Abstract
Background
As vaccine supply and access remain limited in many parts of the world, understanding the duration of protection from reinfection after natural infection is important.
Methods
Distinct individuals testing positive and negative for SARS-CoV-2 between March 6, 2020, and August 31, 2020, in Kentucky, USA, were identified using the Kentucky National Electronic Disease Surveillance System. Individuals were followed for occurrence of a positive test for SARS-CoV-2 from 91 days after their initial test result through December 31, 2020. Protection from reinfection provided by a prior infection was calculated and additional analyses evaluated impact of age, sex, symptom status, long-term care facility connection, testing occurrence and frequency, and time from initial infection.
Results
The protective effect from prior infection was 80.3% (95% CI, 78.2%–82.2%) for those aged 20–59 years and 67.4% (95% CI, 62.8%–71.4%) for those aged ≥60 years. At 30-day time periods through 270 days (with limited exceptions), protection was estimated to be >75% for those aged 20–59 years and >65% for those aged ≥60 years. Factors associated with repeat positive testing included a connection to a long-term care facility, duration of potential exposure, and absence of symptoms during initial infection.
Conclusions
Natural infection provides substantial and persistent immunologic protection for a period of several months for most individuals, although subpopulations may be at greater risk of repeat positive testing and potential poor outcomes associated with reinfection. These subgroups include individuals aged ≥60 years, residents and staff of long-term care facilities, and those who have mild or asymptomatic illness with initial infection. Continued emphasis on vaccination and infection prevention and control strategies remains critically important in reducing the risk of reinfection and associated severe outcomes for these groups.
Keywords: SARS-CoV-2, COVID-19, protective immunity after natural infection, reinfection, COVID-19 risk factors for reinfection
Introduction
Worldwide, as of November 12, 2021, there have been over 251 million infections and more than five million deaths associated with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the virus that causes coronavirus disease-2019 (COVID-19) (World Health Organization, 2021). Protective immunity after natural infection with SARS-CoV-2 has not been fully elucidated. As vaccine supply and access remain limited in many parts of the world, understanding the duration of protection from reinfection after natural infection is important, as is obtaining additional information regarding the impact of host factors such as age, sex, and setting of work or residence.
Immunity from reinfection with seasonal coronaviruses is known to be limited (Edridge et al., 2020, Kellam and Barclay, 2020and Barclay), while antibodies that develop after infection with coronaviruses that are closely related to SARS-CoV-2 (i.e., SARS-CoV and MERS-CoV) may last ≥2 years (Kellam and Barclay, 2020, Mo et al., 2006, Payne et al., 2016). Studies of healthcare workers in the United Kingdom have suggested 84%–92% protection for up to 7 months after initial infection with SARS-CoV-2 (Hall et al., 2021, Lumley et al., 2020). A study from Denmark estimated protection from reinfection of 78%–82% overall, but reported that this decreased to 47% in those aged ≥65 years (Hansen et al., 2021). The current study aimed to provide an estimate of protective immunity against reinfection after initial infection with SARS-CoV-2 in a United States subpopulation (i.e., state of Kentucky), with consideration of the impact of age, sex, association with a long-term care facility (LTCF), symptom status at time of initial infection, duration of potential time for exposure, and testing frequency.
Methods
Kentucky emergency regulation requires that results of all testing for SARS-CoV-2 be reported to the Kentucky Department for Public Health (KDPH). These results are either submitted electronically or via fax, and are entered into the Kentucky National Electronic Disease Surveillance System (NEDSS). Results are reported for both nucleic acid amplification tests (NAATs; e.g., polymerase chain reaction [PCR]) and antigen tests. Additionally, upon identification of a positive result, case investigation, with interview, is performed and additional data are entered into NEDSS as available. Demographic characteristics (e.g., age, sex) and other available data within the NEDSS system, including symptom status at the time of initial infection, connection to an LTCF, and performance of additional testing, are imported into a REDCap database for management.
Early in the pandemic, Kentucky relied upon the Centers for Disease Control and Prevention (CDC) for PCR testing of those who were symptomatic and had known travel or exposure placing them at risk of infection. Subsequently, the state public health laboratory validated the CDC testing protocol and began testing within the state. As testing was expanded to include contacts of known cases and then those living in areas of increased transmission, so was the number of laboratories offering PCR testing. A variety of platforms were utilized for testing, with validation being performed in collaboration with the state laboratory prior to official reporting of results. By July of 2020, testing was available to anyone in Kentucky, regardless of symptoms or exposure status, and all were encouraged to receive testing. Drive-through specimen collection for no-cost PCR testing, as an adjunct to more traditional testing, was made available across the state by state and local public health and by corporate partners in collaboration with the state. Antigen tests were not utilized until late in July, and during the period of March through August 31, 2020, <0.1% of tests performed were antigen tests. Use of antigen tests increased during the remainder of 2020, but never accounted for more than 6% of the tests performed in any given month. Results of at-home tests, unless virtually proctored, were not accepted into the reporting system.
The NEDSS system was queried for distinct individuals testing positive for SARS-CoV-2 between March 6, 2020, and August 31, 2020, and for distinct individuals having at least one negative test and no subsequent positive result within 90 days during the same time period. Because medical facilities and external testing facilities both report SARS-CoV-2 laboratory test results, duplicate tests were removed by reviewing names, dates of birth, NEDSS identification numbers, test date, test type, and test result. Additional positive tests within 90 days of an initial positive were disregarded. For those individuals having more than one negative test during the time period, the initial negative test was the referent. Individuals from the positive testing group who died within 90 days of initial positive test result were excluded from analyses. Both groups were then followed through December 31, 2020, for occurrence of a subsequent positive test >90 days after the initial positive or negative test result.* The test-negative comparison group was utilized in an attempt to reduce the potential impact of test type, test-seeking behavior, and reason for testing (e.g., screening versus diagnostic testing), as both groups would have had similar access and requirements for testing and would have had specimens tested using similar platforms. Protection from reinfection provided by a prior positive test was derived from the relative risk of a positive test result for those with a history of infection versus those with no such history ([1-RR] x 100). Analyses included adults aged ≥20 years, and individuals were grouped into the following age categories: 20–39, 40–49, 50–59, 60–69, 70–79, 80+ years, with summary younger (20–59) and older (≥60 years) age groups also included.
To provide context, monthly case incidence rates per 100,000 population were calculated for those aged ≥20 years for each month, June through December. Additionally, as testing frequency could impact the identification of positive test results, the number of tests performed >90 days after the initial positive test was evaluated by age group, sex, and association with an LTCF.
In an additional analysis, the rate of infection was calculated for the two groups per 100,000 person-days of exposure. Days of potential exposure started on the 91st day after the initial test result and accumulated until the date of a positive specimen, date of death (if known), or until December 31, 2020, whichever came first. Since duration of follow-up was dependent upon date of initial positive (or negative) test, analysis of protective effect relative to time from initial test was also performed. This included graphical illustration of time to positive result by use of event-free survival curves. Time period analysis included individuals who remained in follow-up at that time.
To further understand risk of reinfection, analyses were performed using factors considered to be potentially associated with reinfection and for which data were available. Unadjusted and adjusted relative risks were estimated using log-binomial regression with simultaneous entry of the variables of interest, which included age group (≥60 versus 20–59 years), sex (female versus male), month of initial infection (March–June versus July–August), symptom status (asymptomatic versus symptomatic or unknown during initial infection), testing after 90 days (one or more versus no test prior to the repeat positive), and connection with an LTCF (yes versus no, including both residents and staff). Additional analyses were performed after stratification by LTCF connection. Statistical analyses were performed using R (version 4.1.0, R Foundation for Statistical Computing).
Results
Through December 31, 2020, positive test results were obtained >90 days after an initial positive test for 593 (1.4%) of the 41,647 distinct Kentucky residents aged ≥20 years who tested positive for SARS-CoV-2 from March 6, 2020, through August 31, 2020. For 508,521 distinct individuals with a negative test result and no subsequent positive result within 90 days, 31,842 (6.3%) tested positive >90 days after the initial negative test. Less than 14% of the positive results during the follow-up period were from antigen tests, with a larger percentage from those with no history of prior infection (14%) as compared with those with a history of prior infection (8%). Monthly incidence rates for Kentucky during the months of June through December ranged from 136 cases per 100,000 population in June to 1,530 cases per 100,000 in November.
Overall, individuals aged ≥20 years with no history of a positive SARS-CoV-2 test result were 4.4 (95% CI, 4.1–4.8) times as likely to have a positive test result compared with those with previous infection (Table 1 ). This corresponds to a protective effect of 77.3% (95% CI, 75.4%–79.0%) provided by prior infection. For individuals aged 20–59 years, those without a history of infection were 5.1 (95% CI, 4.6–5.6) times as likely to have a positive test when compared with those with a history of infection, giving a protective effect of 80.3% (95% CI, 78.2%–82.2%) from prior infection. For individuals aged ≥60 years, those without a history of infection were 3.1 (95% CI, 2.7–3.5) times as likely to have a positive test when compared with those with a history of infection, giving a protective effect of 67.4% (95% CI, 62.8%–71.4%) provided by prior infection.
Table 1.
Protection from repeat positive test >90 days after initial infection and repeat positive test results per 100,000 person-days exposure, June–December, 2020, Kentucky, USA.
| Population and age group | No history of infectiona |
History of infectionb |
No history of infection versus history of infection RR (95% CI) | Estimated protection from repeat positive (95% CI)c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number at risk | Number positive (%) | Person-days exposured | Infection ratee | Number at risk | Number positive (%) | Person-days exposured | Infection ratee | |||
| All (female and male) | ||||||||||
| 20–39 | 173,522 | 10,162 (5.9%) | 14,630,087 | 69.5 | 17,815 | 194 (1.1%) | 1,479,352 | 13.1 | 5.4 (4.7–6.2) |
81.4 (78.6–83.9) |
| 40–49 | 79,321 | 4,829 (6.1%) | 7,083,308 | 68.2 | 7,291 | 94 (1.3%) | 636,749 | 14.8 | 4.7 (3.9–5.8) |
78.8 (74.1–82.7) |
| 50–59 | 87,493 | 4,987 (5.7%) | 8,000,157 | 62.3 | 6,807 | 81 (1.2%) | 594,563 | 13.6 | 4.8 (3.9–6.0) |
79.1 (74.0–83.2) |
| 60–69 | 84,353 | 4,399 (5.2%) | 7,954,388 | 55.3 | 5,004 | 77 (1.5%) | 435,004 | 17.7 | 3.4 (2.7–4.2) |
70.5 (63.1–76.4) |
| 70–79 | 54,375 | 3,474 (6.4%) | 5,164,484 | 67.3 | 2,810 | 70 (2.5%) | 249,843 | 28.0 | 2.6 (2.0–3.2) |
61.0 (50.8–69.1) |
| 80+ | 29,457 | 3,991 (13.5%) | 2,716,514 | 146.9 | 1,920 | 77 (4.0%) | 179,230 | 43.0 | 3.4 (2.7–4.2) |
70.4 (63.1–76.3) |
| All (female and male) | ||||||||||
| 20+ | 508,521 | 31,842 (6.3%) | 45,548,938 | 69.9 | 41,647 | 593 (1.4%) | 3,574,741 | 16.6 | 4.4 (4.1–4.8) |
77.3 (75.4–79.0) |
| 20–59 | 340,336 | 19,978 (5.9%) | 29,713,552 | 67.2 | 31,913 | 369 (1.2%) | 2,710,664 | 13.6 | 5.1 (4.6–5.6) |
80.3 (78.2–82.2) |
| 60+ | 168,185 | 11,864 (7.1%) | 15,835,386 | 74.9 | 9,734 | 224 (2.3%) | 864,077 | 25.9 | 3.1 (2.7–3.5) |
67.4 (62.8–71.4) |
| Female | ||||||||||
| 20+ | 297,915 | 20,446 (6.9%) | 26,836,350 | 76.2 | 22,154 | 373 (1.7%) | 1,890,933 | 19.7 | 4.1 (3.7–4.5) |
75.5 (72.8–77.8) |
| 20–59 | 201,763 | 12,965 (6.4%) | 17,790,862 | 72.9 | 16,708 | 227 (1.4%) | 1,409,252 | 16.1 | 4.7 (4.2–5.4) |
78.9 (75.9–81.4) |
| 60+ | 96,152 | 7,481 (7.8%) | 9,045,488 | 82.7 | 5,446 | 146 (2.7%) | 481,681 | 30.3 | 2.9 (2.5–3.4) |
65.5 (59.5–70.7) |
| Male | ||||||||||
| 20+ | 210,606 | 11,396 (5.4%) | 18,712,588 | 60.9 | 19,493 | 220 (1.1%) | 1,683,808 | 13.1 | 4.8 (4.2–5.5) |
79.1 (76.2–81.7) |
| 20–59 | 138,573 | 7,013 (5.1%) | 11,922,690 | 58.8 | 15,205 | 142 (0.9%) | 1,301,412 | 10.9 | 5.4 (4.6–6.4) |
81.5 (78.2–84.4) |
| 60+ | 72,033 | 4,383 (6.1%) | 6,789,898 | 64.6 | 4,288 | 78 (1.8%) | 382,396 | 20.4 | 3.3 (2.7–4.2) |
70.1 (62.7–76.1) |
Abbreviations: RR, relative risk; CI, confidence interval; F, female; M, male; SARS-CoV-2, severe respiratory syndrome coronavirus-2
Individuals who had at least one negative test for SARS-CoV-2 and no subsequent positive result within 90 days during the time period March 6, 2020 through August 31, 2020
Individuals who had a positive PCR or antigen test for SARS-CoV-2 during the time period March 6, 2020 through August 31, 2020
Calculated as (1-(1/RR of no history of infection versus history of infection)) x 100 or (1-RR of history of infection versus no history of infection) x 100
For each individual, person-days of exposure began on day 91 after the referent initial positive or negative test and accumulated until a positive test result or until December 31, 2020, whichever came first
Rate of infection per 100,000 person-days of exposure
The infection rate for those aged ≥20 years with no history of infection was 69.9 cases per 100,000 person-days of exposure (Table 1). Among the same age group with a history of infection, the infection rate was 16.6 cases per 100,000 person-days, which was a reduction of 76%. For those with history of infection, the rate per 100,000 person-days of exposure increased with age, with the lowest rate among those aged 20–39 years (13.1 cases per 100,000 person-days exposure) and the highest rate among those aged ≥80 years (43.0 cases per 100,000 person-days exposure).
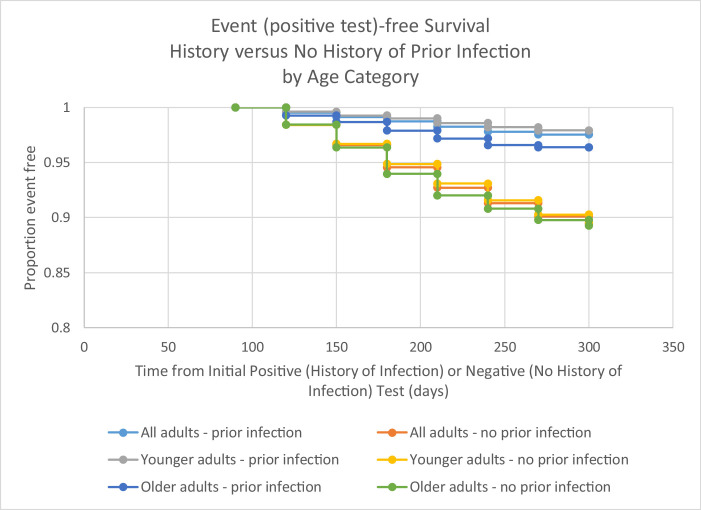
Event (positive test)-free survival was consistently better for those with a history of infection compared with those with no history of infection (Figure 1 , Table 2 ). Although those with a history of infection aged ≥60 years were consistently more likely to have an event after an initial positive test relative to those aged 20–59 years, protection was maintained in comparison with those with no history of infection throughout the period of follow-up. Of the 593 repeat positive tests, the largest percentage (33.1%, n=196) was obtained during days 91–120. This was true for both the younger (20–59) and older (≥60 years) age groups.
Figure 1.
Event (positive test)-free survival for those with and without history of SARS-CoV-2 infection, by younger (ages 20–59) and older (60 years and older) age groups, June–December, 2020, Kentucky, USA
Table 2.
Protection from repeat positive test by time from initial (positive – history of infection or negative – no history of infection) test by age category, June–December, 2020, Kentucky, USA.
| Days from initial test | No history of infectiona |
History of infectionb |
No history of infection versus history of infection RR (95% CI) |
Estimated protection from repeat positive (95% CI)c |
||
|---|---|---|---|---|---|---|
| Number at riskd | Repeat positive(%) | Number at risk | Positive(%) | |||
| All 20+ years of age | ||||||
| 91–120 | 508,521 | 8,002 (1.57%) | 41,647 | 196 (0.47%) | 3.3 (2.9–3.9) | 70.1 (65.6–74.0) |
| 121–150 | 500,414 | 9,341 (1.87%) | 41,429 | 165 (0.40%) | 4.7 (4.0–5.5) | 78.7 (75.1–81.7) |
| 151–180 | 374,624 | 7,878 (2.10%) | 27,359 | 107 (0.39%) | 5.4 (4.4–6.5) | 81.4 (77.5–84.6) |
| 181–210 | 222,120 | 4,337 (1.95%) | 14,198 | 72 (0.51%) | 3.9 (3.1–4.9) | 74.0 (67.2–79.4) |
| 211–240 | 116,800 | 1,763 (1.51%) | 8,954 | 40 (0.45%) | 3.4 (2.5–4.6) | 70.4 (59.5–78.4) |
| 241–270 | 33,629 | 444 (1.32%) | 4,873 | 13 (0.27%) | 4.9 (2.9–8.6) | 79.8 (65.0–88.4) |
| 271–300 | 10,382 | 77 (0.74%) | 1,335 | 0 (0%) | — | — |
| 20–59 years of age | ||||||
| 90–120 | 340,336 | 5,373 (1.58%) | 31,913 | 125 (0.39%) | 4.0 (3.4–4.8) | 75.2 (70.4–79.2) |
| 121–150 | 334,960 | 5,869 (1.75%) | 31,785 | 107 (0.34%) | 5.2 (4.3–6.3) | 80.8 (76.8–84.1) |
| 151–180 | 245,160 | 4,651 (1.90%) | 20,986 | 57 (0.27%) | 7.0 (5.4–9.1) | 85.7 (81.4–89.0) |
| 181–210 | 136,489 | 2,556 (1.87%) | 10,533 | 45 (0.43%) | 4.4 (3.3–5.9) | 77.2 (69.5–83.0) |
| 211–240 | 69,929 | 1,144 (1.64%) | 6,497 | 25 (0.38%) | 4.3 (2.9–6.3) | 76.6 (65.2–84.2) |
| 241–270 | 22,803 | 322 (1.41%) | 3,446 | 10 (0.29%) | 4.9 (2.6–9.1) | 79.5 (61.6–89.1) |
| 271–300 | 7,965 | 63 (0.79%) | 912 | 0 (0%) | — | — |
| 60+ years of age | ||||||
| 90–120 | 168,185 | 2,629 (1.56%) | 9,734 | 71 (0.73%) | 2.1 (1.7–2.7) | 53.3 (41.0–63.1) |
| 121–150 | 165,454 | 3,472 (2.10%) | 9,644 | 58 (0.60%) | 3.5 (2.7–4.5) | 71.3 (62.9–77.9) |
| 151–180 | 129,464 | 3,227 (2.49%) | 6,373 | 50 (0.78%) | 3.2 (2.4–4.2) | 68.5 (58.4–76.2) |
| 181–210 | 85,631 | 1,781 (2.08%) | 3,665 | 27 (0.74%) | 2.8 (1.9–4.1) | 64.6 (48.3–75.7) |
| 211–240 | 46,871 | 619 (1.32%) | 2,457 | 15 (0.61%) | 2.2 (1.3–3.6) | 53.8 (23.0–72.3) |
| 241–270 | 10,826 | 122 (1.13%) | 1,427 | 3 (0.21%) | 5.4 (1.7–16.8) | 81.3 (41.4–94.1) |
| 271–300 | 2,417 | 14 (0.58%) | 423 | 0 (0%) | — | — |
Individuals who had at least one negative test for SARS-CoV-2 and no subsequent positive result within 90 days during the time period March 6, 2020 through August 31, 2020
Individuals who had a positive PCR or antigen test for SARS-CoV-2 during the time period March 6, 2020 through August 31, 2020
Calculated as (1-RR of history of infection versus no history of infection) x 100
Included individuals who remained in follow-up during the time period and had not already tested positive during the follow-up
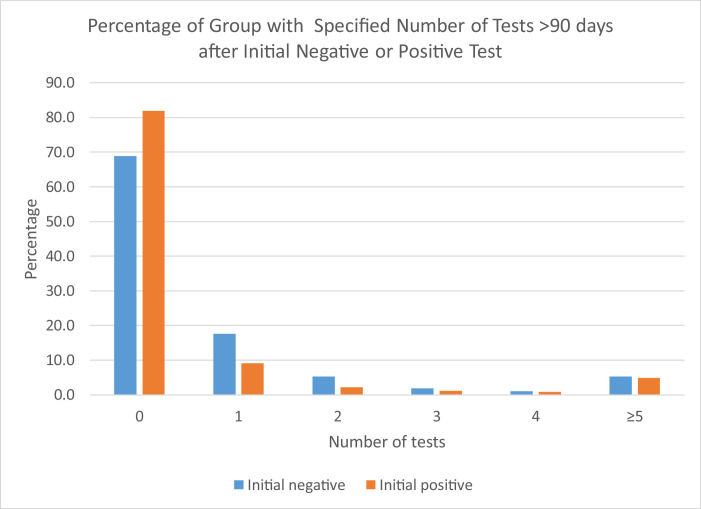
The mean number of negative tests obtained from 90 days after the initial positive test until the repeat positive test result was 1.8 (SD 4.1, median=0) for those aged 20–59 and 3.1 (SD 4.4, median=1) for those aged ≥60 years (P<0.001, t-test; P<0.001, Mann-Whitney non-parametric test). The mean number of negative tests obtained was 2.5 (SD 4.3, median=0) for females and 1.9 (SD 4.2, median=0) for males (P=0.103, t-test; P=0.021, Mann-Whitney non-parametric test). The mean number of negative tests obtained was 4.8 (SD 5.4, median=3) for those associated with LTCFs and 0.5 (SD 1.6, median=0) for those not associated with LTCFs (P<0.001, t-test; P<0.001, Mann-Whitney non-parametric test). A larger proportion of individuals with an initially positive test between March 6 and August 31, 2020, had no follow-up testing performed from day 91 after the initial positive result through the follow-up period in comparison with those identified with a negative test during the baseline period (Figure 2 ).
Figure 2.
Percentage of group (initial negative test versus initial positive test) with specified number of tests performed >90 days after an initial qualifying test result, June–December, 2020, Kentucky, USA
In analysis of factors associated with repeat positive tests results >90 days after an initial positive test, unadjusted relative risks were significant for all at P<0.001 (Table 3 ). After adjusting for covariates, a repeat positive test result was most strongly associated with having a connection to a long-term care facility. Those connected to an LTCF were 4.4 times as likely to have a repeat positive test compared with those without a known connection to an LTCF. Those who were reported to have no symptoms with initial infection were 2.3 (95% CI, 1.9–2.8) times as likely to have a repeat positive test compared with those who reported symptoms or for whom symptom status was unknown. Those with initial infection between March 6 and June 30 were 1.6 (95% CI, 1.4–1.9) times as likely to have a repeat positive test compared with those with initial infection in July or August. Repeat positive test results were obtained from 292/13,504 (2.2%) of those initially infected in March–June and in 301/28,143 (1.1%) of those initially infected in July–August. Relative risks for age, sex, and testing after 90 days did not maintain statistical significance after adjusting for covariates. These factors were all strongly associated with LTCF designation. Females made up 72% of those associated with LTCFs, but 51% of those who were not associated with an LTCF. Additionally, 60% of those associated with an LTCF were aged ≥60 years, while 19% of those not associated with an LTCF were in the older age group. Lastly, while 67% of those associated with LTC had at least one negative test prior to their repeat positive test result, the same was true for 19% of those not associated with an LTCF.
Table 3.
Factors associated with risk of repeat positive test >90 days after initial positive result, with 95% confidence intervals, June–December, 2020, Kentucky, USA
| Variable | Repeat positive test |
Unadjusted estimates |
Adjusted estimates |
|||
|---|---|---|---|---|---|---|
| Yes(n=593) | No(n=41,054) | RR | 95% CI | RR | 95% CI | |
| Agea | 1.99 | 1.69-2.34 | 0.96 | 0.80–1.16 | ||
| 60+ | 224 (37.8) | 9,510 (23.2) | ||||
| 20–59 | 369 (62.2) | 31,544 (76.8) | ||||
| Sexb | 1.49 | 1.27–1.76 | 1.17 | 0.99–1.39 | ||
| Female | 373 (62.9) | 21,781 (53.1) | ||||
| Male | 220 (37.1) | 19,273 (46.9) | ||||
| Month of initial infectionc | 2.02 | 1.72–2.37 | 1.60 | 1.36–1.88 | ||
| March–June | 292 (49.2) | 13,212 (32.2) | ||||
| July–August | 301 (50.8) | 27,842 (67.8) | ||||
| Symptom statusd | 3.43 | 2.89–4.05 | 2.31 | 1.93–2.75 | ||
| Asymptomatic | 196 (33.1) | 5,047 (12.3) | ||||
| Symptoms or unknown | 397 (66.9) | 36,007 (87.7) | ||||
| Testing after 90 dayse | 2.86 | 2.44–3.45 | 1.20 | 0.99–1.45 | ||
| ≥1 test | 239 (40.3) | 7,640 (18.6) | ||||
| No test | 354 (59.7) | 33,414 (81.4) | ||||
| LTCFf | 6.67 | 5.56–7.69 | 4.35 | 3.45–5.26 | ||
| Yes | 247 (41.7) | 3,878 (9.4) | ||||
| No | 346 (58.3) | 37,176 (90.6) | ||||
| Associated with LTCF (n=4,125) | (n=247) | (n=3,878) | ||||
| Age | 1.10 | 0.86–1.42 | 0.93 | 0.72–1.21 | ||
| 60+ | 153 (61.9) | 2,304 (59.4) | ||||
| 20–59 | 94 (38.1) | 1,574 (40.6) | ||||
| Sex | 0.94 | 0.73–1.24 | 0.96 | 0.74–1.26 | ||
| Female | 175 (70.9) | 2,798 (72.2) | ||||
| Male | 72 (29.1) | 1,080 (27.8) | ||||
| Month of initial infection | 1.32 | 1.04–1.69 | 1.36 | 1.06–1.74 | ||
| March–June | 137 (55.5) | 1,866 (48.1) | ||||
| July–August | 110 (44.5) | 2,012 (51.9) | ||||
| Symptom status | 1.89 | 1.48–2.40 | 1.93 | 1.51–2.47 | ||
| Asymptomatic | 110 (44.5) | 1,120 (28.9) | ||||
| Symptoms or unknown | 137 (55.5) | 2,758 (71.1) | ||||
| Testing after 90 days | 0.90 | 0.69–1.17 | 0.94 | 0.72–1.22 | ||
| ≥1 test | 172 (69.6) | 2,611 (67.3) | ||||
| No test | 75 (30.4) | 1,267 (32.7) | ||||
| Not associated with LTCF (n=37,522) | (n=346) | (37,176) | ||||
| Age | 1.07 | 0.82–1.38 | 1.03 | 0.79–1.33 | ||
| 60+ | 71 (20.5) | 7,206 (19.4) | ||||
| 20–59 | 275 (79.5) | 29,970 (80.6) | ||||
| Sex | 1.28 | 1.04–1.58 | 1.32 | 1.07–1.64 | ||
| Female | 198 (57.2) | 18,983 (51.1) | ||||
| Male | 148 (42.8) | 18,193 (48.9) | ||||
| Month of initial infection | 1.84 | 1.49–2.26 | 1.81 | 1.46–2.23 | ||
| March–June | 155 (44.8) | 11,346 (30.5) | ||||
| July–August | 191 (55.2) | 25,830 (69.5) | ||||
| Symptom status | 2.76 | 2.16–3.50 | 2.78 | 2.17–3.52 | ||
| Asymptomatic | 86 (24.9) | 3,927 (10.6) | ||||
| Symptoms or unknown | 260 (75.1) | 33,249 (89.4) | ||||
| Testing after 90 days | 1.54 | 1.16–1.96 | 1.35 | 1.02–1.75 | ||
| ≥1 test | 67 (19.4) | 5,029 (13.5) | ||||
| No test | 279 (80.6) | 32,147 (86.5) | ||||
Age: >60 versus 20–59 years of age
Sex: female versus male; missing data for two individuals with reinfection, 285 individuals without reinfection
Month of initial infection: March–June versus July–August
Symptom status: asymptomatic versus symptomatic or unknown during initial infection
Testing after 90 days: ≥1 test versus no tests prior to repeat positive
When stratifying by connection to LTCF, both symptom status and time period of initial infection were significantly associated with a repeat positive test result after adjusting for covariates. In the group without connection to LTC, sex and any negative testing after 90 days from the initial positive test were additionally associated with a repeat positive test (Table 3).
Discussion
In Kentucky, 41,647 individuals identified with a SARS-CoV-2 infection between March 6, 2020, and August 31, 2020, were followed through December 31, 2020, and 593 (1.4%) had a repeat positive test collected >90 days after the initial positive result. Natural infection with SARS-CoV-2 was shown to provide protection from a repeat positive test result. Overall protection was estimated to be 77.3% for those aged ≥20 years, which was very similar to estimates reported from Denmark and the United States (Hansen et al., 2021; Sheehan et al, 2021). Although the current study found a reduction in protection for older individuals, with estimated protection of 67.4% for those aged ≥60 years compared with 80.3% for those aged 20–59 years, the reduction in protection was not as dramatic as that noted in the study from Denmark (Hansen et al., 2021), where estimated protection was 47.1% in those aged ≥65 years.
A decline in protection with age was further illustrated by the rate per 100,000 days exposure, with rates increasing with age. Older individuals have been shown to have a less robust immunologic response to both natural infection and vaccination (Bajaj et al., 2021; Canaday et al., 2021; Simon et al., 2015). Less effective immune response and dysregulation of the inflammatory response have been implicated in the more severe outcomes associated with infection with SARS-CoV-2 in older individuals (Moderbacher et al., 2020; Zhou et al., 2020). Age, however, was not noted to be associated with a repeat positive test among those with a history of infection when accounting for other measured potential associated factors.
This study also noted a slightly lower protective effect of natural infection among females, which was in contrast to the Danish study (Hansen et al., 2021). Differences in age and sex effects may reflect testing of a larger number of older individuals, the majority of them female, in the current study. Although females have been found to have more vigorous and effective immunologic responses to a number of viruses, there is evidence that this may not be the case for SARS-CoV-2 (Gadi et al., 2020; Grzelak et al., 2021; Markmann et al, 2021; Takahashi et al., 2020; Zeng et al., 2020). For example, Markmann et al., 2020 noted that neutralizing antibody levels were higher in males than females. In the present study, there were potential confounding effects of age, testing frequency, and association with LTCFs, and with adjustment for these factors, female sex was not associated with greater risk of a repeat positive test (Table 3). However, when considering only those individuals with no known connection to an LTCF, an association with female sex and occurrence of a repeat positive test result was noted, with females 1.3 times as likely to have a repeat positive test as compared with males, after accounting for other measured factors.
It has been suggested that repeat positive testing in nursing home residents may exceed that noted in younger individuals living in the community (Armstrong et al., 2021). When considering all of those with an initial positive test through August 31, 2020, the strongest association of a repeat positive test was with connection to an LTCF. Those connected to an LTCF were 4.4 times as likely to have a repeat positive test result compared with those with no connection to an LTCF. As previously noted, those associated with an LTCF were more likely to be aged ≥60 years, female, and to have a negative testing prior to a positive result. The occurrence and frequency of testing were impacted by workplace policies and regulations. For example, testing was mandated by the Centers for Medicare and Medicaid Services (CMS) for staff working in long-term care facilities, and repeated testing of long-term care residents was necessary with the identification of any new case among staff or residents in a facility.
Risk of a repeat positive test was also noted to be higher in those who were reported to be asymptomatic during the primary infection. Several studies have suggested that immune response following infection with no or mild symptoms is less robust than that seen with moderate to severe clinical illness (Carsetti et al., 2020; Gudbjartsson et al., 2020; Ibarrondo et al., 2020; Kutsuna et al., 2020; Legros et al., 2021; Long et al., 2020; Luo et al., 2020; Rijkers et al., 2020; Seow et al., 2020). In the current study, 12.6% of those with primary infection were reported to be asymptomatic.
Additionally, likelihood of repeat positive test was greater in those with a longer period of follow-up and potential at-risk exposure. Adjusted risk of a repeat positive test was 1.6 times higher for those with earlier initial infections, and their at-risk exposure time also included longer exposure during the autumn months, when cases were increasing in Kentucky and across the United States. However, the event-free survival graphs illustrate that protection from a repeat positive test was consistent across the follow-up period in comparison with non-infected peers over the same time period. There was no clear evidence of reduction of protective effect of primary infection over the 6 months of follow-up beyond the first 90 days after an initial positive test result, which was consistent with the findings of others (Hall et al., 2021, Lumley et al., 2020). The greater risk of a repeat positive test result appears more a matter of increased risk of exposure during a longer time period than waning of immunity during that time period.
The time period of greatest likelihood of a positive test result was in the 90–120 day time period after an initial positive test. The event-free survival graph and estimated protection at 30-day increments post 90 days suggest that the apparent decrease in protection during the 90–120 time decrease may be an artifact of detection of persistent virus for some individuals. Protection was 75% in those aged 20–59 during the 90–120 day time period, but was consistently >75% from 120 days onward through the end of follow-up. Similarly, although protection was estimated to be 53% in those aged ≥60 years during the 90–120 day time period, it was consistently >53% from 120 days onward. These data suggest that the overall protection of 77.3% may be an underestimate of the true level of protection offered from primary infection as a portion of the positive results in the 90–120 time period were likely not indicative of active infection.
This study had several limitations. First, the estimate of protection was based on comparison of those receiving initial positive and negative test results during March through August. If initial results differentially impacted risk behaviors and the likelihood of subsequent testing, then estimated protection could have been biased. An initial positive test could lead to increased caution in some individuals, but could also decrease caution if the positive result was believed to eliminate likelihood of a subsequent infection. In any case, those testing positive were noted to be less likely to have testing performed in the follow-up period. Second, cases would have been missed if testing was not performed, and the population tested during the study period was a minority of the residents of Kentucky. Additionally, as noted in Figure 2, the majority of individuals had no testing performed during the follow-up period. Third, antigen testing without confirmation of findings with PCR testing could have resulted in either inclusion of individuals with false positive results in the primary infection group or individuals with false negative results in the no history of prior infection group, with a consequent reduction in the estimate of protection from primary infection. Fourth, although all testing results are to be reported to the Kentucky Department for Public Health, negative antigen testing in some settings was not consistently and reliably reported. This likely resulted in an underestimation of the amount and frequency of testing, including testing performed >90 days after an initial positive result. Fifth, information on symptom status was missing in 40% of those with primary infection in March – August, 2020, so the estimated impact of symptoms on reinfection should be interpreted with caution. Sixth, the follow-up period for this study occurred prior to the widespread occurrence of variants of concern and variants of interest in Kentucky; the protective effect of primary infection with circulating viruses during March through August, 2020, may not accurately predicted protection from infection with newer variants. Seventh, there are potential confounding factors that were not adequately or consistently measured (e.g., race, ethnicity) or that remain unknown, which might have affected the findings and conclusions. The finding of an association between female sex and a repeat positive test result in the non-LTCF sample may be an example of the impact of an unmeasured confounder. Finally, repeat positive tests do not necessarily equate with repeat infections and may have reflected viral persistence or false positive results in some cases (Ladhani et al., 2021); consequently, protection from reinfection may have been higher than the 77% noted in the current study.
In summary, repeat positive results for SARS-CoV-2 after primary infection were relatively infrequent (1.4%) among the group infected from March through August, 2020, and followed through December 31, 2020, in Kentucky, USA. Accumulated evidence, including that from the present study, suggests that natural infection provides immunologic protection for a period of several months (Breathnach et al., 2021; Dan et al., 2021; Hanrath et al., 2021; Pilz et al., 2021; Wajnberg et al., 2020). Although primary infection appears to provide substantial and persistent protection for most individuals, certain subpopulations may be at higher risk of a relatively poorer immune response to initial infection or increased risk of exposure to active cases, and consequently are at greater risk for positive testing and potential poor outcomes associated with reinfection. These subgroups include older individuals, those living or working in LTCFs, those who are asymptomatic or who have mild clinical illness with initial infection, and those with longer duration of potential exposure after natural infection (as a result of increased exposure risk and not necessarily as a reflection of waning immunity). A continued emphasis on vaccination and infection prevention and control strategies remains critically important in reducing the risk for reinfection and associated severe outcomes for these groups. Just as a second dose of vaccine boosts initial immunologic response, there is evidence that vaccination after infection provides a similar benefit and may result in a more robust and sustained immunological response, especially in those with less vigorous initial response to primary infection (Manisty et al., 2021; Saadat et al., 2021).
Conflict of Interest Statement
The authors have declared no conflicts of interest.
Funding
No external funding was received.
Ethics approval
This activity was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy.† Data were collected as part of routine public health surveillance and case investigation, and this project was consequently determined to be a non-research activity.
*The follow-up period ended on December 31, 2020, so that the potential impact of vaccination did not complicate interpretation of results and estimates of protection related directly to immunity after natural infection. Vaccination for healthcare workers and older individuals in long-term care facilities began in Kentucky in mid-December, 2020, and therefore no individual included in the current study would have been fully vaccinated during the follow-up period.
† See e.g., 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the Kentucky Department for Public Health.
REFERENCES
- Armstrong JN, Campbell L, Tabatsky-her T, Leung V, Parikh S. Repeat positive SARS-CoV-2 RNA testing in nursing home residents during the initial 9 months of the COVID-19 pandemic: an observational retrospective analysis. The Lancet Regional Health – Americas. 2021 doi: 10.1016/j.lana.2021.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections. Front Physiol. 2021;11 doi: 10.3389/fphys.2020.571416. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach DAS, Riley PA, Cotter MP, Houston AC, Habibi MS, Planche TB. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J Infect. 2021;82:e11–e12. doi: 10.1016/j.jinf.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday DH, Carias L, Oyebanji OA, Keresztesy D, Wilk D, Payne M, et al. Reduced BNT162b2 mRNA vaccine response in SARS-CoV-2-naïve nursing home residents. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsetti R, Zaffina S, Mortari EP, Terreri S, Corrente F, Capponi C, et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020 doi: 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edridge AWD, Kaczorowska J, Hoste ACR, Bakker M, Klein M, Loens K, et al. Seasonal coronavirus protective immunity is short-lasting. Nature Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- Gadi N, Wu SC, Spihlman AP, Moulton VR. What's sex got to do with COVID-19? Gender-based differences in the host immune response to coronaviruses. Front Immunol. 2020;11:2147. doi: 10.3389/fimmu.2020.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzelak L, Velay A, Madec Y, Gallais F, Staropoli I, Schmidt-Mutter C, et al. Sex differences in the evolution of neutralizing antibodies to SARS-CoV-2. J Infect Dis. 2021 doi: 10.1093/infdis/jiab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson DF, Norddahl GL, Mellsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJ, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicenter, prospective cohort study (SIREN) Lancet. 2021 doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrath AT, Payne BAI, Duncan CJA. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J infect. 2021;82:e29–e30. doi: 10.1016/j.jinf.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CH, Michlmayr D, Gubbels SM, Molbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021 doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:11. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020;101:791–797. doi: 10.1099/jgv.0.001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna S, Asai Y, Matsunaga A. Loss of anti-SARS-CoV-2 antibodies in mild COVID-19. N Engl J Med. 2020;383:1694–1698. doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- Ladhani SN, Chow JY, Atkin S, Brown KE, Ramsay ME, Randell P, et al. Regular mass screening for SARS-CoV-2 infection in care homes already affected by COVID-19 outbreaks: implications of false positive test results. J Infect. 2021;82(2):282–327. doi: 10.1016/j.jinf.2020.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18(2):318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q-X, Tang X-J, Shi Q-L, Li Q, Deng H-J, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Lumley SF, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. New Eng J Med. 2020 doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YR, Chakraborty I, Yun C, Wu AHB, Lynch KL. Kinetics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody avidity maturation and association with severe disease. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisty C, Otter AD, Treibel TA, McKnight A, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmann AJ, Giallourou N, Bhowmik DR, Hou YJ, Lerner A, et al. Sex disparities and neutralizing antibody durability to SARS-CoV-2 infection in convalescent individuals. medRxiv. 2021 doi: 10.1101/2021.02.01.21250493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo H, Zeng G, Ren X, Li H, Ke C, Tan Y, et al. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirol. 2006;11(1):49–53. doi: 10.1111/j.1440-1843.2006.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moderbacher CR, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DC, Iblan I, Rha B, Alqasrawi S, Haddadin A, Al Nsour M, et al. Persistence of antibodies against Middle East Respiratory Syndrome Coronavirus. Emerg Inf Dis. 2016;22(10):1824–1826. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Chakeri A, Ioannidis JPA, Richter L, Theiler-Schwetz V, Trummer C, et al. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. 2021;51:e13520. doi: 10.1111/eci.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkers G, Murk J-L, Wintermans B, van Looy B, van den Berge M, Veenemans J, et al. Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. J Infect Dis. 2020;222:1265–1269. doi: 10.1093/infdis/jiaa463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S, Tehrani ZR, Logue J, Newman M, Frieman MB, Harris AD, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325(14):1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow J, Graham C, Merrick B, Acors S, Steel KJA, Hemmings O, et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. MedRxiv. 2020 doi: 10.1101/2020.07.09.20148429. [DOI] [Google Scholar]
- Sheehan MM, Reddy AJ, Rothberg MB. Reinfection rates among patients who previously tested positive for COVID-19: a retrospective cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B. 2015;282 doi: 10.1098/rspb.2014.3085. https://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed June 21, 2021. https://covid19.who.int/?gclid=CjwKCAiAtK79BRAIEiwA4OskBtAbOw6T6wNgUS76dyazGbUP3AdVbHz7JZLTG4xKNRk_r3W9C9NQ-xoCRDoQAvD_BwE
- Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92:2050–2054. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]