Abstract
Objectives:
Image-guided radiotherapy (IGRT) is a recommended advanced radiation technique that is associated with fewer acute and chronic toxicities. However, one Phase III trial showed worse overall survival in the IGRT arm. The purpose of this observational study is to evaluate the impact of IGRT on overall survival.
Methods:
We used the Taiwan Cancer Registry Database to enroll cT1-4N0M0 prostate cancer patients who received definitive radiotherapy between 2011 and 2015. We used inverse probability treatment weighting (IPW) to construct balanced IGRT and non-IGRT groups. We compared the overall survival of those in the IGRT and non-IGRT groups. Supplementary analyses (SA) were performed with alternative covariates in propensity score (PS) models and PS approaches. The incidence rates of prostate cancer mortality (IPCM), other cancer mortality (IOCM), and cardiovascular mortality (ICVM) were also evaluated.
Results:
There were 360 patients in the IGRT arm and 476 patients in the non-IGRT arm. The median follow-up time was 50 months. The 5-year overall survival was 88% in the IGRT arm and 86% in the non-IGRT arm (adjusted hazard ratio [HR] of death = 0.93; 95% CI, 0.61–1.45; p = 0.77). The SA also showed no significant differences in the overall survival between those in the IGRT and non-IGRT arms. Both groups did not significantly differ in terms of IPCM, IOCM, and ICVM.
Conclusions:
The overall survival of localized prostate cancer patients who underwent IGRT was not inferior to those who did not.
Advances in knowledge:
We demonstrated that the overall survival for prostate cancer patients with IGRT was not worse than those who did not undergo IGRT; this important outcome comparison has not been previously examined in the general population.
Introduction
Prostate cancer (PC) is a common malignancy and radiotherapy is an important treatment modality.1 Definitive prostate radiotherapy (DPR) performed via conventional fractionated external beam radiotherapy (CFEBRT) is commonly used for localized PC (LPC).1,2 Prostate radiotherapy may even be beneficial for cases of PC with low metastatic burden.3
Daily image-guided radiotherapy (IGRT) is an advanced imaging technique that is employed to ensure localization of the target position during radiotherapy (in contrast to weekly verification), is the preferred approach as per the treatment guidelines.2,4
However, a randomized controlled trial (RCT) published in 2018 reported significantly worse overall survival (OS) when daily IGRT was compared to weekly verification.5 While the findings of this RCT were considered a false positive in the guidelines,4 we felt more extensive research was needed because OS was obviously the most important endpoint. Due to the lack of other published RCTs reporting on the OS associated with this treatment modality,4 we aimed to compare the OS for LPC patients treated with DPR via CFEBRT using IGRT versus those without IGRT in this population-based analysis.
Methods and materials
Data source
We obtained data with permission from the Health and Welfare Data Science Center (HWDC) database, which includes the Taiwan Cancer Registry (TCR), death registration, and reimbursement data for the entire population of Taiwan provided by the Bureau of National Health Insurance (NHI). Personal identifiers in the HWDC data were removed. The TCR is a high-quality database that provides comprehensive information including patient, disease, and treatment characteristics, as well as prognostic factor details.6 This study was approved by the research ethics committee at our institute (CRREC-108-080).
study population and design
The study flow chart was designed to conform to the STROBE statement and is depicted in Figure 1.7 The study population consisted of non-operated localized prostate adenocarcinoma patients (age range: 18–80 years) diagnosed from 2011 to 2015, and who received definitive external beam radiotherapy to the prostate using conventional fractionation via IGRT or non-IGRT. We excluded patients with other cancers, those with nodal or distant metastasis, those with brachytherapy constituting all or part of their treatment, or those treated with elective nodal irradiation. We determined the explanatory variable of interest (IGRT versus non-IGRT), the primary outcome of interest (OS), and other supplementary outcomes [incidence of PC mortality (IPCM), other cancer mortality (IOCM), and cardiovascular mortality (ICVM)] based on the TCR and the death registry. The date of diagnosis in the TCR was defined as the index date, and OS was calculated from the date of diagnosis to the date of death, or 31 December 2017 (the censoring date of the death registry). The related covariates were collected based on the literature,5,8 as well as based on our experiences in clinical care9 and TCR studies,10–12 to adjust for potential nonrandomized treatment selection (see below).
Figure 1.
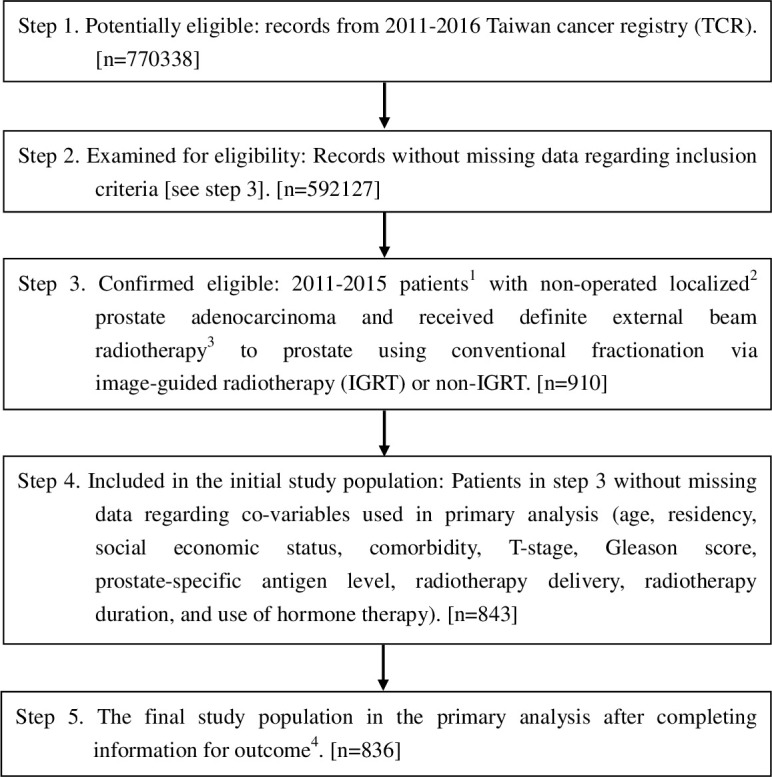
STROBE study flowchart and the number of individuals at each stage of the study. 1We included those treated (class 1–2) with only one record to ensure data consistency. 2The Seventh American Joint Committee on Cancer Staging clinical stage cT1-4N0M0. 3Dose 72–81 Gy ( ± 10%) at 1.8–2 Gy/fraction, as suggested in the guidelines.2 4Without missing information in the TCR and the death registry regarding survival status and cause of death. IGRT: image-guided radiotherapy.
Other explanatory covariates
Patient demographics (age, residency, socioeconomic status), patient characteristics (comorbidity), disease characteristics [Gleason score, T-stage, and prostate-specific antigen (PSA) level in ng/mL], treatment characteristics [radiotherapy (RT) technique and duration], and the use of hormone therapy were included in the primary analysis. The covariates were defined as follows: Patient residency region was classified as “northern Taiwan” or “non-north”. Socioeconomic status was classified as “higher” (an income greater than minimum wage) or “no higher than minimum wage”. Comorbidity was classified as “with” or “without”, as determined by the Charlson comorbidity index score. Clinical T-stage was classified as “T1–T2” or “T3–T4”. RT technique was classified as “three-dimensional conformal radiotherapy” (3DCRT) or ‘intensity-modulated radiotherapy’ (IMRT). The presumed proper RT duration (in weeks) was calculated as the total factions divided by 5. If the actual treatment interval (the first day to the last day of radiotherapy) is one week longer than the proper duration, it was defined as prolongation >1 week; otherwise, it would be classified as ≤1 week. The use of hormone therapy was classified as “with” or “without”.
Statistical and supplementary analyses
In the primary analysis (PA), we used the propensity score (PS) method, as advocated in the literature, to balance the measured potential confounders.13–16 We evaluated the probability of receiving IGRT (versus non-IGRT) via a logistic regression model, as commonly used in the literature,17 based on all the above covariates. We used overlap weights in the PS weighting, as suggested in the literature,18,19 to balance the differences in covariates between groups. The standardized difference (SDif) was used to assess the balance of covariates between groups.9,20,21 We compared the hazard ratio (HR) of death between IGRT and non-IGRT groups during the entire follow-up period using the Cox proportional hazards model in the weighted sample and used the bootstrap method to estimate the 95% confidence interval (95% CI).15,22,23 As suggested in the recent literature,24 we also used the E-factor to evaluate the impact of potential unmeasured confounding factor(s) on OS.
In the supplementary analysis (SA), we performed two separate SAs, as suggested by different reviewers during revisions. In the first SA (SA-1), we used PS matched cohort as an alternative approach to compare IGRT versus non-IGRT.15 We used logistic regression for PS estimation.9,17 When estimating the PS in SA-1, we added two equivocal covariates [radiotherapy dose and risk grouping (classified as high versus intermediate/low)], as considered during the revision, although the RCT had reported a similar OS for high versus low dose,25,26 and the components (Gleason score, T-stage, and PSA level) of risk grouping27,28 were already included as covariates. We compared the HR of death between IGRT and non-IGRT (1:1 matched) groups during the entire follow-up period via a robust variance estimator.15 We adopted the subdistribution HR via the clustered Fine–Gray model to evaluate the IPCM, IOCM, and ICVM.29 In the second SA (SA-2), we adopted PS regression as the third approach, in addition to PS weighting or PS matching.30,31 We also excluded two covariates (“RT technique” and “RT duration prolongation”) in PS estimation during SA-2. These two covariates were included in the primary analyses because we believed they were the “variables of ambiguous status”, which were “perhaps slightly affected by the treatment, but plausibly standing in as a surrogate for an important covariate that was not measured”.10,32,33 After checking the covariate balance, as suggested in the literature,34 we used the Cox regression method, while adjusting this separately estimated PS plus the two excluded covariables (“RT technique” and “RT duration prolongation”) to estimate the effect of IGRT.34 We performed the statistical analyses using SAS 9.4 (SAS Institute, Cary, NC, USA) and R version 3.6.2 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
Population
We identified 836 eligible LPC patients treated with DPR from 2011 to 2015 via CFEBRT (Figure 1). A total of 360 of these patients were treated with IGRT, whereas 476 were treated without IGRT. These two groups of patients were not all balanced in terms of covariates (only one covariate had an SDif >0.25, while others had an SDif <0.25), but were well balanced (i.e. SDif ≤0.25)21 after being weighted by overlapping weights (Table 1).18,19
Table 1.
Patient characteristics of the study population in the primary analysis
| IGRT (n = 360; median follow-up = 50 months) |
non-IGRT (n = 476; median follow-up = 50 months) |
Standardized difference (rounded)a |
|||||
|---|---|---|---|---|---|---|---|
| Number or mean (SD)a | (%)a | Number or mean (SD)a | (%)a | Before PSW |
After PSW |
||
| Age | 72.13 (6.07) | 72.58 (5.61) | 0.08 | ≈ 0 | |||
| Residency | Non-north | 162 | (45) | 134 | (28) | 0.36 | ≈ 0 |
| North | 198 | (55) | 342 | (72) | |||
| Socioeconomic status | No more than minimum wage | 96 | (27) | 162 | (34) | 0.16 | ≈ 0 |
| Higher | 264 | (73) | 314 | (66) | |||
| Comorbidity | Without | 126 | (35) | 172 | (36) | 0.02 | ≈ 0 |
| Withb | 234 | (65) | 304 | (64) | |||
| T-stage | T1–T2 | 285 | (79) | 364 | (76) | 0.07 | ≈ 0 |
| T3–T4 | 75 | (21) | 112 | (24) | |||
| Gleason score | 6.77 (1.02) | 6.93 (1.09) | 0.16 | ≈ 0 | |||
| Prostate-specific antigen level | 19.26 (21.85) | 20.57 (22.70) | 0.06 | ≈ 0 | |||
| RT technique | 3DCRT | c | c | c | c | 0.16 | ≈ 0 |
| IMRT | c | c | c | c | |||
| RT duration prolongation | ≤1 week | 343 | (95) | 439 | (92) | 0.13 | ≈ 0 |
| >1 week | 17 | (5) | 37 | (8) | |||
| Use of hormone therapy | Without | 99 | (28) | 122 | (26) | 0.04 | ≈ 0 |
| With | 261 | (72) | 354 | (74) | |||
3DCRT, Three-dimensional radiotherapy; IGRT, Image-guided radiotherapy; IMRT, Intensity-modulated radiotherapy; PSW, Propensity-score weighting; RT, Radiotherapy; SD, Standard deviation.
Rounded at the second.
Modified Charlson comorbidity score ≥1.
The exact numbers were not reported because of a Health and Welfare Data Science Center (HWDC) database center policy to avoid numbers in single cells (≤2).
Primary analysis
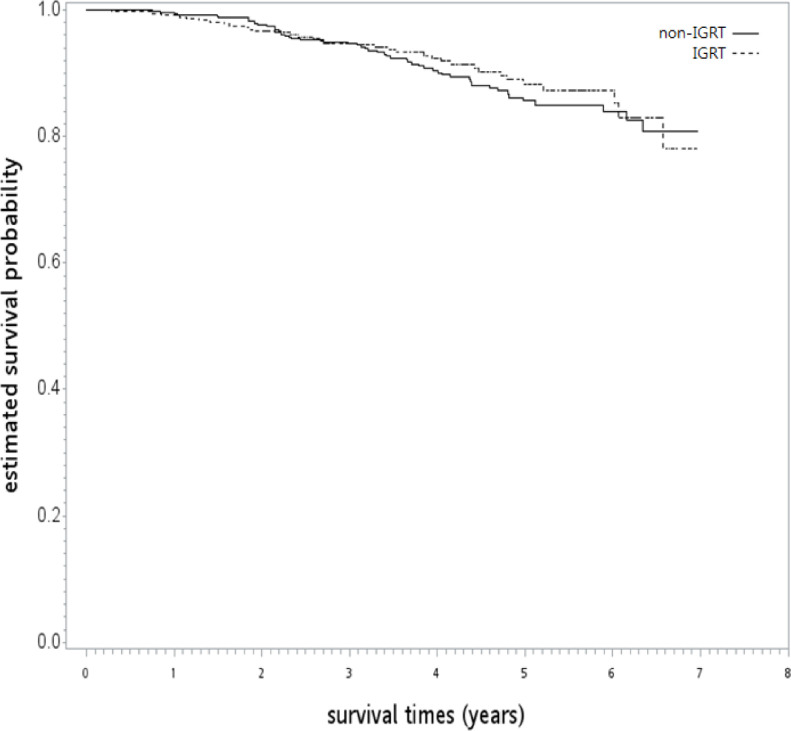
After a median follow-up of 50 months (range: 4–83 months), 35 and 53 patients were dead in the IGRT and non-IGRT groups, respectively. The 5-year OS rate was 88% [95% confidence interval (95% CI), 83–92] and 86% (95% CI, 81–89) for IGRT and non-IGRT in the unadjusted analysis (log-rank test, p = 0.56; Figure 2). After being adjusted by overlapping weights, the HR of death when IGRT was compared to non-IGRT was 0.93 (95% CI, 0.61–1.45; p = 0.77). The observed HR of 0.93 for OS could be explained by an unmeasured confounder associated with the selection of treatment (IGRT or non-IGRT) and survival by a risk ratio of 1.28 (E-value)-fold each, but weaker confounding factors could not. The overlapping weight-adjusted OS curve is shown in Figure 3.
Figure 2.
Kaplan–Meier overall survival curve (in years) in the primary analysis. IGRT: image-guided radiotherapy.
Figure 3.
The overlapping weight-adjusted overall survival curve (in years) in the primary analysis. IGRT: image-guided radiotherapy.
Supplementary analysis
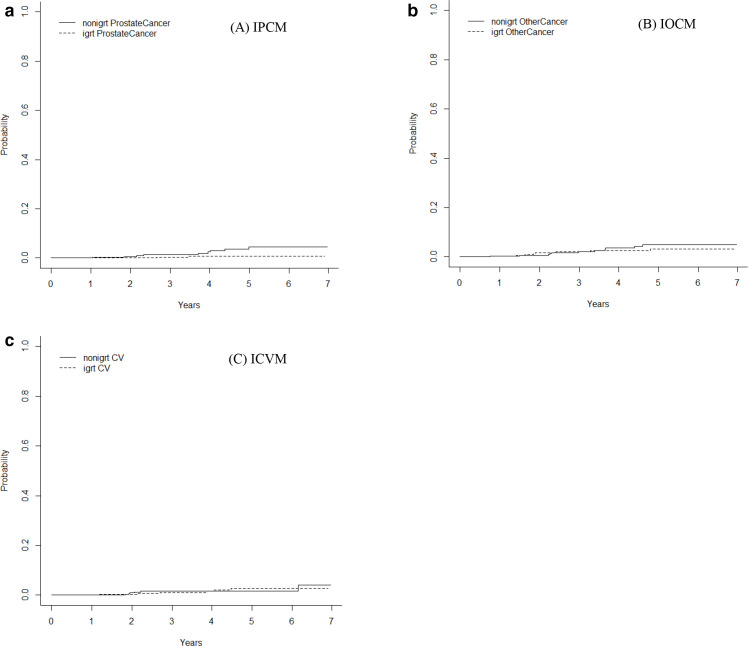
In the first SA, we constructed a PS-matched subgroup (N = 606; Table 2). The HR of death when IGRT was compared to non-IGRT in this PS-matched analysis was 0.84 (95% CI, 0.51–1.39; p = 0.50). The HRs (with a 95% CI) for IPCM, IOCM, and ICVM were 0.21 (95% CI, 0.05–1.00; p = 0.05), 0.71 (95% CI, 0.28–1.80; p = 0.47), and 1 (95% CI, 0.33–3.1; p = 0.99), respectively, when IGRT was compared to non-IGRT (Figure 4a–4c). In the second SA, we found all covariates were well balanced between the two groups (weighted standardized difference <0.1)21,34 and the HR of death when IGRT was compared to non-IGRT in this PS-regression analysis was 0.93 (95% CI, 0.60–1.43; p = 0.73).
Table 2.
Patient characteristics of the PS-matched subgroup in this supplementary analysis
| IGRT (n = 303; median follow-up = 51 months) |
non-IGRT (n = 303; median follow-up = 50 months) |
Standardized difference (rounded)a |
||||
|---|---|---|---|---|---|---|
| Number or mean (SD)a | (%)a | Number or mean (SD)a | (%)a | |||
| Age | 72.22 (6.04) | 72.25 (5.62) | 0 | |||
| Residency | Non-north | 115 | (38) | 122 | (40) | 0.05 |
| North | 188 | (62) | 181 | (60) | ||
| Socioeconomic status | No more than minimum wage | 88 | (29) | 91 | (30) | 0.02 |
| Higher | 215 | (71) | 212 | (70) | ||
| Comorbidity | Without | 108 | (36) | 110 | (36) | 0.01 |
| Withb | 195 | (64) | 193 | (64) | ||
| T-stage | T1–T2 | 229 | (76) | 233 | (77) | 0.03 |
| T3–T4 | 74 | (24) | 70 | (23) | ||
| Gleason score | 6.80 (1.05) | 6.79 (1.01) | 0.01 | |||
| Prostate-specific antigen level | 19.54 (22.27) | 19.87 (21.64) | 0.02 | |||
| RT technique | 3DCRT | c | c | c | c | 0 |
| IMRT | c | c | c | c | ||
| RT duration prolongation | ≤1 week | 287 | (95) | 282 | (93) | 0.07 |
| >1 week | 16 | (5) | 21 | (7) | ||
| Use of hormone therapy | Without | 81 | (27) | 81 | (27) | 0 |
| With | 222 | (73) | 222 | (73) | ||
| Radiotherapy dose | 76.77 (2.34) | 76.65 (2.12) | 0.06 | |||
| Risk grouping | Intermediate/low | 106 | (35) | 105 | (35) | 0.01 |
| High | 197 | (65) | 198 | (65) | ||
3DCRT, Three-dimensional radiotherapy; IGRT, Image-guided radiotherapy; IMRT, Intensity-modulated radiotherapy; PS, Propensity score; RT, Radiotherapy; SD, Standard deviation.
Rounded at the second.
Modified Charlson comorbidity score ≥1.
The exact numbers were not reported because of a Health and Welfare Data Science Center (HWDC) database center policy to avoid numbers in single cells (≤2).
Figure 4.
Estimated cumulative incidence for (a) the incidence rates of prostate cancer mortality (IPCM), (b) the incidence rates of other cancer mortality (IOCM), and (c) the incidence rates of cardiovascular mortality (ICVM).
Discussion
Our study is the first to use a real-world population database to investigate the impact of IGRT on PC patients’ OS. To our knowledge, our study was the largest to evaluate the effect of IGRT on the number of enrolled patients.35,36 With balanced baseline characteristics of the IGRT and non-IGRT arms, the OS was not significantly different. This result implicates the safety of adding IGRT to standard DPR for PC.
In our study, the crude 5-year OS rate was 88 and 86% for IGRT and non-IGRT, respectively. After balancing the baseline covariates with IPW, the HR of death with IGRT was 0.93 (95% CI, 0.61–1.45). A statistically significant difference was not noted. Our 5-year OS was comparable to that of the IGRT group in a French Phase III trial (83%).5 In the French trial, there were more second primary malignancy- and cardiovascular-related deaths in the IGRT arm. However, most second primary malignancies at that trial occurred shortly after radiation, and they mostly originated from the radiation fields. The relationship between the extra events and daily IGRT was not compatible with most carcinogenesis studies due to irradiation. In the first SA of our study, we used the well-advocated PS-matching method to evaluate the OS between the IGRT group and non-IGRT group. In line with the literature,18 covariate balancing was better with PS weighting than with PS matching. However, the OS was still not significantly different between the two groups. Similar results were seen in SA-2 as well.
In addition, the mortality rate due to the second primary malignancy (IOCM) in the IGRT arm was not significantly different from that of the non-IGRT arm (p = 0.17). In the French trial, more cardiovascular-related deaths were also observed; our SA-1 showed a larger risk of cardiovascular-related deaths in the IGRT arm, but this did not reach statistical significance (HR = 2.39; 95% CI, 0.63–9.03; p = 0.20). The most commonly found studies related to radiotherapy and cardiovascular events examine the effects of thoracic irradiation, such as in cases of Hodgkin disease or breast cancer. More cardiovascular events were noted between surgery and radiotherapy on PC patients.37 In addition, the duration and regimen of hormone treatment38 might serve as potential confounders of the cardiovascular events. However, the interaction between these systemic agents and IGRT was not known. Furthermore, the results on the secondary endpoints (such as ICVM or IOCM) must be interpreted with caution because the comorbidity assessment we used (the Charlson comorbidity score) is a general composite score that may not be specific for these endpoints.
Overall, it has been shown that IGRT can provide margin reduction39 and fewer radiotherapy-related side-effects in PC and other malignancies,40,41 although one prospective study showed no differences in patient-reported outcomes.8 Precise dose delivery with IGRT is advocated, which might be of benefit when attempting to ensure better tumor control. As IGRT offers benefits associated with decreasing toxicity, the additional cost was thought to be acceptable.42 The French Phase III trial showed better biochemical and clinical progression-free survival in the daily IGRT group. One retrospective study also demonstrated the benefit of biochemical failure with IGRT in the high-risk group.36 In our study, we could not evaluate the difference in biochemical failure between the IGRT and non-IGRT arms given the lack of laboratory exam results in the database. However, the HR of PC mortality with IGRT was 0.41 (95% CI, 0.11–1.60) in SA-1. Patients with IGRT may be treated with higher biologically effective doses to the prostate; however, it was also shown that dose escalation to PC patients yields better biochemical-free survival, but does not lead to better OS.25,26 This might be due to the positive therapeutic effects of salvage hormone treatment.
There are several obvious limitations in our study. First, this study is a non-randomized retrospective study; as such, there might be unmeasured confounders between the two groups. We used the PS methods to ensure baseline covariate balance and to avoid the risks associated with model misspecification. For the potentially unmeasured confounders, we further used the E-factor to measure the strength of our results. The OS outcomes of other RCTs are also forthcoming and eagerly awaited.8 Second, due to the fact that the data were derived from real-world practice and obtained from most hospitals in Taiwan, it was noted that there are no unified protocols for contouring, treatment, or IGRT technique. In TCR, IGRT was simply coded as ‘with’ or ‘without’ in the item “external beam radiotherapy”, without further detail regarding IGRT technique. Therefore, the intervention (IGRT) was actually heterogeneous (including but not limited to radio-opaque fiducial markers, cone beam computed tomography, and megavoltage computed tomography in tomotherapy), and imaging dose was not the same between techniques,4 which can be a confounding factor for OS, IOCM, or ICVM. However, there was no universal preferred IGRT technique to our knowledge.
In the European guidelines, there were four types of techniques recommended for IGRT of PC.4 Even in the NCCN guidelines, there are also several techniques recommended for daily prostate localization.2 In addition, the coding details regarding the radiotherapy parameters in TCR were modified from the National Cancer Database.43 A previous validation study showed good accuracy in the details of radiotherapy and chemotherapy treatment.44 Third, due to the lack of laboratory data and imaging data, we could not compare the biochemical failure survival between the IGRT and non-IGRT groups. However, this is not an obstacle for our primary endpoint, OS.
In addition, the similar OS rates imply the safety of using IGRT in daily practice. The lack of comparison of biochemical failure-free survival in the two groups could not explain the effectiveness of IGRT, as the previous French trial showed the efficacy of tumor control with IGRT.5 Fourth, in our study, the median follow-up time was 50 months, which might be relatively short for PC control and the development of a secondary malignancy. However, the benefit of PC control and the difference in the OS were statistically significant after a similar follow-up (median: 4.1 years) in the French trial.5 The lack of a notable difference in OS in comparable follow-up studies showed the safety of IGRT administration and indicated that a longer follow-up time is necessary to further observe this trend.
Fifth, the choice of covariates included in the PS model may be a limitation of our study. Theoretically, only the true confounders were needed for the PS model, whereas the risk factors without an association to IGRT may be skipped in the PS model.45 However, the optimal practical approach for covariate selection in the PS model was debated in the literature.9,21,45 Therefore, we performed two SAs to examine the robustness of our findings, as they pertained to the different covariates included in the PS model.
The final limitation relates to the accessibility of IGRT for our study population (i.e. reflecting a non-zero probability assumption for the PS method). This cannot be definitively confirmed due to data limitations. However, to our knowledge, all of the radiotherapy departments in Taiwan possessed the ability to perform IGRT in accordance with the literature.4
Conclusion
Our study showed how adding IGRT to radical radiotherapy in PC has no obvious impact on OS. Further, IGRT does not increase the risk of death due to other malignancies or cardiovascular events. However, the follow-up period was modest (median: 50 months), and the results related to other cancer- or cardiovascular-related mortality should be interpreted with caution. Further studies are needed to clarify our findings.
Footnotes
Acknowledgements: The data analyzed in this study were provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare, Executive Yuan, Taiwan. We are grateful to the Health Data Science Center, China Medical University Hospital for providing administrative, technical, and funding support. The authors thank the Department of Medical Research CMUHCH (CMUHCH-DMR 109-001 and 109-015 and 110-002) and the Ministry of Science and Technology (MOST 109-2314-B-039-014-) for their administrative and funding support. English-language editing of this manuscript was provided by Journal Prep Services.
The authors Yao-Hung Kuo, Ji-An Liang, Guan-Heng Chen and Chia-Chin Li contributed equally to the work.
Contributor Information
Yao-Hung Kuo, Email: taiwankyh@gmail.com.
Ji-An Liang, Email: d4615@mail.cmuh.org.tw.
Guan-Heng Chen, Email: D18149@mail.cmuhch.org.tw.
Chia-Chin Li, Email: jiajin45@yahoo.com.tw.
Chun-Ru Chien, Email: d16181@gmail.com.
REFERENCES
- 1.Kamran SC, D'Amico AV. Radiation therapy for prostate cancer. Hematol Oncol Clin North Am 2020; 34: 45–69. doi: 10.1016/j.hoc.2019.08.017 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network National Comprehensive Cancer Network guidelines for prostate cancer version 4. 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 3.Ali A, Parker CC, Clarke NW. Prostate radiotherapy in newly diagnosed metastatic prostate cancer. Curr Opin Urol 2019; 29: 620–8. doi: 10.1097/MOU.0000000000000675 [DOI] [PubMed] [Google Scholar]
- 4.Ghadjar P, Fiorino C, Munck Af Rosenschöld P, Pinkawa M, Zilli T, van der Heide UA. ESTRO ACROP consensus guideline on the use of image guided radiation therapy for localized prostate cancer. Radiother Oncol 2019; 141: 5–13. doi: 10.1016/j.radonc.2019.08.027 [DOI] [PubMed] [Google Scholar]
- 5.de Crevoisier R, Bayar MA, Pommier P, Muracciole X, Pêne F, Dudouet P, et al. Daily versus Weekly prostate cancer image guided radiation therapy: phase 3 multicenter randomized trial. Int J Radiat Oncol Biol Phys 2018; 102: 1420–9. doi: 10.1016/j.ijrobp.2018.07.2006 [DOI] [PubMed] [Google Scholar]
- 6.Chiang C-J, Wang Y-W, Lee W-C. Taiwan's nationwide cancer registry system of 40 years: past, present, and future. J Formos Med Assoc 2019; 118: 856–8. doi: 10.1016/j.jfma.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 7.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–9. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 8.Tøndel H, Lund Jo-Åsmund, Lydersen S, Wanderås AD, Aksnessæther B, Jensen CA, et al. Radiotherapy for prostate cancer - Does daily image guidance with tighter margins improve patient reported outcomes compared to weekly orthogonal verified irradiation? Results from a randomized controlled trial. Radiother Oncol 2018; 126: 229–35. doi: 10.1016/j.radonc.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 9.Ali MS, Groenwold RHH, Belitser SV, Pestman WR, Hoes AW, Roes KCB, et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol 2015; 68: 122–31. doi: 10.1016/j.jclinepi.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 10.Kuo Y-H, Fang H-Y, Lin Y-S, Lein M-Y, Yang C-Y, Ho S-C, et al. Effectiveness of image-guided radiotherapy for locally advanced esophageal squamous cell carcinoma patients treated with definitive concurrent chemoradiotherapy. Thorac Cancer 2020; 11: 113–9. doi: 10.1111/1759-7714.13244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C-C, Liang J-A, Chung C-Y, Chen WT-L, Chien C-R. Effectiveness of intensity-modulated radiotherapy for rectal cancer patients treated with neoadjuvant concurrent chemoradiotherapy: a population-based propensity score-matched analysis. Anticancer Res 2019; 39: 1479–84. doi: 10.21873/anticanres.13265 [DOI] [PubMed] [Google Scholar]
- 12.Tu C-Y, Hsia T-C, Fang H-Y, Liang J-A, Yang S-T, Li C-C, et al. A population-based study of the effectiveness of stereotactic ablative radiotherapy versus conventional fractionated radiotherapy for clinical stage I non-small cell lung cancer patients. Radiol Oncol 2018; 52: 181–8. doi: 10.1515/raon-2017-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagsi R, Bekelman JE, Chen A, Chen RC, Hoffman K, Shih Y-CT, et al. Considerations for observational research using large data sets in radiation oncology. Int J Radiat Oncol Biol Phys 2014; 90: 11–24. doi: 10.1016/j.ijrobp.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth CM, Karim S, Mackillop WJ. Real-World data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol 2019; 16: 312–25. doi: 10.1038/s41571-019-0167-7 [DOI] [PubMed] [Google Scholar]
- 15.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33: 1242–58. doi: 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum PR. Observation and experiment an introduction to causal inference. Cambridge, MA USA: Harvard University Press; 2017. [Google Scholar]
- 17.Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst 2017; 109: djw323. doi: 10.1093/jnci/djw323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao H, Li L, Greene T. Propensity score weighting analysis and treatment effect discovery. Stat Methods Med Res 2019; 28: 2439–54. doi: 10.1177/0962280218781171 [DOI] [PubMed] [Google Scholar]
- 19.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc 2018; 113: 390–400. doi: 10.1080/01621459.2016.1260466 [DOI] [Google Scholar]
- 20.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34: 3661–79. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, et al. Methods for constructing and assessing propensity scores. Health Serv Res 2014; 49: 1701–20. doi: 10.1111/1475-6773.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004; 75: 45–9. doi: 10.1016/j.cmpb.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016; 35: 5642–55. doi: 10.1002/sim.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019; 321: 602–3. doi: 10.1001/jama.2018.21554 [DOI] [PubMed] [Google Scholar]
- 25.Al-Mamgani A, van Putten WLJ, Heemsbergen WD, van Leenders GJLH, Slot A, Dielwart MFH, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008; 72: 980–8. doi: 10.1016/j.ijrobp.2008.02.073 [DOI] [PubMed] [Google Scholar]
- 26.Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014; 15: 464–73. doi: 10.1016/S1470-2045(14)70040-3 [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues G, Warde P, Pickles T, Crook J, Brundage M, Souhami L, et al. Pre-Treatment risk stratification of prostate cancer patients: a critical review. Can Urol Assoc J 2012; 6: 121–7. doi: 10.5489/cuaj.11085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280: 969–74. doi: 10.1001/jama.280.11.969 [DOI] [PubMed] [Google Scholar]
- 29.Austin PC, Fine JP. Propensity-score matching with competing risks in survival analysis. Stat Med 2019; 38: 751–77. doi: 10.1002/sim.8008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalani N, Jimenez RB, Yeap B. Understanding propensity score analyses. Int J Radiat Oncol Biol Phys 2020; 107: 404–7. doi: 10.1016/j.ijrobp.2020.02.638 [DOI] [PubMed] [Google Scholar]
- 31.Goetghebeur E, le Cessie S, De Stavola B, Moodie EE, Waernbaum I, .“on behalf of” the topic group Causal Inference (TG7) of the STRATOS initiative . Formulating causal questions and principled statistical answers. Stat Med 2020; 39: 4922–48. doi: 10.1002/sim.8741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum PR. Section 18.2: are analytic adjustments feasible? In: Rosenbaum P. R, ed.Design of observational studies springer series in statistics, vol. New York: Springer; 2010. pp. 317–21. [Google Scholar]
- 33.Rosenbaum PR. Solutions to common problems. In: Rosenbaum P. R, ed.Design of observational studies springer series in statistics, vol. New York: Springer; 2010. pp. 355–6. [Google Scholar]
- 34.Duarez D, Faries DE. Propensity score stratification and regression. In: Faries D. E, Leon A. C, Haro J. M, Obenchain R. L, eds.Analysis of observational health care data using SAS. Cary, NC: SAS Institute, Inc; 2010. pp. 23–46. [Google Scholar]
- 35.Zhong Q, Gao H, Li G, Xiu X, Wu Q, Li M, et al. Significance of image guidance to clinical outcomes for localized prostate cancer. Biomed Res Int 2014; 2014: 1–8. doi: 10.1155/2014/860639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2012; 84: 125–9. doi: 10.1016/j.ijrobp.2011.11.047 [DOI] [PubMed] [Google Scholar]
- 37.Wallis CJD, Satkunasivam R, Herschorn S, Law C, Seth A, Kodama RT, et al. Association between primary local treatment and Non-prostate cancer mortality in men with nonmetastatic prostate cancer. Urology 2018; 114: 147–54. doi: 10.1016/j.urology.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 38.Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer 2018; 16: e645–53. doi: 10.1016/j.clgc.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 39.Maund IF, Benson RJ, Fairfoul J, Cook J, Huddart R, Poynter A. Image-Guided radiotherapy of the prostate using daily CBCT: the feasibility and likely benefit of implementing a margin reduction. Br J Radiol 2014; 87: 20140459. doi: 10.1259/bjr.20140459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D, Zhang Q, Eisenberg BL, Kane JM, Li XA, Lucas D, et al. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: results of radiation therapy Oncology Group RTOG-0630 trial. J Clin Oncol 2015; 33: 2231–8. doi: 10.1200/JCO.2014.58.5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin S. Setup errors in cone-beam computed tomography and their effects on acute radiation toxicity in cervical cancer radiotherapy. Genet Mol Res 2015; 14: 10937–43. doi: 10.4238/2015.September.21.4 [DOI] [PubMed] [Google Scholar]
- 42.Perrier L, Morelle M, Pommier P, Lagrange J-L, Laplanche A, Dudouet P, et al. Cost of prostate image-guided radiation therapy: results of a randomized trial. Radiother Oncol 2013; 106: 50–8. doi: 10.1016/j.radonc.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 43.Shiau C-Y. Facility radiation oncology registry data standards of Taiwan cancer database. Therapeutic Radiology and Oncology 2010; 17: 259–69. [Google Scholar]
- 44.Cheng C-Y, Chiang C-J, Hsieh C-H, Chang Y-K, Lai M-S. Is quality of registry treatment data related to registrar experience and workload? A study of Taiwan cancer registry data. J Formos Med Assoc 2018; 117: 1093–100. doi: 10.1016/j.jfma.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 45.Loke YK, Mattishent K. Propensity score methods in real-world epidemiology: a practical guide for first-time users. Diabetes Obes Metab 2020; 22 Suppl 3(Suppl 3): 13–20. doi: 10.1111/dom.13926 [DOI] [PubMed] [Google Scholar]