Abstract
While three-dimensional spheroids outperform traditional two-dimensional monolayer culture for human adipose-derived stem cells (hASCs), there is not a consensus on the most successful method for enhancing their adipogenic differentiation and minimizing the loss of physiologically relevant, fatty spheroids during culture. To this end, we compared three culture methods, namely, elastin-like polypeptide-polyethyleneimine (ELP-PEI) coated surfaces, ultra-low attachment static culture, and suspension culture for their ability to form and retain productive hASC spheroids. The ELP-PEI coatings used the ELP conjugated to two molecular weights of PEI (800 or 25,000 g/mol). FTIR spectroscopy, atomic force microscopy, and contact angle goniometry revealed that the ELP-PEI coatings had similar chemical structures, surface topography, and hydrophobicity. Time-lapse microscopy showed that increasing the PEI molecular weight resulted in smaller spheroids. Measurement of triglyceride content showed that the three methods induced comparable differentiation of hASCs toward the adipogenic lineage. DNA content and morphometric analysis revealed the merging of spheroids to form larger spheroids in the ultra-low attachment static culture and suspension culture methods. In contrast, the retention of hASC spheroid sizes and numbers with a regular spheroid size (~100 μm) were best atop the ELP-PEI800 coatings. Overall, this research shows that the spheroid culture atop the ELP-PEI coatings is a suitable cell culture model for future studies involving long-term, three-dimensional culture of mature adipocytes derived from hASCs.
Keywords: Human adipose-derived stem cells, elastin-like polypeptide, spheroids, ultra-low attachment static culture, suspension culture
INTRODUCTION
The prevalence of obesity in both adults and children is increasing each year, and in the United States, nearly 37% of the adult population is considered obese.[1] Though diet and exercise work to reduce obesity to a certain extent, cellular mechanisms that determine the behavior of adipocytes (the primary cell type present in the adipose tissue) must be studied to find effective treatments for obesity. As a first step toward this goal, a protocol to differentiate stem cells into adipocytes in a physiologically-relevant in vitro culture is necessary. Human adipose-derived stem cells (hASCs) are a relatively new source for stem cells. Approximately 1% of the adipose tissue cells are stem cells compared to the relatively smaller fractions (~ 0.002%) for mesenchymal stem cells (MSCs) found in the bone marrow. While the hASCs can be easily procured during a minimally invasive surgery as well as elective surgeries including liposuction,[2,3] hASCs have been shown to lose their ability to proliferate and to have decreased expression of pluripotent markers over time in vitro.[4,5] The hASCs are able to maintain the expression of pluripotent markers, along with differentiation potential, for longer periods of time in vitro if they are cultured under a three-dimensional (3D) culture format, instead of the conventional monolayer culture.[2] Multicellular spheroids are widely used as an effective 3D culture method to mimic intracellular in vivo like conditions.[6]
While there are several methods of forming spheroids,[7–12] there is not a general consensus on which methods are the most efficacious for stem cell proliferation, differentiation, and maturation. These methods include 3D printed scaffolds,[7] hydrogel cultures,[8,9] microfluidic devices,[10] hanging drop,[11] ultra-low attachment static culture,[12] and suspension culture.[12] The 3D printed scaffolds and hydrogel cultures use natural, synthetic, or hybrid materials to encapsulate stem cells or preadipocytes. The encapsulated adipocytes remain functionally impaired[13,14] due to the exogenous/artificial nature of scaffolds. The design of porous scaffolds proves especially challenging in adipose tissue engineering because existing scaffolds cannot dynamically adapt their pore size as the maturing adipocytes significantly increase in volume.[15,16] Additionally, the compressive forces from scaffolds restrict adipocyte volume expansion.[17,18] The so-called “Scaffold-Free” approaches, namely the hanging drop, ultra-low attachment static culture, and suspension culture, prohibit cell surface interaction and form 3D spheroids. Recently, Klingelhutz et al. used the hanging drop method to form 3D spheroids of human and mouse pre-adipocytes and successfully differentiated them toward the adipogenic lineage over a five-week culture period.[19]
After their differentiation along the adipogenic lineage, the stem cells begin to increase their triglyceride content during the maturation phase. Such fatty spheroids become more buoyant over time, lift off of the culture surface and are removed during the next media change. To minimize the loss of such physiologically relevant fatty spheroids and to provide a proven, long-term culture method for mature adipocytes in vitro, we investigated the use of elastin-like polypeptide-polyelectrolyte coated surfaces.[6,20–23] Elastin-like polypeptides (ELPs) conjugated to polyethyleneimine (PEI) have been used in coatings as a 3D culture method for many different cell types.[6,24–27] Here, the biocompatible ELP encourages cell attachment, and the PEI repels the cells from the coated surface. This causes the cells to prefer one another to the surface, creating spheroids, while at the same time tethering the spheroids to the surface.[6] PEI has been shown to be cytotoxic on its own, but when conjugated to ELP and when used at low molar concentrations (< 5 mol%), it becomes biocompatible and effectively induces spheroid formation.[24]
In this research, we used the ELP-PEI coated surface for culturing the hASCs to investigate the effect of the molecular weight of the PEI entities on the spheroid formation and subsequent adipogenic differentiation. To this end, we created ELP-PEI coatings using two different molecular weights of PEI (800 and 25,000 g/mol). We hypothesized that the PEI molecular weight (i.e., amine content present in the ELP-PEI coating) would determine the hASC spheroid size and retention. Finally, we compared the ELP-PEI coatings to two popular alternative spheroid formation methods, namely, the ultra-low attachment static culture and suspension culture to determine which method is more effective in hASC spheroid formation, spheroid retention, and adipogenic maturation.
MATERIALS AND METHODS
ELP-PEI Synthesis and Characterization:
ELP (Valine-Proline-Glycine-Valine-Glycine)40 with molecular weight of 17,000 g/mol was produced using genetically modified E. coli and purified using inverse phase transition temperature cycling.[6,20–23] ELP-PEI conjugates were synthesized by carbodiimide activation of the c-terminus carboxyl group on the ELP for 15 minutes and dropwise addition of the activated ELP (MES buffer, pH 6.2, 30 mg ELP) to a PEI solution (MES buffer, pH 6.8, 10:1 PEI to ELP molar ratio) to react at 4 °C for a minimum of 8 hours. Two molecular weights of branched PEI (Mw = 800 and 25,000 g/mol; Sigma Aldrich, St. Louis, MO) were used. The conjugates are referred hereafter as ELP-PEI800 and ELP-PEI25,000. An o-phthalaldehyde (OPA) assay (Thermo Fisher, Waltham, MA) was used to measure the number of primary amine groups (n = 3 per conjugate). Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) was performed (n = 3 per conjugate) to examine any molecular differences within the conjugates using a Spectrum 100 FTIR (Perkin-Elmer, Waltham, MA) over an absorbance range of 650–4000 cm−1 at a spectral resolution of 4 cm-1.
Coating Technique:
The 24-well tissue culture polystyrene (TCPS) plates were coated with 200-μL of 5 mg/mL ELP/ELP-PEI mixture by drying the plates at 37 °C for 24 hours. To compare the effects of the different molecular weights, the two ELP-PEI conjugates were used at the same composition of 1 mol%, with the remaining 99 mol% being neat ELP.
Characterization of ELP-PEI Coatings:
A Bioscope Catalyst atomic force microscope (AFM, Bruker, Billerica, MA) was used to determine the roughness of the ELP-PEI coating surfaces. 30 × 30 µm images (n = 3 per coating) were taken under ScanAsyst mode and analyzed using Gwyddion software. Contact angle goniometry (CAG) was used to quantitatively analyze hydrophobicity of the ELP-PEI coatings, with images taken of 10 µL water droplets (n = 10 per coating) on the surfaces using the sessile drop method (Rame Hart, Succasunna, NJ) and analyzed with ImageJ.
hASC Culture Atop ELP-PEI Coatings:
hASCs were isolated from an unidentified adult female donor according to previously published protocols.[28,29] The hASC isolation and culture protocols were approved by the University of Mississippi Medical Center Institutional Review Board (Approval #2012–0004). Cells were cultured on the ELP-PEI coated surfaces (26,000 cells/cm2) to form 3D spheroids over a three day acclimation period with exposure to stem cell maintenance media (50:50 DMEM:F-12, 1% pen-strep, 10% calf serum). hASC spheroids were then exposed to a differentiation media (50:50 DMEM:F-12, 1% pen-strep, 1 μM Dexamethasone, 0.5 mM IBMX, 0.1 U/mL Insulin, 1 μM Indomethacin) over a three day period, and finally a maturation media (50:50 DMEM:F-12, 1% pen-strep, 2% bovine serum albumin, 10% fetal bovine serum) for up to 10 days. Fresh media was provided every other day.
hASC Culture Using the Alternative Spheroid Formation Methods:
Two alternative spheroid formation methods, namely, static culture and suspension culture atop ultra-low attachment plates (Corning Costar plates, Corning, NY), were used to compare spheroid size and cell function to the ELP-PEI coating method. The same cell seeding, media exposure, and time points were used as described above. The suspension culture was achieved by placing the ultra-low attachment plates on an orbital shaker throughout the duration of the experiment to induce the suspension.
Optical Microscopy:
Spheroid formation kinetics and size were monitored using an Olympus IX81 optical microscope equipped with a Hamamatsu digital camera and LiveCell unit, to allow for time-lapse imaging throughout the experiment. At least three positions per well of triplicate wells were imaged throughout the experiment (n = 9).
Morphometric Analysis:
The spheroid images (n = 9) were analyzed for size using ImageJ software by analyzing the area and diameter of each spheroid formed. The lower and upper diameter limits of 30 μm and 200 μm were used to categories an object to be a spheroid.
Biochemical Analyses:
Spheroids were removed from the various culture plates by carefully removing the culture medium and rinsing with PBS. Aliquots were centrifuged for 2 min at 1000 rpm and resuspended in PBS. Subsequently, a Branson Digital Sonifier 450 (Danbury, CT, USA) was used for 30 s at 10% amplitude to lyse the cells. The DNA levels were measured using the Cyquant cell proliferation assay (#C7026, Thermo Fisher), and the triglyceride content was measured by a triglyceride reagent assay (#T2449, Sigma Aldrich) according to the manufacturers’ protocols on days 0, 5, and 10. Three measurements were taken from three replicate wells (n = 9).
Statistical Analysis:
One-way ANOVA with the Games-Howell post hoc test for unequal variance was performed, and statistical significance was assumed at p ≤ 0.05.
RESULTS
Characterization of ELP-PEI Conjugates
The neat ELP and the ELP-PEI conjugates were characterized using FTIR spectroscopy and the quantitative OPA assay. The FTIR data (Fig. 1a) revealed that the two ELP-PEI conjugates, as well as the neat ELP, had equivalent chemical structures. All spectra show the expected peaks at 1661 cm−1 (amide I), 1550 cm−1 (amide II), and 3000 and 3200 cm−1 (N–H stretching vibrations) for the amide groups present in ELP. PEI molecules contain several amine groups and can show peaks at wavenumbers 3000 and 3200 cm−1 for the N–H stretching vibrations in the amines. As expected for the low (1 mol%) ELP-PEI used to prepare the coatings, there was no substantial difference in the intensity of the peaks at 3000 and 3200 cm-1. The OPA assay (Fig. 1b) showed a significant increase in OPA fluorescence for the two ELP-PEI conjugates compared to the neat ELP (p < 0.05), indicating an increase in the amine content. Only a slight increase in the OPA fluorescence was seen for ELP-PEI25,000 compared to ELP-PEI800 (p > 0.05). This finding indicates that the ELP-PEI mole percentages used in our study are at the lower end of the sensitivity of the OPA assay.
Figure 1.
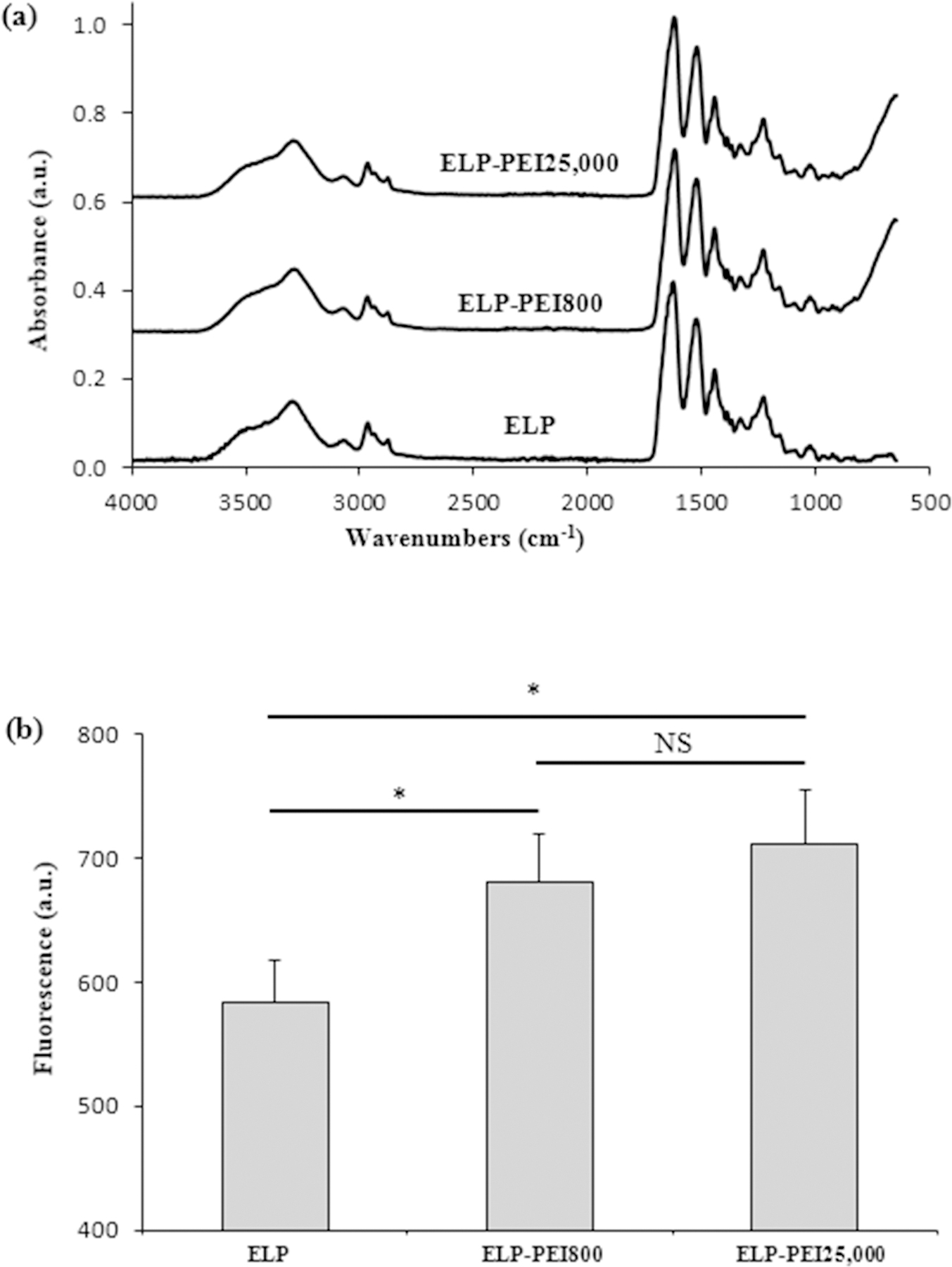
The ELP-PEI conjugates used for surface modification had similar chemical structure and amine content. (a) FTIR spectra of ELP-PEI conjugates with different molecular weights of PEI. The overlapping spectra confirm that the conjugates all have similar chemical structures. (b) The OPA fluorescence of ELP-PEI conjugates with different molecular weights of PEI. * denotes a significant difference (p ≤ 0.05) compared to ELP. The non-significant differences (NS, p > 0.05) among the two ELP-PEIs indicates that the 1 mol% of the ELP-PEIs used in our study is at the lower end of the sensitivity of the OPA assay.
Characterization of ELP-PEI Coatings
AFM indicated that all ELP-PEI coatings had a similar uniform, smooth topography. The average roughness for the ELP-PEI800 coatings was 9.5 ± 3.0 nm, while that for the ELP-PEI25,000 coatings was 6.5 ± 2.4 nm (p > 0.05). Water contact angle measurements were performed on each ELP-PEI coating surface to determine the hydrophobicity of the surface, as the surface hydrophobicity influences a cell’s ability to adhere to the surface. The average water contact angle for the ELP-PEI800 coatings was 39.8 ± 10.2°, while that for the ELP-PEI25,000 coatings was 47.1 ± 10.7° (p > 0.05), which could be attributed to the larger presence (≥ 99%) of hydrophobic ELP.
Microscopic Observations of hASC Culture
Atop uncoated TCPS surfaces, as well as TCPS surfaces coated with neat ELP, hASCs form a 2D monolayer and continue to proliferate until confluence (data not shown). Time-lapse microscopy showed 3D spheroid formation atop the ELP-PEI coatings tested (Fig. 2). The size of the spheroids formed atop the ELP-PEI25,000 coatings appeared smaller compared to those formed atop the ELP-PEI800 coating. The ELP-PEI800 coating showed the formation of regular-shaped spheroids with the best retention and is the only surface in which a single set of spheroids could be traced throughout the time-lapse microscopy. The ELP-PEI25,000 coating appeared to allow the formation of smaller spheroids that appeared to shift across the culture well. Images obtained from the ultra-low attachment static culture and suspension culture methods are shown in Fig. 3. Both the ultra-low attachment static culture and the suspension culture formed spheroids that grew larger in size but reduced in number over time. Though the images give a visual indication as to the retention and maturation of the spheroids, quantitatively these aspects were examined using the DNA assay (Fig. 4), triglyceride assay (Fig. 5), the size of the spheroids formed (Fig. 6), as well as the average number of spheroids that remained after each time point (Fig. 7).
Figure 2.
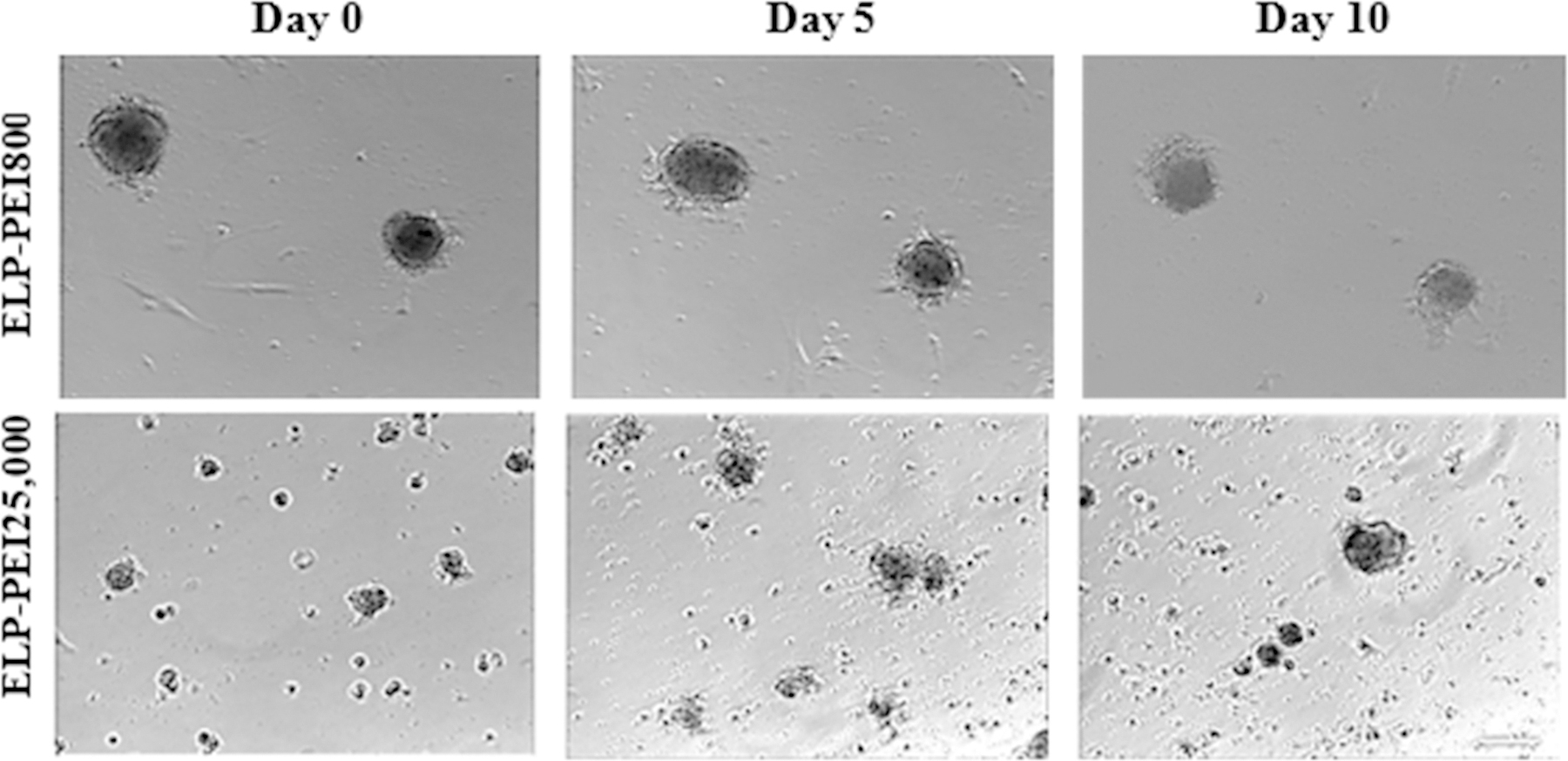
Optical microscopy of hASC culture atop the ELP-PEI800, and ELP-PEI25,000 coatings on day 0, day 5, and day 10 of maturation. The size of the spheroids formed atop the ELP-PEI25,000 coating appears smaller compared to those formed atop the ELP-PEI800 coating. Scale bar = 100 µm.
Figure 3.
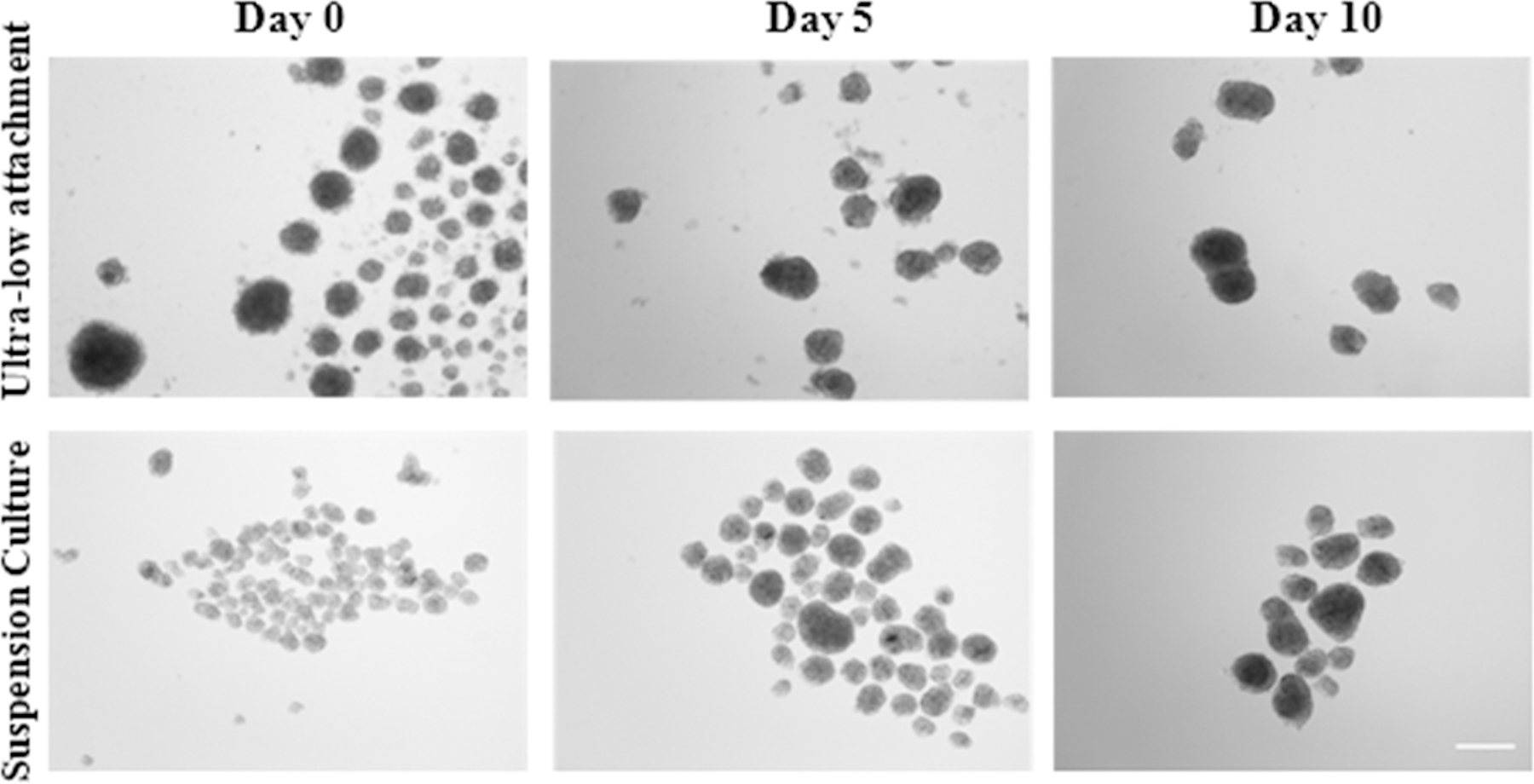
Optical microscopy of the two alternative spheroid formation methods: Ultra-low attachment static culture and Suspension culture on day 0, day 5, and day 10 of maturation. Both methods form spheroids that grow larger in size but reduce in number over time. Scale bars = 100 µm.
Figure 4.

DNA content reduces on ELP-PEI25,000 coating, while the ELP-PEI800 coating and the two alternative methods of spheroid formation show relatively stable DNA content over the course of 10 days of maturation. (a) ELP-PEI coatings and (b) the alternative spheroid formation methods. * denotes a significant difference (p ≤ 0.05) compared to day 0. # denotes a significant difference (p ≤ 0.05) compared to ELP-PEI800 on the same day.
Figure 5.

Triglyceride content increases over the course of 10 days of maturation for all spheroid formation methods, signifying the continued maturation of the hASCs along the adipogenic lineage. (a) ELP-PEI coatings and (b) the alternative spheroid formation methods. * denotes a significant difference (p ≤ 0.05) compared to day 0. # denotes a significant difference (p ≤ 0.05) compared to ELP-PEI800 on the same day.
Figure 6.

The average spheroid sizes over the 10-day maturation indicate that smaller spheroids are formed atop the ELP-PEI25,000 coating compared to the ELP-PEI800 coating and spheroids grow larger in size in the alternative spheroid formation methods. (a) compares the ELP-PEI coatings and (b) compares the alternative spheroid formation methods. * denotes a significant difference (p ≤ 0.05) compared to day 0. # denotes a significant difference (p ≤ 0.05) compared to ELP-PEI800 on the same day.
Figure 7.

The average spheroid numbers over the 10-day maturation indicate that the number of spheroids remain relatively stable atop the ELP-PEI800 coating, but reduce atop the ELP-PEI25,000 coating and in the alternative spheroid formation methods. (a) compares the ELP-PEI coatings and (b) compares the alternative spheroid formation methods. * denotes a significant difference (p ≤ 0.05) compared to day 0. # denotes a significant difference (p ≤ 0.05) compared to ELP-PEI800 on the same day.
Biochemical Characterization of hASC Culture
DNA levels (Fig. 4) of the spheroids were measured on days 0, 5, and 10 of the maturation phase to monitor spheroid retention. It should be noted that any differences in the DNA content on day 0 of the maturation phase between the various culture conditions is a result of the proliferation that occurs during the 3 days of acclimation and 3 days of differentiation that precede the beginning of the maturation phase. As shown in Fig. 4a, the DNA content of cells cultured on the ELP-PEI800 coating was 9.7 ± 1.7 ng/mL on day 0 of the maturation phase, which significantly decreased to 4.8 ± 1.0 ng/mL by day 5 (p < 0.05). The DNA content increased back to the original level by day 10, most likely by individual cells attached to the surface continuing to proliferate. The DNA content on the ELP-PEI25,000 coating showed a significant decrease from 18.0 ± 2.2 ng/mL on day 0 of the maturation phase to 4.9 ± 0.6 ng/mL by day 5 (p < 0.05), similar to the ELP-PEI800 coating. Unlike the ELP-PEI800 coating, the DNA content on the ELP-PEI25,000 coating remained at this low level till day 10. These results indicate that the 25,000 g/mol molecular weight PEI induced a significant spheroid loss. The two alternative methods of spheroid formation showed relatively steady DNA levels over the 10-day maturation period. As shown in Fig. 4b, the suspension culture method showed lower DNA levels (~ 10 ng/mL) than the ultra-low attachment static culture (~ 15 ng/mL). These DNA levels are comparable to those obtained atop the ELP-PEI800 coating.
Triglyceride content (Fig. 5a) of the spheroids was measured to monitor maturation. The triglyceride levels increased over time as expected for all culture methods, signifying that although the cell aggregation kinetics may change, the continued maturation of the hASCs along the adipogenic lineage remains similar. The triglyceride content of the cells atop the ELP-PEI coatings steadily increased from ~1 to ~15 μg/ng DNA over the 10-day maturation period. The alternative spheroid formation methods showed a much higher increase in the triglyceride content of the cells from ~1 to ~30 μg/ng DNA over the 10-day maturation period (Fig. 5b). This is surprising as the DNA content and condition of the spheroids present in the ultra-low attachment static culture and the suspension culture appear to be comparable to those on the ELP-PEI coatings.
Retention of hASC spheroids
Figures 6 and 7 display the average size and the average number of spheroids formed on the ELP-PEI coatings and the two alternative spheroid formation methods. A quantitative analysis of retention and spheroid size is important because it does not matter how mature one spheroid is over the other if the spheroids are not retained in the culture method over the long term.
As shown in Fig. 6a, the spheroid size atop the various ELP-PEI coatings depends on the PEI molecular weight. The ELP-PEI800 coating had spheroids with an average diameter of 105 ± 11 μm on day 0 of the maturation phase, which remained statistically unchanged over the 5-day maturation period (p > 0.05), but decreased slightly to 80 ± 7 μm by day 10 of the maturation phase (p < 0.05). With ELP-PEI25,000, smaller spheroids with average diameters of ~50 μm were formed on day 0 of the maturation phase compared to those formed atop the ELP-PEI800 coating (p < 0.05). The average spheroid size remained relatively constant on the ELP-PEI25,000 coating throughout the 10-day maturation period (p > 0.05). As shown in Fig. 6b, the ultra-low attachment static culture and the suspension culture produced spheroids of similar sizes (~110 μm) on day 0 of the maturation phase. These spheroids sizes on day 0 of the maturation phase for either method are statistically indistinct from those produced atop the ELP-PEI800 coating (p > 0.05; Fig. 6a). The spheroid sizes remained relatively the same until day 5 of the maturation phase (p > 0.05) but increased significantly by day 10 of the maturation phase (p < 0.05). By day 10, the average spheroid diameter produced in the ultra-low attachment static and suspension cultures increased to 154 ± 15 μm and 145 ± 12 μm, respectively.
Figure 7 shows the average number of spheroids, as a quantitative evaluation of spheroid retention and/or merging in the different methods. The ELP-PEI800 coating had a small number of spheroids (3.8 ± 1.5 spheroids per 0.53 mm2 image) on day 0 of the maturation phase, which remained statistically unchanged over the 10-day maturation period (p > 0.05). The ELP-PEI25,000 coatings had 12.0 ± 3.2 spheroids per 0.53 mm2 image on day 0 of the maturation phase (p < 0.05 compared to ELP-PEI800), which gradually reduced to 6.9 ± 2.6 spheroids per 0.53 mm2 image over the 10-day maturation period (p < 0.05). As shown in Fig. 7b, the ultra-low attachment static and suspension cultures formed a significantly greater number of spheroids (~ 30 spheroids per 0.53 mm2 image) on day 0 of the maturation phase, which significantly decreased to ~ 10 spheroids per 0.53 mm2 image by day 10 of the maturation phase. Recalling that, in these alternative spheroid formation methods, the DNA content of the cells remained relatively constant (Fig. 4b), while the spheroid size increased (Fig. 6b) over the 10-day maturation period, the decrease in the number of spheroids in the ultra-low attachment static and suspension cultures indicates merging of spheroids to form larger spheroids. Overall, the ELP-PEI coatings (Fig. 7a) retain spheroid numbers better than the alternative methods (Fig. 7b).
DISCUSSION
With the increasing popularity of hASCs for tissue engineering applications, researchers have used multiple natural materials as coatings to promote spheroid formation and differentiation of hASCs. Stillaert et al. formed spheroids of non-processed fat biopsy specimens atop Matrigel coating.[30] Choi et al. differentiated hASCs along the adipogenic lineage in vitro atop the porous silk fibroin coatings over a two-week culture period.[31] Modification of polystyrene surface using maltose-binding protein grafted fibroblast-growth factor-2 was shown to induce hASC spheroid formation within 24 hours.[32] Cheng et al. showed hASC spheroid formation and enhanced differentiation toward the adipogenic lineage atop chitosan films which are naturally positively charged due to the presence of the amine groups.[33] They improved the initial cell attachment and reduced spheroid detachment over a 7-day culture period by forming chitosan-gelatin blends.[34] Coatings of chitosan-hyaluronic acid blend,[35] polyelectrolyte multilayers made of chitosan and alginate,[36] and immobilization of chitosan and fibroblast-growth factor-2 in varying ratios[37] were also shown to form hASC spheroids. Several factors affect cellular response to such polymeric coatings. Surface chemistry and surface roughness, which also affect the hydrophilicity/hydrophobicity of the surface, have been shown to influence stem cell attachment, proliferation, and subsequent differentiation.[33,38] Another important aspect of spheroid culture is controlling the size of the spheroids. Jeon et al. prepared micropatterned crosslinked hydrogel coatings of alginate and polyethylene glycol to achieve hASC spheroids of uniform sizes.[39]
Adipose tissue ECM primarily consists of collagen, laminin, fibronectin, and elastin.[40] Hoefner et al. showed that hASCs produce this ECM-like pattern when cultured as 3D spheroids.[41] In this study, we chose to use the ELP-based coating to induce 3D spheroid formation. ELPs are genetically-engineered polypeptides that mimic the amino acid sequence of human elastin segments.[42] The genetic engineering process allows production of ELPs with precise molecular weight and chemistry, minimizing any batch-to-batch variations common in other commercially available ECM proteins. ELP-PEI block copolymers are a class of multifunctional polymers that have been shown to exhibit the inverse phase transition behavior of ELP as well as the mild polyelectrolyte nature of the PEI. Previous work with ELP-PEI has utilized a low molecular weight block of PEI (< 1200 g/mol) which, used in low concentrations in surface coatings, have been shown to elicit cellular aggregations into three-dimensional structures.[6,20–27] The ELP-PEI800 copolymer coating has been used in the past to prepare 3D spheroid cultures of several different cell types, including rat hepatocytes, mouse 3T3-L1 preadipocytes, H35 rat hepatoma, human osteosarcoma, and hASCs.[6,20–27] Specifically, for the mouse 3T3-L1 preadipocyte and hASC cultures, 3D spheroids formed atop the ELP-PEI800 coating have been shown to be a better in vitro model compared to the 2D monolayer in terms of accumulation of triglycerides and sensitivity of cellular responses to nutrients and cytokines.[20–23] However, the effects of the molecular weight of the polyelectrolyte (PEI) block in the coatings on the spheroid organization kinetics and spheroid size of hASCs as they are differentiated along the adipogenic lineage have not been previously investigated. We first characterized the ELP-PEI coatings for any differences in their chemical structures, surface topography, and hydrophilicity/hydrophobicity. We found that the ELP-PEI coatings had identical chemical groups (Fig. 1a) along with similar surface roughness and water contact angles. Having no significant differences eliminates surface topography and hydrophobicity as confounding variables when investigating the effect of the PEI molecular weight on the formation and retention of the spheroids.
In the present study, we determined that hASC spheroid formation and retention atop the two ELP-PEI coatings (Figs. 2,4) are driven by the PEI molecular weight. Though the physical characterization of the ELP-PEI coatings indicated that their chemical structures, surface topography, and hydrophobicity were similar, cell morphology and spheroid formation atop the ELP-PEI coating surfaces were significantly different. We attribute these differences to the PEI molecular weight used to form the conjugates. As we used the same number of ELP-PEI molecules (1 mol%) in the coatings, it is expected that the PEI with a higher molecular weight of 25,000 g/mol will occupy more surface area and be more effective in repelling the cells compared to the PEI with a lower molecular weight of 800 g/mol. Such repulsion between the cells and the PEI was the likely reason that the DNA content of the cells remained stable on the ELP-PEI800 coating and significantly decreased on the ELP-PEI25,000 coating (Fig. 4). As shown in Fig. 6, the cell-PEI repulsion also resulted in the formation of the smallest spheroids with an average diameter ~ 50 μm atop the ELP-PEI25,000 coating, while the ELP-PEI800 coating formed larger spheroids (average diameter ~ 110 μm). Since we seeded the same number of cells on all coatings, larger spheroid size correlated with a lower number of spheroids, with the ELP-PEI25,000 coating forming nearly 3-times as many spheroids compared to the ELP-PEI800 coating (Fig. 7).
We also determined that the differentiation and maturation of hASCs toward the adipogenic lineage were independent of the PEI molecular weight (Fig. 5). We used spheroid size (determined by time-lapse microscopy) and cellular adiposity (determined by the triglyceride assay) to validate the adipogenic nature of the spheroids. In our previous work, which focused more on the biological aspects of hASC culture,[20–23] we have established the relevancy of spheroid size and cellular adiposity by using Oil-Red-O staining and RT-PCR to further confirm the adipogenic differentiation of hASCs.
Mature adipocytes grown as 3D spheroids in vitro may eventually become too buoyant to remain tethered to a surface, due to the less dense lipid the cells accumulate over time. Once such fat-laden spheroids detach from the surface, they may be lost to the media changes and can no longer be studied within the in vitro model. This means that the best spheroids to study adiposity on a cellular level may be lost due to a lack of attachment. For this reason, the retention properties of any method that creates 3D spheroids must be characterized. In the present study, ultra-low attachment static culture and suspension culture were performed for the same time points as for the cultures performed atop the ELP-PEI surface coatings. This comparison study was performed to determine which method of forming spheroids is more effective in their retention and maturation. Table 1 summarizes the performance of the selected scaffold-free methods.
Table 1.
Performance summary of the scaffold-free spheroid forming methods tested over the 10-day maturation period.
| Spheroid Forming Method | Retention |
Differentiation (Triglyceride content) | |
|---|---|---|---|
| DNA Content | Number of Spheroids | ||
| ELP-PEI800 Coating | Stable | Stable | Gradual increase |
| ELP-PEI25,000 Coating | Reduced | Reduced due to loss | Gradual increase |
| Ultra-low Attachment Plate | Stable | Reduced due to merging | Initial increase |
| Suspension Culture | Stable | Reduced due to merging | Gradual increase |
Ultra-low attachment surfaces have been used in the past to prepare 3D spheroids.[12] Circulating or rocking motion in the suspension culture method induces collisions among the floating cells and has been used to induce spheroids formation.[43–47] Such constant fluid motion also prevents settling and subsequent attachment of individual cells to the container surfaces. The hASC spheroids created using such methods showed that the spheroid configuration enhanced the adipogenic, osteogenic, chondrogenic, and angiogenic activities of the hASCs.[43,44] While a lack of surface attachment promotes self-aggregation in the anchorage-dependent cells, it may also lead to loss of the formed spheroids to media changes. In the present study, we did not find any significant loss of hASC spheroids to media changes in the static culture atop the ultra-low attachment plates (Fig. 4b); instead, we found that the spheroids merged together to form fewer, larger spheroids over the culture period (Figs. 6,7), with the hASCs differentiating toward the adipogenic lineage to a similar degree compared to other spheroid forming methods (Fig. 5). Lee et al. suspension cultured hASCs in the ultra-low attachment plate for 7 days and showed that the average spheroid size increased from 40 μm on day 3 to 70 μm on day 7.[43] However, they did not track the number of spheroids to determine spheroid merging or loss during that period. Kapur et al. used the hanging drop method to first form the hASC spheroids, which they later suspension cultured for five weeks. Their results indicated formation of large 400-μm diameter spheroids, which increased in size to 600 μm. This study used optical microscopy, but not quantification, to indicate spheroid merging over the culture period.[44] Baraniak and McDevitt used the suspension culture method to achieve the differentiation of bone marrow-derived stem cells toward multiple lineages. Their work indicated variation in spheroid size over the one-week culture period, indicating spheroid loss and merging.[45] Hammond et al.[48] and Roy et al.[49] have suggested that the shear force experienced by the cells in suspension culture may introduce phenotypic and functional changes and can hinder cell differentiation and cause cell death. We found that the suspension culture method formed fewer, larger hASC spheroids over the culture period through merging the spheroids together (Figs. 6,7), but did not result in any significant loss of spheroids or detriment to their adipogenic differentiation (Figs. 4,5) likely due to the low rotational speed used in our work.
CONCLUSIONS
The PEI molecular weight influenced the formation and retention of hASC spheroids atop the ELP-PEI coatings, with the variation in PEI molecular weight offering a possibility to control the spheroid size. DNA measurement and morphometric analysis indicated that the ELP-PEI800 coating retained the spheroids better compared to the ELP-PEI25,000 coating. The adipogenic differentiation of hASC spheroids as indicated by triglyceride levels, was equal or higher in the ultra-low attachment static culture and suspension culture compared to the ELP-PEI coatings method. However, spheroids gradually merged to form larger spheroids in the ultra-low attachment static culture and suspension culture methods over the 10-day maturation period. The ELP-PEI800 coating showed better retention of hASC spheroid sizes and numbers with adequate adipogenic differentiation, which allows for the more physiologically relevant spheroids to be acquired.
ACKNOWLEDGEMENTS
This work was funded by National Institutes of Health (NIH; R01EB020006). This work made use of instruments in the Department of Biomedical Materials Science Shared Equipment Facility.
Footnotes
The authors have no conflict of interests to disclose.
REFERENCES
- 1.Overweight & Obesity. In: Centers for Disease Control and Prevention. https://www.cdc.gov/obesity/data/prevalence-maps.html. Accessed 28 August 2019.
- 2.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: An Underappreciated Source of Stem Cells for Biotechnology. Trends in Biotechnol 2006;24:150–154. [DOI] [PubMed] [Google Scholar]
- 3.Cheng NC, Chen SY, Li JR, Young TH. Short-Term Spheroid Formation Enhances the Regenerative Capacity of Adipose-Derived Stem Cells by Promoting Stemness, Angiogenesis, and Chemotaxis. Stem Cells Transl Med 2013;2:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai CC, Su PF, Huang YF, Yew TL, Hung SC. Oct4 and Nanog Directly Regulate Dnmt1 to Maintain Self-Renewal and Undifferentiated State in Mesenchymal Stem Cells. Mol Cell 2012;47:169–182. [DOI] [PubMed] [Google Scholar]
- 5.Yew TL, Hung YT, Li HY, Chen HW, Chen LL, Tsai KS, Chiou SH, Chao KC, Huang TF, Chen HL, Hung SC. Enhancement of Wound Healing by Human Multipotent Stromal Cell Conditioned Medium: The Paracrine Factors and p38 MAPK Activation. Cell Transplant 2011;20:693–706. [DOI] [PubMed] [Google Scholar]
- 6.Gurumurthy B, Bierdeman PC, Janorkar AV. Spheroid Model for Functional Osteogenic Evaluation of Human Adipose Derived Stem Cells. J Biomed Mater Res A 2017;105:1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gettler BC, Zakhari JS, Gandhi PS, Williams SK. Formation of Adipose Stromal Vascular Fraction Cell-Laden Spheroids Using a Three-Dimensional Bioprinter and Superhydrophobic Surfaces. Tissue Eng Part C Methods 2017;23:516–524. [DOI] [PubMed] [Google Scholar]
- 8.Sart S, Tomasi RF, Amselem G, Baroud CN. Multiscale Cytometry and Regulation of 3D Cell Cultures on a Chip. Nat Commun 2017;8:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for Generation of Homogeneous Multicellular Tumor Spheroids Applicable to a Wide Variety of Cell Types. Biotechnol Bioeng 2003;83:173–180. [DOI] [PubMed] [Google Scholar]
- 10.Mineda K, Feng J, Ishimine H, Takada H, Doi K, Kuno S, Kinoshita K, Kanayama K, Kato H, Mashiko T, Hashimoto I, Nakanishi H, Kurisaki A, Yoshimura K. Therapeutic Potential of Human Adipose-Derived Stem/Stromal Cell Microspheroids Prepared by Three-Dimensional Culture in Non-Cross-Linked Hyaluronic Acid Gel. Stem Cells Transl Med 2015;4:1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurumurthy B, Bierdeman PC, Janorkar AV. Composition of Elastin Like Polypeptide-Collagen Composite Scaffold Influences In Vitro Osteogenic Activity of Human Adipose Derived Stem Cells. Dent Mater 2016;32:1270–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong JG, Valdes YR, Barrett JW, Bell JC, Stojdl D, McFadden G, McCart JA, DiMattia GE, Shepherd TG. Evidence for Differential Viral Oncolytic Efficacy in an In Vitro Model of Epithelial Ovarian Cancer Metastasis. Mol Ther Oncolytics 2015;2:15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomillion CT, Burg KJL. Stem Cells and Adipose Tissue Engineering. Biomaterials 2006;27:6052–6063. [DOI] [PubMed] [Google Scholar]
- 14.Patrick CW Jr. Tissue Engineering Strategies for Adipose Tissue Repair. Anat Rec 2001;263:361–366. [DOI] [PubMed] [Google Scholar]
- 15.von Heimburg D, Zachariah S, Heschel I, Kühling H, Schoof H, Hafemann B, Pallua N. Human Preadipocytes Seeded on Freeze-dried Collagen Scaffolds Investigated In Vitro and In Vivo. Biomaterials 2001;22:429–438. [DOI] [PubMed] [Google Scholar]
- 16.Hemmrich K, von Heimburg D, Rendchen R, Di Bartolo C, Milella E, Pallua N. Implantation of Preadipocyte-loaded Hyaluronic Acid Based Scaffolds into Nude Mice to Evaluate Potential for Soft Tissue Engineering. Biomaterials 2005;26:7025–7037. [DOI] [PubMed] [Google Scholar]
- 17.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006;126:677–689. [DOI] [PubMed] [Google Scholar]
- 18.Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A Pericellular Collagenase Directs the 3‑Dimensional Development of White Adipose Tissue. Cell 2006;125:577–591. [DOI] [PubMed] [Google Scholar]
- 19.Klingelhutz AJ, Gourronc FA, Chaly A, Wadkins DA, Burand AJ, Markan KR, Idiga SO, Wu M, Potthoff MJ, Ankrum JA. Scaffold-free Generation of Uniform Adipose Spheroids for Metabolism Research and Drug Discovery. Sci Rep 2018;8:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner PA, Harris LM, Purser CA, Baker RC, Janorkar AV. A Surface-tethered Spheroid Model for Functional Evaluation of 3T3-L1 Adipocytes. Biotechnol Bioeng 2014;111:174–183. [DOI] [PubMed] [Google Scholar]
- 21.Turner PA, Tang Y, Weiss SJ, Janorkar AV. 3-D spheroid Cell Model of In Vitro Adipocyte Inflammation. Tissue Eng Part A, 2015;21:1837–1847. [DOI] [PubMed] [Google Scholar]
- 22.Turner PA, Gurumurthy B, Bailey JL, Elks CM, Janorkar AV. Adipogenic Differentiation of Human Adipose-Derived Stem Cells Grown as Spheroids. Proc Biochem 2017;59:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner PA, Garrett MR, Didion SP, Janorkar AV. Spheroid Culture System Confers Differentiated Transcriptome Profile and Functional Advantage to 3T3-L1 Adipocytes. Annals of Biomed Eng 2018;46:772–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner PA, Weeks CA, McMurphy AJ, Janorkar AV. Spheroid Organization Kinetics of H35 Rat Hepatoma Model Cell System on Elastin-Like Polypeptide-Polyethyleneimine Copolymer Substrates. J Biomed Mater Res A 2014;102A:852–861. [DOI] [PubMed] [Google Scholar]
- 25.Weeks CA, Aden B, Zhang J, Singh A, Hickey RD, Kilbey SM, Nyberg SL, Janorkar AV. Effect of Amine Content and Chemistry on Long-Term, Three-Dimensional Hepatocyte Spheroid Culture atop Aminated Elastin-Like Polypeptide Coatings, J Biomed Mater Res A 2017;105A:377–388. [DOI] [PubMed] [Google Scholar]
- 26.Penfornis P, Fernandes JD, Abraham A, Gurumurthy B, Janorkar AV, Pasco D, Pochampally RR. Three-dimensional Spheroid Model Using Cancer and Stromal Cells for In Vitro Drug Screening Assays. J Stem Cell Res Med 2017;2:1–5. [Google Scholar]
- 27.Clark K, Janorkar AV. Milieu for Endothelial Differentiation of Human Adipose-Derived Stem Cells. Bioengineering 2018;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn L, Semple JL, Woodhouse KA. Decellularized Placental Matrices for Adipose Tissue Engineering. J Biomed Mater Res A 2006;79:359–369. [DOI] [PubMed] [Google Scholar]
- 29.Hauner H, Skurk T, Wabitsch M, Cultures of Human Adipose Precursor Cells. From: Methods in Molecular Biology, in: Ailhaud G (Ed.), Adipose Tissue Protocols, Humana Press, Inc, Totawa, NJ, 2001, pp. 239–247. [DOI] [PubMed] [Google Scholar]
- 30.Stillaert FB, Abberton KM, Keramidaris E, Thompson EW, Blondeel PN, Morrison WA. Intrinsics and Dynamics of Fat Grafts: An In Vitro Study. Plast Reconstr Surg 2010;126:1155–1162. [DOI] [PubMed] [Google Scholar]
- 31.Choi JH, Bellas E, Vunjak-Novakovic G, Kaplan DL. Adipogenic Differentiation of Human Adipose-Derived Stem Cells on 3D Silk Scaffolds. Methods Mol Biol 2011;702:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JH, Park IS, Park Y, Jung Y, Kim SH, Kim SH. Therapeutic Angiogenesis of Three-Dimensionally Cultured Adipose-Derived Stem Cells in Rat Infarcted Hearts. Cytotherapy 2013;15:542–556. [DOI] [PubMed] [Google Scholar]
- 33.Cheng NC, Wang S, Young TH. The Influence of Spheroid Formation of Human Adipose-Derived Stem Cells on Chitosan Films on Stemness and Differentiation Capabilities. Biomaterials 2012;33:1748–1758. [DOI] [PubMed] [Google Scholar]
- 34.Cheng NC, Chang HH, Tu YK, Young TH. Efficient Transfer of Human Adipose-Derived Stem Cells by Chitosan/Gelatin Blend Films. J Biomed Mater Res B Appl Biomater 2012;100:1369–1377. [DOI] [PubMed] [Google Scholar]
- 35.Hsu SH, Ho TT, Huang NC, Yao CL, Peng LH, Dai NT. Substrate-dependent Modulation of 3D Spheroid Morphology Self-Assembled in Mesenchymal Stem Cell-Endothelial Progenitor Cell Coculture. Biomaterials 2014;35:7295–7307. [DOI] [PubMed] [Google Scholar]
- 36.Hatami J, Silva SG, Oliveira MB, Costa RR, Reis RL, Mano JF. Multilayered Films Produced by Layer-by-Layer Assembly of Chitosan and Alginate as a Potential Platform for the Formation of Human Adipose-Derived Stem Cell aggregates. Polymers 2017;9. pii: E440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen ST, Wu CY, Chen HY. Enhanced Growth Activities of Stem Cell Spheroids Based on a Durable and Chemically Defined Surface Modification Coating. ACS Appl Mater Interfaces 2018;10:31882–31891. [DOI] [PubMed] [Google Scholar]
- 38.Laschke MW, Schank TE, Scheuer C, Kleer S, Schuler S, Metzger W, Eglin D, Alini M, Menger MD Three-dimensional Spheroids of Adipose-derived Mesenchymal Stem Cells Are Potent Initiators of Blood Vessel Formation in Porous Polyurethane Scaffolds. Acta Biomater 2013;9:6876–6884. [DOI] [PubMed] [Google Scholar]
- 39.Jeon O, Marks R, Wolfson D, Alsberg E. Dual-crosslinked Hydrogel Microwell System for Formation and Culture of Multicellular Human Adipose Tissue-Derived Stem Cell Spheroids. J Mater Chem B 2016;4:3526–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K.. Fibrosis in Human Adipose Tissue: Composition, Distribution, and Link with Lipid Metabolism and Fat Mass Loss. Diabetes 2010;59:2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Höfner C, Muhr C, Horder H, Wiesner M, Wittmann K, Lukaszyk D, Radeloff, Winnefeld M, Becker M, Blunk T, Bauer-Kreisel P. Human ASC Spheroids Possess High Adipogenic Capacity and Acquire an Adipose Tissue-like ECM Pattern. Tissue Eng Part A. 2020. [DOI] [PubMed]
- 42.Arias FJ, Reboto V, Martin S, Lopez I, Rodriguez-Cabello JC. Tailored Recombinant Elastin-like Polymers for Advanced Biomedical and Nano(bio)technological Applications. Biotechnol Lett 2006;28:687–695. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Han YS, Lee SH. Long-Duration Three-Dimensional Spheroid Culture Promotes Angiogenic Activities of Adipose-Derived Mesenchymal Stem Cells. Biomol Ther (Seoul) 2016;24:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapur SK, Wang X, Shang H, Yun S, Li X, Feng G, Khurgel M, Katz AJ. Human Adipose Stem Cells Maintain Proliferative, Synthetic and Multipotential Properties When Suspension Cultured as Self-Assembling Spheroids. Biofabrication 2012;4:025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baraniak PR, McDevitt TC. Scaffold-free Culture of Mesenchymal Stem Cell Spheroids in Suspension Preserves Multilineage Potential. Cell Tissue Res 2012;347:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, McDevitt TC. Homogeneous and Organized Differentiation within Embryoid Bodies Induced by Microsphere-Mediated Delivery of Small Molecules. Biomaterials 2009;30:2507–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shukla S, Nair R, Rolle MW, Braun KR, Chan CK, Johnson PY, Wight TN, McDevitt TC. Synthesis and Organization of Hyaluronan and Versican by Embryonic Stem Cells Undergoing Embryoid Body Differentiation. J Histochem Cytochem 2010;58:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammond TG, Birdsall HH. Hepatocyte CYP2B6 Can Be Expressed in Cell Culture Systems by Exerting Physiological Levels of Shear: Implications for ADME Testing. J Toxicol 2017;2017:1907952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy P, Washizu J, Tilles AW, Yarmush ML, Toner M. Effect of Flow on the Detoxification Function of Rat Hepatocytes in a Bioartificial Liver Reactor. Cell Transplant 2001;10:609–614. [PubMed] [Google Scholar]


