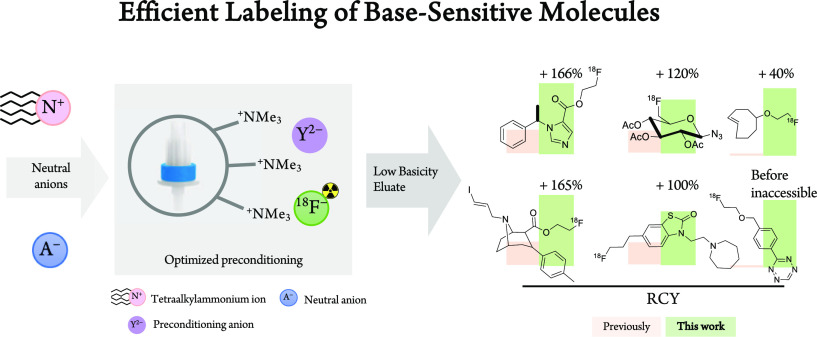
Abstract
Aliphatic nucleophilic substitution (SN2) with [18F]fluoride is the most widely applied method to prepare 18F-labeled positron emission tomography (PET) tracers. Strong basic conditions commonly used during 18F-labeling procedures inherently limit or prohibit labeling of base-sensitive scaffolds. The high basicity stems from the tradition to trap [18F]fluoride on anion exchange cartridges and elute it afterward with basic anions. This sequence is used to facilitate the transfer of [18F]fluoride from an aqueous to an aprotic organic, polar reaction medium, which is beneficial for SN2 reactions. Furthermore, this sequence also removes cationic radioactive contaminations from cyclotron-irradiated [18O]water from which [18F]fluoride is produced. In this study, we developed an efficient elution procedure resulting in low basicity that permits SN2 18F-labeling of base-sensitive scaffolds. Extensive screening of trapping and elution conditions (>1000 experiments) and studying their influence on the radiochemical yield (RCY) allowed us to identify a suitable procedure for this. Using this procedure, four PET tracers and three synthons could be radiolabeled in substantially higher RCYs (up to 2.5-fold) compared to those of previously published procedures, even from lower precursor amounts. Encouraged by these results, we applied our low-basicity method to the radiolabeling of highly base-sensitive tetrazines, which cannot be labeled using state-of-art direct aliphatic 18F-labeling procedures. Labeling succeeded in RCYs of up to 20%. We believe that our findings facilitate PET tracer development by opening the path toward simple and direct SN2 18F fluorination of base-sensitive substrates.
Keywords: fluorine-18, aliphatic radiolabeling, anion-exchange, QMA, base sensitivity, elution conditions
Positron emission tomography (PET) is a powerful and versatile molecular imaging tool to diagnose disease or monitor treatment progress.1−3 The most widely used PET radionuclide is fluorine-18 (18F), as it can be produced in large amounts (>300 GBq) and possesses almost ideal nuclear decay characteristics for molecular imaging.4 Its low positron energy ensures high image resolution, while the half-life of approximately 110 min allows for production of 18F radiopharmaceuticals for a large number of patients and their distribution to remote sites several hundred kilometers away.2,5 Nucleophilic aliphatic 18F fluorination (SN2) is one of the most widely applied 18F-radiolabeling methods.6,7 However, the standard approach (Figure 1) to purify and concentrate [18F]fluoride requires strong bases.8−10 The resulting basic environment hinders (or even prevents) 18F fluorination of base-sensitive substrates while triggering side reactions such as hydrolysis, elimination and/or decomposition of precursors/products.8,11−13 To address this challenge, a wide variety of methods to carry out SN2 18F fluorinations under less basic conditions have been developed over the last decades.8,14−20 However, none of these methods appear to be ideal, as they only utilize a fraction of the available radioactivity, need special and nonstandard precursors/equipment, or are difficult to implement.21−23 Recently, Mossine et al. have shown that replacement of strong basic anions with weak organic bases significantly increased the radiochemical yields (RCY) for Cu-mediated aromatic 18F fluorinations.8 In light of this, we decided to explore the relationships between the [18F]fluoride elution efficiency for a given preconditioning/eluting anion combination and its potential to activate [18F]fluoride for SN2 reactions (Figure 1). We hypothesized that by carefully mapping elution conditions and analyzing the corresponding SN2 18F fluorination yields we would be able to tailor the anion combination in such way as to efficiently radiolabel base-sensitive substrates, which cannot currently be radiolabeled using standard reaction conditions (Figure 1).
Figure 1.
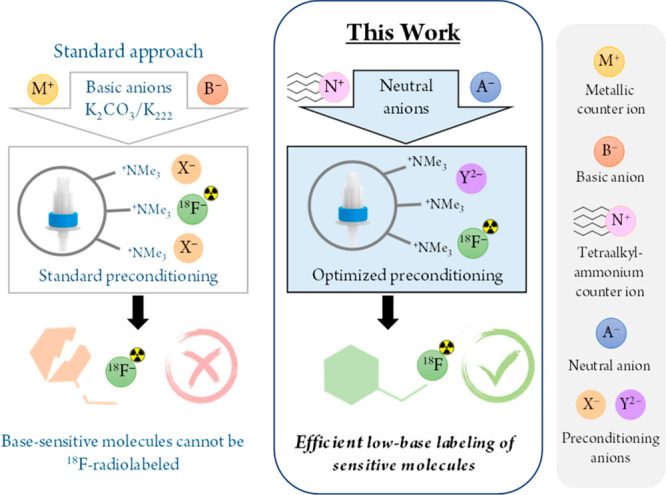
Optimization of [18F]fluoride elution method. Standard approach (left) promotes side reactions and precludes the labeling of base-sensitive molecules, while the careful choice of anions to exchange with [18F]fluoride promotes labeling (right).
Results and Discussion
Nomenclature and Rationale
The RCY of a labeling procedure is a measure of the proportion of decay corrected and isolated product with respect to the starting radioactivity. Consequently, all steps of a labeling procedure contribute to the RCY.24 We reasoned that the RCY of an SN2 18F fluorination is primarily determined by two factors: (1) the elution efficiency (EE) of the trapped [18F]fluoride and (2) the reaction efficiency (radiochemical conversion, RCC) (Figure 2A).25,26 The latter can be optimized with respect to time, precursor amount, solvent, and temperature as well as with respect to the basicity of the reaction medium and the solubility and activation of [18F]fluoride. Whereas the first four parameters are frequently optimized,27 the last three are often neglected, even though they are crucial for the labeling of base-sensitive substrates, as they determine the base concentration of the reaction. The aforementioned parameters are strongly dependent on how the anion exchange cartridge (AEC) is preconditioned and eluted.12 In order to study the influence of preconditioning and elution conditions on the RCY, we decided to investigate the EE and the RCC independently. To approximate the expected efficiency of the whole labeling procedure (RCY), we defined a theoretical measure, which we named the pseudo radiochemical yield (pRCY) − pRCY = EE × RCC. This measure was used to evaluate the applied labeling conditions.
Figure 2.
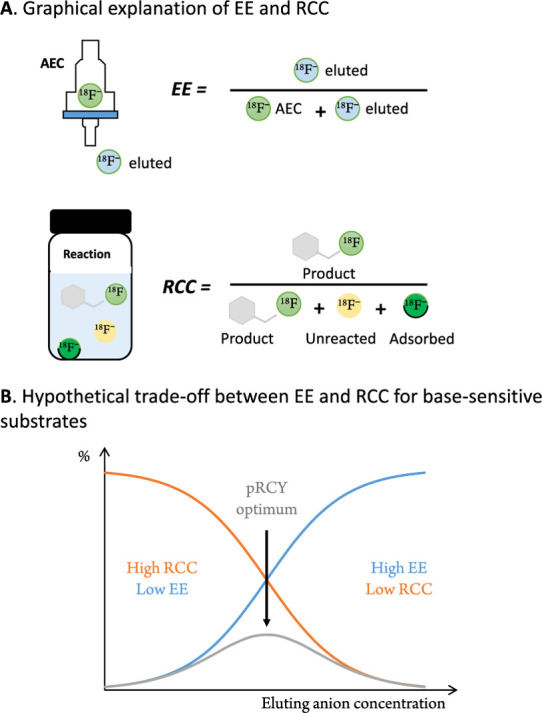
Hypothesized relationship between the elution efficiency (EE), the radiochemical conversion (RCC), and the pseudo radiochemical yield (pRCY) for base-sensitive compounds. (A) Definition of EE and RCC. RCCs were calculated including resolubilization of [18F]fluoride, adsorbed to the glass vessel wall (see the Supporting Information for further information). (B) Typical dependence of EE (sigmoidal curve, blue) and hypothetical dependence of RCC (brown) on the eluting anion concentration for base-sensitive compounds. Highest pRCY is a trade-off between the EE (as an indicator of the anion elution concentration) and the RCC, i.e., at an anion concentration resulting in sufficient elution with minimal influence on the base-sensitive reaction.
Screening of Elution Conditions
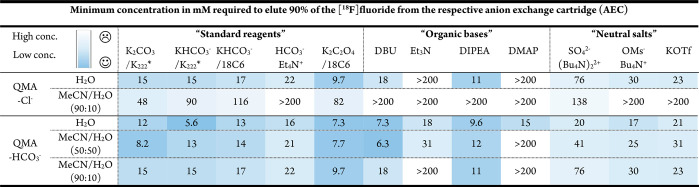
Initially, we screened a broad set of different elution conditions (>500 experiments) with the aim to identify a sufficient EE that simultaneously resulted in a low basicity eluate. For simplicity, only the commonly used Sep-Pak Light QMA (130 mg of resin loading) AEC was investigated.28 Furthermore, we explored how different preconditioning anions influence the EE, as this could be a major contributing factor in subsequent fluorinations (Figure 2A). We decided to precondition the cartridges with relatively nonbasic Cl– and HCO3– anions. As elution solvents, we studied water and two different MeCN/H2O mixtures. These elution solvents were chosen to find the best compromise between the better EEs of a higher water content and the considerably shorter azeotropic distillation process associated with a lower water content.
In all experiments, cyclotron-produced aqueous [18F]fluoride was quantitatively trapped. The concentrations resulting in an EE of 90% were calculated by fitting the Hill equation to the data (Table 1). We decided to use this value as we believe that the initial activity loss during the trapping and elution steps should be minimized to ≤10%. Various types and concentrations of eluting anions were screened to identify minimal concentrations. In addition to commonly applied eluting anions such as carbonates, bicarbonates, or oxalates, we investigated organic bases such as DBU, Et3N, DIPEA, and DMAP. These bases deprotonate water molecules, forming OH– anions in situ, which displace [18F]fluoride from AECs. During the subsequent drying procedure, bases are removed through distillation, resulting in the low basicity of the reaction mixture. We also investigated a range of neutral salts as eluting anions. In all cases, the EE showed a sigmoidal curve progression with a sharp decrease at a specific concentration depending on the preconditioning of the AECs and the eluting anions (Figure 2B). Bicarbonate preconditioned AECs generally required lower concentrations of eluting anion compared to those for chloride preconditioned AECs. This effect is driven by the weaker interaction of chloride with the quaternary methyl groups of the resin compared to bicarbonates.8 As expected, the EE was higher for solvent mixtures containing more water. This improvement in EE was especially pronounced for organic bases, as higher water concentrations promoted in situ formation of OH– ions. The most efficient eluting anions were bivalent “standard reagent” anions (K2CO3/K222 (Kryptofix 222) and K2C2O4/18C6 (18-crown-6) and “in situ formed OH–-anions” with organic bases (DBU and DIPEA) in higher water concentrations, whereas the neutral salts generally required higher concentrations of anions. For this reason, we decided to study the influence of eluting conditions on the RCC.
Table 1. Results from EE Screening Using Different Preconditioning and Eluting Anions over a Range of Concentrationsa.
The table displays concentrations of eluting anions in mM required to elute 90% of [18F]fluoride from the QMA cartridge. These values were determined by fitting the Hill equation to a set of 7 elutions (5–100 mM of the eluting anion in 1 mL of eluting solvent (5–100 μmol). Further details can be found in Table S1. Colors indicate concentrations required to obtain EE 90%, with white representing the lowest concentration and gradually darker blue for higher concentrations. K222 = Kryptofix 222, 18C6 = 18-crown-6.
Trade-Off between the EE and the RCC
In a previous study of elution conditions for aromatic 18F fluorodeboronations, the highest RCCs were achieved using the lowest concentrations of eluting anions.8 The best RCYs could be reached using a trade-off between the concentration of eluting anions that yielded high RCCs and acceptable, but incomplete EE. The authors explained the observed trade-off with the base sensitivity of 18F fluorodeboronations.8,29,30 This observation inspired us to explore if such a trade-off between the EE and the RCC also exists for aliphatic 18F-radiolabeling for base-sensitive compounds (Figure 2B). For this reason, we decided to study the influence of eluting conditions on the RCC and used them together with the EE to determine pRCYs. A model reaction was chosen for this purpose that was not particularly base-sensitive but allowed for fast and efficient screening (Figure 3A).
Figure 3.
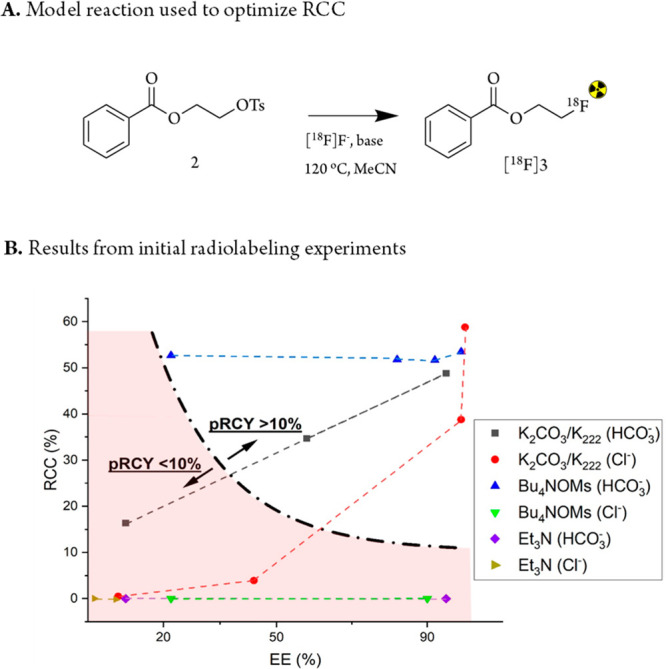
Model radiolabeling reaction using precursor 2 to form [18F]3. (A) Reaction scheme. (B) Results from initial screening of different elution conditions at 120 °C and 5 min in MeCN. Concentration range = 2–200 μmol of eluting anion depending on elution efficiency with preconditioning anions in brackets. A higher EE correlates to a higher eluting anion concentration. Detailed information is provided in Table S2.
We hypothesized that the lowest acceptable base concentration which resulted in reasonable pRCYs of this reaction would allow us to decide on which conditions to test with base-sensitive reactions. A pRCY of 10% was defined as the lowest acceptable limit. This limit was set since it would theoretically allow isolation of 375 MBq of final product from 5 GBq of starting activity with a 45 min synthesis time taken into account. This starting amount is accessible even at radiopharmaceutical centers that lack direct access to a cyclotron and therefore are dependent on 18F deliveries. The radioactivity amount used for a single human PET scan is approximately 300 MBq,31 and as such, 375 MBq of labeled tracer is sufficient as a lower limit for this purpose. To reduce the number of experiments, we decided to determine the pRCY on elution conditions that result in an EE of 20, 50, 90 and ∼100%. From our initial elution experiments (Table 1), we further decided to test only elutions based on a 50:50 MeCN/H2O mixture. This decision is a compromise between the diminishing EE observed with a 90:10 mixture and the prolonged drying procedure (∼30 min compared to 10–15 min) when pure water was used.
Initial Radiolabeling Screen Using a Model Reaction
In order to determine the trade-off between the EE and the RCC, 23 reactions were carried out to determine the minimal anion (base) concentration needed to obtain a pRCY of >10% for our model compound (Figure 3A, Table S2). Details of the workflow for the reactions and analyses can be found in Figure S2. The anion concentration was varied, with one eluting reagent from each category chosen, namely, K2CO3/K222 for standard reagents, Et3N for organic bases, and Bu4NOMs for neutral salts, and two preconditioning anions (HCO3– or Cl–) were tested for each combination. The results are summarized in Figure 3B. No trade-off between the EE (as an indicator of the anion elution concentration) and the RCC could be identified for any of the reactions. For QMAs preconditioned with HCO3– and eluted with K2CO3/K222, a linear dependency between the EE and the RCC was observed. A lower eluting anion concentration, and subsequently lower EE, was accompanied by lower RCC. This can be explained by the increased capability of the vessel’s glass wall to adsorb [18F]fluoride when the (bi)carbonate concentration in the eluate decreases. Adsorbed [18F]fluoride is not accessible for labeling reactions, and consequently, the RCC drops. A pRCY of >10% could be achieved using >3 mM K2CO3/K222 (interpolated from the elution curves, Figure 3B). As such, we suggest that this should be the minimal elution concentration as a starting point to explore if base-sensitive structures can be labeled using HCO3–-preconditioned QMAs and the according K2CO3/K222 elution mixture. Lower K2CO3/K222 concentrations would not be expected to result in acceptable RCYs for base-sensitive substrates. Consequently, 18F-labeling attempts would be futile if they cannot withstand 3 mM HCO3–. QMA cartridges preconditioned with Cl– and eluted with K2CO3/K222 showed a similar trend. However, the RCC was further reduced in an exponential fashion with lower EE. This decrease stems from the capacity of the cationic QMA resin to adsorb (bi)carbonate ions. At lower concentrations, no or very little amounts of (bi)carbonate ions can pass through the QMA and are as such not available in the eluate to promote 18F fluorination. In contrast, Cl– ions from the Cl–-preconditioned QMA are released from the resin during the elution process leading to competing chlorination and thus reducing the RCC further (Figures 3B and S3). Elutions using Et3N or other organic bases such as DBU or DIPEA resulted in the loss of 18F activity (5–50%) during azeotropic distillation of the eluate, and no 18F incorporation of the remaining activity into the precursor was observed (Figure 3B, Table S2). This indicates that the organic bases do not generate conditions that are basic enough to promote SN2 fluorinations. Surprisingly, elution of the HCO3–-preconditioned QMA using Bu4NOMs resulted in stable RCCs of around 50% independent of the elution anion concentration. pRCY values of >10% could be reached for all tested conditions. Since 18F fluorination requires a base and the OMs– eluting anion is nonbasic, the then basicity must stem from the HCO3–-preconditioning anion that coelutes with the [18F]fluoride when eluting the QMA with Bu4NOMs. No product was formed using the same conditions but preconditioning with the nonbasic anions: OMs– and SO42– (Table S3). A previous study reported an 18F-labeling strategy using neutral elution and preconditioning conditions and then subsequently basifying the eluted mixture with KHCO3, KOH, or K2CO3 before 18F fluorination.32 Unfortunately, in our hands, this strategy resulted in diminishing pRCYs for [18F]3 with lower concentrations of base, in line with our previous results using potassium (bi)carbonates. This was due to the fact that up to 50% of [18F]fluoride was adsorbed to the glass wall, despite using a protic solvent and high concentrations of K222. Rigorous stirring during the reaction to promote higher resolubilization of the adsorbed [18F]fluoride did not improve the RCC (Table S4). This prompted us to investigate further how preconditioning of the QMA cartridge combined with neutral elution could promote high pRCYs for low-base conditions.
Investigating the Role of the Preconditioning Anion
Our data suggest that it is possible to utilize the basicity of the QMA cartridge preconditioning anion to promote 18F fluorinations when using nonbasic salts for an efficient elution process. This combination could be used to minimize the base concentration in the reaction and protect base-sensitive precursors/tracers against degradation or to reduce base-promoted side-reactions. Therefore, we decided to test a number of preconditioning anions in combination with Bu4NOMs elution to determine their influence on the EE, RCC, and ultimately, the pRCY (Table 2). Interestingly, the EE was mainly dependent on the valency of the preconditioning anion rather than the pKa, with a higher valency increasing the EE (Tables 2 and S5). Nucleophilic preconditioning anions such as C2O42–, AcO–, or Cl– should be avoided as they lower the RCC by outcompeting the [18F]fluoride nucleophile, as confirmed by LC-MS analysis (Figures S3 and S5). As for any 18F a fluorination, a certain basicity of the preconditioning anion is needed to promote the reaction. In our setup, the reaction could proceed if preconditioning anions with a pKa of around 4 were used. For univalent preconditioning anions, a higher pKa resulted in a higher EE, in line with what has previously been reported.8 This observation follows the electroselectivity theory which is based on the Donnan potential.33 It allows us to determine the electroselectivity of anions in heterogeneous systems, i.e., the selectivity coefficient between ions in solution and bound to the resin. For anions of the same valency at low concentrations, the dominating factor for the affinity to the resin is the Debye–Hückel activity coefficient which in turn is proportional to the pKa, i.e., compounds with higher pKa values bind stronger to the resin.34 As such, preconditioning anions with a higher pKa than the fluoride ion facilitate elution of [18F]fluoride from the QMA cartridge, since eluting anions can more easily displace fluoride from the resin compared to the more strongly bound preconditioning anions.
Table 2. Radiolabeling of Precursor 2 in MeCN at 120°C and 5 min Reaction Time with Bu4NOMs Elution (20 mM in 1 mL of 50% MeCN/H2O) Using Different Preconditioning of the QMA.
| screening
of different preconditioning anions for
elution by Bu4NOMs | ||||
|---|---|---|---|---|
| preconditioning anion | pKa35 | EE (%) | RCC (%) | pRCY (%) |
| Cl– | –7.0 | 24 | 0 | 0 |
| OMs– | –1.9 | 28 | 0 | 0 |
| SO42– | 2.0 (−9.0)a | 96 | 0 | 0 |
| H2PO4– | 2.1 | 86b | 0 | 0 |
| C2O42– | 4.2 (1.3)a | 99 | traces | traces |
| AcO– | 4.7 | 45 | traces | traces |
| HCO3– | 6.4 | 91.0 ± 5.4c | 56.7 ± 8.9c | 52.1 ± 6.9c |
| HPO42– | 7.2 | 95.6 ± 0.9c | 53.4 ± 4.3c | 51.0 ± 5.4c |
| CO32– | 10.3 | 92.0 ± 6.3c | 55.1 ± 1.1c | 50.7 ± 4.3c |
| PO43– | 12.7 | 97.0 ± 0c | 74.3 ± 14.2c | 72.0 ± 11.3c |
pKa for second protonation if only one of the divalent anion was investigated.
Higher elution could be due to a mixture of mono- and divalent anions formed in aqueous solution.
Reactions carried out in triplicates.
Quantifying the Breakthrough of Precondition Anions
Given that the amount of base in the reaction mixture is determined by the EE of the preconditioning anion when nonbasic elution approaches are used, a precise quantification of the amount of preconditioning anion that is eluted into the reaction vessel would allow us to understand more thoroughly how these anions affect 18F fluorinations, especially for base-sensitive structures. In order to quantify the breakthrough of the preconditioning anions from the QMA cartridge, we estimated their concentration in the eluate by (i) pH measurements (Table S6) and (ii) quantitative NMR (qNMR) (Table S7). In general, qNMR measurements provided a higher precision than pH measurements, but this approach could only be applied to the monovalent anions, HCO3–, H2PO4–, and OMs–. Respective quantifications showed that the monovalent HCO3– preconditioning anion was proportionally displaced by OMs–, whereas the di- and trivalent CO32– and PO43– showed only minor displacement, even with high concentrations of OMs–. This observation can be explained by the Donnan potential. Due to their multiple charges, multivalent anions interact with the cationic groups on the anion-exchange resin more strongly than with monovalent anions. This effect is stronger than the one promoted by the pKa-dependent Debye–Hückel activity effect.33 Therefore, perhaps counterintuitively, when the QMA cartridge is preconditioned with more basic multiple-charge anions, e.g., PO43–, the basicity of the final elution mixture is lower than when a less basic anion with a lower charge, e.g., HCO3–, is used for QMA preconditioning. This is because the breakthrough of the multiple-charge anion is considerably lower. Finally, qNMR results also showed that the more acidic H2PO4– anion (pKa: 2.14 in H2O) remained in its diprotonated form after it was eluted from the QMA. As such, it is able to reduce the basicity of the reaction mixture. However, the mixture remains basic enough to promote the 18F-labeling step.
Improved Resolubilization of [18F]Fluoride
Adsorption of [18F]fluoride on the wall of the glass reaction vessels is a commonly observed phenomenon reducing RCCs under low basicity conditions. In comparison to standard systems using cryptands such as [18F]KF/K222, tetraalkylammonium[18F]fluoride is more lipophilic (cLog D7.4 calculated with Chemicalize software for Bu4NF is 1.32 and for KF/K222 is −0.41). Consequently, the solubility of such salts is higher in organic, polar aprotic solvents which are commonly used for fluorinations. For example, the use of Bu4NOMs resulted in 10% less glass adsorption compared to that when using the corresponding K+/K222 mixture (Tables S8 and S9). As a result, [18F]fluoride adsorption to glass walls is minimized, and the amount available in the reaction solution increased. To further explore the potential of tetraalkylammonium salts with respect to reaction basicity and to increase the resolubilization process of [18F]fluoride, three additional salts with different physicochemical properties were studied. Table 3A displays the rationale behind the selection of the respective salts. We decided to study the influence of these tetraalkylammonium salts in combination with the most promising preconditioning anions (carbonate, bicarbonate, phosphate, and hydrogen phosphate) that we identified in the preconditioning screening and three solvents (DMSO, MeCN, and tBuOH) which are commonly used solvents for aliphatic 18F fluorinations.27,36 The selection of solvents was based on their different ability to act as hydrogen bond donors (HBD) and/or hydrogen bond acceptors (HBA) (Table 3B). These factors can affect the solubility of the anions, thus influence the basicity and thus RCYs when labeling base-sensitive structures.
Table 3. Rationales Behind the Choice of Tetraalkylated Eluting Anions and Physiochemical Properties of the Different Solvent Used for the Multiparametric Screen of Elution Conditions.
| tetraalkylammonium salts | rationale |
|---|---|
| Bu4NOTf | The lower pKa of OTf– compared to that of the OMs– of Bu4NOMs should displace lower amounts of preconditioning anions during the elution process, resulting in a less basic eluate and therefore enabling labeling of tracers under milder reaction conditions. |
| Bu4NH2PO4 | Due to the buffering capabilities of Bu4NH2PO4, we decided to test this compound. This salt should neutralize more basic preconditioning anions. |
| Et4NHCO3 | This salt is commonly used for elution in nucleophilic 18F-radiolabeling and is used as a comparison.37,38 |
| solvent | proton affinity | H-bonding properties | |
|---|---|---|---|
| DMSO | aprotic | protophilic | no HBD and HBA exist |
| MeCN | aprotic | protophobic | no HBD and very weak HBA |
| tBuOHa | protic | amphiprotic | both HBD and HBA properties |
Mixed with ∼17% v/v MeCN added to make it liquid at room temperature.
Multiparametric Screening Using Selected Preconditioning Anions and Elution Reagents for the Model Reaction
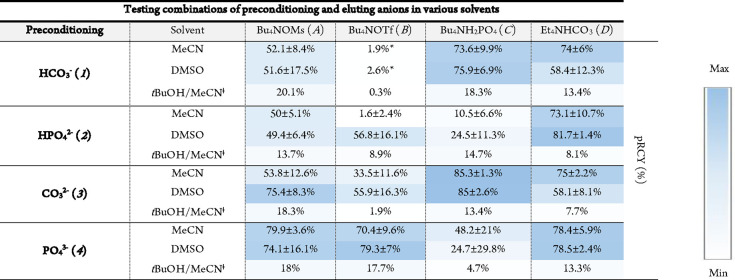
All possible combinations of preconditioning anions, eluting reagents, and reaction solvent were tested (Figure 3A, Table 4). The nonbasic eluting anions OMs– and OTf– resulted in the highest pRCY in combination with multicharged preconditioning anions, especially phosphates. These conditions led to a very low preconditioning anion breakthrough and consequently to a lower base concentration in the eluate. This resulted in surprisingly high pRCYs while retaining high amounts of intact precursor (2) (Figures S14 and S15). H2PO4–, as an acidic eluting anion, only resulted in good pRCYs when applied with carbonate or bicarbonate preconditioning anions. As indicated by the qNMR experiments, H2PO4– can lower the basicity of the carbonates and act as a buffer. Consequently, increasing the selected concentration of H2PO4– from 20 to ≥50 mM diminished the pRCY with the (bi)carbonate preconditioning anions as the eluate became too acidic to promote 18F-labeling of [18F]3. This could explain the inconsistent pRCYs of the same elution of the phosphate preconditioned QMAs considering the possible variations in elution of the basic preconditioning anion (PO43–) at this concentration range. The balance of acidic elution and basic preconditioning is regulated for the carbonates by H2CO3 formation escaping as CO2. However, for phosphate preconditioning all acidity from the elution remains in the eluate. This could probably be optimized with lower concentrations of Bu4NH2PO4 but then at the expense of a lower EE. Finally, the more frequently used elution reagent Et4NHCO3 resulted, as expected for base-insensitive precursors/tracers, in good pRCY for all preconditioning anions, comparable with those of the aforementioned high-yielding elution conditions.
Table 4. Pseudo Radiochemical Yields (pRCY) of the Model Compound ([18F]3, Figure 3A) Using Different Tetraalkylammonium Salts in Combination with Various Preconditioning Anions in Either MeCN, DMSO, or t-BuOH/MeCN (5:1)a.
Values given as mean values with standard deviation, n = 3. Italic numbers and letters are used to indicate combinations of elution and preconditioning, for example, 1A representing HCO3– preconditioning with Bu4NOMs elution.
Previous studies have reported efficient 18F fluorinations using t-BuOH.12 Surprisingly, the use of t-BuOH/MeCN in this case only resulted in relatively low RCC for all tested elution conditions. However, the amount of intact precursor at the end of the reaction was significantly higher compared to reactions using MeCN or DMSO and otherwise identical conditions (Figure S15). This indicates that the use of t-BuOH in the solvent could be beneficial for very base-sensitive substrates, since the resulting mild labeling conditions lead to less degradation of the precursor/tracer, but at the expense of less efficient 18F incorporation.40
Reaction Time, Temperature, Precursor Concentration, and Leaving Groups
From the literature it is known that the reaction time, temperature, precursor concentration, and the chosen leaving group have a strong, but structure-dependent, influence on RCYs. Therefore, we decided not to optimize these parameters for our model reaction and recommend that this should be investigated for individual syntheses.
Intermediate Findings
In order to minimize the base content during SN2 18F fluorinations while simultaneously maintaining good pRCYs, the following key parameters should be followed: (i) Nonbasic anions should be used for the elution process. (ii) Multicharged, non-nucleophilic preconditioning anions should be used. (iii) Tetrabutylammonium counterions should be used for elution to increase resolubilization. (iv) For very base-sensitive compounds, t-BuOH could be used in the reaction solvent to reduce degradation (with the expense of lowered reaction efficiency).
Improving the Labeling Procedures of Known Radiopharmaceuticals/Synthons
Next, we aimed to apply our findings (from Table 4) to the synthesis of a set of well-described PET tracers and radiolabeled building blocks and increase the RCYs of those structures thereby. We set out two criteria for compounds to be studied: (I) Selected structures should possess a reported RCY < 50%, and more importantly, (II) base-insensitive and -sensitive structures should be included to study the beneficial effect of the identified conditions. We were also interested to cover a broad set of structural motifs which could be affected by a basic environment (Table 5). Preconditioning and elution conditions were selected on a rational analysis or by reported data of the base-sensitivity of compounds to be labeled and selected from Table 4.
Table 5. Tracers Tested with the Derived Conditions from the Model Reactiona.
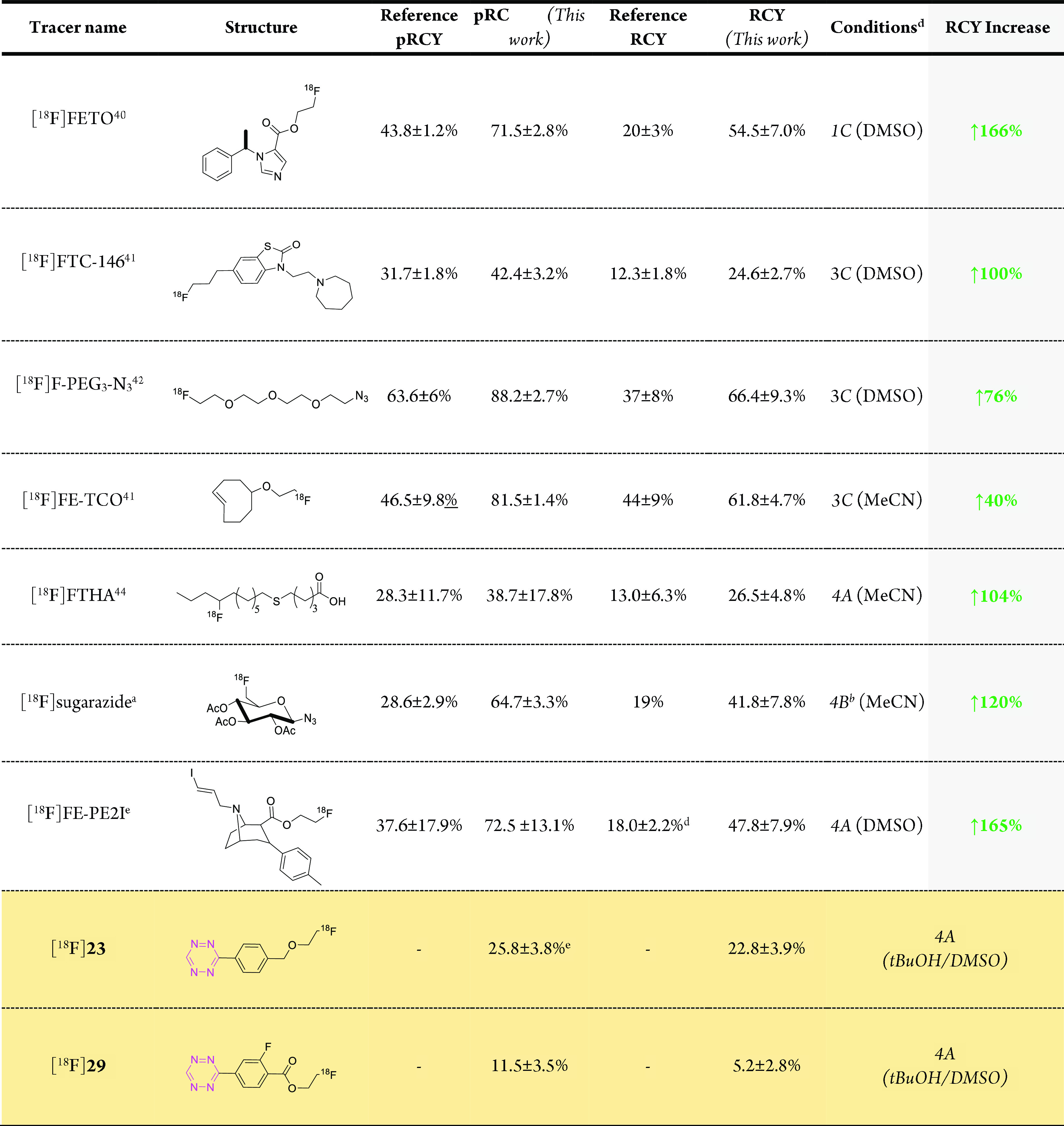
Reference procedures were reproduced manually and compared to derived conditions. Automated synthesis was carried out and isolated RCY was compared to references. All results created within this work are based on n = 3. Synthetic schemes for precursors can be found in the Schemes S3–S5. Further details on the syntheses can be found in the Figures S20–S49 and Tables S11–S20.
Earlier reported syntheses do not use quantitative analysis methods (only HPLC) and do not report isolated RCY and was therefore not suitable for comparison.39
MeCN/H2O, (50:50) was used for elution instead of methanol.
tBuOH/MeCN used instead.
In-house data (n = 7). Tracers that were not accessible via standard 18F-labeling approaches are colored beige.
First, four relatively base-insensitive tracers were tested. We hypothesized that even these structures could benefit from elution with tetraalkylammonium salts in respect to increasing the 18F–-resolubilization from the glass wall into the reaction solvent. [18F]FETO, [18F]FTC-146, [18F]F-PEG3-N3, and [18F]FE-TCO have been reported to be stable under “standard” basic labeling conditions.41−43 No degradation adducts were observed using those conditions. We assumed that eluting a QMA which was preconditioned with HCO3– or the slightly more basic CO32– (conditions 1C or 3C, Table 4) with tetralkylammonium salts would result in higher 18F–resolubilization, while simultaneously the preconditioning anion would provide enough basicity to promote the labeling step. For all four compounds, an increased isolated RCY was achieved spanning from approximately 40 to 170% increase using the optimized conditions (Table 5). Retrospective analysis of the 18F–-resolubilization data showed that this parameter was indeed in all reaction increased and significant contributed to the improvement RCY (10–30% of the observed increase). One additional factor that might have improved the yields is the lower base content used. This condition rather favors SN2 labeling over E2 elimination, a possible side reaction which is typically facilitated at higher base concentrations.
The first relatively base-sensitive structure that was investigated in this study was [18F]FTHA. This compound is labeled at a secondary carbon atom and thus, is more prone to undergo E2 elimination, especially under strong basic conditions.44 We hypothesized that less basic conditions should consequently lead to a high RCY. Preconditioning with PO43– and using Bu4OMs for elution resulted in the lowest basicity of the eluent (conditions 4A, Table 4). Applying these conditions doubled the isolated RCY compared to the reference procedure using “standard” conditions (Table 5).45 The next compound tested was a building block which can be used to label a broad set of radiopharmaceuticals.39 [18F](2R,3R,4S,5R,6R)-2-Azido-6-(fluoromethyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate ([18F]sugarazide) is labeled via a two-step labeling procedure. First, a hydroxy-group protected precursor is 18F-labeled and then deprotected. The acetyl protection groups are base-labile. In the reported labeling procedure, partial hydrolysis of those protecting groups occurred using “standard” basic labeling conditions. Free hydroxy groups typically form H-bonds with 18F– and reduce its nucleophilicity, thus decreasing RCYs. We applied our low-basicity conditions using a PO43– preconditioned QMA and Bu4NOTf (4B) for elution in order to reduce the basicity and consequently reduce premature deprotection. No deprotection was observed using these conditions, and the isolated RCY increased approximately 2-fold to 41.8 ± 7.8% (Table 5, Figure S33).55
Finally, we directed our focus toward [18F]FE-PE2I. This tracer is regularly produced for clinical applications. The complex cocaine-scaffold along with a vinylic iodine has been shown to degrade in the reaction crude, presumably due to basic conditions.46 To investigate if lower basicity leads to higher RCYs, we used our low-basicity conditions applying a PO43– preconditioned QMA and Bu4NOMs for elution (4A). The final isolated RCY was increased by over 150% using this setup (Table 5).
Labeling of Base-Sensitive Structures That Are Not Accessible via “Standard” Aliphatic 18F-Labeling Conditions
Tetrazines (Tz) are a class of compounds which can be applied in pretargeted imaging.47−53 Currently, only low-reactivity Tzs can be radiolabeled via direct aliphatic SN2.54 Unfortunately, these structures display too low a reactivity for in vivo bioorthogonal chemistry approaches.55,56 Highly reactive structures such as monounsubstituted tetrazines (H-Tzs) have been reported to be highly sensitive to base.57 Extensive degradation is observed which prevents isolation of meaningful amounts for imaging studies. Using “standard” conditions, no or only trace amounts of the radiolabeled product could be observed (Figure S50 and Li et al.).57 We hypothesized that our mildest labeling conditions (4A) in combination with a t-BuOH-mixture could provide sufficiently low basicity labeling conditions to label a H-Tz. Initial attempts using a mesylate precursor resulted in an increase from traces of labeled product to a pRCY of approximately 2% of [18F]23. In a next step, we investigated the influence of different leaving groups. In addition to the mesylate (OMs) group, we tested tosylate (OTs)- and nosylate (ONs)-based precursors.
As mentioned previously, different leaving groups can influence the labeling yield substantially, but no trend with respect to increased RCY has been observed. The yields varied on a case-by-case basis depending on the individual molecular structure of the precursor.58 To facilitate the reaction, the solvent was further changed to t-BuOH mixed with DMSO which decreased the evaporation of solvent during automated synthesis while maintaining the RCC (Table S20). The nosylate precursor with t-BuOH/DMSO and the low basic elution condition (4A) resulted in a RCY of approximately 22% (Figure 4). Control experiments were also carried out to investigate if low-basicity conditions (4A in combination with a t-BuOH solvent) are needed to promote the reaction. These experiments yielded no or only trace amount of the 18F-labeled Tz ([18F]23). In order to test the applicability of the identified conditions to label H-Tzs, we decided to radiolabel an even more reactive Tz. The chosen structure displays a 4-fold increased reactivity toward TCO (2676 vs 682 M–1 s–1Table S21)54 and should as such be even more difficult to label, since reactivity is proportional to the Tz’s base instability (Tables 5 and S20). In line with the aforementioned observations, the more reactive Tz ([18F]29) could only be radiolabeled using the mildest labeling conditions. As expected, the compound could be isolated from the ONs precursor in a lower RCY (ca. 5% RCY) than the less reactive Tz. This reflects the higher base sensitivity of the structure.
Figure 4.
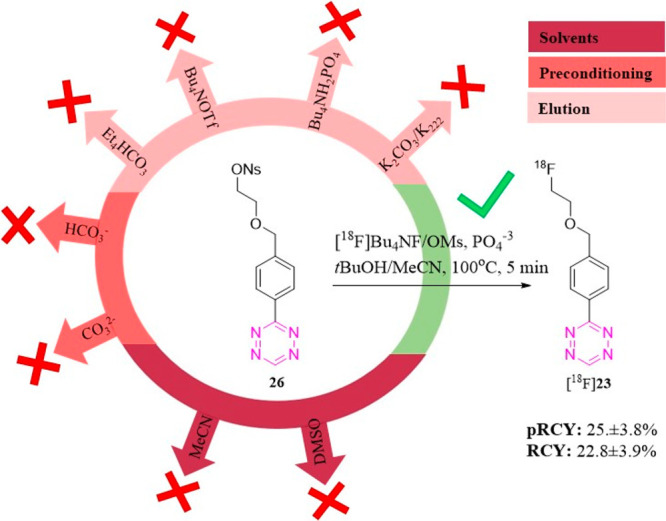
18F-Labeling of a base-sensitive structure that is not accessible using “standard” aliphatic labeling conditions. The H-Tz ([18F]23) could only be labeled using low basic conditions identified within this work, i.e., 4A in combination with t-BuOH/MeCN.
Recommendations for Aliphatic 18F-Radiolabeling Attempts
Our results indicate that the following labeling conditions should be used as a starting point to label aliphatic structures (Figure 5): (i) Base-sensitive tracers/precursors: t-BuOH-mixtures or similar hindered protic solvents should be used in combination with condition 4A or 4B. (ii) Moderately base-sensitive compounds: Conditions 4A or 4B in combination with MeCN or DMSO should be used. (iii) Base-insensitive structures: 1C or 3C in DMSO are robust high-yield conditions; alternatively conventional methods using tetraalkylated carbonates should be used.
Figure 5.

(A) Recommendations for 18F-labeling of aliphatic substrates. (B) Conditions represent parameters that we suggest to apply as a starting point before further optimization with respect to reaction time, temperature, precursor concentration and leaving groups. Detailed reaction conditions can be found in Tables 4 and S20.
Conclusions
By carefully studying the key parameters involved in the trapping of [18F]fluoride on an anion exchange cartridge and its subsequent elution, we were able to identify conditions that result in low basic elutions. These conditions enable us to radiolabel base-sensitive structures with significantly improved RCYs. Even structures that were previously not accessible by applying “standard” aliphatic 18F-labeling strategies could be radiolabeled. The developed methodology can easily be implemented on all synthesis modules and is only dependent on the preconditioning of the anion exchange cartridge, its nonbasic elution, and on the selection of the right reaction solvent. This places new classes of 18F-fluorinated compounds within the reach of classical labeling approaches (SN2 labeling).
Acknowledgments
We thank the staff at the Department of Radiation Physics (Skåne University Hospital) and the Department of Clinical Physiology, Nuclear Medicine & PET, Rigshospitalet, for the production of fluorine-18, technical assistance, and support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00133.
Detailed experimental procedures, characterization of novel structures and labeling protocols (PDF)
Author Contributions
The elution screening experiments were conducted by K.B., V.S., and I.N.P. Precursor synthesis was done by K.B., U.B., and S.L.B., and subsequent radiolabeling experiments were carried out by K.B. The study concept was designed and the manuscript written by K.B., V.S., and M.H.H. with contribution from all authors. All authors have given approval to the final version of the manuscript.
This project received funding from European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant Agreement No. 813528. M.M.H. received funding from the European Union’s EU Framework Program for Research and Innovation Horizon 2020 (grant agreement no. 670261). V.S. was supported by BRIDGE – Translational Excellence Program at the Faculty of Health and Medical Sciences, University of Copenhagen, funded by the Novo Nordisk Foundation (grant agreement no. NNF18SA0034956). The Lundbeck Foundation, the Innovation Fund Denmark, and the Research Council for Independent Research are further acknowledged.
The authors declare no competing financial interest.
Supplementary Material
References
- Piel M.; Vernaleken I.; Rösch F. (2014) Positron Emission Tomography in CNS Drug Discovery and Drug Monitoring. J. Med. Chem. 57 (22), 9232–9258. 10.1021/jm5001858. [DOI] [PubMed] [Google Scholar]
- Ametamey S. M.; Honer M.; Schubiger P. A. (2008) Molecular Imaging with PET. Chem. Rev. 108 (5), 1501–1516. 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- Varlow C.; Szames D.; Dahl K.; Bernard-Gauthier V.; Vasdev N. (2018) Fluorine-18: An Untapped Resource in Inorganic Chemistry. Chem. Commun. 54 (84), 11835–11842. 10.1039/C8CC04751K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen J. L., and Herth M. M. (2017) In Vivo Imaging in Drug Discovery, in Textbook of Drug Design and Discovery (Strømgaard K., Krogsgaard-Larsen P., and Madsen U., Eds.), CRC Press, Copenhagen. [Google Scholar]
- Edem P. E., Steen E. J. L., Kjær A., and Herth M. M. (2019) Fluorine-18 Radiolabeling Strategies—Advantages and Disadvantages of Currently Applied Labeling Methods, in Late-Stage Fluorination of Bioactive Molecules and Biologically-Relevant Substrates, pp 29–103, Elsevier Inc., Buenos Aires. [Google Scholar]
- Beejot R., and Gouverneur V. (2012) Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications (Gouverneur V., and Muller K., Eds.), Vol. 6, World Scientific. [Google Scholar]
- Deng X.; Rong J.; Wang L.; Vasdev N.; Zhang L.; Josephson L.; Liang S. H. (2019) Chemistry for Positron Emission Tomography: Recent Advances in 11C-, 18F-, 13N-, and 15O-Labeling Reactions. Angew. Chem., Int. Ed. 58 (9), 2580–2605. 10.1002/anie.201805501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossine A. V.; Brooks A. F.; Ichiishi N.; Makaravage K. J.; Sanford M. S.; Scott P. J. H. (2017) Development of Customized [18F]Fluoride Elution Techniques for the Enhancement of Copper-Mediated Late-Stage Radiofluorination. Sci. Rep. 7 (1), 1–9. 10.1038/s41598-017-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P.; Haswell S. J.; Pamme N.; Archibald S. J. (2014) Advances in Processes for PET Radiotracer Synthesis: Separation of [18F]Fluoride from Enriched [18O]Water. Appl. Radiat. Isot. 91, 64–70. 10.1016/j.apradiso.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Katsifis A.; Hamacher K.; Schnitter J.; Stöcklin G. (1993) Optimization Studies Concerning the Direct Nucleophilic Fluorination of Butyrophenone Neuroleptics. Appl. Radiat. Isot. 44 (7), 1015–1020. 10.1016/0969-8043(93)90005-U. [DOI] [Google Scholar]
- Pees A.; Windhorst A. D.; Vosjan M. J. W. D.; Tadino V.; Vugts D. J. (2020) Synthesis of [18F]Fluoroform with High Molar Activity. Eur. J. Org. Chem. 2020 (9), 1177–1185. 10.1002/ejoc.202000056. [DOI] [Google Scholar]
- Seo J. W.; Lee B. S.; Lee S. J.; Oh S. J.; Chi D. Y. (2011) Fast and Easy Drying Method for the Preparation of Activated. [18F]Fluoride Using Polymer Cartridge. Bull. Korean Chem. Soc. 32 (1), 71–76. 10.5012/bkcs.2011.32.1.71. [DOI] [Google Scholar]
- Iwata R.; Pascali C.; Terasaki K.; Ishikawa Y.; Furumoto S.; Yanai K. (2017) Minimization of the Amount of Kryptofix 222 - KHCO3 for Applications to Microscale 18F-Radiolabeling. Appl. Radiat. Isot. 125, 113–118. 10.1016/j.apradiso.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Lemaire C. F.; Aerts J. J.; Voccia S.; Libert L. C.; Mercier F.; Goblet D.; Plenevaux A. R.; Luxen A. J. (2010) Fast Production of Highly Reactive No-Carrier-Added [18F]Fluoride for the Labeling of Radiopharmaceuticals. Angew. Chem., Int. Ed. 49 (18), 3161–3164. 10.1002/anie.200906341. [DOI] [PubMed] [Google Scholar]
- Maisonial-Besset A.; Serre A.; Ouadi A.; Schmitt S.; Canitrot D.; Léal F.; Miot-Noirault E.; Brasse D.; Marchand P.; Chezal J. M. (2018) Base/Cryptand/Metal-Free Automated Nucleophilic Radiofluorination of [18F]FDOPA from Iodonium Salts: Importance of Hydrogen Carbonate Counterion. Eur. J. Org. Chem. 2018 (48), 7058–7065. 10.1002/ejoc.201801608. [DOI] [Google Scholar]
- Richarz R.; Krapf P.; Zarrad F.; Urusova E. A.; Neumaier B.; Zlatopolskiy B. D. (2014) Neither Azeotropic Drying, nor Base nor Other Additives: A Minimalist Approach to 18F-Labeling. Org. Biomol. Chem. 12 (40), 8094–8099. 10.1039/C4OB01336K. [DOI] [PubMed] [Google Scholar]
- Pees A.; Sewing C.; Vosjan M. J. W. D.; Tadino V.; Herscheid J. D. M.; Windhorst A. D.; Vugts D. J. (2018) Fast and Reliable Generation of [18F]Triflyl Fluoride, a Gaseous [18F]Fluoride Source. Chem. Commun. 54 (72), 10179–10182. 10.1039/C8CC03206H. [DOI] [PubMed] [Google Scholar]
- Petersen I. N.; Kristensen J. L.; Herth M. M. (2017) Nucleophilic 18F-Labeling of Spirocyclic Iodonium Ylide or Boronic Pinacol Ester Precursors: Advantages and Disadvantages. Eur. J. Org. Chem. 2017 (3), 453–458. 10.1002/ejoc.201601448. [DOI] [Google Scholar]
- Orlovskaya V.; Antuganov D.; Fedorova O.; Timofeev V.; Krasikova R. (2020) Tetrabutylammonium Tosylate as Inert Phase-Transfer Catalyst: The Key to High Efficiency SN2 Radiofluorinations. Appl. Radiat. Isot. 163, 109195. 10.1016/j.apradiso.2020.109195. [DOI] [PubMed] [Google Scholar]
- Fedorova O.; Orlovskaya V.; Nadporojskii M.; Krasikova R. (2020) Automated Synthesis of the 16α-[18F]Fluoroestradiol ([18F]FES): Minimization of Precursor Amount and Resulting Benefits. Radiochim. Acta 108 (12), 979–988. 10.1515/ract-2020-0058. [DOI] [Google Scholar]
- van der Born D.; Pees A.; Poot A. J.; Orru R. V. A.; Windhorst A. D.; Vugts D. J. (2017) Fluorine-18 Labelled Building Blocks for PET Tracer Synthesis. Chem. Soc. Rev. 46 (15), 4709–4773. 10.1039/C6CS00492J. [DOI] [PubMed] [Google Scholar]
- Taylor N. J.; Emer E.; Preshlock S.; Schedler M.; Tredwell M.; Verhoog S.; Mercier J.; Genicot C.; Gouverneur V. (2017) Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 139 (24), 8267–8276. 10.1021/jacs.7b03131. [DOI] [PubMed] [Google Scholar]
- Graham T. J. A.; Lambert R. F.; Ploessl K.; Kung H. F.; Doyle A. G. (2014) Enantioselective Radiosynthesis of Positron Emission Tomography (PET) Tracers Containing [18F]Fluorohydrins. J. Am. Chem. Soc. 136 (14), 5291–5294. 10.1021/ja5025645. [DOI] [PubMed] [Google Scholar]
- Coenen H. H.; Gee A. D.; Adam M.; Antoni G.; Cutler C. S.; Fujibayashi Y.; Jeong J. M.; Mach R. H.; Mindt T. L.; Pike V. W.; Windhorst A. D. (2017) Consensus Nomenclature Rules for Radiopharmaceutical Chemistry — Setting the Record Straight. Nucl. Med. Biol. 55, v–xi. 10.1016/j.nucmedbio.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Basuli F.; Swenson R. E. (2019) An Azeotropic Drying-Free Approach for Copper-Mediated Radiofluorination without Addition of Base. J. Labelled Compd. Radiopharm. 62 (3), 139–145. 10.1002/jlcr.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herth M. M.; Ametamey S.; Antuganov D.; Bauman A.; Berndt M.; Brooks A. F.; Bormans G.; Choe Y. S.; Gillings N.; Häfeli U. O.; et al. (2021) On the Consensus Nomenclature Rules for Radiopharmaceutical Chemistry – Reconsideration of Radiochemical Conversion. Nucl. Med. Biol. 93, 19–21. 10.1016/j.nucmedbio.2020.11.003. [DOI] [PubMed] [Google Scholar]
- Roeda D.; Dolle F. (2010) Aliphatic Nucleophilic Radiofluorination. Curr. Radiopharm. 3 (2), 81–108. 10.2174/1874471011003020081. [DOI] [Google Scholar]
- Antuganov D.; Zykov M.; Timofeev V.; Timofeeva K.; Antuganova Y.; Orlovskaya V.; Fedorova O.; Krasikova R. (2019) Copper-Mediated Radiofluorination of Aryl Pinacolboronate Esters: A Straightforward Protocol by Using Pyridinium Sulfonates. Eur. J. Org. Chem. 2019 (5), 918–922. 10.1002/ejoc.201801514. [DOI] [Google Scholar]
- Mossine A. V.; Brooks A. F.; Makaravage K. J.; Miller J. M.; Ichiishi N.; Sanford M. S.; Scott P. J. H. (2015) Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett. 17 (23), 5780–5783. 10.1021/acs.orglett.5b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatopolskiy B. D.; Zischler J.; Krapf P.; Zarrad F.; Urusova E. A.; Kordys E.; Endepols H.; Neumaier B. (2015) Copper-Mediated Aromatic Radiofluorination Revisited: Efficient Production of PET Tracers on a Preparative Scale. Chem. - Eur. J. 21 (15), 5972–5979. 10.1002/chem.201405586. [DOI] [PubMed] [Google Scholar]
- Khamwan K.; Krisanachinda A.; Pasawang P. (2010) The Determination of Patient Dose from 18F-FDG PET/CT Examination. Radiat. Prot. Dosim. 141 (1), 50–55. 10.1093/rpd/ncq140. [DOI] [PubMed] [Google Scholar]
- Lee S. J.; Oh S. J.; Chi D. Y.; Moon D. H.; Ryu J. S. (2012) High Yielding [18F]Fluorination Method by Fine Control of the Base. Bull. Korean Chem. Soc. 33 (7), 2177–2180. 10.5012/bkcs.2012.33.7.2177. [DOI] [Google Scholar]
- Harland C. E. (1994) Ion Exchange: Theory and Practice, 2nd ed., Royal Society of Chemistry, Cambridge, U.K. [Google Scholar]
- Reijenga J.; van Hoof A.; van Loon A.; Teunissen B. (2013) Development of Methods for the Determination of PKa Values. Anal. Chem. Insights 8 (1), 53–71. 10.4137/ACI.S12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripin D. H., and Evans D. A. Aqueous pKa compilation with some dimethyl sulfoxide values. http://ccc.chem.pitt.edu/wipf/MechOMs/evans_pKa_table.pdf (accessed Dec 11, 2020).
- Kütt A.; Selberg S.; Kaljurand I.; Tshepelevitsh S.; Heering A.; Darnell A.; Kaupmees K.; Piirsalu M.; Leito I. (2018) PKa Values in Organic Chemistry – Making Maximum Use of the Available Data. Tetrahedron Lett. 59 (42), 3738–3748. 10.1016/j.tetlet.2018.08.054. [DOI] [Google Scholar]
- Reed C. D.; Launay G. G.; Carroll M. A. (2012) Evaluation of Tetraethylammonium Bicarbonate as a Phase-Transfer Agent in the Formation of [18F]Fluoroarenes. J. Fluorine Chem. 143, 231–237. 10.1016/j.jfluchem.2012.07.015. [DOI] [Google Scholar]
- Brichard L.; Aigbirhio F. I. (2014) An Efficient Method for Enhancing the Reactivity and Flexibility of [18F]Fluoride towards Nucleophilic Substitution Using Tetraethyl-ammonium Bicarbonate. Eur. J. Org. Chem. 2014 (28), 6145–6149. 10.1002/ejoc.201402587. [DOI] [Google Scholar]
- Maschauer S.; Haubner R.; Kuwert T.; Prante O. (2014) 18F-Glyco-RGD Peptides for PET Imaging of Integrin Expression: Efficient Radiosynthesis by Click Chemistry and Modulation of Biodistribution by Glycosylation. Mol. Pharmaceutics 11 (2), 505–515. 10.1021/mp4004817. [DOI] [PubMed] [Google Scholar]
- Kim D. W.; Ahn D. S.; Oh Y. H.; Lee S.; Kil H. S.; Oh S. J.; Lee S. J.; Kim J. S.; Ryu J. S.; Moon D. H.; Chi D. Y. (2006) A New Class of SN2 Reactions Catalyzed by Protic Solvents: Facile Fluorination for Isotopic Labeling of Diagnostic Molecules. J. Am. Chem. Soc. 128 (50), 16394–16397. 10.1021/ja0646895. [DOI] [PubMed] [Google Scholar]
- Rahman O.; Erlandsson M.; Blom E.; Långström B. (2010) Automated Synthesis Of 18F-Labelled Analogs of Metomidate, Vorozole and Harmine Using Commercial Platform. J. Labelled Compd. Radiopharm. 53 (4), 169–171. 10.1002/jlcr.1742. [DOI] [Google Scholar]
- Collins J.; Waldmann C. M.; Drake C.; Slavik R.; Ha N. S.; Sergeev M.; Lazari M.; Shen B.; Chin F. T.; Moore M.; Sadeghi S.; Phelps M. E.; Murphy J. M.; Van Dam R. M. (2017) Production of Diverse PET Probes with Limited Resources: 24 18F-Labeled Compounds Prepared with a Single Radiosynthesizer. Proc. Natl. Acad. Sci. U. S. A. 114 (43), 11309–11314. 10.1073/pnas.1710466114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B.; Jeon J.; Palner M.; Ye D.; Shuhendler A.; Chin F. T.; Rao J. (2013) Positron Emission Tomography Imaging of Drug-Induced Tumor Apoptosis with a Caspase-Triggered Nanoaggregation Probe. Angew. Chem., Int. Ed. 52 (40), 10511–10514. 10.1002/anie.201303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliego J. R.; Piló-Veloso D. (2007) Chemoselective Nucleophilic Fluorination Induced by Selective Solvation of the SN2 Transition State. J. Phys. Chem. B 111 (7), 1752–1758. 10.1021/jp066580p. [DOI] [PubMed] [Google Scholar]
- Savisto N.; Viljanen T.; Kokkomäki E.; Bergman J.; Solin O. (2018) Automated Production of [18F]FTHA According to GMP. J. Labelled Compd. Radiopharm. 61 (2), 84–93. 10.1002/jlcr.3589. [DOI] [PubMed] [Google Scholar]
- Stepanov V.; Krasikova R.; Raus L.; Loog O.; Hiltunen J.; Halldin C. (2012) An Efficient One-Step Radiosynthesis of [18F]FE-PE2I, a PET Radioligand for Imaging of Dopamine Transporters. J. Labelled Compd. Radiopharm. 55 (6), 206–210. 10.1002/jlcr.2927. [DOI] [Google Scholar]
- Stéen E. J. L.; Edem P. E.; Nørregaard K.; Jørgensen J. T.; Shalgunov V.; Kjaer A.; Herth M. M. (2018) Pretargeting in Nuclear Imaging and Radionuclide Therapy: Improving Efficacy of Theranostics and Nanomedicines. Biomaterials 179, 209–245. 10.1016/j.biomaterials.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Rossin R.; Renart Verkerk P.; Van Den Bosch S. M.; Vulders R. C. M.; Verel I.; Lub J.; Robillard M. S. (2010) In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew. Chem., Int. Ed. 49 (19), 3375–3378. 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- Meyer J. P.; Houghton J. L.; Kozlowski P.; Abdel-Atti D.; Reiner T.; Pillarsetty N. V. K.; Scholz W. W.; Zeglis B. M.; Lewis J. S. (2016) 18F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels-Alder Click Chemistry. Bioconjugate Chem. 27 (2), 298–301. 10.1021/acs.bioconjchem.5b00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S.; Choi J. S.; Garcia M. A.; Xing Y.; Chen K. J.; Chen Y. M.; Jiang Z. K.; Ro T.; Wu L.; Stout D. B.; Tomlinson J. S.; Wang H.; Chen K.; Tseng H. R.; Lin W. Y. (2016) Pretargeted Positron Emission Tomography Imaging That Employs Supramolecular Nanoparticles with in Vivo Bioorthogonal Chemistry. ACS Nano 10 (1), 1417–1424. 10.1021/acsnano.5b06860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edem P. E.; Jørgensen J. T.; Nørregaard K.; Rossin R.; Yazdani A.; Valliant J. F.; Robillard M.; Herth M. M.; Kjaer A. (2020) Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging. Molecules 25 (3), 463. 10.3390/molecules25030463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herth M. M.; Andersen V. L.; Lehel S.; Madsen J.; Knudsen G. M.; Kristensen J. L. (2013) Development of a 11C-Labeled Tetrazine for Rapid Tetrazine-Trans-Cyclooctene Ligation. Chem. Commun. 49 (36), 3805–3807. 10.1039/c3cc41027g. [DOI] [PubMed] [Google Scholar]
- Denk C.; Svatunek D.; Mairinger S.; Stanek J.; Filip T.; Matscheko D.; Kuntner C.; Wanek T.; Mikula H. (2016) Design, Synthesis, and Evaluation of a Low-Molecular-Weight 11C-Labeled Tetrazine for Pretargeted PET Imaging Applying Bioorthogonal in Vivo Click Chemistry. Bioconjugate Chem. 27 (7), 1707–1712. 10.1021/acs.bioconjchem.6b00234. [DOI] [PubMed] [Google Scholar]
- Denk C.; Svatunek D.; Filip T.; Wanek T.; Lumpi D.; Fröhlich J.; Kuntner C.; Mikula H. (2014) Development of a 18F-Labeled Tetrazine with Favorable Pharmacokinetics for Bioorthogonal PET Imaging. Angew. Chem., Int. Ed. 53 (36), 9655–9659. 10.1002/anie.201404277. [DOI] [PubMed] [Google Scholar]
- Rossin R.; Robillard M. S. (2014) Pretargeted Imaging Using Bioorthogonal Chemistry in Mice. Curr. Opin. Chem. Biol. 21, 161–169. 10.1016/j.cbpa.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Stéen E. J. L.; Jørgensen J. T.; Denk C.; Battisti U. M.; Nørregaard K.; Edem P. E.; Bratteby K.; Shalgunov V.; Wilkovitsch M.; Svatunek D.; Poulie C. B. M.; Hvass L.; Simón M.; Wanek T.; Rossin R.; Robillard M.; Kristensen J. L.; Mikula H.; Kjaer A.; Herth M. M. (2021) Lipophilicity and Click Reactivity Determine the Performance of Bioorthogonal Tetrazine Tools in Pretargeted in Vivo Chemistry. ACS Pharmacol. Transl. Sci. 4 (2), 824–833. 10.1021/acsptsci.1c00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Cai H.; Hassink M.; Blackman M. L.; Brown R. C. D.; Conti P. S.; Fox J. M. (2010) Tetrazine-Trans-Cyclooctene Ligation for the Rapid Construction of 18F-Labeled Probes. Chem. Commun. 46 (42), 8043–8045. 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötering S.; Scheunemann M.; Günther R.; Löser R.; Hiller A.; Peters D.; Brust P.; Fischer S.; Steinbach J. (2016) Tos-Nos-Mos: Synthesis of Different Aryl Sulfonate Precursors for the Radiosynthesis of the Alpha7 Nicotinic Acetylcholine Receptor Radioligand [18F]NS14490. Appl. Radiat. Isot. 114, 57–62. 10.1016/j.apradiso.2016.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.