Abstract
Farnesyltransferase inhibitors (FTIs) represent a novel class of anticancer drugs that exhibit a remarkable ability to inhibit malignant transformation without toxicity to normal cells. However, the mechanism by which FTIs inhibit tumor growth is not well understood. Here, we demonstrate that FTI-277 inhibits phosphatidylinositol 3-OH kinase (PI 3-kinase)/AKT2-mediated growth factor- and adhesion-dependent survival pathways and induces apoptosis in human cancer cells that overexpress AKT2. Furthermore, overexpression of AKT2, but not oncogenic H-Ras, sensitizes NIH 3T3 cells to FTI-277, and a high serum level prevents FTI-277-induced apoptosis in H-Ras- but not AKT2-transformed NIH 3T3 cells. A constitutively active form of AKT2 rescues human cancer cells from FTI-277-induced apoptosis. FTI-277 inhibits insulin-like growth factor 1-induced PI 3-kinase and AKT2 activation and subsequent phosphorylation of the proapoptotic protein BAD. Integrin-dependent activation of AKT2 is also blocked by FTI-277. Thus, a mechanism for FTI inhibition of human tumor growth is by inducing apoptosis through inhibition of PI 3-kinase/AKT2-mediated cell survival and adhesion pathway.
Small G proteins such as Ras, Rho, and Rac have been shown to regulate a wide spectrum of cellular functions, including cytoskeletal organization, membrane trafficking, transcriptional activation, and cellular transformation. Recent studies demonstrated that the effects of Ras proteins on cytoskeleton and membrane trafficking are important in establishing and maintaining the transformed phenotype (61, 73). Many types of extracellular signals, especially those involving activation of receptor tyrosine kinase and integrin receptor, trigger activation of small G proteins, which in turn activate a variety of signalings. Ras, which is frequently mutated in human tumors, activates several signaling pathways, including the Raf/mitogen-activated protein kinase cascade and the phosphatidylinositol 3-kinase (PI 3-kinase)/Akt pathway, resulting in malignant transformation in rodent fibroblasts (57, 60, 73, 74). It has also been documented that Rac, Rho, and Cdc42 stimulate cell cycle progression and display transforming and oncogenic potential in some cell lines (72). Cells expressing constitutively active mutants of Rac and Rho exhibited a malignant transformation phenotype (37). In addition, Rho and Rac have been shown to be essential for Ras transformation (37, 58). Recent studies demonstrated that small G proteins are pivotal mediators in integrin-mediated cell motility and invasiveness of human tumor cell lines (36, 72). Therefore, pharmacological inhibition of small G protein function is a rational approach for the prevention and treatment of human malignancies.
One approach is inhibition of small G protein prenylation, a lipid posttranslational modification that is critical to cellular localization and function of the proteins (19, 28, 64). Farnesyltransferase (FTase) and geranylgeranyltransferase (GGTase) I have been shown to catalyze protein prenylation (19, 28, 64, 77). FTase catalyzes the transfer of farnesyl from farnesylpyrophosphate to a cysteine at the carboxyl terminus of proteins ending in CAAX, where C is cysteine, A is an aliphatic amino acid, and X is methionine, serine, cysteine, or glutamine. GGTase I, on the other hand, transfers geranylgeranyl from geranylgeranylpyrophosphate to CAAX terminal sequences where X is leucine or isoleucine. We and others developed two types of inhibitor, FTIs and GGTIs, to specifically target FTase and GGTase I, respectively (19, 28, 46, 47, 52, 64). FTIs show promise in blocking the tumor growth without toxicity to normal cells (19, 64, 69, 70). However, the mechanism by which FTIs contribute to inhibition of tumor cell growth is not known.
Several lines of evidence indicate that PI 3-kinase is required for Ras transformation and Ras-induced cytoskeletal reorganization (38, 61). Dominant-negative PI 3-kinase (p85Δ iSH2-N) strongly inhibits transformation by RasV12 (61). Moreover, active PI 3-kinase is sufficient to transform cells (13, 32, 42). Akt/protein kinase B (PKB), a subfamily of serine/threonine protein kinases, has been identified as a direct target of PI 3-kinase (21, 27). All three members, Akt/AKT1/PKBα, AKT2/PKBβ, and AKT3/PKBγ (8, 15, 33, 34, 42), of this family are activated by growth factors in a PI 3-kinase-dependent manner (3, 11, 27, 48, 51). Phosphorylation of Thr-308 (Thr-309 in AKT2) in the activation loop and Ser-473 (Ser-474 in AKT2) in the C-terminal activation domain is required for full activation of Akt and AKT2. 3-Phosphoinositide-dependent protein kinase 1 and integrin link kinase (ILK) have been found to phosphorylate Thr-308 and Ser-473 of Akt, respectively (2, 3, 23, 68). Recent studies demonstrated that the tumor suppressor PTEN/MMAC1 and SHIP, encoding dual-specificity {phosphatidylinositol-3,4,5-triphosphate [PI(3,4,5)P3] and tyrosine/threonine} phosphatases and inositol phosphatase, negatively regulate intracellular levels of PI(3,4,5)P3 in cells and thus inhibit the PI 3-kinase/Akt signaling pathway (5, 50, 67).
It has been shown that Akt induces cell survival and suppresses the apoptotic death of a number of cell types induced by a variety of stimuli, including growth factor withdrawal, cell cycle disruption, and loss of cell adhesion. Several downstream targets, containing the Akt phosphorylation consensus sequence R-X-R-X-X-S/T, have been identified as possible mechanisms by which Akt promotes cell survival and blocks apoptosis. One is glycogen synthase kinase 3 (GSK-3): Akt phosphorylates GSK-3 and leads to inactivation of GSK-3, accumulation of β-catenin, activation of c-myc transcription, and stabilization of cyclin D1 (20, 25, 30). Akt was also shown to phosphorylate the proapoptotic proteins BAD and caspase 9 and transcription factor FKHRL1, resulting in reduced binding of BAD to Bcl-XL, inhibition of caspase 9 protease activity, and decreased Fas ligand transcription, respectively (10, 12, 22, 24).
Among the three members of the Akt/PKB family, only AKT2 has been implicated in several types of human malignancy. In particular, alterations of AKT2 have been detected in 10 to 20% of ovarian carcinomas and pancreatic cancers (7, 15, 16, 54, 62). Overexpression of AKT2 in NIH 3T3 cells resulted in a transformed phenotype (17). Moreover, the antisense of AKT2 can significantly inhibit the invasiveness and tumorigenesis of pancreatic cancer cells overexpressing this gene (16). We have recently demonstrated that AKT2 is significantly activated by the active form of Ras, and this activation is partially inhibited by wortmannin. Moreover, dominant-negative Ras N17 blocks growth factor-induced activation of AKT2 (48), suggesting that Ras is an essential mediator for AKT2 activation. In this report, we provide evidence that FTI-277 targets PI 3-kinase/AKT2 cell survival and cell adhesion pathway and induces apoptosis in human cancer cell lines that overexpress AKT2.
MATERIALS AND METHODS
Cell lines and transfection.
Human ovarian epithelial cancer cell lines OVCAR-3, OVCAR-4, OVCAR-5, OVCAR-8, and A2780 and pancreatic cancer cell lines PANC-1, ASPC-1, CAPAN-2, and COLO-357 were provided by T. C. Hamilton and A. Klein-Szanto (Fox Chase Cancer Center). The COS7 cell line was obtained from the American Type Culture Collection. The cells were cultured at 37°C in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS). Transfections were performed by the calcium phosphate method. For stable transfection, transfected OVCAR-3 cells were selected with G418 at the final concentration of 600 μg/ml.
Plasmid constructs.
Hemagglutin epitope (HA)-tagged AKT2 (HA-AKT2) and HA-E299K-AKT2 were prepared as described previously (48, 53). The constitutively active pcDNA3-m/p-HA-AKT2 construct was made by PCR to add 12 amino acids derived from the N terminus of the Lck tyrosine kinase to the N terminus of HA-AKT2. The PCR fragment was subcloned as an EcoRI/XbaI fragment into pcDNA3 vector. The glutathione S-transferase (GST)-BAD, GST-BADS112A, GST-BADS136A, and GST-BADS2A constructs were kindly provided by Michael E. Greenberg (Harvard Medical School). Wild-type and constitutively active (RhoB-V14), forms of RhoB were obtained from Alan Hall (University College, London, United Kingdom).
TUNEL assay and DNA fragmentation.
Cells were seeded into 60-mm-diameter dishes and grown in DMEM supplemented with 5% FCS for 24 h and then treated with FTI-277 at concentration of 30 μM for 48 h. Apoptosis was determined by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) by using an in situ cell death detection kit (Boehringer Mannheim, Indianapolis, Ind.). The cells were trypsinized, and cytospin preparations were obtained. Cells were fixed with freshly prepared paraformaldehyde (4% in phosphate-buffered saline [PBS], pH 7.4). Slides were rinsed with PBS, incubated in permeabilization solution, and cross-reacted with TUNEL reaction mixture for 60 min at 37°C in a humidified chamber. Following a rinse, the slides were reacted with converter-alkaline phosphatase solution for 30 min at 37°C and then detected with alkaline phosphatase substrate solution (Vector Laboratories, Burlingame, Calif.) for 10 min. After an additional rinse, the slides were mounted and analyzed under a light microscope. These experiments were performed in duplicate. To detect DNA fragmentation, cellular DNA was prepared by using a blood and cell culture mini DNA kit (Qiagen). The DNA was analyzed on 1.5% agarose gel and visualized by ethidium bromide staining.
GST fusion proteins.
GST-BAD fusion proteins were purified as previously described (17). Briefly, logarithmically growing cultures of Escherichia coli JM83 transformed with the pGEX-3X recombinants were incubated with 0.1 mM isopropyl-β-d-thiogalactopyranoside at 37°C for 6 h. The cells were pelleted, resuspended in cold PBS, and sonicated on ice. Debris was removed by centrifugation, and the supernatant was applied to a glutathione-Sepharose 4B column (Pharmacia). GST-BAD fusion proteins were eluted and used as the substrate (5 μg/reaction) for AKT2 in vitro kinase assay.
Immunoprecipitation and immunoblotting.
Cells were lysed in a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 15% (vol/vol) glycerol, 1% NP-40, 2 mM phenylmethylsulfonyl fluoride, 2 μg each of aprotinin and leupeptin per ml, 2 mM benzamidine, 20 mM NaF, 10 mM NaPPi, 1 mM sodium vanadate, and 25 mM β-glycerolphosphate. Lysates were centrifuged at 12,000 × g for 15 min at 4°C prior to immunoprecipitation or Western blotting. Equal amounts of the lysates were analyzed for protein expression and enzyme activity. For immunoprecipitation, lysates were precleared with protein A-protein G (2:1) agarose beads at 4°C for 20 min. Following removal of the beads by centrifugation, lysates were incubated with anti-AKT2 monoclonal antibody (17), anti-HA monoclonal antibody 12CA5 (Boehringer Mannheim), anti-p85 antibody (Santa Cruz), or anti-BAD antibody (Santa Cruz) in the presence of 30 μl of protein A-protein G (2:1) agarose beads (GibcoBRL) for 2 h at 4°C. The beads were washed once with 50 mM Tris-HCl (pH 7.5)–0.5 M LiCl–0.5% Triton X-100, twice with PBS, and once with 10 mM Tris-HCl (pH 7.5)–10 mM MgCl2–10 mM MnCl2–1 mM dithiothreitol, all containing 20 mM β-glycerolphosphate and 0.1 mM sodium vanadate. Immunoprecipitates were subjected to in vitro kinase assay or Western blotting analysis. Protein expression and phosphorylation were determined by probing Western blots of immunoprecipitates or total cell lysate with the anti-HA, anti-AKT2, antiphosphotyrosine (anti-p-Tyr; 4G10; Upstate Biotechnology, Inc.), or anti-phospho-BAD (New England Biolabs) antibody. Antigen-bound antibody was detected by enhanced chemiluminescence Western blotting analysis (Amersham).
In vitro protein kinase assay.
The AKT2 kinase assay was performed as previously described (17). Briefly, the reaction was carried out in the presence of 10 μCi of [γ-32P]ATP (NEN) and 3 μM unlabeled ATP in 30 μl of buffer containing 20 mM HEPES (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, and 1 mM dithiothreitol. Histone H2B was used as the exogenous substrate. After incubation at room temperature for 30 min, the reaction was stopped by adding protein loading buffer and the mixture was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Each experiment was repeated three times. The relative amounts of incorporated radioactivity were determined by autoradiography and quantitated with a PhosphorImager (Molecular Dynamics).
PI 3-kinase assay.
The cells were washed, lysed, and immunoprecipitated with pan-p85 or anti-P-Tyr (Ab-4; Oncogene) antibody (40). The immunoprecipitates were washed once with cold PBS, twice with 0.5 M LiCl–0.1 M Tris (pH 7.4), and finally with 10 mM Tris–100 mM NaCl–1 mM EDTA. The presence of PI 3-kinase activity in immunoprecipitates was determined by incubating the beads with reaction buffer containing 10 mM HEPES (pH 7.4), 10 mM MgCl2, 50 μM ATP, 20 μCi of [γ-32p]ATP, and 10 μg of l-α-phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2; Biomol] for 20 min at 25°C. The reactions were stopped by adding 100 μl of 1 M HCl. Phospholipids were extracted with 200 μl of CHCl3-MeOH. Phosphorylated products were separated by thin-layer chromatography as previously described (75). The conversion of PI(4,5)P2 to PI 3-phosphate was determined by autoradiography and quantitated with a PhosphorImager.
RESULTS
FTI-277 induces apoptosis in human cancer cells.
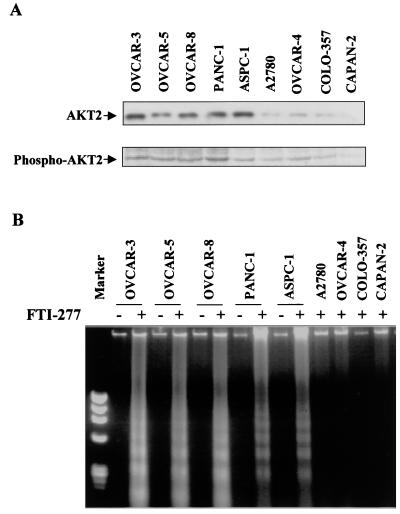
Previous studies showed that FTIs have a profound inhibitory effect on human tumor cell growth (19, 28, 64). However, the mechanism by which FTIs exert this effect is not well understood. The fact that AKT2 is frequently overexpressed in human ovarian and pancreatic carcinoma and significantly activated by growth factor and active Ras through PI 3-kinase prompted us to examine the involvement of the PI 3-kinase/AKT2 pathway in inhibition of tumor cell growth by FTIs. We initially treated two human ovarian cancer cell lines, one (OVCAR-3) overexpressing AKT2 and the other (A2780) expressing a low level of AKT2 (15) (Fig. 1A), with FTI-277 (30 μM) in DMEM medium supplemented with 5% FCS. After 48 h of treatment, OVCAR-3 cells underwent apoptosis detected by DNA ladder assay (Fig. 1B). However, apoptosis was not observed in A2780 cells (Fig. 1B), even though cell growth was inhibited (data not shown). We extended our study to another seven human cancer cell lines, consisting of three ovarian carcinoma and four pancreatic cancer lines. FTI-277-induced apoptosis was detected in all of the four AKT2-overexpressing cell lines (OVCAR-5, OVCAR-8, PANC-1, and ASPC-1) but not in cell lines expressing low levels of AKT2 (OVCAR-4, CAPAN-2, and COLO-357) (15, 16) (Fig. 1). These results suggest that the cancer cells overexpressing AKT2 are sensitive to FTI-277 and that FTI-277-induced apoptosis may result from inhibition of the PI 3-kinase/AKT2 pathway.
FIG. 1.
FTI-277 induces apoptosis in AKT2-overexpressing human cancer cell lines. (A) (Top) Western blot analysis of AKT2 expression in nine ovarian and pancreatic cancer cell lines. Equal amounts of protein were separated by SDS-PAGE and probed with an anti-AKT2 monoclonal antibody. Overexpression of AKT2 was observed in five cell lines (lanes 1 to 5). (Bottom) Western blot analyses of immunoprecipitates prepared from each cell line with monoclonal AKT2 antibody. The blots were detected with polyclonal anti-phospho-Akt-S473 antibody. Elevated levels of phosphorylated AKT2 were detected in AKT2-overexpressing cell lines. (B) Internucleosomal DNA fragmentation. The cells were seeded at 5 × 105 cells/60-mm-diameter dish in 5% FCS medium. After 24 h, the cells were treated with 30 μM FTI-277 (+) or DMSO (−) for 48 h. Genomic DNA was prepared and analyzed on a 1.5% agarose gel as described in Materials and Methods. Lane 1 shows DNA size markers (φX174 replicative-form DNA/HaeIII fragments; GibcoBRL). DNA fragmentation was detected in OVCAR-3, OVCAR-5, OVCAR-8, PANC-1, and ASPC-1 cell lines after FTI-277 treatment.
Ecotopic expression of wild-type AKT2 renders cells sensitive to FTI-277.
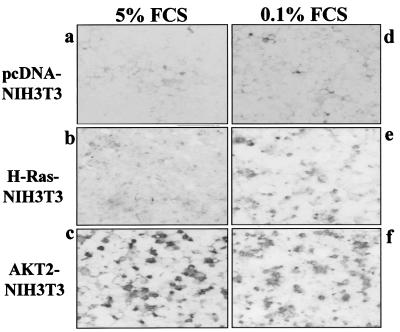
Recent reports demonstrated that FTIs are capable of inducing apoptosis of ras-transformed but not untransformed Rat1 and rat kidney cells only when the cells are deprived of serum or substratum attachment (44, 71). The percentages of FTI-induced apoptotic cells were 50 and 56 in v-H-ras-transformed Rat1 cells and v-K-ras-transformed rat kidney cells, respectively (44, 71). We previously showed that overexpression of wild-type AKT2 in NIH 3T3 cells resulted in a malignant phenotype (17). To test the hypothesis that overexpression of AKT2 renders the cells sensitive to FTIs, AKT2-transformed NIH 3T3 cells and NIH 3T3 cells transfected with the pcDNA3 vector alone were treated with FTI-277 (30 μM) in medium containing 0.1 or 5% FCS. H-ras-transformed NIH 3T3 cells were used as a control. Apoptosis was observed in AKT2- but not pcDNA3-transfected NIH 3T3 cells after 48 h of FTI-277 treatment at serum concentrations of both 0.1 and 5% (Fig. 2). Percentages of apoptotic cells were approximately 90 at 0.1% serum and 60 at 5% serum. However, apoptotic cells accounted for only ∼15% of H-ras-transformed NIH 3T3 cells at 0.1% FCS, and no apoptosis was detected in 5% FCS culture medium (Fig. 2). These data indicate that overexpressed wild-type AKT2 is more effective than oncogenic H-Ras at sensitizing cells to FTI-277-induced apoptosis. Furthermore, under high-serum (5% FCS) conditions, AKT2 but not H-Ras sensitizes cells to FTI-277. The effect of FTI-277 appears to be specific for AKT2 in NIH 3T3 cells since it was unable to induce apoptosis in Akt1-transfected NIH 3T3 cells (data not shown). This is possibly due to the fact that overexpression of Akt1 in NIH 3T3 cells does not result in malignant transformation (1, 17).
FIG. 2.
Overexpression of wild-type AKT2 sensitizes NIH 3T3 cells to FTI-277-induced apoptosis. A TUNEL assay was used to detect FTI-277-induced apoptosis in AKT2- or H-ras-transformed NIH 3T3 cells in a medium containing 5% FCS (a to c) or 0.1% FCS (d to f). After 48 h of FTI-277 treatment, apoptosis was detected in AKT2-transformed (c) but not H-ras-transformed (b) and pcDNA3-transfected (a) NIH 3T3 cells in 5% FCS medium. In 0.1% FCS culture medium, FTI-277-induced apoptosis was more prominent in AKT2 (f)- than H-ras (e)-transformed NIH 3T3 cells.
FTI-277 inhibits growth factor-induced PI 3-kinase/AKT2 activation in vitro and in vivo.
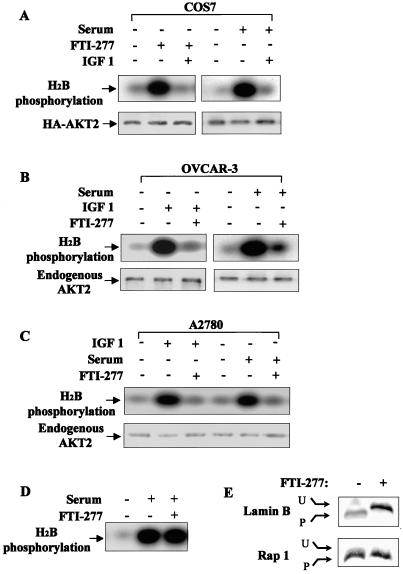
Several lines of evidence have shown that PI 3-kinase is required for Ras transformation (38, 61). Because FTI-277 preferentially induced apoptosis in AKT2-overexpressing cancer cells, we next examined whether FTI-277 inhibits growth factor-induced PI 3-kinase/AKT2 activation. COS7 cells were transiently transfected with pcDNA3-HA-AKT2. Serum-starved cells were treated with FTI-277 (30 μM) for 12 h prior to insulin-like growth factor 1 (IGF-1) stimulation for 10 min. Immunoprecipitation was carried out with an anti-HA monoclonal antibody, and the immunoprecipitates were subjected to in vitro kinase assay using histone H2B as the substrate. Repeated experiments revealed that IGF-1-induced AKT2 activation was effectively blocked by FTI-277 (Fig. 3A).
FIG. 3.
FTI-277 inhibits AKT2 activation. (A to C) In vitro kinase assay of immunoprecipitates from COS7 cells transfected with HA-AKT2 (A), OVCAR-3 cells (B), and A2780 cells (C). After serum starvation overnight, the cells were treated with or without FTI-277 for 12 h prior to IGF-1 (50 ng/ml) or 5% FCS stimulation for 10 min. (D) FTI-277 does not directly inhibit serum-induced AKT2 activation. An in vitro kinase assay was carried out with immunoprecipitates from OVCAR-3 cells. After serum starvation and restimulation, FTI-277 (30 μM) was directly added into kinase reaction (lane 3). (E) OVCAR-3 cell lysates were analyzed by SDS-PAGE followed by immunoblotting with an anti-lamin B or anti-Rap1A antibody. Lamin B is a substrate for FTase, whereas Rap1A is a substrate for GGTase I. FTI-277 prevented lamin B farnesylation, resulting in a band shift. Rap1A prenylation was unaffected by FTI-277. U, unprenylated form; P, prenylated form (prenylated proteins migrate faster in an SDS-polyacrylamide gel).
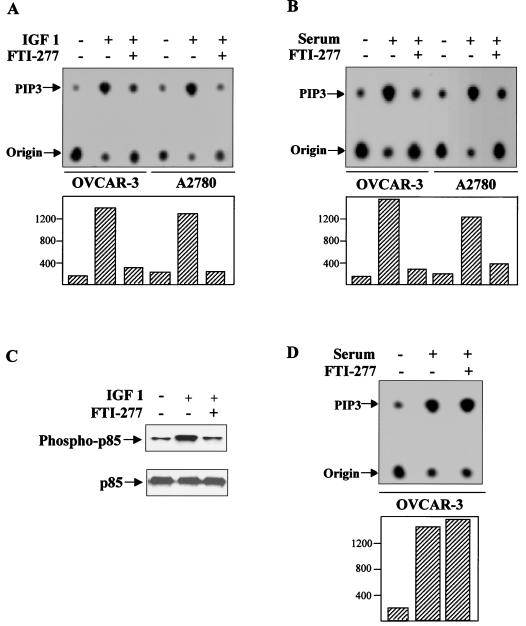
Furthermore, we evaluated the effect of FTI-277 on endogenous PI 3-kinase and AKT2 activation by IGF-1. OVCAR-3 cells were serum starved and treated with FTI-277 for 12 h prior to IGF-1 stimulation for 10 min. Cells were then lysed and immunoprecipitated with an anti-AKT2 or anti-P-Tyr monoclonal antibody or anti-p85 (the regulatory subunit of PI 3-kinase) polyclonal antibody. The AKT2 immunoprecipitates were subjected to in vitro kinase assays. The p85 immunoprecipitates were divided into two aliquots. One aliquot was separated by SDS-PAGE, transferred to a membrane, and probed with the anti-p-Tyr monoclonal antibody. The other was subjected to an in vitro PI 3-kinase assay (40). Results showed that FTI-277 abrogated IGF-1-induced endogenous AKT2 activation, p85 phosphorylation, and PI 3-kinase activity (Fig. 3B, 4A, and 4C). The efficacy of FTI-277 to inhibit selectively protein farnesylation in OVCAR-3 cells was verified by analyzing the prenylation status of lamin B and Rap1A in these cells. Aliquots of cell lysate were analyzed by SDS-PAGE followed by immunoblotting with anti-lamin B antibody or anti-Rap1A antibody. Lamin B serves as a positive control for the FTI-277 effect, as it is known to be strictly farnesylated. Rap1A serves as a negative control because it is only geranylgeranylated. Figure 3E shows that lamin B farnesylation, but not Rap1A geranylgeranylation, was inhibited in FTI-277-treated OVCAR-3 cells.
FIG. 4.
FTI-277 inhibits PI 3-kinase activity. (A and B) In vitro PI 3-kinase assay of the anti-p85 immunoprecipitates from OVCAR-3 and A2780 cells. Following serum starvation overnight, the cells were treated with or without FTI-277 for 12 h prior to IGF-1 (A) or 5% FCS (B) stimulation for 15 min. Basal levels of PI 3-kinase are not significantly different between OVCAR-3 and A2780 cells. However, IGF1- or serum-induced PI 3-kinase activity in both cell lines was inhibited by FTI-277. (C) Western blot analyses of immunoprecipitates from OVCAR-3 cells. Following FTI-277 treatment, the cells were lysed, immunoprecipitated with anti-p85 polyclonal antibody, and detected with an anti-p-Tyr antibody. The blots were reprobed with anti-p85 antibody. (D) FTI-277 does not directly inhibit serum-induced PI 3-kinase activation. An in vitro PI 3-kinase assay was carried out with immunoprecipitates from OVCAR-3 cells. After serum starvation and restimulation, FTI-277 (30 μM) was directly added to the kinase reaction (lane 3).
We also examined AKT2 phosphorylation and PI 3-kinase activity in ovarian and pancreatic cancer cell lines used in this study under 5% FCS culture conditions. We have recently demonstrated that phospho-Akt-Ser473 antibody is able to detect phosphorylated AKT2 (W. Yuan and J. Q. Cheng, submitted for publication). The levels of AKT2 phosphorylation in AKT2-overexpressing cell lines are higher than those in cells expressing low levels of AKT2 (Fig. 1A). However, the activity of PI 3-kinase is not significantly different between these two groups of cell lines (Fig. 4A and B and data not shown). These results, in combination with those of a previous study using antisense RNA (16), suggest that cell survival in AKT2-overexpressing cell lines may, at least in part, rely on high levels of AKT2 activity in normal cell culture conditions.
To determine the effects of FTI-277 on PI 3-kinase/AKT2 activation under the conditions (5% FCS medium) in which FTI-277 induces apoptosis, OVCAR-3 and A2780 cells were cultured in a serum-free medium containing either FTI-277 (30 μM) or vehicle (dimethyl sulfoxide [DMSO]) overnight and then stimulated with 5% FCS for 20 min. The AKT2 and P-Tyr or p85 immunoprecipitates were subjected to in vitro protein kinase and PI 3-kinase assays, respectively. Figures 3B, 3C, and 4B illustrate that serum-induced AKT2 and PI 3-kinase activation was also abolished by FTI-277. However, FTI-277 does not directly inhibit PI 3-kinase and AKT2 activities, as determined by adding FTI-277 to the kinase reactions (Fig. 3D and 4D).
AKT2 phosphorylates BAD, and the phosphorylation is blocked by FTI-277.
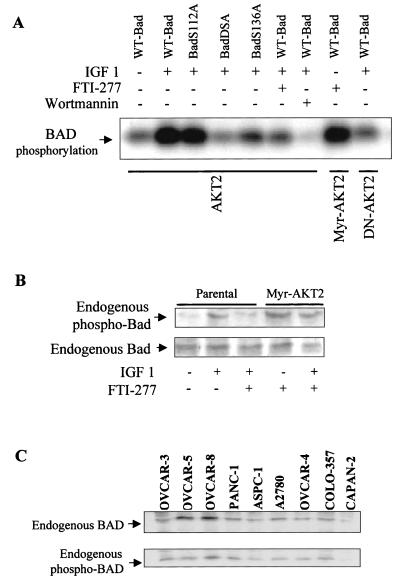
Previous studies demonstrated that Akt phosphorylates the proapoptotic protein BAD to suppress apoptosis and promote cell survival (22, 24). However, to date there is no report showing that AKT2 phosphorylates BAD even though it is assumed that BAD may be phosphorylated by AKT2, based on the sequence homology between Akt and AKT2. To test whether AKT2 phosphorylates BAD, wild-type and mutant forms of HA-AKT2 were expressed in COS7 cells, immunoprecipitated with an anti-HA antibody after serum starvation and IGF-1 stimulation, and assayed in an immunocomplex kinase assay for the ability to phosphorylate recombinant BAD. Repeated experiments revealed that IGF-1-induced AKT2 activation or constitutively active AKT2 (Myr-AKT2) resulted in BAD phosphorylation, whereas dominant-negative AKT2 (AKT2-E299K) failed to do so (Fig. 5A). AKT2-mediated BAD phosphorylation was inhibited by the PI 3-kinase inhibitor wortmannin and FTI-277, suggesting that the phosphorylation of BAD by AKT2 is regulated by PI 3-kinase and a farnesylated protein(s). BAD is phosphorylated at two sites, Ser-112 and Ser-136, in response to interleukin-3 (76). Three recombinant BAD mutant proteins, in which Ser-112 (BADS112A), Ser-136 (BADS136A), or both (BADS2A) were converted to alanine, were used to identify the AKT2-mediated BAD phosphorylation site. As shown in Fig. 5A, IGF-1-induced AKT2 activation results in the phosphorylation of BADSer112A. In contrast, BADS136A and BADS2A were not phosphorylated by AKT2 (Fig. 5A), indicating that the Ser-136 is an AKT2-mediated phosphorylation site of BAD.
FIG. 5.
AKT2 phosphorylates recombinant BAD and BADS112A but not BADS136A or BAD2SA: inhibition by FTI-277 in vitro and in vivo. (A) In vitro kinase assays using anti-HA immunoprecipitates from COS7 cells transfected transiently with HA-tagged AKT2 constructs expressing wild-type AKT2 (HA-AKT2), constitutively active AKT2 (Myr-AKT2), and dominant-negative mutant AKT2 (AKT2-E299K). Following serum starvation, the cells were treated with or without FTI-277 (3 h) or wortmannin (30 min) prior to IGF-1 (50 ng/ml) stimulation. Anti-HA immunoprecipitates were subjected to an in vitro kinase assay using wild-type BAD (WT-Bad), BADS112A, BADS136A, or BAD2SA as the substrate. Note that wortmannin inhibited BAD phosphorylation more efficiently than FTI-277 due to relatively short time of treatment of the cells with FTI-277 (3 h; see Fig. 8). (B) Western blot analysis of phosphorylation (top) and expression (bottom) of endogenous BAD from parental OVCAR-3 cells and the stably transfected cell clones expressing Myr-AKT2 treated with or without FTI-277 before stimulation with IGF-1 (50 ng/ml) for 10 min. (C) Western blot analyses of cell lysates from ovarian and pancreatic cancer cell lines. The blots were detected by anti-BAD (top) or anti-p-BAD (bottom) antibody.
We next examined whether AKT2 phosphorylates endogenous BAD and whether this phosphorylation is blocked by FTI-277. Following serum starvation and FTI-277 treatment, OVCAR-3 and Myr-AKT2-transfected OVCAR-3 cells were stimulated with or without IGF-1, lysed, and immunoprecipitated with an anti-BAD antibody. The immunoprecipitates were separated by SDS-PAGE, and the filters were probed with anti-phospho-BAD antibody. As shown in Fig. 5B, endogenous AKT2 and constitutively active AKT2 were capable of phosphorylating BAD in vivo. Wild-type AKT2-mediated, but not Myr-AKT2-mediated, BAD phosphorylation was abrogated by FTI-277. These results suggest that FTI-277 targets a farnesylated protein(s) upstream of AKT2 and inhibits AKT2-mediated BAD phosphorylation, resulting in programmed cell death.
Integrin-induced AKT2 activation is inhibited by FTI-277.
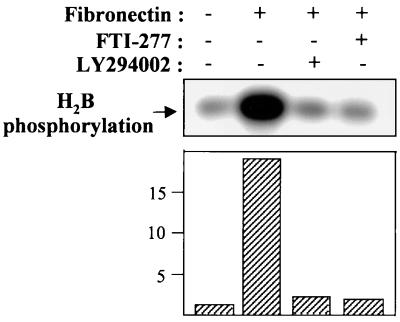
A recent study demonstrated that cell attachment is a critical factor for FTI-induced apoptosis in ras-transformed cell (44). We next tested whether adhesion to fibronectin activates AKT2 and whether the integrin-mediated AKT2 activation is blocked by FTI-277. After serum starvation and treatment with or without FTI-277 or LY294002, OVCAR-3 cells were trypsinized and held in suspension for 30 min prior to attachment to tissue culture plates precoated with fibronectin or polylysine. Following a 30-min exposure, AKT2 was immunoprecipitated from cell lysates, and AKT2 kinase activity was assayed with histone H2B as the substrate. As shown in Fig. 6, AKT2 kinase activity was 20-fold higher in cells exposed to fibronectin than in cells plated on polylysine dishes or kept in suspension. Importantly, this integrin-dependent activation of AKT2 was abrogated by FTI-277 and LY294002 (Fig. 6). These data indicate that AKT2 is activated by integrin in a PI 3-kinase-dependent manner and that the integrin pathway is involved in FTI-277-induced apoptosis in human cancer cells.
FIG. 6.
Activation of AKT2 following attachment to fibronectin: inhibition by FTI-277. OVCAR-3 cells were serum starved, treated with or without FTI-277 or LY294002, and replated on fibronectin- or polylysine-coated plates. AKT2 was immunoprecipitated with an anti-AKT2 monoclonal antibody, and the immunoprecipitates were subjected to an in vitro kinase assay using histone H2B as the substrate. The autoradiogram (top) and quantitation by phosphorimaging (bottom) show that AKT2 is activated by cell adhesion to fibronectin (lane 2) but not to polylysine (lane 1). Integrin-mediated AKT2 activation is inhibited by LY294002 (lane 3) and FTI-277 (lane 4).
Constitutively active AKT2 rescues OVCAR-3 cells from FTI-277-induced apoptosis.
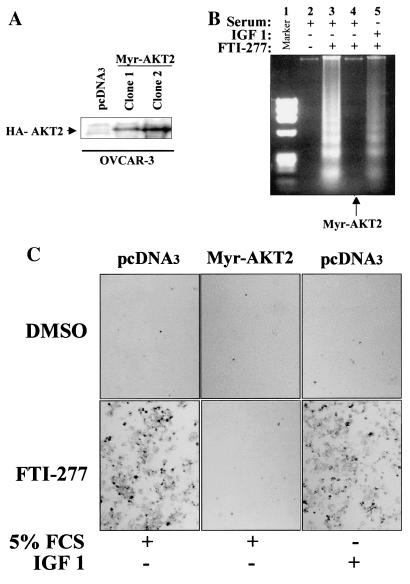
We reasoned that if FTI-277 inhibits a farnesylated protein upstream of AKT2, then constitutively active AKT2 should overcome FTI-277-induced apoptosis in the cancer cells. A constitutively active AKT2 expression construct (Myr-HA-AKT2) or pcDNA3 vector alone was stably transfected into OVCAR-3 cells. Western blot analyses with anti-HA antibody revealed expression of Myr-HA-AKT2 in the transfectants (Fig. 7A). In vivo BAD phosphorylation experiments confirmed the presence of constitutively active AKT2 kinase in the Myr-HA-AKT2-transfected cells (Fig. 5B). After 48 h of treatment with FTI-277 in the presence of 5% FCS or IGF-1 (50 ng/ml), DNA fragmentation and apoptotic cells were observed in pcDNA3- but not Myr-HA-AKT2-transfected OVCAR-3 cells (Fig. 7B and C), indicating that constitutively active AKT2, but not serum or IGF-1, can rescues FTI-277-induced apoptosis.
FIG. 7.
A constitutively activated form of AKT2, but not treatment with IGF-1, rescues human cancer cells from FTI-277-induced apoptosis. (A) OVCAR-3 cells were transfected with pcDNA3 or constitutively active AKT2 (Myr-HA-AKT2), and two stable clones were established. Western blot analyses with an anti-HA antibody revealed expression of Myr-AKT2 in these two clonal cell lines but not in cells transfected with pcDNA3 vector alone. (B and C) DNA fragmentation and TUNEL assay. After treatment with FTI-277, the DNA ladder and apoptotic cells were not observed in Myr-AKT2-transfected cells (lane 4 of panel B and middle column of panel C). However, FTI-277-induced apoptosis was detected in pcDNA3-transfected OVCAR-3 cells cultured in medium containing either IGF-1 (50 ng/ml; lane 5 of panel B and rightmost column of panel C) or 5% FCS (lane 3 of panel B and leftmost column of panel C). Lane 1, φX174 replicative-form DNA/HaeIII markers; lanes 2 and 3, parental OVCAR-3 cells treated with vehicle (DMSO) and FTI-277, respectively.
A short-lived farnesylated protein, but not RhoB, mediates FTI-277 inhibition of PI 3-kinase/AKT2 activation.
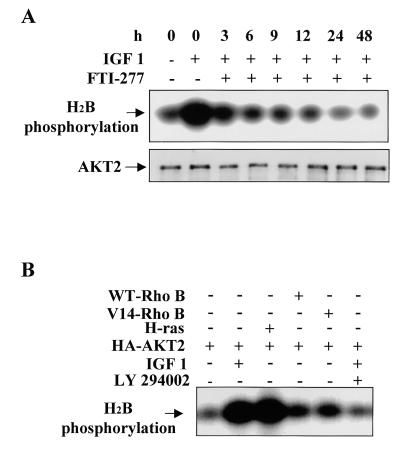
Farnesylated proteins generally have different half-lives; for example, the half-lives of RhoB and Ras are ∼2 and 24 h, respectively. To determine whether a short- or a long-half-life protein is involved in regulation of the PI 3-kinase/AKT2 pathway, OVCAR-3 cells were serum starved and treated with FTI-277 at different times from 1 to 48 h prior to IGF-1 stimulation. AKT2 was immunoprecipitated from cell lysates with an anti-AKT2 monoclonal antibody, and the kinase activity of AKT2 was assayed with histone H2B as the substrate. Repeated experiments revealed that IGF-1-induced AKT2 activation rapidly declined after 3 h of FTI-277 treatment and reached a basal level at 9 h (Fig. 8A), indicating that a short-lived farnesylated protein(s) is predominantly involved in regulation of the PI 3-kinase/AKT2 pathway. To examine whether the short-lived protein RhoB activates the PI 3-kinase/AKT2 pathway, COS7 cells were cotransfected with HA-AKT2 and two different forms of RhoB. After serum starvation, HA-AKT2 was immunoprecipitated with an anti-HA antibody, and the immunoprecipitates were subjected to an in vitro kinase assay. Figure 8B shows that neither wild-type nor active (RhoB-V14) RhoB activates AKT2, suggesting that RhoB is not the FTI-277 target that regulates the PI 3-kinase/AKT2 pathway. As a positive control, v-H-ras activates AKT2 (Fig. 8B).
FIG. 8.
FTI-277 targets a short-lived farnesylated protein, but not RhoB, upon inhibition of AKT2 activation. (A) In vitro kinase assay of the AKT2 immunoprecipitates from OVCAR-3 cells. The cells were treated with FTI-277 at different time points as indicated at the top, serum starved, stimulated with IGF-1, lysed, and immunoprecipitated with monoclonal anti-AKT2 antibody. The immunoprecipitates were subjected to in vitro kinase assay (top), and the filter was detected with polyclonal anti-AKT2 antibody (bottom). (B) In vitro kinase assays of HA-AKT2 immunoprecipitated from lysates of COS7 cells transfected with HA-AKT2 and different combinations of v-Ha-Ras or wild-type (WT) and active (V14) forms of RhoB.
DISCUSSION
In this study, we demonstrate that inhibition of the PI 3-kinase/AKT2 pathway by FTI-277 induces apoptosis in human cancer cells under conditions where cells are attached to a substratum and serum is present. Addition of IGF-1 fails to rescue FTI-277-induced apoptosis, whereas constitutively active AKT2 prevents programmed cell death. Growth factor-induced AKT2 activation phosphorylates BAD. Furthermore, we documented that engagement of the fibronectin receptor in OVCAR-3 cells results in activation of AKT2 and that FTI-277 inhibits both growth factor-induced and integrin-mediated AKT2 activation and subsequently blocks AKT2-mediated BAD phosphorylation. We have also demonstrated that AKT2- but not ras-transformed NIH 3T3 cells are sensitive to FTI-277.
PI 3-kinase has been implicated in the regulation of a variety of different cellular responses, including cytoskeletal organization, mitogenesis, membrane trafficking, cell survival, and transformation. A number of downstream targets of PI 3-kinase have been identified, including p70S6K Akt/PKB, PKCξ, PKCδ, JNK1, p38, and Etk (6, 9, 11, 21, 27, 41, 45, 49, 59), but only the Akt/PKB family was shown to be involved in malignant transformation. Previous studies have shown that AKT2 is frequently altered in several types of human malignancies (7, 15, 16, 54, 62) and that overexpression of AKT2 in NIH 3T3 cells results in a malignant phenotype (17). Alterations of Akt or AKT3, however, have not been consistently observed in human malignancy. A previous study showed that Akt is nononcogenic in nude mice when overexpressed in a nontumorigenic rat T-cell lymphoma cell line (1). Therefore, AKT2 appears to play a more important role than Akt and AKT3 in malignant transformation. Previously, we have documented that AKT2 is a downstream target of PI 3-kinase and mediates mitogenic signals to promote cell proliferation and transformation (17, 48). In this report, we provide further evidence that the PI 3-kinase/AKT2 pathway is essential for cell survival and that inhibition of this pathway by FTI-277 results in induction of apoptosis in a subset of human ovarian and pancreatic cancer cell lines.
Akt and AKT2 have similar upstream regulators and downstream targets. However, there are clear differences between Akt and AKT2 in terms of biological and physiological function. In addition to the more prominent role of AKT2 in human malignancy and transformation, the expression patterns of Akt and AKT2 in normal adult tissues as well as during development are quite different (4). All of the cell lines used in this study exhibit low levels of Akt expression (data not shown). However, the five cell lines that are sensitive to FTI-277 exhibited high levels of AKT2 protein. The preferential effect of FTI-277 on AKT2-overexpressing cells is further supported by our observation that ecotopic expression of wild-type AKT2 in NIH 3T3 cells renders the cells sensitive to FTI-277. Recently, Suzuki et al. (71) showed that FTIs induce apoptosis of ras-transformed but not untransformed rat kidney cells. In the present report, we show that AKT2-transformed NIH 3T3 cells are more sensitive to FTI-277 treatment than ras-transformed cells (Fig. 2). This finding suggests that FTIs selectively inhibit AKT2-dependent cell transformation, that farnesylated proteins, in addition to Ras, are involved in the activation of AKT2, and that their inhibition by FTI-277 induces apoptosis.
Previous studies demonstrated that FTIs are capable of inducing apoptosis in ras-transformed rodent cells only under low-serum (0.1% FCS) conditions. In this report, we show that FTI-277 induces apoptosis in AKT2-overexpressing cancer cells under high-serum (5% FCS) conditions and that IGF-1, a major cellular survival factor protecting cells from apoptosis induced by a wide variety of agents (35, 55), fails to rescue FTI-277-induced apoptosis in these cancer cells. FTI-277 effectively inhibits IGF-1- or serum-induced PI 3-kinase/AKT2 activation, resulting in programmed cell death. Furthermore, constitutively active AKT2 blocks FTI-277-induced apoptosis. These data indicate that serum/IGF-1 survival signals are predominantly mediated by the PI 3-kinase/AKT2 pathway in these cells and that activation of AKT2 is sufficient for the antiapoptotic signaling.
We have demonstrated that IGF-1-induced AKT2 activation phosphorylates BAD both in vitro and in vivo and that this phosphorylation is inhibited by FTI-277 and wortmannin. Moreover, constitutively active AKT2-mediated phosphorylation of BAD effectively blocks FTI-277-induced cell death. These findings indicate that the PI 3-kinase/AKT2/BAD pathway represents a general mechanism by which growth factors promote cell survival and that inhibition of this pathway leads to apoptosis. AKT2 triggers BAD phosphorylation at Ser-136, creating a binding site for 14-3-3 protein (22, 24). When BAD forms a complex with 14-3-3, it is unable to heterodimerize with and inhibit the survival activity of Bcl-XL or Bcl-2. However, BAD is expressed in only a limited range of tissues and cell lines. All nine ovarian or pancreatic cancer cell lines studied exhibit moderate levels of expression of BAD, and the phosphorylation levels of BAD are slightly higher in all AKT2-overexpressing cell lines except ASPC-1 (Fig. 5C). These results indicate that PI 3-kinase/AKT2-mediated BAD phosphorylation is important for maintaining cell survival in these cell lines. However, other downstream targets of Akt, such as FKHRL, caspase 9, GSK-3β, and 4E-BP1, could be also important for cell growth in these cell lines. Investigations of the involvement of these targets are required for a better understanding of the mechanism of FTI-induced apoptosis via inhibition of the PI 3-kinase/AKT2 pathway.
Integrins, a diverse class of αβ heterodimeric receptors, have been implicated in cellular adhesion and transduction of signals within the cell to regulate intracellular events, including cytoskeletal rearrangements and cell spreading, migration, differentiation, survival, and growth (18, 29, 31, 56, 65, 66, 78). Recently, PI 3-kinase was found to associate with the integrin-dependent focal adhesion kinase (FAK) and to regulate ILK (14, 23, 36). FAK and ILK are rapidly activated following integrin-mediated attachment to the extracellular matrix (23, 63). It has been documented that integrin-mediated adhesion to fibronectin results in accumulation of the PI 3-kinase products PI(3,4)P2 and PI(3,4,5)P3 as well as PI 3-kinase-dependent activation of ILK and Akt (23, 39). A recent study demonstrated that at normal serum concentrations, FTIs induce cell death only in cells detached from the substratum, suggesting that they affect cellular adhesion pathways (44). In the present study, we provide direct evidence that integrin pathway is targeted in FTI-277-induced apoptosis in human cancer cells. We have shown that AKT2 is highly activated by integrin and that this activation is completely blocked by FTI-277 and LY294002, indicating that integrin-mediated AKT2 activation is via PI 3-kinase pathway and that FTI-277 targets a farnesylated protein(s) directly regulating the integrin/PI 3-kinase/AKT2 pathway.
Our time course experiments indicate that inhibition of PI 3-kinase/AKT2 activation by FTI-277 takes place at very early time points, approximately 3 h after FTI-277 treatment (Fig. 8A). It has been shown that FTIs may inhibit Ras phenylation and blocks Ras signaling and transformation (19, 64). However, the half-life of Ras is approximately 24 h, suggesting that FTI-277 targets a short-lived farnesylated protein that regulates the PI 3-kinase/AKT2 pathway. Among identified small G proteins, only RhoB has a short half-life (∼2 h). Recent studies demonstrated that FTIs inhibit cell growth and Ras transformation in Rat1 cells by targeting the RhoB protein (26, 43). However, we showed in this report that neither wild-type nor constitutively active RhoB activates AKT2. Moreover, we found that two other small G proteins, Rac1 and RhoA, were not involved in the activation of AKT2 (data not shown).
In conclusion, our data demonstrate that inhibition of PI 3-kinase/AKT2 pathway by FTI-277 induces apoptosis in human cancer cells. FTI-277 effectively inhibits growth factor-induced and integrin-mediated AKT2 activation and AKT2-mediated BAD phosphorylation. Further studies are required to identify and characterize a farnesylated protein(s) that activates the PI 3-kinase/AKT2 pathway and is inhibited by FTI-277.
ACKNOWLEDGMENTS
We are grateful to Michael E. Greenberg for GST-Bad plasmids; Alan Hall for RhoB constructs; Thomas C. Hamilton for ovarian cancer cell lines; Andres J. P. Klein-Szanto for pancreatic cancer cell lines; Sue A. Shelley and Richard I. Feldman for constructive comments; June E. Paciga, Ai-xie Liu and Jie-liu Tang for technical support; and Wen-ching Lee for critical reading and comments on the manuscript. We are also grateful to DNA Sequence Facility at H. Lee Moffitt Cancer Center for sequencing AKT2 expression constructs.
This work was supported by grants CA-77935 (J.Q.C.) and CA-67771 (S.M.S. and A.D.H.) from the National Cancer Institute and grant 6115-000-20 (S.V.N.) from University of South Florida.
REFERENCES
- 1.Ahmed N N, Franke T F, Bellacosa A, Datta K, Gonzalez-Portal M-E, Taguchi T, Testa J R, Tsichlis P N. The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- 2.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 4.Altomare D A, Lyons G E, Mitsuuchi Y, Cheng J Q, Testa J R. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene. 1998;16:2407–2411. doi: 10.1038/sj.onc.1201750. [DOI] [PubMed] [Google Scholar]
- 5.Aman M J, Lamkin T D, Okada H, Kurosaki T, Ravichandran K S. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J Biol Chem. 1998;273:33922–33928. doi: 10.1074/jbc.273.51.33922. [DOI] [PubMed] [Google Scholar]
- 6.Barthel A, Nakatani K, Dandekar A A, Roth R A. Protein kinase C modulates the insulin-stimulated increase in Akt1 and Akt3 activity in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1998;243:509–513. doi: 10.1006/bbrc.1998.8134. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Feo D, Godwin A K, Bell D W, Cheng J Q, Altomare D, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Panici P B, Mancuso S, Neri G, Testa J R. Molecular alteration of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 8.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 9.Berra E, Diaz-Meco M T, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–10797. doi: 10.1074/jbc.273.17.10792. [DOI] [PubMed] [Google Scholar]
- 10.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 11.Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 12.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 13.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chichen cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 14.Chen H C, Appeddu P A, Isoda H, Guan J L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J Q, Godwin A K, Bellacosa A, Taguchi T, Franke T F, Hamilton T C, Tsichlis P N, Testa J R. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng J Q, Ruggeri B, Klein W M, Sonoda G, Altomare D A, Watson D K, Testa J R. Amplification of AKT2 in human pancreatic cancer cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng J Q, Altomare D A, Klein W M, Lee W-C, Kruh G D, Lissy N A, Testa J R. Transforming activity and mitosis-dependent expression of the AKT2 oncogene: evidence suggesting a link between cell cycle regulation and oncogenesis. Oncogene. 1997;14:2793–2801. doi: 10.1038/sj.onc.1201121. [DOI] [PubMed] [Google Scholar]
- 18.Choquet D, Felsenfeld D P, Sheetz M P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 19.Cox A D, Der C J. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim Biophys Acta. 1997;1333:F51–F71. doi: 10.1016/s0304-419x(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 20.Cross D A E, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 21.Datta K, Bellacosa A, Chan T O, Tsichlis P N. Akt is a direct target of the phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:30835–30839. doi: 10.1074/jbc.271.48.30835. [DOI] [PubMed] [Google Scholar]
- 22.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 23.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 25.Diehl J A, Cheng M G, Roussel M F, Sherr C J. Glycogen synthase kinase 3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du W, Lebowitz P F, Prendergast G C. Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol Cell Biol. 1999;19:1831–1840. doi: 10.1128/mcb.19.3.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franke T F, Yang S L, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 28.Gibbs J B, Oliff A. The potential of farnesyltransferase inhibitors as cancer chemotherapeutics. Annu Rev Pharmacol Toxicol. 1997;37:143–166. doi: 10.1146/annurev.pharmtox.37.1.143. [DOI] [PubMed] [Google Scholar]
- 29.Hannigan G E, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino M G, Radeva G, Filmus J, Bell J C, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 30.He T C, Sparks A B, Rago C, Hermeking H, Zawel L, da Costa L T, Morin P J, Vogelstein B, Kinzler K W. Identification of c-MYC as a target of the APC pathway. Science. 1998;28:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 31.Howe A, Aplin A E, Alahari S K, Juliano R L. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez C, Jones D R, Rodriguez-Viciana P, Gonzalez-Garcia A, Leonardo E, Wennstrom S, von Kobbe C, Toran J L, Borlado L R-, Calvo V, Copin S G, Albar J P, Gaspar M L, Diez E, Marcos M A, Downward J, Martinez-A C, Merida I, Carrera A C. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones P F, Jakubowicz T, Hemmings B A. Molecular cloning of a second form of rac protein kinase. Cell Regul. 1991;2:1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung Y K, Miura M, Yuan J. Suppression of interleukin-1β-converting enzyme-mediated cell death by insulin-like growth factor. J Biol Chem. 1996;271:5112–5117. doi: 10.1074/jbc.271.9.5112. [DOI] [PubMed] [Google Scholar]
- 36.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 37.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klippel A, Escobedo J A, Hu Q, Williams L T. A region of the 85-kilodalton (kDa) subunit of phosphatidylinositol 3-kinase binds the 110-kDa catalytic subunit in vivo. Mol Cell Biol. 1993;13:5560–5566. doi: 10.1128/mcb.13.9.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klippel A, Escobedo M A, Wachowicz M S, Apell G, Brown T W, Giedlin M A, Kavanaugh W M, Williams L T. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konishi H, Kuroda S, Tanaca M, Ono Y, Kameyama K, Haga T, Kikkawa U. Molecular cloning and characterization of a new member of the Rac protein kinase family: association of the pleckstrin homology domain of three types of Rac protein kinase with protein kinase C subspecies and βγ subunits of G proteins. Biochem Biophys Res Commun. 1995;216:526–534. doi: 10.1006/bbrc.1995.2654. [DOI] [PubMed] [Google Scholar]
- 43.Lebowitz P F, Davide J P, Prendergast G C. Evidence that farnesyltransferase inhibitors suppress Ras transformation by interfering with Rho activity. Mol Cell Biol. 1995;15:6613–6622. doi: 10.1128/mcb.15.12.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lebowitz P F, Sakamuro D, Prendergast G C. Farnesyl transferase inhibitors induce apoptosis of Ras-transformed cells denied substratum attachment. Cancer Res. 1997;57:708–713. [PubMed] [Google Scholar]
- 45.Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 46.Lerner E C, Qian Y, Blaskovich M A, Fossum R D, Vogt A, Sun J, Cox A D, Der C J, Hamilton A D, Sebti S M. Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J Biol Chem. 1995;270:26802–26806. doi: 10.1074/jbc.270.45.26802. [DOI] [PubMed] [Google Scholar]
- 47.Lerner E C, Zhang T T, Knowles D B, Qian Y, Hamilton A D, Sebti S M. Inhibition of the prenylation of K-Ras, but not H- or N-Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene. 1997;15:1283–1288. doi: 10.1038/sj.onc.1201296. [DOI] [PubMed] [Google Scholar]
- 48.Liu A-X, Testa J R, Hamilton T C, Jove R, Nicosia S V, Cheng J Q. AKT2, a member of the protein kinase B family, is activated by growth factors, v-Ha-ras, and v-src through phosphatidylinositol 3-kinase in human ovarian epithelial cancer cells. Cancer Res. 1998;58:2973–2977. [PubMed] [Google Scholar]
- 49.Logan S K, Falasca M, Hu P, Schlessinger J. Phosphatidylinositol 3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol. 1997;17:5784–5790. doi: 10.1128/mcb.17.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maehama T, Dixon J E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 51.Meier R, Alessi D R, Cron P, Andjelkovic M, Hemmings B A. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 52.Miquel K, Pradines A, Sun J, Qian Y, Hamilton A D, Sebti S M, Favre G. GGTI-298 induces G0-G1 block and apoptosis whereas FTI-277 causes G2-M enrichment in A549 cells. Cancer Res. 1997;57:1846–1850. [PubMed] [Google Scholar]
- 53.Mitsuuchi Y, Johnson S W, Moonblatt S, Testa J R. Translocation and activation of AKT2 in response to stimulation by insulin. J Cell Biochem. 1998;70:433–441. [PubMed] [Google Scholar]
- 54.Miwa W, Yasuda J, Murakami Y, Yashima K, Sugano K, Sekine T, Kono A, Egawa S, Yamaguchi K, Hayashizaki Y, Sekiya T. Isolation of DNA sequences amplified at chromosome 19q13.1-q13.2 including the AKT2 locus in human pancreatic cancer. Biochem Biophys Res Commun. 1996;225:968–974. doi: 10.1006/bbrc.1996.1280. [DOI] [PubMed] [Google Scholar]
- 55.Muta K, Krantz S B. Apoptosis of human erythroid colony-forming cells is decreased by stem cell factor and insulin-like growth factor 1 as well as erythropoietin. J Cell Physiol. 1993;156:264–271. doi: 10.1002/jcp.1041560207. [DOI] [PubMed] [Google Scholar]
- 56.Palecek S P, Loftus J C, Ginsberg M H, Lauffenburger D A, Horwitz A F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 57.Porter A C, Vaillancourt R R. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17:1343–1352. doi: 10.1038/sj.onc.1202171. [DOI] [PubMed] [Google Scholar]
- 58.Qiu R G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 59.Qiu Y, Robinson D, Pretlow T G, Kung H J. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 62.Ruggeri B, Huang L, Wood M, Cheng J Q, Testa J R. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinoma. Mol Carcinog. 1998;21:81–86. [PubMed] [Google Scholar]
- 63.Schaller M D, Parsons J T. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994;6:705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 64.Sebti S M, Hamilton A D. Inhibition of Ras prenylation: a novel approach to cancer chemotherapy. Pharmacol Ther. 1997;74:103–114. doi: 10.1016/s0163-7258(97)00014-4. [DOI] [PubMed] [Google Scholar]
- 65.Shaw L M, Rabinovitz I, Wang H H, Toker A, Mercurio A M. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 66.Short S M, Talbott G A, Juliano R L. Integrin-mediated signaling events in human endothelial cells. Mol Biol Cell. 1998;9:1969–1980. doi: 10.1091/mbc.9.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 68.Stokoe D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 69.Sun J, Qian Y, Hamilton A D, Sebti S M. Ras CAAX peptidomimetic FTI 276 selectively blocks tumor growth in nude mice of a human lung carcinoma with K-Ras mutation and p53 deletion. Cancer Res. 1995;55:4243–4247. [PubMed] [Google Scholar]
- 70.Sun J, Qian Y, Hamilton A D, Sebti S M. Both farnesyltransferase and geranylgeranyltransferase I inhibitors are required for inhibition of oncogenic K-Ras prenylation but each alone is sufficient to suppress human tumor growth in nude mouse xenografts. Oncogene. 1998;16:1467–1473. doi: 10.1038/sj.onc.1201656. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki N, Urano J, Tamanoi F. Farnesyltransferase inhibitors induce cytochrome c release and caspase 3 activation preferentially in transformed cells. Proc Natl Acad Sci USA. 1998;95:15356–15361. doi: 10.1073/pnas.95.26.15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 73.van Weering D H, de Rooij J, Marte B, Downward J, Bos J L, Burgering B M. Protein kinase B activation and lamellipodium formation are independent phosphoinositide 3-kinase-mediated events differentially regulated by endogenous Ras. Mol Cell Biol. 1998;18:1802–1811. doi: 10.1128/mcb.18.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warne P H, Viciana P R, Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993;364:352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- 75.Whitman M, Kaplan D R, Schaffhausen B, Cantley L, Roberts T M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 76.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 77.Zhang F L, Casey P J. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z, Vuori K, Wang H, Reed J C, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]