Abstract
Objectives
Computerised physician order entry (CPOE) systems facilitate the review of medication orders by pharmacists. Reports have emerged that show conception flaws or the misuse of CPOE systems generate prescribing errors. We aimed to characterise pharmacist interventions (PIs) triggered by prescribing errors identified as system-related errors (PISREs) in French hospitals.
Design
This was a cross-sectional observational study based on PIs prospectively documented in the Act-IP observatory database from January 2014 to December 2018.
Setting
PISREs from 319 French computerised healthcare facilities were analysed.
Participants
Among the 319 French hospitals, 232 (72.7%) performed SRE interventions, involving 652 (51%) pharmacists.
Results
Among the 331 678 PIs recorded, 27 058 were qualified as due to SREs (8.2%). The main drug-related problems associated with PISREs were supratherapeutic (27.5%) and subtherapeutic dosage (17.2%), non-conformity with guidelines/contraindications (22.4%) and improper administration (17.9%). The PI prescriber acceptation rate was 78.9% for SREs vs 67.6% for other types of errors. The PISRE ratio was estimated relative to the total number of PIs. Concerning the certification status of CPOE systems, the PISRE ratio was 9.4% for non-certified systems vs 5.5% for certified systems (p<0.001). The PISRE ratio for senior pharmacists was 9.2% and that for pharmacy residents 5.4% (p<0.001). Concerning prescriptions made by graduate prescribers and those made by residents, the PISRE ratio was 8.4% and 7.8%, respectively (p<0.001).
Conclusion
Computer-related prescribing errors are common. The PI acceptance rate by prescribers was higher than that observed for PIs that were not CPOE related. This suggests that physicians consider the potential clinical consequences of SREs for patients to be more frequently serious than interventions unrelated to CPOE. CPOE medication review requires continual pharmacist diligence to catch these errors. The significantly lower PISRE ratio for certified software should prompt patient safety agencies to undertake studies to identify the safest software and discard software that is potentially dangerous.
Keywords: health & safety, adverse events, health informatics
Strengths and limitations of this study.
This study provides an overview of prescribing issues related to the use of computerised physician order entry (CPOE) systems at the national level.
Beyond this large register of prescribing problems related to CPOE use, this is the first study to evaluate pharmacist interventions in daily practice for such a large sample of interventions, pharmacists and hospitals.
This study focuses on declarative data based on interventions performed by hospital pharmacists.
These pharmacist interventions highlight prescription problems, but they are not exhaustive.
Introduction
Every day, numerous hospitalised patients are subject to drug-related problems (DRPs), resulting in suboptimal therapy, suffering and decreased quality of life, as well as high healthcare costs for society.1 2 Computerised physician order entry (CPOE) systems, along with clinical decision support systems (CDSS), improve the safety, quality and value of patient care.3 According to a meta-analysis, CPOE systems have reduced hospital medication errors by approximately 12.5% (95% CI 10.6% to 14.4%).4 However, CPOE systems also have the potential to introduce or contribute to errors. Indeed, new mechanisms that lead to prescription errors have been identified with CPOE: wrong patient selection, failure to report drug allergies, incorrect entry or wrong selection of medication, dose, route or time of administration and confusing free-text comments.5–10
In France, as in other countries, various incentives and requirements have been put in place to encourage computerised drug prescribing, such as France’s ‘Digital Hospital’ programme.11 Since the 2000s, prescribing errors associated with the use of CPOE have been slowly coming to light as healthcare has become increasingly computerised.9 Compared with handwritten prescriptions, the analysis of electronic prescriptions requires a particular effort on the part of pharmacists and other health professionals to detect errors.9 System-related errors (SREs) are defined as those in which the electronic prescribing system functionality or design contributed to the error, with little possibility that another cause, such as lack of knowledge, produced the error. For example, an order for an inappropriate drug located on a drop-down menu next to a likely drug selection is an SRE.12
A pharmacist intervention (PI) due to an SRE is defined as any PI resulting from the identification of a prescribing error by a pharmacist that would probably not have occurred in the context of a handwritten prescription and of which at least one cause is related to the use of a computer (software system configuration issue, software functionality issue or software misuse).13–16
Most studies concerning PIs triggered by system-related prescribing errors were conducted within a single hospital.17–19 As a result, it is not possible to assess the extent of prescribing errors related to electronic systems or draw conclusions about subsequent PIs at a national level.
In 2003, the French Society of Clinical Pharmacy (SFPC) developed and validated a tool for classifying and documenting clinical PIs.20 This tool allows the reporting of DRPs and PIs performed during the daily review of medication orders.21 In 2006, a website, Act-IP, was created with the objectives to (A) create a documentation system that is freely accessible to any pharmacist, through the SFPC Web site (http://www.actip.sfpc.eu/actip/index/ficheip/) and (B) pool the data recorded by all pharmacists to conduct epidemiological studies concerning DRPs detected by pharmacists.22 The data recording is on a voluntary basis. The pooling of PIs constitutes an observatory of clinical pharmacy practices, called the ‘Act-IP Observatory’.
The aim of this study was to characterise PIs triggered due to SREs in French hospitals between 2014 and 2018. Our secondary objective was to determine the physician acceptance rate and its frequency according to the certification status (certified vs non-certified) of the CPOE systems.
Methods
Study design
This was a cross-sectional observational study using PIs prospectively documented in the Act-IP observatory over a 5-year period from 1 January, 2014 to 31 December 2018. The main outcome was a PI due to an SRE (PISRE) reported by French hospital pharmacists on the Act-IP observatory.
Data sources
The data come from PIs registered in the Act-IP Observatory from January 2014 to December 2018. Based on the SFPC criteria, using the report form developed and validated for routine documentation of the PIs, Act-IP users completed the online report form notifying the date, type of DRP, PI, type of drug involved (according to the ATC (Anatomical Therapeutic Chemical) classification), acceptance of the intervention by the prescriber and free-text details of the context. Ten categories were determined for DRPs and seven for PIs (online supplemental appendix 1). A PI was considered to be ‘accepted’ if the physician took it into account and modified the prescription as suggested by the pharmacist or ‘refused’ if the prescription remained unchanged, including cases of expressed refusal by the prescriber. If acceptance of the intervention was impossible to ascertain (ie, discharged patients or those transferred to another ward before acceptance), the PI was noted as ‘not assessable’. The pharmacist’s academic background, hospital characteristics and software used were documented online by the pharmacist when he/she registered onto the Act-IP website. To be registered onto the Act-IP website, pharmacists had prior to accept terms and conditions and allowed the use of their data for analysis. Since July 2013, pharmacists have been able to indicate whether the DRP was ‘related to the electronic system’ or not for each registered PI. For the purpose of this study, PISREs were DRPs rated by each pharmacist as ‘related to the electronic system’ in the Act-IP website.
bmjopen-2020-045778supp001.pdf (1.1MB, pdf)
The reliability of the classification of the type of drug therapy problem and intervention according to the SFPC classification was determined in a previous study by assessing the degree of agreement between 12 pharmacists using the kappa concordance coefficient (kappa=0.76 for drug problems and kappa=0.89 for drug interventions).20 Database quality controls were performed by an independent pharmacist to ensure that data coding and entry errors were minimal.22
French law made the certification of CPOE systems mandatory on 29 December 2011. However, two decrees abolished this obligation in 2017. Certification is now based on the sole initiative of the software developer. Forty-eight hospital CPOE software packages are currently certified by the agency for patient safety (Haute Autorité de Santé (HAS)).23 For our analysis, PISREs were classified according to the HAS status of the CPOE system (certified vs not certified).
Analysis
The PISRE ratio was estimated relative to the total number of PIs. Proportions were compared using the χ2 test. PISREs coded as ‘refused’ or ‘not assessable’ were combined and compared with the accepted PISREs. Values of p<0.001 were considered to be statistically significant. Statistical analyses were performed using Stata V.13 (Stata). Several qualitative examples are given to illustrate PISREs.
Study participants and public involvement
This research was done without study participant involvement. Patients and/or the public were not involved in the design, or conduct, or dissemination plans of this research.
Results
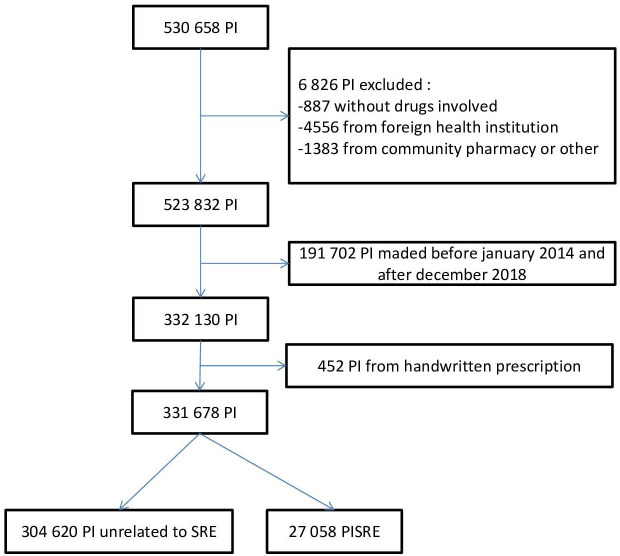
From January 2014 to December 2018, 331,678 PIs were entered into the Act-IP observatory. Among them, 27 058 (8.2%) were indicated to be system-related prescribing errors (figure 1).
Figure 1.
Flow chart, PISRE selection in Act-IP observatory (extraction on 11 February 2019). PI, pharmacist intervention; PISRE, prescribing errors identified as system-related error; SRE, system-related error.
Over the study period, 1219 pharmacists from 319 hospitals recorded PIs in the Act-IP observatory database. The geographical location of the hospitals involved is shown in figure 2. Among them, 232 (72.7%), involving 652 (51%) pharmacists, performed SRE interventions. Among the 319 hospitals, 87 (27.3%) did not qualify any PIs as being due to an SRE. PIs come from 82 software involving 19 certified systems.
Figure 2.
Geographical location of French hospitals that entered data into the Act-IP observatory between 2014 and 2018.
The characteristics of the PISREs are summarised in table 1. The most commonly identified type of DRP was ‘supratherapeutic dosage’, followed by ‘non-conformity with guidelines/contraindications’ and ‘improper administration’. Among the 27 058 PISREs, 78.9% (n=21 356) were accepted. The PISRE ratio was 9.4% for non-certified systems vs 5.5% for certified systems (p<0.001). Online supplemental appendix 2 presents examples of DRPs classified as being triggered by prescribing errors due to the CPOE system. For example: Prescription errors can be the same whether they are handwritten prescriptions or computer-assisted prescriptions. Indeed, the combination of amiodarone and escitalopram can appear on handwritten prescription because of prescriber’s lack of knowledge. With CPOE, CDSS tool can alert on drug–drug interaction. However, high frequency of alerts and dozens of daily interruptions for clinicians are responsible of ‘alert fatigue’ and practitioners override alerts.24 We can also find duplicate orders, meaning the same drug is prescribed twice. With predefined order set, it is common to have 8 g of paracetamol per day prescribed. Duplication errors are partially explained by the fact that many screens are required to view patient medications, making intrinsically difficult to spot duplicates.25
Table 1.
Characteristics of all Act-IP observatory PISREs and PIs between 2014 and 2018
| Characteristics | PISRE (N=27 058) |
PI total (N=3 31 678) |
Ratio | |
| n | n | % | P value | |
| Drug-related problem | ||||
| Supratherapeutic dosage | 7436 | 72 912 | 10.2 | <0.001 |
| Non-conformity with guidelines/hospital formulary | 6069 | 86 072 | 7.1 | – |
| Improper administration | 4838 | 49 184 | 9.8 | <0.001 |
| Subtherapeutic dosage | 4646 | 29 105 | 16.0 | <0.001 |
| Untreated indication | 2366 | 30 138 | 7.9 | <0.001 |
| Drug without indication | 1302 | 27 690 | 4.7 | <0.001 |
| Drug interaction | 161 | 18 267 | 0.9 | <0.001 |
| Drug monitoring | 111 | 10 303 | 1.1 | <0.001 |
| Adverse drug reaction | 65 | 5854 | 1.1 | <0.001 |
| Failure to receive drug | 64 | 2153 | 3.0 | <0.001 |
| Type of intervention | ||||
| Dose adjustment | 7447 | 89 390 | 8.3 | – |
| Drug switch | 6649 | 85 033 | 7.8 | <0.001 |
| Drug discontinuation | 5220 | 62 715 | 8.3 | <0.001 |
| Optimisation of administration | 4123 | 32 558 | 12.7 | <0.001 |
| Addition of new drug | 3228 | 34 198 | 9.4 | <0.001 |
| Change of administration route | 213 | 6978 | 3.1 | <0.001 |
| Drug monitoring | 178 | 20 806 | 0.9 | <0.001 |
| Prescriber acceptance | ||||
| Interventions accepted | 21 356 | 227 223 | 9.4 | <0.001* |
| Interventions not accepted | 3068 | 51 957 | 5.9 | |
| Not assessable | 2634 | 52 498 | 5.0 | |
| Prescriber’s status | ||||
| Senior | 15 152 | 180 863 | 8.4 | <0.001 |
| Resident | 11 765 | 150 136 | 7.8 | |
| Midwife† | 141 | 679 | 20.8 | |
| Pharmacist’s status | ||||
| Senior | 21 271 | 231 519 | 9.2 | <0.001 |
| Resident | 4640 | 86 728 | 5.4 | |
| Not assessable† | 1147 | 13 431 | 8.5 | |
| CPOE system status | ||||
| Not certified | 21 385 | 226 878 | 9.4 | <0.001 |
| Certified | 5549 | 101 516 | 5.5 | |
| Not assessable† | 124 | 3284 | 3.8 | |
| Total | 27 058 | 331 678 | 8.2 | |
*Not accepted and not assessable interventions have been regrouped for χ2 test.
†Excluded from the χ2 analysis.
CPOE, computerised prescriber order entry; PI, pharmacist intervention; PISRE, prescribing errors identified as system-related errors.
bmjopen-2020-045778supp002.pdf (65.3KB, pdf)
Discussion
This study provides an overview of prescription problems related to CPOE systems used in French hospitals. It provides insights into the main situations and medications involved in computer-related prescribing problems detected by pharmacists by providing a broad description of PIs performed during the daily review of routine medication orders. Thus one strength of this study is that it is based on a large number of hospitals scattered throughout France, as no prior study of such extent evaluating PIs in daily practice has been published.
PISRE rate
Our PISRE rate (8.2%) is within the range reported by Korb-Savoldelli et al.19 They analysed peer-reviewed studies (n=14) that quantitatively reported medication-prescription errors related to CPOE. The prevalence of CPOE system-related medication errors relative to all prescription medication errors ranged from 6.1% to 77.7% (median = 26.1% (IQR: 17.6–42.1)) and was less than 6.3% relative to the number of prescriptions reviewed. Ours is the first large-scale descriptive study using an observatory hospital pharmacy practice database to study computer-related prescribing errors.
DRPs induced by CPOE
The main category of DRPs identified as PISREs were supratherapeutic (27.5%, 7436) and subtherapeutic dosage (17.2%, 4646), non-conformity to guidelines/hospitals’ drug formularies (22.4%, 6069) (ie, medication selection non-compliant with the hospital drug formulary) and improper administration (17.9%, 4838) (ie, incorrect or no formulation, wrong timing). According to Korb-Savoldelli et al all studies reported ‘wrong dose’ and ‘wrong drug’ errors,19 with the ‘wrong dose’ error being that most frequently reported (from 7% to 67.4%, median = 31.5% (IQR: 20.5–44.5)). Many of the prescription errors due to CPOE systems can have serious consequences for patients, depending on the clinical circumstances. Although some of are unlikely to occur (eg, IV ketoprofen 150 ampoules/day instead of 150 mg/day), they nevertheless illustrate flaws in certain CPOE systems.26 However, our data do not allow the discrimination between software errors, connection problems and human error.
CPOE systems
The proportion of PIs triggered by software-related prescription errors was higher for non-certified (9.4%) than certified software (5.5%). In France, certification tests produced by the HAS are intended to technically assess the functionality of the software in various situations, as the CPOE evaluation methodology simulates various clinical scenarios.27 French regulations do not require CPOE developers to carry out usability studies before the systems are marketed. Nevertheless, despite the limitations of this type of certification criteria, which have already been highlighted,28 our results show that prescribing with CPOE-certified systems results in fewer prescription errors than prescribing with non-certified software. These results are consistent with those of other studies, i.e. all software is not equal and some is safer than others.29–31
Prescribers
The PISRE ratio was higher for prescriptions made by graduate prescribers (8.4%) than medical residents (7.8%) (p<0.001). This finding is, at first glance, counterintuitive, as one would expect that a prescriber who has been practising for several years in the same health facility would make fewer CPOE-related prescription errors with the software than a resident who has only been using the software for a few months. Observational studies show that medical residents make most prescriptions and transcribe them to the software prescription instructions of senior prescribers during the medical examination.32 It is thus possible that, in some hospitals, senior physicians are only occasional users of the prescription software. According to Nerich et al the occasional use of software (<1 prescription per day) is a risk factor for prescription error (OR=3.85, 95% CI 2.08 to 7.14).33 Tolley described how a junior doctor remarked that there was no one he could ask for help with using the ePrescribing system, as he was ‘the most experienced person on this floor with regards to the ePrescribing system’. She also described how one consultant admitted she had not ‘learnt how to prescribe properly’ because she did not ‘use the system often enough and regularly enough to know the quirks and tweaks’. This consultant relied on her junior staff to prescribe on the system.34
Act-IP pharmacist’ users
The PISRE ratio for senior pharmacists (9.2%) was higher than that of pharmacy residents (5.4%). This is consistent with the results of a study performed in a UK teaching hospital showing that the likelihood of senior pharmacists identifying errors was greater than that of junior pharmacists35 and in accordance with our expectations. A study concerning French pharmacy students showed that they trust the contribution of computerisation to healthcare without critical analysis. This results in overconfidence in the computer tool, perceived to be reliable, and makes users less willing to search for the errors produced by this tool.36 They are therefore not aware that the review of computerised prescription orders requires additional effort to identify prescription errors. This is the consequence of the lack of teaching/training about this subject in French pharmacy schools. This situation contrasts strikingly with the content of the curricula taught in the UK and USA, for example.37 38
Prescriber acceptance rate
The rate of acceptance of PISREs by prescribers was 78.9% vs 67.6% for other PIs. This suggests that prescribers recognise the relevance of such interventions due to the potential clinical consequences of such prescription errors. This rate varies from 65.9% to 92% in studies of drug errors induced by computerised prescription,10 14 suggesting that physicians consider the potential clinical consequences of SRE to patients to be more frequently serious than interventions unrelated to CPOE. In light of our findings, a CPOE-related prescription error is a factor that favours acceptance of the PI. These points warrant further studies.
Limits
Our study had several limitations. First, our work is based on declarative data. These interventions are performed by hospital pharmacist and entered on Act-IP website on a voluntary basis. Therefore, these PIs highlight prescription problems, but are not exhaustive. Moreover, our team annually analyses the quantitative and qualitative evolution of the data recorded on the Act-IP website (unpublished data). We observed that data entry can be irregular or performed with a delay. Indeed, data can be conditioned by pharmacist workload. For example, many pharmacists record prospectively their data on paper on a daily basis and thereafter register them by series on Act-IP. Data entry can also be total on a given period and can stop during a change of assignment. We consider that these elements have consequences on the quantity of recorded data but not on their quality. However, as illustrated by publications related to other databases on information technology incidents, despite their limitations, studies based on voluntary reports remain relevant to examine the nature of technology safety problems.39 40 Moreover, the large sample size probably provides a relatively precise vision of the problem at the national level. Second, several pharmacists analysing the same drug prescriptions may not all track down the same problems. One of major determinant of a PI is the knowledge of the pharmacist who analyses the prescription. It is this knowledge that enables him to detect a problem. Thus, a PI that is considered as necessary and is not performed means that it is not recorded and will be absent from the database. This happens when a doctor routinely makes a certain type of prescribing error and the pharmacist fails to detect it.41 It has been shown that, if several pharmacists analyse the same drug prescriptions, they do not all track down the same problems. In a study involving 57 hospital pharmacies, the mean percentage of detected prescribing errors was 59%, with a broad range of 7%–88% between pharmacies.42 In the absence of specific studies to determine the performance of pharmacists in detecting prescription errors induced by CPOE-system flaws and misuse, we are reduced to simply assuming that such variation may be observed. In addition, there are various definitions of PISREs in the literature.13–16 This suggests that there is a certain level of subjectivity when a pharmacist characterises a PI as being related to a computer-generated prescription. Among hospitals that entered the PIs on Act-IP, 87 never qualified a PI as being an SRE. There are two possible explanations for this observation. The first, and relatively unlikely, is that the software is near perfect and that there was no misuse by prescribers. For example, the absence of PISREs for these hospitals could result from the absence of computer-related errors due to the use of high-performance software and/or appropriately trained prescribers. The second possibility is that pharmacists do not establish a link between certain prescription errors and misuse of the prescription software and/or its design flaws. Conversely, a high rate of PISREs for a given hospital may result from software conception flaws and/or misuse of the software by prescribers and pharmacists who are very aware of the role of CPOE-systems in generating prescription errors. Regardless of the considered scenario, it is important to remember that differences in PISRE rates may also be due to the quality of the training provided. Studies have shown that insufficient training on an ePrescribing system can contribute to errors.43 44 Tolley illustrated how pharmacists did not receive any formal training about the system after starting at a hospital trust and observed that no formal training was offered when pharmacists changed roles. It has been shown that training plays a role in the users’ experience but there is a lack of published research in this area.34 Thus, further research is warranted to lift the veil on these unknowns.
Our results highlight that prescribing problems related to computer software are common in France. This is a concern that affects most (if not all) CPOE systems currently being used and therefore all hospitals, to varying degrees. Identifying the most dangerous software appears to be a priority to improve the quality and safety of patient care.
Conclusion
Computer-related prescribing errors are common, with wrong dose being the most frequent type of error. Such errors concern all drug classes and have potentially serious adverse clinical consequences if they are not intercepted by pharmacists when performing their daily medication review. The message appears to be well received by prescribers who agree to change their prescription more frequently than for PIs not related to CPOE use. CPOE medication review requires additional pharmacist diligence to catch such errors. As the PISRE ratio is significantly lower for certified software, patient safety agencies should undertake studies to identify the safest software so as to discard software that is potentially dangerous.
Supplementary Material
Acknowledgments
The authors would like to thank the team of THEMAS and VIP working group for assistance in this project. We thank the clinical pharmacists of the SFPC Act-IP group who participated in the data collection. Members of the working group ‘Valorization of Pharmaceutical Interventions/ Valorisation des Interventions Pharmaceutiques – Act-IP’ of the French Society for Clinical Pharmacy: Pierrick Bedouch (Grenoble), Magalie Bourdelin (Villefranche-sur-Saone), Bruno Charpiat (Lyon), Ornella Conort (Paris), Julien Gravoulet (Leyr), Audrey Janoly-Dumenil (Lyon), Michel Juste (Epernay), and Céline Mongaret (Reims). We thank Kévin Mastrorillo, technical consultant of the Act-IP observatory, for his contribution to the data extraction and data management.
Footnotes
Collaborators: Clinical pharmacists of the SFPC Act-IP group who participated in the data collection: S. Abkhtaoui-Couriat (Corbie), B. Allard-Latour (Saint-Genis-Laval), C. Andrieu (Saint-Etienne), X. Armoiry (Lyon), E. Armoiry (Villeurbanne), D. Attivi (Neufchâteau), L. Audibert (Alix), A. Barbet (Amiens), M. Bascoulergue (Aulnay sous bois), C. Basselin (Saint-Genis-Laval), F. Baud (Paris), P. Bedouch (Grenoble), M. Belhout (Amiens), S. Benhaoua (Saint Denis), J. Beny (Alix), S. Berthet (Lyon), J. Berthou (Besancon), D. Bichard (Besancon), A.C. Blandin (Besancon), E. Blondel (Aix les Bains), S. Bonn Loue (Luneville), A. Bonvin (Lyon), F. Bouchand (Garches), P. Bouniot (Francheville), M. Bourdelin (Besancon), C. Bouret (Lyon), L. Bourguignon (Lyon), C. Bourne (Saint-Egrève), M. Bouteille (Lyon), J. Burdin (Lyon), C. Bureau (Alix), C. Bureau (Villeurbanne), M. Burgin (Luneville), M. Buyse (Paris), E. Cabaret (Hyeres), D. Cabelguenne (Pierre Benite), C.Capele (Saint André lez Lille), D. Carli (Vienne), I. Carpentier (Saint-Genis-Laval), E. Chambrey (Rang-du-Fliers), S. Chantel (Pierre Benite), N. Charhon (Vienne), B. Charpiat (Lyon), M. Chaumont (Le Chesnay), K. Civiletti (Martigues), B. Clerc (Besancon), M. Cleve (Vienne), R. Colomb (Saint-Etienne), C. Combe (Saint-Etienne), O. Conort (Paris), R. Contreras (Besancon), S. Crepin (Limoges), M. Creusat-Aube (Illkirch-Graffenstaden), A. Cuoq (Lyon), C. Decourcelle (Lomme), T. Delanoy (Vienne), C. Derharoutunian (Vienne), A. Deronze (Lyon); M. Desseignet (Lyon), S. Diallo (Le Chesnay), L. Dietrich (Strasbourg), A. Dory (Strasbourg), J. Dos-Reis (Paris), N. Duarte (Draveil), M.O. Duzanski (Strasbourg), L. Escofier (Mayenne), F. Fabre (Clermont-Ferrand), S. Fare (Paris), J. Fillon (Paris), A. Fonteneau (Amiens), A. Fouquet (Vienne); A. Gadot (Lyon), H. Galtier (Vienne); I. Garreau (Epernay), C. Gerard (Francheville), R. Gervais (Saint Denis), O. Gloulou (Saint Denis), I. GraguebChatti (Vienne), A. Grass (Lyon), I. Gremeau (Clermont-Ferrand), P.Y. Grosse (Grasse), C. Guenaire (Rennes), F. Guerin (Aix les Bains), A. Guillermet (Lyon), S. Hannou (Illkirch-Graffenstaden), A. Henry (Lyon), G. Herbin (Bayeaux), N. Herment (Epernay), A. JanolyDumenil (Pierre Benite), C. Jarre (Vienne), L. Jovenaux (Martigues), M. Juste (Epernay), A.S. Kaczmarek (Clermont-Ferrand), W. KiniMatondo (Saint Denis), H. Labrosse (Lyon), C. Laillier (Strasbourg), E. Lamarre (Saint-Etienne), J. Lamoureux (Lyon), M. Laurent (Lyon), A. Le Bris (Le Chesnay), M. Le Duff (Rennes), R. Lecointre (Saint-Etienne), J. Lecompte (Grasse), M. Lefebvre (Lyon), A.L. Lepetit (Epernay), H. Lepont-Gilardi (Rennes), A. Lescoat (Villeurbanne), J.P. Levillain (Migennes), G. Liguori (Clermont-Ferrand), C. Lohier (Villeurbanne), C. Lupo (Lyon), J. Machon (Lyon), K. Maes (Vienne), G. Magerand (Villeurbanne), K. Mangerel (Epernay), S. Martelet (Saint-Etienne), D. Matanza (Francheville), V. Mermet (Saint-Genis-Laval), C. Mouchoux (Villeurbanne), Y. Nivoix (Strasbourg), A. Orly (Paris), E. Orng (Lyon), A. Oufella (Aulnay Sous Bois), I. Paillole (Toulouse), D. Pallot (Saint Denis), A. Papon (Lyon), L. Parnet (Paris), M. Paysant (Saint-Genis-Laval), E. Perrier-Cornet (Illkirch-Graffenstaden), S. Perrin (Besancon), D. Peynaud (Lyon), B.N. Pham (Vienne), D. Piney (Luneville), A. Pohyer (Montpellier), C. Porot (Besancon), J. Pouzoulet (Créteil), L. Poy (Lyon), E. Prevost (Epernay), E. Prunier (Besancon), F. Ranchon (Lyon), M. Rave (Besancon), C. Remonnay (Besancon), M. Remy (Ho-Chi-Minh Ville), M. Rhalimi (Chaumont-en-Vexin), C. Rioufol (Pierre Benite), A. Robelet (Paris), S. Roche (Epernay), F.X. Rose (Saint-Avé), R. Roubille (Vienne), A. Sambarino (Bourgoin Jallieu), D. Sankhare (Saint Denis), R. Santucci (Strasbourg), J. Scholler (Strasbourg), R. Selmi (Saint Denis), C. Stamm (Pierre Benite), C. Tanguy (Brest), D. Tessier (Saint Denis), H. Thery (Rang-du-Fliers), N. Thiriat (Paris), C. Turci (Saint-Genis-Laval), N. Vantard (Lyon), N. Vauvarin (Joigny), S.Vernardet (Annonay), D. Viard (Besancon), C. Vignand (Lyon), C. Villa (Vienne), P. Vonna (Epernay), S. Wacker (Strasbourg), N. Wereszczynski (Grasse), and L. Zerhouni (Paris).
Contributors: MV and BC designed the study, performed the statistical analyses, interpreted the results and wrote the first version of the manuscript. CV contributed to the design of the study, performed the statistical analyses and revised the manuscript. J-LB contributed to the design of the study and revised the manuscript. OC contributed substantially to the interpretation of the data and contributed to the revision of the manuscript. PB designed the study, performed the statistical analyses, interpreted the results and revised the manuscript.
Funding: This study was supported by The French Society of Clinical Pharmacy, a nonprofit and independent foundation for clinical pharmacy research and development.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the SFPC VIP– Act-IP group:
S. Abkhtaoui-Couriat, B. Allard-Latour, C. Andrieu, X. Armoiry, E. Armoiry, D. Attivi, L. Audibert, A. Barbet, M. Bascoulergue, C. Basselin, F. Baud, P. Bedouch, M. Belhout, S. Benhaoua, J. Beny, S. Berthet, J. Berthou, D. Bichard, A.C. Blandin, E. Blondel, S. Bonn Loue, A. Bonvin, F. Bouchand, P. Bouniot, M. Bourdelin, C. Bouret, L. Bourguignon, C. Bourne, M. Bouteille, J. Burdin, C. Bureau, C. Bureau, M. Burgin, M. Buyse, E. Cabaret, D. Cabelguenne, D. Carli, I. Carpentier, E. Chambrey, S. Chantel, N. Charhon, B. Charpiat, M. Chaumont, K. Civiletti, B. Clerc, M. Cleve, R. Colomb, C. Combe, O. Conort, R. Contreras, S. Crepin, M. Creusat-Aube, A. Cuoq, C. Decourcelle, T. Delanoy, C. Derharoutunian, A. Deronze, M. Desseignet, S. Diallo, L. Dietrich, A. Dory, J. Dos-Reis, N. Duarte, M.O. Duzanski, L. Escofier, F. Fabre, S. Fare, J. Fillon, A. Fonteneau, A. Fouquet, A. Gadot, H. Galtier, I. Garreau, C. Gerard, R. Gervais, O. Gloulou, I. GraguebChatti, A. Grass, I. Gremeau, P.Y. Grosse, C. Guenaire, F. Guerin, A. Guillermet, S. Hannou, A. Henry, G. Herbin, N. Herment, A. JanolyDumenil, C. Jarre, L. Jovenaux, M. Juste, A.S. Kaczmarek, W. KiniMatondo, H. Labrosse, C. Laillier, E. Lamarre, J. Lamoureux, M. Laurent, A. Le Bris, M. Le Duff, R. Lecointre, J. Lecompte, M. Lefebvre, A.L. Lepetit, H. Lepont-Gilardi, A. Lescoat, J.P. Levillain, G. Liguori, C. Lohier, C. Lupo, J. Machon, K. Maes, G. Magerand, K. Mangerel, S. Martelet, D. Matanza, V. Mermet, C. Mouchoux, Y. Nivoix, A. Orly, E. Orng, A. Oufella, I. Paillole, D. Pallot, A. Papon, L. Parnet, M. Paysant, E. Perrier-Cornet, S. Perrin, D. Peynaud, B.N. Pham, D. Piney, A. Pohyer, C. Porot, J. Pouzoulet, L. Poy, E. Prevost, E. Prunier, F. Ranchon, M. Rave, C. Remonnay, M. Remy, M. Rhalimi, C. Rioufol, A. Robelet, S. Roche, F.X. Rose, R. Roubille, A. Sambarino, D. Sankhare, R. Santucci, J. Scholler, R. Selmi, C. Stamm, C. Tanguy, D. Tessier, H. Thery, N. Thiriat, C. Turci, N. Vantard, N. Vauvarin, D. Viard, C. Vignand, C. Villa, P. Vonna, S. Wacker, N. Wereszczynski, and L. Zerhouni
Data availability statement
Data are available on reasonable request. Deidentified participant data are available upon reasonable request to Act-IP© Administrator (email address : actip@sfpc.eu).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical approval for the study was obtained on 19 February 2020 (CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand, IRB 5891).
References
- 1. Lewis PJ, Dornan T, Taylor D, et al. Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf 2009;32:379–89. 10.2165/00002018-200932050-00002 [DOI] [PubMed] [Google Scholar]
- 2. Ashcroft DM, Lewis PJ, Tully MP, et al. Prevalence, nature, severity and risk factors for prescribing errors in hospital inpatients: prospective study in 20 UK hospitals. Drug Saf 2015;38:833–43. 10.1007/s40264-015-0320-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vélez-Díaz-Pallarés M, Álvarez Díaz AM, Gramage Caro T, et al. Technology-induced errors associated with computerized provider order entry software for older patients. Int J Clin Pharm 2017;39:729–42. 10.1007/s11096-017-0474-y [DOI] [PubMed] [Google Scholar]
- 4. Radley DC, Wasserman MR, Olsho LE, et al. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc 2013;20:470–6. 10.1136/amiajnl-2012-001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nuckols TK, Smith-Spangler C, Morton SC, et al. The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev 2014;3:56. 10.1186/2046-4053-3-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA 2005;293:1197–203. 10.1001/jama.293.10.1197 [DOI] [PubMed] [Google Scholar]
- 7. Brown CL, Mulcaster HL, Triffitt KL, et al. A systematic review of the types and causes of prescribing errors generated from using computerized provider order entry systems in primary and secondary care. J Am Med Inform Assoc 2017;24:432–40. 10.1093/jamia/ocw119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horsky J, Kuperman GJ, Patel VL. Comprehensive analysis of a medication dosing error related to CPOE. J Am Med Inform Assoc 2005;12:377–82. 10.1197/jamia.M1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Staff PE. Electronic prescribing: the risk of errors and adverse effects. Prescrire Int 2016;25:24–7. [PubMed] [Google Scholar]
- 10. Vialle V, Tiphine T, Poirier Y, et al. [To know, understand and combating medication errors related to computerized physician order entry]. Ann Pharm Fr 2011;69:165–76. 10.1016/j.pharma.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 11. Direction Générale de l’offre de Soins . 2018 HIS Atlas. State of the art of hospital information systems [online], 2018. Available: https://solidarites-sante.gouv.fr/IMG/pdf/dgos_atlas_sih_2018.pdf [Accessed 9 Nov 2019].
- 12. Westbrook JI, Reckmann M, Li L, et al. Effects of two commercial electronic prescribing systems on prescribing error rates in hospital in-patients: a before and after study. PLoS Med 2012;9:e1001164. 10.1371/journal.pmed.1001164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouchand F, Thomas A, Zerhouni L. Pharmaceutical interventions before and after computerization of the prescription in an internal medicine department. Presse Médicale 2007;36:410–8. [DOI] [PubMed] [Google Scholar]
- 14. Hellot-Guersing M, Jarre C, Molina C, et al. [Medication errors related to computerized physician order entry at the hospital: Record and analysis over a period of 4 years]. Ann Pharm Fr 2016;74:61–70. 10.1016/j.pharma.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 15. Westbrook JI, Baysari MT, Li L, et al. The safety of electronic prescribing: manifestations, mechanisms, and rates of system-related errors associated with two commercial systems in hospitals. J Am Med Inform Assoc 2013;20:1159–67. 10.1136/amiajnl-2013-001745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sittig DF, Ash JS, Zhang J, et al. Lessons from "Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system". Pediatrics 2006;118:797–801. 10.1542/peds.2005-3132 [DOI] [PubMed] [Google Scholar]
- 17. Charpiat B, Bedouch P, Conort O, et al. [Opportunities for medication errors and pharmacist’s interventions in the context of computerized prescription order entry: a review of data published by French hospital pharmacists]. Ann Pharm Fr 2012;70:62–74. 10.1016/j.pharma.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 18. Ranji SR, Rennke S, Wachter RM. Computerised provider order entry combined with clinical decision support systems to improve medication safety: a narrative review. BMJ Qual Saf 2014;23:773–80. 10.1136/bmjqs-2013-002165 [DOI] [PubMed] [Google Scholar]
- 19. Korb-Savoldelli V, Boussadi A, Durieux P, et al. Prevalence of computerized physician order entry systems-related medication prescription errors: a systematic review. Int J Med Inform 2018;111:112–22. 10.1016/j.ijmedinf.2017.12.022 [DOI] [PubMed] [Google Scholar]
- 20. Allenet B, Bedouch P, Rose F-X, et al. Validation of an instrument for the documentation of clinical pharmacists’ interventions. Pharm World Sci 2006;28:181–8. 10.1007/s11096-006-9027-5 [DOI] [PubMed] [Google Scholar]
- 21. Act-IP . Standardisation et Valorisation des Activités de Pharmacie Clinique – Descriptif [online]. Available: http://www.actip.sfpc.eu/actip/index/ficheip/ [Accessed 1 Apr 2010].
- 22. Bedouch P, Sylvoz N, Charpiat B, et al. Trends in pharmacists' medication order review in French hospitals from 2006 to 2009: analysis of pharmacists' interventions from the Act-IP© website observatory. J Clin Pharm Ther 2015;40:32–40. 10.1111/jcpt.12214 [DOI] [PubMed] [Google Scholar]
- 23. Haute Autorité de Santé . Logiciels d'Aide la Prescription hospitaliers [online], 2019. Available: https://www.has-sante.fr/jcms/c_1751516/fr/logiciels-d-aide-a-la-prescription-hospitaliers [Accessed 9 Nov 2019].
- 24. van der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006;13:138–47. 10.1197/jamia.M1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wetterneck TB, Walker JM, Blosky MA, et al. Factors contributing to an increase in duplicate medication order errors after CPOE implementation. J Am Med Inform Assoc 2011;18:774–82. 10.1136/amiajnl-2011-000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marcilly R, Ammenwerth E, Vasseur F, et al. Usability flaws of medication-related alerting functions: a systematic qualitative review. J Biomed Inform 2015;55:260–71. 10.1016/j.jbi.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 27. Haute Autorité de Santé . Référentiel de certification des logiciels hospitaliers par essai de type [online], 2012. Available: https://www.has-sante.fr/upload/docs/application/pdf/2012-06/referentiel_certification_lap_hospitalier_juin12.pdf [Accessed 6 Oct 2019].
- 28. Prescrire Rédaction . Prescription informatisée: un outil encore expérimental, trop peu régulé. Rev Prescrire 2015;35:938–9. [Google Scholar]
- 29. Nanji KC, Rothschild JM, Salzberg C, et al. Errors associated with outpatient computerized prescribing systems. J Am Med Inform Assoc 2011;18:767–73. 10.1136/amiajnl-2011-000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holmgren AJ, Adler-Milstein J, McCullough J. Are all certified EHRs created equal? Assessing the relationship between EHR vendor and hospital meaningful use performance. J Am Med Inform Assoc 2018;25:654–60. 10.1093/jamia/ocx135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ratwani RM, Fairbanks RJ, Hettinger AZ, et al. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc 2015;22:1179–82. 10.1093/jamia/ocv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaensch SL, Baysari MT, Day RO, et al. Junior doctors’ prescribing work after-hours and the impact of computerized decision support. Int J Med Inform 2013;82:980–6. 10.1016/j.ijmedinf.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 33. Nerich V, Limat S, Demarchi M, et al. Computerized physician order entry of injectable antineoplastic drugs: an epidemiologic study of prescribing medication errors. Int J Med Inform 2010;79:699–706. 10.1016/j.ijmedinf.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 34. Tolley CL. An investigation of healthcare professionals' experiences of training and using electronic prescribing systems: four literature reviews and two qualitative studies undertaken in the UK hospital context, Durham theses, Durham University. available at Durham E-Theses online, 2018. Available: http://etheses.dur.ac.uk/12845/
- 35. Tully MP, Buchan IE. Prescribing errors during hospital inpatient care: factors influencing identification by pharmacists. Pharm World Sci 2009;31:682–8. 10.1007/s11096-009-9332-x [DOI] [PubMed] [Google Scholar]
- 36. Charpiat B, Derfoufi S, Larger M, et al. [Identification of knowledge deficits of pharmacy students at the beginning of the fifth year of pharmacy practice experience: Proposals to change the content of academic programs]. Ann Pharm Fr 2016;74:404–12. 10.1016/j.pharma.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 37. Pontefract SK, Wilson K. Using electronic patient records: defining learning outcomes for undergraduate education. BMC Med Educ 2019;19:30. 10.1186/s12909-019-1466-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Accreditation Council of Pharmacy Education . Accreditation standards and key elements for the professional program in pharmacy leading to the doctor of pharmacy degree standards [online], 2016. Available: https://www.acpe-accredit.org/pdf/Standards2016FINAL.pdf [Accessed 13 Oct 2019].
- 39. Magrabi F, Ong M-sing, Runciman W, et al. Patient safety problems associated with heathcare information technology: an analysis of adverse events reported to the US food and drug administration. AMIA Annu Symp Proc 2011;2011:853–7. [PMC free article] [PubMed] [Google Scholar]
- 40. Magrabi F, Ong M-S, Runciman W, et al. Using FDA reports to inform a classification for health information technology safety problems. J Am Med Inform Assoc 2012;19:10.1136/amiajnl-2011-000369:45–53. 10.1136/amiajnl-2011-000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Charpiat B, Bedouch P, Tod M, et al. Classifying pharmacists' interventions recorded in observational databases: are they all necessary and appropriate? Res Social Adm Pharm 2017;13:1184–5. 10.1016/j.sapharm.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 42. Beex-Oosterhuis MM, de Vogel EM, van der Sijs H, et al. Detection and correct handling of prescribing errors in Dutch Hospital pharmacies using test patients. Int J Clin Pharm 2013;35:1188–202. 10.1007/s11096-013-9848-y [DOI] [PubMed] [Google Scholar]
- 43. Schiff GD, Amato MG, Eguale T, et al. Computerised physician order entry-related medication errors: analysis of reported errors and vulnerability testing of current systems. BMJ Qual Saf 2015;24:264–71. 10.1136/bmjqs-2014-003555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Condren M, Honey BL, Carter SM, et al. Influence of a systems-based approach to prescribing errors in a pediatric resident clinic. Acad Pediatr 2014;14:485–90. 10.1016/j.acap.2014.03.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-045778supp001.pdf (1.1MB, pdf)
bmjopen-2020-045778supp002.pdf (65.3KB, pdf)
Data Availability Statement
Data are available on reasonable request. Deidentified participant data are available upon reasonable request to Act-IP© Administrator (email address : actip@sfpc.eu).