Abstract
Insulin receptor substrate (IRS) proteins are tyrosine phosphorylated and mediate multiple signals during activation of the receptors for insulin, insulin-like growth factor 1 (IGF-1), and various cytokines. In order to distinguish common and unique functions of IRS-1, IRS-2, and IRS-4, we expressed them individually in 32D myeloid progenitor cells containing the human insulin receptor (32DIR). Insulin promoted the association of Grb-2 with IRS-1 and IRS-4, whereas IRS-2 weakly bound Grb-2; consequently, IRS-1 and IRS-4 enhanced insulin-stimulated mitogen-activated protein kinase activity. During insulin stimulation, IRS-1 and IRS-2 strongly bound p85α/β, which activated phosphatidylinositol (PI) 3-kinase, protein kinase B (PKB)/Akt, and p70s6k, and promoted the phosphorylation of BAD. IRS-4 also promoted the activation of PKB/Akt and BAD phosphorylation during insulin stimulation; however, it weakly bound or activated p85-associated PI 3-kinase and failed to mediate the activation of p70s6k. Insulin strongly inhibited apoptosis of interleukin-3 (IL-3)-deprived 32DIR cells expressing IRS-1 or IRS-2 but failed to inhibit apoptosis of cells expressing IRS-4. Consequently, 32DIR cells expressing IRS-4 proliferated slowly during insulin stimulation. Thus, the activation of PKB/Akt and BAD phosphorylation might not be sufficient to inhibit the apoptosis of IL-3-deprived 32DIR cells unless p85-associated PI 3-kinase or p70s6k are strongly activated.
Insulin receptor substrate (IRS) proteins play a central role in signal transduction by the receptors for insulin, insulin-like growth factor 1 (IGF-1), and a growing number of cytokines and integrins (19, 34, 58). IRS proteins are composed of an NH2-terminal pleckstrin homology (PH) domain and a phosphotyrosine binding (PTB) domain, followed by a COOH-terminal tail containing multiple tyrosine phosphorylation motifs (48). The PH and PTB domains are well conserved in each IRS protein and have a common tertiary structure but function differently to couple IRS proteins to the activated insulin receptor (10, 26, 57). PH domains are thought to bind membrane phospholipids or acidic motifs of various proteins, whereas the PTB domain interacts with the phosphorylated NPEY motif in the juxtamembrane region of the insulin receptor β-subunit; both interactions promote tyrosine phosphorylation of multiple tyrosine residues in the COOH terminus. The COOH terminus is poorly conserved among the IRS proteins; in IRS-1 (the first cloned and best-characterized IRS protein) it contains 18 potential tyrosine phosphorylation sites in amino acid sequence motifs that directly bind to Src homology-2 domains in several proteins, including phosphatidylinositol (PI) 3-kinase regulatory subunits (p85α, p55α p50α, p85β, and p55PIK), Grb-2, Nck, c-fyn, and SHP-2 (58). The other IRS proteins contain common and unique phosphorylation motifs, suggesting that they mediate overlapping and distinct biological signals. One region of predicted overlap in the signals mediated by IRS-1, IRS-2, and IRS-4 is the binding and activation of PI 3-kinase; a conspicuous example of dissimilarity is the absence of SHP-2 binding motifs in IRS-4.
PI 3-kinase is a heterodimeric signaling enzyme composed of a catalytic subunit (p110) associated with an SH2 domain-containing regulatory subunit (e.g., p55PIK, p85α, and p85β) that binds to tyrosine-phosphorylated YXXM motifs on signaling proteins. Since all of the IRS proteins contain numerous YXXM motifs, they are all expected to bind and activate p85-associated PI 3-kinase. The lipid products of PI 3-kinase mediate the activation of several serine/threonine kinases, including PDK1/PDK2, protein kinase B (PKB)/Akt, PKCζ and PKCλ, p70s6k, and others (3, 29, 32, 52, 53). These serine/threonine kinases play various roles in the regulation of the many biological responses linked to the activation of PI 3-kinase, including activation of glucose transport and glycogen synthesis, protein synthesis, lipogenesis and antilipolysis, and mitogenesis and antiapoptosis (12, 14, 16, 22, 38).
In vivo and in vitro experiments reveal important differences in the signaling capacity of IRS-1 and IRS-2. IRS-1 and IRS-2 display differential sensitivities for binding various SH2 proteins (46). Moreover, IRS-2 expressed in fibroblasts lacking IRS-1 does not reconstitute normal insulin–IGF-1 signaling (9). Mice lacking IRS-1 are small and mildly insulin resistant but never develop diabetes (5). In contrast, mice lacking IRS-2 are insulin resistant with abnormal glucose tolerance at birth and progressively develop fasting hyperglycemia with reduced β-cell mass (54; D. J. Withers, D. J. Burks, H. H. Towery, S. L. Altamuro, C. L. Flint, and M. F. White, unpublished data). Together, these results suggest that IRS-2 is critical for normal carbohydrate metabolism and that IRS-1 is important for somatic cell growth (55). Previous results suggest that IRS-4 has similar signaling potential to IRS-1 and IRS-2; however, our results with 32DIR cells revealed that IRS-4 activates PKB/Akt and promotes BAD phosphorylation without the activation of p70s6k or the inhibition of apoptosis. These differences might have important consequences for the specific tissues that express IRS-4.
MATERIALS AND METHODS
Cloning of human IRS-4.
To isolate the gene encoding IRS-4, a 540-bp DNA fragment of IRS-4 was amplified by RT-PCR with total RNA extracted from HEK293 cells. The sense and antisense primers were 5′-CCGCTCGAGCCGGGAGGCT-3′ (nucleotides 1171 to 1193) and 5′-ATCTCTAGAGCACTGGTTTC-3′ (nucleotides 1703 to 1722), respectively. The nucleotide numbers used here correspond to the numbering used by Lavan et al. (24). This DNA fragment was labeled with [α-32P]dCTP and used to screen a human fibroblast genomic library (Stratagene). Four independent clones were obtained, and a 4.5-kb portion of EcoRI DNA fragment was subcloned into pBluescript II for subsequent sequencing. Sequence analysis revealed that human IRS-4 had no introns like IRS-1 and IRS-2 and that this EcoRI fragment contained the full length of the coding region.
Cell culture.
32D cell lines were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS) and 5% WEHI-3-conditioned RPMI 1640 medium as a source of interleukin-3 (IL-3). 32DIR cells expressing IRS-1, IRS-2, or IRS-4 cell lines were maintained in the same medium containing, in addition, 5 mM histidinol (Bachem Bioscience, Inc.). HEK293 cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS.
Generation of 32D cell line.
32DIR cells and 32DIR cells expressing IRS-1 and IRS-2 are described elsewhere (48). A 4.5-kb EcoRI fragment of IRS-4 was inserted into pCMVhis expression vector, and then 10 μg of purified vector was used to transfect 5 × 106 32DIR cells by electroporation. Antibiotic-resistant cells were selected in the RPMI 1640 containing 5 mM histidinol for 10 days. Resistant colonies were then expanded and screened for protein expression by Western blotting.
Immunoprecipitation and immunoblotting.
Anti-IRS-4 polyclonal antibodies were raised against the carboxyl-terminal 17 amino acids of human IRS-4 (MDFARRDNQFDSPKRGR) coupled to keyhole limpet hemocyanin. The antibodies immunoprecipitated and immunoblotted both endogenous and recombinant IRS-4 from HEK293 cells and 32DIR/IRS-4 cells without any cross-reactivity with IRS-1 or IRS-2 (data not shown). Phospho-PKB/Akt polyclonal antibodies and phospho-BAD polyclonal antibodies (Ser112) were purchased from New England Biolabs (numbers 9271 and 9291, respectively). PY20 monoclonal antiphosphotyrosine antibody was purchased from Transduction Laboratories (P11120). Polyclonal or monoclonal antibodies against Grb-2 (C-23), PKB/Akt (C-20), BAD (C-20), 14-3-3 (H-8), and Bcl-2 (C-2) were purchased from Santa Cruz Biotechnology. Polyclonal antibodies against insulin receptor, p85 PI 3-Kpan, p85 PI 3-Kα, p85 PI 3-Kβ, mitogen-activated protein kinase (MAPK), and p70s6k were as described elsewhere (59).
Each cell line (108 cells) was serum starved in 10 ml of RPMI 1640 in a 50-ml conical tube for 4 h. The cells were treated with or without insulin, and ice-cold phosphate-buffered saline (PBS) was added to stop the reaction. The cells were collected by centrifugation and then lysed in 1 ml of TNE lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 5 mM EDTA, 5% glycerol, 10 μg leupeptin per ml, 10 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 2 mM sodium vanadate). Then, 10-mm plates of HEK293 cells were serum starved in 10 ml of DMEM for 4 h, treated with or without insulin, and lysed as described above. Insoluble material was removed by centrifugation, and the resulting supernatants were subjected to immunoprecipitation at 4°C for 2 h. Immune complex was collected and washed with TNE lysis buffer three times and then resuspended in 2 × sodium dodecyl sulfate (SDS) sample buffer. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to Protran nitrocellulose membrane (Schleicher & Schuell). The blots were blocked with 5% skim milk in TBST buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% Tween 20) and then incubated with antibodies in TBST containing 2% bovine serum albumin (BSA), followed by incubation with either secondary antibodies conjugated to horseradish peroxidase or 125I-protein A. The immunoreactive bands were visualized by either enhanced chemiluminescence or a Bio-Rad Molecular Imager.
PI 3-kinase assay.
32D cell lines treated with or without insulin for 1 min were lysed in 1 ml of PI 3-kinase lysis buffer (20 mM Tris-HCl [pH 7.4], 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10% glycerol, 1% NP-40, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 mM PMSF, and 2 mM sodium vanadate) and immunoprecipitated. The resulting immune complex was washed with PBS containing 1% NP-40 and 2 mM sodium vanadate (three times); 100 mM Tris-HCl (pH 7.4) containing 500 mM LiCl and 2 mM sodium vanadate (three times); and 10 mM Tris-HCl containing 100 mM NaCl, 1 mM EDTA, and 1 mM sodium vanadate (twice). The pellets were resuspended in 60 μl of 10 mM Tris-HCl (pH 7.4) containing 100 mM NaCl and 1 mM EDTA and then combined with 10 μl of 100 mM MgCl2 and 10 μl of a 2-μg/ml concentration of PI (Avanti) sonicated in 10 mM Tris-HCl (pH 7.4) containing 1 mM EGTA. The phosphorylation reaction was started by adding 5 μl of 65 μM ATP containing 3 μCi of [γ-32P]ATP. After 15 min at room temperature, the reaction was stopped with 20 μl of 8 N HCl and 160 μl of CHCl3-methanol (1:1). The samples were centrifuged, and the lower organic phase was applied to a silica gel thin-layer chromatography (TLC) plate (Merck). TLC plates were developed in CHCl3-CH3OH-H2O-NH4OH (60:47:11.3:2), dried, and visualized and quantified on a Bio-Rad Molecular Imager.
PKB/Akt kinase activity assay.
32D cell lines were serum starved 4 h, followed by stimulation with 100 nM insulin for 30 min. Cells were lysed in 1 ml of Akt lysis buffer (20 mM Tris-HCl [pH 7.4], 5 mM EDTA, 1% NP-40, 10 mM Na4P2O7, 100 mM NaF, 2 mM sodium vanadate, 1 mM PMSF, 10 μg of aprotinin per ml, and 10 μg of leupeptin per ml). Then, 1.5 mg of total protein was subjected to immunoprecipitation with anti-Akt polyclonal antibodies for 1 h at 4°C. Immune complexes were washed three times in Akt lysis buffer and twice in kinase buffer (20 mM Tris-HCl [pH 7.4], 10 mM MgCl2, and 1 mM dithiothreitol [DTT]). The in vitro kinase reaction was performed in 30 μl of 50 mM Tris-HCl (pH 7.4) containing 10 mM MgCl2, 1 mM DTT, 1 mg of BSA per ml, 50 μM ATP, 1 μM protein kinase inhibitor (Sigma), 0.2 mg of histone 2B, and 3 μCi of [γ-32P]dATP at room temperature for 15 min. Reactions were stopped by adding 10 μl of 5 × SDS sample buffer and then boiled for 5 min. Supernatants were electrophoresed on SDS–15% gel, and the radioactivity incorporated into substrate was determined by using a Bio-Rad Molecular Imager.
MAPK and p70s6k activity assay.
The cells were stimulated with or without insulin for 5 min (MAPK activity) or 30 min (p70s6k) and then lysed in 10 mM KPO4 (pH 7.05) containing 0.5% NP-40, 1 mM EDTA, 5 mM EGTA, 10 mM MgCl2, 2 mM DTT, 50 mM β-glycerophosphate, 2 mM sodium vanadate, 1 mM PMSF, 10 μg of aprotinin per ml, and 10 μg of leupeptin per ml. The supernatant was subjected to immunoprecipitation with polyclonal antibodies against MAPK or p70s6k. After being washed with buffer A (10 mM Tris-HCl [pH 7.2], 1% NP-40, 0.5% deoxycholate, 100 mM NaCl, 1 mM EDTA, 1 mM sodium vanadate, 2 mM DTT) twice and buffer B (10 mM Tris-HCl [pH 7.2], 0.1% NP-40, 1 M NaCl, 1 mM sodium vanadate, 2 mM DTT) twice, the immunoprecipitates were incubated for 15 min at room temperature in a reaction mixture (30 μl) containing 20 mM HEPES (pH 7.2), 10 mM MgCl2, 3 mM β-mercaptoethanol, 1 mg of BSA (MAPK) per ml, 50 μM cold ATP, and 3 μCi of [γ-32P]ATP. A total of 67 μg of MBP (MAPK) per ml or 250 μM S6 kinase substrate peptide (Upstate Biotech, Inc.) was used as a substrate. After the reaction was stopped with 10 μl of 5× SDS sample buffer (MAPK) or 10 μl of stop buffer (S6 kinase, 1% BSA, 1 mM ATP, 0.6% HCl), the resulting supernatants (20 μl) were resolved by electrophoresis on an SDS–12% gel (MAPK) or transferred to P81 filter paper (S6 kinase; Whatman, Hillsboro, Oreg.), followed by extensive washing in 75 mM phosphoric acid. Radioactivity incorporated into the substrate was determined by using the Bio-Rad Molecular Imager (MAPK) or by scintillation counting (S6 kinase).
[3H]thymidine incorporation assay.
The cells in log-phase growth were washed twice in RPMI 1640 containing 10% FBS, and 2 × 105 cells were incubated in 1 ml of RPMI 1640 containing 10% in the absence or presence of various concentration of insulin or 5% WEHI for 48 h. [3H]thymidine (0.5 mCi; ICN) was spiked into the medium and incubated for 3 h. The cells were collected onto glass microfiber filters and washed three times with water to remove unincorporated nucleotide. Incorporation of labeled thymidine into DNA was quantitated by liquid scintillation counting.
Gel fragmentation assay.
Cells (5 × 106) in log-phase growth were incubated for 18 h in RPMI 1640 containing either 10% FBS with IL-3 (5% WEHI supplement) or 10% FBS with the indicated insulin doses. Cells were lysed in 400 μl of Apo lysis buffer (10 mM Tris-HCl [pH 7.4], 10 mM EDTA, 0.2% Triton X-100), and then insoluble material was removed by centrifugation. The resulting supernatant was extracted once with an equal volume of phenol and once with phenol-chloroform (1:1). Fragmented DNA was collected by ethanol precipitation in the presence of glycogen carrier and then treated with RNase at 37°C for 30 min. Samples were loaded onto a 1.5% agarose gel and stained with ethidium bromide. The relative intensity of DNA bands was determined by using Molecular Analyst (Bio-Rad).
RESULTS
Isolation of the human IRS-4 open reading frame (ORF) and expression in 32DIR cells.
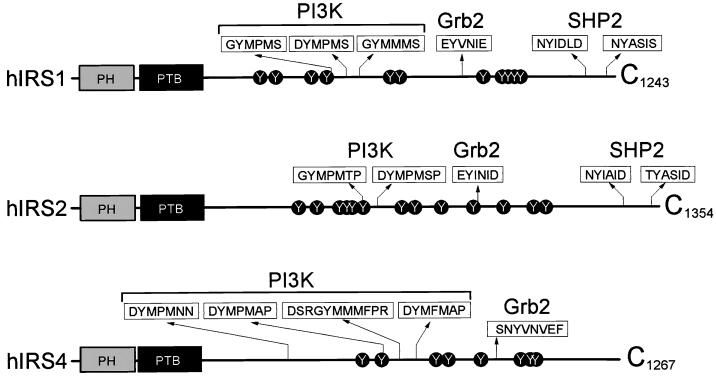
IRS-1, IRS-2, and IRS-4 have similar structures, including an NH2-terminal PH domain and a PTB domain, and multiple tyrosine phosphorylation sites that are expected to bind the SH2 domain of various signaling proteins (Fig. 1). Although the structural similarities suggest that IRS-1, IRS-2, and IRS-4 have certain common functions, recent observations suggest divergent signaling roles for each IRS protein. For instance, the number and type of SH2 domain-binding motifs differ among the IRS proteins, and mice null for IRS-1, IRS-2, or IRS-4 display radically different phenotypes (54). Moreover, IRS-1 and IRS-2 are expressed ubiquitously in human tissues (8), whereas human IRS-4 is expressed mainly in pituitary and thyroid glands and detected at lower levels in brain, spinal cord, trachea, and lymph nodes (Fig. 2A). IRS-4 transcripts were almost undetectable in the major insulin-sensitive tissues, suggesting that IRS-4 plays a unique physiologic role.
FIG. 1.
Schematic diagram of various landmarks in the IRS-1, IRS-2, and IRS-4. The PH domain and the PTB domain are shown. Tyrosine phosphorylation sites are indicated and, where appropriate, the sequence around the site is shown to indicate presumed binding sites for specific SH2 proteins.
FIG. 2.
IRS-4 mRNA expression in various tissues and protein expression in HEK293 cells and 32DIR cells. (A) Tissue distribution of IRS-4 transcripts was analyzed by Northern blotting. A genomic DNA insert (nucleotides 1714 to 3220) was used as a probe for analysis of human poly(A)+ RNA blot (Clontech Laboratories) according to the manufacturer's instructions. (B) 32D cells lysates, expressing insulin receptor (32DIR) or IRS-4 (32DIR/IRS-4), or HEK293 cell lysates were analyzed by immunoblotting by using anti-IRS-4 polyclonal antibodies. (C) One clone of 32DIR or 32DIR/IRS-2 cells, two different clones of 32DIR/IRS-1 cells, and four different clones of 32DIR/IRS-4 cell lysates were immunoblotted with the indicated antibodies. (D) The indicated cell lines were incubated with or without 100 nM insulin for 1 min. Cell lysates were immunoblotted with antiphosphotyrosine monoclonal antibody PY20.
Because they contain few insulin receptors and no endogenous IRS proteins, 32D myeloid progenitor cells provide an ideal system for comparing the signaling potential of IRS-1, IRS-2, and IRS-4 (51). Stable 32D cell lines expressing insulin receptors alone (32DIR) or together with IRS-1 (32DIR/IRS-1) or IRS-2 (32DIR/IRS-2) were described previously (48). We subcloned the human IRS-4 ORF into the mammalian expression vector pCMVhis and introduced it into 32DIR cells (32DIR/IRS-4). Following selection of histidinol-resistant clones, IRS-4 expression was determined by immunoblotting with αIRS-4 (Fig. 2B). While 32DIR cells expressed no IRS-4, a 160-kDa αIRS-4-reactive protein, which comigrated with endogenous IRS-4 from HEK293 cells, was detected in 32DIR/IRS-4 cells. Four independent 32DIR/IRS-4 clones were selected for further study based on apparently equal expression levels compared to IRS-1 and IRS-2 (Fig. 2C). Using the selected cell lines, insulin stimulated approximately equal amounts of tyrosine phosphorylation of IRS-1, IRS-2, and IRS-4 (Fig. 2D). Since the number of predicted tyrosine phosphorylation sites of each IRS protein was similar, the protein expression level of each IRS protein was thought to be comparable.
Insulin-stimulated phosphorylation of IRS proteins in 32DIR cells.
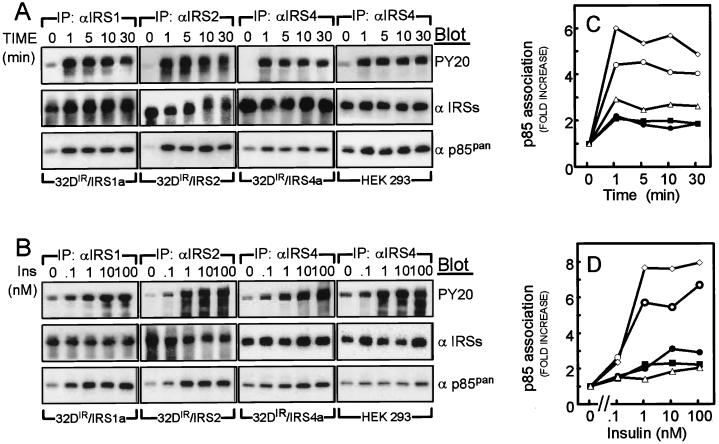
We analyzed specific IRS protein immunoprecipitates from insulin-stimulated 32DIR cell lines or HEK293 cells by immunoblotting with PY20 in order to compare their rates of tyrosine phosphorylation. IRS-1, IRS-2, and IRS-4 were all maximally tyrosine phosphorylated during the first minute of insulin stimulation, with half-maximal phosphorylation occurring at or near a 1 nM insulin concentration (Fig. 3A and B). During prolonged insulin stimulation, the tyrosine phosphorylation of IRS-1 and IRS-2 decreased somewhat, whereas the tyrosine phosphorylation of IRS-4 remained elevated during 30 min of stimulation in both 32DIR and HEK293 cells. Furthermore, the migration of IRS-1 and IRS-2 during SDS-PAGE was progressively retarded during insulin stimulation, whereas the migration of IRS-4 did not change in either 32DIR or HEK293 cells (Fig. 3A). The retarded migration of IRS-1 and IRS-2 was attributed previously to increased insulin-stimulated serine phosphorylation, which may inhibit tyrosine phosphorylation and reduce downstream signaling by IRS proteins (18, 21). Thus, IRS-4 may be less sensitive to heterologous serine phosphorylation than IRS-1 and IRS-2.
FIG. 3.
Insulin-induced tyrosine phosphorylation of IRS-1, IRS-2, and IRS-4 and their association with p85 PI-3 kinase. (A) 32DIR/IRS-1, 32DIR/IRS-2, 32DIR/IRS-4, and HEK293 cells were stimulated by 100 nM insulin for the indicated time intervals. Cell lysates were immunoprecipitated with the indicated antibodies and immunoblotted with PY20 (upper panel), anti-IRS protein antibodies (middle panel), and anti-p85 PI 3-Kpan antibodies (lower panel). (B) Each cell line was incubated with the indicated dose of insulin for 1 min. Cell lysates were immunoprecipitated and immunoblotted as described above. (C and D) Relative increase in p85 associated with each IRS protein was estimated by phosphorimager analysis. Symbols: ○, 32DIR/IRS-1-a; ◊, 32DIR/IRS-2; ● and ■, 32DIR/IRS-4-a and 32DIR/IRS-4-b, respectively; ▵, HEK293. These results are representative of at least three experiments.
Insulin-stimulated association of IRS proteins with PI 3-kinase.
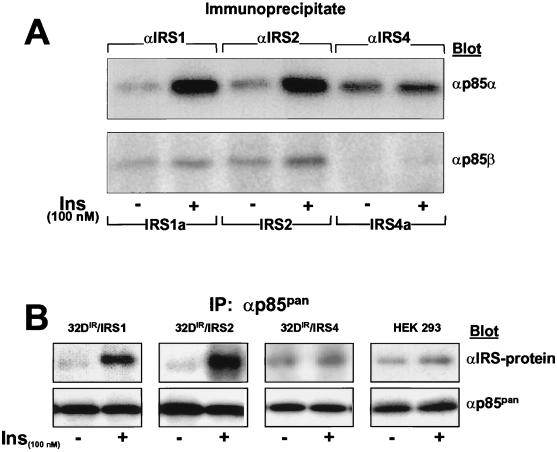
Activation of PI 3-kinase by insulin regulates a variety of metabolic and mitogenic effects (41). The binding of p85 isoforms to tyrosine phosphorylated YMXM motifs on IRS proteins is a principal pathway by which insulin activates PI 3-kinase (6, 40). Before insulin stimulation, p85 barely associated with immunoprecipitates of IRS-1, IRS-2, or IRS-4 (Fig. 3A and B). However, during the first minute of insulin stimulation, p85 binding to IRS-1 or IRS-2 increased approximately fivefold and remained elevated for 30 min; half-maximal stimulation occurred at a concentration of ca. 1 nM insulin (Fig. 3C and D). By contrast, the association between p85 and IRS-4 in 32DIR or HEK293 cells was less sensitive to insulin (50% effective dose [ED50] of >10 nM) and barely increased twofold (Fig. 3C and D). With isoform-specific antibodies, insulin-stimulated association of p85α with IRS-4 was 10-fold weaker than with IRS-1 or IRS-2, and the association of p85β with IRS-4 was undetectable (Fig. 4A). Consistent with these results, insulin barely stimulated the association of IRS-4 with isoform-independent p85 immunoprecipitates (αp85pan), although p85 expression was approximately equal in each cell line tested (Fig. 4B). Thus, p85α and p85β weakly engage IRS-4 compared to IRS-1 or IRS-2.
FIG. 4.
Effects of IRS-1, IRS-2, and IRS-4 on the insulin-induced association of IRS proteins with p85. (A) Each cell line was incubated with or without insulin for 1 min, and each IRS protein was immunoprecipitated with the indicated antibodies and then immunoblotted with anti-p85α or anti-p85β antibodies. (B) Each cell line was incubated with or without 100 nM insulin for 1 min, and the cell lysates were subjected to immunoprecipitation with anti-p85pan antibodies and then immunoblotted with anti-IRS protein or anti-p85pan antibodies.
Insulin-stimulated activation of PI 3-kinase by IRS proteins.
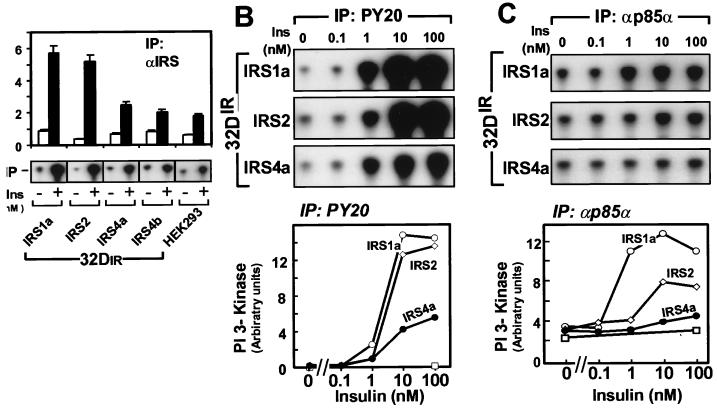
The binding of p85 to phosphorylated IRS-1 activates the associated PI 3-kinase catalytic subunit p110 (6, 40). Consistent with the strong association of IRS-1 and IRS-2 with p85α and p85β, insulin increased the PI 3-kinase activity 7- to 10-fold in αIRS-1 or αIRS-2 immunoprecipitates; by contrast, PI 3-kinase activity in IRS-4 immunoprecipitates increased about 2-fold (Fig. 5A). To ensure that this difference did not merely reflect differing efficiencies of immunoprecipitation by the various αIRS protein antibodies, we assessed PI 3-kinase activity in PY20 immunoprecipitates. Before expression of IRS proteins, PI 3-kinase activity was barely detected in PY20 immunoprecipitates from untreated or insulin-stimulated 32DIR cells, which is consistent with the inability of the insulin receptor to engage PI 3-kinase directly (Fig. 5B). As expected, PI-3 kinase activity was strongly associated with PY20 immunoprecipitates from 32DIR cells expressing IRS-1 or IRS-2, whereas this activity was reduced 60% in 32DIR/IRS-4 cells.
FIG. 5.
Effects of overexpression of IRS proteins on IRS protein, phosphotyrosine, and p85α-associated PI 3-K activity. (A) PI 3-kinase activity associated with IRS protein immunocomplexes was determined by in vitro kinase assay before or after stimulation with 100 nM insulin for 1 min. Phosphorylated PI was quantified (upper panel) and visualized (lower panel) on a Bio-Rad Molecular Imager. The data represent the averages and standard errors of the mean from two independent determinations. (B and C) 32DIR cells were incubated in the absence or presence of insulin for 1 min. PI 3-kinase activity associated with PY20 (B) or αp85α (C) immunoprecipitates was determined. Phosphorylated PI was visualized (upper panel) and quantified (lower panel). Symbols: □, 32DIR; ○, 32DIR/IRS-1; ◊, 32DIR/IRS-2; ●, 32DIR/IRS-4. These results are representative of three independent experiments.
To measure directly whether the p85-associated PI 3-kinase was activated in 32DIR cells expressing IRS-1, IRS-2, or IRS-4, we prepared specific immunoprecipitates of p85α from control and insulin-stimulated cells. As expected, PI 3-kinase in p85α immunoprecipitates was strongly activated by insulin in 32DIR cells expressing IRS-1 (Fig. 5C). IRS-2 also mediated PI 3-kinase activation in 32DIR cells, although the response was less robust and less sensitive to insulin. Consistent with the reduced association of p85α with IRS-4, insulin weakly activated PI 3-kinase in p85α immunoprecipitates from 32DIR cells expressing IRS-4 (Fig. 5C). Together, these results suggest that reduced activation of PI 3-kinase is a consequence of the weak interaction between IRS-4 and p85.
IRS-4 mediates insulin-stimulated PKB/Akt signaling cascade.
PKB/Akt is an important signaling element downstream of PI 3-kinase (2). Activation of PI 3-kinase leads to the formation of PtdIns(3,4,5)P3 and/or PtdIns(3,4)P2, thereby recruiting PKB/Akt to membranes and promoting its phosphorylation by other membrane-associated phospholipid-dependent kinases (PDKs). Phosphorylation of Ser473 plays an important role in the activation of PKB/Akt, which is measured directly with a phospho-specific antibody (αPhosphoAkt). Without IRS proteins, insulin had a negligible effect on the phosphorylation of Ser473 in PKB/Akt. By contrast, after 10 min Ser473 was maximally phosphorylated with equal insulin sensitivity in 32DIR in cells containing IRS-1, IRS-2, or IRS-4 (Fig. 6A and B).
FIG. 6.
Effects of IRS-1, IRS-2, or IRS-4 on phosphorylation and activation of PKB/Akt. (A) Equal amounts of cell lysates from insulin (100 nM)-treated 32D cell lines for the indicated times were analyzed by immunoblotting with phospho-Akt antibodies. (B) Cells were incubated in the indicated concentrations of insulin for 30 min, and the cell lysates were analyzed as described above. (C) Cells were incubated in the absence (□) or presence (■) of 100 nM insulin for 30 min. Whole-cell lysates were immunoprecipitated with PKB/Akt antibodies and subjected to in vitro kinase assay as described in Materials and Methods. The results represent averages and standard errors of mean from three independent experiments. (D) Prior to insulin (100 nM) stimulation for 30 min, cells were incubated with or without LY294002 (LY) or dimethyl sulfoxide (DM) for 30 min. PKB/Akt was immunoprecipitated and subjected to in vitro kinase assay.
Since PKB/Akt activation requires PI 3-kinase activation, we were initially surprised that IRS-4 mediated activation of PKB/Akt as robustly as IRS-1 and IRS-2. To confirm this result, we measured PKB/Akt activity with histone H2B in αPKB immunoprecipitates prepared from untreated and insulin-stimulated 32DIR cell lines. Consistent with Ser473 phosphorylation, insulin strongly activated PKB/Akt in 32DIR cells expressing IRS-1, IRS-2, or IRS-4, whereas it failed to stimulate PKB in 32DIR cells without IRS proteins (Fig. 6C). To confirm that PKB/Akt activation was mediated by products of the PI 3-kinase, 32DIR cells were incubated with LY294002, an inhibitor of PI 3-kinase. In each cell line tested, LY294002 inhibited insulin-stimulated activation of PKB/Akt, suggesting that the weak PI 3-kinase activation by IRS-4 might be sufficient to promote stimulation of PKB/Akt (Fig. 6D).
The proapoptotic protein BAD is an important target of PKB/Akt. PKB/Akt catalyzed phosphorylation of BAD to link extracellular signals to the inhibition of apoptosis (13, 20). During phosphorylation, BAD associates with 14-3-3, which prevents the formation of a proapoptotic heterodimer between BAD and Bcl-2/Bcl-XL (14, 16). The phosphorylation of BAD was detected in 32DIR cells with a phosphospecific antibody. In 32DIR cells lacking IRS proteins, insulin weakly stimulated BAD phosphorylation and slightly promoted BAD association with Bcl-2 but not with 14-3-3 (Fig. 7A). By contrast, the expression of IRS-1, IRS-2, or IRS-4 in 32DIR cells promoted strong insulin-stimulated BAD phosphorylation. Moreover, as expected, insulin promoted the concurrent association of 14-3-3 and dissociation of Bcl-2 from BAD (Fig. 7A). Moreover, wortmannin inhibited the effects of insulin on the regulation of BAD in 32DIR cells expressing IRS-1, IRS-2, or IRS-4, whereas it had no effect in cells lacking an IRS protein (Fig. 7B).
FIG. 7.
Phosphorylation of BAD and association of Bcl-2 and 14-3-3. (A) Each cell line was incubated without or with 100 nM insulin for the indicated time intervals. The cell lysates were subjected to immunoprecipitation with αBAD antibodies, and immunoblotted with αPhosphoBAD, αBcl-2, α14-3-3, or αBAD antibodies. (B) Prior to insulin (100 nM) stimulation for 10 min, cells were incubated with or without wortmannin (Wort) for 30 min. BAD was immunoprecipitated and immunoblotted with the indicated antibodies.
Activation of p70s6k by 32DIR cells.
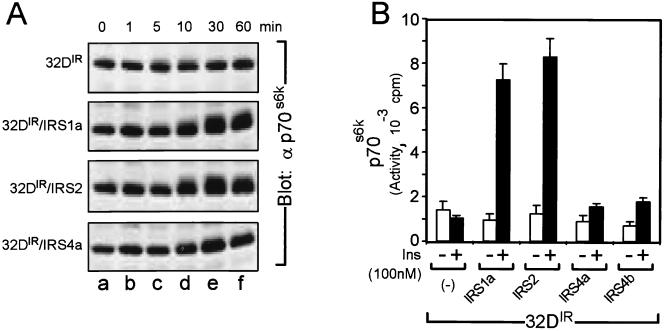
The p70s6k is a serine/threonine kinase involved in insulin-stimulated cell proliferation and protein synthesis which is activated by PI 3-kinase-dependent phosphorylation of Thr252 and Thr412 (15, 23, 32, 37, 42, 53, 56, 59). In order to compare the abilities of IRS-1, IRS-2, and IRS-4 to activate p70s6k, we initially monitored the migration rate of p70s6k during SDS-PAGE (32). Insulin stimulation (up to 30 min) reduced the migration rate of p70s6k from 32DIR cells expressing IRS-1 or IRS-2 but not from cells expressing IRS-4 (Fig. 8A). These results suggest that insulin failed to activate p70s6k in 32DIR/IRS-4 cells. We confirmed these results with immunoprecipitated p70s6k by using in vitro kinase assays. Robust insulin-stimulated activation of p70s6k was observed in 32DIR cells expressing IRS-1 or IRS-2 cells but not in cells expressing IRS-4 (Fig. 8B). Thus, during insulin stimulation, IRS-4 differentially activated p70s6k and PKB/Akt in 32DIR cells.
FIG. 8.
Effects of overexpression of IRS-1, IRS-2, and IRS-4 on phosphorylation and activation of p70s6k. (A) Equal amounts of cell lysates from insulin (100 nM)-treated 32D cell line for the indicated times were analyzed by immunoblotting with αp70s6k antibodies. (B) The cells were incubated in the absence (□) or presence (■) of 100 nM insulin for 30 min. Whole-cell lysates were immunoprecipitated with αp70s6k antibodies and subjected to in vitro kinase assay as described in Materials and Methods. The results represent averages and standard errors of mean from three independent experiments.
Differential activation of the MAPK cascade by the various IRS proteins.
Each IRS protein contains a tyrosine phosphorylation site in an amino acid sequence motif predicted to bind the SH2 domain of Grb-2 (see Fig. 1, above), an upstream mediator of p21ras→Erk kinase signaling (33). While the insulin receptor mediates the insulin-stimulated activation of Erk kinases in the absence of IRS proteins (likely via the Shc→Grb-2 pathway), the recruitment of Grb-2 by IRS-1 enhances insulin-stimulated Erk kinase activation (33, 36, 43). In order to assess differences in the regulation of this pathway among the various IRS proteins, we compared the abilities of IRS-1, IRS-2, and IRS-4 to bind Grb-2 and enhance the insulin-stimulated activity of Erk kinases in 32DIR cells. αGrb-2 immunoblotting of αIRS protein immunoprecipitates from insulin-stimulated 32DIR cell extracts revealed similar amounts of Grb-2 associated with IRS-1 and IRS-4 during maximal insulin stimulation; by contrast, approximately 40% less Grb-2 associated with IRS-2 (Fig. 9A and B). The weak association of IRS-2 with Grb-2 is consistent with previous results (46).
FIG. 9.
Insulin-induced IRS proteins and Grb-2 association and effects of overexpression of IRS proteins on the activation of MAPK. (A) 32DIR/IRS-1, 32DIR/IRS-2, and 32DIR/IRS-4 cells were incubated with or without 100 nM insulin for 2 min. Whole-cell lysates were prepared, immunoprecipitated with indicated anti-IRS antibodies, and immunoblotted with PY20 (upper panel), anti-IRS protein antibodies (middle panel), and anti-Grb-2 antibodies (lower panel). (B) Grb-2 associated with each IRS protein was quantified on a Bio-Rad Molecular Imager. (C and D) Cells were incubated in the absence or presence of different doses of insulin for 5 min. Whole-cell lysates were immunoprecipitated with MAPK antibodies and subjected to in vitro kinase assay as described in Materials and Methods. Radioactivity incorporated into myelin basic protein was estimated with a phosphorimager. The results represent averages from two independent experiments. Symbols: □, 32DIR; ●, 32DIR/IRS-1-b; ◊, 32DIR/IRS-2; ■, 32DIR/IRS-4-b.
We measured Erk kinase activity in αErk immunoprecipitates prepared from cells stimulated for 5 min with various insulin concentrations. As previously shown, the IRS proteins were not required for insulin-stimulated Erk kinase activation in 32DIR cells, although expression of IRS-1 or IRS-4 enhanced the insulin response (Fig. 9C and D). However, IRS-2 failed to enhance insulin-stimulated Erk kinase activity above the level measured in 32DIR cells lacking IRS proteins (Fig. 9C and D). Similar results were observed when Erk kinase activity was assessed by immunoblotting cell lysates with activated Erk-specific antiphosphorylated Erk antibodies (data not shown). Thus, IRS-1 and IRS-4 associated strongly with Grb-2 and enhanced insulin-stimulated Erk kinase activity.
Differential signaling by IRS proteins on DNA synthesis and cell growth.
The activation of both Erk and PI-3 kinase cascades are essential for insulin-stimulated proliferative signaling (29). In the 32DIR cells, IRS proteins are required for insulin-stimulated proliferation; however, previous work demonstrated that IRS protein-mediated activation of Erk kinases is not required (33, 59). By contrast, the IRS protein-mediated activation of PI 3-kinase and its downstream mediators (e.g., PKB/Akt and p70s6k) are critical for proliferation. Since IRS-4 failed to bind p85-associated PI 3-kinase or activate p70s6k, it may only weakly promote growth of 32DIR cells. To test this hypothesis, we measured insulin-stimulated [3H]thymidine incorporation into DNA (a measure of proliferation) in the 32DIR cell lines (Fig. 10A). As previously shown, insulin had little or no effect on thymidine incorporation in 32DIR cells (31, 51). IRS-1 strongly promoted insulin-stimulated thymidine incorporation, with a half-maximal effect at a 0.3 nM insulin concentration; 32DIR/IRS-2 cells were 10-fold less sensitive to insulin, but the maximal response was the same (Fig. 10A). By contrast, [3H]thymidine incorporation in 32DIR/IRS-4 cells was both less sensitive to insulin and significantly less intense.
FIG. 10.
Effects of IRS-1, IRS-2, and IRS-4 on cell growth. (A) [3H]thymidine incorporation induced by different concentrations of insulin was determined by a 2-h pulse given 48 h after removal of IL-3, as described in Materials and Methods. A portion (2,000 cells/ml) of each cell line was seeded into medium containing 10% FBS without additional growth factor (B) or with IL-3 (C) or 100 nM insulin (D). Cell number was counted by using a Coulter Counter on days 1, 3, 5, and 7. The same medium was replaced, and the cells were split 1:10 on day 4. Symbols: □, 32DIR; ○ and ▵, 32DIR/IRS-1-a and -b, respectively; ◊, 32DIR/IRS-2; ■, ●, ▴, and ⧫, 32DIR/IRS-4-a, -b, -c, and -d, respectively. The results represent the average of two independent experiments.
In order to confirm that the [3H]thymidine incorporation data reliably reflected cell proliferation in the 32DIR cell lines, we measured proliferation by determining the absolute number of cells for each line during a 7-day interval in the presence or absence of IL-3 or insulin (Fig. 10B to D). Consistent with the induction of apoptosis that occurs upon IL-3 withdrawal (59), all cells from all cell lines died within 3 to 5 days in the absence of IL-3 and insulin (Fig. 10B). In the presence of IL-3, all cell lines increased approximately 1,000-fold over 7 days; however, three independent clones of 32DIR/IRS-4 cells proliferated slightly more slowly than the other lines (Fig. 10C). As previously shown, insulin promoted proliferation of IL-3-deprived 32DIR cells expressing IRS-1 or IRS-2, although the 32DIR/IRS-2 cells responded slightly less robustly than the 32DIR/IRS-1 cells; the replication of 32DIR/IRS-4 cells was weakly stimulated by insulin. Thus, the differing abilities of the IRS proteins to mediate the growth of 32DIR cells correlated strongly with the ability of the IRS-1 and IRS-2 to activate the p85-associated PI 3-kinase and p70s6k, rather than the ability to activate PKB/Akt.
IRS-4 fails to mediate antiapoptosis in 32DIR cells.
The relative rates of cell division and death determine cell number. To assess the role of apoptosis, we examined the ability of the various IRS proteins to prevent DNA fragmentation in 32DIR cells incubated without IL-3. DNA from apoptotic cells characteristically displays a ladder of DNA fragments when analyzed by ethidium bromide-agarose gel electrophoresis, owing to the cleavage of genomic DNA between the ∼200-bp nucleosome repeats (55). By this assay, all 32DIR cell lines undergo apoptosis between 8 and 12 h in the absence of IL-3 or insulin (7, 28, 59). As previously shown, insulin protected 32DIR/IRS-1 and 32DIR/IRS-2 cells from apoptosis during IL-3 deprivation (59); however, IRS-4 failed to inhibit apoptosis during insulin stimulation (Fig. 11A).
FIG. 11.
Effects of IRS-1, IRS-2, IRS-4, and IRS-1 PH+PTB (PP) domain on apoptosis. (A) Each cell line was assayed for apoptosis by gel fragmentation. Cells were incubated with 10% FBS in the absence or presence of different doses of insulin or IL-3 for 18 h. Fragmented DNA was isolated and analyzed by 1.5% agarose gel. (B and C) The relative intensity of DNA bands was determined by using Molecular Analyst (Bio-Rad), and the ED50 for each cell line was determined.
IL-3 typically prevents apoptosis of 32D cells, but the level of DNA fragmentation was consistently elevated in four independent clones of 32DIR/IRS-4 cells even during incubation with IL-3; upon IL-3 withdrawal, DNA fragmentation was not inhibited by insulin (Fig. 11A). Thus, not only did IRS-4 poorly inhibit apoptosis during growth in insulin, it slightly increased apoptosis during IL-3-dependent growth. This result is even more striking compared to 32DIR cells expressing PPIRS-1. PPIRS-1 lacks the COOH tail of tyrosine phosphorylation sites and contains only the NH2-terminal PH and PTB domains of IRS-1 (59). PPIRS-1 fails to bind or activate PI 3-kinase but inhibits apoptosis of 32DIR cells during insulin stimulation. Compared to intact IRS-1, PPIRS-1 effectively inhibited apoptosis of 32DIR cells, although it was about 10-fold less sensitive to insulin (Fig. 11B and C). Thus, signaling pathways engaged by the NH2-terminal portion of IRS-1 that prevent apoptosis during insulin stimulation may not be engaged by the PH and PTB domains of IRS-4.
DISCUSSION
Tyrosine phosphorylated IRS proteins bind SH2 domain-containing proteins to assemble complexes that mediate the insulin response. The four known IRS proteins share similar overall structure, including an NH2-terminal PH domain and a PTB domain, as well as a COOH-terminal tail with numerous tyrosine phosphorylation sites (17, 25, 47, 48). The common structure suggests some similarity of function among the IRS proteins. Indeed, each IRS protein binds various SH2 proteins following tyrosine phosphorylation by the insulin receptor (58). Beyond this basic paradigm, the details of signaling specificity diverge somewhat. The number, placement, and even presence of tyrosine phosphorylation motifs within the tails of IRS proteins differ; the COOH-terminal tail of IRS-3 has one-third the length of the other IRS proteins; and IRS-4 lacks both consensus SHP-2 binding motifs present in IRS-1, IRS-2, and IRS-3. In terms of function, the various IRS proteins appear to differ, as well. IRS-2 cannot substitute for IRS-1 in fibroblasts lacking IRS-1 (9). Similarly, the phenotypes of mice null for the various IRS proteins vary widely. The IRS-1 null is small and insulin resistant, although never diabetic, whereas the IRS-2 null mouse is diabetic due to insulin resistance and β-cell failure. No phenotype has yet been ascribed to the IRS-3 and IRS-4 null mice (27).
While IRS-1 and IRS-2 are expressed relatively ubiquitously, expression of IRS-4 is restricted almost exclusively to the pituitary gland and brain; such differences in expression pattern provide one possible explanation for specificity. However, the overlapping expression pattern suggests that IRS proteins possess distinct functions as well. Indeed, by contrasting the functions of IRS-1, IRS-2, and IRS-4 in 32D cells, several differences in the signal transmission emerge. IRS-1 and IRS-4 strongly bind Grb-2 and enhance the activation of the MAPK during insulin stimulation; IRS-2 performs these functions weakly. During insulin stimulation, IRS-1 and IRS-2 mediate antiapoptosis, DNA synthesis, and replication of IL-3-deprived 32DIR; however, under these conditions IRS-4 expression fails to inhibit apoptosis of 32DIR cells and, as a consequence, promotes cell replications weakly. Moreover, in the presence of IL-3, IRS-4 expression promotes apoptosis through an unknown mechanism. Thus, each IRS protein is a distinct molecular platform coupling insulin receptors to a unique cohort of regulatory signals.
The class I PI 3-kinase is composed of a catalytic (p110α, p110β, and p110δ) subunit which heterodimerizes with one of many SH2 domain-containing regulatory subunits (p85α, p85β, p55α, and p55PIK). The SH2 domain-containing regulatory subunits recognize tyrosine phosphorylated YXXM motifs in signaling proteins such as IRS-1 and IRS-2. Occupancy of the SH2 domains by these motifs activates the associated p110 catalytic subunit (6). These interactions cause a strong association of activated PI 3-kinase with the IRS proteins and mediate the stimulation of PKB/Akt, PKCζ, and p70s6k and other serine/threonine kinases (29, 30, 32, 33, 35, 49; M. G. Myers, Jr., Y. Zhang, L. Yenush, et al., abstr., Diabetes 44:49A, 1995). PI 3-kinase may also interact positively with the p21ras pathway, although we have not detected such an interaction in the 32D cells (32, 33, 39).
IRS-4 contains at least six YXXM motifs that should bind p85α and/or p85β (17). In contrast to IRS-1 and IRS-2, IRS-4 binding to p85 is barely detected during insulin stimulation; and while IRS-4 binds p85α weakly, it completely fails to bind p85β. Furthermore, IRS-4 lacks the COOH-terminal SHP-2 binding sites, which should enhance the binding and activation of PI 3-kinase and PI 3-kinase-dependent responses by IRS-4 (31). Thus, the poor activation of PI 3-kinase by IRS-4 may arise from the inability of the insulin receptor to phosphorylate the YXXM motifs in IRS-4. Since YXXM motifs are ideal phosphorylation sites for the insulin receptor in vitro (44, 45), the tail of IRS-4 may have a conformation that precludes the phosphorylation of its YXXM motifs by the insulin receptor. In light of our results, a direct analysis of tyrosine phosphorylation site utilization in IRS-4 needs to be conducted.
Although IRS-4 weakly binds p85α and fails to bind p85β, it promotes the PKB/Akt signaling pathway during insulin stimulation. This effect is sensitive to LY294002, an inhibitor of PI 3-kinase, indicating that products of the PI 3-kinase are required. While we cannot exclude the possibility that constitutive levels of PI 3-kinase products in combination with unique IRS-4-mediated signals are sufficient to promote activation of PKB/Akt during insulin stimulation, our results suggest that IRS-4 stimulates sufficient PI 3-kinase activity to activate PKB/Akt. Some PI 3-kinase activity is immunoprecipitated from insulin-stimulated cells with antibodies against IRS-4 or phosphotyrosine (PY20), but this does not appear to be associated with p85α or p85β. The interaction between IRS-4 and p85 is so weak that αp85 immunoprecipitates from insulin-stimulated cells do not contain increased PI 3-kinase activity, as usually observed in cells expressing IRS-1 or IRS-2. Perhaps IRS-4 fails to bind to both SH2 domains in p85, which is ordinarily required for full activation (40). Nevertheless, the activation of PI 3-kinase by association with IRS-4 is significantly reduced, although sufficient to fully stimulate PKB/Akt as evaluated by our in vitro kinase assays and BAD phosphorylation in cells.
Although IRS-4 mediates insulin-stimulated activation of PKB/Akt and BAD phosphorylation, it fails promote the activation of p70s6k. This unexpected result is one of the clearest demonstrations of the different signaling capacity of IRS-4 compared to IRS-1 or IRS-2. Previous reports revealed that YMXM motifs in IRS-1 are required for insulin to stimulated p70s6k, suggesting that a direct association between PI 3-kinase and IRS-1 is required (32, 35). Although increased PI 3-kinase activity is detected in antiphosphotyrosine immunoprecipitates from IRS-4-expressing 32DIR cells, this is poorly associated with p85, suggesting that p85-associated PI 3-kinase is essential for promoting activation of p70s6k during insulin stimulation. Thus, the regulation of p70s6k diverges early from the pathways that activate PKB/Akt, which is activated by non-p85-associated PI 3-kinase and IRS-4.
The regulation of PKB/Akt involves the recruitment of several kinases to the plasma membrane. The PH domain in PKB/Akt binds to PI 3-kinase products, recruiting PKB/Akt to the plasma membrane and exposing it to membrane-associated phospholipid-dependent kinases (1). Phospholipid-dependent kinase-1 (PDK1) also associates with the plasma membrane through interaction of its PH domain with PI 3-kinase products; membrane-associated PDK1 catalyzes phosphorylation of Thr308 in PKB/Akt (3, 50). The phosphorylation of Ser473, which is required for full activation, is thought to be catalyzed by a similar membrane-associated kinase, called PDK2 (2). Thus, activation of PKB/Akt depends on PI 3-kinase activity to recruit interacting kinases to the plasma membrane. This regulatory mechanism may be independent of the exact mechanism that stimulates the production of phospholipids.
PI 3-kinase activity is also critical for the activation of p70s6k. The PI 3-kinase-dependent phosphorylation of Thr252 and Thr412 fully activates p70s6k, although the exact kinases involved are not well defined (11, 52). Based on our results with IRS-4, activation of PKB/Akt is not the only critical step in p70s6k activation during insulin stimulation. PDK1 partially activates p70s6k by phosphorylating Thr252 (4). Whether Thr252 is phosphorylated in 32DIR cells expressing IRS-4 is not known. There exist several potential explanations for the failure of IRS-4 to mediate activation of p70s6k while mediating activation of PKB/Akt normally. It is possible that IRS-1 or IRS-2 mediate PI 3-kinase-independent signals that are not engaged by IRS-4 but which are essential for activation of p70s6k. However, this mechanism is unlikely because the Drosophila insulin receptor or a chimeric mammalian insulin receptor containing an extended tail of YXXM motifs that bind p85-associated PI 3-kinase and activate p70s6k without IRS proteins (56, 59). Full activation of p85-associated PI 3-kinase may be the critical step in p70s6k activation.
32D cells are ordinarily dependent on IL-3 for long-term growth, since withdrawal of IL-3 induces apoptosis within 12 h (59). In the absence of IRS proteins, IL3-deprived 32DIR cells undergo apoptosis and fail to proliferate during insulin stimulation. In contrast, insulin strongly stimulates DNA synthesis and suppresses apoptosis in 32DIR cells expressing IRS-1 or IRS-2. However, both insulin responses are significantly reduced in 32DIR cells expressing IRS-4. The inability of IRS-4 to form a complex with PI 3-kinase and activate p70s6k may in part explain the weak insulin-stimulated DNA synthesis promoted by IRS-4.
Persistent apoptosis in insulin- or IL3-treated 32DIR/IRS-4 cells is unexpected since signaling pathways that promote survival are thought to be downstream of PKB/Akt. BAD is one of the cellular targets that PKB/Akt phosphorylates to protect cultured neurons from apoptosis (13). Consistent with this hypothesis, insulin stimulates wortmannin-sensitive BAD phosphorylation equally in 32DIR cells expressing IRS-1, IRS-2, or IRS-4. Moreover, Bcl-2 dissociates from BAD and 14-3-3 associates with BAD in each of these cell lines, suggesting that BAD phosphorylation may not be the critical step to inhibit apoptosis in IL-3-deprived 32DIR cells.
IRS-4 fails to activate p70s6k, but the possibility that this is essential for antiapoptosis is inconsistent with our previous results. Our previous work with 32D cells expressing mammalian insulin receptor chimeras that directly bind p85 suggests that activation of PI 3-kinase, PKB/Akt, and p70s6k is not sufficient to inhibit apoptosis during IL-3 withdrawal (56, 59). Moreover, a truncated IRS-1 molecule (PPIRS1) lacking the entire tail of tyrosine phosphorylation sites just after the PTB domain inhibits apoptosis during insulin stimulation (59). In these cells, p70s6k was not activated after insulin stimulation for up to 60 min, although PKB/Akt was weakly activated (59). Therefore, activation of p70s6k is unlikely to be involved in insulin-induced antiapoptosis. Moreover, like the expression of IRS-4 in 32DIR cells, direct binding of p85-associated PI 3-kinase to these chimeric insulin receptors promotes apoptosis during IL-3 stimulation and accelerates apoptosis during IL-3 withdrawal (56, 59). Interestingly, IRS-1 promotes survival of 32D cells expressing the chimeric insulin receptors, suggesting that the presence of IRS-1, but not necessarily the activation of PI 3-kinase-dependent pathways, is a critical element for insulin-stimulated antiapoptosis.
The signaling pathways regulated by IRS-1 to inhibit apoptosis during insulin stimulation may be independent of PI 3-kinase. A mutant IRS-1 molecule lacking all 18 tyrosine phosphorylation sites (IRS-1F18) reduces significantly the ability of insulin to stimulate proliferation of 32DIR cells; however, IRS-1F18 inhibits apoptosis of IL-3-deprived 32DIR cells during insulin stimulation (L. Yenush, M. F. White, et al., unpublished data). The replacement of a few PI 3-kinase binding motifs increases the sensitivity of the signal to insulin, suggesting that products of the PI 3-kinase may amplify the otherwise phosphotyrosine-independent antiapoptotic signal by recruiting essential elements to the plasma membrane. However, it should be kept in mind that p85 binding sites are not required for the biological response.
In summary, IRS-4 either fails to mediate an antiapoptotic (PI 3-kinase-independent) signal mediated by other IRS-1 or IRS-2 or it promotes a proapoptotic signal. Given the increased rate of apoptosis exhibited by 32DIR/IRS-4 cells when grown in IL-3, the latter explanation might be correct. The phenotypes of IRS-1, IRS-2, and IRS-4 in 32DIR cells highlight dramatically the functional differences among the various IRS proteins. The ability of IRS-4 to mediate activation of PKB/Akt in the absence of p70s6k activation suggests an antiapoptotic mechanism that diverges at the level of the IRS proteins. A full understanding of these pathways has certain physiological significance, since IRS-2 is critical for pancreatic β-cell proliferation and IRS-1 is essential for somatic cell growth.
ACKNOWLEDGMENTS
This work was supported by DK38712 (M.F.W.) and Juvenile Diabetes Foundation Research grant 197043 (M.G.M.). T.U. was supported by an American Diabetes Association Mentor-Based Fellowship and the Nakatomi Foundation Research Grant from Japan.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Gene. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 3.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R J, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 4.Alessi D R, Kozlowski M T, Weng Q P, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. doi: 10.1016/s0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- 5.Araki E, Lipes M A, Patti M E, Brüning J C, Haag III B L, Johnson R S, Kahn C R. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 6.Backer J M, Myers M G, Jr, Shoelson S E, Chin D J, Sun X J, Miralpeix M, Hu P, Margolis B, Skolnik E Y, Schlessinger J, et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baffy G, Miyashita T, Williamson J R, Reed J C. Apoptosis induced by withdrawal of IL-3 from an IL-3 dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–6519. [PubMed] [Google Scholar]
- 8.Bernal D, Almind K, Yenush L, Ayoub M, Zhang Y, Rosshani L, Larsson C, Pedersen O, White M F. IRS-2 Amino acid polymorphisms are not associated with random type 2 diabetes among caucasians. Diabetes. 1998;47:976–979. doi: 10.2337/diabetes.47.6.976. [DOI] [PubMed] [Google Scholar]
- 9.Bruning J C, Winnay J, Cheatham B, Kahn C R. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17:1513–1521. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burks D J, Pons S, Towery H, Smith-Hall J, Myers M G, Jr, Yenush L, White M F. Heterologous PH domains do not mediate coupling of IRS-1 to the insulin receptor. J Biol Chem. 1997;272:27716–27721. doi: 10.1074/jbc.272.44.27716. [DOI] [PubMed] [Google Scholar]
- 11.Chung J, Grammer T C, Lemon K P, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 12.Collins M K, Perkins G R, Rodriguez Tarduchy G, Nieto M A, Lopez-Rivas A. Growth factors as survival factors: regulation of apoptosis. Bioessays. 1994;16:133–138. doi: 10.1002/bies.950160210. [DOI] [PubMed] [Google Scholar]
- 13.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 15.Dennis P B, Pullen N, Pearson R B, Kozma S C, Thomas G. Phosphorylation sites in the autoinhibitory domain participate in p70(s6k) activation loop phosphorylation. J Biol Chem. 1998;273:14845–14852. doi: 10.1074/jbc.273.24.14845. [DOI] [PubMed] [Google Scholar]
- 16.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 17.Fantin V R, Sparling J D, Slot J W, Keller S R, Lienhard G E, Lavan B E. Characterization of insulin receptor substrate 4 in human embryonic kidney 293 cells. J Biol Chem. 1998;273:10726–10732. doi: 10.1074/jbc.273.17.10726. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein R, Kanety H, Papa M Z, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- 19.Guilherme A, Czech M P. Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by β1-integrin signaling in rat adipocytes. J Biol Chem. 1998;273:33119–33122. doi: 10.1074/jbc.273.50.33119. [DOI] [PubMed] [Google Scholar]
- 20.Hemmings B A. Akt signaling linked membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 21.Hotamisligil G S, Peraldi P, Budvari A, Ellis R W, White M F, Spiegelman B M. IRS-1 mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 22.Kapeller R, Cantley L C., Jr Phosphatidylinositol 3-kinase. Bioessays. 1994;16:565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- 23.Lane H A, Fernandez A, Lamb N J C, Thomas G. p70s6k function is essential for G1 progression. Nature. 1993;363:170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- 24.Lavan B E, Fantin V R, Chang E T, Lane W S, Keller S R, Lienhard G E. A novel 160 kDa phosphotyrosine protein in insulin-treated embryonic kidney cells is a new member of the insulin receptor substrate family. J Biol Chem. 1997;272:21403–21407. doi: 10.1074/jbc.272.34.21403. [DOI] [PubMed] [Google Scholar]
- 25.Lavan B E, Lane W S, Lienhard G E. The 60-kDa phosphotyrosine protein in insulin-treated adipocytes is a new member of the insulin receptor substrate family. J Biol Chem. 1997;272:11439–11443. doi: 10.1074/jbc.272.17.11439. [DOI] [PubMed] [Google Scholar]
- 26.Lemmon M A, Ferguson K M, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 27.Liu S C, Wang Q, Lienhard G E, Keller S R. Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. J Biol Chem. 1999;274:18093–18099. doi: 10.1074/jbc.274.25.18093. [DOI] [PubMed] [Google Scholar]
- 28.Magnelli L, Cinelli M, Turchetti A, Chiarugi V P. Apoptosis induction in 32D cells by IL-3 withdrawal is preceded by a drop in the intracellular calcium level. Biochem Biophys Res Commun. 1993;194:1394–1397. doi: 10.1006/bbrc.1993.1979. [DOI] [PubMed] [Google Scholar]
- 29.Mendez R, Kollmorgen G, White M F, Rhoads R E. Requirement of protein kinase C zeta for stimulation of protein synthesis by insulin. Mol Cell Biol. 1997;17:5184–5192. doi: 10.1128/mcb.17.9.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez R, Myers M G, Jr, White M F, Rhoads R E. Stimulation of protein synthesis, eukaryotic translation initiation factor 4E phosphorylation, and PHAS-1 phosphorylation by insulin requires insulin receptor substrate-1 and phosphotidylinositol-3-kinase. Mol Cell Biol. 1996;16:2857–2864. doi: 10.1128/mcb.16.6.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers M G J, Mendez R, Shi P, Pierce J H, Rhoads R, White M F. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J Biol Chem. 1998;273:26908–26914. doi: 10.1074/jbc.273.41.26908. [DOI] [PubMed] [Google Scholar]
- 32.Myers M G, Jr, Grammer T C, Wang L M, Sun X J, Pierce J H, Blenis J, White M F. IRS-1 mediates PI 3′-kinase and p70s6k signaling during insulin, IGF-1 and IL-4 stimulation. J Biol Chem. 1994;269:28783–28789. [PubMed] [Google Scholar]
- 33.Myers M G, Jr, Wang L M, Sun X J, Zhang Y, Yenush L, Schlessinger J, Pierce J H, White M F. The role of IRS-1/GRB2 complexes in insulin signaling. Mol Cell Biol. 1994;14:3577–3587. doi: 10.1128/mcb.14.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers M G, Jr, White M F. The new elements in insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993;42:643–650. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- 35.Myers M G, Jr, Zhang Y, Aldaz G A I, Grammer T C, Glasheen E M, Yenush L, Wang L M, Sun X J, Blenis J, Pierce J H, White M F. YMXM motifs and signaling by an insulin receptor substrate 1 molecule without tyrosine phosphorylation sites. Mol Cell Biol. 1996;16:4147–4155. doi: 10.1128/mcb.16.8.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruett W, Yuan Y, Rose E, Batzer A G, Harada N, Skolnik E Y. Association between GRB2/Sos and insulin receptor substrate 1 is not sufficient for activation of extracellular signal-regulated kinases by interleukin-4: Implications for ras activation by insulin. Mol Cell Biol. 1995;15:1778–1785. doi: 10.1128/mcb.15.3.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pullen N, Dennis P B, Andjelkovic M, Dufner A, Kozma S C, Hemmings B A, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 38.Reiss K, Porcu P, Sell C, Pietrzkowski Z, Baserga R. The insulin-like growth factor 1 receptor is required for the proliferation of hemopoietic cells. Oncogene. 1992;7:2243–2248. [PubMed] [Google Scholar]
- 39.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 40.Rordorf-Nikolic T, Van Horn D J, Chen D, White M F, Backer J M. Regulation of phosphatidylinositol 3-kinase by tyrosyl phosphoproteins. Full activation requires occupancy of both SH2 domains in the 85 kDa regulatory subunit. J Biol Chem. 1995;270:3662–3666. doi: 10.1074/jbc.270.8.3662. [DOI] [PubMed] [Google Scholar]
- 41.Shepherd P R, Withers D J, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma S C. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skolnik E Y, Batzer A G, Li N, Lee C H, Lowenstein E J, Mohammadi M, Margolis B, Schlessinger J. The function of GRB2 in linking the insulin receptor to ras signaling pathways. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- 44.Songyang Z, Cantley L C. Recognition and specificity in protein tyrosine kinase-mediated signaling. Trends Biochem Sci. 1995;20:470–475. doi: 10.1016/s0968-0004(00)89103-3. [DOI] [PubMed] [Google Scholar]
- 45.Songyang Z, Carraway III K L, Eck M J, Harrison S C, Feldman R A, Mohammadi M, Schlessinger J, Hubbard S R, Lorenzo M J, Ponder B A J, Mayer B J, Cantley L C. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995;373:536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- 46.Sun X J, Pons S, Wang L M, Zhang Y, Yenush L, Burks D, Myers M G, Jr, Glasheen E, Copeland N G, Jenkins N A, Pierce J H, White M F. The IRS-2 gene on murine chromosome 8 encodes a unique signaling adapter for insulin and cytokine action. Mol Endocrinol. 1997;11:251–262. doi: 10.1210/mend.11.2.9885. [DOI] [PubMed] [Google Scholar]
- 47.Sun X J, Rothenberg P L, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. The structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 48.Sun X J, Wang L M, Zhang Y, Yenush L, Myers M G, Jr, Glasheen E M, Lane W S, Pierce J H, White M F. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 49.Toker A, Cantley L C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 50.Walker K S, Deak M, Paterson A, Hudson K, Cohen P, Alessi D R. Activation of protein kinase B β and γ isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B α. Biochem J. 1998;331:299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L M, Myers M G, Jr, Sun X J, Aaronson S A, White M F, Pierce J H. IRS-1: essential for insulin and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993;261:1591–1594. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- 52.Weng Q P, Andrabi K, Klippel A, Kozlowski M T, Williams L T, Avruch J. Phosphatidylinositiol 3-kinase signals activation of p70 S6 kinase in situ through site-specific p70 phosphorylation. Proc Natl Acad Sci USA. 1995;92:5744–5748. doi: 10.1073/pnas.92.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng Q P, Kozlowski M, Belham C, Zhang A, Comb M J, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- 54.Withers D J, Gutierrez J S, Towery H, Burks D J, Ren J M, Previs S, Zhang Y, Bernal D, Pons S, Shulman G I, Bonner-Weir S, White M F. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–903. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 55.Wyllie A H, Kerr J F, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 56.Yenush L, Fernandez R, Myers M G, Jr, Grammer T C, Sun X J, Blenis J, Pierce J H, Schlessinger J, White M F. The Drosophila insulin receptor activates multiple signaling pathways but requires insulin receptor substrate proteins for DNA synthesis. Mol Cell Biol. 1996;16:2509–2517. doi: 10.1128/mcb.16.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yenush L, Makati K J, Smith-Hall J, Ishibashi O, Myers M G, Jr, White M F. The pleckstrin homology domain is the principle link between the insulin receptor and IRS-1. J Biol Chem. 1996;271:24300–24306. doi: 10.1074/jbc.271.39.24300. [DOI] [PubMed] [Google Scholar]
- 58.Yenush L, White M F. The IRS-signaling system during insulin and cytokine action. Bioessays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]
- 59.Yenush L, Zanella C, Uchida T, Bernal D, White M F. The pleckstrin homology and phosphotyrosine binding domains of insulin receptor substrate 1 mediate inhibition of apoptosis by insulin. Mol Cell Biol. 1998;18:6784–6794. doi: 10.1128/mcb.18.11.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]