic arch and an 8 sec monitoring delay. Images were acquired at 120 kVp and 250 mAs, with 128 × 0.6 mm slices reconstructed to 1 mm, and 5 mL/sec contrast injection for CTA acquisition.
All non-contrast CT and CTA head studies performed at presentation were reviewed by two neuroradiologists, with 7 and 6 years of experience, respectively, in consensus who were blinded to patient clinical information and additional imaging. On non-contrast CT, the presence and distribution of aSAH was evaluated and assigned a score according to the mFS.4 Intracranial arterial calcification was identified and categorized as ≥50% or <50% circumferential wall involvement.9 Review of the involved segments was done in a plane orthogonal to the lumen course, for a true cross-sectional evaluation, in order to best evaluate the degree of circumferential involvement.
On CTA images, the contour and caliber of intracranial arteries were inspected for evidence of focal fixed non-calcified stenosis, defined as stable narrowing present on prior and follow-up imaging, and undulation, defined as any visible minimal segmental arterial narrowing or luminal irregularity with intervening normal segments that was not fixed on prior or subsequent studies, an appearance similar to minimal early vasospasm. For the purpose of inter-reader agreement evaluation, a third board-certified neuroradiologist rater with 15 years of experience reviewed the first 48 consecutive cases for calcification pattern, arterial undulation and fixed non-calcified stenosis using the same criteria described above. The arterial segments evaluated included the intracranial internal carotid artery, V4 vertebral arteries, basilar artery, and proximal and distal segments of the middle, anterior, and posterior cerebral arteries. All available angiographic imaging studies (longitudinal CTA, DSA, MRA and transcranial Doppler (TCD)) during the post-intervention time period prior to discharge, and follow-up studies after discharge, were then reviewed for evidence of vasospasm and stenoses, respectively. Vasospasm was defined as reversible stenosis >30% of one or more intracranial arterial segments during the post-interventional period during the same hospitalization after aneurysm repair, and appearing no sooner than 72 h after hemorrhage. Focal fixed stenosis was defined based on the Warfarin-Aspirin Symptomatic Intracranial Disease Trial in arteries with cross-sectional luminal narrowing of >30% [(most stenosed segment/closest proximal normal segment) x 100].10 Imaging in the post-intervention period was based on clinical course, but at our institution (Harborview Medical Center, Seattle, WA, all patients post-intervention undergo serial TCD, and all patients had at least one follow-up luminal imaging study performed in the post-intervention period.
Etiology of cerebral infarct was dichotomized into infarcts related to vasospasm and infarcts related to other etiologies (e.g. ischemia associated with aneurysm endovascular or open surgical repair). This review was performed by an independent board-certified radiologist blinded to the imaging review. Vasospasm-related infarcts were defined as new regions of hypoattenuation on CT or restricted diffusion on MRI that were not present on initial imaging, and following a pattern typically seen with vasospasm (e.g. single cortical infarction, typically near the ruptured aneurysm, or multiple widespread subcortical and deep gray lesions) and congruent with the angiographic distribution of vasospasm. Treatment-related infarcts, identified on available CT and MRIs within 4 days following intervention, followed a pattern of ischemia consistent with mechanism of aneurysm repair such as localized traction edema in cases of surgical treatment, and catheter-related emboli in cases of endovascular treatment.11
Statistical analysis
Categorical variables were summarized as count (percentage) and continuous variables were summarized as mean ± standard deviation. Associations of clinical factors and CTA findings with development of vasospasm were initially assessed using univariate logistic regression. The LASSO (least absolute shrinkage and selection operator), a machine learning technique, was used to generate multivariate logistic models for predicting vasospasm based on the mFS, other clinical variables (age, sex, smoking status, use of stimulants, type of aneurysm treatment), and CT/CTA findings (intracranial arterial calcification, undulation and non-calcified stenosis) at presentation.12 The LASSO simultaneously performs variable selection and fits regression coefficients subject to a constraint that stabilizes estimates and allows for lower events per variable ratios than standard unpenalized models.13 The LASSO penalty parameter was selected by picking the largest value with a cross-validated prediction error within one standard error of the minimum. Internal validation of the model was performed by summarizing overall performance using the c-statistic after bootstrap optimism-adjustment to account for training and testing the model using the same data set.14 χ2 test was used to evaluate the association between vasospasm development and anterior vs posterior circulation aneurysms, as well as vasospasm comparison between the excluded and included subjects. Inter-rater agreement was calculated for arterial undulation, calcification pattern and fixed non-calcified stenosis using Cohen’s κ statistics. Statistical significance was defined as a two-sided p-value (p) less than 0.05. All statistical analyses were conducted using STATA/SE v. 15.1 software (StataCorp; College Station, TX) and R v. 3.5.1 (R Foundation for Statistical Computing; Vienna, Austria).
Results
A total of 195 patients with aSAH fulfilled study criteria and were included in the study (Figure 1). The majority of patients were female (73%; n = 143), with a population mean age of 54 ± 14 years. 86 (44%) were current smokers, and 9 (5%) were current stimulant users. MFS of 4 was assigned in nearly half of all patients (48%; n = 93), with a score of 3 in 32 (16%), and 1–2 in the remaining 70 (36%). Surgical clipping was the most common method for aneurysm repair, performed in 120 (62%) patients, followed by endovascular coiling in 58 (30%) and mixed, stent or pipeline treatment methods in 17 (9%) (Supplementary Table 1). In comparing the excluded and included patients, there was no significant difference in vasospasm frequency [36% (14/40) of excluded vs 47% included (93/195), p = .1].
Figure 1.
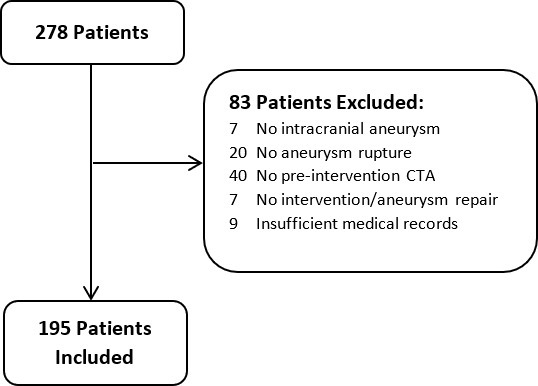
Included patients and exclusion criteria. CTA, CT angiography.
93 (48%) patients developed vasospasm during their hospital stay. The overall incidence of DCI was 57 (29%), most (n = 40) of which occurred in patients with vasospasm. Cerebral infarcts were identified in 64 (33%) patients, which were vasospasm-related in 39 patients and treatment-related in 25 (Supplementary Table 1).
The pre-intervention CTA findings are summarized in Online Supplementary Table 1. Over half of the patients (52%; n = 101) demonstrated intracranial arterial calcification on non-contrast head CT, with involvement of <50% circumference in 71 patients. On CTA, undulation of at least one intracranial arterial segment was identified in 46 (24%) patients. 10 (5%) patients had non-calcified focal stenosis, which was confirmed to persist on post-discharge follow-up MRA (n = 5), CTA (n = 4), and/or DSA (n = 4). The cardiovascular risk factors in patients with non-calcified focal stenosis included hypertension (n = 10), obesity (n = 6), diabetes (n = 4), hyperlipidemia (n = 4), smoking (n = 3), coronary artery disease (n = 3) and prior stroke (n = 1).
There were 30 posterior circulation and 165 anterior circulation aneurysms. Of these, 47% (14/30) of posterior circulation and 48% (79/165) of anterior circulation aneurysms developed vasospasm, which was not significantly different (p = 0.9).
Univariate analysis
Younger age (OR-0.7 per 10 year increase; 95% CI-0.5–0.9; p = 0.001), current smokers (OR-1.8; 95% CI-1.0–3.2; p = 0.044), and patients with a higher mFS (OR-1.4 per 1-level increase, 95% CI-1.1–1.8, p = 0.003) were at significantly increased risk of vasospasm (Table 1). Intracranial arterial calcification (p = 0.009) was associated with a significantly lower risk of vasospasm, particularly in patients with calcification involving ≥50% circumference (OR-0.3 vs no calcification; 95% CI-0.1–0.7). Conversely, arterial undulation on CTA (OR-2.6; 95% CI-1.3–5.1; p = 0.007) and non-calcified stenosis (OR-4.7, 95% CI-1.0–22.8, p = 0.054) were associated with a higher risk of vasospasm. Associations were similar after adjusting for mFS.
Table 1.
Association of treatment, demographics, mFS and CT/CTA findings with vasospasm
| Vasospasma | Multivariate | ||||
|---|---|---|---|---|---|
| No | Yes | Univariate models | LASSO model | ||
| Variable | (n = 102) | (n = 93) | OR (95% CI) | p value | OR |
| Intervention | 0.21 | ||||
| Clipping only | 58 (57) | 62 (67) | (ref) | (ref) | |
| Coiling only | 36 (35) | 22 (24) | 0.6 (0.3,1.1) | 0.98 | |
| Mixed or Othersb | 8 (8) | 9 (10) | 1.3 (0.5,3.4) | – | |
| Age, yearsc | 57 ± 14 | 50 ± 13 | 0.7 (0.5,0.9) | 0.001 | 0.75 |
| Male sex | 29(28) | 23(25) | 0.8 (0.4,1.6) | 0.56 | – |
| Current smoker | 38(37) | 48(52) | 1.8 (1.0,3.2) | 0.044 | 1.10 |
| Current stimulant use | 4 (4) | 5 (5) | 1.4 (0.4,5.3) | 0.63 | – |
| Modified Fisher score | 0.003d | 1.29e | |||
| 1 | 29(28) | 11(12) | (ref) | ||
| 2 | 18(18) | 12(13) | 1.8 (0.6,4.8) | ||
| 3 | 13(13) | 19(20) | 3.9 (1.4,10.4) | ||
| 4 | 42(41) | 51(55) | 3.2 (1.4,7.2) | ||
| Vessel calcification | 0.009d | 0.88e | |||
| None | 42(41) | 52(56) | (ref) | ||
| <50% circumferential | 38(37) | 33(35) | 0.7 (0.4,1.3) | ||
| ≥50% | 22(22) | 8 (9) | 0.3 (0.1,0.7) | ||
| Arterial undulation | 16(16) | 30(32) | 2.6 (1.3,5.1) | 0.007 | 2.19 |
| Non-calcified stenosis | 2 (2) | 8 (9) | 4.7 (1.0,22.8) | 0.054 | 1.60 |
CI, Confidence interval; OR, Odds ratio; mFS, Modified fisher score.
Dashes indicate variables de-selected by the LASSO (i.e. OR = 1).
Values are no. (%, rounded to nearest integer) or mean ± SD.
Mixed intervention includes endovascular treatment other than coiling (e.g. stent, pipeline), or clipping in addition to coiling or other endovascular treatment.
The OR for age is scaled to correspond to 10 year increase.
Test of trend.
OR per 1-level increase.
Multivariate analysis
The LASSO selected vessel calcification (OR-0.88 per 1-level increase; no calcification, <50% circumferential calcification, >50% circumferential), arterial undulation (OR-2.19), and non-calcified stenosis (OR-1.60) as predictors of vasospasm in addition to mFS (OR-1.29 per 1-level increase), current smoker (OR-1.10), age (OR-0.75 per 10 year increase) and coiling vs clipping (OR-0.98) in a multivariate predictive model for vasospasm (Table 1). The bootstrap-adjusted c-index for the multivariate model was 0.74 (95% CI-0.69–0.82), which was significantly higher than a model using mFS only (c-index-0.61; 95% CI-0.53–0.68; Δc-index-0.13; 95% CI-0.07–0.23, p < 0.001) and a LASSO model using all of the clinical predictors (c-index-0.66; 95% CI-0.57–0.75; Δc-index-0.07; 95% CI-0.02–0.16, p = 0.010; Figure 2). Similarly, a prediction model based on mFS only was outperformed by a model using a combination of mFS and CT/CTA findings (c-index-0.61 vs 0.71, p = 0.028).
Figure 2.
Cross-validated ROC curves of the multivariate models for predicting vasospasm. CTA, CT angiography; ROC, receiver operating characteristic
Inter-rater agreement
Overall agreement between the consensus raters and the separate rater was 83.3% for arterial undulation, 93.8% for fixed non-calcified stenosis and 89.6% for calcification pattern. Agreement for undulation was substantial (κ = 0.66), and excellent for fixed non-calcified stenosis (κ = 0.85) and calcification pattern (κ = 0.84), respectively.
Discussion
The current study examined imaging and clinical characteristics in patients presenting with aSAH, and found multiple variables that were associated with future development of vasospasm. Specifically, circumferential intracranial arterial calcification was associated with a decreased risk of vasospasm in univariate analysis, while arterial undulation significantly predisposed to vasospasm development. These imaging findings, in addition to non-calcified stenosis, when combined with conventional biomarkers to create multivariate prediction models of vasospasm outperformed models based on mFS and clinical variables alone. The greatest performance for predicting vasospasm was found in a model combining CT/CTA findings, mFS and clinical variables (AUC = 0.74; 95% CI: 0.69–0.82). The potential value of better predictive models for angiographic vasospasm after aSAH, is improved tools for future clinical trials, investigating treatments to prevent the development of vasospasm and other related complications. Upon prospective confirmation in larger data sets, our model can potentially identify aSAH patients at risk of vasospasm as candidates for early proactive intervention.
Frontera et al4 found significant value of mFS for predicting vasospasm in 1355 patients with aSAH (OR-1.28; 95% CI-1.06–1.54; p = 0.010). Our study found similar statistically significant increased risk of vasospasm in patients with thicker clots on CT and with mFS scores of 3 or more. Compared to a prediction model based on mFS alone, we found superior predictive capability in a multivariate prediction model that was based on CT/CTA findings of intracranial arterial calcification, non-calcified stenosis and undulation in addition to mFS (p = 0.028). These results indicate that findings on CTA at the time of admission can improve upon current methods for assessing risk of vasospasm without additional testing beyond standard of care.
Studies have demonstrated that multiple demographic factors may carry prognostic value in aSAH patients, including an association between younger age and vasospasm in a study of 108 patients,15 which was also found in the current study in both univariate and multivariate models. This may reflect a trend of decreasing vessel reactivity with age.16 Likewise, cigarette smoking has been found to be an independent predictor of symptomatic vasospasm,17 which was also confirmed in our study in the univariate and multivariate models.
To our knowledge, arterial undulation on presenting CTA after aSAH has not been previously investigated in relation to vasospasm. Of the imaging findings examined in our study, this was associated with the highest risk of subsequent vasospasm, seen on 46 (24%) initial CTAs, 30 of whom subsequently developed vasospasm (OR-2.6; 95% CI-1.3–5.1; p = 0.007) (Figure 3). Furthermore, undulation proved useful in the multivariate prediction model (OR-2.2; Table 1), suggesting that it can improve vasospasm prediction over mFS and clinical factors alone. Given that vasospasm does not typically present prior to 5 days post-hemorrhage, this observation may represent very early mild vasospasm that persists and leads to worsened vasospasm later. It may also potentially predict hyperreactivity of the arteries, that could be a contributing factor to subsequent vasospasm. Further investigation to better understand this process is needed. However, its frequent incidence may warrant reconsideration of the temporal manifestation of angiographic vasospasm in future studies. Advanced MRI techniques, specifically vessel wall MRI, could better lend understanding to the pathophysiological basis of undulation, and has already shown association with future vasospasm events in aSAH.18
Figure 3.
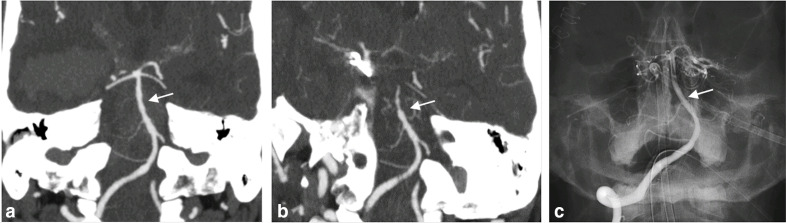
Intracranial arterial undulation and subsequent vasospasm in a patient with sudden onset of severe headache. When presenting to the hospital later in the day, CT (not shown) showed thick subarachnoid hemorrhage in the basilar cisterns and ventricles with mFS of 4. Concurrently performed CTA (a) revealed a ruptured right posterior communicating artery aneurysm, and subtle undulation of the basilar artery (arrow). Following surgical clipping 9 days later, the patient experienced a sudden decline in neurological status that correlated with (b) new vasospasm (arrow) on repeat CTA. (c) Post-intervention catheter angiogram images demonstrate normalization of the basilar artery contour and caliber (arrow) after intra-arterial nicardipine administration.
Intracranial arterial calcification was seen in nearly half of all patients (48%; 95/195) and similar to the 52% incidence in 172 patients with aSAH from a study by Hussein et al19. These calcifications primarily represent non-atherosclerotic calcification, predominantly of the internal elastic lamina and tunica media layers of the vessel wall.20 The presence of intracranial arterial calcifications in our cohort was associated with a statistically significant lower rate of vasospasm, as demonstrated in univariate analysis of overall calcifications, as well as a subset of those calcifications with 50% or more of circumferential wall involvement. In multivariate analysis, increased intracranial arterial calcification was a selected tuning parameter in all three models that included CTA findings. These findings suggest that calcifications may serve as an imaging marker for identifying patients with cerebrovasculature less susceptible to vasospasm. This protective effect against downstream vasospasm has been investigated in prior studies examining intra- and extracranial arterial calcifications and is likely related to a process of vessel stiffening.19,21
In a study examining nearly 1300 intracranial arterial segments on CTAs from 99 patients, Quiney et al22 found an associated increased risk of downstream ischemic events (e.g. stroke, TIA) in patients with intracranial atherosclerotic stenosis. In the current study, we investigated the potential influence of focal non-calcified fixed intracranial arterial stenosis in patients with aSAH, and found that the majority (80%; 8/10) of patients with this finding on CTA developed vasospasm. This association fell just short of statistical significance for risk of downstream vasospasm in univariate models (p = 0.054), possibly due to the relatively low incidence of this finding in our study population (n = 10) with a mean age of 54 years. Although some predictive value was demonstrated in the multivariate models in which it was included, the relationship of stenotic intracranial atherosclerosis and vasospasm warrants additional investigation, in a larger cohort.
The 48.0% (93/195) incidence of vasospasm in our cohort is similar to 53.1% (n = 182) in a study of 343 consecutive patients with aSAH by Mijiti et al,23 and centered within the 30–70% range of incidence from prior studies.2 The variability of vasospasm amongst studies could reflect differences in the timing and choice of modality (e.g. CTA, DSA, Doppler ultrasound) used to identify vasospasm, as well as treatments and preventative measures that have evolved over time. The rate of cerebral infarction of any etiology occurring in our cohort was 32%, compared to 23–30% in prior studies.24–26 The one out of five patients in our cohort with infarcts secondary to vasospasm is considerably less than 39% of 143 patients from a study by Rabinstein et al11, however, the latter identified infarctions only on CT, while the current study was better able to differentiate vasospasm-related infarction from treatment-related infarction with the added specificity of MRI. Lastly, the 29% incidence of DCI in our cohort falls within previously observed rates of the 20–30%.27–29
A limitation in this study is its retrospective design with varying degrees of available follow-up imaging and medical records data. The treatment approaches in our study were heterogeneous, and although we distinguished between surgical and endovascular types of treatment, their application in individual cases is not controlled for. As alluded to above with the wide range of rates of vasospasm in cohort studies of aSAH, the relationship of angiographic vasospasm and symptomatic vasospasm is not uniform and should also be taken into consideration with the clinical application of our findings. Likewise, the complex nature of DCI presents additional challenge to our study due to its multifactorial causes in patients with aSAH and could be due to multiple causes other than vasospasm that remain undetected that are not differentiated by the GCS. DCI, however, is a heterogeneous metric, with many different definitions used in the literature, and is a hard to assess metric clinically, making its application difficult.
Conclusion
A combination of clinical variables and CT/CTA findings at presentation may better predict subsequent vasospasm in patients with aSAH compared to current prognostic models. Intracranial arterial calcifications confer a protective effect against vasospasm, while undulation is associated with an increased risk of subsequent vasospasm. Consideration of imaging findings in addition to mFS and clinical measures such as patient age and smoking status may improve prognostication of patients undergoing aneurysmal repair.
Contributor Information
Charles G Colip, Email: charcoli@uw.edu.
Sean Wo, Email: seanwo@uw.edu.
Daniel S Hippe, Email: dhippe@uw.edu.
Hiroko Watase, Email: hiroko7@uw.edu.
Alfonso R Urdaneta-Moncada, Email: alfonso.r.urdaneta@gmail.com.
Chengcheng Zhu, Email: zhucheng@uw.edu.
Lei Wu, Email: wulei@uw.edu.
Justin E Vranic, Email: JVRANIC@mgh.harvard.edu.
Cory M Kelly, Email: kellycm@uw.edu.
Michael R Levitt, Email: mlevitt@uw.edu.
Mahmud Mossa-Basha, Email: mmossab@uw.edu.
REFERENCES
- 1.Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart Association/american stroke association. Stroke 2012; 43: 1711–37. doi: 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 2.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1985; 16: 562–72. doi: 10.1161/01.STR.16.4.562 [DOI] [PubMed] [Google Scholar]
- 3.Crowley RW, Medel R, Dumont AS, Ilodigwe D, Kassell NF, Mayer SA, et al. Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke 2011; 42: 919–23. doi: 10.1161/STROKEAHA.110.597005 [DOI] [PubMed] [Google Scholar]
- 4.Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES. Prediction of symptomatic Vasospasmafter subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 2006; 59: 21–7. doi: 10.1227/01.NEU.0000218821.34014.1B [DOI] [PubMed] [Google Scholar]
- 5.Eagles ME, Jaja BNR, Macdonald RL. Incorporating a modified Graeb score to the modified Fisher scale for improved risk prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Neurosurgery 2018; 82: 299–305. doi: 10.1093/neuros/nyx165 [DOI] [PubMed] [Google Scholar]
- 6.Platz J, Güresir E, Wagner M, Seifert V, Konczalla J. Increased risk of delayed cerebral ischemia in subarachnoid hemorrhage patients with additional intracerebral hematoma. J Neurosurg 2017; 126: 504–10. doi: 10.3171/2015.12.JNS151563 [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary SR, Ko N, Dillon WP, Yu MB, Liu S, Criqui GI, et al. Prospective evaluation of multidetector-row CT angiography for the diagnosis of vasospasm following subarachnoid hemorrhage: a comparison with digital subtraction angiography. Cerebrovasc Dis 2008; 25(1-2): 144–50. doi: 10.1159/000112325 [DOI] [PubMed] [Google Scholar]
- 8.Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies. Stroke 2010; 41: 2391–5. doi: 10.1161/STROKEAHA.110.589275 [DOI] [PubMed] [Google Scholar]
- 9.Koton S, Tashlykov V, Schwammenthal Y, Molshatzki N, Merzeliak O, Tsabari R, et al. Cerebral artery calcification in patients with acute cerebrovascular diseases: determinants and long-term clinical outcome. European Journal of Neurology 2012; 19: 739–45. doi: 10.1111/j.1468-1331.2011.03620.x [DOI] [PubMed] [Google Scholar]
- 10.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000; 21: 643–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinstein AA, Weigand S, Atkinson JLD, Wijdicks EFM. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 2005; 36: 992–7. doi: 10.1161/01.STR.0000163090.59350.5a [DOI] [PubMed] [Google Scholar]
- 12.T R. Regression shrinkage and selection via the LASSO. Journal of the Royal Statistical Society Series B 1996; 58: 267–88. [Google Scholar]
- 13.Pavlou M, Ambler G, Seaman S, De Iorio M, Omar RZ. Review and evaluation of penalised regression methods for risk prediction in low‐dimensional data with few events. Stat Med 2016; 35: 1159–77. doi: 10.1002/sim.6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001; 54: 774–81. doi: 10.1016/s0895-4356(01)00341-9 [DOI] [PubMed] [Google Scholar]
- 15.Kale SP, Edgell RC, Alshekhlee A, Borhani Haghighi A, Sweeny J, Felton J, et al. Age-Associated vasospasm in aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 2013; 22: 22–7. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.024 [DOI] [PubMed] [Google Scholar]
- 16.Macdonald RL, Rosengart A, Huo D, Karrison T. Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J Neurosurg 2003; 99: 644–52. doi: 10.3171/jns.2003.99.4.0644 [DOI] [PubMed] [Google Scholar]
- 17.Lasner TM, Weil RJ, Riina HA, King JT, Zager EL, Raps EC, et al. Cigarette smoking—induced increase in the risk of symptomatic vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 1997; 87: 381–4. doi: 10.3171/jns.1997.87.3.0381 [DOI] [PubMed] [Google Scholar]
- 18.Mossa-Basha M, Huynh TJ, Hippe DS, Fata P, Morton RP, Levitt MR. Vessel wall MRI characteristics of endovascularly treated aneurysms: association with angiographic vasospasm. J Neurosurg 2018; 131: 859–67. doi: 10.3171/2018.4.JNS172829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussein HM, Zacharatos H, Cordina S, Lakshminarayan K, Ezzeddine MA. Intracranial vascular calcification is protective from vasospasm after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 2014; 23: 2687–93. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 20.Vos A, Van Hecke W, Spliet WGM, Goldschmeding R, Isgum I, Kockelkoren R, et al. Predominance of Nonatherosclerotic internal elastic lamina calcification in the intracranial internal carotid artery. Stroke 2016; 47: 221–3. doi: 10.1161/STROKEAHA.115.011196 [DOI] [PubMed] [Google Scholar]
- 21.Park K-Y, Chung P-W, Kim YB, Moon H-S, Suh B-C, Yoon WT. Increased Pulsatility index is associated with intracranial arterial calcification. Eur Neurol 2013; 69: 83–8. doi: 10.1159/000342889 [DOI] [PubMed] [Google Scholar]
- 22.Quiney B, Ying SM, Hippe DS, Balu N, Urdaneta-Moncada AR, Mossa-Basha M. The association of intracranial vascular calcification and stenosis with acute ischemic cerebrovascular events. J Comput Assist Tomogr 2017; 41: 849–53. doi: 10.1097/RCT.0000000000000629 [DOI] [PubMed] [Google Scholar]
- 23.Mijiti M, Mijiti P, Axier A, Amuti M, Guohua Z, Xiaojiang C, et al. Incidence and predictors of angiographic vasospasm, symptomatic vasospasm and cerebral infarction in Chinese patients with aneurysmal subarachnoid hemorrhage. PLoS One 2016; 11: e0168657. doi: 10.1371/journal.pone.0168657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Brown R, Dhar R, Sampson T, Derdeyn CP, Moran CJ, et al. Early vs. delayed cerebral infarction after aneurysm repair after subarachnoid hemorrhage. Neurosurgery 2013; 73: 617–23. doi: 10.1227/NEU.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 25.Ferguson S, Macdonald RL. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2007; 60: 658–67. doi: 10.1227/01.NEU.0000255396.23280.31 [DOI] [PubMed] [Google Scholar]
- 26.FW S, Lin YJ, Chang WN, JT H, Wang HC, Yang TM. Predictors and outcome of acute symptomatic cerebral infarctions following aneurysmal subarachnoid hemorrhage. J Neurol 2010; 257: 264–70. [DOI] [PubMed] [Google Scholar]
- 27.Dorsch N. A clinical review of cerebral vasospasm and delayed ischaemia following aneurysm rupture. Acta Neurochir Suppl 2011; 110(Pt 1): 5–6. doi: 10.1007/978-3-7091-0353-1_1 [DOI] [PubMed] [Google Scholar]
- 28.SB K, Choi HA, Carpenter AM, Helbok R, Schmidt JM, Badjatia N. Quantitative analysis of hemorrhage volume for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 2011; 42: 669–74. [DOI] [PubMed] [Google Scholar]
- 29.Sundt TM, Whisnant JP. Subarachnoid hemorrhage from intracranial aneurysms. surgical management and natural history of disease. N Engl J Med 1978; 299: 116–22. doi: 10.1056/NEJM197807202990303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.