Abstract
Objectives:
To evaluate the role of contrast-enhanced ultrasound (CEUS) quantitative parameters in predicting neoadjuvant chemotherapy (NACT) response in patients with locally advanced breast cancer (LABC).
Methods:
30 patients with histologically proven LABC scheduled for NACT were recruited. CEUS was performed using a contrast bolus of 4.8 ml and time intensity curves (TICs) were obtained by contrast dynamics software. CEUS quantitative parameters assessed were peak enhancement (PE), time-to-peak (TTP), area under the curve (AUC) and mean transit time (MTT). The parameters were documented on four consecutive instances: before NACT and 3 weeks after each of the three cycles. The gold-standard was pathological response using Miller Payne Score obtained pre NACT and post-surgery.
Results:
A decrease in mean values of PE and an increase in mean values of TTP and MTT was observed with each cycle of NACT among responders. Post each cycle of NACT (compared with baseline pre-NACT), there was a statistically significant difference in % change of mean values of PE, TTP and MTT between good responders and poor responders (p-value < 0.05). The diagnostic accuracy of TTP post-third cycle was 87.2% (p = 0.03), and MTT post--second and third cycle was 76.7% (p = 0.004) and 86.7% (p = 0.006) respectively.
Conclusion:
In responders, a decrease in the tumor vascularity was reflected in the CEUS quantitative parameters as a reduction in PE, and a prolongation in TTP, MTT.
Advances in knowledge:
Prediction of NACT response by CEUS has the potential to serve as a diagnostic modality for modification of chemotherapy regimens during ongoing NACT among patients with LABC, thus affecting patient prognosis.
Introduction
Breast cancer is the most common malignancy in females globally. The age standardized incidence rate for breast cancer in the world is reported to be 46.3 per 100,000 females and in the year 2018, the mortality was estimated at 13.0 per 100,000 females.1 Locally advanced breast cancer (LABC) is one of the common presentations of breast cancer, and refers to a subset of Stage IIB (T3N0) and Stage III. LABC is frequently characterized by a much poorer prognosis as compared to early stage breast cancer, due of its high rate of its locoregional recurrence (10–20%).2 Currently, the recommended protocol for management of LABC is an initial neoadjuvant chemotherapy (NACT) regime, followed by lumpectomy or mastectomy with axillary nodal clearance, which is subsequently supplemented by radiation therapy, adjuvant chemotherapy and/or immunotherapy depending on ER/PR status. The reference standard for patient outcome is based on determining pathological complete response (pCR).3,4 The assessment of tumor response to ongoing NACT, which allows for timely modification of regime and contributes to improving patient prognosis, remains a crucial factor in the management cycle. Therefore, there is a need for development of reliable non-invasive methods to determine tumor response early in the course of NACT, during the pre-operative stage.
Conventional methods for assessing response to NACT include physical examination, mammography and ultrasonography. Each of these modalities have their known limitations.5–7 Ultrasound elastography, both strain and shear wave, have also been explored by few investigators, however, the reported results show a variable accuracy.8,9 Modalities such as dynamic contrast-enhanced MRI (DCE-MRI), positron emission tomography (PET) CT reflecting neo-angiogenesis and providing functional information of the tumors, do hold a potential in NACT response evaluation. Although DCE-MRI is the current standard for assessment of response to NACT, it has inherent limitations of contraindication in patients with metallic implants and deranged renal functions. PET-CT is limited by poor spatial resolution.5 Therefore, color Doppler although appearing relevant in assessing neo-angiogenesis, is restricted by the ability to explore low flow microvasculature.10 The constraints of these modalities can be effectively overcome by contrast-enhanced ultrasound (CEUS). Ultrasound contrast agents are exclusively blood pool agents, owing to the minute size of microbubbles (2–4 microns), and therefore effectively assess microvascular status of cancers and provide functional information. Furthermore, as these contrast agents are excreted through the lungs, they can be safely used even in patients with compromised renal function.11
Although the studies using CEUS for assessing NACT response are few, those reported by Saracco et al12 in 2016, and Wan et al13 in 2018, have shown encouraging results. Based on these studies, we hypothesized that alterations in CEUS quantitative parameters would serve as objective data for indicating a decrease in tumor vascularity (or otherwise) in patients undergoing NACT, therefore facilitating effective differentiation of responders from non-responders amongst breast cancer patients. The present study was designed to evaluate the role of CEUS quantitative parameters in predicting NACT response in LABC patients. We believe that the present study is amongst the very few in which a sequential quantitative CEUS evaluation has been performed before NACT, and after each NACT cycle, comprising a total of four instances.
Methods and materials
Patients and neoadjuvant chemotherapy
This Institutional Review Board approved prospective observational study was aimed at determining the role of CEUS quantitative parameters in assessing the response to NACT. The quantitative parameters were documented on four consecutive instances: before NACT, and 3 weeks after each of the three cycles. The gold-standard was pathological response using Miller Payne Score obtained pre-NACT and post-surgery. The calculated sample size for our study was 30, based on the study by Lee et al14. 30 patients with biopsy-proven LABC, planned for NACT were recruited in the study, between October 2018 and March 2020. Patients with prior history of breast surgery, chemotherapy, allergic drug reaction, poor pulmonary or cardiac function or a poor Karnofsky’s performance status were excluded from the study. All the patients received three cycles of NACT which consisted of Cyclophosphamide (500 mg m−2), Adriamycin (50 mg m−2) and 5 Fluorouracil (500 mg m−2).
Contrast-enhanced ultrasound examination and image analysis
The CEUS study was performed by Author 2, who has more than 20 years of experience in breast imaging and more than 3 years of experience in CEUS imaging of other organs. Author 2 was ably assisted by Author 1, who had undergone one year of supervised training in breast imaging and CEUS. Any disagreements between the investigators on region of interest (ROI) selection, were resolved by discussion. For the CEUS study, patients were instructed to abstain from oral intake for 4 h, in order to prevent emetic side-effects. An intravenous cannula was inserted in the antecubital vein and fixed in position prior to the procedure. The equipment used was Siemens ACUSON S-3000 with contrast capability. A linear-array transducer L9-5 MHz was used for all examinations, and low mechanical index settings was chosen for the CEUS procedure. The contrast agent used was a second generation ultrasonography contrast, sulphur hexafluoride (SonoVue; Bracco, Milano, Italy) in a dose of 4.8 ml as recommended by Cao et al15. The ultrasound procedure was initiated with grayscale ultrasonography to localize the most homogeneous solid part of the tumor, avoiding necrotic areas for the CEUS study. After identifying the best view in split mode for the CEUS study, the tumor area being interrogated was fixed under the transducer (using minimal hand pressure by the examiner), in order to eliminate motion artifacts. As soon as the area of interest was satisfactorily immobilized, the contrast agent was injected, followed by a flush of 10 ml normal saline, and the video clips were recorded for 4 min. The patients were instructed to shallow breathe, in order to reduce the breathing artifacts during the video recording. A ROI was selected by placing the cursor on the most homogeneous part of the tumor showing maximum enhancement and TICs were obtained by contrast dynamics software. Quantitative parameters generated by the equipment’s software, were PE (%), TTP (s), AUC (%s) and MTT (s).
Pathological examination and response evaluation
Following NACT, all patients underwent modified radical mastectomy, and specimens were examined by the pathologist of our team. Information regarding tumor grade, histologic subtype, size, and pathological response was recorded. Pathological response was graded according to the Miller-Payne system of five grades.14 A good histological response had a grade score of 4–5 and a poor histological response had a grade score of 1–3. Changes in the Miller Payne score (pre-NACT and post-surgery), were correlated with trends in the CEUS quantitative parameters documented at each cycle, to determine response to NACT.
Statistical analysis
The changes in the values of quantitative parameters were used as predictors in the analysis, which examined the best separation between the two groups. The data were entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) v. 22.0. Normality of data was tested by Kolmogorov–Smirnov test. If the normality was rejected, then non-parametric test was used. Quantitative variables between the two groups (good responders and poor responders), were compared using Unpaired t-test, Mann–Whitney est and Wilcoxon test. A p < 0.05 was considered statistically significant.
Results
Clinical profile and pathological profile
The clinical profile of the patients included in the study is summarized in Table 1. The mean age in years was 49, and majority (46.7%) of the patients were in the age Group 41–50 Years. More than half (63.3%) of the patients had a left sided breast tumor, with the upper outer quadrant being most commonly involved (40%). Luminal A (43.3%) and triple negative (23.3%) were the most common molecular classes. All of the patients had infiltrating ductal carcinoma (IDC) on histopathology.
Table 1.
Clinical profile of patients included in the study
| Basic details | Number of patients || Frequency (%) |
|---|---|
| Age (Years) | 49.37 ± 7 (Mean) |
| Side | |
| Left | 19 (63.3%) |
| Right | 11 (36.7%) |
| Quadrant Involved | |
| Upper outer | 12 (40.0%) |
| Upper inner | 7 (23.3%) |
| Lower outer | 5 (16.7%) |
| Lower inner | 2 (6.7%) |
| Retroareolar | 4 (13.3%) |
| T stage | |
| 2 | 2 (6.7%) |
| 3 | 15 (50.0%) |
| 4a | 2 (6.7%) |
| 4b | 11 (36.7%) |
| N stage | |
| 0 | 9 (30.0%) |
| 1 | 12 (40.0%) |
| 2 | 9 (30.0%) |
| ER (Positive) | 17 (56.7%) |
| PR (Positive) | 11 (36.7%) |
| Her2neu (Positive) | 5 (16.7%) |
| Luminal A | 13 (43.3%) |
| Luminal B | 5 (16.7%) |
| Her2 Type | 5 (16.7%) |
| Triple Negative | 7 (23.3%) |
Statistical analysis of CEUS quantitative parameters before and after each NACT cycle
The comparison of the change in quantitative parameters, between good and poor responders was computed using Wilcoxon test. The Tables 2–5 show the p values for the change in PE (Table 2), TTP (Table 3), AUC (Table 4) and MTT (Table 5) compared pre-NACT and post each cycle of NACT. Green background denotes statistically significant difference at p < 0.05. Figures 1 and 2 are representative consecutive CEUS studies (four instances) in two different patients with LABC.
Table 2.
Comparison of good responders and poor responders, in terms of change in mean PE (E1%), post each cycle of NACT compared with baseline (pre-NACT)
| Time point comparison | Change in PE (E1%) from pre-NACT to third cycle of NACT | Comparison of the two groups in terms of difference of PE (E1%) from pre-NACT to follow-up NACT cycles | ||||
|---|---|---|---|---|---|---|
| Good responder | Poor responder | |||||
| Mean (SD) of absolute change | Mean (SD) of % change | Mean (SD) of absolute change | Mean (SD) of % change | p-value of absolute change | p-value of % change | |
| Post first cycle - Pre-NACT | −0.71 (1.03) | −8.0% (19.5) | 0.65 (1.07) | 18.5% (31.2) | <0.001 | <0.001 |
| Post second cycle - Pre-NACT | −1.52 (1.34) | −19.8% (21.7) | 1.06 (1.36) | 30.1% (38.7) | <0.001 | <0.001 |
| Post third cycle - Pre-NACT | −2.03 (1.24) | −28.5% (20.4) | 0.98 (1.84) | 29.2% (49.1) | <0.001 | <0.001 |
NACT, neoadjuvant chemotherapy; PE, peak enhancement; SD, standard deviation.
Table 3.
Comparison of good responders and poor responders, in terms of change in mean TTP (E1s), post each cycle of NACT compared with baseline (pre-NACT)
| Time point comparison | Change in TTP (E1s) from pre-NACT to third cycle of NACT | Comparison of the two groups in terms of difference of TTP (E1s) from pre-NACT to follow-up NACT cycles | ||||
|---|---|---|---|---|---|---|
| Good responder | Poor responder | |||||
| Mean (SD) of absolute change | Mean (SD) of % change | Mean (SD) of absolute change | Mean (SD) of % change | p-value of absolute change | p-value of % change | |
| Post first cycle - pre-NACT | 0.75 (1.02) | 38.4% (55.5) | −0.16 (3.36) | 7.6% (55.9) | 0.054 | 0.031 |
| Post second cycle - pre-NACT | 1.72 (1.90) | 83.6% (89.4) | 0.14 (4.11) | 22.3% (100.1) | 0.014 | 0.013 |
| Post third cycle - pre-NACT | 5.19 (4.04) | 205.0% (151.2) | 0.26 (6.09) | 38.1% (153.8) | 0.008 | 0.004 |
NACT, neoadjuvant chemotherapy; SD, standard deviation; TTP, time-to-peak.
Table 4.
Comparison of good responders and poor responders, in terms of change in mean AUC (E3%s), post each cycle of NACT compared with baseline (pre-NACT)
| Time point comparison | Change in AUC (E3%s) from pre-NACT to third cycle of NACT | Comparison of the two groups in terms of difference of AUC (E3%s) from pre-NACT to follow-up NACT cycles | ||||
|---|---|---|---|---|---|---|
| Good responder | Poor responder | |||||
| Mean (SD) of absolute change | Mean (SD) of % change | Mean (SD) of absolute change | Mean (SD) of % change | p-value of absolute change | p-value of % Change | |
| Post first cycle - pre-NACT | −2.15 (3.61) | −1.1% (85.9) | −1.29 (4.87) | 45.0% (130.0) | 0.752 | 0.411 |
| Post second Cycle - pre-NACT | 0.12 (3.99) | 53.3% (114.8) | −0.37 (3.93) | 67.9% (121.7) | 0.916 | 0.689 |
| Post third cycle - pre-NACT | 2.77 (6.35) | 103.4% (139.0) | 1.41 (2.51) | 96.1% (95.4) | 0.784 | 0.817 |
AUC, area under the curve; NACT, neoadjuvant chemotherapy; SD, standard deviation.
Table 5.
Comparison of good responders and poor responders, in terms of change in mean MTT (E1s), post each cycle of NACT compared with baseline (pre-NACT)
| Time point comparison | Change in MTT (E1s) from pre-NACT to third cycle of NACT | Comparison of the two groups in terms of difference of MTT (E1s) from pre-NACT to follow-up NACT cycles | ||||
|---|---|---|---|---|---|---|
| Good responder | Poor responder | |||||
| Mean (SD) of absolute change | Mean (SD) of % change | Mean (SD) of absolute change | Mean (SD) of % change | p-value of absolute change | p-value of % change | |
| Post first cycle - pre-NACT | 0.75 (0.95) | 24.1% (26.9) | −3.44 (3.59) | −25.8% (36.7) | <0.001 | 0.001 |
| Post second cycle - pre-NACT | 5.35 (3.96) | 148.0% (139.9) | −3.26 (6.50) | 3.2% (130.4) | <0.001 | <0.001 |
| Post third cycle - pre-NACT | 8.08 (4.91) | 209.8% (133.6) | −1.96 (7.99) | 12.5% (130.0) | 0.001 | <0.001 |
MTT, mean transit time; NACT, neoadjuvant chemotherapy; SD, standard deviation.
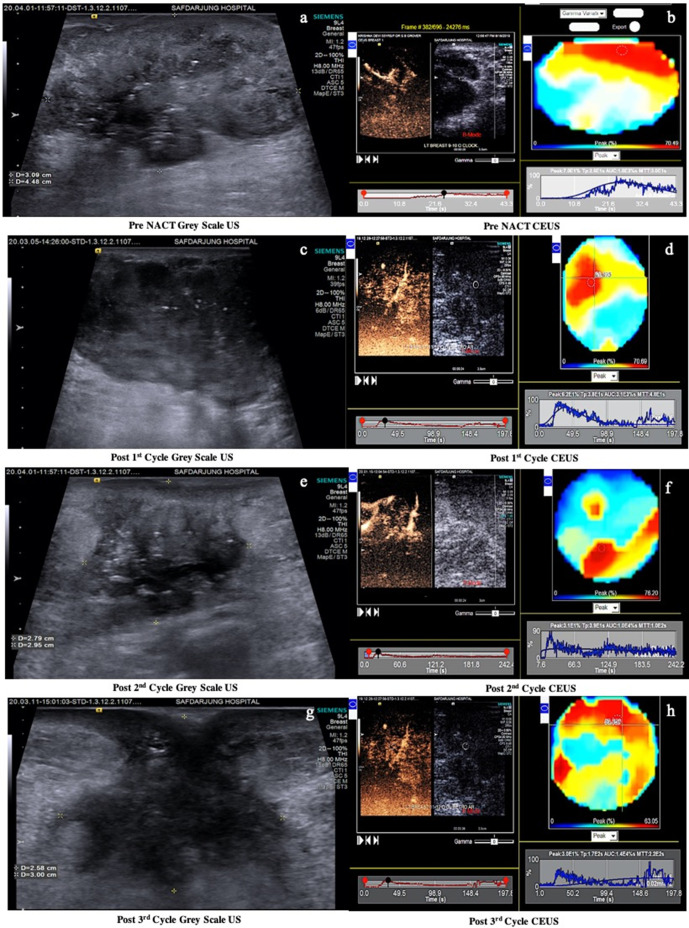
Figure 1.
(a-h) are the sequential CEUS studies in a 46 years female with biopsy proven IDC located at 11-12 o’clock position in the left breast (a). Pre NACT CEUS (b) reveals PE 70%, TTP 26s, AUC 1800%s and MTT 30s. Post 1st cycle CEUS (d) shows PE 62%, TTP 38s, AUC 3100%s and MTT 48s. Post 2nd cycle CEUS (f) shows PE 31%, TTP 39s, AUC 10000%s and MTT 100s. Post 3rd cycle CEUS (h) shows PE 30%, TTP 170s, AUC 14000%s and MTT 220s. The CEUS studies post 1st, 2nd and 3rd cycles of NACT show a progressive decrease in peak enhancement and an increase in AUC, TTP and MTT, indicating a good response to NACT. The correlating histopathological outcome is shown in fig 2.
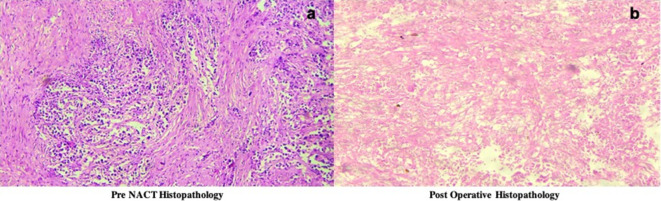
Figure 2.
(a-b) Histopathological outcome of patient 1, (shown in fig 1) revealed that the patient was a good responder with loss of more than 90% malignant cells (Miller Payne Score of 4).
Histopathological response assessment post-surgery
Post-operative histopathological response was determined by the Miller Payne scoring system, with a good response score range of 4–5 and a poor response score range of 1–3. The distribution of patients showing good response and poor response, is depicted in Table 6. Based on the Miller Payne scoring system, 43.3% of the participants had good response, and 56.7% of the participants had poor response. Figures 3 and 4 illustrate the histopathological changes in pre- and post-NACT Miller Payne score, for the patients illustrated in Figures 1 and 2 respectively.
Table 6.
Pathological response of patients according to MPS
| Response | Frequency | Percentage |
|---|---|---|
| Good (MPS score 4–5) | 13 | 43.3% |
| Poor (MPS score 1–3) | 17 | 56.7% |
| Total | 30 | 100.0% |
MPS, Miller Payne Score.
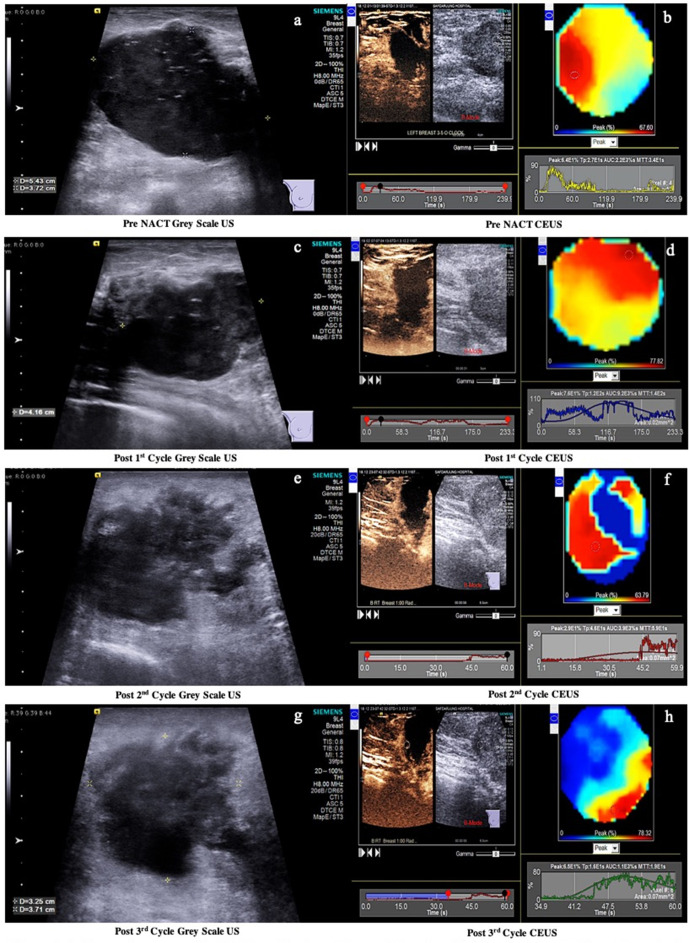
Figure 3.
(a-h) are the sequential CEUS studies in a 50years’ female with biopsy proven IDC at 5-7 o’clock position in the rightbreast. Pre NACT CEUS (b) reveals PE 64%, TTP 27s, AUC 2200%s and MTT 34s. Post1st cycle CEUS (d) shows PE 76%, TTP 120s, AUC 9200%s and MTT 140s.Post 2nd cycle CEUS (f) shows PE 29%, TTP 46s, AUC 3900%s and MTT59s. Post 3rd cycle CEUS (h) shows PE 65%, TTP 16s, AUC 1100%s andMTT 19s. Pre NACT and post 2nd cycle of NACT, CEUS (d, h) revealed no significant change in Peak Enhancement. The TTP and MTT increased post 1st cycle, then decreased subsequently post 2nd and 3rd cycles. The CEUS parameters show a non-responder trend. The correlating histopathological outcome is shown in fig 4.
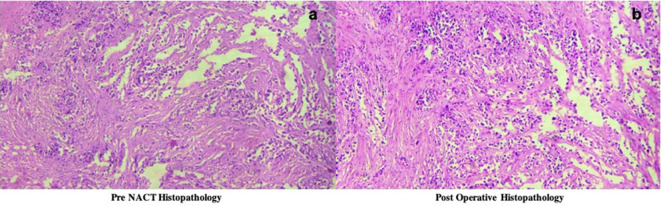
Figure 4.
(a-b) Histopathological outcome of patient 2(shown in figure 3), revealed that the patient was a poor responder with loss of less than 30% malignant cells (Miller Payne Score of 2).
Statistical correlation of histopathological response with CEUS quantitative parameters
A decrease in mean values of PE and an increase in mean values of TTP and MTT was observed with each cycle of NACT among responders. PE, TTP and MTT had a p-value < 0.05 in assessing response to NACT (as shown in Tables 2, 3 and 5). The AUC was found to decrease following the first cycle and increase post-second and third cycles of NACT, among responders and had a p > 0.05. There was no significant trend of CEUS quantitative parameters observed in non-responders.
The cyclewise diagnostic accuracy of CEUS quantitative parameters in predicting response to NACT is shown in Table 7. The diagnostic accuracy of TTP post third cycle was 87.2% (p = 0.03), MTT post second and third cycles 76.7% (p = 0.004) and 86.7% (p = 0.006) respectively. Hence, tumors showing good response to NACT revealed a decrease in the tumor vascularity reflected in the CEUS quantitative parameters as a reduction in PE, a prolongation in TTP and MTT.
Table 7.
NACT cycle-wise diagnostic accuracy of CEUS quantitative parameters in predicting response to NACT
| Variable | Instance | Sensitivity | Specificity | PPV | NPV | Diagnostic Accuracy | p-value |
|---|---|---|---|---|---|---|---|
| PE (E1%) | Post first cycle | 88.2% | 38.5% | 65.2% | 71.4% | 66.7% | 0.152 |
| Post second cycle | 52.9% | 76.9% | 75.0% | 55.6% | 63.3% | 0.414 | |
| Post third cycle | 47.1% | 100.0% | 100.0% | 59.1% | 70.0% | 0.106 | |
| TTP (E1s) | Post first cycle | 41.2% | 76.9% | 70.0% | 50.0% | 56.7% | 0.950 |
| Post second cycle | 58.8% | 76.9% | 76.9% | 58.8% | 66.7% | 0.124 | |
| Post third cycle | 84.2% | 94.5% | 93.7% | 81.0% | 87.2% | 0.003 | |
| AUC (E3%s) | Post first cycle | 82.4% | 38.5% | 63.6% | 62.5% | 63.3% | 0.720 |
| Post second cycle | 82.4% | 38.5% | 63.6% | 62.5% | 63.3% | 0.933 | |
| Post third cycle | 64.7% | 69.2% | 73.3% | 60.0% | 66.7% | 0.212 | |
| MTT (E1s) | Post first cycle | 41.2% | 84.6% | 77.8% | 52.4% | 60.0% | 0.513 |
| Post second cycle | 70.6% | 84.6% | 85.7% | 68.8% | 76.7% | 0.004 | |
| Post third cycle | 82.4% | 92.3% | 93.3% | 80.0% | 86.7% | 0.006 |
AUC, area under the curve; CEUS, contrast-enhanced ultrasound; NACT, neoadjuvant chemotherapy; NPV, negative predictive value; PE, peak enhancement; PPV, positive predictive value; TTP, time-to-peak.
Discussion
The standard protocol for management of LABC includes, NACT followed by Modified Radical Mastectomy with axillary nodal clearance, radiation therapy and adjuvant chemotherapy. The effects of NACT are downsizing the tumor, enabling more conservative breast surgery techniques and reducing distant metastasis. The ideal outcome of NACT is a pCR, which is known to be associated with better long-term survival rates. When the response is poor, second line therapies such as monoclonal antibodies/hormonal therapy depending upon receptor status, or immediate surgery have to be considered.16 Furthermore, it has been documented in various studies that the response to NACT can be improved by adjusting the ongoing therapy and instituting early change in chemotherapy in the non-responders, so as to achieve better results prior to surgery.12,16 The critical issue in being able to achieve ideal response to NACT, is the opportunity for early assessment of the tumor characteristics. Therefore, there is a necessity to explore modalities which can achieve this goal during the course of NACT, rather than being able to achieve only a post-NACT pathology evaluation. Ideally, the modality for intra-NACT assessment should be capable of providing objective parameters.
Since conventional methods utilizing changes in size, documented by clinical, mammography or grayscale ultrasound examination have already been proven to have their limitations, techniques providing functional assessment of neovascularity are now considered more accurate. The imaging modalities which are capable of providing tumor neovascularity and functional information are DCE MRI (which provides contrast flow dynamics) and PET CT (which provides information of tumor glucose metabolism), respectively.17–19 While MRI is a superior modality, its contraindication in patients with deranged renal function and metallic implants precludes its routine use as a modality for detection of response to NACT. Various studies have been performed evaluating FDG PET/CT for predicting pathologic response to NACT, and a few have reported false-negative results in small residual tumors measuring <1 cm, due to the limited spatial resolution of PET scanners.19 Moreover, biologic factors affecting tumor glucose metabolism, which is the basis of using FDG PET as an early indicator of response, may result in false-positive uptake from inflammatory changes if repeat tissue biopsy is obtained for histopathological response evaluation.20The pooled data analysis of a few recent studies on the role of CEUS in assessing chemotherapy response had been reported as demonstrating a positive trend, with conclusions to further explore the technique.21 22
We hypothesized that CEUS studies utilizing second generation of contrast media which are pure intravascular agents, would provide a true reflection of tumor vascularity and alterations in tumor vasculature before and after NACT. The change in vascularity assessed as a change in CEUS quantitative parameters would serve as objective data for indicating a decrease in tumor vascularity in patients undergoing NACT, therefore facilitating effective differentiation of responders from non-responders amongst breast cancer patients.
We conducted a prospective study on 30 patients to assess the role of CEUS in the evaluation of response to NACT in patients with LABC. Consecutive CEUS examinations were performed prior to NACT and post each of the three cycles of NACT. Post-surgical histopathological response was assessed by Miller Payne Score. CEUS qualitative parameters were not assessed in the study as they are operator-dependant and do not provide reproducible information. Therefore, in the current study quantitative parameters were used, which are objective, and were self-generated by the equipment: PE, TTP, AUC and MTT. As highlighted above, these parameters were obtained pre-NACT and 3 weeks post each of the three cycles of NACT. The results of the study revealed that PE, TTP and MTT had a p-value of < 0.05 in assessing response to NACT. The highest diagnostic accuracy amongst the quantitative parameters, was that of TTP post third cycle (87.2%, p = 0.003) and MTT post second and third cycles (76.7%, p = 0.004 and 86.7%, p = 0.006) respectively. The AUC was found to decrease among responders following the first cycle and increase post second and third cycle of NACT (p > 0.05).
There are a few studies in the literature reporting the role of CEUS in assessing NACT response among breast cancer patients. The notable ones amongst these are by Cao et al15, Saracco et al12, Amioka et al23 and Wan et al13.
Amongst the earliest studies reporting CEUS quantitative parameters as a reliable modality are attributed to Cao et al15 in 2012, who evaluated 31 patients. They showed that the CEUS TIC obtained prior to NACT, presented a steep rising period and a smooth descent period, indicating a blood perfusion process of a rapid rise and a slow fall. After NACT, CEUS quantitative parameters: TTP increased, peak intensity (PI) decreased, and wash in slope (WIS) decreased. The differences in the rising time and MTT were not statistically significant in the two groups (p > 0.05). However, CEUS was performed only on two instances, before NACT and after completion of three cycles of NACT.
The study done by Amioka et al23 comprised of 63 patients, who were also evaluated after completion of 4 cycles of NACT. Their results showed PI to have the highest diagnostic accuracy for predicting pCR with a sensitivity, specificity and accuracy of 95.7%, 77.5 and 84.1% respectively. PI and Ascending slope (AS) were smaller and TTP was longer in patients who achieved pCR than in those who did not; with both PI (13.9 ± 8.0 vs 38.0 ± 19.6, p value < 0.001) and AS (1.0 ± 0.7 vs 3.7 ± 2.8, p value < 0.001) being significantly lower in responders. The TTP of pCR and non-pCR did not significantly differ among tumors (16.6 ± 8.1 vs 15.3 ± 11.5 s; p-value 0.65). The sensitivity of CEUS for predicting pCR was significantly greater than that of MRI (95.7% vs 69.6%, p-value 0.047). The specificity and accuracy for predicting pCR were significantly greater and tended to be greater, respectively, for CEUS than PET/CT (specificity 77.5% vs 52.5%, p = 0.02; accuracy 84.1% vs 69.8% and p-value 0.057).23
Wan et al13 recorded CEUS quantitative parameters at baseline, after NACT and 1 week before surgery. After four cycles of the NAC, compared with non-pCR tumors, the kinetic parameters PE and Regional Blood Flow were lower, and TTP, MTT were higher in pCR tumors. The area under the ROC curve for quantitative parameters peak and TTP, was 0.927, and the sensitivity and specificity to predict pCR were 81.2 and 94.3%, respectively.
There are few studies which have evaluated the CEUS quantitative parameters intra NACT. The study conducted by Saracco et al12 in 2016 is probably one of the few studies which is similar to ours, in which CEUS was performed intra-NACT, however with a smaller sample size of 19 patients. In the study, data was collected at weeks 2 and 5, after the first cycle of NACT. A significant increase in TTP was observed among the responders, when compared with non-responders (p = 0.027). The increase in TTP in responders was attributed to a decrease in blood perfusion, which in turn lead to a slower wash-in of contrast agent indicating good efficacy of NACT. However, Saracco et al12 found no statistical difference between responders and non-responders for AUC, Cmax (PE), MTT, and wash-out parameters.
The results of the present study are largely in agreement with that of those enumerated above and a decrease in PE and prolongation in TTP, have been found to be reproducible quantitative parameters for assessing NACT response. Although the studies by Cao et al15, Amioka et al23, Wan et al13 and Sarcco et al12, have not found MTT to be a reliable parameter, in contradistinction, the results in our study show a statistically significant prolongation of MTT post second and third cycles. Of the enumerated studies, the only one which evaluated CEUS quantitative parameters intra NACT was by Saracco et al12. In their study, MTT at week 2 (comparable to evaluation post first cycle of NACT, of our study) showed a p-value of 0.629 and data collected at week 5 (comparable with evaluation post second cycle of NACT, in our study) showed a p-value of 0.027. The statistical significance for evaluation of MTT at the corresponding intra NACT cycles in the present study, were reflected as p-value 0.513 and 0.004 respectively. Furthermore, an additional evaluation of MTT post third cycle had a p-value of 0.006 in our study.
The results of our study show that PE, TTP and MTT show statistically significant change following second cycle of NACT. Therefore, if the test has to be performed minimum number of times intra-NACT and compared to the pre-NACT, then post second cycle is probably the best time to evaluate response to NACT and continue or alter the treatment regime, if necessary.
The unique feature whereby our study scores over previous studies is that the data were recorded after each of the three cycles, whereas most other studies have the data only on completion of NACT, when it is obviously too late to alter the NACT regime. The limitations of our study were a small sample size and a relatively short time span available to recruit patients and complete the project. It would be useful to perform the study in larger cohorts and perhaps at multiple Institutions so as to try and establish guidelines for the minimum alterations in the CEUS parameters which could be considered clinically significant.
Conclusion
The results of our study highlight that the documentation of intra NACT changes in CEUS quantitative parameters can significantly contribute to assessment of response to NACT in LABC patients. The results of our study show that post second cycle is probably the best time to assess with CEUS. Since timely alteration of the NACT regime is known to impact prognosis of LABC patients, the clinical importance of further exploring CEUS as a reliable technique is aptly justified by the results of our study.
Footnotes
The authors Anant Sharma and Shabnam Bhandari Grover contributed equally to the work.
Anant Sharma and Shabnam Bhandari Grover have contributed equally to this study and should be considered as co-first authors.
Contributor Information
Anant Sharma, Email: anante266@gmail.com.
Shabnam Bhandari Grover, Email: shabnamgrover@yahoo.com.
Chinta Mani, Email: drchintamani7@gmail.com.
Charanjeet Ahluwalia, Email: charanjeet.ahluwalia@rediffmail.com.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-A Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Giordano SH. Update on locally advanced breast cancer. Oncologist 2003; 8: 521–30. doi: 10.1634/theoncologist.8-6-521 [DOI] [PubMed] [Google Scholar]
- 3.Vasudevan D, Jayalakshmy PS, Kumar S, Mathew S. Assessment of pathological response of breast carcinoma in modified radical mastectomy specimens after neoadjuvant chemotherapy. Int J Breast Cancer 2015; 2015: 536145: 1–8. doi: 10.1155/2015/536145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss A, Bashour SI, Hess K, Thompson AM, Ibrahim NK. Effect of neoadjuvant chemotherapy regimen on relapse-free survival among patients with breast cancer achieving a pathologic complete response: an early step in the de-escalation of neoadjuvant chemotherapy. Breast Cancer Res 2018; 20: 27–37. doi: 10.1186/s13058-018-0945-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler AM, Mankoff DA, Joe BN. Imaging neoadjuvant therapy response in breast cancer. Radiology 2017; 285: 358–75. doi: 10.1148/radiol.2017170180 [DOI] [PubMed] [Google Scholar]
- 6.Chagpar AB, Middleton LP, Sahin AA, Dempsey P, Buzdar AU, Mirza AN, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg 2006; 243: 257–64. doi: 10.1097/01.sla.0000197714.14318.6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keune JD, Jeffe DB, Schootman M, Hoffman A, Gillanders WE, Aft RL. Accuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancer. Am J Surg 2010; 199: 477–84. doi: 10.1016/j.amjsurg.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katyan A, Mittal MK, Mani C, Mandal AK. Strain wave elastography in response assessment to neo-adjuvant chemotherapy in patients with locally advanced breast cancer. Br J Radiol 2019; 92: 20180515. doi: 10.1259/bjr.20180515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans A, Whelehan P, Thompson A, Purdie C, Jordan L, Macaskill J, et al. Identification of pathological complete response after neoadjuvant chemotherapy for breast cancer: comparison of greyscale ultrasound, shear wave elastography, and MRI. Clin Radiol 2018; 73: 910.e1–910.e6. doi: 10.1016/j.crad.2018.05.030 [DOI] [PubMed] [Google Scholar]
- 10.Ricci P, Cantisani V, Ballesio L, Pagliara E, Sallusti E, Drudi FM, et al. Benign and malignant breast lesions: efficacy of real time contrast-enhanced ultrasound vs. magnetic resonance imaging. Ultraschall Med 2007; 28: 57–62. doi: 10.1055/s-2006-927226 [DOI] [PubMed] [Google Scholar]
- 11.Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol 2006; 60: 324–30. doi: 10.1016/j.ejrad.2006.06.022 [DOI] [PubMed] [Google Scholar]
- 12.Saracco A, Szabó BK, Tánczos E, Bergh J, Hatschek T. Contrast-Enhanced ultrasound (CEUS) in assessing early response among patients with invasive breast cancer undergoing neoadjuvant chemotherapy. Acta Radiol 2017; 58: 394–402. doi: 10.1177/0284185116658322 [DOI] [PubMed] [Google Scholar]
- 13.Wan C-F, Liu X-S, Wang L, Zhang J, Lu J-S, Li F-H. Quantitative contrast-enhanced ultrasound evaluation of pathological complete response in patients with locally advanced breast cancer receiving neoadjuvant chemotherapy. Eur J Radiol 2018; 103: 118–23. doi: 10.1016/j.ejrad.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 14.Lee YJ, Kim SH, Kang BJ, Kim YJ. Contrast-enhanced ultrasound for early prediction of response of breast cancer to neoadjuvant chemotherapy. Ultraschall Med 2019; 40: 194–204. doi: 10.1055/a-0637-1601 [DOI] [PubMed] [Google Scholar]
- 15.Cao X-L, Bao W, Zhu S-G, Wang L-H, Sun M-H, Wang L, et al. Contrast-Enhanced ultrasound characteristics of breast cancer: correlation with prognostic factors. Ultrasound Med Biol 2014; 40: 11–17. doi: 10.1016/j.ultrasmedbio.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 16.Fernandes J, Sannachi L, Tran WT, Koven A, Watkins E, Hadizad F, et al. Monitoring breast cancer response to neoadjuvant chemotherapy using ultrasound strain elastography. Transl Oncol 2019; 12: 1177–84. doi: 10.1016/j.tranon.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Kim JJ, Hwangbo L, Suh HB, Kim S, Choo KS, et al. Kinetic heterogeneity of breast cancer determined using computer-aided diagnosis of preoperative MRI scans: relationship to distant metastasis-free survival. Radiology 2020; 295: 517–26. doi: 10.1148/radiol.2020192039 [DOI] [PubMed] [Google Scholar]
- 18.Hulikal N, Gajjala SR, Kalawat T, Kadiyala S, Kottu R. Predicting response to neoadjuvant chemotherapy using 18F FDG PET-CT in patients with locally advanced breast cancer. Asian Pac J Cancer Prev 2020; 21: 93–8. doi: 10.31557/APJCP.2020.21.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JS, Moon WK, Lyou CY, Cho N, Kang KW, Chung J-K. The assessment of breast cancer response to neoadjuvant chemotherapy: comparison of magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography. Acta Radiol 2011; 52: 21–8. doi: 10.1258/ar.2010.100142 [DOI] [PubMed] [Google Scholar]
- 20.Mghanga FP, Lan X, Bakari KH, Li C, Zhang Y. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in monitoring the response of breast cancer to neoadjuvant chemotherapy: a meta-analysis. Clin Breast Cancer 2013; 13: 271–9. doi: 10.1016/j.clbc.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 21.Dong T. Early response assessed by contrast-enhanced ultrasound in breast cancer patients undergoing neoadjuvant chemotherapy. Ultrasound Q 2018; 34: 84–7. doi: 10.1097/RUQ.0000000000000333 [DOI] [PubMed] [Google Scholar]
- 22.Jia K, Li L, Wu XJ, Hao MJ, Xue HY. Contrast-Enhanced ultrasound for evaluating the pathologic response of breast cancer to neoadjuvant chemotherapy: a meta-analysis. Medicine 2019; 98: e14258–58. doi: 10.1097/MD.0000000000014258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amioka A, Masumoto N, Gouda N, Kajitani K, Shigematsu H, Emi A, et al. Ability of contrast-enhanced ultrasonography to determine clinical responses of breast cancer to neoadjuvant chemotherapy. Jpn J Clin Oncol 2016; 46: 303–9. doi: 10.1093/jjco/hyv215 [DOI] [PMC free article] [PubMed] [Google Scholar]