Key Points
Question
What is the association of mitral annular disjunction (MAD) with cardiovascular outcomes among patients with Marfan syndrome (MFS)?
Findings
In this cohort study of 142 patients with MFS, MAD was found in 34% and was associated with a higher need for mitral valve intervention, occurrence of arrhythmic events (defined as sustained ventricular tachycardia and sudden cardiac death), and, among patients with extensive MAD, more aortic events. In addition, ventricular arrhythmia, but not atrial arrhythmia, was more often observed among patients with MAD.
Meaning
This study suggests that reporting on the presence and extent of MAD should be considered in the routine cardiac evaluation of patients with MFS as a potential marker for adverse outcomes.
Abstract
Importance
Mitral annular disjunction (MAD) has received particular interest in patients with mitral valve prolapse, ventricular tachycardia, and sudden cardiac death. The clinical significance of MAD for patients with Marfan syndrome (MFS) remains largely unexplored.
Objective
To define the prevalence of MAD and examine its association with cardiovascular outcomes and arrhythmia among patients with MFS.
Design, Setting, and Participants
This retrospective, single-center cohort study included 142 patients with a diagnosis of MFS based on the revised Ghent criteria and a confirmed (likely) pathogenic variant in the FBN1 gene who underwent regular follow-up between January 1, 2004, and December 31, 2019.
Main Outcomes and Measures
The presence of MAD was assessed by echocardiography, and the extent of MAD was categorized in tertiles. Patients also underwent resting electrocardiography and 24-hour Holter monitoring. Outcomes included aortic events (aortic dissection or prophylactic aortic surgery), arrhythmic events (defined as sustained ventricular tachycardia or sudden cardiac death), and mitral valve surgery.
Results
A total of 142 patients (72 female patients [51%]; median age at first examination, 25 years [range, 2-64 years]) were evaluated. Forty-eight patients (34%) had MAD. Patients with MAD had larger aortic root z scores than patients without MAD (4.1 [interquartile range, 2.8-5.7] vs 3.0 [interquartile range, 1.8-4.0]; P < .001) and more often had mitral valve prolapse (34 of 48 [71%] vs 14 of 94 [15%]; P < .001), ventricular ectopy (14 of 33 [42%] vs 15 of 70 [21%]; P = .03), and nonsustained ventricular tachycardia (13 of 33 [39%] vs 12 of 70 [17%]; P = .01). During follow-up, aortic events occurred at similar rates among patients with vs without MAD (15 of 43 [35%] vs 21 of 84 [25%]; P = .24), but patients in the upper MAD tertile (>10 mm) showed a higher occurrence of aortic events compared with patients with MAD of 10 mm or smaller (9 of 15 [60%] vs 6 of 28 [21%]; P = .01). Patients with arrhythmic events (n = 5) and patients requiring mitral valve surgery (n = 7) were observed exclusively in the group displaying MAD.
Conclusions and Relevance
This study suggests that MAD among patients with MFS is associated with the occurrence of arrhythmic events, a higher need for mitral valve intervention, and, among patients with extensive MAD, more aortic events. Cardiac imaging for patients with MFS should consider the assessment of MAD as a potential marker for adverse outcomes.
This cohort study examines the prevalence of mitral annular disjunction and its association with cardiovascular outcomes and arrhythmia among patients with Marfan syndrome.
Introduction
Marfan syndrome (MFS) (OMIM 154700, ORPHA 558) is a heritable connective tissue disorder with a reported prevalence ranging from 1.5 to 17.2 per 100 000 individuals.1 Most patients with MFS carry a (likely) pathogenic variant in the fibrillin-1 (FBN1) gene (OMIM 134797), encoding the extracellular matrix glycoprotein fibrillin-1. The cardinal features of MFS occur in the cardiovascular, ocular, and skeletal organ systems. Aortic disease is the most common cardiovascular manifestation of MFS, presenting as aortic root dilatation, with aortic dissection and rupture as life-threatening complications.2 Other cardiovascular manifestations of MFS include mitral valve prolapse (MVP) (occurring in 28%-58% of patients) and myocardial dysfunction.3,4,5,6 In addition, ventricular arrhythmia has been increasingly reported in patients with MFS, presenting as significant ventricular ectopy, nonsustained ventricular tachycardia (NSVT), and sustained ventricular tachycardia (VT) or sudden cardiac death (SCD).7,8,9,10,11 Owing to the difficulties in predicting outcomes among patients with MFS, there is an unmet need for additional prognostic indicators. Recently, mitral annular disjunction (MAD), defined as a separation between the mitral valve hinge point and the left ventricular (LV) myocardium, in patients with or without MVP has received particular interest as a potential marker or substrate for ventricular arrhythmia and SCD.12,13,14,15,16 A recent report on aortic outcomes in a surgical population of patients with MFS suggests that aortic events occur at a younger age among patients with MAD.17 However, data on the prevalence of MAD and its association with cardiovascular outcomes and arrhythmia among a nonsurgical population of patients with MFS are lacking.
Methods
Study Design
We conducted a retrospective, single-center cohort study at the Ghent University Hospital among patients with a diagnosis of MFS based on the revised Ghent criteria2 who underwent regular follow-up between January 1, 2004, and December 31, 2019. Patients with suspected connective tissue disease (presenting with aortic aneurysms, dissections, and/or clinical signs suggestive of connective tissue disease) were genetically tested, and their family members were identified through cascade genetic screening. Patients with a (likely) pathogenic FBN1 gene variant were eligible for inclusion. Pediatric patients were defined as those younger than 16 years; patients with neonatal MFS were excluded. Follow-up was performed annually among patients with an aortic root diameter of less than 45 mm and semiannually among those with diameters between 45 and 50 mm. Echocardiographic, electrocardiographic, and 24-hour Holter monitoring data were collected. Clinical data obtained at the first and last patient visits were extracted from electronic medical records. The Ghent University Hospital Ethics Committee approved the study and waived the need for patient informed consent because the data were deidentified and the rights and welfare of the patients would not be adversely affected by the study.
Outcome
Outcome measures included aortic events, mitral valve surgery, arrhythmic events, and all-cause mortality. Aortic events were defined as aortic dissection (Stanford type A or B) and prophylactic aortic surgery. Indications for prophylactic surgery were standardized and in accordance with the European Society of Cardiology guidelines on aortic disease in all patients.18 Mitral events included any mitral valve surgery (isolated or in combination with aortic surgery). Arrhythmic events were defined as sustained VT and SCD. Sustained VT was defined as a documented wide complex (QRS duration >120 milliseconds) tachycardia at a rate of more than 100 beats per minute, lasting more than 30 seconds or accompanied by hemodynamic instability within 30 seconds. Sudden cardiac death was defined as a witnessed cardiac arrest or death within 1 hour after the onset of acute symptoms or an unexpected death in a patient known to have been well within the last 24 hours.19 Sudden cardiac death was considered arrhythmogenic if the results of an autopsy showed no signs of dissection or if other potential causes were deemed highly unlikely based on findings and the timing of the last visit.
Echocardiography
All patients underwent a (semi)annual transthoracic echocardiographic examination (GE Vivid systems; GE Healthcare). Image acquisitions and standard measurements were carried out by experienced echocardiographers in accordance with the guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging.20 Data on aortic diameters, valvular function, cardiac dimensions, and cardiac function were collected from the most recent patient visits (or from the last available examination prior to any surgery). Aortic diameters were measured in diastole using the leading edge–to–leading edge convention and are expressed as absolute values and z scores using the standardized reference values of Campens et al.21 For patients who underwent aortic surgery, the last available z score prior to surgery was considered for analysis, whereas for patients who did not undergo surgery, the z score from the most recent echocardiography was taken into account. The mean annual aortic root growth rate (in millimeters per year) was defined as the difference in the aortic root diameter between the first echocardiography and last echocardiography (prior to aortic surgery), divided by the time difference. Valvular regurgitation was graded by color Doppler imaging,22 and data were obtained prior to any valvular surgery. Mitral valve prolapse was defined as a mitral leaflet or scallop displacement of 2 mm or more beyond the mitral annulus toward the left atrium in systole.20 The anteroposterior mitral annular diameter was measured during end systole and end diastole in parasternal long-axis view.15 Left ventricular dimensions were expressed as values indexed for body surface area (BSA). Left ventricular ejection fraction (LVEF) was calculated according to the methods of Quinones et al23 (LVEF = LVEDD2 − LVESD2/LVEDD2 × 100 + k, where LVEDD is the LV end-diastolic diameter, LVESD is the LV end-systolic diameter, and k is correction for apical contraction).
The first available echocardiographic images were assessed (by A.D.) for the presence of MAD (EchoPAC, version 201; GE Healthcare). In our analyses, MAD was considered as both a qualitative and a quantitative trait: present or absent based on the presence or absence of a separation (≥3 mm) between the left atrial wall–mitral valve junction and the base of the LV wall (Video). Mitral annular disjunction distance was measured during end systole in parasternal long-axis views from the left atrial wall–mitral valve leaflet junction to the base of the LV posterolateral wall.13 The median value of 3 consecutive measurements was considered. The earliest echocardiographic images were compared with those obtained at the last patient visit (or prior to mitral valve surgery) to assess the evolution of MAD distance over time. Stratified random sampling was used to obtain 30 representative cases to assess the reproducibility of the MAD diagnosis and MAD distance measurements. A second observer (L.M.-M.) independently examined the sample population to assess the interobserver agreement.
Video. Mitral Annular Disjunction in Marfan Syndrome.
Parasternal long-axis view of an echocardiogram in a patient with Marfan syndrome presenting with mitral disjunction.
12-Lead Electrocardiography
The 12-lead electrocardiogram closest to the most recent echocardiography or prior to any cardiac surgery was reviewed. Time intervals were evaluated for diagnosis of conduction abnormalities. Baseline electrocardiographic characteristics included PR interval, QRS duration, and QT and QTc intervals (using the Bazett formula24).
24-Hour Holter Monitoring
Twenty-four–hour Holter monitoring was performed as clinically indicated or annually within a research context since 2016.11 Data were analyzed using 2 semiautomatic software packages (Philips DigiTrak XT; Philips and Trillium Platinum; Forest Medical). Premature atrial complexes (PACs), premature ventricular complexes (PVCs), and other arrhythmias were automatically identified and manually overread. Atrial ectopy was defined as more than 10 PACs, and ventricular ectopy was defined as more than 10 PVCs per hour.9 Atrial runs were defined as 3 or more consecutive premature atrial beats at a rate of more than 100 beats per minute, and NSVT was defined as 3 or more consecutive premature ventricular beats at a rate of more than 100 beats per minute. All 24-hour Holter monitoring reports were examined; the numbers of PACs, PVCs, atrial runs, and NSVT episodes observed were noted for each 24-hour Holter monitoring report, and the highest number was considered for analysis.
Genetic Variant Classification
Variants in the FBN1 gene were classified based on their alterations in DNA structure as missense (single-nucleotide change), nonsense (single-nucleotide change causing a premature stop-codon), frameshift (indels causing an alteration in the reading frame), splice-site variants (indels or single-nucleotide change in a place where splicing occurs), and in-frame variants (indels not causing alteration in the reading frame). Variants were also classified as dominant negative (leading to a shorter or a structurally abnormal but stable protein) or haploinsufficient (leading to the production of a reduced amount of normal fibrillin-1 derived from the nonvariant allele) depending on the anticipated effect at the protein level.11,25 Criteria for haploinsufficiency and for a dominant negative effect have been previously described.11 Variants affecting exons 24 to 32 and variants affecting cysteine (missense variants substituting or creating cysteine) were considered separately based on previous reports of genotype-phenotype correlations.
Statistical Analysis
Continuous data are reported as mean (SD) values or median values with interquartile ranges (IQRs). The Shapiro-Wilk test was used to test for normality. The Spearman test was used to assess correlations between MAD, age, and BSA. Comparisons between groups were performed using either the t test or the Mann-Whitney test. Categorical data were reported as proportions or percentages, and comparisons between groups were performed using the χ2 test or the Fisher exact test. The Wilcoxon signed rank test was chosen to compare MAD distance over time. Linear regression was used to assess indexed ventricular dimensions and ejection fraction. Interobserver agreement and intraobserver agreement on MAD classification were assessed using Cohen κ. The intraclass correlation coefficient was used to assess interobserver and intraobserver variability of MAD distance measurements. Statistical tests were 2 tailed, and P < .05 was considered statistically significant. Analyses were conducted using SPSS, version 26.0 (IBM Corp).
Results
For this study, 154 patients with MFS and regular follow-up between January 1, 2004, and December 31, 2019, were considered. Poor echocardiographic image quality resulted in the exclusion of 12 patients. A total of 142 patients (72 female patients [51%]; median age at first examination, 25 years [range, 2-64 years]) were selected for MAD assessment, of whom 48 (34%) were pediatric patients. Data on baseline characteristics and clinical evolution are shown in Table 1. The median follow-up was 12.0 years (IQR, 6.2-15.4 years), and the median time between the first available and the most recent echocardiographic images for MAD assessment was 5.8 years (IQR, 4.2-12.0 years). Fifteen patients (11%) had an aortic event prior to inclusion; 5 had aortic dissection, and 10 had prophylactic aortic surgery, resulting in available aortic root z scores for 127 patients (89%).
Table 1. Characteristics of Patients.
| Baseline characteristic | Total (N = 142) | Patients without MAD (n = 94) | Patients with MAD (n = 48) | P value |
|---|---|---|---|---|
| Age at first examination, median (IQR), y | 25 (12-35) | 28 (15-37) | 16 (8-32) | .005 |
| Pediatric patients, No. (%) | 48 (34) | 24 (26) | 24 (50) | .004 |
| Female, No. (%) | 72 (51) | 52 (55) | 20 (42) | .12 |
| Proband, No. (%) | 70 (49) | 43 (46) | 27 (56) | .24 |
| Baseline MAD distance, median (IQR), mm | 6 (4-12) | NA | 6 (4-12) | NA |
| Bicuspid aortic valve, No. (%) | 6 (4) | 4 (4) | 2 (4) | >.99 |
| Prophylactic aortic surgery prior to inclusion, No. (%) | 10 (7) | 6 (6) | 4 (8) | .73 |
| Aortic dissection prior to inclusion, No. (%) | 5 (4) | 4 (4) | 1 (2) | .66 |
| Type A dissection | 4 (3) | 3 (3) | 1 (2) | >.99 |
| Type B dissection | 1 (1) | 1 (1) | 0 | >.99 |
| Clinical evolution of the population | ||||
| Age at most recent examination, median (IQR), y | 35 (23-49) | 38 (26-50) | 27 (18-48) | .009 |
| Follow-up duration, median (IQR), y | 12.0 (6.2-15.4) | 11.9 (6.0-14.9) | 12.1 (6.5-15.5) | .71 |
| Prophylactic aortic surgery during follow-up, No. (%) | 30/27 (24) | 16/84 (19) | 14/43 (33) | .09 |
| Height, median (IQR), cm | 182 (174-191) | 183 (174-192) | 180 (174-188) | .30 |
| Weight, mean (SD), kg | 74 (21) | 79 (21) | 63 (18) | <.001 |
| BSA, median (IQR), m2 | 1.9 (1.7-2.1) | 2 (1.8-2.2) | 1.8 (1.6-1.9) | <.001 |
| Antihypertensive medication, No. (%) | ||||
| None | 31 (22) | 23 (25) | 8 (17) | .29 |
| β-Blocker | 100 (70) | 66 (70) | 34 (71) | .93 |
| ARB | 30 (21) | 19 (20) | 11 (23) | .71 |
| Other | 7 (5) | 5 (5) | 2 (4) | >.99 |
| Received at least one 24-h Holter monitoring, No. (%) | 103 (73) | 70 (75) | 33 (69) | .47 |
| Echocardiographic data (most recent or prior to surgery) | ||||
| LA diameter, median (IQR), mm | 34 (30-39) | 33 (30-37) | 34 (30-40) | .45 |
| LVESDi, median (IQR), mm/m2 | 17 (14-19) | 16 (14-17) | 19 (17-22) | <.001a |
| LVEDDi, median (IQR), mm/m2 | 25 (23-28) | 24 (22-27) | 29 (25-31) | <.001a |
| LVEF, median (IQR), % | 69 (63-74) | 69 (64-75) | 66 (60-71) | .03a |
| LVEF <55%, No. (%) | 10 (7) | 4 (4) | 6 (13) | .09 |
| MVP, No. (%) | 48 (34) | 14 (15) | 34 (71) | <.001 |
| Bileaflet MVP, No. (%) | 35 (25) | 7 (7) | 28 (58) | <.001 |
| MR severity, No. (%) | ||||
| None | 62 (44) | 52 (55) | 10 (21) | <.001 |
| Grade I or II | 71 (50) | 42 (45) | 29 (60) | |
| Grade III or IV | 9 (6) | 0 | 9 (19) | |
| Mitral annular diameter, mean (SD) | ||||
| End-systolic, mm | 34 (6) | 32 (5) | 38 (6) | <.001 |
| End-diastolic, mm | 32 (6) | 31 (5) | 34 (6) | .007 |
| Aortic root, median (IQR), mmb,c | 42 (37-46) | 41 (36-46) | 42 (37-48) | .34 |
| Aortic root z score, median (IQR)b | 3.3 (2.2-4.3) | 3.0 (1.8-4.0) | 4.1 (2.8-5.7) | <.001 |
| Aortic root z score >3, No. (%)b | 71/127 (56) | 41/84 (49) | 30/43 (70) | .02 |
| AR severity, No. (%)b | ||||
| None | 88/127 (69) | 59/84 (70) | 29/43 (67) | .47 |
| Grade I or II | 34/127 (27) | 23/84 (27) | 11/43 (26) | |
| Grade III or IV | 5/127 (4) | 2/84 (2) | 3/43 (7) |
Abbreviations: AR, aortic regurgitation; ARB, angiotensin receptor blocker; BSA, body surface area; IQR, interquartile range; LA, left atrium; LVEDDi, left ventricular end-diastolic diameter indexed; LVEF, left ventricular ejection fraction; LVESDi, left ventricular end-systolic diameter indexed; MAD, mitral annular disjunction; MR, mitral regurgitation; MVP, mitral valve prolapse; NA, not applicable.
Corrected for the presence of aortic and mitral valve regurgitation.
Excluding 15 patients with aortic events prior to inclusion.
Measured at the aortic sinus.
Diagnosis of MAD
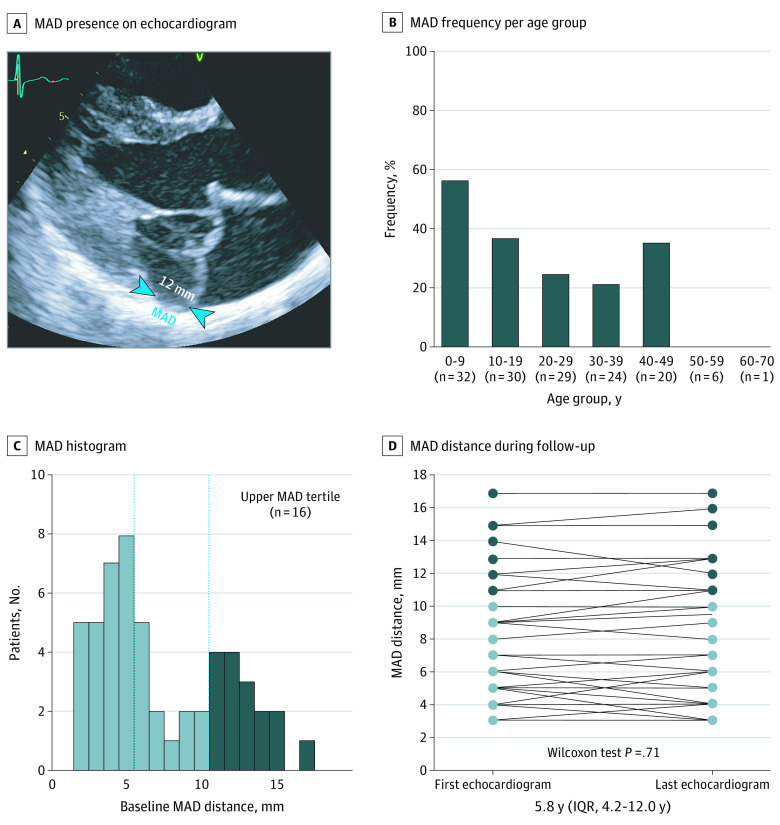
Mitral annular disjunction was present in 48 patients (34%) in the general cohort and was more frequent in pediatric patients than adult patients (24 of 48 [50%] vs 24 of 94 [26%]; P = .004) (Figure 1). Among probands (n = 70), pediatric patients more often had MAD compared with adult patients (14 of 24 [58%] vs 13 of 46 [28%]; P = .01). After excluding pediatric patients, the presence of MAD did not increase across age groups. No correlation was found between MAD distance and BSA (r = –0.004; P = .98) or MAD distance and age (r = 0.054; P = .72), even after excluding pediatric patients (MAD distance and BSA, r = 0.120; P = .58; MAD distance and age, r = –0.046; P = .83). No statistically significant increase in MAD distance was noted between the first and most recent echocardiographic images among the general cohort (median, 6 mm [IQR, 4-12 mm] vs 6 mm [IQR, 4-12 mm]; P = .71), pediatric patients (median, 6 mm [IQR, 5-10 mm] vs 6 mm [IQR, 5-10 mm]; P = .66), or patients requiring aortic surgery (median, 8 mm [IQR, 4-12 mm] vs 9 mm [IQR, 4-12 mm]; P = .71). Interobserver agreement and intraobserver agreement of MAD classification were high (eTable 1 in the Supplement). Both interobserver and intraobserver variability of measured MAD distance showed good concordance.
Figure 1. Mitral Annular Disjunction (MAD) in Patients With Marfan Syndrome.
A, Representative echocardiographic image of a patient with extensive MAD (blue arrowheads). B, Prevalence of MAD per age group: overall prevalence, 34%; adult (>16 years) prevalence, 26%; and pediatric prevalence, 50%. C, Histogram showing the frequency distribution of MAD distance. Dashed vertical lines indicate the tertile cutoff points. D, Evolution of MAD distance between initial and last echocardiography. IQR indicates interquartile range.
Characteristics of Patients With vs Without MAD
The characteristics of patients with vs without MAD are shown in Table 1. Patients with MAD had a younger median age at diagnosis (16 years [IQR, 8-32 years] vs 28 years [IQR, 15-37]), more frequent MVP (34 of 48 [71%] vs 14 of 94 [15%]; P < .001), more mitral regurgitation (MR) of any degree (38 of 48 [79%] vs 42 of 94 [45%]), larger mean (SD) mitral annular diameters (end-systolic diameter, 38 [6] vs 32 [5] mm; end-diastolic diamter, 34 [6] vs 31 [5] mm), increased median indexed LV diameters (also after adjusting for the presence of mitral and aortic valve regurgitation; end-systolic diameter, 19 mm/m2 [IQR, 17-22 mm/m2] vs 16 mm/m2 [IQR, 14-17 mm/m2]; end-diastolic diameter, 29 mm/m2 [IQR, 25-31 mm/m2] vs 24 mm/m2 [IQR, 22-27 mm/m2]), lower median LVEF (also after adjusting for the presence of mitral and aortic valve regurgitation; 66% [IQR, 60%-71%] vs 69% [IQR, 64%-75%]), and higher aortic root z scores (4.1 [IQR, 2.8-5.7] vs 3.0 [IQR, 1.8-4.0]; P < .001). The results of 12-lead electrocardiogram analysis showed no differences in PR interval, QRS duration, or QTc duration (eTable 2 in the Supplement). The presence of MAD was independent of any type of FBN1 variant and was not associated with predicted haploinsufficiency or a dominant negative effect at the protein level (eTable 3 in the Supplement).
Cardiovascular Outcomes
The results of cardiovascular outcomes are shown in Figure 2 and Table 2. During follow-up, aortic events occurred in 36 of 127 patients (28%); 30 of 127 (24%) underwent prophylactic aortic surgery, while 7 of 127 (6%) presented with aortic dissection (5 type A aortic dissections and 2 type B aortic dissections). One patient without MAD had prophylactic aortic surgery with type B dissection later. Aortic events occurred at similar rates among patients with vs patients without MAD (15 of 43 [35%] vs 21 of 84 [25%]; P = .24). No significant differences in the aortic root growth rate were found after adjusting for age at the initial echocardiography. With the categorization of patients in MAD distance tertiles (eFigure in the Supplement), patients in the upper tertile (MAD >10 mm [16 of whom 1 had an aortic event prior to inclusion]) showed larger aortic root z scores (4.7 [4.0-6.3] vs 3.8 [2.5-4.9]; P = .07) and an increased presence of aortic events (limited to prophylactic aortic surgery) (9 of 15 [60%] vs 6 of 28 [21%]; P = .01) compared with patients with MAD of 10 mm or smaller. Patients requiring mitral valve surgery (n = 7) were observed exclusively in the group with MAD (Figure 2).
Figure 2. Cardiovascular Outcomes.
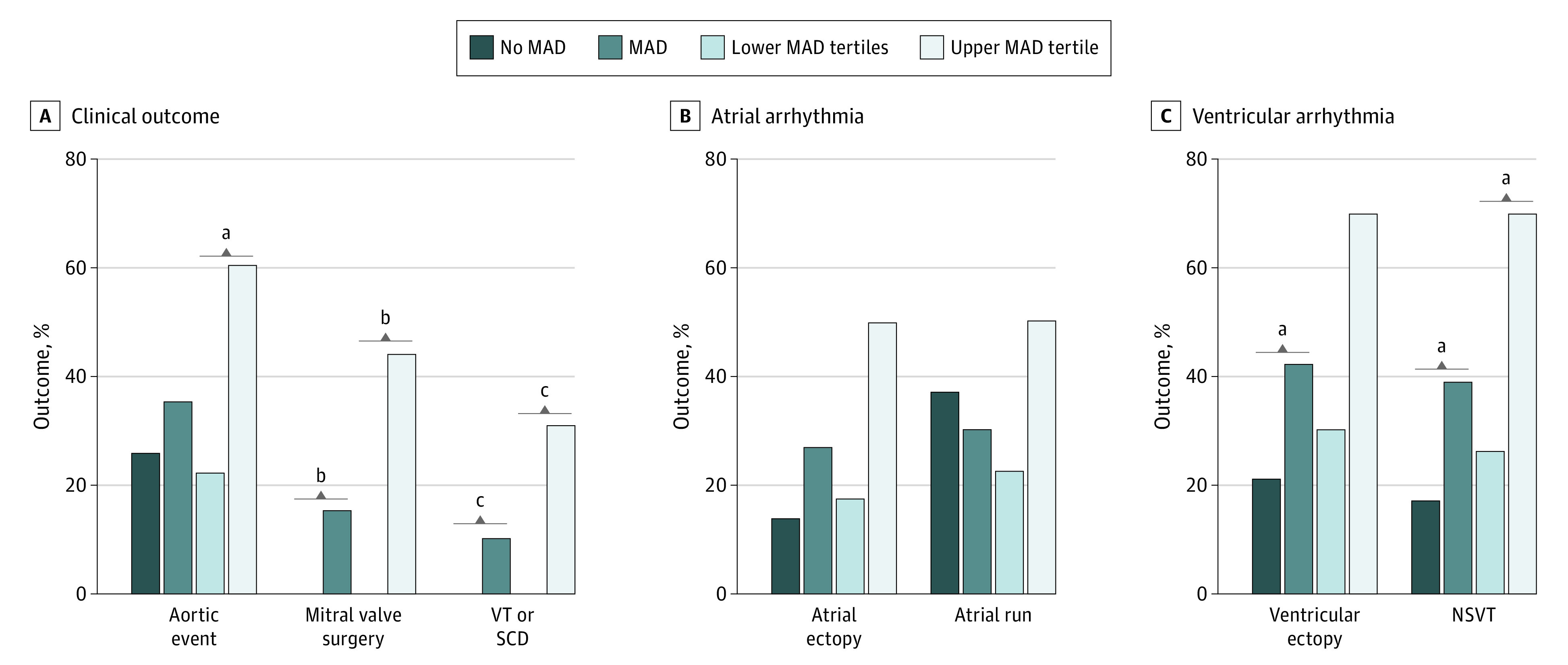
A, Clinical outcomes among patients with Marfan syndrome. B, Atrial arrhythmia observed during 24-hour Holter monitoring. Atrial ectopy was defined as more than 10 premature atrial complexes per hour, and an atrial run was defined as 3 or more consecutive premature atrial beats at a rate of more than 100 beats per minute. C, Ventricular arrhythmia observed during 24-hour Holter monitoring. Ventricular ectopy was defined as more than 10 premature ventricular complexes per hour, and nonsustained ventricular tachycardia (NSVT) was defined as 3 or more consecutive premature ventricular beats at a rate of more than 100 beats per minute. MAD indicates mitral annular disjunction; SCD, sudden cardiac death; and VT, ventricular tachycardia.
aP < .05.
bP < .001.
cP < .01.
Table 2. Cardiovascular Outcomes Observed During Follow-up.
| Cardiovascular outcome | Patients, No./total No. (%) | P value | ||
|---|---|---|---|---|
| Total (N = 142) | Without MAD (n = 94) | With MAD (n = 48) | ||
| Aortic root growth rate, median (IQR), mm/ya | 0.49 (0.19-1.13) | 0.45 (0.15-0.95) | 0.68 (0.27-1.57) | .14b |
| Aortic eventa | 36/127 (28) | 21/84 (25) | 15/43 (35) | .24 |
| Prophylactic aortic surgerya | 30/127 (24) | 16/84 (19) | 14/43 (33) | .09 |
| Aortic dissectiona | 7/127 (6) | 6/84 (7) | 1/43 (2) | .42 |
| Type A dissection | 5/127 (4) | 4/84 (5) | 1/43 (2) | .66 |
| Type B dissection | 2/127 (2) | 2/84 (2) | 0 | .55 |
| Mitral valve surgery, No. (%) | 7 (5) | 0 | 7 (15) | <.001 |
| Sustained VT and SCD, No. (%) | 5 (4) | 0 | 5 (10) | .004 |
| All-cause mortality, No. (%) | 9 (6) | 6 (6) | 3 (6) | >.99 |
Abbreviations: IQR, interquartile range; MAD, mitral annular disjunction; SCD, sudden cardiac death; VT, ventricular tachycardia.
Excluding 15 patients with aortic events prior to inclusion.
Corrected for age at first examination.
Arrhythmias
Arrhythmic events were observed in 5 of 142 patients (4%); sustained VT was documented for 3 patients, and SCD was documented for 2 patients. Sustained VT and SCD were confined to patients with MAD and occurred only among patients in the highest tertile of MAD severity. Patients with sustained VT and SCD showed no severe MR prior to the event, and MVP was not always present (eTable 4 in the Supplement). Four of the 5 patients had a history of aortic surgery prior to the arrhythmic event. Two of the 5 patients had a mildly reduced ejection fraction (47% and 52%) prior to the arrhythmic event. In the present study cohort, 103 patients (73%) received a median of 3 Holter recordings (IQR, 2-4 Holter recordings). The results are summarized in Table 3 and Figure 2. In total, 286 Holter recordings were performed; 43 patients (42%) received 77 clinically indicated Holter recordings (27%), and 60 patients (58%) received 209 Holter recordings (73%) within a research context. The most common clinical indication for Holter monitoring was palpitations (32 of 43 [74%]), which were more often reported among patients with MAD than among patients without MAD (20 of 23 [87%] vs 12 of 20 [60%]; P = .04). Patients with MAD were characterized by a similar burden of atrial ectopy, atrial couplets, and atrial runs. In contrast, in comparison with patients without MAD, those with MAD more often had ventricular ectopy (14 of 33 [42%] vs 15 of 70 [21%]; P = .03), ventricular couplets, and NSVT (13 of 33 [39%] vs 12 of 70 [17%]; P = .01).
Table 3. Data on 24-Hour Holter Monitoring.
| 24-h Holter monitoring | Total (N = 103) | Patients without MAD (n = 70) | Patients with MAD (n = 33) | P value |
|---|---|---|---|---|
| Holter recordings per patient, median (IQR), No. | 3 (2-4) | 3 (2-4) | 3 (2-4) | .29 |
| Patients with clinically indicated Holter monitoring, No. (%) | 43 (42) | 20 (29) | 23 (70) | <.001 |
| Clinical indication, No. (%) | ||||
| Palpitations | 32/43 (74) | 12/20 (60) | 20/23 (87) | .04 |
| Syncope | 2/43 (5) | 1/20 (5) | 1/23 (4) | >.99 |
| Vertigo or presyncope | 6/43 (14) | 5/20 (25) | 1/23 (4) | .08 |
| TIA | 3/43 (7) | 2/20 (10) | 1/23 (4) | .59 |
| Atrial arrhythmia | ||||
| AF, AFL, or AT, No. (%) | 11 (11) | 6 (9) | 5 (15) | .32 |
| Atrial ectopy, No. (%) | 19 (18) | 10 (14) | 9 (27) | .11 |
| PACs, median (IQR), No. | 25 (6-141) | 20 (4-95) | 27 (9-383) | .08 |
| PAC burden, median (IQR), % | 0 (0-0.1) | 0 (0-0.1) | 0 (0-0.3) | .09 |
| Presence of atrial runs, No. (%) | 36 (35) | 26 (37) | 10 (30) | .50 |
| Atrial runs, median (IQR), No. | 2 (1-3) | 2 (1-2) | 4 (1-6) | .06 |
| Longest atrial run, median (IQR), beats | 5 (3-6) | 4 (3-6) | 5 (4-7) | .32 |
| Ventricular arrhythmia | ||||
| Ventricular ectopy, No. (%) | 29 (28) | 15 (21) | 14 (42) | .03 |
| PVCs, median (IQR), No. | 24 (6-429) | 16 (4-210) | 107 (20-822) | .01 |
| PVC burden, median (IQR), % | 0 (0-0.4) | 0 (0-0.2) | 0.1 (0-0.8) | .048 |
| Presence of NSVT, No. (%) | 25 (24) | 12 (17) | 13 (39) | .01 |
| NSVT episodes, median (IQR), No. | 1 (1-4) | 1 (1-3) | 2 (1-8) | .61 |
| Longest NSVT episode, median (IQR), beats | 4 (3-6) | 4 (3-5) | 4 (3-6) | >.99 |
Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; IQR, interquartile range; MAD, mitral annular disjunction; NSVT, nonsustained ventricular tachycardia; PAC, premature atrial complex; PVC, premature ventricular complex; TIA, transient ischemic attack.
Discussion
In this study, we report the prevalence and extent of MAD and its associated cardiovascular outcomes in a large cohort of patients with MFS. We present novel findings on arrhythmia, including Holter monitoring data obtained in 73% of the cohort. Mitral annular disjunction was present in 34% of the cohort and was associated with the occurrence of sustained VT and SCD and a higher PVC burden. Atrial arrhythmia was not observed more frequently in patients with MAD. Aortic root z scores were higher among patients with MAD. We observed a higher need for mitral valve intervention among patients with MAD and no differences in aortic events, which corroborates the findings from a recent study by Chivulescu et al.17 However, our study did demonstrate differences from previous studies as well as new findings.
First, the availability of non–symptom-driven Holter data for most patients allowed for a more objective assessment of arrhythmia. Similar to previous reports on ventricular arrhythmia in patients with MFS, we observed NSVT in 25 of 103 patients (24%) and VT and SCD in 5 of 142 patients (4%).7,9,11,26,27 The pathophysiology of and the factors associated with ventricular arrhythmia in patients with MFS remain unclear. Mitral valve prolapse, mitral valve surgery, LV dimension, and N-terminal prohormone B-type natriuretic protein have been associated with ventricular arrhythmia in patients with MFS, but conflicting evidence exists on the association with MVP and MR.7,9,11,26,27 The lack of association between MVP and arrhythmic events is in line with Dejgaard et al13 reporting increased arrhythmic events among patients with MAD in the absence of MVP. Therefore, MAD appears to be more strongly associated with arrhythmic events than MVP. In the present study, arrhythmic events were observed exclusively among patients exhibiting extensive MAD (>10 mm). Although the extent of MAD has been associated with increased arrhythmic risk in MVP,12,13,14,15,28 additional studies are required to establish MAD distance thresholds for predicting arrhythmic events.13,14,15,29 Considering the pathophysiology of ventricular arrhythmia, MAD has been hypothesized to induce mechanical stress on the inferobasal myocardial wall and papillary muscles through the abnormal systolic curling motion of the subvalvular region of the LV free wall, eventually leading to myocardial hypertrophy and fibrosis, thereby adding to a proarrhythmogenic substrate.13,14,16 Perazzolo Marra et al14 have shown correlations between LV fibrosis in the papillary muscles and MAD on histologic and cardiovascular magnetic resonance (CMR) imaging, supporting this hypothesis. However, studies on arrhythmic MVP have also implicated other coexisting pathophysiological risk factors associated with malignant ventricular arrhythmia, making it difficult to consider MAD as a cause or a marker of disease, or both.12,30,31 Another potential predisposing factor could be cardiovascular surgery. It has been reported that some patients with MFS develop cardiomyopathy over time after undergoing cardiovascular surgery.32 In our cohort, 4 of the 5 patients with arrhythmic events had a history of aortic surgery, but it remains uncertain whether such interventions are associated with arrhythmogenesis.
Second, our study population was selected using diagnostic criteria and genetic testing for MFS in contrast to the surgically selected population from Chivulescu et al,17 in which nearly 70% of patients presented with aortic events. The observation of higher aortic z scores for patients with MAD vs those without MAD in our study is probably related to lower BSA and age in the MAD group. This finding was not observed in the study by Chivulescu et al17 and may be related to a more widespread phenotypic spectrum in our group, which is likely more representative of the general population with MFS. The fact that this difference is not reflected in a higher rate of aortic root surgery among patients with MAD is explained by the recommendation to use absolute aortic diameters and aortic growth to define surgical thresholds.
No differences in aortic events or aortic growth rate were seen in our cohort. In addition, there was no statistically significant difference in aortic dissections (6 of 7 dissections occurred among patients without MAD). Although patients in the upper MAD tertile (>10 mm) showed a higher prevalence of prophylactic aortic surgery, the low number of patients in this group precludes definite conclusions. Whether the severity of MAD may be associated with the need for prophylactic aortic surgery requires further investigation.
The observation that pediatric probands in our study more often showed MAD compared with adult probands suggests a more severe phenotype when receiving a diagnosis of MFS at a younger age and corroborates observations of more severe MFS phenotypes in probands who received a diagnosis in childhood. Another determinant of phenotypic severity may be the underlying genotype, as suggested in recent literature.33 Our results did not show an association between MAD and the underlying type or location of the FBN1 variant, which may also be related to the small sample sizes and requires confirmation in larger cohorts.
Mitral annular disjunction distance was independent of age or BSA and showed no significant increase over time, possibly related to a slow progression rate and/or echocardiographic variability. Mitral valve prolapse is frequently observed among patients with MFS,3 suggesting a potential association between MAD and connective tissue alterations. The pathophysiology of MAD and the association with MVP and MFS are still incompletely understood and warrant further investigation.
In our study, the presence and extent of MAD were strongly associated with the presence and severity of MR, and mitral valve surgery was confined to patients with MAD. This finding contrasts with a study from Konda et al34 reporting no association between the degree of MR and the presence of MAD in patients with MVP. However, differences in the presence and severity of MR between patients with and patients without MAD in both our study and the study by Chivulescu et al17 suggest that the association between MAD and MR may be more specific for MFS, perhaps showing more extensive MAD. Increasing MAD and associated changes in the mitral annular plane and annular dynamics, including paradoxical systolic expansion and flattening, have been demonstrated, thereby altering mitral coaptation and resulting in loss of the optimal mitral valve geometry.14,15,35 In the study by Lee et al,35 the extent of disjunction among patients with MAD correlated with MR severity assessed by the proximal isovelocity surface area method. This correlation may explain why MR is more common in the presence of (extensive) MAD. Patients with MAD also demonstrated increased LV diameters and reduced LVEF, even after adjusting for valvular regurgitation. Since primary myocardial dysfunction has been reported in patients with MFS,6 it remains to be investigated whether patients with MFS and MAD more often present with primary dysfunction of the myocardium.
Our results suggest that patients with MFS and significant MAD may require close clinical follow-up and Holter monitoring. A recent case report of a young patient with MFS presenting with extensive MAD, ventricular arrhythmia, and the need for mitral valve surgery is indicative of our findings.36 The presence of MAD should be reported in the routine cardiac evaluation, potentially requiring further assessment with CMR imaging. However, therapeutic implications remain unclear, and more evidence from outcome studies and risk stratification is mandatory.
Limitations
This study has several limitations. First, evident limitations related to a retrospective study design can be expected. Second, the frequency of certain observations was relatively low, impacting the robustness of the statistical analysis. Third, the Holter monitoring data did not allow for characterization of the morphologic characteristics and origin of PVCs, which may be associated with the prognostic significance of PVCs.16 Fourth, we did not report CMR imaging data on MAD because the image acquisitions were not available for all patients. Discrete MAD may not be identified by echocardiography,29 and CMR imaging may be required for a more careful assessment.13 However, Mantegazza et al37 demonstrated that the diagnostic accuracy of echocardiography improved with increasing MAD distance, suggesting that patients with more extensive MAD distance will be captured by echocardiography. Fifth, late gadolinium enhancement by CMR imaging was not performed; therefore, myocardial fibrosis was not assessed.
Conclusions
This study of patients with MFS suggests that the presence of MAD in such patients is associated with arrhythmic events and a higher need for mitral valve intervention, without significant differences in aortic events. For patients with extensive MAD, consistently worse cardiovascular outcomes were noted (ie, higher occurrence of aortic events, arrhythmic events, and the need for mitral valve surgery). Assessment of the presence and extent of MAD may be useful to better assess the risk of adverse cardiovascular outcomes among patients with MFS.
eFigure. Echocardiographic Examples of Mitral Annular Disjunction
eTable 1. Interobserver and Intraobserver Statistics
eTable 2. ECG Characteristics
eTable 3. Genotype Comparison
eTable 4. Clinical Characteristics of Patients With Sustained VT/SCD, Prior to the Arrhythmic Event
References
- 1.von Kodolitsch Y, De Backer J, Schüler H, et al. Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome. Appl Clin Genet. 2015;8:137-155. doi: 10.2147/TACG.S60472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476-485. doi: 10.1136/jmg.2009.072785 [DOI] [PubMed] [Google Scholar]
- 3.Rybczynski M, Mir TS, Sheikhzadeh S, et al. Frequency and age-related course of mitral valve dysfunction in the Marfan syndrome. Am J Cardiol. 2010;106(7):1048-1053. doi: 10.1016/j.amjcard.2010.05.038 [DOI] [PubMed] [Google Scholar]
- 4.Taub CC, Stoler JM, Perez-Sanz T, et al. Mitral valve prolapse in Marfan syndrome: an old topic revisited. Echocardiography. 2009;26(4):357-364. doi: 10.1111/j.1540-8175.2008.00825.x [DOI] [PubMed] [Google Scholar]
- 5.Mühlstädt K, De Backer J, von Kodolitsch Y, et al. Case-matched comparison of cardiovascular outcome in Loeys-Dietz syndrome versus Marfan syndrome. J Clin Med. 2019;8(12):E2079. doi: 10.3390/jcm8122079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alpendurada F, Wong J, Kiotsekoglou A, et al. Evidence for Marfan cardiomyopathy. Eur J Heart Fail. 2010;12(10):1085-1091. doi: 10.1093/eurjhf/hfq127 [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann BA, Rybczynski M, Rostock T, et al. Prospective risk stratification of sudden cardiac death in Marfan’s syndrome. Int J Cardiol. 2013;167(6):2539-2545. doi: 10.1016/j.ijcard.2012.06.036 [DOI] [PubMed] [Google Scholar]
- 8.Schaeffer BN, Rybczynski M, Sheikhzadeh S, et al. Heart rate turbulence and deceleration capacity for risk prediction of serious arrhythmic events in Marfan syndrome. Clin Res Cardiol. 2015;104(12):1054-1063. doi: 10.1007/s00392-015-0873-9 [DOI] [PubMed] [Google Scholar]
- 9.Yetman AT, Bornemeier RA, McCrindle BW. Long-term outcome in patients with Marfan syndrome: is aortic dissection the only cause of sudden death? J Am Coll Cardiol. 2003;41(2):329-332. doi: 10.1016/S0735-1097(02)02699-2 [DOI] [PubMed] [Google Scholar]
- 10.von Kodolitsch Y, Demolder A, Girdauskas E, et al. Features of Marfan syndrome not listed in the Ghent nosology—the dark side of the disease. Expert Rev Cardiovasc Ther. 2019;17(12):883-915. doi: 10.1080/14779072.2019.1704625 [DOI] [PubMed] [Google Scholar]
- 11.Muiño-Mosquera L, De Wilde H, Devos D, et al. Myocardial disease and ventricular arrhythmia in Marfan syndrome: a prospective study. Orphanet J Rare Dis. 2020;15(1):300. doi: 10.1186/s13023-020-01581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Essayagh B, Sabbag A, Antoine C, et al. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2020;76(6):637-649. doi: 10.1016/j.jacc.2020.06.029 [DOI] [PubMed] [Google Scholar]
- 13.Dejgaard LA, Skjølsvik ET, Lie ØH, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72(14):1600-1609. doi: 10.1016/j.jacc.2018.07.070 [DOI] [PubMed] [Google Scholar]
- 14.Perazzolo Marra M, Basso C, De Lazzari M, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9(8):e005030. doi: 10.1161/CIRCIMAGING.116.005030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound. 2010;8:53. doi: 10.1186/1476-7120-8-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basso C, Perazzolo Marra M, Rizzo S, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132(7):556-566. doi: 10.1161/CIRCULATIONAHA.115.016291 [DOI] [PubMed] [Google Scholar]
- 17.Chivulescu M, Krohg-Sørensen K, Scheirlynck E, et al. Mitral annulus disjunction is associated with adverse outcome in Marfan and Loeys-Dietz syndromes. Eur Heart J Cardiovasc Imaging. 2020;jeaa324. Published online December 6, 2020. doi: 10.1093/ehjci/jeaa324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erbel R, Aboyans V, Boileau C, et al. ; ESC Committee for Practice Guidelines; The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) . 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873-2926. doi: 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 19.Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. ; ESC Scientific Document Group . 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793-2867. doi: 10.1093/eurheartj/ehv316 [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233-270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 21.Campens L, Demulier L, De Groote K, et al. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am J Cardiol. 2014;114(6):914-920. doi: 10.1016/j.amjcard.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 22.Kamoen V, Calle S, El Haddad M, De Backer T, De Buyzere M, Timmermans F. Diagnostic and prognostic value of several color Doppler jet grading methods in patients with mitral regurgitation. Am J Cardiol. 2021;143:111-117. doi: 10.1016/j.amjcard.2020.12.027 [DOI] [PubMed] [Google Scholar]
- 23.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64(4):744-753. doi: 10.1161/01.cir.64.4.744 [DOI] [PubMed] [Google Scholar]
- 24.Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2(2):117-194. doi: 10.1111/j.1542-474x.1997.tb00325.x [DOI] [Google Scholar]
- 25.Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet. 2015;8(2):383-388. doi: 10.1161/CIRCGENETICS.114.000950 [DOI] [PubMed] [Google Scholar]
- 26.Aydin A, Adsay BA, Sheikhzadeh S, et al. Observational cohort study of ventricular arrhythmia in adults with Marfan syndrome caused by FBN1 mutations. PLoS One. 2013;8(12):e81281. doi: 10.1371/journal.pone.0081281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savolainen A, Kupari M, Toivonen L, Kaitila I, Viitasalo M. Abnormal ambulatory electrocardiographic findings in patients with the Marfan syndrome. J Intern Med. 1997;241(3):221-226. doi: 10.1046/j.1365-2796.1997.115125000.x [DOI] [PubMed] [Google Scholar]
- 28.Torras O, Hourdain J, Deharo JC, et al. Exhaustive echocardiographic phenotyping of mitral valve prolapse in a single center: “severe myxomatous mitral valve disease” as a specific entity? Arch Cardiovasc Dis Suppl. 2019;11(1):52. doi: 10.1016/j.acvdsp.2018.10.112 [DOI] [Google Scholar]
- 29.Essayagh B, Iacuzio L, Civaia F, Avierinos JF, Tribouilloy C, Levy F. Usefulness of 3-Tesla cardiac magnetic resonance to detect mitral annular disjunction in patients with mitral valve prolapse. Am J Cardiol. 2019;124(11):1725-1730. doi: 10.1016/j.amjcard.2019.08.047 [DOI] [PubMed] [Google Scholar]
- 30.Lancellotti P, Garbi M. Malignant mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9(8):e005248. doi: 10.1161/CIRCIMAGING.116.005248 [DOI] [PubMed] [Google Scholar]
- 31.Rybczynski M, Koschyk DH, Aydin MA, et al. Tissue Doppler imaging identifies myocardial dysfunction in adults with Marfan syndrome. Clin Cardiol. 2007;30(1):19-24. doi: 10.1002/clc.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hetzer R, Siegel G, Delmo Walter EM. Cardiomyopathy in Marfan syndrome. Eur J Cardio-thoracic Surg. 2016;49(2):561-568. doi: 10.1093/ejcts/ezv073 [DOI] [PubMed] [Google Scholar]
- 33.Arnaud P, Milleron O, Hanna N, et al. Clinical relevance of genotype-phenotype correlations beyond vascular events in a cohort study of 1500 Marfan syndrome patients with FBN1 pathogenic variants. Genet Med. Published online March 17, 2021. doi: 10.1038/s41436-021-01132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konda T, Tani T, Suganuma N, et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J Echocardiogr. 2017;15(4):176-185. doi: 10.1007/s12574-017-0349-1 [DOI] [PubMed] [Google Scholar]
- 35.Lee APW, Jin CN, Fan Y, Wong RHL, Underwood MJ, Wan S. Functional implication of mitral annular disjunction in mitral valve prolapse: a quantitative dynamic 3D echocardiographic study. JACC Cardiovasc Imaging. 2017;10(12):1424-1433. doi: 10.1016/j.jcmg.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 36.Motwani M, Venetucci L. Mitral annular disjunction arrhythmia syndrome in Marfan syndrome. Eur Heart J Case Rep. 2020;4(5):1-2. doi: 10.1093/ehjcr/ytaa306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantegazza V, Volpato V, Gripari P, et al. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart. 2020;107(1):25-32. doi: 10.1136/heartjnl-2020-317330 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Echocardiographic Examples of Mitral Annular Disjunction
eTable 1. Interobserver and Intraobserver Statistics
eTable 2. ECG Characteristics
eTable 3. Genotype Comparison
eTable 4. Clinical Characteristics of Patients With Sustained VT/SCD, Prior to the Arrhythmic Event