Abstract
Objectives
This study aimed to determine the effect of melatonin on thrombosis, sepsis, and mortality rate in adult patients with severe coronavirus infection (COVID-19).
Methods
This single-center, prospective, randomized clinical trial was conducted from 1 December 2020 to 1 June 2021 at Al-Shifaa hospital in Mosul, Iraq. There were 158 patients with severe COVID-19 included in the study: 82 in the melatonin group (who received 10 mg melatonin in addition to standard therapeutic care) and 76 in the control group (given standard therapeutic care only). Patients were chosen by a blocked randomization design. The physician then evaluated and recorded the incidence of thrombosis, sepsis, and mortality rate on days 5, 11, and 17 of symptoms.
Results
The intervention group consisted of 82 patients, while the control group consisted of 76 patients. In comparison to the control group, thrombosis and sepsis developed significantly less frequently (P < 0.05) in the melatonin group during the second week of infection, while mortality was significantly higher in the control group (P < 0.05).
Conclusions
Adjuvant use of melatonin may help to reduce thrombosis, sepsis, and mortality in COVID-19 patients.
Keywords: COVID-19, Melatonin, Clinical trial
Introduction
Since December 31, 2019, when the People's Republic of China's health authorities notified the World Health Organization of several cases of pneumonia with an unknown etiology in the city of Wuhan, called Coronavirus 2019 (COVID-19), the infection has spread throughout the world, with 170 million confirmed cases and more than 3.5 million deaths at the time of article writing (on June, 2020) (according to Worldometer 2021).
Thrombotic complications are common in COVID-19 and associated with a significant increase in mortality and morbidity. COVID-19 may also enhance sepsis-induced hypercoagulability (Barnes, et al. 2020).
While COVID-19 infection continues to spread globally and the need for urgent adjuvant treatment increases, clinical trials are underway to investigate the use of appropriate immunosuppressive and immunomodulatory agents, and also specific drugs to target individual pro-inflammatory cytokines (Soy et al., 2020). There is a need for drugs that could diminish some of the effects of the potentially deadly immune response.
Melatonin is a multifunctional molecule that has been shown to have antioxidant, anti-inflammatory, and immunomodulatory properties. Melatonin has been shown to be involved in the regulation of sleep and blood pressure, as well as in the improvement of viral respiratory disorders. As a result of these properties, recent publications have recommended melatonin for its potentially beneficial effects on clinical outcomes in patients with COVID-19 (Huang, et al. 2020; Slominski, et al. 2018). However, there are few clinical and laboratory studies on the adjunctive use of melatonin in COVID-19 infection: one used melatonin 9 mg/day (Gholamreza, et al. 2020) and there are two ongoing registered trials (Ameri et al., 2021, Rodríguez-Rubio and Figueira, 2020). Thus, the current randomized clinical trial was designed and conducted to compare the efficacy of adding oral melatonin to standard treatment in hospitalized adult patients with severe COVID-19 infection.
Methods
This study was a single-center, open-label, randomized clinical trial to determine the effect of melatonin on thrombosis, sepsis, and mortality in adults with COVID-19 admitted to Al-Shifaa Hospital in Mosul, Iraq, from 1 December 2020 to 1 June 2021. The diagnosis of COVID-19 was confirmed using reverse transcriptase-polymerase chain reaction (RT-PCR), as well as findings consistent with COVID-19 pneumonia on computed tomography (CT) or chest radiography. Thompson's equation was used to determine the sample size (Thompson, 2012). Patients who met the inclusion and exclusion criteria were eligible. Hospitalized patients with confirmed severe COVID-19 infection and aged >18 years or <80 years were included in the study. The following criteria were used to exclude individuals: patients aged <18 or >80 years, known melatonin allergy, pregnancy, lactating female, renal impairment, liver impairment, autoimmune disease, cancer, or terminal medical illness. The ethical committee of Baghdad university/college of medicine approved the study protocol (approval number: 20201110872).
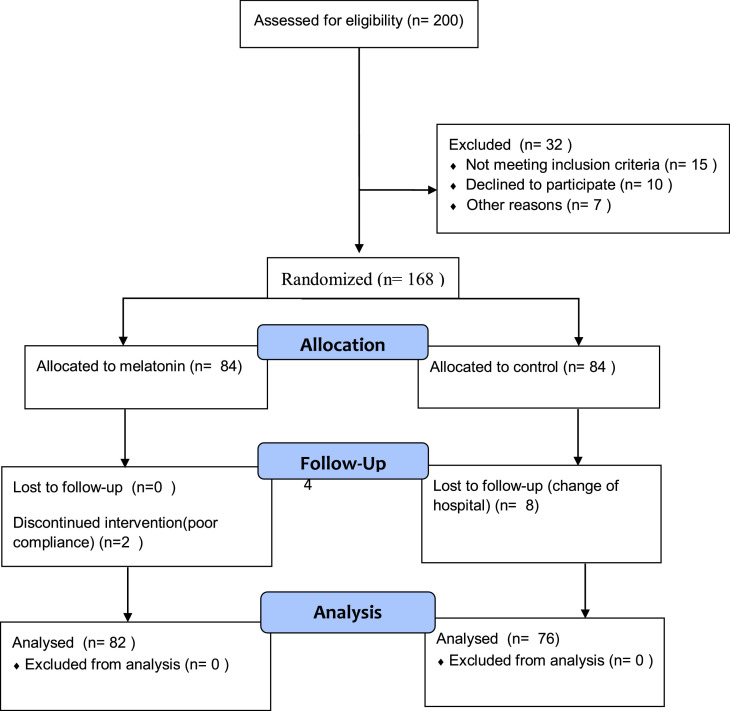
A total of 158 patients were enrolled in the current study (Figure 1 ). They were randomly assigned to intervention or control groups using a random block design. The two treatment groups were (A) melatonin and (B) control, and the block size was 2 × 2=4. Within each block, the treatment allocation was as follows: (1) AABB, (2) BBAA, (3) ABAB, (4) BABA, (5) ABBA, and (6) BAAB. All patients in the control group received standard therapy: oxygen therapy; antiviral agents: remdesivir (day 1, 200 mg intravenous infusion over 1 hour, then 100 mg intravenous infusion over 1 hour on days 2, 3, 4 and 5); antibacterial agents: levofloxacin 500 mg/day intravenously was used empirically for secondary bacterial infections; corticosteroids: dexamethasone 24 mg/day intravenously; and anticoagulants: enoxaparin 6000 units once daily for prophylaxis and twice daily for therapeutic treatment of thrombosis. All of the intervention group patients received standard therapy plus 10 mg melatonin (Natrol®) once daily 20-30 minutes before bed time for 14 days following diagnosis. The physician who administered melatonin was also responsible for treatment and assessment of each patient's condition.
Figure 1.
Flow diagram illustrating the progress of patients through the trial.
Thrombosis was diagnosed clinically and in the laboratory as pulmonary embolism, deep venous thrombosis (DVT), and ischemic stroke: imaging studies (computed tomography or magnetic resonance imaging of the brain), doppler ultrasound for DVT, CT angiography, and D-dimer were ordered in accordance with the clinical presentation, and sepsis was diagnosed on days 5, 11, and 17 of symptoms by a physician. All data were entered into a computer database.
Statistical analysis
SPSS software was used to conduct statistical analyses (version 20.0; IBM). The categorical variables (age, gender, and comorbidities such as hypertension, ischemic heart disease, diabetes mellitus, and asthma) were expressed as counts and percentages of patients in the melatonin and control groups, respectively. Chi-square and Fisher exact tests were used to compare these variables. The Kolmogorov-Smirnov test was used to determine the normality of the data. Non-parametric statistical methods were employed for not normally distributed continuous variables, Mann-Whitney U test was used in variable between-group differences. Chi-square and Fisher's exact tests were used to compare categorical variables between the two groups (thrombosis, sepsis, and death). All analyses were conducted on a two-sided basis, with P ≤ 0.05 considered as statistically significant.
Results
The baseline demographic and clinical characteristics of the patients in both groups are shown in (Table 1 ). Males made up 72.2% of the patients in the current study, while females made up 27.8%, and there was no significant difference in gender between the two groups (P > 0.05). The study population was 56.3±7.3 years old (mean±SD), with a range of 32–78 years, and there was no significant difference in age between the two groups (P > 0.05). Other comorbidities were present in 70.3% of patients, and there was no significant difference between the two groups in relation to hypertension, ischemic heart disease, asthma, and diabetes mellitus (P > 0.05) (see Table 1).
Table 1.
Demographic and clinical characteristics of COVID-19 patients in melatonin and control groups.
| Patient characteristics | All patients (n=158) | Control group (n=76) | Melatonin group (n=82) | df | P-value |
|---|---|---|---|---|---|
| Age (mean±SD) | 56.3±7.7 | 55.7±8.0 | 56.8±7.5 | . | 0.393 ͣ |
| Gender | |||||
| Male, n (%) | 114 (72.2%) | 56 (73.7%) | 58 (70.7%) | 1 | 0.725° |
| Female, n (%) | 44 (27.8%) | 20 (26.3%) | 24 (29.3%) | ||
| Other comorbidities Hypertension | |||||
| 84 (53.2%) | 34 (44.7%) | 50 (61.0%) | 1 | 0.055° | |
| Ischemic heart disease | 25 (15.8%) | 10 (13.2%) | 15 (18.3%) | 0.394° | |
| 47 (29.7%) | 22 (28.9%) | 25 (30.5%) | 0.863° | ||
| Diabetes mellitus | |||||
| Asthma | 16 (10.1%) | 11 (14.5%) | 5 (6.1%) | 0.113° |
df, degree of freedom; SD, standard deviation; n, number of patients; %, percentage of patients in each group; °, no significant difference (P > 0.05) using Chi-square test; ͣ, no significant difference (P > 0.05) using Mann-Whitney U test
Effect of melatonin on developing thrombosis in COVID-19 patients
The Fisher's exact test revealed no significant difference between the two groups at baseline (day 5 of symptoms) (P = 1.000). Additionally, no significant difference in developing thrombosis was observed between the two groups on day 11 (P = 1.000), while developing thrombosis was significantly greater in the control group than in the melatonin group on day 17 (P < 0.05) (see Table 2 ).
Table 2.
Effect of melatonin on thrombosis in COVID-19 patients.
| All patients (n=158) | Melatonin group (n=82) | Control group (n=76) | df | P-value | |
|---|---|---|---|---|---|
| Day 5 | 1 (0.6%) | 1 (1.2%) | 0 (0.0%) | 1 | 1.000 |
| Day11 | 6 (3.8%) | 3 (3.7%) | 3 (3.9%) | 1 | 1.000 |
| Day17 | 27 (17.1%) | 9 (11.0%) | 18 (23.7%) | 0.037* |
n, number of patients; df, degree of freedom
*Significant using Fisher's exact test
A binary logistic regression model was used, in which melatonin use, age, gender, hypertension, ischemic heart disease, diabetes mellitus, and asthma were considered as explanatory variables, and thrombosis as the dependent variable. Considering the Exp (β), an odds ratio = 1 showed no effect; an odds ratio >1 showed a variable increase in the odds of outcome target level; and an odds ratio <1 indicated the variable in question decreased the odds of the outcome target level (Garson, 2013). From the odds ratio evaluation, the probability of developing thrombosis in patients using melatonin (in the melatonin group) was 0.309 times less likely compared to patients who didn't use melatonin (in the control group) (see Table 3 ).
Table 3.
Logistic regression analysis to determine melatonin use associated with thrombosis in COVID-19 patients.
| B | SE | Wald | df | Sig. | Exp. (B) | 95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Melatonin | -1.174 | 0.504 | 5.428 | 1 | 0.020* | 0.309 | 0.115 | 0.830 |
| Age | 0.099 | 0.039 | 6.419 | 1 | 0.011* | 1.105 | 1.023 | 1.193 |
| Gender | -0.635 | 0.579 | 1.201 | 1 | 0.273 | 0.530 | 0.170 | 1.650 |
| Hypertension | -0.577 | 0.511 | 1.274 | 1 | 0.259 | 0.561 | 0.206 | 1.530 |
| Ischemic heart disease | 1.335 | 0.564 | 5.599 | 1 | 0.018* | 3.800 | 1.258 | 11.481 |
| Diabetes mellitus | 0.735 | 0.508 | 2.093 | 1 | 0.148 | 2.086 | 0.770 | 5.648 |
| Asthma | 0.649 | 0.688 | 0.891 | 1 | 0.345 | 1.914 | 0.497 | 7.367 |
| Constant | -7.048 | 2.217 | 10.103 | 1 | 0.001* | 0.001 | ||
B, coefficient; SE, standard error of B; df, degree of freedom; Exp. (B), estimated odds ratio; CI, confidence interval for exp (B)
*Significant using binary logistic regression
Effect of melatonin on developing sepsis in COVID-19 patients
No patients developed sepsis during the baseline period (day 5 of symptoms) (no between-group difference). On day 11, two patients (2.4%) in the melatonin group developed sepsis, compared to eight patients (10.5%) in the control group. Chi-square analysis revealed a significant difference between the two groups (P = 0.050). On day 17, sepsis developed significantly more frequently in patients in the control group (35.5%) than in patients in the melatonin group (8.5%) (P = 0.000) (see Table 4 ).
Table 4.
Effect of melatonin on sepsis in COVID-19 patients.
| All patients (n=158) | Melatonin group (n=82) | Control group (n=76) | Df | P-value | |
|---|---|---|---|---|---|
| Day 5 | 0 (0%) | 0 (0%) | (0%) | 1 | 1.000 |
| Day11 | 10 (6.3%) | 2 (2.4%) | 8 (10.5%) | 1 | 0.050* |
| Day17 | 34 (21.5%) | 7 (8.5%) | 27 (35.5%) | 1 | 0.000* |
n, number of patients; df, degree of freedom
*Significant using Fisher's exact test
From the odds ratio evaluation in the binary logistic regression analysis, the probability of developing sepsis in patients who used melatonin (in the melatonin group) was 0.071 times less likely compared with those who did not use melatonin (in the control group) (Table 5 ).
Table 5.
Logistic regression analysis to determine melatonin use associated with sepsis in COVID-19 patients.
| B | SE | Wald | df | Sig. | Exp. (B) | 95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Melatonin | -2.642 | 0.624 | 17.949 | 1 | 0.000* | 0.071 | 0.021 | 0.242 |
| Age | 0.157 | 0.046 | 11.766 | 1 | 0.001* | 1.170 | 1.070 | 1.279 |
| Gender | -0.563 | 0.620 | 0.825 | 1 | 0.364 | 0.569 | 0.169 | 1.920 |
| Hypertension | -0.520 | 0.556 | 0.876 | 1 | 0.349 | 0.594 | 0.200 | 1.766 |
| Ischemic heart disease | 0.622 | 0.661 | 0.885 | 1 | 0.347 | 1.862 | 0.510 | 6.803 |
| Diabetes mellitus | 2.243 | 0.597 | 14.131 | 1 | 0.000* | 9.418 | 2.925 | 30.322 |
| Asthma | 1.829 | 0.798 | 5.250 | 1 | 0.022* | 6.225 | 1.303 | 29.744 |
| Constant | -12.918 | 2.910 | 19.704 | 1 | 0.000* | 0.000 | ||
B, coefficient; SE, standard error of B; df, degree of freedom; Exp (B), estimated odds ratio; CI, confidence interval for exp (B)
*Significant using binary logistic regression
Effect of melatonin on mortality rate in COVID-19 patients
According to the Chi-square test, the mortality rate was significantly higher in the control group (17.1%) than in the melatonin group (1.2%), df = 1, (P = 0.001) (see Table 6 ).
Table 6.
Effect of melatonin on mortality in COVID-19 patients.
| All patients (n=158) | Melatonin group (n=82) | Control group (n=76) | df | P-value | |
|---|---|---|---|---|---|
| Death | 14 (8.9%) | 1 (1.2%) | 13 (17.1%) | 1 | 0.000* |
n, number of patients; df, degree of freedom
*Significant using Fisher's exact test
Discussion
The COVID-19 pandemic has infected and killed millions of people worldwide. These large numbers have necessitated rapid development of clinical trials to evaluate therapies capable of lowering the alarmingly high death rate; as a result, a large number of drugs have been studied in COVID-19 patients. Melatonin's efficacy as an adjunctive therapy has been demonstrated in a variety of diseases (Biancatelli, et al. 2020; Zhang, et al. 2020); however, there are few trials evaluating the use of melatonin in patients with COVID-19 (Gholamreza, et al. 2020; Ameri et al., 2021, Rodríguez-Rubio and Figueira, 2020). The current randomized trial evaluated the efficacy and safety of 10 mg oral melatonin as an adjunctive therapy in patients hospitalized with severe COVID-19. It has been found that melatonin administration could alleviate viral infection-induced oxidative stress as well as increase antioxidant activity (Habtemariam, et al. 2017). The current study evaluated three clinical complications: thrombosis, sepsis, and mortality rate.
Effect of melatonin on thrombosis in COVID-19 patients
Coronaviruses have been shown to enter cells via angiotensin-converting enzyme 2 (ACE-2) receptors, which are predominantly found on the alveolar epithelium and endothelium. Endothelial cell activation is thought to be the primary cause of thrombosis. Inclusion bodies from viruses have been identified in endothelial cells from a variety of organs, including the lungs and gastrointestinal tract (Varga, et al. 2020). Rapid viral replication results in the release of large amounts of inflammatory mediators. One theory has proposed that neutrophil extracellular traps (NETs) could be the source of hypercoagulation in severe COVID-19 patients. High NET levels in the blood are associated with elevated thrombin levels, which are predictive of adverse cardiac events that can result in major organ damage (Barnes, et al. 2020). In severe cases of COVID-19, a retrospective analysis of 452 patients revealed a significant increase in neutrophil counts (Qin, et al. 2020). NETs have the ability to alter endothelial barrier structures, resulting in an increase in vascular endothelial permeability and a decrease in anti-thrombotic and anti-inflammatory properties (Ma, et al. 2019; Hernández-Reséndiz, et al. 2018). Local injection of melatonin (140 pg) into endothelial cells effectively reduced endothelial cell vascular permeability induced by leukotriene B4-activated neutrophils, as demonstrated in an in vivo rodent experiment. Melatonin's inhibition of endothelial cell hyper-adhesiveness likely mediated the decrease in vascular permeability (Lotufo, et al. 2006). In 46 healthy young men, oral melatonin (3 mg) administration resulted in an inverse relationship between procoagulant measures, with increased plasma melatonin predicting lower FVIII:C (P = 0.037) and fibrinogen (P = 0.022) levels (Wirtz, et al. 2008).
An increased D-dimer level is one of the most consistent findings. Although numerous inflammatory processes can affect D-dimer levels, they almost certainly reflect intravascular thrombosis to some extent in patients with COVID-19 (Leonard-Lorant, et al. 2020; Cui, et al. 2020). An elevated D-dimer level (>1000 ng/mL) at admission was associated with an increased risk of in-hospital death in the early studies emerging from China (Zhou, et al. 2020). The true prevalence of COVID-19-associated thrombosis is unknown, as the majority of studies to date have lacked systematic and comprehensive investigation protocols. As a result, individuals infected with COVID-19 face a risk of venous thromboembolism of up to 25% (Bikdeli, et al. 2020; Chen, et al 2020).
At day 17 of symptoms, 23.7% of patients in the control group developed thrombosis, compared with 11% in the melatonin group (P < 0.05). The aforementioned data are consistent with this study, which established a significant effect of melatonin use on thrombosis.
Effect of melatonin on sepsis in COVID-19 patients
Sepsis is defined as organ dysfunction that is potentially fatal as a result of an abnormal host response to infection (Singer, et al. 2016). Sepsis may be caused by a number of different pathogens but is primarily caused by bacterial infection. However, up to 42% of sepsis patients have negative cultures, implying a nonbacterial cause (Phua, et al. 2013). Although almost any virus can cause sepsis in susceptible patients, viral sepsis is a very rare clinical diagnosis. Increased awareness, early detection of viral sepsis, rapid administration of appropriate antiviral medications, and prompt treatment can significantly reduce viral sepsis-related deaths (Reinhart Konrad, et al. 2017). With time, a significant difference in the development of sepsis was observed in the current study between the melatonin and control groups (at day 17 of symptoms, 35.5% of patients in the control group developed sepsis versus 8.5% in the melatonin group) (P < 0.001). Although no study has been conducted on the effects of melatonin on sepsis in COVID-19 patients, a recent clinical trial found that intravenous administration of 60 mg/day of a melatonin formulation improved septic patients, decreased their mortality to zero, and decreased their hospital stay by 40% (Dario, et al. 2020). Another trial used 9 mg of melatonin per day in COVID-19 patients and showed a significant reduction in hospital stay duration (P < 0.05) and improved clinical symptoms as well as the level of CRP and pulmonary involvement in the melatonin group (P < 0.05) (Gholamreza, et al. 2020). This trial tested the effect of a 10 mg melatonin tablet, as it is the available dosage form in Turkey and assured good compliance.
Effect of melatonin on mortality rate in COVID 19 patients
As previously demonstrated, thrombosis and sepsis are associated with a higher mortality rate and were improved by melatonin administration. As a result, it is entirely reasonable that melatonin resulted in a lower mortality rate in COVID-19 patients. This implied that the effect of melatonin was quantified directly in this study, which found that 17.1% of in-hospital patients in the control group died compared with 1.2% of patients in the melatonin group (P < 0.001).
Limitations of this trial were the open labeled and single-centere design, which could have restricted the generalizability of the results.
Conclusion
The results of this study demonstrated that oral melatonin, when added to standard of care, was more effective than standard of care alone in patients hospitalized with severe COVID-19. Improved thrombosis, sepsis, and mortality rates support the adjuvant of melatonin's efficacy in mitigating this infectious disease. Given melatonin's superior performance as a cheap, highly safe, and readily available medication, it is strongly recommended that this be addressed in future studies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The patients provided informed consent to provide specimen and clinical data, and the study received approval by the ethical committee of Baghdad University/college of medicine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I would like to express my deep thanks to assistant professor Dr. Mohammed Qasim Yahya for his great and unlimited support and a special thanks to Dr. Ahmed Kayes Mehuaiden for his contribution.
References
- Ameri A, Frouz M, Manoochehr, et al. Evaluation of the effect of melatonin in patients with COVID-19-induced pneumonia admitted to the Intensive Care Unit: A structured summary of a study protocol for a randomized controlled trial. Iranian registry of clinical trial. 2021 doi: 10.1186/s13063-021-05162-3. the registration number (IRCT20200506047323N7). available at https://doi. org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;(6):217. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancatelli RMLC, Berrill M, Mohammed YH, Marik PE. Melatonin for the treatment of sepsis: The scientific rationale. J Thorac Dis. 2020;12(Suppl 1):54–S65. doi: 10.21037/jtd.2019.12.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dario A, Germaine E, Juan C, et al. Clinical trial to test the efficacy of melatonin in COVID-19. J Pineal Res. 2020;20:83. doi: 10.1111/jpi.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson DG. Statistical Associates Publishers; Asheboro, NC, USA: 2013. Logistic Regression: Binary & Multinomial; pp. 1–52. [Google Scholar]
- Gholamreza, Mostafa, Taleb, et al. Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial. (2020) Clinical Trials.gov Identifier: NCT0440952 [DOI] [PMC free article] [PubMed]
- Habtemariam S, Daglia M, Sureda A, Selamoglu Z, Fuat Gulhan M, Mohammad Nabavi S. Melatonin and respiratory diseases: a review. Curr Top Med Chem. 2017;17:467–488. doi: 10.2174/1568026616666160824120338. [DOI] [PubMed] [Google Scholar]
- Hernández-Reséndiz S, Muñoz-Vega M, Contreras WE, et al. Responses of endothelial cells towards ischemic conditioning following acute myocardial infarction. Cond Med. 2018;1(5):247–258. [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;95:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard-Lorant I, Delabranche X, Severac F, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;296(3) doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotufo CM, Yamashita CE, Farsky SH, et al. Melatonin effect on endothelial cells reduces vascular permeability increase induced by leukotriene B4. Eur J Pharmacol. 2006:258–263. doi: 10.1016/j.ejphar.2006.01.050. 534 534 (1-3) [DOI] [PubMed] [Google Scholar]
- Ma Y, Yang X, Chatterjee V, et al. Role of neutrophil extracellular traps and vesicles in regulating vascular endothelial permeability. Front Immunol. 2019;10:1037. doi: 10.3389/fimmu.2019.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 2013;17:202. doi: 10.1186/cc12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan. China. Clin Infect. 2020;10:248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart KR, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rubio M, Figueira JC, et al. A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial. EU Clinical Trials Register. Date of trial registration: 10 July 2020. URL: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001808-42/ES. [DOI] [PMC free article] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J Invest Dermatol. 2018;138:490–499. doi: 10.1016/j.jid.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SK. Sampling. 2012;3:59–60. [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz PH, Spillmann M, Bärtschi C, et al. Oral melatonin reduces blood coagulation activity: a placebo-controlled study in healthy young men. J. Pineal Res. 2008;44(2):127–133. doi: 10.1111/j.1600-079X.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- Worldometer. COVID-19 Coronavirus Pandemic. Available at: https://www.worldometers.info/coronavirus /. Accessed 1 June 2021.
- Zhang R, Wang X, Ni L, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]