Key Points
Question
For children and adolescents with type 1 diabetes, does a hybrid closed-loop (HCL) system improve glycemic and psychosocial outcomes compared with conventional management?
Findings
In this 6-month randomized clinical trial including 135 children and adolescents, mean percent time in target glucose range was 62.5% with HCL therapy and 56.1% with standard therapy with a mean adjusted difference of 6.7%. Diabetes-specific quality of life was higher with HCL therapy than with standard therapy.
Meaning
The HCL system improved glycemic control and quality of life compared with conventional diabetes management in youth with type 1 diabetes.
This randomized clinical trial investigates the use of a hybrid closed-loop system to improve both glycemic control and psychosocial outcomes in children and adolescents with type 1 diabetes as compared with conventional diabetes management.
Abstract
Importance
Hybrid closed-loop (HCL) therapy has improved glycemic control in children and adolescents with type 1 diabetes; however, the efficacy of HCL on glycemic and psychosocial outcomes has not yet been established in a long-term randomized clinical trial.
Objective
To determine the percentage of time spent in the target glucose range using HCL vs current conventional therapies of continuous subcutaneous insulin infusion or multiple daily insulin injections with or without continuous glucose monitoring (CGM).
Design, Setting, and Participants
This 6-month, multicenter, randomized clinical trial included 172 children and adolescents with type 1 diabetes; patients were recruited between April 18, 2017, and October 4, 2019, in Australia. Data were analyzed from July 25, 2020, to February 26, 2021.
Interventions
Eligible participants were randomly assigned to either the control group for conventional therapy (continuous subcutaneous insulin infusion or multiple daily insulin injections with or without CGM) or the intervention group for HCL therapy.
Main Outcomes and Measures
The primary outcome was the percentage of time in range (TIR) within a glucose range of 70 to 180 mg/dL, measured by 3-week masked CGM collected at the end of the study in both groups. Secondary outcomes included CGM metrics for hypoglycemia, hyperglycemia, and glycemic variability and psychosocial measures collected by validated questionnaires.
Results
A total of 135 patients (mean [SD] age, 15.3 [3.1] years; 76 girls [56%]) were included, with 68 randomized to the control group and 67 to the HCL group. Patients had a mean (SD) diabetes duration of 7.7 (4.3) years and mean hemoglobin A1c of 64 (11) mmol/mol, with 110 participants (81%) receiving continuous subcutaneous insulin infusion and 72 (53%) receiving CGM. In the intention-to-treat analyses, TIR increased from a mean (SD) of 53.1% (13.0%) at baseline to 62.5% (12.0%) at the end of the study in the HCL group and from 54.6% (12.5%) to 56.1% (12.2%) in the control group, with a mean adjusted difference between the 2 groups of 6.7% (95% CI, 2.7%-10.8%; P = .002). Hybrid closed-loop therapy also reduced the time that patients spent in a hypoglycemic (<70 mg/dL) range (difference, −1.9%; 95% CI, −2.5% to −1.3%) and improved glycemic variability (coefficient of variation difference, −5.7%; 95% CI, −10.2% to −0.9%). Hybrid closed-loop therapy was associated with improved diabetes-specific quality of life (difference, 4.4 points; 95% CI, 0.4-8.4 points), with no change in diabetes distress. There were no episodes of severe hypoglycemia or diabetic ketoacidosis in either group.
Conclusions and Relevance
In this randomized clinical trial, 6 months of HCL therapy significantly improved glycemic control and quality of life compared with conventional therapy in children and adolescents with type 1 diabetes.
Trial Registration
ANZCTR identifier: ACTRN12616000753459
Introduction
Over the past 2 decades, there have been significant innovations in the management of type 1 diabetes (T1D). Automation in closed-loop systems offers the potential to reduce diabetes burden and improve glucose profile.1 There are several automated insulin delivery platforms using various combinations of insulin pumps, continuous glucose monitor (CGM) systems, and algorithms. The MiniMed 670G (Medtronic) insulin pump was the first commercial system to be available after regulatory approval, based on findings from a 3-month single-arm observational study.2 The study also reported improved glycemic control, with increased time in range (TIR) and reductions in hemoglobin A1c (HbA1c), compared with baseline.3 The hybrid closed-loop (HCL) system uses a control algorithm that delivers insulin based on CGM glucose measures. However, meals must still be announced and an insulin bolus delivered according to meal carbohydrate content.
A recent 6-month randomized clinical trial (RCT) in adults evaluating this system demonstrated improvement in all glycemic metrics with HCL compared with standard therapy with multiple daily insulin injections (MDI) or continuous subcutaneous insulin infusion (CSII).4 Because there were no RCTs, to our knowledge, that had evaluated the effectiveness of this system in adolescents and young adults, a cohort with suboptimal glycemic control,5 we performed an RCT to investigate whether the system is effective in long-term use in children and adolescents with T1D. Furthermore, the effect of the system on psychosocial outcomes remains less recognized6 mainly owing to the short-term duration of studies designed primarily to analyze glycemic outcomes. This gap in knowledge is relevant especially with a high discontinuation rate demonstrated with long-term use of the system in youth with T1D.7 This 6-month trial in children and adolescents attempted to address these clinically important questions.
In Australia, most children and adolescents with T1D are receiving CSII or MDI with access to subsidized CGM for their management.8,9 However, less than one-quarter meet the international glycemic targets,10 which highlights the need to evaluate these newer therapies. The primary objective of this RCT was to compare the percentage of time in the target glucose range (70 to 180 mg/dL; to convert to mmol/L, multiply by 0.0555) using HCL vs the currently available conventional therapies (CSII or MDI with or without CGM).
Methods
Study Design
A detailed description of the protocol (Supplement 1) has been published.11 This was a parallel-group, randomized, controlled, phase 3 home clinical trial conducted at 5 tertiary pediatric diabetes centers in Australia. Participants were recruited between April 18, 2017, and October 4, 2019. Information on patient race and ethnicity was not available; therefore, it was not included in the study data. This was a free-living study, and all participants continued to undertake their routine activities. The study was coordinated by the Children’s Diabetes Centre, Perth, Australia, supported by the National Health and Medical Research Council Clinical Trials Centre for randomization and data collection (collected using Rave EDC software [Medidata]) and the statistical analysis plan (Supplement 2), which were codesigned. Alignment of study design and data elements with the similar trial in adults12 was included to aid later cross-cohort comparisons. Ethics approval was received at each site. The study recruited participants aged between 12 and 25 years with T1D for at least 1 year, with a fasting C-peptide level of less than 0.30 ng/mL (to convert to nmol/L, multiply by 0.331) and HbA1c of less than or equal to 91 mmol/mol (10.5%) on standard therapy, which comprised either CSII or MDI with or without CGM. Participants were excluded if they had used any noninsulin glucose-lowering agent or corticosteroid within the preceding 3 months; were using any form of closed-loop systems; had uncontrolled celiac or thyroid disease, clinically significant gastroparesis, uncontrolled hypertension, or cardiac or neurologic complications; were pregnant or planned pregnancy; had an unstable medical or psychological condition; were not able to comply with the protocol requirements; or did not speak English. Written informed consent was obtained from participants aged 18 years or older, and written parental consent and participant assent was obtained for those younger than 18 years. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Run-in Period
A 5-week run-in period included 2 weeks that were an education review by the diabetes educator and dietician to refresh participants on diabetes self-management, including carbohydrate counting skills. This period was followed by a 3-week period of masked CGM (Guardian Sensor 3 [Medtronic]) to capture baseline glycemic data. An additional 1 to 2 weeks of masked CGM were offered to participants who did not meet the required CGM data for more than 70% of the time.13
Randomization
After the run-in phase, participants were randomly assigned either to the control group (conventional therapy of continued CSII or MDI with or without CGM) or to the intervention group (HCL) for 26 weeks. To ensure optimal balance between treatment groups,14 participants were allocated using minimization with the following factors: TIR (above or below 55%), age, sex, diabetes duration, and trial site. As this was an open-label study requiring device training and use, study participants and study researchers were not blinded to treatment. However, treatment allocation was blinded to the trial statistical team and to investigators undertaking data analyses.
Study Treatment
After randomization, control group participants continued standard therapy (CSII or MDI with or without CGM). Participants in the HCL group received training, which for participants on MDI also included education on insulin pump therapy. The HCL group received training on the MiniMed 670G pump, glucose sensor (Guardian sensor 3), and transmitter (Guardian Link 3 [Medtronic]). Participants in both study groups had a clinical review 4 weeks after randomization and were also followed up at 13 weeks (midstudy) and 26 weeks (study end) after randomization. Clinical and technical support was always available. A schematic representation of a closed-loop system is provided in eFigure 1 in Supplement 3.
Data Collection
Masked CGM data were collected at baseline (3 weeks), midstudy (2 weeks), and study end (3 weeks). The participant had no access to these CGM data (blinded), which were collected via an independent system that did not communicate with the participant’s sensor or pump. As the masked CGM data were used for glycemic outcomes, this supplementary CGM was worn by both control and intervention groups to provide the same platform for data collection. Real-time CGM data were also collected in HCL participants during the study period. Point-of-care HbA1c was measured at randomization, midstudy, and study end. Age-appropriate validated psychosocial questionnaires were administered at baseline, midstudy, and study end.
Outcomes
Glycemic outcomes were reported according to the recommendation for artificial pancreas clinical trials.15,16 The primary outcome was the percentage of TIR (70-180 mg/dL) measured at 5-minute intervals by the 3-week masked CGM data collected at study end in both groups. Secondary CGM glycemic outcomes included the percentage of time in hypoglycemia (<70 mg/dL, <60 mg/dL, <54 mg/dL, and <50 mg/dL) and hyperglycemia (>180 mg/dL, >250 mg/dL, and >300 mg/dL), the number of hypoglycemic events (<54 mg/dL for more than 20 minutes), mean sensor glucose, SD of sensor glucose, and coefficient of variation along with fasting capillary glucose and HbA1c. The study also evaluated CGM metrics separately for day (06:00 to 24:00) and night (00:00 to 06:00). Other nonglycemic clinical outcomes included change in auxological parameters, change in insulin doses (total daily insulin, proportion of basal insulin, and insulin-carbohydrate ratio). Validated questionnaires were also administered at baseline, midstudy, and study end to evaluate diabetes-specific quality of life,17 diabetes distress,18 treatment satisfaction,19 fear of hypoglycemia,20 anxiety,21 and hypoglycemia awareness.22
Safety outcomes were the frequency of severe hypoglycemia, diabetic ketoacidosis, device-related adverse events, and any other untoward medical occurrence. An independent data and safety monitoring board provided trial oversight. Study outcomes were centrally assessed. The trial was prospectively registered with the Australian New Zealand Clinical Trials.
Statistical Analysis
We aimed to recruit 158 trial participants to detect an absolute difference of 7% in TIR between conventional therapy and HCL, assuming an SD of 13%,23 a power of 85%, and a type I error rate (2-sided) of .05 allowing for 20% predicted dropout.
To test for an effect of treatment group on the primary outcome, an analysis of covariance, including treatment group and baseline percentage TIR, was conducted on an intention-to-treat basis. Other continuous secondary outcomes were analyzed using analysis of covariance including treatment group and baseline value of the relevant outcome. Where analysis of covariance assumptions were violated and unable to be addressed through log transformation of the outcome measure, rank-sum tests were performed. An independent biostatistician validated the analyses of the key outcomes.
Multiple imputations using multivariate normal regression under a “missing at random” assumption were employed separately for each group to account for missing data; auxiliary variables in the regression models were selected based on correlation with the outcome.24 Sensitivity analyses were conducted under a “missing not at random” assumption and “complete case population.” A prespecified subgroup analysis examining treatment effect based on treatment regimen at baseline was conducted. An exploratory regression analysis within the HCL group examining the relationship between the percentage of time the closed loop was active and TIR after adjusting for baseline was also conducted.
Tests of significance were 2-sided, and P < .05 was considered statistically significant. All analyses were conducted from July 25, 2020, to February 26, 2021, in Stata, version 16.125 (StataCorp LLC), and figures were produced in R, version 4.0.2 (R Foundation).26
Results
Participants
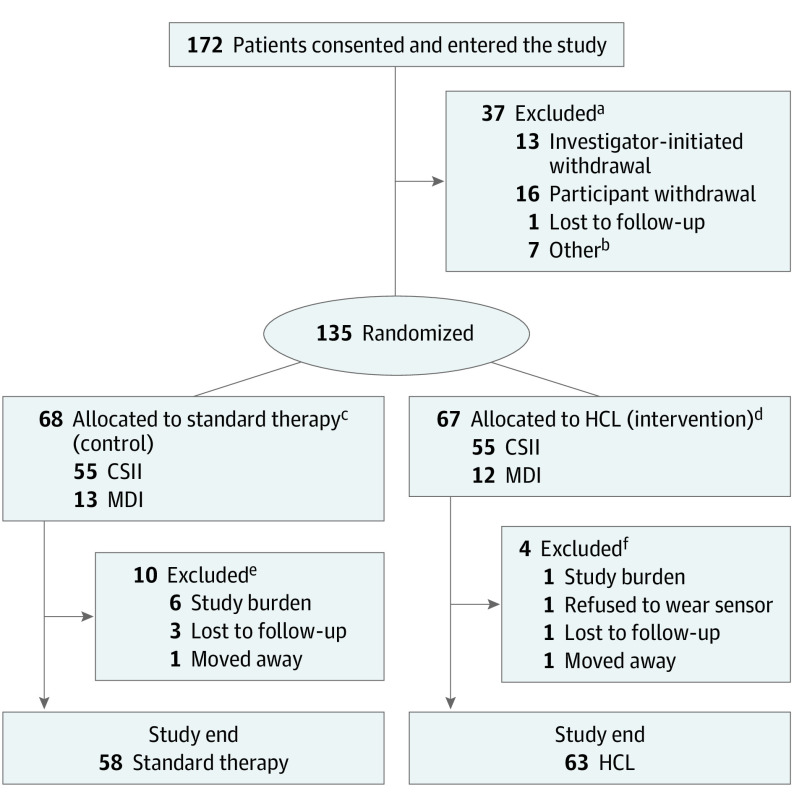
Study enrollment, randomization, and retention are shown in Figure 1. Of the 172 participants recruited between April 18, 2017, and October 4, 2019, a total of 135 patients (mean [SD] age, 15.3 (3.1) years; 76 girls [56%]) were randomized to the control group (n = 68) and the HCL group (n = 67). Participant characteristics according to the randomized group are shown in Table 1. The mean (SD) diabetes duration was 7.7 (4.3) years. The mean (SD) HbA1c level was 64 (11) mmol/mol (8.0% [1.0%]), with 110 participants (81%) receiving CSII and 72 (53%) receiving CGM. Of the 72 participants receiving CGM, 32 (44%) were using stand-alone CGM systems and 40 (56%) were using systems with automated suspends to reduce hypoglycemia.
Figure 1. Study Flow Diagram.
CSII indicates continuous subcutaneous insulin infusion; HCL, hybrid closed loop; MDI, multiple daily insulin.
aData on insulin regimen were available in 31 patients (21 with CSII/10 MDI and 15 who were sensor naive).
bOther indicates that 6 withdrawals were participant initiated and 1 was investigator initiated.
cTwenty-eight sensor naive.
dThirty-five sensor naive.
eSeven CSII/3 MDI; 4 sensor naive.
fThree CSII/1 MDI; 3 sensor naive.
Table 1. Baseline Characteristics of Participants in the Trial.
| Characteristic | Mean (SD) | |
|---|---|---|
| HCL (n = 67) | Control (n = 68) | |
| Age, y | 15.2 (3.3) | 15.4 (3.0) |
| Age group, No. (%) | ||
| <18 y | 55 (82) | 55 (81) |
| ≥18 y | 12 (18) | 13 (19) |
| Boys, No. (%) | 30 (45) | 29 (43) |
| Girls, No. (%) | 37 (55) | 39 (57) |
| Duration of diabetes, y | 7.9 (4.2) | 7.6 (3.4) |
| Body mass index z score | 0.7 (0.8) | 0.7 (0.7) |
| Insulin delivery modality, No. (%) | ||
| CSIIa | 55 (82) | 55 (81) |
| MDI | 12 (18) | 13 (19) |
| CGM at enrollment, No. (%) | 32 (48) | 40 (59) |
| HbA1c at enrollmentb | ||
| mmol/mol | 64 (10) | 63 (11) |
| % | 8.0 (1.0) | 7.9 (1.0) |
| HbA1c at randomization | ||
| mmol/mol | 62 (11) | 60 (9) |
| % | 7.8 (1.0) | 7.7 (0.8) |
| Total daily insulin, units/kg/d | 0.8 (0.2) | 0.9 (1.2) |
| Insulin:carbohydrate ratio | ||
| 8 am | 7.8 (3.0) | 7.2 (2.6) |
| 12 pm | 9.0 (3.6) | 8.3 (3.5) |
| 6 pm | 8.6 (3.0) | 8.1 (3.4) |
| Diabetic ketoacidosis in past 12 mo, No. (%) | 3 (4.5) | 3 (4.4) |
| Severe hypoglycemia events in past 12 mo, No. (%) | 3 (3) | 3 (4.4) |
| Severe diabetes distress (PAID score ≥40 points), No. (%) | 22 (33.8) | 16 (24.2) |
Abbreviations: CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; HbA1c, hemoglobin A1c; HCL, hybrid closed loop; MDI, multiple daily insulin; PAID, Problem Areas in Diabetes.
CSII, Medtronic pumps (83%), Animas (15%).
Hemoglobin A1c is reported as % (National Glycohemoglobin Standardization Program; to convert to proportion of total hemoglobin, multiply by 0.01) and mmol/mol (International Federation of Clinical Chemistry).
Fourteen participants (10.4%) withdrew after randomization, with 4 withdrawals in the HCL group. One HCL withdrawal occurred because a participant did not want to wear a sensor; another 2 HCL participants continued to the end of the study, although they reverted to their standard therapy (CSII) owing to burden related to sensor wear and alarms interrupting school and sleep. Missing data for each of the outcomes are presented in eTable 1 in Supplement 3.
Efficacy Outcomes
The mean (SD) percentage of time that glucose level was in the target range increased from 53.1% (13.0%) at baseline to 62.5% (12.0%) at study end in the HCL group and from 54.6% (12.5%) to 56.1% (12.2%) in the control group, with a mean adjusted difference between the 2 groups at study end of 6.7% (95% CI, 2.7%-10.8%; P = .002). This mean difference equated to an additional 1.6 hours per day in target range on HCL. The treatment effect was also evident at 3 months (Figure 2).
Figure 2. Percentage of Time With Glucose Level in Target Range.

A, Percentage time in range at baseline, midstudy, and at the end of the study from masked continuous glucose monitoring in the participants of the control and intervention group. Whiskers represent 95% CIs. B, Envelope plot of time in range by the hour of the day from the masked continuous glucose monitoring during the last 3 weeks of the trial in the participants of the control and intervention group. Solid lines denote median values, shaded regions represent the IQR, and dashed lines represent the boundaries for target glycemia. HCL indicates hybrid closed loop.
This improvement in TIR with HCL was seen across both day and night with a mean difference of 5.4% (95% CI, 1.6%-9.3%) during the day and 9.3% (95% CI, 4.9%-13.8%) at night. Continuous glucose monitoring outcomes for day and night are provided in eFigure 2 in Supplement 3. The results of the sensitivity analyses were similar to those of the primary analysis. In the complete case analysis, using a “missing completely at random” assumption, the between-group difference in TIR at study end was 6.8% (95% CI, 3.2%-10.4%; P < .001). The between-group difference with missing data imputed using a “missing not at random” assumption was 7.7% (95% CI, 3.4%-12.2%; P = .001) for −1 SD and 5.7% (95% CI, 1.4%-10.0%) P = .01) for +1 SD. After adjustment for stratification variables, the difference was 6.5% (95% CI, 2.4%-10.6%; P = .002).
The treatment effect was also seen for most of the secondary outcomes, as in Table 2 and eFigure 3 in Supplement 3, with less time spent in all levels of hypoglycemia and reduced glycemic variability. There was no difference in time spent in hyperglycemia between HCL and standard therapy. The HCL therapy also reduced the time that the patient spent in a hypoglycemic (<70 mg/dL) range (difference, −1.9%; 95% CI, −2.5% to −1.3%) and improved glycemic variability (coefficient of variation difference, −5.7%; 95% CI, −10.2% to −0.9%).
Table 2. Clinical, Glycemic, and Psychosocial Outcomes.
| Outcome | Baseline | Study end | HCL-control | P value | ||
|---|---|---|---|---|---|---|
| HCL (n = 67) | Control (n = 68) | HCL (n = 58) | Control (n = 53) | |||
| Clinical and glycemic outcomes | ||||||
| Primary outcome, % time | ||||||
| 70-180 mg/dLa | 53.1 (13.0) | 54.6 (12.5) | 62.5 (12.0) | 56.1 (12.2) | 6.7 (2.7 to 10.8) | .002 |
| Secondary outcomes, % time | ||||||
| 70-140 mg/dLa | 33.7 (11.3) | 34.7 (11.2) | 40.4 (10.3) | 36.0 (11.2) | 4.9 (1.1 to 8.7) | .01 |
| <70 mg/dLb | 2.9 (1.7 to 6.4) | 4.8 (2.6 to 9.0) | 2.2 (1.1 to 4.2) | 4.1 (2.6 to 8.2) | −1.9 (−2.5 to −1.3) | <.001 |
| <60 mg/dLb | 1.1 (0.6 to 3.2) | 2.2 (0.8 to 4.6) | 0.8 (0.4 to 2.0) | 1.8 (0.7 to 4.1) | −1.0 (−1.2 to −0.5) | <.001 |
| <54 mg/dLb | 0.6 (0.2 to 1.8) | 1.3 (0.3 to 2.8) | 0.4 (0.2 to 0.9) | 1.0 (0.4 to 2.3) | −0.5 (−0.7 to −0.3) | <.001 |
| <50 mg/dLb | 0.4 (0.1 to 1.0) | 0.7 (0.2 to 1.7) | 0.3 (0.1 to 0.5) | 0.6 (0.2 to 1.6) | −0.3 (−0.4 to −0.2) | <.001 |
| >180 mg/dLa | 41.8 (15.4) | 39.4 (14.5) | 34.4 (13.0) | 37.9 (13.8) | −4.3 (−8.8 to 0.2) | .06 |
| >250 mg/dLb | 15.2 (8.1 to 21.3) | 13.8 (8.1 to 18.5) | 13.0 (4.1 to 17.6) | 12.6 (6.8 to 18.3) | −0.6 (−4.0 to 1.4) | .75 |
| >300 mg/dLb | 5.0 (2.6 to 9.0) | 4.9 (2.0 to 8.0) | 5.0 (1.2 to 7.4) | 4.3 (1.9 to 8.3) | 0.3 (−1.2 to 1.2) | .70 |
| Mean SGL, mg/dLa | 174 (29) | 170 (27) | 166 (21) | 167 (26) | −2.9 (−11.5 to 5.7) | .51 |
| SD SGL, mg/dLa | 70 (12) | 70 (14) | 64 (13) | 69 (14) | −4.9 (−9.2 to −0.6) | .03 |
| CEV SGLc | 40 (37 to 45) | 41 (38 to 45) | 38 (35 to 42) | 41 (37 to 45) | −5.7 (−10.2 to −0.9) | .02 |
| Fasting blood glucose (mg/dL)a | 162 (33) | 152 (34) | 147 (21) | 149 (26) | −5.3 (−14.2 to 3.6) | .24 |
| Hypoglycemia rate, events/wkb | 0.9 (0.4 to 3.5) | 1.9 (0.4 to 4.4) | 1.1 (0.4 to 2.0) | 2.1 (0.8 to 4.1) | −1.1 (−1.5 to −0.7) | <.001 |
| HbA1ca | ||||||
| %d | 7.8 (1.0) | 7.7 (0.8) | 7.5 (1.1) | 7.6 (0.8) | −0.3 (−0.5 to 0.0) | .045 |
| mmol/mol | 62 (11) | 60 (9) | 58 (12) | 60 (9) | −2.9 (−5.8 to −0.1) | |
| TDI, u/kg/db | 0.87 (0.74 to 1.09) | 0.87 (0.73 to 1.02) | 0.89 (0.77 to 1.14) | 0.85 (0.71 to 0.99) | NA | NA |
| Change in TDI | NA | NA | 0.03 (−0.14 to 0.18) | 0.01 (−0.07 to 0.08) | 0.02 (−0.04 to 0.06) | .498 |
| Basal percentage (%)a | 43.5 (9.4) | 44.8 (10.4) | 42.9 (8.7) | 46 (9.7) | NA | NA |
| Change in basal insulin | NA | NA | −0.8 (12.3) | 1.5 (8.8) | −2.8 (−6.1 to 0.4) | .09 |
| ICR weightedb | 7.4 (6.0 to 10.0) | 7.7 (6.0 to 10.0) | 6.8 (5.4 to 8.3) | 7.5 (5.3 to 9.1) | NA | NA |
| Change in ICR | NA | NA | −0.7 (−2.0 to 0.0) | −0.5 (1.3 to 0.0) | −0.30 (−.73 to 0.02) | .08 |
| BMI z scoreb | 0.73 (0.27 to 1.22) | 0.81 (0.26 to 1.23) | 0.72 (0.25 to 1.29) | 0.81 (0.38 to 1.26) | NA | NA |
| Change in BMI z score | NA | NA | −0.03 (−0.15 to 0.18) | −0.05 (−0.18 to 0.11) | 0.03 (−0.03 to 0.11) | .24 |
| Psychosocial outcomes | ||||||
| Diabetes-specific PedsQL V3a | 70.7 (13.5) | 69.3 (14.6) | 72.3 (14.8) | 67.7 (13.6) | 4.4 (0.4 to 8.4) | .03 |
| Diabetes distress PAID score, pointsa | 30.3 (19.7) | 28.9 (18.9) | 27.9 (21.8) | 31.1 (20.7) | −4.5 (−10.6 to 1.6) | .14 |
| Fear of hypoglycemia HFS-II worrya | 16.2 (9.8) | 16.9 (9.3) | 14.0 (9.7) | 16.4 (11.4) | −1.8 (−4.9 to 1.3) | .24 |
| Hypoglycemia awareness Gold scoreb | 2 (1 to 3) | 2 (2 to 3) | 2 (1 to 3) | 2 (2 to 3) | 0 (0 to 0) | .45 |
| Diabetes treatment satisfaction, DTSQa | ||||||
| Child | 21.3 (7.5) | 20.4 (7.2) | 17.6 (7.3) | 19.8 (7.2) | −3.3 (−6.0 to −0.6) | .02 |
| Adult | 13.9 (5.2) | 14.9 (5.1) | 8.8 (2.8) | 14.5 (6.1) | −5.2 (−9.2 to −1.2) | .01 |
| State, STAI-Sa | ||||||
| Child | 29.0 (5.9) | 28.6 (4.6) | 29.5 (5.2) | 28.7 (5.4) | −0.8 (−3.6 to 2.0) | .57 |
| Adult | 16.2 (7.7) | 19.3 (8.4) | 15.6 (10.1) | 19.8 (10.2) | −1.1 (−4.5 to 2.2) | .51 |
| Trait, STAI-Ta | ||||||
| Child | 31.3 (8.1) | 29.7 (7.0) | 30.1 (9.8) | 27.9 (6.1) | −1.8 (−6.4 to 2.7) | .76 |
| Adult | 16.6 (9.1) | 21.3 (9.4) | 16.2 (10.2) | 20.3 (10.7) | 0.5 (−2.7 to 3.7) | .42 |
Abbreviations: BMI, body mass index; CEV, coefficient of variation; DTSQ, Diabetes Treatment Satisfaction Questionnaire; HCL, hybrid closed loop; HFS-II, Hypoglycemia Fear Survey 2; ICR, insulin-carbohydrate ratio; MDI, multiple daily insulin; NA, not applicable; PAID, Problem Areas in Diabetes; PedsQL V3, Pediatric Quality of Life Inventory, version 3; SGL, sensor glucose level; STAI-S, State-Trait Anxiety Inventory, State Scale; STAI-T, State-Trait Anxiety Inventory, Trait Scale; TDI, total daily insulin.
Results presented as raw mean (SD) and mean difference (95% CI), analyzed using analysis of covariance with adjustment for baseline with multiple imputations.
Results presented as raw median (IQR) and median difference (95% CI), analyzed using rank-sum test with simple imputation.
Results presented as median (IQR), percent difference (95% CI), analysis using analysis of covariance with adjustment for baseline with outcome log transformed with multiple imputations.
To convert to proportion of total hemoglobin, multiply by 0.01.
The mean adjusted between-group difference in HbA1c at study end was −2.9 mmol/mol (95% CI, −5.8 to −0.1 mmol/mol) lower with HCL. There was an improvement in diabetes-specific quality of life (difference, 4.4 points; 95% CI, 0.4-8.4 points) and treatment satisfaction in the HCL group, with no change in diabetes distress and fear of hypoglycemia between groups. At study end, there were no significant differences between groups in the daily insulin requirement, basal insulin, insulin-carbohydrate ratio, or body mass index.
A post hoc analysis of the percentage of participants achieving glycemic targets was conducted. The recommended target of TIR above 70%16 was met in 31% of participants receiving HCL and 15% of participants receiving standard therapy (eFigure 4 in Supplement 3). Likewise, the glycemic target of HbA1c less than 7%27 was achieved in 16 participants (25.4%) receiving HCL and 11 participants (19.0%) receiving standard therapy.
A post hoc analysis of real-time CGM data from HCL users during the 6-month trial was also conducted. The treatment effect was evident in the first month and remained throughout the study, as shown in eFigure 5 in Supplement 3. The TIR was proportional to the percent time spent in closed loop; the baseline-adjusted coefficient for time spent in closed loop was 0.35% (95% CI, 0.16%-0.55%).
The primary outcome, TIR, was evaluated in a prestated subgroup analysis of the standard therapy (CSII and MDI), although caution should be used in interpreting these results owing to the small number of MDI participants (n = 37). Regression analysis with multiple imputation showed a treatment effect of 8.0% TIR with HCL use over CSII (baseline vs study end: control, 54.4 [11.4] mg/dL vs 55.9 [12.0] mg/dL; HCL, 53.1 [13.7] mg/dL vs 63.3 [11.7] mg/dL) whereas the difference was 1.2% with HCL compared with MDI, largely driven by an improvement in the control group (control, 49.8 [12.2] mg/dL vs 57.5 [14] mg/dL; HCL, 51.4 [12.9] mg/dL vs 58.1 [12.9] mg/dL).
Safety Outcomes
There were no episodes of severe hypoglycemia or diabetic ketoacidosis in either therapy group. There were no serious adverse events related to the trial device. A list of adverse events is provided in eTable 2 in Supplement 3 with device-related skin reactions in both the control and intervention groups.
Discussion
Although the MiniMed 670G HCL system is commercially available, this multicenter RCT study was, to our knowledge, the first RCT in children and adolescents with T1D to provide robust scientific evidence on the effectiveness of the system in improving glycemic outcomes and quality of life. In the study, HCL therapy resulted in a 6.7% improvement in TIR—a gain of an additional 1.6 hours in the target glucose range compared with conventional therapy, which comprised adolescents using CSII or MDI with or without CGM. The improvement in glycemia with HCL use aligns with findings from other observational studies in children and adolescents with T1D.3,28,29 An improvement in TIR from 60.4% to 67.2% at 3 months and from 50.7% to 56.9% at 6 months was reported by Garg et al3 and Berget et al,28 respectively. In our study, the improvement in TIR was seen across both day and night and was associated with a reduction in hypoglycemia and glycemic variability, albeit with no reduction in hyperglycemia. The clinical targets for CGM metrics, as recommended by the international consensus statement,16 were not uniformly reached in this trial, although the HCL system improved most CGM metrics.
Although closed-loop systems have improved glycemic control, the psychosocial effects remain less understood,6 with barriers to HCL use being recognized in real-world use.7 In our 6-month study, HCL users had higher scores than the control group on the diabetes-specific pediatric quality-of-life measure. Likewise, diabetes treatment satisfaction also improved with the HCL system. This improvement in outcomes could be due to the reduced worry, increased confidence and trust in the system with improved glycemic control, and increased ownership of diabetes management that were experienced by HCL users.30 This is a vital observation, as although there may be difficulties encountered in engaging with the system, users found it beneficial in their day-to-day management.
The recommended TIR of greater than 70% was achieved in fewer than 10% of the participants at baseline on conventional therapy, highlighting the complexities of diabetes management in this cohort. With the HCL system, 31% met this target. Although there was an improvement, this study highlights the existing manual interface and the conservative algorithm of the 670G that was the first commercially available version, designed for safety. The improvement in TIR is reported to be proportional to the time spent in closed loop,31 as was the case in our trial. However, over time, Berget et al reported that 30% of children and adolescents discontinued HCL in the first 6 months of use28 with the workload required to use the HCL system being cited as an important underlying reason for discontinuation.7 Sensor-related issues were also recognized as a primary concern in a 12-month observational study that reported high discontinuation rates.32 The need to maintain frequent blood glucose checks for sensor calibrations to stay in closed-loop mode and the frustration in dealing with alerts contribute to the decline in HCL use.28 The increasing trust in automation and user experience have led to further advancements in algorithms. A randomized crossover study in 60 individuals demonstrated a 12.5% improvement in TIR primarily owing to a reduction in hyperglycemia with the advanced HCL system compared with the control group using predictive low glucose suspend technology.33 This improved algorithm therefore has the potential to improve the long-term use and the health of people with T1D.34
The HCL system was safe, with no device-related adverse events. Sensor-related skin reactions were common. Most youth in the trial continued to wear the sensor despite these issues for the perceived benefits of the sensor and system, but the ability to wear and tolerate a sensor can be seen as a critical step in a wider adoption of closed-loop systems. Sensor-wear issues highlight the need for strategies in preserving skin integrity with long-term use of technological devices.35
This RCT was designed in 2016, commenced recruitment in 2017, and was completed in 2020; this timeline highlights the challenges in conducting RCTs with newer technological interventions. The landscape of technology use is constantly evolving. Newer closed-loop systems now deliver autocorrections and permit remote monitoring of glucose levels. Few RCTs have assessed glycemic outcomes with closed-loop systems in children for longer than 3 months. The improvement in TIR by 11% in 168 children (aged >14 years) and adults in a 6-month trial36 and in 101 children (aged 6-13 years) in a 4-month trial37 with the Control-IQ system (Tandem Diabetes Care) is reflective of the further advancements in algorithms. A subanalysis in the 63 adolescents and young adults (aged 14-24 years) in the 6-month study also showed a significant improvement in TIR by 13%,38 reflecting the more aggressive parameters in the algorithm settings. Thabit et al39 also reported an 8.9% TIR difference between the closed-loop system and sensor-augmented pump in a 12-week crossover trial in 25 children. The Fuzzy Logic Automated Insulin Regulation (FLAIR) study compared the commercially available MiniMed 670G pump to an investigational advanced HCL system incorporating the MD-Logic algorithm and reported a reduction in hyperglycemia without increased hypoglycemia with the newer system.40 In other words, as algorithms become more advanced with more flexible settings for glucose targets and active insulin time, the clinical availability of these systems should increase the opportunities available to choose a device that best suits the needs of children and adolescents with diabetes.
Strengths and Limitations
The strength of our study was the 6-month multicenter RCT design. The intensity of RCTs and time to completion may not be acceptable for the fast pace of rapidly evolving technology; however, there remains a paucity of robust data with predominantly short-term observational studies that can be translated into real-world outcomes. Likewise, psychosocial outcomes also require longer studies to effectively analyze the effect of the intervention on the daily life of the individual with T1D. The control group in this study represented the currently available contemporary therapies.
There were also limitations to the trial, as the trial population was not fully representative of the general T1D population with respect to device usage (higher representation of CSII) and was conducted by tertiary centers with technology expertise. Further, the study excluded participants with higher glycated hemoglobin levels and those who could not speak English. This study was originally designed to have an equal number of participants using CSII and MDI; however, recruitment of MDI participants was limited owing to the relatively high use of CSII in children in Australia (41%).9 We were also unable to ascertain the degree of engagement of participants in the control group with their CGM devices.
Conclusions
To our knowledge, this is the first RCT in youth with T1D to provide conclusive evidence that HCL improves glycemic outcomes and quality of life in youth with T1D. The HCL system represents an important step in the pursuit of technological advancements toward a fully automated closed-loop system.
Trial Protocol
Statistical Analysis Plan
eTable 1. Missing Data for Outcomes in Trial
eTable 2. Adverse Events in the Trial
eFigure 1. A Schematic Representation of the HCL System
eFigure 2. Subgroup Analysis by Day and Night
eFigure 3. Analysis of Glycemic Outcomes
eFigure 4. Cumulative Distribution of Time in Range in Participants Assigned to HCL and Control Group
eFigure 5. Real-Time CGM Data From HCL Participants
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501-512. doi: 10.1016/S2213-8587(17)30167-5 [DOI] [PubMed] [Google Scholar]
- 2.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. doi: 10.1001/jama.2016.11708 [DOI] [PubMed] [Google Scholar]
- 3.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155-163. doi: 10.1089/dia.2016.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAuley SA, Lee MH, Paldus B, et al. ; Australian JDRF Closed-Loop Research Group . Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020;43(12):3024-3033. doi: 10.2337/dc20-1447 [DOI] [PubMed] [Google Scholar]
- 5.Miller KM, Foster NC, Beck RW, et al. ; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971-978. doi: 10.2337/dc15-0078 [DOI] [PubMed] [Google Scholar]
- 6.Farrington C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Diabet Med. 2018;35(4):436-449. doi: 10.1111/dme.13567 [DOI] [PubMed] [Google Scholar]
- 7.Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21(2):319-327. doi: 10.1111/pedi.12971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt G. Free continuous glucose monitoring devices for young Australians. April 1, 2017. Accessed July 7, 2020. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/free-continuous-glucose-monitoring-devices-for-young-australians
- 9.Australasian Diabetes Data Network . Accessed September 8, 2021. https://www.addn.org.au/research
- 10.Phelan H, Clapin H, Bruns L, et al. The Australasian Diabetes Data Network: first national audit of children and adolescents with type 1 diabetes. Med J Aust. 2017;206(3):121-125. doi: 10.5694/mja16.00737 [DOI] [PubMed] [Google Scholar]
- 11.de Bock M, McAuley SA, Abraham MB, et al. ; Australian JDRF Closed-Loop Research Group . Effect of 6 months hybrid closed-loop insulin delivery in young people with type 1 diabetes: a randomised controlled trial protocol. BMJ Open. 2018;8(8):e020275. doi: 10.1136/bmjopen-2017-020275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAuley SA, de Bock MI, Sundararajan V, et al. Effect of 6 months of hybrid closed-loop insulin delivery in adults with type 1 diabetes: a randomised controlled trial protocol. BMJ Open. 2018;8(6):e020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20(4):314-316. doi: 10.1089/dia.2017.0455 [DOI] [PubMed] [Google Scholar]
- 14.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330(7495):843. doi: 10.1136/bmj.330.7495.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care. 2016;39(7):1175-1179. doi: 10.2337/dc15-2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. doi: 10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 diabetes module. Diabetes Care. 2003;26(3):631-637. doi: 10.2337/diacare.26.3.631 [DOI] [PubMed] [Google Scholar]
- 18.Weissberg-Benchell J, Antisdel-Lomaglio J. Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatr Diabetes. 2011;12(4 Pt 1):341-344. doi: 10.1111/j.1399-5448.2010.00720.x [DOI] [PubMed] [Google Scholar]
- 19.Bradley C. The diabetes treatment satisfaction questionnaire: DTSQ. In: Bradley C, ed. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Harwood Academic Publishers; 1994:111-132. [Google Scholar]
- 20.Gonder-Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with type 1 diabetes and their parents. Diabetes Manag (Lond). 2011;1(6):627-639. doi: 10.2217/dmt.11.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. 1998;36(7-8):777-788. doi: 10.1016/S0005-7967(98)00023-0 [DOI] [PubMed] [Google Scholar]
- 22.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17(7):697-703. doi: 10.2337/diacare.17.7.697 [DOI] [PubMed] [Google Scholar]
- 23.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33(1):17-22. doi: 10.2337/dc09-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardt J, Herke M, Leonhart R. Auxiliary variables in multiple imputation in regression with missing X: a warning against including too many in small sample research. BMC Med Res Methodol. 2012;12:184. doi: 10.1186/1471-2288-12-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp . Stata Statistical Software: Release 16. 2019; StataCorp LLC. [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2020.
- 27.DiMeglio LA, Acerini CL, Codner E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(suppl 27):105-114. doi: 10.1111/pedi.12737 [DOI] [PubMed] [Google Scholar]
- 28.Berget C, Messer LH, Vigers T, et al. Six months of hybrid closed loop in the real-world: An evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21(2):310-318. doi: 10.1111/pedi.12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. Safety evaluation of the MiniMed 670G system in children 7-13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):11-19. doi: 10.1089/dia.2018.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts A, Abraham M, Fried L, Dart J, Davis E, Jones T. Experiences learned from individuals managing type 1 diabetes using closed loop systems. Diabetes Technol Ther J. 2021;23(S2):A1-A206. doi: 10.1089/dia.2021.2525.abstracts [DOI]
- 31.Messer LH, Forlenza GP, Sherr JL, et al. Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care. 2018;41(4):789-796. doi: 10.2337/dc17-1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42(12):2190-2196. doi: 10.2337/dc19-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969-975. [DOI] [PubMed] [Google Scholar]
- 34.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400-405. doi: 10.2337/dc18-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messer LH, Berget C, Beatson C, Polsky S, Forlenza GP. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol Ther. 2018;20(S2):S254-S264. doi: 10.1089/dia.2018.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SA, Kovatchev BP, Raghinaru D, et al. ; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. doi: 10.1056/NEJMoa1907863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breton MD, Kanapka LG, Beck RW, et al. ; iDCL Trial Research Group . A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020;383(9):836-845. doi: 10.1056/NEJMoa2004736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isganaitis E, Raghinaru D, Ambler-Osborn L, et al. ; iDCL Trial Research Group . Closed-loop insulin therapy improves glycemic control in adolescents and young adults: outcomes from the international diabetes closed-loop trial. Diabetes Technol Ther. 2021;23(5):342-349. doi: 10.1089/dia.2020.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thanit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129-2140. doi: 10.1056/NEJMoa1509351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergenstal RM, Nimri R, Beck RW, et al. ; FLAIR Study Group . A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397(10270):208-219. doi: 10.1016/S0140-6736(20)32514-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Missing Data for Outcomes in Trial
eTable 2. Adverse Events in the Trial
eFigure 1. A Schematic Representation of the HCL System
eFigure 2. Subgroup Analysis by Day and Night
eFigure 3. Analysis of Glycemic Outcomes
eFigure 4. Cumulative Distribution of Time in Range in Participants Assigned to HCL and Control Group
eFigure 5. Real-Time CGM Data From HCL Participants
Nonauthor Collaborators
Data Sharing Statement