Key Points
Question
When compared with triptans, are 5-hydroxytryptamine1F receptor agonists (ie, ditans) and calcitonin gene-related peptide antagonists (ie, gepants) associated with reduced pain and fewer adverse events for acute management of migraine headache?
Findings
This systematic review and network meta-analysis including 64 randomized clinical trials with a total of 46442 participants found that triptans, ditans, and gepants were associated with reduced pain at 2 hours compared with placebo; however, most triptans were associated with reduced pain when compared with ditans and gepants. Ditans were associated with the highest risk of adverse events among all treatments, and certain triptans were associated with a higher risk of adverse events compared with gepants.
Meaning
Ditans and gepants were associated with less efficacy compared with triptans, whereas gepants were associated with fewer adverse events compared with triptans.
Abstract
Importance
New therapeutic classes of migraine-specific treatment have been developed, including 5-hydroxytryptamine1F receptor agonists (lasmiditan) and calcitonin gene-related peptide antagonists (rimegepant and ubrogepant).
Objective
To compare outcomes associated with the use of lasmiditan, rimegepant, and ubrogepant vs triptans for acute management of migraine headaches.
Data Sources
The Cochrane Register of Controlled Trials, Embase, and PubMed were searched from inception to March 5, 2020.
Study Selection
Double-blind randomized clinical trials examining current available migraine-specific acute treatments were included.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was applied to extract the data according to a predetermined list of variables of interest, and all network meta-analyses were conducted using a random-effects model.
Main Outcomes and Measures
The primary outcome was the odds ratio (OR) for freedom from pain (hereafter referred to as pain freedom) at 2 hours after the dose, and the secondary outcomes were ORs for pain relief at 2 hours after the dose and any adverse events.
Results
A total of 64 randomized clinical trials were included (46 442 participants; 74%-87% women; age range, 36-43 years). Most of the included treatments were associated with reduced pain at 2 hours compared with placebo. Most triptans were associated with higher ORs for pain freedom at 2 hours compared with lasmiditan (range: OR, 1.72 [95% CI, 1.06-2.80] to OR, 3.40 [95% CI, 2.12-5.44]), rimegepant (range: OR, 1.58 [95% CI, 1.07-2.33] to OR, 3.13 [95% CI, 2.16-4.52]), and ubrogepant (range: OR, 1.54 [95% CI, 1.00-2.37] to OR, 3.05 [95% CI, 2.02-4.60]). Most triptans were associated with higher ORs for pain relief at 2 hours compared with lasmiditan (range: OR, 1.46 [95% CI, 1.09-1.96] to OR, 3.31 [95% CI, 2.41-4.55]), rimegepant (range: OR, 1.33 [95% CI, 1.01-1.76] to OR, 3.01 [95% CI, 2.33-3.88]), and ubrogepant (range: OR, 1.38 [95% CI, 1.02-1.88] to OR, 3.13 [95% CI, 2.35-4.15]). The comparisons between lasmiditan, rimegepant, and ubrogepant were not statistically significant for both pain freedom and pain relief at 2 hours. Lasmiditan was associated with the highest risk of any adverse events, and certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any adverse events than the calcitonin gene-related peptide antagonists.
Conclusions and Relevance
For pain freedom or pain relief at 2 hours after the dose, lasmiditan, rimegepant, and ubrogepant were associated with higher ORs compared with placebo but lower ORs compared with most triptans. However, the lack of cardiovascular risks for these new classes of migraine-specific treatments may offer an alternative to triptans.
This systematic review and network meta-analysis of randomized clinical trials compares outcomes associated with the use of lasmiditan, rimegepant, and ubrogepant vs triptans for acute management of migraine headaches.
Introduction
Triptans, regarded as the standard of care for treating acute migraine, are selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonists that have replaced ergot preparations because these preparations have been associated with considerable adverse events (AEs) and limited benefits.1 However, only 27% to 30% of patients treated with triptans were pain free at 2 hours after the dose, and the discontinuation rate was up to 55.2% to 81.5%.1,2,3 A further concern is that frequent use of triptans may lead to headaches associated with medication overuse, which further points to the necessity of searching for alternative treatments. Moreover, triptans are contraindicated for patients with cardiovascular risks owing to 5-HT1B receptor–mediated vasoconstriction.
New drug discovery programs shifted the focus to the development of 2 classes of drugs: ditans, a group of antimigraine drugs targeting 5-hydroxytryptamine1F (5-HT1F) receptors, and gepants, a group of calcitonin gene-related peptide (CGRP) receptor antagonists. These 2 new generations of antimigraine drugs have demonstrated efficacy and tolerability for the acute treatment of migraine in either phase 2 or phase 3 randomized clinical trials (RCTs).4,5,6,7,8,9,10 A better understanding of the comparisons between these new treatments and currently available specific acute pharmacologic treatments may help clinicians in relevant decision-making and provide a guide for further studies. Nevertheless, uncertainties remain regarding the efficacy and safety of 5-HT1F receptor agonists and CGRP antagonists compared with existing migraine-specific acute medications. The aim of the present study was to perform a systematic review and network meta-analysis (NMA) to compare the benefits of 5-HT1F receptor agonists and CGRP antagonists with those of triptans for the treatment of acute migraine attacks.
Methods
Literature Search
The algorithm of the NMA follows the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline checklist.11 We conducted a search of the Cochrane Register of Controlled Trials, PubMed, and Embase without language restriction from the databases’ inception through March 5, 2020. The search strategy is illustrated in detail in the eAppendix in the Supplement. The present study has been registered in PROSPERO (CRD42021242145).
Study Selection
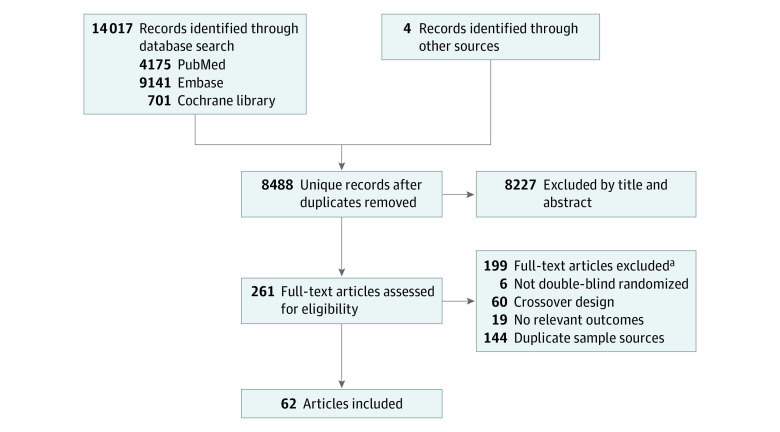
Three of us (C.-P.Y., C.-M.C., and K.-T.T.) independently assessed the titles and abstracts and retrieved the relevant full-text articles for final eligibility. Discrepancies between 2 of the authors regarding the eligibility of the full-text articles were resolved through discussion or consultation with the third author. Studies were included if they (1) were double-blind RCTs examining relevant clinical outcomes for participants aged 18 years or older, (2) included current available migraine-specific acute treatments (ergotamine, dihydroergotamine [DHE 45], sumatriptan [GR-43175], zolmitriptan [311C90], naratriptan [GR 85548A], rizatriptan [MK 0462], almotriptan, eletriptan [UK-166044], frovatriptan [VML-251, SB 209509], lasmiditan, rimegepant [BMS-927711], and/or ubrogepant [MK-1602]), (3) included comparisons between different specific monotherapies and/or placebo, and (4) used the International Headache Society criteria for a diagnosis of migraine.12 We excluded RCTs comparing only the same treatment via different administration routes (eg, oral sumatriptan vs inhaled sumatriptan). A total of 261 articles were considered eligible for a full-text review (Figure 1), and 62 articles met our inclusion criteria. A total of 64 trials were included because 2 articles provided the results of 2 studies each.13,14 The network graphs are shown in Figure 2.
Figure 1. Selection of Studies to be Included in the Network Meta-analysis.
A total of 64 trials were included because 2 articles provided the results of 2 studies each.13,14
aNumbers are not mutually exclusive.
Figure 2. Network Structures of the Outcomes.
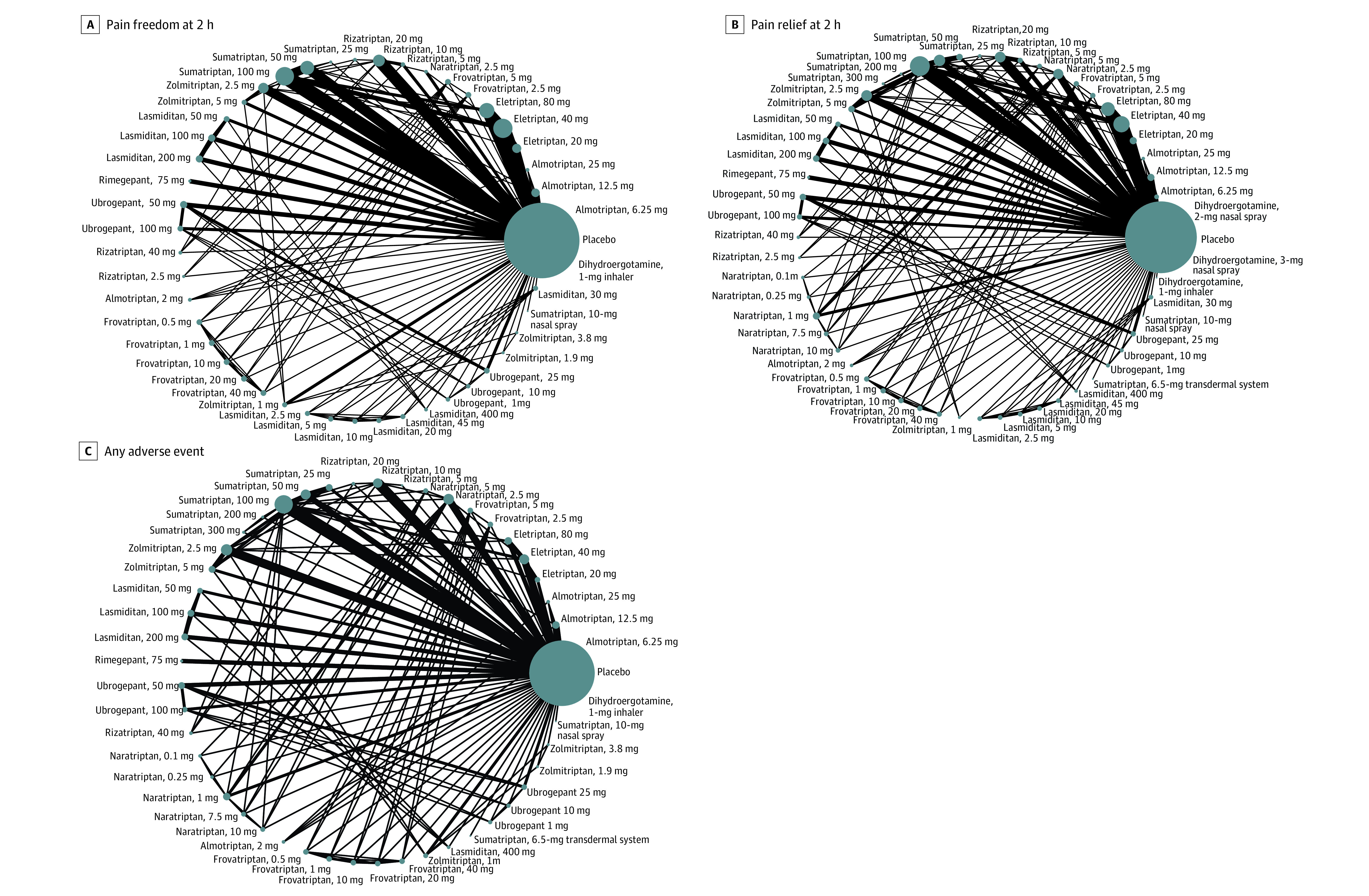
A, Primary outcome: pain freedom at 2 hours. B, Secondary outcome: pain relief at 2 hours. C, Secondary outcome: any adverse event. The lines between nodes represent direct comparisons in various trials, and the size of each circle is proportional to the size of the population involved in each specific treatment. The thickness of the lines is proportional to the number of trials connected to the network. One study was excluded from our analysis because it compared subcutaneous dihydroergotamine and subcutaneous sumatriptan and did not connect with the other studies in the network.
Data Collection and Outcomes
Two of us (C.-C.Y. and P.-H.S.) independently extracted data using a standardized form. Disagreements were resolved through consensus and, if necessary, consultation with a third reviewer (C.-S.L.). The primary outcome was freedom from pain (hereafter referred to as pain freedom; rated on a 4-point global scale) 2 hours after treatment. The secondary outcomes were (1) pain relief (no pain or mild pain, rated on a 4-point global scale) 2 hours after treatment and (2) tolerability, as determined by any AEs and withdrawal due to AEs.
Study Quality
Two of us (C.-P.Y. and C.-M.C.) independently assessed each trial based on version 2 of the Cochrane risk-of-bias tool for randomized trials.15 We compared the data extracted and resolved discrepancies through discussion or consultation with a third author (K.-T.T.).
Statistical Analysis
The statistical analysis was performed using Stata, version 14.0 (StataCorp) based on the frequentist models using the “network” command. For each specified outcome, we estimated the odds ratios (ORs) with 95% CIs with random-effects models. We ranked the treatment using the surface under the cumulative ranking curve (SUCRA), which is the cumulative relative probability of a treatment being the best option.16 The potential inconsistency of the model was evaluated using the loop-specific approach and the node-splitting method. The design-by-treatment model was applied to evaluate global inconsistency.
We also restricted our analysis to the doses that were in widespread clinical use: dihydroergotamine, 2-mg nasal spray; dihydroergotamine, 3-mg nasal spray; almotriptan, 6.25 mg; almotriptan, 12.5 mg; eletriptan, 20 mg; eletriptan, 40 mg; frovatriptan, 2.5 mg; naratriptan, 1 mg; naratriptan, 2.5 mg; rizatriptan, 5 mg; rizatriptan, 10 mg; sumatriptan, 10-mg nasal spray; sumatriptan, 50 mg; sumatriptan, 100 mg; zolmitriptan, 2.5 mg; lasmiditan, 50 mg; lasmiditan, 100 mg; rimegepant, 75 mg; ubrogepant, 50 mg; and ubrogepant, 100 mg.
We performed a sensitivity analysis after the exclusion of studies with a high risk of bias. We also performed a meta-regression of treatment efficacy by adjusting for the percentage of participants with severe headache at baseline among all studies with the percentage reported.
Results
Search Results and Study Characteristics
A total of 64 RCTs (46 442 participants) were included in the analysis (74%-87% women across studies; age range, 36-43 years). Most (59 [92%]) of the studies did not allow for the concurrent use of preventive medications. The details of the enrolled studies are shown in eTable 1 in the Supplement.
Efficacy Analysis
All specific treatments, in some doses, were associated with a higher OR for pain freedom at 2 hours compared with placebo (Figure 3A). For example, lasmiditan (dose range, 2.5-400 mg) was not associated with a higher OR for pain freedom in comparison with placebo when the dose was lower than 50 mg. For doses with widespread clinical use, all treatments were associated with a higher OR for pain freedom compared with placebo (range: OR, 1.65 [95% CI, 1.08-2.50]; lowest for lasmiditan, 50 mg] to OR, 5.59 [95% CI, 4.50-6.94]; highest for eletriptan, 40 mg]), except for sumatriptan, 10-mg nasal spray (eFigure 1A in the Supplement). Most of the triptans were also associated with a significantly higher OR compared with lasmiditan (range: OR, 1.72 [95% CI, 1.06-2.80] to OR, 3.40 [95% CI, 2.12-5.44]), rimegepant (range: OR, 1.58 [95% CI, 1.07-2.33] to OR, 3.13 [95% CI, 2.16-4.52]), and ubrogepant (range: OR, 1.54 [95% CI, 1.00-2.37] to OR, 3.05 [95% CI, 2.02-4.60]); however, the comparisons between lasmiditan, rimegepant, and ubrogepant were not significant (Figure 4). According to the SUCRA (eTable 2 in the Supplement), eletriptan, 40 mg, was ranked the best treatment for pain freedom, with a likelihood of pain freedom of 95.4%.
Figure 3. Forest Plots of the Outcomes.
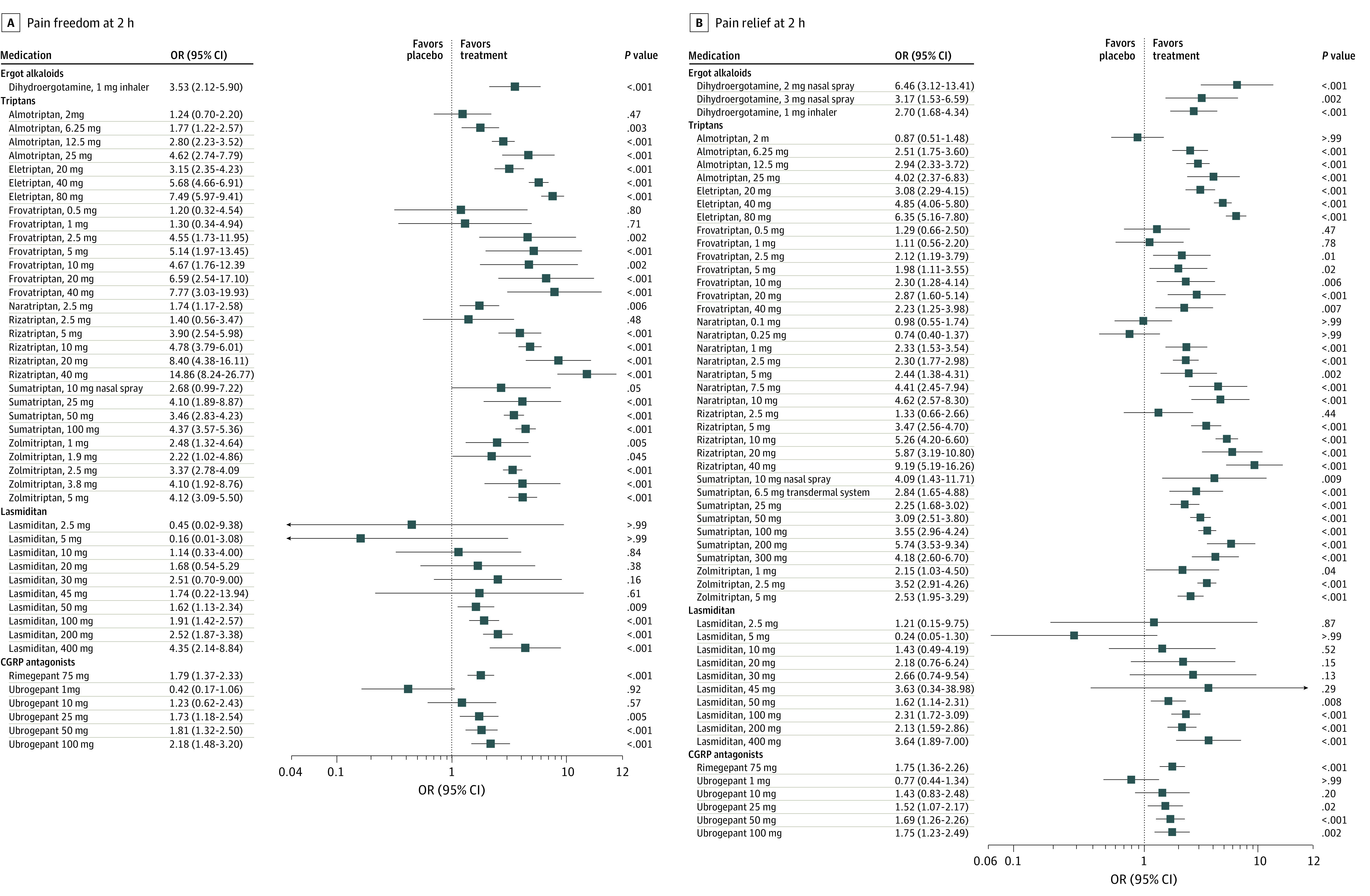
A, Primary outcome: pain freedom at 2 hours. B, Secondary outcomes: pain relief at 2 hours. CGRP indicates calcitonin gene-related peptide. Error bars indicate 95% CI.
Figure 4. Specific Antimigraine Treatment in Dosages With Widespread Clinical Use as Regards to Pain Freedom and Pain Relief at 2 Hours.
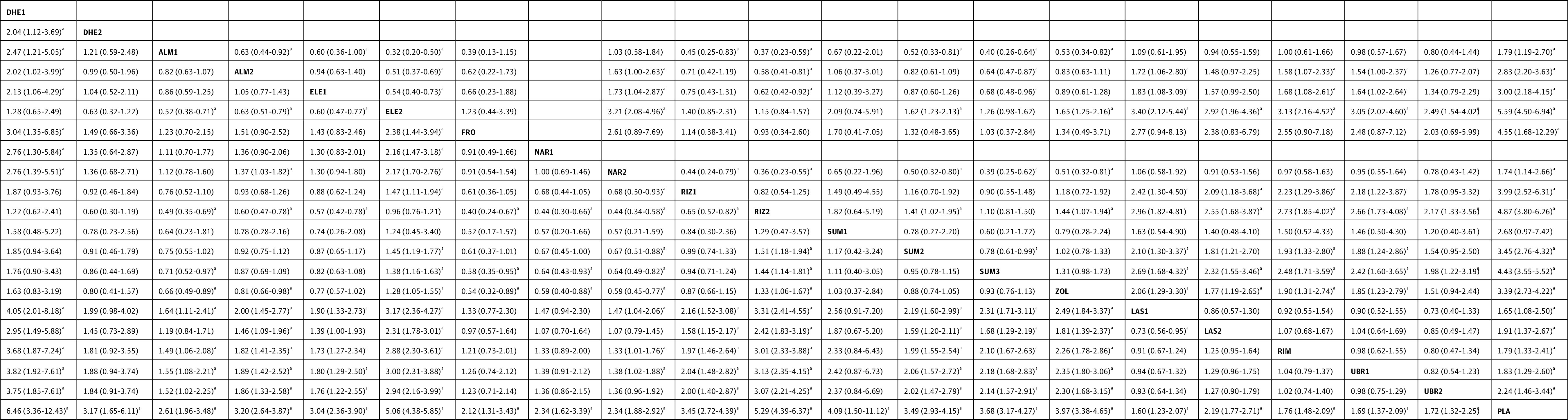
Results of pain relief at 2 hours are presented in the left lower half and results of pain freedom at 2 hours in the upper right half, if available. Comparisons between treatment must be read from left to right. In the left lower half (pain relief), the estimates (odds ratio with 95% CI) higher than 1 favor the column-defining treatment. In the right upper half (pain freedom), the estimates higher than 1 favor the row-defining treatment. ALM1 indicates almotriptan, 6.25 mg; ALM2, almotriptan, 12.5 mg; DHE1, dihydroergotamine, 2 mg nasal spray; DHE2, dihydroergotamine, 3 mg nasal spray; ELE1, eletriptan, 20 mg; ELE2, eletriptan, 40 mg; FRO, frovatriptan, 2.5 mg; NAR1, naratriptan, 1 mg; LAS1, lasmiditan, 50 mg; LAS2, lasmiditan, 100 mg; NAR2, naratriptan, 2.5 mg; PLA, placebo; RIM, rimegepant, 75 mg; RIZ1, rizatriptan, 5 mg; RIZ2, rizatriptan, 10 mg; SUM1, sumatriptan, 10 mg nasal spray; SUM2, sumatriptan, 50 mg; SUM3, sumatriptan, 100 mg; UBR1, ubrogegant, 50 mg; UBR2, ubrogepant, 100 mg; and ZOL, zolmitriptan, 2.5 mg.
aP < .05.
For pain relief at 2 hours, we excluded 1 study from our analysis because it compared subcutaneous dihydroergotamine with subcutaneous sumatriptan but did not connect with the other studies in the network.17 All specific treatments were associated with a higher OR for pain relief compared with placebo (Figure 3B). Rizatriptan, 40 mg, was associated with the highest OR in pain relief (9.19 [95% CI, 5.19-16.26]). For doses with widespread clinical use, all treatments were associated with a higher OR compared with placebo (range: OR, 1.60 [95% CI, 1.23-2.07]; lowest for lasmiditan, 50 mg] to OR, 6.46 [95% CI, 3.36-12.43]; highest for dihydroergotamine, 2-mg nasal spray]) (eFigure 1B in the Supplement). Triptans were associated with significantly higher ORs than lasmiditan (range: OR, 1.46 [95% CI, 1.09-1.96] to OR, 3.31 [95% CI, 2.41-4.55]), rimegepant (range: OR, 1.33 [95% CI, 1.01-1.76] to OR, 3.01 [95% CI, 2.33-3.88]), and ubrogepant (range: OR, 1.38 [95% CI, 1.02-1.88] to OR, 3.13 [95% CI, 2.35-4.15]) (Figure 4); however, the comparisons between lasmiditan, rimegepant, and ubrogepant were not significant for pain relief.
Tolerability Analysis
Based on our NMA, lasmiditan, 400 mg, was associated with the highest OR for any AEs (9.66 [95% CI, 4.03-23.16]) compared with placebo (eFigure 2 in the Supplement), and most triptans were associated with higher ORs for any AEs with a trend of dose-response relationship, including eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan. Dihydroergotamine, almotriptan, rimegepant, and ubrogepant were not associated with a higher OR compared with placebo. Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%; eTable 3 in the Supplement). Chest symptoms, including chest pain, tightness, heaviness, and pressure, accounted for 0% to 20% of the AEs for each specific treatment (eTable 4 in the Supplement). In the analysis of treatment doses in widespread clinical use, lasmiditan, 50 mg and 100 mg, were associated with higher ORs for any AEs than most of the other treatments (OR, 3.12 [95% CI, 1.86-5.24] and OR, 4.30 [95% CI, 2.80, 6.58]; eFigure 1C in the Supplement), and certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher OR for any AEs than the CGRP antagonists (OR, 1.96 [95% CI, 1.14-3.35] for rizatriptan; OR, 1.83 [95% CI, 1.09-3.09] for sumatriptan; and OR, 2.34 [95% CI, 1.39-3.95] for zolmitriptan; Figure 5). According to the SUCRA, lasmiditan, 100 mg, was most likely to be associated with the highest OR with respect to any AEs (95.1% likelihood; eTable 2 in the Supplement).
Figure 5. Specific Antimigraine Treatment in Dosages With Widespread Clinical Use as Regards to Adverse Events.
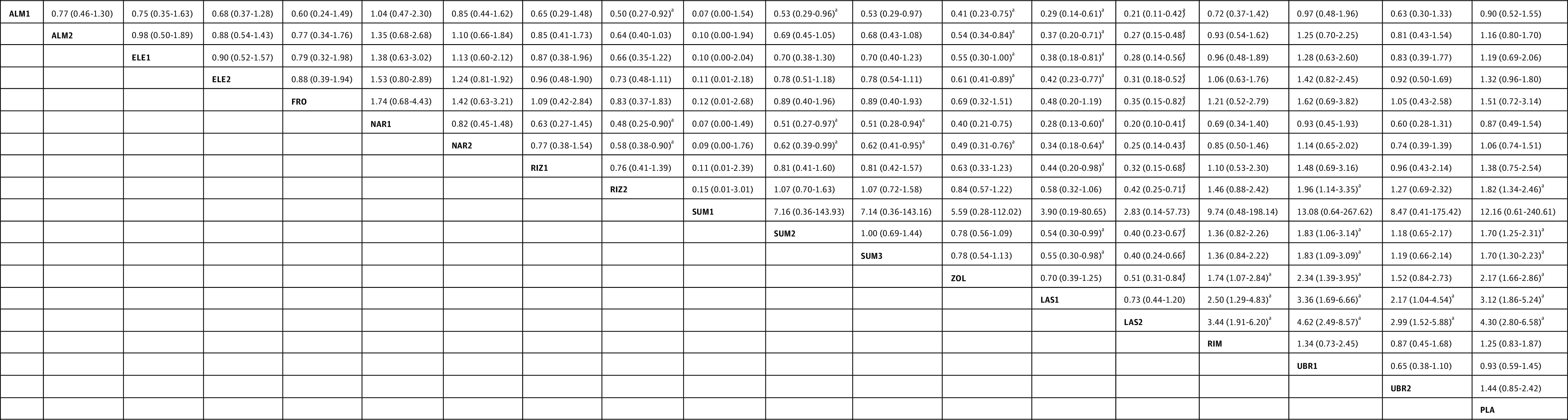
Comparisons between treatment must be read from left to right and the estimates (odds ratio with 95% CI) lower than 1 favor column-defining treatment. ALM1 indicates almotriptan, 6.25 mg; ALM2, almotriptan, 12.5 mg; ELE1, eletriptan, 20 mg; ELE2, eletriptan, 40 mg; FRO, frovatriptan, 2.5 mg; LAS1, lasmiditan, 50 mg; LAS2, lasmiditan, 100 mg; NAR1, naratriptan, 1 mg; NAR2, naratriptan, 2.5 mg; PLA, placebo; RIM, rimegepant, 75 mg; RIZ1, rizatriptan, 5 mg; RIZ2, rizatriptan, 10 mg; SUM1, sumatriptan, 10 mg nasal spray; SUM2, sumatriptan, 50 mg; SUM3, sumatriptan, 100 mg; UBR1, ubrogegant, 50 mg; UBR2, ubrogepant, 100 mg; and ZOL, zolmitriptan, 2.5 mg.
aP < .05.
Risk of Bias
As shown in eFigure 3 in the Supplement, we found that 6.3% of the included studies (4 of 64) had overall risks of bias determined to be low, 87.5% (56 of 64) had overall risks of bias determined to be of some concern, and 6.3% (4 of 64) had overall risks of bias determined to be high. Selection of the reported results and the randomization process were the domains that may be of concern in most studies.
Sensitivity Analyses
After exclusion of studies with a high risk of bias, most of the results were similar, except that lasmiditan was associated with AEs at lower ORs (eg, lasmiditan, 50 mg: OR, 2.31 [95% CI, 1.34-3.99]; lasmiditan, 100 mg: OR, 3.52 [95% CI, 2.31-5.39]). After adjustment for the percentage of patients with severe headache at baseline, the efficacy of dihydroergotamine, 2-mg nasal spray, and the efficacy of rizatriptan, 5 mg and 10 mg, regarding pain relief at 2 hours were attenuated (OR, 2.98 [95% CI, 1.73-5.15] for dihydroergotamine, 2-mg nasal spray; OR, 1.05 [95% CI, 1.01-1.08] for rizatriptan, 5 mg; and OR, 1.33 [95% CI, 0.42-4.16] for rizatriptan, 10 mg), whereas the efficacy of CGRP antagonists regarding pain relief at 2 hours was enhanced (range: OR, 1.00 [95% CI, 0.97-1.02] to OR, 9.46 [95% CI, 5.40, 16.57]; eFigure 4 in the Supplement).
Publication Bias and Inconsistency
Funnel plots revealed general symmetry (eFigure 5 in the Supplement). Results of the Egger tests (eFigure 6 in the Supplement) indicated significant publication bias in the analysis of pain freedom. There were 2 triangular loops, with loop-specific inconsistencies with regard to pain relief (placebo-zolmitriptan 2.5 mg-zolmitriptan 5 mg and placebo-sumatriptan 50 mg-zomitriptan 2.5mg; eTable 5 in the Supplement). Some side-splitting inconsistencies were also noted, especially for pain relief (eTable 6 in the Supplement). We conducted a sensitivity analysis by removing studies that seemed to introduce inconsistencies, and the results were similar. There was no significant global inconsistency in the design-by-treatment model (eTable 7 in the Supplement).
Discussion
To our knowledge, this is the first NMA addressing the outcomes of 5-HT1F receptor agonists (lasmiditan) and CGRP antagonists (rimegepant and ubrogepant) compared with current specific acute medications for the treatment of acute migraine attacks. We found that most of the triptans were associated with higher ORs for pain freedom or pain relief at 2 hours compared with the 5-HT1F receptor agonist and the CGRP antagonists, whereas the comparisons between lasmiditan, rimegepant, and ubrogepant were not significantly different. For tolerability, lasmiditan was associated with the highest risk of any AEs among all treatments, and certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs than rimegepant and ubrogepant. However, most of the AEs were mild to moderate.
To date, few RCTs have compared 5-HT1F receptor agonists or CGRP antagonists with triptans, to our knowledge. A major strength of our study is the use of an NMA, which is unique for estimating multiple direct and indirect comparisons, ranking the efficacy and safety of individual pharmacologic interventions and providing more precise estimates than those of RCTs and traditional meta-analyses.18,19 Our findings provide some reference for clinical applications in the acute treatment of migraine. First, although the 5-HT1F receptor agonist lasmiditan and the CGRP antagonists rimegepant and ubrogepant have enriched the therapies available for acute migraine treatment, most triptans are associated with higher ORs for pain freedom or pain relief at 2 hours compared with these 2 new classes of antimigraine drugs, which may imply that triptans will remain the current mainstay of specific acute migraine treatment. Second, although these 2 new classes of antimigraine drugs may not be as efficacious as triptans, these novel abortive agents without cardiovascular risks might offer an alternative to current specific migraine treatments for patients at risk of cardiovascular disease. Third, successful treatment with the 5-HT1F receptor agonist and the CGRP antagonists compared with placebo reveals that vasoconstriction is not essential for antimigraine therapy, which suggests a direction for future pharmaceutical development of specific acute migraine treatment.
According to the latest International Headache Society Guidelines (2019)20 for controlled trials of acute treatment of migraine attacks in adults, the primary end point to determine effectiveness should be either pain freedom at 2 hours or the absence of the most bothersome migraine-associated symptom at 2 hours as a coprimary end point. These end points also meet patients’ expectations for rapid and complete relief of headache and bothersome migraine-associated symptoms. Because none of the included earlier triptan studies evaluated freedom from migraine-associated symptoms as an outcome, we selected pain freedom at 2 hours after treatment as the primary end point. In the present study, the NMA showed that eletriptan, 40 mg, was associated with the best therapeutic efficacy in pain freedom at 2 hours. Eletriptan is a 5-HT1B/1D/1F–selective receptor agonist with a higher affinity for receptors than other triptans.21 In addition, the structural design of eletriptan allows for more rapid and stable absorption and enables the drug to cross the blood-brain barrier. Owing to its enhanced hydrophobicity, eletriptan has also been reported to have a higher bioavailability and longer plasma half-life than other triptans.22 The ability of eletriptan to cross the blood-brain barrier also explained its better efficacy and faster and more consistent absorption than sumatriptan and other triptans.23 Ergot derivatives represent another specific migraine treatment. Our study showed that dihydroergotamine, 2-mg nasal spray, was associated with the best therapeutic efficacy in terms of pain relief at 2 hours; however, the acute and long-term AEs of ergot derivatives have resulted in their being replaced by triptans and not recommended for routine use.24,25
Lasmiditan, a novel selective 5-HT1F receptor agonist, has demonstrated significant efficacy in phase 2 and 3 clinical trials and was approved as 50-mg and 100-mg tablets by the US Food and Drug Administration (FDA) in October 2019 for the acute treatment of migraine in adults with or without aura.4,5 The findings of our NMA found that lasmiditan was associated with higher ORs of pain freedom and pain relief at 2 hours compared with placebo; however, lasmiditan was associated with the highest risk of any AEs. This result was consistent with that of a previous meta-analysis, demonstrating a relatively high incidence of central nervous system–related AEs (especially dizziness, nausea, and fatigue) in individuals taking lasmiditan.26 However, the AEs were tolerable and were not considered serious. In fact, central nervous system–related AEs appeared to be dose related and were reported in all published studies addressing lasmiditan.4,5 These AEs might be associated with the drug’s lipophilic structure, leading to high permeability through the blood-brain barrier.26 Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan.
The pathogenesis of migraine is multifactorial, and CGRP is now considered a key element in the pathophysiology of migraine. A total of 6 different small-molecule CGRP receptor antagonists were created for the acute treatment of migraine attacks. Four gepants (olcegepant, telcagepant, MK-3207, and BI 44370) were discontinued primarily for either safety concerns due to liver toxicity or poor oral availability.27,28 The current NMA included ubrogepant and rimegepant, which received US FDA approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively.29,30 The findings of our NMA showed that these 2 novel medications were efficacious for the acute treatment of migraine compared with placebo; however, these 2 medications are associated with lower ORs for pain freedom or pain relief compared with certain triptans at 2 hours after the dose. The issue has been widely discussed, and some investigators noted a late benefit, in the 3-hour to 8-hour postdose period, for ubrogepant and rimegepant because of their relatively long half-lives (5-7 hours and 10-12 hours, respectively), which could not be demonstrated in these included studies.31,32 Triptans appeared to be associated with higher risks of any AEs than rimegepant and ubrogepant. Therefore, future studies are encouraged to compare gepants with triptans in efficacy at a later point, as well as tolerability.
One hypothesis suggests that acute treatment failure leads to more frequent migraine attacks as well as greater disability, which may lower the threshold for subsequent attacks through a neuroplastic mechanism.33 In fact, ineffective acute treatment is associated with a 2-fold increased risk of new-onset chronic migraine compared with effective acute treatment.34 In the US, 1 large survey revealed that 19.2% of individuals with episodic migraine used specific treatment (triptans, 18.7%; ergotamine, 0.5%), and 11.1% of individuals routinely used opioids for migraine, whereas 6% used compounds with barbiturates.35 However, a specific treatment was used by 22% of individuals with chronic migraine, and 34.3% of individuals used opioids and barbiturates.35 Opioids and barbiturates are not evidence-based treatments and are therefore not recommended by the US Headache Consortium Guideline.36 However, the use of these 2 classes of drugs for migraine seems to be disproportionately high.35,36 For patients who did not respond to current available abortive treatment and turned to barbiturate or opioid misuse, these new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction.
Limitations
This study has some limitations. First, we focused only on short-term headache responses and AEs after a single dose during the double-blinded period. The long-term safety and tolerability of gepants and ditans remain to be investigated in future clinical studies. Second, although all included trials used similar criteria based on the International Headache Society guidelines, some differences in study design were present (eg, the presence or absence of migraine aura and the concomitant use of preventive medications). As such, the findings of this study should be interpreted with caution. Third, most studies presented results based on a single migraine attack, and information about the consistency of the medications’ effectiveness when used for repeated attacks within a longer period is unknown. Further studies are needed to evaluate the outcomes of these medications, such as decreased response over time, the potential occurrence of medication overuse headaches, and AEs with repeated use. Fourth, although NMA allows comparisons between different treatments, the results still depend on the quality and heterogeneity of the included studies. The proportion of studies with an overall high risk of bias is low in the present study. In addition, we obtained similar results after exclusion of these studies. Fifth, indirect comparisons in the NMA could not replace direct comparisons between treatments. Future studies are needed for head-to-head comparisons between different drugs.
Conclusions
This systematic review and meta-analysis compared new pharmacologic agents with triptans for migraine treatment. For pain freedom and pain relief at 2 hours after the dose, lasmiditan, rimegepant, and ubrogepant were associated with a higher OR compared with placebo, but with a lower OR compared with most triptans. However, the lack of cardiovascular risks of these new classes of migraine-specific treatments may provide alternative treatment options for individuals for whom currently available acute treatments have failed or for those with cardiovascular contraindications.
eAppendix. Search Strategies
eTable 1. Study Characteristics
eReferences.
eTable 2. SUCRA of Outcomes for Anti-Migraine Specific Treatments in Dosages With Widespread Clinical Use
eTable 3. The Percentage of Serious Adverse Events in All Adverse Events With Respect to Specific Anti-Migraine Treatment
eTable 4. The Percentage of Chest Symptoms in all Adverse Events With Respect to Specific Anti-Migraine Treatment
eTable 5. Significant Loop-Specific Inconsistencies of Network Meta-Analysis
eTable 6. Significant Side-Splitting Inconsistencies of Network Meta-Analysis
eTable 7. Design‐by‐Treatment Interaction Model for Inconsistency of Network Meta-Analysis
eFigure 1A. Forest Plot of the Primary Outcome: Pain Freedom at 2 Hours for Specific Anti-Migraine Treatments in Dosages With Widespread Clinical Use
eFigure 1B. Forest Plot of the Secondary Outcome: Pain Relief at 2 Hours for Specific Anti-Migraine Treatments in Dosages With Widespread Clinical Use
eFigure 1C. Forest Plot of the Secondary Outcome: Adverse Events for Specific Anti-Migraine Treatments in Dosages With Widespread Clinical Use
eFigure 2. Forest Plot of the Secondary Outcome: Adverse Events
eFigure 3. Risk of Bias
eFigure 4A. Forest Plot of the Primary Outcome: Pain Freedom at 2 Hours for Specific Anti-Migraine Treatments After Adjustment for the Percentage of Severe Headache at Baseline
eFigure 4B. Forest Plot of the Secondary Outcome: Pain Relief at 2 Hours for Specific Anti-Migraine Treatments After Adjustment for the Percentage of Severe Headache at Baseline
eFigure 5A. Funnel Plot of the Primary Outcome: Pain Freedom at 2 Hours for Specific Anti-Migraine Treatments
eFigure 5B. Funnel Plot of the Secondary Outcome: Pain Relief at 2 Hours for Specific Anti-Migraine Treatments
eFigure 5C. Funnel Plot of the Secondary Outcome: Adverse Events for Specific Anti-Migraine Treatments
eFigure 6A. Egger’s Regression of All Specific Anti-Migraine Treatments for the Primary Outcome: Pain Freedom at 2 Hours
eFigure 6B. Egger’s Regression of All Specific Anti-Migraine Treatments for the Secondary Outcome: Pain Relief at 2 Hours
eFigure 6C. Egger’s Regression of All Specific Anti-Migraine Treatments for the Secondary Outcome: Adverse Events
References
- 1.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22(8):633-658. doi: 10.1046/j.1468-2982.2002.00404.x [DOI] [PubMed] [Google Scholar]
- 2.Dodick DW. Migraine. Lancet. 2018;391(10127):1315-1330. doi: 10.1016/S0140-6736(18)30478-1 [DOI] [PubMed] [Google Scholar]
- 3.Chen TB, Chen YT, Fuh JL, Tang CH, Wang SJ. Treatment adherence among new triptan users: a 2-year cohort study in Taiwan. J Headache Pain. 2014;15:48. doi: 10.1186/1129-2377-15-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Wietecha LA, Dennehy EB, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894-1904. doi: 10.1093/brain/awz134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB; COL MIG-301 Study Group . Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91(24):e2222-e2232. doi: 10.1212/WNL.0000000000006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381(23):2230-2241. doi: 10.1056/NEJMoa1813049 [DOI] [PubMed] [Google Scholar]
- 7.Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322(19):1887-1898. doi: 10.1001/jama.2019.16711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipton RB, Conway CM, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant 75 mg, an oral CGRP receptor antagonist, for the acute treatment of migraine: results from a double-blind, randomized, placebo-controlled trial, study 301. Headache. 2018;58(8):1336-1337. doi: 10.1111/head.13411 [DOI] [Google Scholar]
- 9.Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381(2):142-149. doi: 10.1056/NEJMoa1811090 [DOI] [PubMed] [Google Scholar]
- 10.Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737-745. doi: 10.1016/S0140-6736(19)31606-X [DOI] [PubMed] [Google Scholar]
- 11.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 12.Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 13.Landy S, Savani N, Shackelford S, Loftus J, Jones M. Efficacy and tolerability of sumatriptan tablets administered during the mild-pain phase of menstrually associated migraine. Int J Clin Pract. 2004;58(10):913-919. doi: 10.1111/j.1368-5031.2004.00295.x [DOI] [PubMed] [Google Scholar]
- 14.Cady R, Martin V, Mauskop A, et al. Efficacy of rizatriptan 10 mg administered early in a migraine attack. Headache. 2006;46(6):914-924. doi: 10.1111/j.1526-4610.2006.00466.x [DOI] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 16.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. doi: 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 17.Winner P, Ricalde O, Le Force B, Saper J, Margul B. A double-blind study of subcutaneous dihydroergotamine vs subcutaneous sumatriptan in the treatment of acute migraine. Arch Neurol. 1996;53(2):180-184. doi: 10.1001/archneur.1996.00550020092020 [DOI] [PubMed] [Google Scholar]
- 18.Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One. 2013;8(7):e69930. doi: 10.1371/journal.pone.0069930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu CS, Li CT, Brunoni AR, et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: a component network meta-analysis. J Neurol Neurosurg Psychiatry. 2021;92(2):195-203. doi: 10.1136/jnnp-2020-323870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diener HC, Tassorelli C, Dodick DW; International Headache Society Clinical Trials Standing Committee. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: fourth edition. Cephalalgia. 2019;39(6):687-710. doi: 10.1177/0333102419828967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Napier C, Stewart M, Melrose H, Hopkins B, McHarg A, Wallis R. Characterisation of the 5-HT receptor binding profile of eletriptan and kinetics of [3H]eletriptan binding at human 5-HT1B and 5-HT1D receptors. Eur J Pharmacol. 1999;368(2-3):259-268. doi: 10.1016/S0014-2999(99)00026-6 [DOI] [PubMed] [Google Scholar]
- 22.Johnson DE, Rollema H, Schmidt AW, McHarg AD. Serotonergic effects and extracellular brain levels of eletriptan, zolmitriptan and sumatriptan in rat brain. Eur J Pharmacol. 2001;425(3):203-210. doi: 10.1016/S0014-2999(01)01151-7 [DOI] [PubMed] [Google Scholar]
- 23.Knyihár-Csillik E, Tajti J, Csillik AE, Chadaide Z, Mihály A, Vécsei L. Effects of eletriptan on the peptidergic innervation of the cerebral dura mater and trigeminal ganglion, and on the expression of c-fos and c-jun in the trigeminal complex of the rat in an experimental migraine model. Eur J Neurosci. 2000;12(11):3991-4002. doi: 10.1046/j.1460-9568.2000.00299.x [DOI] [PubMed] [Google Scholar]
- 24.Worthington I, Pringsheim T, Gawel MJ, et al. ; Canadian Headache Society Acute Migraine Treatment Guideline Development Group . Canadian Headache Society Guideline: acute drug therapy for migraine headache. Can J Neurol Sci. 2013;40(5)(suppl 3):S1-S80. doi: 10.1017/S0317167100118943 [DOI] [PubMed] [Google Scholar]
- 25.Kennis K, Kernick D, O’Flynn N. Diagnosis and management of headaches in young people and adults: NICE guideline. Br J Gen Pract. 2013;63(613):443-445. doi: 10.3399/bjgp13X670895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou M, Xing H, Li C, et al. Short-term efficacy and safety of lasmiditan, a novel 5-HT1F receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis. J Headache Pain. 2020;21(1):66. doi: 10.1186/s10194-020-01138-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messina R, Goadsby PJ. CGRP—a target for acute therapy in migraine: clinical data. Cephalalgia. 2019;39(3):420-427. doi: 10.1177/0333102418768095 [DOI] [PubMed] [Google Scholar]
- 28.Holland PR, Goadsby PJ. Targeted CGRP small molecule antagonists for acute migraine therapy. Neurotherapeutics. 2018;15(2):304-312. doi: 10.1007/s13311-018-0617-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Food and Drug Administration. FDA approves new treatment for adults with migraine. Published December 23, 2019. Accessed October 15, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-adults-migraine
- 30.U.S. Food and Drug Administration. NDA approval: NDA 212728. Published February 27, 2020. Accessed October 16, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/212728Orig1s000ltr.pdf
- 31.Institute for Clinical and Economic Review . Migraine: acute therapies. Accessed October 19, 2020. https://icer-review.org/material/acute-migraine-evidence- report/
- 32.Doty EG, Krege JH, Pohl G, Case M, Dowsett SA, Tepper SJ. Pain freedom at 2 to 8 hours with lasmiditan: a comparison with rimegepant and ubrogepant. Headache. 2020;60(8):1793-1796. doi: 10.1111/head.13899 [DOI] [PubMed] [Google Scholar]
- 33.Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol. 2012;8(2):89-99. doi: 10.3988/jcn.2012.8.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. 2015;84(7):688-695. doi: 10.1212/WNL.0000000000001256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigal ME, Borucho S, Serrano D, Lipton RB. The acute treatment of episodic and chronic migraine in the USA. Cephalalgia. 2009;29(8):891-897. doi: 10.1111/j.1468-2982.2008.01819.x [DOI] [PubMed] [Google Scholar]
- 36.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3-20. doi: 10.1111/head.12499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Strategies
eTable 1. Study Characteristics
eReferences.
eTable 2. SUCRA of Outcomes for Anti-Migraine Specific Treatments in Dosages With Widespread Clinical Use
eTable 3. The Percentage of Serious Adverse Events in All Adverse Events With Respect to Specific Anti-Migraine Treatment
eTable 4. The Percentage of Chest Symptoms in all Adverse Events With Respect to Specific Anti-Migraine Treatment
eTable 5. Significant Loop-Specific Inconsistencies of Network Meta-Analysis
eTable 6. Significant Side-Splitting Inconsistencies of Network Meta-Analysis
eTable 7. Design‐by‐Treatment Interaction Model for Inconsistency of Network Meta-Analysis
eFigure 1A. Forest Plot of the Primary Outcome: Pain Freedom at 2 Hours for Specific Anti-Migraine Treatments in Dosages With Widespread Clinical Use
eFigure 1B. Forest Plot of the Secondary Outcome: Pain Relief at 2 Hours for Specific Anti-Migraine Treatments in Dosages With Widespread Clinical Use
eFigure 1C. Forest Plot of the Secondary Outcome: Adverse Events for Specific Anti-Migraine Treatments in Dosages With Widespread Clinical Use
eFigure 2. Forest Plot of the Secondary Outcome: Adverse Events
eFigure 3. Risk of Bias
eFigure 4A. Forest Plot of the Primary Outcome: Pain Freedom at 2 Hours for Specific Anti-Migraine Treatments After Adjustment for the Percentage of Severe Headache at Baseline
eFigure 4B. Forest Plot of the Secondary Outcome: Pain Relief at 2 Hours for Specific Anti-Migraine Treatments After Adjustment for the Percentage of Severe Headache at Baseline
eFigure 5A. Funnel Plot of the Primary Outcome: Pain Freedom at 2 Hours for Specific Anti-Migraine Treatments
eFigure 5B. Funnel Plot of the Secondary Outcome: Pain Relief at 2 Hours for Specific Anti-Migraine Treatments
eFigure 5C. Funnel Plot of the Secondary Outcome: Adverse Events for Specific Anti-Migraine Treatments
eFigure 6A. Egger’s Regression of All Specific Anti-Migraine Treatments for the Primary Outcome: Pain Freedom at 2 Hours
eFigure 6B. Egger’s Regression of All Specific Anti-Migraine Treatments for the Secondary Outcome: Pain Relief at 2 Hours
eFigure 6C. Egger’s Regression of All Specific Anti-Migraine Treatments for the Secondary Outcome: Adverse Events