Abstract
Objectives:
Studies show insufficient sensitivity of virtual non-contrast (VNC) reconstructions for stone detection in dual-energy CT urography (DE-CTU). The aim of this study was to investigate if side-by-side-evaluation of both VNC and post-contrast images could increase the sensitivity of single-phase split bolus DE-CTU.
Methods:
Consecutive patients with haematuria who underwent split bolus DE-CTU on the same dual-source DE-CT scanner were retrospectively enrolled in the study. Intravenous furosemide and oral hydration were employed. Two readers, independently and then jointly in two separate sessions, recorded the location and the longest axial stone diameter on three randomised sets of images: separate VNC and post-contrast images, and side-by-side-reconstructions. True non-contrast (TNC) images served as the standard of reference.
Results:
A total of 83 urinary stones were detected on TNC images. Independent reader side-by-side-evaluation of VNC and post-contrast images yielded higher stone detection sensitivity (76 and 84%, respectively) compared to evaluation of only VNC (71 and 81%, respectively) or post-contrast images (64 and 80%, respectively). The sensitivity of joint reader evaluation of side-by-side-images reached almost 86% and was not significantly different from TNC images (p = 0.77). All stones larger than 3 mm were correctly detected by side-by-side-evaluation. Dose reduction of 55% could be achieved by omitting TNC scans.
Conclusion:
Side-by-side-VNC and post-contrast image evaluation enable detection of clinically significant urolithiasis on single-phase split bolus DE-CTU with significant dose reduction.
Advances in knowledge:
This study shows that single-phase DE-CTU is feasible if VNC imaging is simultaneously utilised with post-contrast images.
Introduction
Urinary stones are the most common cause of haematuria with increasing prevalence in industrialised countries affecting about 12% of the population.1–4 Computed Tomography Urography (CTU), consisting of a non-contrast, nephrographic and excretory phase, is the modality of choice for evaluating the underlying cause.1,5–7 Furthermore, the EAU Urolithiasis Guideline Panel recommends a contrast study for assessing the urinary tract anatomy prior to stone treatment.8 However, multiphasic CTU is heavily burdened by high radiation dose, especially detrimental in children and younger patients, and those requiring multiple follow-up examinations: a study by Yecies et al9 emphasises that the potential diagnostic information from conventional CTU in patients with asymptomatic micro-haematuria does not justify the additional radiation burden and the risk of secondary malignancy.
Split bolus CTU and dual-energy CTU (DE-CTU) are two potential means for radiation reduction; however, they have to retain an acceptable level of diagnostic accuracy compared to triple phase CTU. Dose reductions of 30–50% are achieved by splitting the contrast bolus and combining nephrographic and excretory phases in a single image set, without any reported loss of diagnostic accuracy of the entire CTU study.10,11 Reconstructing virtual unenhanced (VNC) from post-contrast DE-CTU images potentially enables omission of the true unenhanced (TNC) scans, and a further 50% dose reduction, provided the study retains its diagnostic value.12 Moreover, information on the chemical composition of the urinary stones and better display of the urinary tract anatomy on 3D volume rendered reconstructions prior to treatment renders DE-CTU the modality of choice for imaging patients with urolithiasis.13–16
The sensitivity of stone detection on VNC images derived from split bolus DE-CTU reported in previous studies varied from 53 to 95%.17,18 The accidental subtraction of urinary stones is the main cause of false-negative results on VNC images and occurs in small, low attenuating stones surrounded by dense contrast urine.17,19,20 On the other hand, pockets of dense contrast urine unevenly accumulated within the urinary tract may be falsely recognised as urinary stones and included on VNC reconstructions.21,22 Research showed that the dilution of contrast urine by oral hydration, diuretics and lower contrast volumes in the first bolus, as well as using 100–140 kVp instead of 80–140 kVp pairs increases the accuracy of iodine subtraction on VNC DE-CT images.18,23–26 Moreover, some earlier studies report that the detection of stones on post-contrast images alone surpasses the accuracy of VNC reconstructions.17,22
Therefore, we hypothesised that assessment of urinary stones on both VNC and post-contrast images on a study with sufficiently diluted contrast urine would improve their detection compared to solemnly assessing the VNC reconstructions.
The aim of this study was to investigate if side-by-side-evaluation of both VNC and post-contrast images compared to sole evaluation of VNC reconstructions could increase the sensitivity of single-phase split bolus DE-CTU in detecting clinically significant urolithiasis. To the best of our knowledge, no previous study reported on the contribution of post-contrast images on the diagnostic accuracy of single-phase split bolus DE-CTU.
Methods and patients
Study design
The institutional review board approved this retrospective study and the informed consent was waived.
Patients
Consecutive adult patients with visible and non-visible haematuria that underwent split bolus DE-CTU between May 2018 and May 2019 on the same dual-source dual-energy CT scanner (Somatom Definition Flash, Siemens, Erlangen, Germany) were included in the study. Exclusion criteria were: patients without urinary stones, with anatomic anomalies or iatrogenic urinary tract distortions, non-excreting or hypo-functional kidney, and studies with significant artefacts.
Radiology resident (T.S.M) collected and recorded demographic and clinical patient data: sex, age, referring diagnosis and dose parameters.
Patient preparation and DE-CTU protocol
Patients had to empty their bladder and drink 750 ml of water 45 min before scanning and were instructed not to void afterwards. After the unenhanced scan and i.v. administration of 10 mg of furosemide, 50 ml of non-ionic iodinated contrast bolus was injected at the rate of 3 ml s−1. Patients were then instructed to walk around the CT table. After approximately 5–12 min, a second 90-ml contrast bolus, followed by a 20-ml saline flush, was applied at the same rate and the combined post-contrast phase was obtained after a 100 s delay.
Scanning parameters
True unenhanced images were scanned with a 120 kV tube voltage and referent tube current at 150 mAs. DE-CT scans were performed with referent tube current/tube voltage of 100 mAs/100 kV and 77 mAs/Sn140 kV. Automatic tube current modulation (CareDose® 4D) was activated and iterative reconstruction software (SAFIRE®) at level 3 was applied to all single- and dual-energy reconstructions, with the detector configuration of 32 × 0.6 mm. Virtual unenhanced reconstructions were generated on a working station (SyngoVia, Siemens Medical Solutions, Malvern PA) in appropriate Liver VNC application. Soft tissue (I26f reconstruction kernel) 1-mm thick multiplanar reconstructions were sent and stored on the working station.
Image analysis
Fully anonymised images were evaluated in two separate sessions by two readers (Reader 1: I.Ž., 5 years of uroradiology experience and 3 years’ experience in DE-CT imaging, and Reader 2: I.P., 15 years of uroradiology experience and limited DE-CT experience) who recorded the location of urinary stones, and their longest axial diameter (mean value of three measurements) on a fixed bone window (window level 300 HU; window width 1,500 HU). The urinary tract was divided into six anatomical sections: calyceal system, renal pelvis, proximal ureter (to the tip of the iliac crest), middle ureter (from the iliac crest to the level of the distal end of the sacroiliac joint), distal ureter (from the level of the distal end of sacroiliac joint to vesico-ureteral junction) and urinary bladder. Initially, each reader independently reviewed three randomised sets of images: separate VNC and post-contrast images, and side-by-side-VNC and post-contrast images. In the next session, they jointly evaluated the same sets of randomised images: discrepancies were resolved in consensus. Reading sessions were 8 weeks apart to minimise recollection bias. Data were recorded and image sets presented to the reviewers by a radiology resident (T.S.M.) in random order determined by a random number table generated on https://www.randomizer.org/ on the 20 August 2019. Evaluation was performed on the same diagnostic monitors: the readers freely changed the window settings and used multiplanar reconstructions. TNC images served as the standard of reference and were subsequently reviewed by the resident (T.S.M) who correlated true stone location with readers findings and measured longest axial diameter (mean value of three measurements on a fixed bone window). If the stones were inseparable, the longest axial diameter of all stones was measured together. Potential reasons for false-negative (FN) and false-positive (FP) findings were identified by comparing the VNC, post-contrast and TNC images.
Radiation dose
The total and the dose-length product (DLP) of each individual scanning phase was recorded from patient protocol data, and the effective dose (ED) was calculated by multiplying the DLP by the abdominal weighting factor of 0.0015.27
Statistical analysis
Sensitivity, specificity, positive and negative predictive values were calculated per stone and per patient basis. McNemar test for paired nominal variables was used to compare differences in stone detection for VNC, post-contrast and side-by-side-evaluation to TNC images that served as a referent standard. Differences between measured axial stone diameter on TNC, VNC and post-contrast images were determined by paired t-test. Interrater agreement was calculated by κ test. The k values were as follows: 0 to 0.19, poor; 0.20 to 0.39, fair; 0.40 to 0.59, moderate; 0.60 to 0.79, substantial; and 0.80 to 1.00, excellent agreement.17 A two-sided p value < 0.05 was considered statistically significant.
Results
Patient population
A flow of participants in the study is summarised in Figure 1. Finally, 37 patients with 83 urinary stones were included in the study: 23 males (62%), age ranging from 30 to 81 years [average 62(15) years]. The referring diagnoses were: suspected urolithiasis (n = 15, 40.5%), haematuria (n = 5, 13.5%), suspected carcinoma (n = 4, 10.8%) and unknown in 13 (35.1%) cases. Additional relevant findings were present in 14 patients: obstructive urolithiasis in nine patients and one case of ureterocele, bladder diverticula, renal, bladder, prostate and sigmoid cancer.
Figure 1.

Selection of study sample presented using STARD participant flow chart DE-CTU, dual-energy CT urography
Urinary stones
A total of 83 urinary stones were detected on TNC images: 69 (83.1%) in the calyces and renal pelvis [37 (53.6%) on the right and 32 (46.4%) on the left], 11 (13.2%) in the ureters [3 (27.3%) in the proximal and 8 (72.7%) in the distal segment], and 3 (3.6%) in the urinary bladder. Fifteen patients (40.5%) had only one urinary stone (diameter range 2.1 to 35.3 mm), and 22 (59.5%) had multiple stones (diameter range 1.2 to 24.6 mm). The overall median (IQR) stone size was 3.5 (5) mm, ranging from 1.2 to 35.3 mm, mode 1.9 mm.
Diagnostic accuracy
There was complete correlation between the location of stones detected by the readers on VNC and post-contrast compared to TNC images. The sensitivity of stone detection in independent readings was highest for both readers when VNC and post-contrast images were evaluated side-by side (per reader sensitivity 76 and 84%, respectively) compared to separate evaluation of VNC (per reader sensitivity 71 and 81%, respectively) and post-contrast images (per reader sensitivity 64 and 80%, respectively). Furthermore, the sensitivity of jointly evaluated side-by-side-VNC and post-contrast images reached almost 86% and was not significantly different from the standard of reference (p = 0.77). Results are displayed in Table 1.
Table 1.
Diagnostic performance of urinary stone detection on virtual non-contrast (VNC), post-contrast (CE) and side-by-side (VNC +CE) images on per stone basis for both readers in individual and joint sessions
| SE (%) | 95% CI | PPV (%) | 95% CI | Accuracy (%) | 95% CI | p | ||
|---|---|---|---|---|---|---|---|---|
| Reader 1 | ||||||||
| TNC vs | VNC | 80.71 | 70.59–88.56 | 98.53 | 92.44–99.73 | 80.46 | 70.57–88.19 | .000 |
| CE | 79.52 | 69.24–87.59 | 94.29 | 93.67–94.85 | 75.86 | 65.5–84.40 | .007 | |
| VNC + CE | 84.34 | 74.71–91.39 | 94.59 | 94.10–95.05 | 80.46 | 70.57–88.19 | .049 | |
| Reader 2 | ||||||||
| TNC vs | VNC | 71.08 | 60.09–80.52 | 100 | / | 72.41 | 61.79–81.46 | .000 |
| CE | 63.86 | 52.57–74.12 | 94.64 | 90.75–96.95 | 62.07 | 51.03–72.26 | .000 | |
| VNC + CE | 75.90 | 65.27–84.62 | 95.45 | 92.17–97.40 | 73.56 | 63.02–82.45 | .000 | |
| Joint evaluation | ||||||||
| TNC vs | VNC | 83.13 | 73.32–90.46 | 98.57 | 92.65–99.74 | 82.14 | 72.26–89.65 | .001 |
| CE | 80.72 | 70.59–88.56 | 95.71 | 95.26–96.13 | 77.91 | 67.67–86.10 | .004 | |
| VNC + CE | 85.54 | 76.11–92.30 | 94.67 | 94.20–95.10 | 81.61 | 71.86–89.11 | .077 |
PPV, Positive predictive value; SE, Sensitivity; SP, Specificity;TNC, True non-contrast.
Seventy-one (71/83, 85.5%) stone was correctly detected on side-by-side-evaluation compared to 67 (67/83, 80.7%) and 69 (69/83, 83.1%) on post-contrast and VNC images, respectively. Side-by-side-evaluation had the lowest rate of false-negative results of 14.4%, missing 12/83 stones, all smaller than 3 mm [mean (SD), 2 (0.39) mm; range 1.3 to 3 mm]. Eleven (91.6%) missed stones were located in the calyceal system, and the remaining one, measuring 1.9 mm, was in the distal ureter. Stones missed on VNC were on average 1.9 (0.39) mm (range 1.2 to 3 mm),and 2.4 (0.84) mm (range 1.2 to 7.6 mm) on post-contrast images. Figure 2 demonstrates an example of a small stone falsely subtracted on VNC images and correctly diagnosed by side-by-side-evaluation. Stones larger than 3 mm were detected on VNC and side-by-side images with 100% (95%CI 94.13–100%) sensitivity, and 98.61% (95%CI 92.5–99.96%) accuracy, while one stone measuring 7.6 mm was not detected on post-contrast images yielding the sensitivity of 98.55% (95%CI 92.19–99.96%) and accuracy of 97.14% (95%CI 90.06–99.65%). There were three false-positive findings on post-contrast images all located in the calyces; however, joint evaluation with VNC reconstructions finally yielded only one false-positive result. Per patient sensitivity of all readings was 94.6% with one or more stones detected in 35/37 patients and two false-negative results. Results are summarised in Table 2.
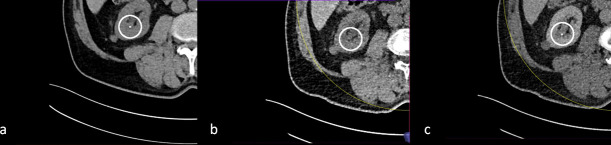
Figure 2.
Small stone in the right kidney visible on true non-contrast (a) and post-contrast images (c). The stone is not visible on virtual non-contrast reconstructions (b) due to over subtraction.
Table 2.
Summary of diagnostic performance of overall stone detection on virtual non-contrast (VNC), post-contrast (CE) and side-by-side (VNC +CE) evaluation on per stone and per patient basis
| Per stone | Per patient | |||||
|---|---|---|---|---|---|---|
| VNC | CE | VNC + CE | VNC | CE | VNC + CE | |
| Sensitivity | 83% | 81% | 86% | 95% | 95% | 95% |
| Accuracy | 82% | 78% | 82% | / | / | / |
| TP | 69 (83.1%) | 67 (80.7%) | 71 (85.5%) | 35 | 35 | 35 |
| FN | 14 (16.9%) | 16 (19.3%) | 12 (14.5%) | 2 | 2 | 2 |
| FP | 1 | 3 | 1 | 0 | 0 | 0 |
FN, false negative; FP, False positive;TP, True positive.
Stone size
There was no statistically significant difference in the measured longest axial stone diameter on VNC and post-contrast images compared to stone size determined on TNC images in both readers, as shown in Table 3.
Table 3.
Comparison of measured urinary stone diameter (r) on true non-contrast (TNC), virtual non-contrast (VNC) and post-contrast (CE) images
| Reader 1 | r/mm | SD | p | |
|---|---|---|---|---|
| TNC vs | VNC | 6.90 | 6,586 | .763 |
| CE | 7.05 | 6,500 | .327 | |
| VNC vs | CE | 7.19 | 6,546 | .175 |
| Reader 2 | ||||
| TNC vs | VNC | 7.45 | 6,501 | .556 |
| CE | 7.33 | 6,366 | .097 | |
| VNC vs | CE | 7.67 | 6,496 | .105 |
Inter-rater agreement
Inter-reader agreement was substantial for urinary stone detection on VNC and side-by-side-evaluation (κ value 0.62 and 0.64, 95% CI 45–81% and 44–84%, respectively) and moderate for the post-contrast evaluation (κ value 0.55, 95% CI 37–73%).
Radiation dose
The mean total DLP was 570.8 (177.4) mGycm, range 320 to 1133 mGycm: mean TNC DLP 319.7 (133) mGycm, range 165.2 to 824.2 mGycm, and mean post-contrast DLP 251 (69.8) mGycm, range 154.8 to 496.2 mGycm. The total mean effective dose was 8.6 (2.66) mSv, range 4.8 to 17 mSv: TNC ED 4.8 (1.99) mSv, range 2.48 to 12.36 mSv and post-contrast ED 3.77 (1.05) mSv, range 2.32 to 7.44 mSv. A radiation dose reduction of 55% could be achieved by omitting the unenhanced scans.
Discussion
This study shows that joint evaluation of both VNC and post-contrast images is beneficial and raises the total sensitivity of single-phase split bolus DE-CTU in detecting urinary stones to 86%: the drop of false-negative findings on side-by-side-evaluation significantly increased the overall sensitivity by up to 5%.
Stone detection was least accurate on post-contrast images alone, with three false-positive and 16 (19%) false-negative findings; however, side-by-side-evaluation finally resulted in one false-positive and 12 (14%) missed stones, all smaller than 3 mm. Contrary to our results, in previous studies by Botsikas and Scheffel sensitivity of post-contrast images trumped VNC by 8.7 and 20%, respectively.22,28 However, the sensitivity of VNC reconstructions in both studies was lower compared to our results (78 and 74 vs 83%, respectively) primarily due to inferior DE-CT technology, although Scheffel et al28 reconstructed VNC from nephrographic images of a triple-phase DE-CTU which are more accurate than excretory or combined phase VNC reconstructions.29,30 Additionally, Scheffel et al28 evaluated stone detection on nephrographic images with minimal amounts of contrast within the urinary tract to obscure small calculi, compared to combined post-contrast phase in our study, which probably caused a 20% sensitivity difference between post-contrast and VNC images in their study. They did not investigate the effect of a side-by-side-evaluation.
According to most recent studies on third-generation DE-CT scanners, the sensitivity of stone detection on VNC reconstructions varies from 83 to 95%.13,17,23,29 Yeo et al had the most similar DE-CTU protocol to ours with oral hydration, fixed amount of contrast material (50 ml for the first, and 100 ml for the second bolus), and a 100 s delay for the nephrographic phase, however, without the intravenous furosemide.17 They reported the highest true-positive detection rate on a large sample size (537 stones in 223 patients) at 93% (499/537 stones), which is higher than our findings at 85.5% (71/83 stones), although a significant difference in sample size might have contributed to the percentage disparity. Moreover, a side-by-side-evaluation of VNC and post-contrast images in our study led to a correct diagnosis of all stones over 3 mm, while 9/362 stones in the range of 3 to 6 mm with potential implications on clinical management were missed in the study by Yeo et al.17 The most recent study by Manoharan et al. reported the sensitivity of VNC of little over 91%; however, they too missed larger stones up to 6.4 mm, similar to the findings of Toepker et al. whose overall sensitivity was around 83%, but the detection of stones smaller than 3 mm was between 43 and 71%, and only the stones larger than 6 mm were diagnosed with a 100% sensitivity.13,23
Substituting TNC with VNC reconstructions would significantly reduce the radiation dose of DE-CTU, by 55% in our study. Substantial dose reduction could also be achieved by replacing TNC with low- and ultra-low dose non-contrast CT (LD-CT), with effective doses below 1 mSv, and reported stone detection sensitivity between 90 and 100% by two systematic reviews.31,32 However, the authors constrict the validity of these findings to stones bigger than 3 mm. More specifically, Xiang et al. demonstrated pooled LD-CT sensitivity of 93.3% for clinically relevant stones over 3 mm, which is significantly lower compared to 100% detection rate of >3 mm stones on side-by-side-evaluation of VNC and post-contrast images in our study.31 Furthermore, Rob et al. concluded that LD-CT might not be sufficiently accurate in detecting stones < 3 mm, which correlates with our results of 14% missed small stones at side-by-side-evaluation on VNC and post-contrast images.32 Our results show that single-phase split bolus DE-CTU with side-by-side-evaluation is superior to other dose reducing CTU techniques in detecting clinically relevant urolithiasis. Additionally, image quality in obese patients and detection of other pathology by both methods remains under investigated, with a recommendation that standard dose TNC be used when the pre-test probability of other pathology is moderate to high.31 Nonetheless, we argue that VNC reconstructions derived from full-dose scans would be superior to LD-CT in this regard, and that single-phase split bolus DE-CTU could yield superior diagnostic accuracy at significantly lower dose. Therefore, single-phase DE-CTU could be the modality of choice in clinical setting of symptomatic patients with known urolithiasis, prior to treatment, in patients requiring frequent follow up, and for evaluation of visible or non-visible haematuria in patients with low probability of other sinister causes.
Decreasing the density of contrast urine is an essential prerequisite for accurate iodine subtraction on VNC reconstructions.33 This can be achieved by oral/i.v. hydration or diuretics which dilute the contrast urine or by lowering the amount of the first contrast bolus and/or shortening the scan delay after the second bolus to scan in the portal venous instead of nephrographic phase thus minimising the amount of excreted contrast in the urinary tract.25,26,28 The combined phase in all of the aforementioned studies was scanned within 60 to 65 s after the second contrast bolus, Toepker et al. used only 15 ml for the first contrast bolus, Manoharan et al. tailored the amount of contrast to patient weight, and all except Manoharan et al. used oral hydration, while none of them used intravenous diuretics.13,17,23,29 Therefore, we argue that the accuracy of a single-phase split bolus DE-CTU in our study could be further improved by adjusting the contrast volume to patients’ weight and shortening the scan delay from 100 to 60 s in addition to existing oral hydration and i.v. furosemide. This would potentially improve both the iodine subtraction on VNC reconstructions and evaluation of stones on post-contrast images due to a lesser amount of contrast medium excreted in the urinary tract.
Determining stone size, as a decisive factor in urolithiasis treatment, proved to be robust in our study on both VNC and post-contrast images in contrast to previous studies which underestimated stone diameter on VNC reconstructions.13,17,21,29 Stones located in the ureters are likely to cause symptoms and instigate treatment, while those in the pelvicalyceal system will remain clinically silent. A side-by-side-evaluation of VNC and post-contrast images in this study correctly detected and measured all but one small ureteral stone (1.9 mm in axial diameter) which would most likely pass spontaneously without treatment, while all the missed stones were in the pelvicalyceal system, causing no obstruction.33 Similar findings were reported by other authors.17,22,23,30
Senior uroradiologist with limited DECT training consistently detected urinary stones much more accurately than the first reader with greater DECT familiarity, but less uroradiology background. Given that all other reading parameters were identical for both readers, these results show the importance of expert experience in adopting new technologies.
Our study yields several limitations. A retrospective analysis potentially contributed to selection bias, while two consecutive evaluation sessions by the readers might have caused recollection bias, although a time interval of 8 weeks should have sufficed. Furthermore, we did not evaluate stone composition which might have influenced stone detection and diameter measurements: in the study by Manoharan et al., all falsely missed stones due to iodine over subtraction were non-uric acid calculi, and a phantom study by Takahashi et al. found that the size of uric acid stones was overestimated while non-uric acid stones were underestimated on VNC images.23,34 Additionally, although we argue that contrast urine dilution by oral hydration and i.v. diuretics would improve stone detection on both VNC and post-contrast images, we did not investigate other possibilities; however, we recognise that supplementing nephrographic with portal venous phase would be the easiest way to further improve stone detection on single-phase split bolus DE-CTU. The probability of missing stones smaller than 3 mm by our technique is real, but as they will most certainly be spontaneously eliminated, they are considered clinically irrelevant. However, we acknowledge that they could have been the undetected cause of haematuria, possibly leading to further unnecessary diagnostic procedures and patient stress. Finally, evaluation of detection and characterisation of other pathologies on DE-CTU without TNC, consisting of only VNC and post-contrast images, was not within the scope of our study. However, it can be presumed that sinister changes would be recognised on post-contrast images, and that the difference in attenuation values between TNC and VNC might influence their characterisation.
In conclusion, this study showed that a side-by-side-evaluation of both VNC and post-contrast images improves diagnostic accuracy of single-phase split bolus DE-CTU in detection of clinically significant urolithiasis. TNC scans can safely be omitted without pertinent diagnostic loss, especially in follow-up patients or prior to treatment, with a significant dose reduction of 55%.
Contributor Information
Doris Dodig, Email: doris_5na5@yahoo.com.
Tereza Solocki Matić, Email: tsolocki@gmail.com.
Iva Žuža, Email: iva.zuza276@gmail.com.
Ivan Pavlović, Email: ivan.pavlovic@ri.t-com.hr.
Damir Miletić, Email: damir.miletic@medri.uniri.hr.
Dean Markić, Email: dean.markic@ri.htnet.hr.
REFERENCES
- 1.Kambadakone AR, Eisner BH, Catalano OA, Sahani DV. New and Evolving Concepts in the Imaging and Management of Urolithiasis: Urologists’ Perspective. RadioGraphics 2010; 30: 603–23. doi: 10.1148/rg.303095146 [DOI] [PubMed] [Google Scholar]
- 2.Alelign T, Petros B. Kidney stone disease: an update on current concepts. Adv Urol 2018; 2018: 1–12. doi: 10.1155/2018/3068365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinchieri A. Epidemiology of urolithiasis: an update. Clin Cases Miner Bone Metab 2008; 5: 101e6. [PMC free article] [PubMed] [Google Scholar]
- 4.Pawar AS, Thongprayoon C, Cheungpasitporn W, Sakhuja A, Mao MA, Erickson SB. Incidence and characteristics of kidney stones in patients with horseshoe kidney: a systematic review and meta-analysis. Urol Ann 2018; 10: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linder BJ, Bass EJ, Mostafid H, Boorjian SA. Guideline of guidelines: asymptomatic microscopic haematuria. BJU Int 2018; 121: 176–83. doi: 10.1111/bju.14016 [DOI] [PubMed] [Google Scholar]
- 6.O'Connor OJ, Fitzgerald E, Maher MM. Imaging of hematuria. AJR Am of Roentgenol 2010; 195: W263–7. doi: 10.2214/AJR.09.4181 [DOI] [PubMed] [Google Scholar]
- 7.Moloney F, Murphy KP, Twomey M, O'Connor OJ, Maher MM. Haematuria: an imaging guide. Adv Urol 2014; 2014: 1–9. doi: 10.1155/2014/414125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol 2016; 69: 468–74. doi: 10.1016/j.eururo.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 9.Yecies T, Bandari J, Fam M, Macleod L, Jacobs B, Davies B. Risk of radiation from computerized tomography urography in the evaluation of asymptomatic microscopic hematuria. J Urol 2018; 200: 967–72. doi: 10.1016/j.juro.2018.05.118 [DOI] [PubMed] [Google Scholar]
- 10.Dillman JR, Caoili EM, Cohan RH, Ellis JH, Francis IR, Nan B, et al. Comparison of urinary tract distension and opacification using single-bolus 3-Phase vs split-bolus 2-phase multidetector row CT urography. J Comput Assist Tomogr 2007; 31: 750–7. doi: 10.1097/RCT.0b013e318033df36 [DOI] [PubMed] [Google Scholar]
- 11.Maheshwari E, O'Malley ME, Ghai S, Staunton M, Massey C. Split-bolus MDCT urography: upper tract opacification and performance for upper tract tumors in patients with hematuria. AJR Am of Roentgenol 2010; 194: 453–8. doi: 10.2214/AJR.09.3228 [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi M, Kawai T, Ito M, Ogawa M, Ohashi K, Hara M, et al. Split-bolus CT-urography using dual-energy CT: feasibility, image quality and dose reduction. Eur J Radiol 2012; 81: 3160–5. doi: 10.1016/j.ejrad.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 13.Toepker M, Kuehas F, Kienzl D, Herwig R, Spazierer E, Krauss B, et al. Dual energy computerized tomography with a split Bolus—A 1-Stop shop for patients with suspected urinary stones? J Urol 2014; 191: 792–7. doi: 10.1016/j.juro.2013.10.057 [DOI] [PubMed] [Google Scholar]
- 14.Ascenti G, Mileto A, Gaeta M, Blandino A, Mazziotti S, Scribano E. Single-Phase dual-energy CT urography in the evaluation of haematuria. Clin Radiol 2013; 68: e87–94. doi: 10.1016/j.crad.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Manglaviti G, Tresoldi S, Guerrer CS, Di Leo G, Montanari E, Sardanelli F, et al. In vivo evaluation of the chemical composition of urinary stones using dual-energy CT. AJR Am of Roentgenol 2011; 197: W76–83. doi: 10.2214/AJR.10.5217 [DOI] [PubMed] [Google Scholar]
- 16.Acharya S, Goyal A, Bhalla AS, Sharma R, Seth A, Gupta AK. In vivo characterization of urinary calculi on dual-energy CT: going a step ahead with sub-differentiation of calcium stones. Acta radiol 2015; 56: 881–9. doi: 10.1177/0284185114538251 [DOI] [PubMed] [Google Scholar]
- 17.Yeo YJ, Kim SH, Kim MJ, Kim YH, Cho SH, Lee EJ. Diagnostic efficiency of split-bolus dual-energy computed tomography for patients with suspected urinary stones. J Comput Assist Tomogr 2015; 39: 25–31. doi: 10.1097/RCT.0000000000000151 [DOI] [PubMed] [Google Scholar]
- 18.Chen C-Y, Hsu J-S, Jaw T-S, Shih M-CP, Lee L-J, Tsai T-H, et al. Split-Bolus portal venous phase dual-energy CT urography: protocol design, image quality, and dose reduction. AJR Am of Roentgenol 2015; 205: W492–501. doi: 10.2214/AJR.14.13687 [DOI] [PubMed] [Google Scholar]
- 19.Mangold S, Thomas C, Fenchel M, Vuust M, Krauss B, Ketelsen D, et al. Virtual nonenhanced dual-energy CT urography with tin-filter technology: determinants of detection of urinary calculi in the renal collecting system. Radiology 2012; 264: 119–25. doi: 10.1148/radiol.12110851 [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Qu M, Duan X, Takahashi N, Kawashima A, Leng S, et al. Characterisation of urinary stones in the presence of iodinated contrast medium using dual-energy CT: a phantom study. Eur Radiol 2012; 22: 2589–96. doi: 10.1007/s00330-012-2532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlo CA, Gnannt R, Winklehner A, Fischer MA, Donati OF, Eberli D, et al. Split-bolus dual-energy CT urography: protocol optimization and diagnostic performance for the detection of urinary stones. Abdom Imaging 2013; 38: 1136–43. doi: 10.1007/s00261-013-9992-9 [DOI] [PubMed] [Google Scholar]
- 22.Botsikas D, Hansen C, Stefanelli S, Becker CD, Montet X. Urinary stone detection and characterisation with dual-energy CT urography after furosemide intravenous injection: preliminary results. Eur Radiol 2014; 24: 709–14. doi: 10.1007/s00330-013-3033-5 [DOI] [PubMed] [Google Scholar]
- 23.Manoharan D, Sharma S, Das CJ, Kumar R, Singh G, Kumar P. Single-Acquisition Triple-Bolus dual-energy CT protocol for comprehensive evaluation of renal masses: a single-center randomized Noninferiority trial. AJR Am of Roentgenol 2018; 211: W22–32. doi: 10.2214/AJR.17.18786 [DOI] [PubMed] [Google Scholar]
- 24.Takahashi N, Vrtiska TJ, Kawashima A, Hartman RP, Primak AN, Fletcher JG, et al. Detectability of urinary stones on virtual nonenhanced images generated at pyelographic-phase dual-energy CT. Radiology 2010; 256: 184–90. doi: 10.1148/radiol.10091411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai RY, Jhaveri K, Saini S, Hahn PF, Nichols S, Mueller PR. Comprehensive evaluation of patients with haematuria on multi-slice computed tomography scanner: protocol design and preliminary observations. Australas Radiol 2001; 45: 536–8. doi: 10.1046/j.1440-1673.2001.00978.x [DOI] [PubMed] [Google Scholar]
- 26.Sahni VA, Shinagare AB, Silverman SG. Virtual unenhanced CT images acquired from dual-energy CT urography: accuracy of attenuation values and variation with contrast material phase. Clin Radiol 2013; 68: 264–71. doi: 10.1016/j.crad.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 27.Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning [published correction appears in AJR Am J Roentgenol. 2010 May;194(5):1404. AJR Am J Roentgenol 2010: 881–9. [DOI] [PubMed] [Google Scholar]
- 28.Scheffel H, Stolzmann P, Frauenfelder T, Schertler T, Desbiolles L, Leschka S, et al. Dual-energy contrast-enhanced computed tomography for the detection of urinary stone disease. Invest Radiol 2007; 42: 823–9. doi: 10.1097/RLI.0b013e3181379bac [DOI] [PubMed] [Google Scholar]
- 29.Chen C-Y, Tsai T-H, Jaw T-S, Lai M-L, Chao M-F, Liu G-C, et al. Diagnostic performance of Split-Bolus portal venous phase dual-energy CT urography in patients with hematuria. AJR Am of Roentgenol 2016; 206: 1013–22. doi: 10.2214/AJR.15.15112 [DOI] [PubMed] [Google Scholar]
- 30.Park JJ, Park BK, Kim CK. Single-phase DECT with VNCT compared with three-phase CTU in patients with haematuria. Eur Radiol 2016; 26: 3550–7. doi: 10.1007/s00330-016-4206-9 [DOI] [PubMed] [Google Scholar]
- 31.Xiang H, Chan M, Brown V, Huo YR, Chan L, Ridley L. Systematic review and meta-analysis of the diagnostic accuracy of low-dose computed tomography of the kidneys, ureters and bladder for urolithiasis. J Med Imaging Radiat Oncol 2017; 61: 582–90. doi: 10.1111/1754-9485.12587 [DOI] [PubMed] [Google Scholar]
- 32.Rob S, Bryant T, Wilson I, Somani BK. Ultra-low-dose, low-dose, and standard-dose CT of the kidney, ureters, and bladder: is there a difference? results from a systematic review of the literature. Clin Radiol 2017; 72: 11–15. doi: 10.1016/j.crad.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 33.Takahashi N, Kawashima A, Ernst RD, Boridy IC, Goldman SM, Benson GS, et al. Ureterolithiasis: can clinical outcome be predicted with unenhanced helical CT? Radiology 1998; 208: 97–102. doi: 10.1148/radiology.208.1.9646798 [DOI] [PubMed] [Google Scholar]
- 34.Takahashi S, Itoh T, Niikawa H, Shikata A, Murakami E, Tsunoda H, et al. Two-pass dual-energy CT imaging for simultaneous detection, characterization, and volume measurement of urinary stones with excretory-phase CT urography alone: a phantom study. Jpn J Radiol 2013; 31: 393–400. doi: 10.1007/s11604-013-0209-5 [DOI] [PubMed] [Google Scholar]