Abstract
Background:
Pain during the neonatal period has been associated with immediate and long-term adverse effects. One of the most frequent painful procedures that neonates face in neonatal intensive care unit is the endotracheal intubation. Midazolam has been a candidate for premedication before neonatal intubation. Our aim was to evaluate the effects of midazolam as the premedication on endotracheal intubation of premature infants during surfactant administration.
Materials and Methods:
In a double-blind clinical trial, 80 preterm infants were undertaken for tracheal intubation following the use of atropine associated to either midazolam or placebo. Patient's vital signs and general conditions were constantly monitored, and pain was assessed using premature infant pain profile (PIPP) score.
Results:
The mean ± standard deviation for postnatal age was 95.38 ± 50.04 and 111.63 ± 49.4 min in control and midazolam groups, respectively. The patients in the midazolam group had significantly better outcomes across several intubation outcome measures such as duration of endotracheal intubation (23.5 ± 6.7 vs. 18.8 ± 4.8 s, P = 0.001), oxygen saturation level (88.05% ±13.7 vs. 95.1 ± 1.8%, P = 0.002), intubation failure (34.2% vs. 2.5%, P = 0.0001), awake and resistance during intubation (95% vs. 20%, P = 0.0001), and excellent patient condition during intubation (0% vs. 82.5%, P = 0.0001). In addition, PIPP score was significantly lower in the midazolam group (5.2 ± 2.06 vs. 12.9 ± 2.9, P = 0.0001).
Conclusion:
Premedication with midazolam in newborns before intubation, can hold promising effects that manifests as better overall outcomes, less complications, better vital signs, more comfortable situation, and lesser pain for these patients.
Keywords: Intubation, midazolam, premature infant, premedication
INTRODUCTION
Pain during the neonatal period has been associated with immediate and long-term adverse effects.[1] Various studies have suggested that the presence of pain during neonatal period is accompanied by long-term behavioral and developmental effects.[2]
Such effects are more severe in premature newborns because of immature and vulnerable nervous system.[3] One of the most frequent painful procedures that neonates face in neonatal intensive care unit (NICU) is the endotracheal intubation. Endotracheal intubation, especially if performed in awake and active newborns has many adverse consequences including hypoxia, bradycardia, systemic hypertension, intraventricular hemorrhage and facial, laryngeal or pharyngeal trauma.[4]
It has been indicated by recent researches that intubation in the absence of a proper premedication raises the number of attempts for a successful procedure and elongate the intubation duration among neonates and thus raising the risk of its complications.[5,6]
Respiratory distress syndrome (RDS) is a common condition among NICU patients that frequently requires intubation for surfactant administration through intubation-surfactant-extubation (INSURE) method.[7]
The current recommendations suggest use of premedication before elective and semi-urgent intubations.[8] Nevertheless, there is not any evidence-based consensus about the best drug for premedication of newborn infants. Midazolam has been a candidate for premedication primarily because of single administration, rapid onset of action, and short half-life. The drug relaxes muscles while sparing the spontaneous respiratory drive and patient's hemodynamics.[9]
We aimed to evaluate the effects of midazolam administration as the premedication on endotracheal intubation among newborn infants during INSURE procedure. The quality of INSURE in these patients was also assessed.
MATERIALS AND METHODS
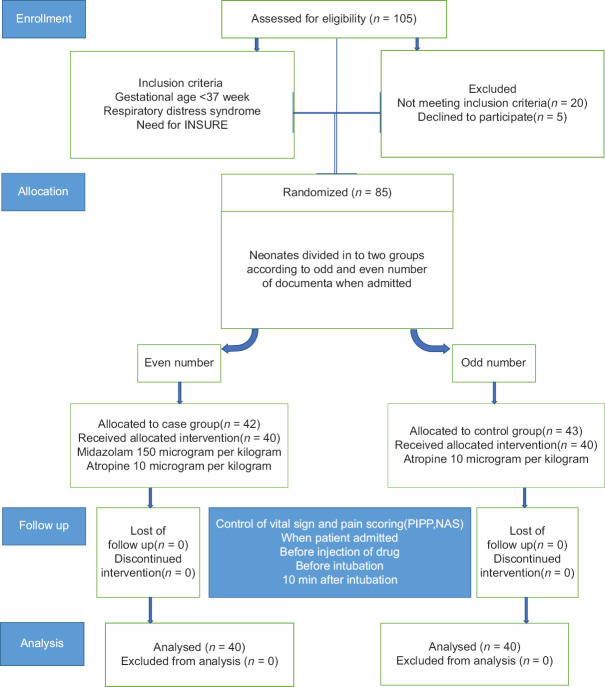
We conducted a double-blind randomized controlled trial from September 2015 to February 2016. Samples were consecutively enrolled in the study from patients admitted to the NICU ward at Alzahra and Shahid Beheshti University Hospitals, Isfahan, Iran. Clinical trial number was IRCT20130329012869N2. Patients were premature newborns with gestational age of <37 weeks who had RDS and needed nasal continuous positive airway pressure with a fraction of inspiratory oxygen of at least 40% to maintain oxygen saturation between 90 to95% and were candidates for INSURE treatment. Randomization was achieved through consecutive random allocation of patients alternatively to the cases or controls group. Enrolled patients with maxillofacial anomalies, congenital heart diseases or previous intubation were not included in the study. Patients who underwent 2 or more failed attempts for intubation were excluded from the study. The stages of selection of newborns are shown in Figure 1. This study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences Ethical Approval Number: (IR.mui.rec. 1395.3.018) and written informed consent was taken from the parents before the enrollment.
Figure 1.
Study flow
Both groups received 10 μg/kg Atropine intravenously 2 min before intubation. Patients in the cases group received 150 μg/kg Midazolam and patients in the control group received the equivalent volume of normal saline 3 min before the intubation. A pharmacist received a box containing the sequence of treatment group and for ensuring that normal saline and midazolam could not be differentiated. We enveloped the entire venous line and syringes with aluminum foil. We used a four-point scale for the assessment of intubation condition which includes: Ease of laryngoscopy, position of vocal cords, coughing, jaw relaxation, and movement of the limbs.[10]
The quality of sedation in patients was assessed using the Hans-Cooper scale (jaw relaxation, vocal cord position and movement, coughing).[11] After preoxygenation and maintaining a blood oxygen level of 94% a neonatologist blinded to the patient groups performed intubation and checked for correct tube placement. The patients then received surfactant through endotracheal tube which was replaced by CPAP after the spontaneous breathing was achieved. Patients who had oxygen level drop to <70%, heart rate drop of more than 20% of the baseline heart rate or failure of intubation procedure more than 30 s (intubation failure) put under positive pressure ventilation using a T-piece resuscitator (NeoPuff infant resuscitator, Fisher-Paykel, Oakland, New Zealand). After two failed intubation attempts, patients were excluded from the study.
The heart rate, blood oxygenation level, and blood pressure were monitored throughout the procedure using a Masimo pulse oximeter (Irvine, CA) and recorded through a fixed camera (Panasonic, NV-VZ10, Japan). Behaviors and facial expressions of the patients were also recorded, starting before administration of the medications all the way to 10 min after INSURE. All data were recorded in the following four steps: Step 1: Basic information, step 2: Before midazolam or normal saline injection, step 3: Immediately before intubation, and step 4: 10 min after intubation.
Using a predesigned form, gestational age, weight, breathing rate before and after intubation and INSURE, mean blood pressure, oxygen saturation level and heart rate before and after INSURE were recorded from all participating patients. Intubation duration was measured using a timer from the insertion of laryngoscope to the mouth until removal of the laryngoscope from the mouth after successful intubation.
The severity of pain perceived by patients was assessed by premature infant pain profile (PIPP) scores. The PIPP score includes points for changes in heart rate, oxygen saturation level, brow bulge, eye squeeze, and nasolabial furrow. Score ranging from 0–6, 7–12, to above 12 were considered as mild, moderate, and severe pain, respectively.
The primary outcome of the study was comparison of quality of sedation using Hans-Cooper scale in midazolam and control groups and secondary outcomes compared physiological measurements including heart rate, mean blood pressure, spo2, number of attempts for and duration of intubation and pain scores between two groups.
On the basis of a former study, a 3 points reduction in the PIPP scores during painful procedures could be considered as clinically significant.[12] Assuming a pooled SD of 3.3, a sample size of 27 newborns was needed to attain a power of 90% to detect a mean difference of 3 points in the PIPP scores with a 0.05 significance level. We recruited additional newborns for data collection errors.
Data were analyzed using the SPSS software (version 18.0, Chicago, IL, USA). Categorical data were reported using frequency reporting measures and compared using Chi-square test. Comparison of means was done using Student's t-test and Mann–Whitney U-test for quantitative data with normal and abnormal distribution, respectively. Statistical significance was defined as a two-tail P < 0.05.
RESULTS
During the study, 105 newborn infants were assessed for eligibility and eventually 80 newborns were included in the study, 40 infants in each group. The mean ± standard deviation (SD) for postnatal age was 95.38 ± 50.04 and 111.63 ± 49.4 min in control and midazolam group respectively. There was no significant differences between the two groups regarding their postnatal age (P > 0.05). Regarding their gender distribution, 57.5% and 72.5% of patients in control and midazolam group were male, respectively. The mean ± SD of birth body weight were 1435.75 ± 519.69 and 1794 ± 673.3 in control and midazolam group, respectively (P = 0.09). The mean ± SD of gestational age at birth was 29.8 ± 3.2 and 31.8 ± 2.6 in control and midazolam group, respectively (P = 0.06). Baseline characteristics of two groups are shown in Table 1.
Table 1.
Baseline characteristics of patients
| Characteristics | Control group (n=40) | Midazolam group (n=40) | P |
|---|---|---|---|
| Birth weight (g) (mean±SD) | 1435±519 | 1794±673 | 0.07 |
| Postnatal age (min) | 95.38±50 | 111.6±49 | 0.14 |
| Gestational age (weeks) (mean±SD) | 29.8±0.3 | 31.8±2.6 | 0.06 |
| Sex (male %) | 57.5 | 72.5 | 0.237 |
| Baseline heart rate (beat/min) (mean±SD) | 143.9±2.7 | 145.5±14.5 | 0.59 |
| Baseline mean blood pressure (mm Hg) (mean±SD) | 45.8±11.4 | 41.6±7.4 | 0.057 |
| Baseline SpO2 (%) (mean±SD) | 90.6±2.6 | 89.9±4.8 | 0.39 |
SD=Standard deviation
The patients in the midazolam group had significantly better outcomes across several intubation outcome measures such as duration of intubation, oxygen level and heart rate plummet, patient comfort, frequency of procedure-related trauma and patient condition. There was significant improvement of Oxygen levels after intubation and after INSURE in the midazolam group. However, we did not find any significant differences between the two groups regarding heart rate and blood pressure after treatment. Table 2 represents the data on the intubation and INSURE efficacy among each group.
Table 2.
Comparison of clinical parameters between two groups
| Parameter | Midazolam group (n=40) | Control group (n=40) | P |
|---|---|---|---|
| Duration of intubation (mean±SD) | 23.5±6.7 | 18.8±4.8 | 0.001 |
| SpO2<70% during intubation (%) | 55 | 5 | 0.0001 |
| Duration of intubation of>30 s (%) | 34.2 | 2.5 | 0.0001 |
| Mouth and laryngeal bleeding (%) | 37.5 | 2.5 | 0.0001 |
| Awake and resisting patients (%) | 80 | 5 | 0.0001 |
| Successful intubation | |||
| At first trying (%) | 66.7 | 92.5 | 0.004 |
| At second trying (%) | 33.3 | 7.5 | |
| Intubation condition (%) | |||
| Excellent | 0 | 82.5 | 0.0001 |
| Good | 0 | 10 | |
| Acceptable | 30 | 2.5 | |
| Weak | 70 | 5 | |
| Neonatal condition 10 min after INSURE (%) | |||
| Excellent | 0 | 82.5 | 0.0001 |
| Good | 0 | 15 | |
| Acceptable | 30 | 2.5 | |
| Weak | 70 | 0 | |
| Blood pressure (mm Hg) (±SD) | |||
| Before intubation | 45.6±9.5 | 43.2±6.3 | 0.19 |
| After intubation | 46.7±9.6 | 43.8±6.3 | 0.12 |
| 10 min after INSURE | 45.6±9.9 | 44.6±6 | 0.15 |
| SpO2 before intubation (%) (±SD) | 83.3±13.1 | 92.3±4.3 | 0.0001 |
| SPO2 after intubation (%) (±SD) | 88.05±13.7 | 95.1±1.8 | 0.002 |
| SpO2 10 min after INSURE (%) (±SD) | 94.4±2.1 | 95.2±1.8 | 0.03 |
| Heart rate before intubation (beat/minute) (±SD) | 143.08±12.7 | 146.9±15.1 | 0.41 |
| Heart rate after intubation (beat/minute) (±SD) | 143.9±12.6 | 146.5±14.7 | 0.21 |
| Heart rate 10 min after INSURE intubation (beat/minute) (±SD) | 143±22.5 | 147±13.4 | 0.11 |
SD=Standard deviation
There was also a significant difference between the two groups regarding their PIPP and FANS; the midazolam group showed significantly better results. Data are presented in Tables 2 and 3.
Table 3.
Comparison of pain score between two groups
| Parameter | Control group (n=40) | Midazolam group (n=40) | P |
|---|---|---|---|
| Baseline PIPP score | 2.6±1.4 | 1.85±1.09 | 0.29 |
| PIPP score after intubation | 12.9±2.9 | 5.2±2.06 | 0.0001 |
| Baseline FANS score | 0.58±0.1 | 0.25±0.09 | 0.1 |
| FANS score after intubation | 6.1±1.5 | 0.68±1.7 | 0.0001 |
PIPP=Premature infant pain profile; FANS=Faceless acute neonatal pain scale
DISCUSSION
Until now, in spite of many published studies on various regimens as premedication for endotracheal intubation, there is no consensus about the best drug for nonemergent endotracheal intubation in newborn infants. Our findings suggest that premedication with Midazolam not only could relieve pain but also reduces duration, number of attempts and complications of endotracheal intubation in premature neonates. These effects allowed for a faster intubation with less failures and less procedural adverse effects that resulted in a better patient conditions and more stable vital signs.
There has been previous studies on various premedication regimens for better outcomes in intubations. Penido et al. performed a study to compare propofol versus midazolam in addition to remifentanil for endotracheal intubation of premature newborns to find which drug could provide better intubation condition. Although they did not find any significant differences between two groups with regard to intubation condition, the intubation condition was good or excellent in 70% and 80% of patients in midazolam and propofol groups, respectively. In addition the pain scores was similar in both the groups.[13]
In another study, 70 newborn infants were randomized to receive either remifentanil or morphine (100 μ/kg) and midazolam (50 μ/kg) before endotracheal intubation. Intubation condition was poor in 25% and 28.6% of the remifentanil and morphine-midazolam groups, respectively. The median time for successful intubation was 33 and 36 s for the remifentanil and morphine-midazolam groups, respectively.[14] In our study, 92.5% of infants who received midazolam have had successful intubation at the first attempt and the mean duration of intubation was about 19 s which is wonderful. The reason for these surprising differences may be related to experience of the neonatologist who perform the intubation. Moreover, the intubation time in our study calculated from insertion to removal of the laryngoscope but in the study of Avino et al., it was calculated from insertion of the laryngoscope to confirmation of intubation.
Papoff and colleague assessed the effectiveness of a combination of propofol and fentanyl to facilitate tracheal intubation in newborn infants. They found successful intubation rate at first attempt of 86% which is close to our results.[15] Penido et al. also reported a successful intubation rate at first attempt of 80%.[13]
Durrmeyer et al. were used a combination of atropine, sufentanil, and atracurium as premedication for endotracheal intubation. They showed 74% success rate for the first attempt.[16] It is worth nothing that the incidence of successful intubation has direct relationship to the moment that endotracheal intubation is done and the peak plasma concentration of the drugs used as premedication.
Baleine et al. assessed the neonatal comfort after the use of nasal midazolam (100 μ/kg) as premedication for intubation of preterm newborns in the delivery room and found adequate comfort during the procedure in 85% of neonates based on FANS score. In addition, they showed 70% success rate for the first attempt of intubation.[17]
Another study evaluated the PIPP score after premedication with remifentanil for the intubation of premature infants and reported a lower PIPP score with the use of remifentanil compared to control group.[18] Although midazolam is a hypnotic drug and has not analgesic effects, our study showed that midazolam premedication could reduce PIPP score significantly which is comparable with remifentanil. One explanation for reduction of pain score with midazolam is that midazolam reduces stress and resistance to intubation and thereby reduces pain during intubation.
On the best of our knowledge, our study is the first one that assesses the effects of intravenous midazolam alone and without combination with analgesics as premedication for endotracheal intubation of premature infants. However, this study has some limitations including the lack of measurement of plasma drug concentration and relatively small sample size.
CONCLUSION
Based on our results, we have shown that premedication with intravenous midazolam before endotracheal intubation of premature infants, not only reduces pain score and duration of intubation but also could lead to better intubation rate at first attempt, better intubation condition, and more stable vital signs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;21:1321–29. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- 2.Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11:268–75. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: Sensitization, receptive fields, and the effects of contralateral stimulation. Pain. 1994;56:95–101. doi: 10.1016/0304-3959(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh V, Ponnusamy V, Anandaraj J, Chaudhary R, Malviya M, Clarke P, et al. Endotracheal intubation in a neonatal population remains associated with a high risk of adverse events. Eur J Pediatr. 2011;170:223–7. doi: 10.1007/s00431-010-1290-8. [DOI] [PubMed] [Google Scholar]
- 5.Muniraman HK, Yaari J, Hand I. Premedication use before nonemergent intubation in the newborn infant. Am J Perinatol. 2015;32:821–4. doi: 10.1055/s-0034-1543987. [DOI] [PubMed] [Google Scholar]
- 6.Allen KA. Premedication for neonatal intubation: Which medications are recommended and why. Adv Neonatal Care. 2012;12:107–11. doi: 10.1097/ANC.0b013e31824c1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European Consensus Guidelines on the management of neonatal respiratory distress syndrome in preterm infants-2013 update. Neonatology. 2013;103:353–68. doi: 10.1159/000349928. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P, Denson SE. Committee on Fetus and Newborn, Section on Anesthesiology and Pain Medicine. Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics. 2010;125:608–15. doi: 10.1542/peds.2009-2863. [DOI] [PubMed] [Google Scholar]
- 9.Durrmeyer X, Daoud P, Decobert F, Boileau P, Renolleau S, Zana-Taieb E, et al. Premedication for neonatal endotracheal intubation: Results from the epidemiology of procedural pain in neonates study. Pediatr Crit Care Med. 2013;14:e169–75. doi: 10.1097/PCC.0b013e3182720616. [DOI] [PubMed] [Google Scholar]
- 10.Viby-Mogensen J, Engbaek J, Eriksson LI, Gramstad L, Jensen E, Jensen FS, et al. Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents. Acta Anaesthesiol Scand. 1996;40:59–74. doi: 10.1111/j.1399-6576.1996.tb04389.x. [DOI] [PubMed] [Google Scholar]
- 11.Hans P, Brichant JF, Hubert B, Dewandre PY, Lamy M. Influence of induction of anaesthesia on intubating conditions one minute after rocuronium administration: Comparison of ketamine and thiopentone. Anaesthesia. 1999;54:276–9. doi: 10.1046/j.1365-2044.1999.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.Axelin A, Salanterä S, Kirjavainen J, Lehtonen L. Oral glucose and parental holding preferable to opioid in pain management in preterm infants. Clin J Pain. 2009;25:138–45. doi: 10.1097/AJP.0b013e318181ad81. [DOI] [PubMed] [Google Scholar]
- 13.Penido MG, de Oliveira Silva DF, Tavares EC, Silva YP. Propofol versus midazolam for intubating preterm neonates: A randomized controlled trial. J Perinatol. 2011;31:356–60. doi: 10.1038/jp.2010.135. [DOI] [PubMed] [Google Scholar]
- 14.Avino D, Zhang WH, De Villé A, Johansson AB. Remifentanil versus morphine-midazolam premedication on the quality of endotracheal intubation in neonates: A noninferiority randomized trial. J Pediatr. 2014;164:1032–7. doi: 10.1016/j.jpeds.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Papoff P, Mancuso M, Caresta E, Moretti C. Effectiveness and safety of propofol in newborn infants. Pediatrics. 2008;121:448. doi: 10.1542/peds.2007-3132. [DOI] [PubMed] [Google Scholar]
- 16.Durrmeyer X, Dahan S, Delorme P, Blary S, Dassieu G, Caeymaex L, et al. Assessment of atropine-sufentanil-atracurium anaesthesia for endotracheal intubation: An observational study in very premature infants. BMC Pediatr. 2014;14:120. doi: 10.1186/1471-2431-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baleine J, Milési C, Mesnage R, Rideau Batista Novais A, Combes C, Durand S, et al. Intubation in the delivery room: Experience with nasal midazolam. Early Hum Dev. 2014;90:39–43. doi: 10.1016/j.earlhumdev.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Badiee Z, Vakiliamini M, Mohammadizadeh M. Remifentanil for endotracheal intubation in premature infants: A randomized controlled trial. J Res Pharm Pract. 2013;2:75–82. doi: 10.4103/2279-042X.117387. [DOI] [PMC free article] [PubMed] [Google Scholar]