Abstract
Objectives:
The aim of this observational study was ultrasound evaluation of peripheral nerves cross-sectional area (CSA) in subjects with probable diabetic peripheral sensorimotor neuropathy (DPN). CSA was analyzed with reference to clinical and nerve conduction study’s (NCS) parameters for early diagnosis and pattern of involvement.
Methods:
A total of 50 patients with probable DPN due to Type 2 diabetes and 50 age-matched healthy controls underwent sonographic examinations of ulnar nerve at the lower arm, median nerve proximal to carpal tunnel, the common peroneal nerve proximal to fibular head, tibial nerve proximal to the tarsal tunnel, and sural nerve at lower third leg.
Results:
CSA was increased in cases of DPN as compared to healthy controls. Area changes were more marked with demyelinating pattern. Probable DPN cases with normal NCS had significantly higher number of peripheral nerves showing increased CSA as compared to healthy control. A cut-off of >4 nerve thickening showed a sensitivity of 86 %, and specificity of 56%. The neuropathy pattern in the lower limb was axonal, whereas in the upper limb, it was demyelinating with the majority showing sonographic feature of associated compressive neuropathy.
Conclusion:
There is an increase in CSA of peripheral nerve in diabetic patients. It can be used as a morphological marker for classifying DPN with changes being picked up earlier to NCS abnormality. Clinical neurological presentation in probable DPN can also be due to compressive neuropathy in early phases, and ultrasound can be a useful tool.
Advances in knowledge:
Early pick up of DPN cases shall be useful for early therapy and motivating the patients to actively participate in the treatment. Morphological changes on ultrasonography precedes the electrodiagnostic change in DPN. Symptoms of DPN is not only due to metabolic changes but also compressive neuropathy.
Introduction
Diabetes is one of the major causes of early illness and morbidity worldwide. Prevalence of diabetes may double globally from 171 million in 2000 to 366 million in 2030, with a maximum increase in India affecting up to 79.4 million individuals.1 In India, there are more than 62 million individuals currently diagnosed with diabetes.2
The most common chronic complication of diabetes mellitus (DM) is diabetic peripheral sensorimotor neuropathy (DPN), found in about 50% of patients. DPN includes peripheral nerve dysfunction in people with diabetes after excluding other probable causes. DPN is broadly classified into generalized typical polyneuropathy, generalized atypical polyneuropathy, and focal neuropathy. Generalized typical DPN is a symmetrical, length-dependent sensorimotor polyneuropathy, and has been attributed to metabolic and microvessel alterations from chronic hyperglycemia exposure and cardiovascular risk covariates. An abnormality of nerve conduction, which is frequently subclinical, appears to be the first objective quantitative indication of this condition.
Symptoms of distal polyneuropathy usually appear after many years of chronic prolonged hyperglycaemia in Type I DM. In Type II, it may appear after only a few years of known poor glycemic control or even at diagnosis.3
Magnetic resonance neurography and high-resolution ultrasonography (HRUS) are imaging techniques that play an important role in evaluating peripheral nerve disorders. However, magnetic resonance neurography for routine use is constrained with its limited availability, cost, and time requirement.3–6 HRUS provides a spatial resolution that enables detailed visualization of even the smallest peripheral nerves. Also, HRUS is a real-time dynamic process and allows the examiner to examine the entire course of the nerve. HRUS, being easily accessible even at rural setup, is less time-consuming, non-invasive procedure, cost-effective, and can be a great boon to be used as a screening tool in resource constrained societies.7
The diagnosis of DPN is done based on clinical and electrophysiological examination based on the American Diabetes Association (ADA) criteria where classification was done as possible, probable, confirmed, and subclinical DPN. For epidemiologic surveys or controlled clinical trials of DPN, it advocates the use of nerve conduction study (NCS) as an early and reliable indicator of the occurrence of this neuropathy.8 However, electrophysiology is non-rewarding for cases presenting with small fiber neuropathy, where changes due to metabolic insults are ongoing in peripheral nerve but is in subclinical stage.
The recent development in peripheral nerve ultrasonography has tried to develop further insight into understanding the pathophysiology of the symptoms in DPN cases, notably excluding the associated conditions like compressive neuropathy as the cause of the symptom and classifying the case in DPN.9
Moreover, there is a large gap between cases of probable DPN and confirmed DPN as per ADA guidelines and morphological changes appreciated on HRUS can be used as a tool to narrow the gap.
The aim of this study was HRUS evaluation of peripheral nerves cross-sectional area (CSA) at multiple sites in cases with probable DPN and correlating the findings with NCS parameters. Therefore, presenting the data of this pilot study to contribute further into morphological diagnosis of early DPN.
Methods and materials
Subjects
This pilot study was designed as a cross-sectional observational study. The study was approved by the institutional ethics committee. Sampling of convenience was decided by the institutional review board with 50 subjects in each arm. Patients of Type II diabetes mellitus with probable DPN presenting as generalised typical polyneuropathy and age-matched healthy volunteers were enrolled after informed written consent was obtained from each participant. Probable DPN was a clinical diagnosis based on cases presenting with clinical symptoms and finding but has not been confirmed by the electrodiagnostic criteria defined by ADA.8 There were 100 each of median, ulnar, common peroneal, tibial nerve, and 96 of sural nerves in cases. In controls, 100 of each nerve were assessed. General baseline characteristics such as age, sex, height, weight, and body mass index (BMI) were recorded. Additionally, time since diagnosis, glycosylated hemoglobin (HbA1c) levels, and NCS parameters of cases were recorded. Subjects who had any clinical and electrophysiological evidence of polyneuropathy other than the DPN (such as inflammatory neuropathy, hereditary neuropathy, metabolic neuropathy), mononeuropathy or traumatic nerve injury were excluded. Also, Pregnant females with lactation, Type I / secondary diabetes, end organ failure, H/o drug (causing neuropathy), thyroid disorders (Hypo/Hyperthyroid patients), Age <18 years, H/o severe trauma to limb were also excluded.
Electrodiagnostic (EDX) examinations
NCS was performed for all patients with diabetes using a Synergy electrodiagnostic software v. 2012 (Gnatus) on both lower and upper extremities with skin surface temperatures of ≥30°C (legs) and ≥32°C (arms). The study was done by a senior technician and results were interpreted by a neurologist of more than 15 years of experience. Both were blinded to the clinical and ultrasonographic findings of the patient. Each sensory NCS was performed for the sural nerve, median nerve, and ulnar nerve. Motor NCS was performed for the common peroneal nerve, tibial nerve, median nerve, and ulnar nerve. Based on NCS cases were grouped into normal, axonal, demyelinating, mixed, and non-recordable.10
Sonographic examinations
Sonographic examinations were performed with SuperSonic Imagine- Aixplorer®- Innovative UltraFast™ Ultrasound Imaging system with SuperLinear™ SLH20-6 probe of bandwidth 6–20 MHz and SuperLinear™ SL18-5 probe of bandwidth 5–18 MHz by a radiologist of more than 10 years of experience in peripheral nerve ultrasonography. Examiner was blinded to the clinical condition of the subjects. CSAs of peripheral nerves were measured by planimetry (tracing inner margin of the hyperechoic perineural stroma). To prevent the overestimation of the nerve CSA by the inclusion of blood vessels, the color doppler mode was used before nerve tracing. The transducer was placed on the skin with minimal pressure perpendicularly to the nerve being measured to minimize anisotropy. Probe was tilted to scan the plane where the area appears smallest. The median nerve was measured just proximal to carpal tunnel inlet (at the scaphoid–pisiform level) and 3 cm proximal to the wrist crease; ulnar nerve at the cubital tunnel inlet (proximal to medial epicondyle); and 3 cm proximal; the tibial nerve posterior to the tip of the medial malleolus and 3 cm proximal to it; common peroneal nerve at fibular head and 3 cm proximal to it; and the sural nerve at the distal third of the leg (Figures 1 and 2). In nerves where two measurements were taken proximal measurement was denoted by letter P and distal by D. The difference between the two measurements was mentioned as δ.
Figure 1.
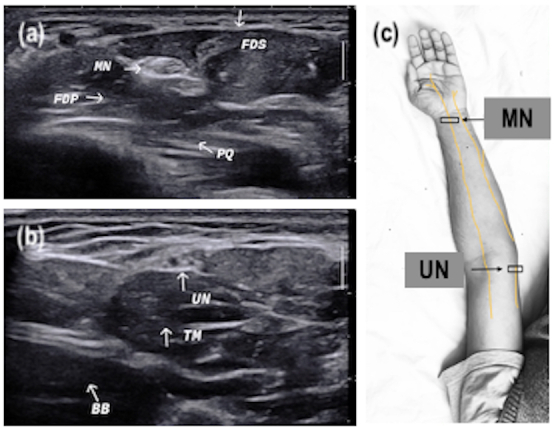
Transverse ultrasound section of median and ulnar nerves (marked with white arrows) at different locations with anatomical landmarks. (a) MN distal forearm between FDS and FDP at the level of PQ proximal end. (b) UN distal arm next to medial head of TM and nearby BB muscle. (c) Image showing course of nerve with probe position (black box). BB, bicep brachii; FDP, forearm deep digital flexor; FDS, forearm superficial digital flexor; MN, median nerve; PQ, pronator quadratus; TM, tricep muscle; UN, ulnar nerve
Figure 2.
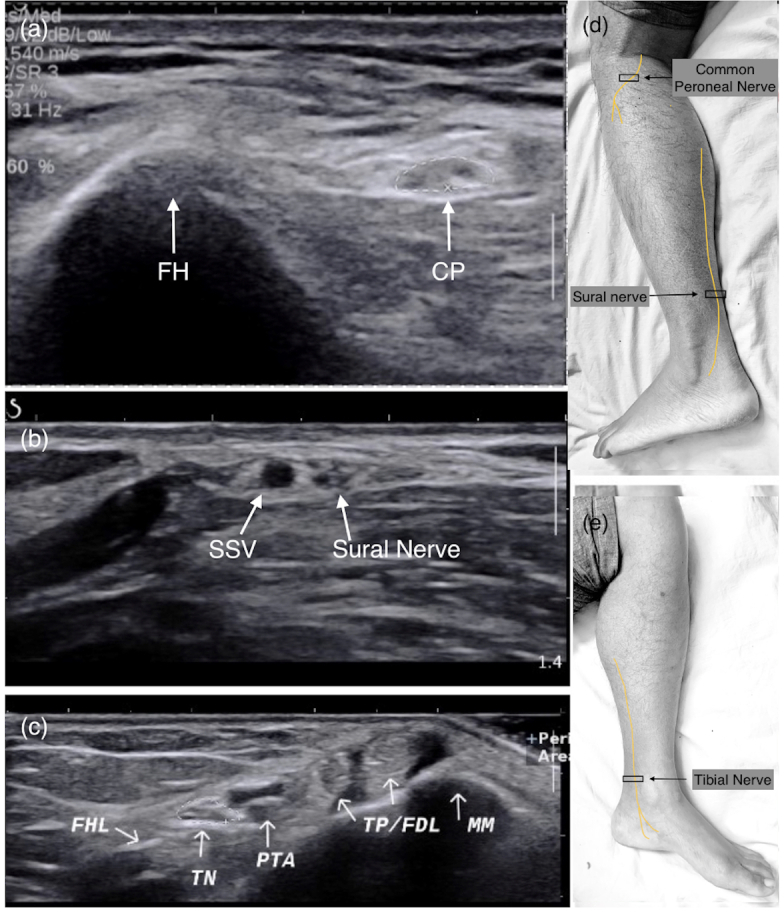
Transverse ultrasound section of common peroneal, sural and tibial nerves (marked with white arrows) at different locations with anatomical landmarks. (a) CP nerve near the FH. (b) Sural nerve distal leg adjacent to SSV. (c) TN at ankle (MM, TP/FDL, PTA and veins, FHL tendon). (d, e) Image showing course of nerve with probe position (black box). CP, common peroneal; FDL, flexor tendons; FH, fibular head; FHL, flexor hallucis longus; MM, medial malleolus; PTA, posterior tibial artery; SSV, short saphenous vein; TN, tibial nerve.
Statistical tools employed
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) v. 21.0 statistical Analysis Software. The values were represented in Number (%) and Mean ± SD. The various test employed was the χ2 test, Student’s 't' test and ANOVA test.
Results
Demographic and clinical parameters
The demographic and clinical characteristics of the study population are shown in Table 1. The mean age of cases was 55.98 years (range 28–75). The mean body weight and height of cases were 67.85 ± 14.08 kg and 161.42 ± 8.81 cm. Body mass index (BMI) of cases ranged from 17.88 to 38.33 kg/m2. Duration of illness since diagnosis ranged from 1 to 25 years. Maximum patients (n = 23; 46%) had diabetes for <5 years. Mean duration since diagnosis was 7.38 ± 5.97 years. Glycated hemoglobin (HbA1c) levels ranged from 4.4 to 15.3%. The mean HbA1c level of cases was 8.42±2.52%. Most of the patients (n = 47; 94%) were on oral hypoglycemic agents (OHAs).
Table 1.
Characteristics of the study population
| SN | Characteristic | Cases (n = 50) | Controls (n = 50) | Significance of difference |
|---|---|---|---|---|
| 1. | Mean age ±SD (Range) in years |
55.98 ± 11.00 (28-75) |
53.66 ± 9.88 (31-75) | ‘t’=1.109; p = 0.270 |
| 2. | Gender Male Female |
34 (68.0%) 16 (32.0%) |
40 (80.0%) 10 (20.0%) |
χ2=1.871; p = 0.171 |
| 3. | Mean weight ± SD in kg | 67.85 ± 14.08 | 61.46 ± 6.17 | ‘t’=2.940; p = 0.004 |
| 4. | Mean height ±SD in cm | 161.42 ± 8.81 | 161.92 ± 6.68 | ‘t’=0.320; p = 0.750 |
| 5. | Mean BMI ±SD (range) in kg/m² |
25.90 ± 4.60 (17.88–38.33) |
23.46 ± 2.16 (19.05–31.64) | ‘t’=3.386; p = 0.001 |
| 6. | Duration of illness <5 years 5–10 years >10 years Mean duration ± SD (Range) in years |
23 (46%) 14 (28%) 13 (26%) 7.38 ± 5.97 (1-25) |
|
|
| 7. | Level of glycemic control Good (<7%) Intermediate (7–8.5%) Poor (>8.5%) Mean HbA1c ± SD (Range) in % |
17 (34%) 5 (10%) 28 (56%) 8.42 ± 2.52 (4.4–15.3) |
|
|
| 8. | Drug regimen OHA Insulin |
47 (94%) 3 (6%) |
|
BMI, body mass index; OHA, oral hypoglycemic agent; SD, standard deviation.
The comparison of cases and healthy controls were done using Student ‘t’-test. There was no statistically significant association between the CSA of all the nerves at all the mentioned locations and levels of glycemic control, duration of illness, and height of cases.
Table 2 describes the correlation of CSA with BMI. When compared to different BMI groups in cases using ANOVA, the mean CSA showed a significant increasing trend in ulnar, common peroneal, tibial, and proximal median nerve(p < 0.05). However, the mean CSA of the sural nerve and median nerve at the carpal tunnel inlet was not significant.
Table 2.
Association of peripheral nerve cross-sectional area measurements at different locations with BMI category in cases (n = 100)
| SN | Nerve/Location | BMI category (kg/m²) | Significance of difference (ANOVA) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal (18.5–24.9) (n = 46)* | Overweight (25.0–29.9) (n = 36) | Obese (>30) (n = 18) | |||||||
| Mean | SD | Mean | SD | Mean | SD | ‘F’ | ‘p’ | ||
| Median nerve | |||||||||
| 1 | Proximal | 7.09 | 1.8 | 7.75 | 1.59 | 8.5 | 2.73 | 3.69 | 0.029 |
| 2 | Distal | 8.63 | 2.43 | 9.17 | 2.42 | 10.22 | 3.56 | 2.328 | 0.103 |
| Ulnar nerve | |||||||||
| 1 | Proximal | 5.41 | 1.54 | 6 | 1.47 | 6.56 | 1.54 | 4.016 | 0.021 |
| 2 | Distal | 6.65 | 2.33 | 7.36 | 3.2 | 7.78 | 3.15 | 1.253 | 0.29 |
| Common peroneal nerve | |||||||||
| 1 | Proximal | 8.85 | 3.34 | 11.67 | 3.94 | 10.89 | 3.89 | 6.334 | 0.003 |
| 2 | Distal | 10 | 3.66 | 12.19 | 4.33 | 11.94 | 4.32 | 3.433 | 0.036 |
| Tibial nerve | |||||||||
| 1 | Proximal | 11.57 | 3.4 | 13.92 | 4.14 | 15.5 | 5.79 | 6.771 | 0.002 |
| 2 | Distal | 11.76 | 4.05 | 15 | 4.76 | 15.56 | 6.14 | 6.59 | 0.002 |
| Sural nerve | |||||||||
| 1 | At lower third leg | 1.31 | 0.56 | 1.53 | 0.65 | 1.56 | 0.86 | 1.418 | 0.247 |
BMI, body mass index.
Includes one patient (n = 2) with BMI 17.88 kg/m2
Table 3 describes the mean CSA of cases and age-matched healthy controls and data were compared using Student ‘t’- Test. The mean CSA at both proximal and distal sites as well as δ value, showed higher value at all locations (p = 0.001) except for the sural nerve.
Table 3.
Comparison of cross-sectional area of peripheral nerves at different locations between cases and controls
| SN | Nerve/Location | Cases | Controls | Significance of difference | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ‘t’ | ‘p’ | ||
| Median nerve | (n = 100)a | (n = 100) | |||||
| 1. | Proximal | 7.58 | 1.98 | 6.68 | 1.45 | 3.668 | <0.001 |
| 2. | Distal | 9.11 | 2.70 | 7.36 | 1.41 | 5.751 | <0.001 |
| 3. | ΔDelta | 1.87 | 2.03 | 1.04 | 0.92 | 3.719 | <0.001 |
| Ulnar nerve | (n = 100) | (n = 100) | |||||
| 1. | Proximal | 5.83 | 1.56 | 4.74 | 0.99 | 5.887 | <0.001 |
| 2. | Distal | 7.11 | 2.83 | 4.90 | 1.24 | 7.161 | <0.001 |
| 3. | ΔDelta | 1.52 | 1.97 | 0.74 | 0.69 | 3.742 | <0.001 |
| Common peroneal nerve | (n = 100) | (n = 100) | |||||
| 1. | Proximal | 10.23 | 3.86 | 7.01 | 1.80 | 7.567 | <0.001 |
| 2. | Distal | 11.14 | 4.13 | 7.83 | 1.79 | 7.360 | <0.001 |
| 3. | ΔDelta | 1.65 | 1.45 | 1.12 | 0.84 | 3.155 | 0.002 |
| Tibial nerve | (n = 100) | (n = 100) | |||||
| 1. | Proximal | 13.12 | 4.41 | 9.18 | 1.48 | 8.468 | <0.001 |
| 2. | Distal | 13.61 | 4.99 | 9.27 | 1.53 | 8.314 | <0.001 |
| 3. | ΔDelta | 1.95 | 2.21 | 0.81 | 0.77 | 4.872 | <0.001 |
| Sural nerve | (n = 96) | (n = 100) | |||||
| 1. | Lower third leg | 1.44 | 0.66 | 1.22 | 0.42 | 2.112 | 0.036 |
SD, standard deviation.
n is no of nerves evaluated
Correlation between CSA and NCS findings
CSA analysis with different pattern noted on NCS for each nerve has been tabulated in Table 4. The CSA in diverse types of neuropathy was analyzed using ANOVA. In all nerves, the CSA with abnormal NCS findings was more than those with normal with the maximum interclass difference in distal and delta measurement of upper limb nerves (p < 0.05)
Table 4.
Nerve-wise correlation between NCS pattern and USG measured cross-sectional areas among cases
| SN | Nerve | Normal NCS | Axonal | Demyelinating | Mixed | Not recordable | Not done | Statistical significance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | F | ‘p’ | ||
| Median Nerve | |||||||||||||||||||||
| 1. | Proximal | 59 | 7.61 | 1.96 | 5 | 7.20 | 1.10 | 23 | 7.43 | 1.97 | 7 | 8.29 | 2.69 | 0 | - | - | 6 | 7.33 | 2.34 | 0.317 | 0.866 |
| 2. | Distal | 59 | 8.59 | 2.31 | 5 | 8.20 | 1.30 | 23 | 9.39 | 2.48 | 7 | 12.57 | 2.88 | 0 | - | - | 6 | 9.83 | 4.71 | 4.226 | 0.003 |
| 3. | δ | 59 | 1.39 | 1.52 | 5 | 1.00 | 0.71 | 23 | 2.22 | 2.04 | 7 | 4.29 | 1.98 | 0 | - | - | 6 | 3.17 | 4.17 | 4.993 | 0.001 |
| Ulnar Nerve | |||||||||||||||||||||
| 1. | Proximal | 63 | 5.68 | 1.54 | 6 | 5.67 | 1.37 | 16 | 5.50 | 1.15 | 8 | 7.75 | 1.58 | 1 | 7.00 | - | 6 | 5.67 | 1.63 | 3.102 | 0.012 |
| 2. | Distal | 63 | 6.89 | 2.30 | 6 | 6.17 | 1.83 | 16 | 6.31 | 1.30 | 8 | 11.50 | 5.55 | 1 | 11.00 | - | 6 | 6.00 | 1.41 | 6.155 | <0.001 |
| 3. | δ | 63 | 1.40 | 1.54 | 6 | 0.83 | 0.75 | 16 | 1.06 | 1.00 | 8 | 4.00 | 4.66 | 1 | 4.00 | - | 6 | 1.00 | 0.63 | 3.780 | 0.004 |
| Common Peroneal Nerve | |||||||||||||||||||||
| 1. | Proximal | 53 | 9.42 | 3.79 | 18 | 9.94 | 2.90 | 2 | 11.50 | 0.71 | 2 | 10.00 | 2.83 | 18 | 11.33 | 4.45 | 7 | 14.00 | 3.56 | 2.316 | 0.050 |
| 2. | Distal | 53 | 10.13 | 3.82 | 18 | 10.89 | 3.71 | 2 | 13.50 | 0.71 | 2 | 11.50 | 0.71 | 18 | 12.22 | 4.45 | 7 | 15.86 | 4.30 | 3.169 | 0.011 |
| 3. | δ | 53 | 1.55 | 1.28 | 18 | 1.50 | 1.29 | 2 | 2.00 | 1.41 | 2 | 1.50 | 2.12 | 18 | 2.00 | 2.09 | 7 | 1.86 | 1.35 | 0.345 | 0.884 |
| Tibial Nerve | |||||||||||||||||||||
| 1. | Proximal | 55 | 11.75 | 4.20 | 11 | 15.55 | 5.18 | 8 | 14.75 | 3.65 | 4 | 17.75 | 4.86 | 15 | 12.87 | 3.42 | 7 | 16.14 | 2.54 | 4.037 | 0.002 |
| 2. | Distal | 55 | 11.89 | 4.06 | 11 | 17.00 | 6.20 | 8 | 15.50 | 4.84 | 4 | 17.00 | 7.30 | 15 | 13.60 | 4.93 | 7 | 17.71 | 2.81 | 4.561 | 0.001 |
| 3. | δ | 55 | 1.75 | 2.18 | 11 | 2.00 | 2.05 | 8 | 2.50 | 1.93 | 4 | 2.25 | 2.63 | 15 | 2.47 | 2.90 | 7 | 1.57 | 1.27 | 0.402 | 0.846 |
| Sural Nerve | |||||||||||||||||||||
| 1. | At Lower third leg | 46 | 1.24 | 0.57 | 24 | 1.50 | 0.66 | 0 | - | - | 0 | - | - | 19 | 1.68 | 0.75 | 7 | 1.86 | 0.69 | 3.527 | 0.018 |
NCS, nerve conduction study; SD, standard deviation.
The CSA of cases with normal NCS and control was analysed using Student’s ‘t’- test and showed significantly higher (p < 0.05) CSA in cases for all nerve except sural nerve (Table 5).
Table 5.
Comparison of cross-sectional area of peripheral nerves at different locations between cases with normal NCS and controls
| Nerve | Location | Cases | Controls (n = 100) | Significance of difference | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Mean | SD | ‘t’ | ‘p’ | ||
| Median nerve | ||||||||
| 1 | Proximal | 59 | 7.61 | 1.96 | 6.68 | 1.45 | 3.419 | 0.001 |
| 2 | Distal | 59 | 8.59 | 2.31 | 7.36 | 1.41 | 4.172 | <0.001 |
| 3 | Delta | 59 | 1.39 | 1.52 | 1.04 | 0.92 | 1.81 | 0.072 |
| Ulnar nerve | ||||||||
| 1 | Proximal | 63 | 5.68 | 1.54 | 4.74 | 0.99 | 4.746 | <0.001 |
| 2 | Distal | 63 | 6.89 | 2.3 | 4.9 | 1.24 | 7.164 | <0.001 |
| 3 | Delta | 63 | 1.4 | 1.54 | 0.74 | 0.69 | 3.736 | <0.001 |
| Common peroneal nerve | ||||||||
| 1 | Proximal | 53 | 9.42 | 3.79 | 7.01 | 1.8 | 5.334 | <0.001 |
| 2 | Distal | 53 | 10.13 | 3.82 | 7.83 | 1.79 | 5.071 | <0.001 |
| 3 | Delta | 53 | 1.55 | 1.28 | 1.12 | 0.84 | 2.498 | 0.014 |
| Tibial nerve | ||||||||
| 1 | Proximal | 55 | 11.75 | 4.2 | 9.18 | 1.48 | 5.537 | <0.001 |
| 2 | Distal | 55 | 11.89 | 4.06 | 9.27 | 1.53 | 5.764 | <0.001 |
| 3 | Delta | 55 | 1.75 | 2.18 | 0.81 | 0.77 | 3.9 | <0.001 |
| Sural nerve | ||||||||
| 1 | At calf | 46 | 1.24 | 0.57 | 1.22 | 0.42 | 0.197 | 0.844 |
NCS, nerve conduction study; SD, standard deviation.
In the median nerve, 59% of cases showed normal NCS, followed by 23% showing a demyelinating pattern. Median nerve (P) mean CSA in cases with DPN was 7.58 mm2 with high delta value 1.87 mm2. However, when individually correlated in each group, the delta value was highest in demyelinating and mixed (4.29 mm2) and was least in axonal pattern (1.00 mm2). Value was intermediate in cases with normal NCS. Evaluation of delta measurement in cases with demyelinating and mixed pattern reveal 18 cases with value >2 mm2.
In ulnar nerve, about 63% cases showed normal NCS followed by 16% showing demyelinating pattern. Ulnar nerve (P) mean CSA in cases with DPN was 5.83 mm2 with a high delta value of 1.52 mm2. However, this delta value was highest in mixed pattern (4.00 mm2) and was least in axonal pattern (0.83 mm2). Value was intermediate in cases with normal NCS. Evaluation of CSA ratio (D/P) in cases with demyelinating and mixed pattern of NCS, there were 9 cases out of 24 showing value >1.4.
In common peroneal nerve, 53% of cases showed normal NCS followed by 18% showing axonal and 18% NCS was not recordable. Common peroneal nerve (P) mean CSA in cases with DPN was 10.23 mm2 with a delta value of 1.65 mm2. However, this delta value was highest in the demyelinating pattern (2.0 mm2) and was least in the axonal pattern (1.5 mm2). The value was intermediate in cases with normal NCS.
In the tibial nerve, 55% of cases showed normal NCS followed by 11% showing axonal, and 19% NCS was not recordable. Tibial nerve (P) mean CSA in cases with DPN was 13.12 mm2 with a delta value of 1.95 mm2. However, delta value was highest in demyelinating/mixed pattern (2.5 mm2) and was least in the axonal pattern (2.0 mm2). The value was low in cases with normal NCS.
In the sural nerve, 24% had axonal neuropathy, 19% were non-recordable, none nerve showed demyelinating or mixed component in abnormal NCS cases.
Receiver operating characteristic (ROC) analysis of CSA (P) showed acceptable performance (AUC 0.73–0.79) of ulnar nerve, common peroneal nerve, and tibial nerve, whereas for median and sural nerve, AUC was lower (0.64 & 0.61 respectively) (Figure 3). Based on the ROC analysis cut-off CSA(P) for the studied nerve with their respective sensitivity and specificity are listed in Table 6. These values were used to evaluate the no of nerves involved in diabetics with all normal NCS and control. The result is depicted in box plot (Figure 4). The average no of nerves involved in control was 3.44 as against 6.5 in cases. In cases with all nerves showing normal NCS, the average no of nerves involvement was 5.80. This difference was statistically significant (p < 0.05).
Figure 3.
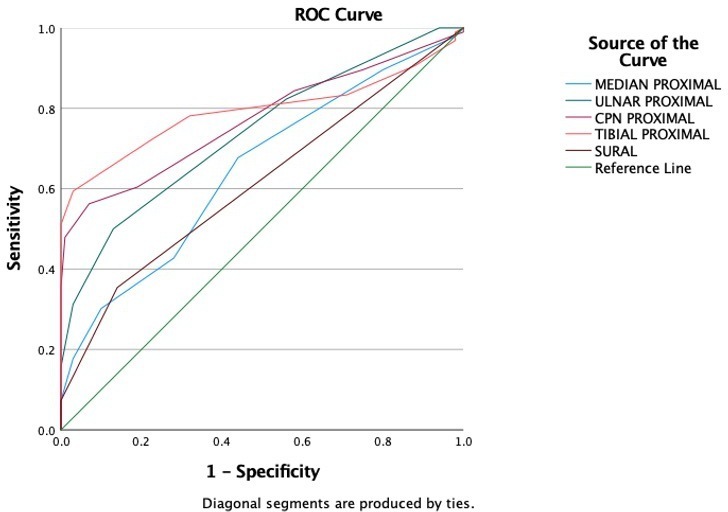
ROC curve for nerve CSA in cases and control. CSA, cross-sectional area; ROC, receiver operating characteristic.
Table 6.
Area under curve for peripheral nerves at proximal site of measurment and cut off point with corresponding sensitivity and specificity for each nerve
| Test result variable(s) | Area | Std. errora | Cut-off value sq mm | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| MEDIAN PROXIMAL | .646 | .039 | 6.5 | 67 | 56 |
| ULNAR PROXIMAL | .738 | .035 | 4.5 | 82 | 44 |
| CPN PROXIMAL | .771 | .034 | 7.5 | 70 | 64 |
| TIBIAL PROXIMAL | .793 | .035 | 10.5 | 72 | 78 |
| SURAL | .612 | .040 | 1.5 | 35 | 86 |
Figure 4.
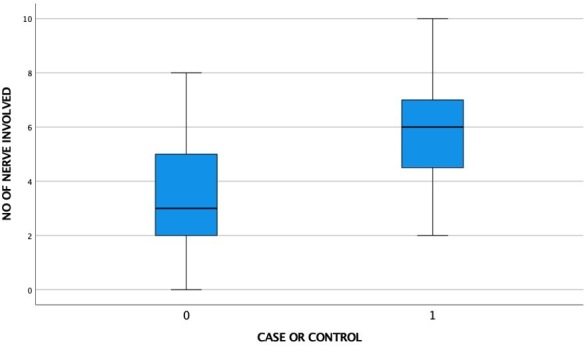
Box plot distribution of number of nerves involved in control vs cases with normal NCS in all nerves {control (0)=50; cases with all normal NCS (1)=15}. NCS, nerve conduction study.
Discussion
The present study was undertaken to evaluate the CSA of peripheral nerves (median nerve at the wrist, the ulnar nerve at the elbow, the common peroneal nerve at knee, tibial nerve at ankle and sural nerve at lower leg) in cases of DPN to evaluate the pattern of involvement with clinical and NCS parameters.
In a landmark study, around 4400 patients with diabetes were serially evaluated for 25 years. The onset of neuropathy correlated positively with the duration of diabetes, and by 25 years, 50% of patients had developed neuropathy.11 This slow progression of the disease is reflected in our study in which the mean age of presentation was 55.9 years (28–71 years). A similar observation has been made in other studies.5,12–14
Our study group showed a significantly higher percentage (68%) of the male population in cases with DPN. Though a similar pattern has been reflected in other studies, contrasting result has been presented in the study conducted by Kelle et al in which there were 26.4% male cases. This variation among these studies may be due to demographic variations of DPN presentation among different study populations. As this subtle chronic problem needs a repeated visit to tertiary care hospitals, it may hinder females from bringing them to the point of care especially in rural populations in developing nations.13,15,16
BMI in cases was higher in our study group, which may be attributable to the high incidence of obesity in diabetic patients. Moreover, there is an incremental trend across increasing BMI and nerve CSA of all nerves, except that of the sural nerve. The reasons for this may be due to higher BMI is related to poor glycaemic control. However, Li et al., Kelle et al, found no correlation of increased CSA with that of BMI. The mean BMI of the participants in our study was 25.9 kg/m2. This value was significantly lower than that of participants in a study conducted by Kelle et al., (30.43 kg/m2), and this difference may explain the discrepancy between the two studies.14,17–19 However, as the BMI is higher in our study group than the control group, and there is an increasing trend in CSA with increasing BMI, so there are chances of BMI as a confounder. This is the limitation that needs further study. There was no correlation of CSA with the different height of the patients. Similar observation has been made in other studies.7,17,20
Onset of late-onset diabetes and DPN is insidious, and the exact duration of illness is not possible to ascertain. There was no significant correlation found between the duration (time since diagnosis) of the disease and the CSA of cases of nerve studied. As this time duration does not quantify the duration of disease, this may be responsible for the insignificant association between nerve CSA and duration of DM. Kang et al., Hobson‐Webb et al., Kelle et al., Attah et al, in their studies also found no correlation between the CSA of various nerves in DPN patients and the duration of the disease.7,14,17,20
A long-term hyperglycemic state has been implicated in the occurrence of DPN. The long-term monitor (HbA1c) gives a good estimate of glycemic control, but only for 3 months duration. So, it does not reflect a sufficient long control to correlate with neuropathy as seen in our study. Riazi et al, also did not find any significant correlation between the nerve CSA and the values of HbA1c. Kang et al, found that HbA1c (mean value: 7.91) was significantly correlated with sural nerve CSA, but this finding was not observed with CSA of any other nerve. This finding of the sural nerve may be due to its nature of being most sensitive to diabetic neuropathy, acute short-term changes, leading to a correlation between the HbA1c values and its enlargement. Kelle et al, also did not find any correlation between the nerve CSA and the HbA1c levels (p value = 0.46). This observation contrasts the findings of Watanabe et al, who reported a significant correlation between median nerve CSA and HbA1c levels despite fewer sample size in their study relative to ours. Genetic and racial differences and small sample size may have contributed to this.7,12,14,16,20
The structural changes in DPN has been presented in various studies as oxidative stress triggered by microangiopathy in the nerve and accumulation of glucose metabolite sorbitol in Schwann cells are the key pathological process that induces axonal and demyelinating nerve changes.21 And, the course of various nerve through an osteofibrous tunnel where the extra space to accommodate the oedematous nerve is limited leading to associated compression and then feature of compressive neuropathy that reflects on sonography as edematous thickened nerve proximal to the site of compression.22 Considering the above pathophysiology, the measurement of the CSA was done for nerves at two sites. Sural nerve being an exception as it does not pass through any fibro-osseous tunnel. However, as the nerve has been significantly studied both in diabetic and non-diabetic conditions, it has been included in the study.
Site of proximal measurement at 3 cm was done based on the previous study by Riazi et al, who concluded this measurement as the optimal site for DPN.16 Though for applying the criteria for compressive neuropathy this may not be optimal, and changes can go beyond. Still literature is silent over the exact site for measurement to define the delta value. Klauser et al presented measurement in the proximal third of pronator quadratus muscle with value showing high sensitivity and specificity for the diagnosis of carpal tunnel, whereas Yoon et al, proposed measurement of ulnar nerve two cm proximally for cubital tunnel syndrome.23,24
There was an overlapping of measurement of the CSA for all the nerves when compared to various published data on the normal values from other studies. Comparing our results of mean CSA in healthy control with other studies (Table 7), there is a significant similarity in measurement in upper limb nerves. However, our observation shows lower values for tibial and common peroneal nerves. This discrepancy might be attributed to studies from different racial groups, and thus need to have normative data for each geographical/ ethnic group for objectivity in interpretation.25–29
Table 7.
Comparison of results of reference values of our study with other studies
| Nerve/site | Present study (DPN cases) | Present study (control) | Bedewi et al | Boehm J et al | Qrimli et al | Won et al |
|---|---|---|---|---|---|---|
| MN/ CT | 9.11 ± 2.70 | 7.36 ± 1.41 | 9.77 ± 2.88 | 8.5 ± 1.8 | 10 ± 2.4 (Ra) | 8.3 ± 1.5(Ra) |
| MN/ P | 7.58 ± 1.98 | 6.68 ± 1.44 | 6.46 ± 2.04 | 5.7 ± 1.3 | 7.3 ± 1.7 (R) | 6.5 ± 1.1(R) |
| UN/ D | 7.11 ± 2.83 | 4.90 ± 1.23 | 7.49 ± 2.35 | 7.6 ± 2.1 | 6.9 ± 2.3 (R) | 7.2 ± 1.4(R) |
| UN/ P | 5.83 ± 1.56 | 4.74 ± 0.99 | 7.55 ± 2.60 | 6.3 ± 1.7 | 6.8 ± 2.3 (R) | 5.9 ± 1.1(R) |
| CPN/ D | 11.14 ± 4.13 | 7.83 ± 1.78 | 8.89 ± 3.23 | 8.9 ± 2.0 | 11.1 ± 3.5 (R) | |
| CPN/ P | 10.23 ± 3.86 | 7.01 ± 1.80 | 11.8 ± 3.8 (R) | |||
| TN/ D | 13.61 ± 4.99 | 9.27 ± 1.53 | 12.66 ± 4.45 | 9.6 ± 2.2 | 12.7 ± 3.4 (R) | |
| TN /P | 13.12 ± 4.41 | 9.18 ± 1.48 | ||||
| SN/ LL | 1.44 ± 0.66 | 1.15 ± 0.35 | 3.52 ± 1.40 | 1.8 ± 0.6 |
DPN, diabetic peripheral sensorimotor neuropathy.
R: Measurement done on the right limb only
Mean CSA in cases of DPN shows observations suggesting a significant increase in the CSA when compared with our healthy control. Other studies have made similar observation.5,7,12,14,16,30
Contrary to our observation, Arumugam et al, reported a statistically significant difference in CSA between healthy controls and diabetic patients at wrist crease only. This may be due to a high percentage of early cases in the study group.5 Hobson‐Webb et al did not find any significant difference in CSA of the common peroneal nerve between diabetic patients and controls when measured at three different sites, fibular head, popliteal fossa and at the ankle. This result may be because they selected the control group who presented to the EMG lab for radiculopathy testing without a history of DM or DPN symptoms.
The difference of CSAs measured from the common compressive sites, and proximal locations were measured in the median, ulnar, common peroneal, tibial nerve that showed higher value in DPN cases as compared to controls (p-value < 0.001 at all sites) suggesting that the enlargement is not uniform and due to the pathological thickening, there is a component of compression at these sites. Similar observations have been reported in other studies that concluded that diabetic patients are susceptible to compressive neuropathy, such as carpal tunnel syndrome and ulnar neuropathy at the elbow.13,31,32
The DPN cases were categorized into four categories based on NCS pattern into axonal, demyelinating, mixed, and non-recordable and their CSAs were analyzed. For all sites, the nerves with normal NCS patterns showed significantly lower CSA than in abnormal NCS cases.
CSA difference in different patterns of involvement was more marked with demyelinating and mixed patterns in the median, ulnar, and common peroneal nerve. This can be explained as nerves are relatively thicker in demyelinating nerve. A similar observation has been made by Grimm et al where nerves showed higher CSA in demyelinating neuropathy as compared to axonal neuropathy in which nerve enlargement was not significant.33 Observations made by Di Pasquale et al in a series of chronic inflammatory demyelinating polyneuroradiculopathy cases were also similar.34 However, this observation did not hold for tibial nerve. This is possibly due to the overestimation of margin in the axonal pattern where the overall echogenicity of the nerve is increased with the loss of fascicular definition and so a clear line of demarcation of epineurium is absent.
In the sural nerve, no such difference could be appreciated. This can be explained with small CSA of the nerve, so minor differences could not be picked up. However, in more advanced conditions (non-recordable) CSA measurements were of higher order compared to healthy control (p < 0.05).
However, Rajabally et al in their study on the median nerve, found that CSA was increased in both demyelinating and axonal neuropathies, and there was no significant difference observed between these two findings.35 Scheidl et al included ulnar neuropathy cases in their study and found that CSA was significantly increased in cases with axonal neuropathy as compared to predominant demyelinating.36
Regarding CSA changes in diverse types of neuropathy, some studies have reported differently. These can be explained as we have included probable DPN patients. In contrast, Grimm et al included patients with axonal, demyelinating, and mixed neuropathy, cause of which were not known, Schiendl et al included undefined patients of ulnar neuropathy and Rajabally et al included patients that had chronic inflammatory demyelinating polyneuropathy (CIDP) and sensory axonal neuropathy (SAN).33,35,36
The nerve CSA of cases with normal NCS was compared to control. All the nerves except sural showed higher CSA in cases. This suggest the possible morphological changes in early DPN before the NCS abnormality is evident.
The AUC of CSA at proximal sites showed acceptable performance in ulnar, common peroneal and tibial nerves, but the values in median and sural nerve were relatively lower. Sural nerve being a very thin nerve with variability of extremely low order might be the reason for the same. Median nerve relatively poor performance may be attributed to late involvement of median nerve as well as selection of site very near to compression site. Distal CSA and delta values were not considered as these can be affected by compressive changes as well; a common feature in DPN.32 Cases with all nerve showing normal NCS and control when evaluated for number of nerves involved. The number of nerves involved in diabetics were significantly higher in diabetics (p < 0.05). These cases may represent the early cases with small fiber neuropathy. A cut-off value of 4 or more than 4 nerve involvement gave a sensitivity of 86% and specificity of 56%. This observation was slightly different from that reported by Breiner et al who used cut point of >3 nerve involvement with sensitivity of 64% and specificity of 77%. This can be attributed to different population characteristics.37
The percentage of cases with abnormal NCS was higher in the lower limb as against the upper limb. Moreover, if we look at the involvement in terms of axonopathy which is the primary involvement in DPN in cases there is only 5% and 7% cases of median and ulnar nerve primary axonopathy on NCS as against 36%, 26% and 50% of Common peroneal, tibial and sural nerve respectively. On the other hand, only 9% nerves of lower limb show primary demyelination (major contribution from tibial) as against 59% of upper limb.
Though demyelinating changes of DPN may be due to ischemic, immune-mediated or compressive; there is high incidence of compressive neuropathy in carpal and cubital tunnel followed by tarsal tunnel.22 In cases presenting with demyelinating and mixed NCS physiology in the ulnar nerve at the elbow and median nerve at carpal tunnel inlet CSA was analyzed in reference to ultrasonographic diagnostic criteria proposed by Klauser et al for carpal tunnel syndrome, and Cartwright et al, Yoon et al for cubital tunnel syndrome.23,24,38 There were 58.1% (18 of 31) cases in the median nerve and 37.5% cases of ulnar nerve (9 of 24) qualified the ultrasonographic criteria of compressive neuropathy at respective sites.
Thus, the demyelinating changes in upper limb and some of the cases of lower limb at compression site may be due to compressive neuropathy in osteofibrous tunnels of thickened nerve and may the reversible component of presenting symptoms. This is further supported by the fact that in sural nerve NCS, none of them presented with primary demyelinating pattern. However, this need further evaluation by documenting the demyelination neurophysiological changes to be only focally present and is normal in the more proximal part of nerve by inching technique.
Limitations
There were some limitations in our study.
Single sonologist performed all evaluations and interobserver agreement was not calculated in our study.
The nerve echogenicity was not analyzed in our study that might be an important parameter in DPN.
Nerve enlargement is not specific for DPN and may be found in other neuropathies. This being a pilot study, bigger sample along with follow-up study is needed for use of HRUS objectively in defining the specific pattern in cases of different polyneuropathy.
Lack of obese control to evaluate the confounding effect of BMI on CSA.
Conclusion
In cases of DPN, there is enlargement of peripheral nerves, both at compression as well as non-compression sites. This enlargement is statistically significant when compared with healthy controls in a similar setting. The difference in the CSA is also noted in cases with normal NCS when compared with healthy control. Enlargement of multiple nerves may serve an effective diagnosis of DPN in cases with normal NCS. In cases with abnormal NCS, lower limb nerves predominantly showed axonopathy. Whereas demyelinating patterns were more commonly seen in upper limb nerves of which a large percentage showing sonographic feature of compressive neuropathy. Thus, clinical presentation in DPN is not only due to primary diabetes but associated compressive neuropathy in early phases. This will lead to better clinical staging/classification of DPN and appropriate intervention for early secondary prevention.
What this study adds
In the case of DPN, there is increased CSA of peripheral nerve as compared to healthy control in similar settings. Changes are appreciable even in DPN cases with normal NCS findings suggesting morphological changes on USG precedes an electrodiagnostic change. Thus, it promises to be useful in early pick up of DPN cases motivating the patients to actively participate in the treatment. This also setup the agenda for future research, as small fiber neuropathy may be associated with peripheral nerve enlargement without NCS abnormality
Predominant changes in the lower limb nerves are axonal. The upper limb involvement shows predominantly demyelinating pattern.
Symptoms of DPN is not only due to metabolic changes but also compressive neuropathy due to morphological changes, especially in cases with upper limb symptoms. And thus, routine use of HRUS helps excluding alternative diagnosis.
Contributor Information
Shamrendra Narayan, Email: samarnarayan@yahoo.co.in.
Amit Goel, Email: amitgoel23@gmail.com.
Ajai Kumar Singh, Email: ajai.shreshtha@gmail.com.
Anup Kumar Thacker, Email: dranupthacker14@gmail.com.
Neha Singh, Email: neha.singh.dr@gmail.com.
Manish Gutch, Email: manish07gutch@gmail.com.
REFERENCES
- 1.Wild S, Bchir MB, Roglic G, Green A, Sci M, Sicree R. . . Global Prevalence of Diabetes Estimates for the year 2000 and projections for 2030 [Internet]. 2004. Available from: http://care.diabetesjournals [cited 2020 Jul 16]. [DOI] [PubMed]
- 2.Kaveeshwar S, Cornwall J. The current state of diabetes management. Australas Med J 2014; 7: 45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulton AJ. End-Stage complications of diabetic neuropathy: foot ulceration. Can J Neurol Sci 1994; 21(S4): S18–22. doi: 10.1017/S0317167100040701 [DOI] [PubMed] [Google Scholar]
- 4.Simon NG, Narvid J, Cage T, Banerjee S, Ralph JW, Engstrom JW. Clinical/scientific notes. Neurology. Lippincott Williams and Wilkins 2014; 83: 1382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arumugam T, Razali SNO, Vethakkan SR, Rozalli FI, Shahrizaila N. Relationship between ultrasonographic nerve morphology and severity of diabetic sensorimotor polyneuropathy. Eur J Neurol 2016; 23: 354–60. doi: 10.1111/ene.12836 [DOI] [PubMed] [Google Scholar]
- 6.Fornage BD. Peripheral nerves of the extremities: imaging with us. Radiology 1988; 167: 179–82. doi: 10.1148/radiology.167.1.3279453 [DOI] [PubMed] [Google Scholar]
- 7.Kang S, Kim SH, Yang SN, Yoon JS. Sonographic features of peripheral nerves at multiple sites in patients with diabetic polyneuropathy. J Diabetes Complications 2016; 30: 518–23. doi: 10.1016/j.jdiacomp.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 8.Tesfaye S, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–93. doi: 10.2337/dc10-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitarokoili K, Kerasnoudis A, Behrendt V, Labedi A, Ayzenberg I, Gold R, et al. Facing the diagnostic challenge: nerve ultrasound in diabetic patients with neuropathic symptoms. Muscle Nerve 2016; 54: 18–24. doi: 10.1002/mus.24981 [DOI] [PubMed] [Google Scholar]
- 10.Goodin DS. Electromyography and neuromuscular disorders. Arch Neurol 1998; 55: 1150–1. doi: 10.1001/archneur.55.8.1150 [DOI] [Google Scholar]
- 11.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care 1978; 1: 252–63. doi: 10.2337/diacare.1.4.252 [DOI] [Google Scholar]
- 12.Watanabe T, Ito H, Sekine A, Katano Y, Nishimura T, Kato Y, et al. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med 2010; 29: 697–708. doi: 10.7863/jum.2010.29.5.697 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Wang C-L, Wu S, He S, Ren J. The feasibility of using high-resolution ultrasonography to assess ulnar nerve in patients with diabetes mellitus. J Ultrason 2017; 17: 160–6. doi: 10.15557/JoU.2017.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelle B, Evran M, Ballı T, Yavuz F. Diabetic peripheral neuropathy: correlation between nerve cross-sectional area on ultrasound and clinical features. J Back Musculoskelet Rehabil 2016; 29: 717–22. doi: 10.3233/BMR-160676 [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi F, Taniguchi M, Kojima R, Kawasaki A, Kosaka A, Uetake H. Morphological changes of the peripheral nerves evaluated by high-resolution ultrasonography are associated with the severity of diabetic neuropathy, but not corneal nerve fiber pathology in patients with type 2 diabetes. J Diabetes Investig 2015; 6: 334–42. doi: 10.1111/jdi.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riazi S, Bril V, Perkins BA, Abbas S, Chan VWS, Ngo M, et al. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? A cross-sectional study. Diabetes Care 2012; 35: 2575–9. doi: 10.2337/dc12-0739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobson-Webb LD, Massey JM, Juel VC. Nerve ultrasound in diabetic polyneuropathy: correlation with clinical characteristics and electrodiagnostic testing. Muscle Nerve 2013; 47: 379–84. doi: 10.1002/mus.23625 [DOI] [PubMed] [Google Scholar]
- 18.Li L, Chen J, Wang J, Cai D. Prevalence and risk factors of diabetic peripheral neuropathy in type 2 diabetes mellitus patients with overweight/obese in Guangdong Province, China. Prim Care Diabetes 2015; 9: 191–5. doi: 10.1016/j.pcd.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 19.Cartwright MS, Passmore LV, Yoon J-S, Brown ME, Caress JB, Walker FO. Cross-Sectional area reference values for nerve ultrasonography. Muscle Nerve 2008; 37: 566–71. doi: 10.1002/mus.21009 [DOI] [PubMed] [Google Scholar]
- 20.Attah FA, Asaleye CM, Omisore AD, Kolawole BA, Aderibigbe AS, Alo M. Relationship between sonographically measured median nerve cross-sectional area and presence of peripheral neuropathy in diabetic subjects. World J Diabetes 2019; 10: 47–56. doi: 10.4239/wjd.v10.i1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deli G, Bosnyak E, Pusch G, Komoly S, Feher G. Diabetic neuropathies: diagnosis and management. Neuroendocrinology 2013; 98: 267–80. doi: 10.1159/000358728 [DOI] [PubMed] [Google Scholar]
- 22.Martinoli C, Bianchi S, Gandolfo N, Valle M, Simonetti S, Derchi LE. US of Nerve Entrap-ments in Osteofibrous Tunnels of the Upper and Lower Limbs 1 LEARNING OBJECTIVES FOR TEST 5 [Internet].. . 2000. Available from: www.rsna.org [cited 2020 Jul 16]. [DOI] [PubMed]
- 23.Klauser AS, Halpern EJ, De Zordo T, Feuchtner GM, Arora R, Gruber J, et al. Carpal tunnel syndrome assessment with us: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology 2009; 250: 171–7. doi: 10.1148/radiol.2501080397 [DOI] [PubMed] [Google Scholar]
- 24.Yoon JS, Walker FO, Cartwright MS. Ultrasonographic swelling ratio in the diagnosis of ulnar neuropathy at the elbow. Muscle Nerve 2008; 38: 1231–5. doi: 10.1002/mus.21094 [DOI] [PubMed] [Google Scholar]
- 25.Won SJ, Kim B-J, Park KS, Yoon JS, Choi H. Reference values for nerve ultrasonography in the upper extremity. Muscle Nerve 2013; 47: 864–71. doi: 10.1002/mus.23691 [DOI] [PubMed] [Google Scholar]
- 26.Qrimli M, Ebadi H, Breiner A, Siddiqui H, Alabdali M, Abraham A, et al. Reference values for ultrasonograpy of peripheral nerves. Muscle Nerve 2016; 53: 538–44. doi: 10.1002/mus.24888 [DOI] [PubMed] [Google Scholar]
- 27.Boehm J, Scheidl E, Bereczki D, Schelle T, Arányi Z. High-Resolution ultrasonography of peripheral nerves: measurements on 14 nerve segments in 56 healthy subjects and reliability assessments. Ultraschall Med 2014; 35: 459–67. doi: 10.1055/s-0033-1356385 [DOI] [PubMed] [Google Scholar]
- 28.Bedewi MA, Abodonya A, Kotb M, Mahmoud G, Kamal S, Alqabbani A, et al. Estimation of ultrasound reference values for the upper limb peripheral nerves in adults: a cross-sectional study. Medicine 2017; 96: e9306. doi: 10.1097/MD.0000000000009306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedewi MA, Abodonya A, Kotb M, Kamal S, Mahmoud G, Aldossari K, et al. Estimation of ultrasound reference values for the lower limb peripheral nerves in adults: a cross-sectional study. Medicine 2018; 97: e0179. doi: 10.1097/MD.0000000000010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdel Latief Mahmoud M, Yousef A, Said S, Abd El-Sameea D. The role of ultrasound in the diagnosis and evaluation of diabetic neuropathy in nerve roots of the foot. Sci J Al-Azhar Med Fac Girls 2018; 2: 1. [Google Scholar]
- 31.Agirman M, Yagci I, Leblebicier MA, Ozturk D, Akyuz GD. Is ultrasonography useful in the diagnosis of the polyneuropathy in diabetic patients? J Phys Ther Sci 2016; 28: 2620–4. doi: 10.1589/jpts.28.2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horinouchi S, Deguchi T, Arimura K, Arimura A, Dochi Y, Uto T, et al. Median neuropathy at the wrist as an early manifestation of diabetic neuropathy. J Diabetes Investig 2014; 5: 709–13. doi: 10.1111/jdi.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimm A, Heiling B, Schumacher U, Witte OW, Axer H. Ultrasound differentiation of axonal and demyelinating neuropathies. Muscle Nerve 2014; 50: 976–83. doi: 10.1002/mus.24238 [DOI] [PubMed] [Google Scholar]
- 34.Di Pasquale A, Morino S, Loreti S, Bucci E, Vanacore N, Antonini G. Peripheral nerve ultrasound changes in CIDP and correlations with nerve conduction velocity. Neurology 2015; 84: 803–9. doi: 10.1212/WNL.0000000000001291 [DOI] [PubMed] [Google Scholar]
- 35.Rajabally YA, Morlese J, Kathuria D, Khan A. Median nerve ultrasonography in distinguishing neuropathy sub-types: a pilot study. Acta Neurol Scand 2012; 125: 254–9. doi: 10.1111/j.1600-0404.2011.01527.x [DOI] [PubMed] [Google Scholar]
- 36.Scheidl E, Böhm J, Farbaky Z, Simó M, Bereczki D, Arányi Z. Ultrasonography of ulnar neuropathy at the elbow: axonal involvement leads to greater nerve swelling than demyelinating nerve lesion. Clin Neurophysiol 2013; 124: 619–25. doi: 10.1016/j.clinph.2012.08.027 [DOI] [PubMed] [Google Scholar]
- 37.Breiner A, Qrimli M, Ebadi H, Alabdali M, Lovblom LE, Abraham A, et al. Peripheral nerve high-resolution ultrasound in diabetes. Muscle Nerve 2017; 55: 171–8. doi: 10.1002/mus.25223 [DOI] [PubMed] [Google Scholar]
- 38.Cartwright MS, Walker FO. Neuromuscular ultrasound in common entrapment neuropathies. Muscle Nerve 2013; 48: 696–704. doi: 10.1002/mus.23900 [DOI] [PubMed] [Google Scholar]


