SUMMARY
People often forget information because they fail to effectively encode it. Here, we test the hypothesis that targeted electrical stimulation can modulate neural encoding states and subsequent memory outcomes. Using recordings from neurosurgical epilepsy patients with intracranially implanted electrodes, we trained multivariate classifiers to discriminate spectral activity during learning that predicted remembering from forgetting, then decoded neural activity in later sessions in which we applied stimulation during learning. Stimulation increased encoding-state estimates and recall if delivered when the classifier indicated low encoding efficiency but had the reverse effect if stimulation was delivered when the classifier indicated high encoding efficiency. Higher encoding-state estimates from stimulation were associated with greater evidence of neural activity linked to contextual memory encoding. In identifying the conditions under which stimulation modulates memory, the data suggest strategies for therapeutically treating memory dysfunction.
In Brief
Direct brain stimulation is a promising tool for modulating cognitive function. Ezzyat et al. show that stimulation differentially affects episodic memory encoding depending on its timing relative to the brain’s encoding state. The data suggest applications for closed-loop treatment of memory dysfunction.
INTRODUCTION
Memory depends on encoding processes that lay down neural representations of experiences for long-term storage [1]. Recordings taken during laboratory memory tasks demonstrate that neural activity in the hippocampus, medial temporal lobe (MTL) cortex, frontal lobe, and parietal lobe [2, 3] differentiates learned information that is likely to be remembered from information likely to be forgotten. These effects extend to other brain areas [4] and exist both during and prior to when a to-be-remembered stimulus is present [5–8]. This suggests that coordinated activity in a distributed neural network generates states that are responsible for effective memory encoding.
If variability in distributed neural network activity reflects fluctuation of encoding states that leads to differences in memory performance, then it should be possible to modulate memory by perturbing the brain’s encoding state directly [9]. We test this hypothesis using electrical stimulation delivered through electrodes implanted in the brains of epilepsy patients. Direct electrical stimulation allows for targeting focal brain structures in order to modulate activity in complex neural networks [10–12] and can be precisely timed to target specific encoding events, offering some advantages over non-invasive methods [13].
We predicted that stimulation’s effects on memory would depend on the brain’s encoding state at the time it is delivered. If the memory network is operating efficiently, stimulation should interfere with the encoding process and thus later memory. However, if the memory network is not operating efficiently, we predicted that stimulation should disrupt dysfunctional encoding activity and therefore facilitate memory. A mechanism whereby stimulation disrupts dysfunctional brain networks is thought to explain the success in using deep brain stimulation (DBS) of thalamocortical circuits in treating motor dysfunction in Parkinson’s disease [14, 15].
To use stimulation to modulate encoding states, we first needed to reliably identify neural activity conducive to successful memory. There is evidence that theta activity in the hippocampus and MTL cortex prior to stimulus presentation predicts memory [5, 6, 8], and pre-stimulus theta activity has been used to trigger learning trials and improve performance in animal models of classical conditioning [16]. However, similar approaches in humans using MTL activity in the form of intracranial theta [17] have not reliably modulated memory performance. We hypothesized that we could derive a more sensitive index of memory function by estimating encoding states that reflect global memory function, as opposed to specific operations carried out in focal brain areas.
To do so, we simultaneously measured neural activity across the brain. We recorded intracranial electroencephalography (iEEG) signals from subdural and depth electrodes implanted in patients with medically refractory epilepsy undergoing clinical monitoring to determine seizure onset foci. Subjects performed free recall, a memory task sensitive to many types of neurological dysfunction [18, 19] and whose cognitive basis has been modeled by multiple computational mechanisms [20]. We then used multivariate classification to test whether a classifier could predict the probability of recall success from patterns of neural activity recorded across the brain during encoding. In this way, we took advantage of our access to many recording channels to derive a subject-specific model that could differentiate encoding states likely to lead to remembering from states likely to lead to forgetting.
Using multivariate classification was advantageous in another way. Direct electrical stimulation has multifaceted effects on neural activity that are local and remote relative to the site of stimulation [21, 22], and that depend on the baseline excitability of the targeted neural population at the time stimulation is delivered [23–26]. This poses a challenge when trying to predict the brain structures stimulation is likely to excite or inhibit and its consequent effects on behavior. Stimulating hippocampal and MTL cortical targets in humans, for example, leads to inconsistent and modest effects on memory, with some studies suggesting memory facilitation [27–29] and others showing memory disruption [30–34].
We addressed this problem by using each subject’s classifier trained on record-only sessions to decode patterns of neural activity during later stimulation sessions. The classifier served as a model that allowed us to assess evidence for the presence of good encoding states before and after stimulation and control periods. The estimates from the classifier integrate information across electrodes and frequencies, which we predicted would account for heterogeneity in stimulation’s physiological effects across people. We targeted stimulation to electrodes placed in nodes of the memory network: if available within the electrode montage, we stimulated a single MTL structure (hippocampus or entorhinal, perirhinal, or parahippocampal cortex) in a given session. For subjects without MTL contacts, we stimulated other structures linked to memory encoding, such as the prefrontal and parietal cortex [3], which we selected by identifying the contact that showed the largest subsequent memory effect in the high-frequency range (70–200 Hz), a marker of successful memory encoding that correlates with multi-unit neural firing [35].
RESULTS
Multivariate Classification of Encoding Activity Predicts Later Recall
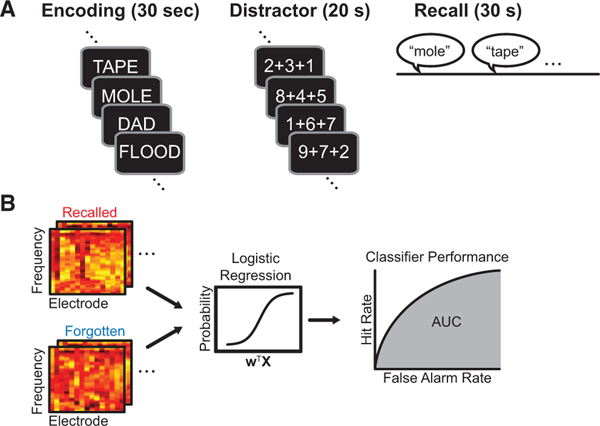
One hundred and two subjects participated in the record-only phase of the study. Subjects performed a free recall memory task during which they studied at least 25 lists of 12 unrelated words, with each list followed by a 20 s mental arithmetic distractor task (a subset of subjects also performed additional sessions of free recall with categorized word lists). Subjects then freely recalled the words from the list in any order (Figure 1A; mean recall = 27.2% ± 1.2%; SEM). For each encoded word, we computed the time-frequency decomposition of the iEEG signal for each bipolar electrode pair (50 frequencies between 1 and 200 Hz;Figure 1B). We used these estimates of spectral power at each frequency and electrode, for each encoded word, as input for training a logistic regression classifier. We employed L2 penalization to avoid overfitting, then assessed performance using N − 1 cross-validation by experimental session and area under the receiver-operating characteristic curve (AUC), a standard measure of a classifier’s ability to generate true positives while avoiding false positives.
Figure 1. Experimental Design and Analysis.
(A) Subjects performed delayed free recall while intracranially implantedelectrodes recorded local field potentials simultaneously across multiple regions of the brain.
(B) The electrode frequency pattern of spectral power for each word-encodingperiod was used as input (X) to fit a classifier to discriminate recalled from forgotten patterns (resulting weight; w). We assessed classifier performance using area under the receiver-operating characteristic curve (AUC).
See also Figure S1.
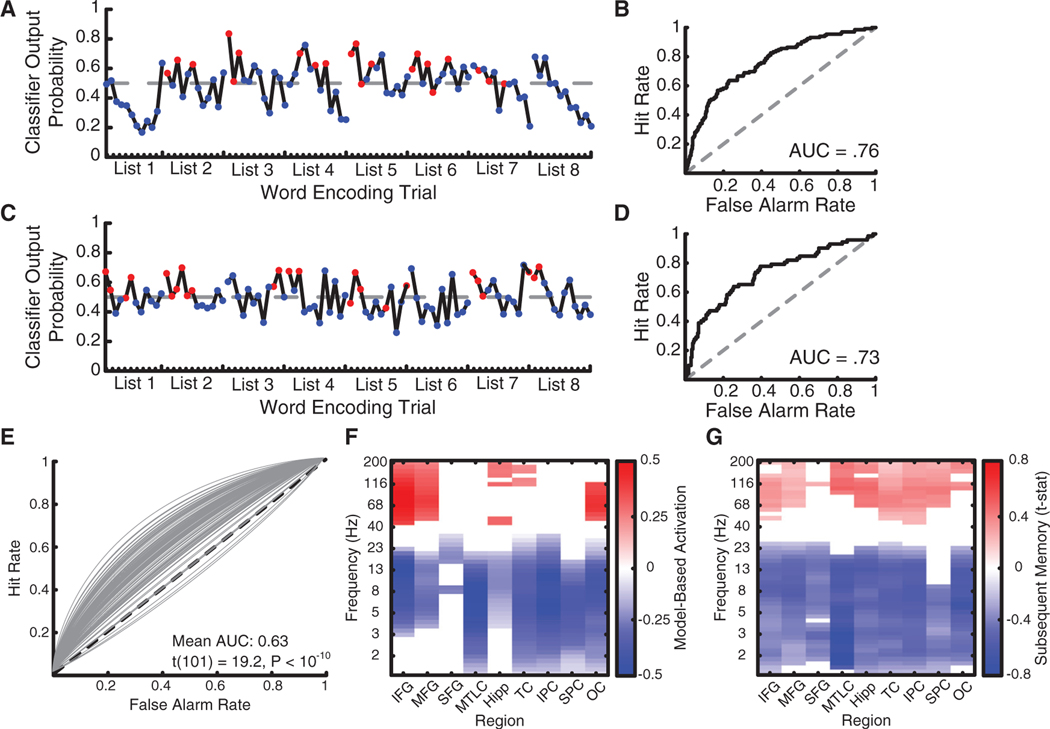
Figures 2A–2D show data from two subjects for eight example encoding lists. The classifier generated higher probabilities for recalled than forgotten items in these periods (Figures 2A and 2C) and across all encoded words as measured using AUC (Figures 2B and 2D). Across subjects, classification performance exceeded chance (mean AUC 0.63 ± 0.07, t(101) = 19.2, p < 10−10; Figure 2E), which indicates that our approach can identify subject-specific features of brain activity during encoding that predict memory. We next asked whether the features that were important to classification were idiosyncratic or instead reflected consistent activity in similar brain regions and at similar frequencies.
Figure 2. Classifier Performance.
(A and C) Classifier output probability for an eight-list period of the delayed free recall task in two example subjects. Dashed lines indicate the optimal decisionthreshold dividing recalled from forgotten trials. Red, later recalled words; blue, later forgotten words. (A) Example patient 1. (C) Example patient 2.
(B and D) AUC for both subjects was significantly greater than chance. (B) Example patient 1. (D) Example patient 2.
(E) Individual receiver-operating characteristic (ROC) curves plotted for all subjects.
(F) Forward-model-derived estimates of classification importance for each electrode region × frequency feature, grouped into anatomical regions of interest.
(G) Subsequent memory analysis contrasting encoding power for later recalled words with later not recalled words.
(F and G) IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus; MTLC, medial temporal lobe cortex; Hipp, hippocampus; TC, temporal cortex; IPC, inferior parietal cortex; SPC, superior parietal cortex; OC, occipital cortex. Data are multiple comparisons corrected using false discovery rate (FDR) at q = 0.05.
See also Figures S2 and S3.
We derived a forward model [36] for each subject using classifier weights, accounting for covariance between features in the input data, to estimate the relative importance of each region × frequency feature for classifier performance. This showed that the classifier relied on widespread low-frequency power decreases simultaneous with high-frequency power increases across the frontal, temporal, and occipital cortex, as well as in the hippocampus, to predict successful recall (Figure 2F). We observed a similar pattern when contrasting power for remembered and forgotten words (Figure 2G), with the MTL and parietal cortex also showing high-frequency power increases. This echoes prior work in intracranial and scalp EEG [37] and suggests consistency in the features that predict efficient memory function across people.
Stimulation Has Heterogeneous Effects on Memory Performance
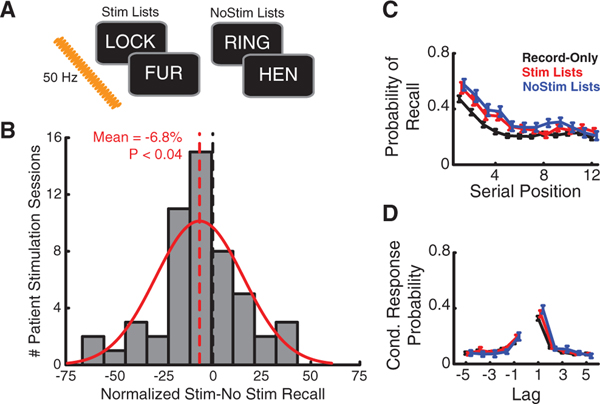
Having established that classification discriminates encoding states, we asked whether stimulation modulates these states in a way that influences memory performance. On stimulation (Stim) lists, we applied 50-Hz trains across a single pair of electrodes at parameters previously used to modulate spatial memory in humans [28]. We then used each subject’s record-only classifier to decode neural activity during stimulation sessions (N = 52 stimulation datasets from 36 subjects). We first tested classifier generalization to the stimulation sessions. Our experimental design included lists without stimulation (NoStim lists) to serve as a baseline for behavioral performance and for testing between-session classifier generalization (Figure 3A). The classifier significantly discriminated encoding activity for recalled and forgotten words (mean AUC on NoStim lists 0.61 ± 0.01, t(51) = 10.4, p < 10−10), even though recall performance was slightly higher for NoStim lists compared to record-only sessions (record-only 30.6% ± 1.7%, NoStim lists 33.5% ± 1.9%, t(51) = 2.6, p < 0.02). This suggests that the relation between neural activity and memory states was stable from the record-only to the stimulation sessions.
Figure 3. Classifier Output Predicts the Effect of Stimulation on Memory.
(A) We applied stimulation across alternating pairs of words on Stim lists;NoStim lists were devoid of stimulation.
(B) The effect of stimulation on memory performance varied across subjects(SD 22.5%); mean, red dashed line.
(C and D) Recall probability as a function of serial position (C) and inter-item lag (D) does not significantly differ as a function of stimulation condition.
See also Table S1.
We next asked whether encoding stimulation tended to facilitate or disrupt recall performance. Within-subject, stimulation significantly increased recall performance in two subjects and decreased recall performance in six subjects (Χ2 test, p < 0.05). Across the group, stimulation reliably decreased memory performance (D normalized recall −6.8% ± 3.2%, p < 0.04; Figure 3B), but there was considerable variability in stimulation’s effects across individuals, ranging from large memory disruption to facilitation (SD = 22.5%). This heterogeneity is consistent with past work [27–30, 32–34], and meant that the small difference in overall recall performance was not accompanied by specific group-level differences in recall organization, measured using two traditional assays of human memory performance, the serial position curve and lag conditional response probability curve (Figures 3C and 3D).
Stimulation’s Behavioral and Neural Effects Are State Dependent
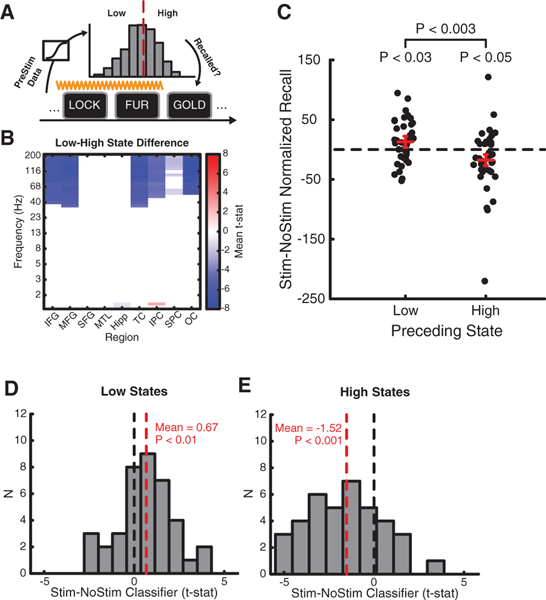
Although stimulation had inconsistent effects overall, we predicted the pre-stimulation encoding state would account for some of this variability. In subjects who showed above-chance classifier generalization (N = 39 datasets from 27 subjects), we applied the classifiers to intervals just prior to each stimulation train (Figure 4A) and split the resulting distribution of classifier outputs into low and high bins, based on the optimal classification threshold from the previous record-only sessions. Low pre-stimulation encoding states were associated with decreased high-frequency power in widespread brain areas that predicted memory performance during word encoding, including the frontal, temporal, and parietal cortex (Figure 4B).
Figure 4. The Effect of Stimulation Depends on Brain State.
(A) Classifier decoding prior to stimulation onset allowed us to analyze memory performance based on pre-stimulation brain state.
(B) Spectral power prior to stimulation onset was significantly lower at high frequencies in frontal, temporal, parietal, and occipital cortex (FDR corrected at q = 0.05).
(C) Recall performance increased if stimulation was delivered when the brain was in a low encoding state (p < 0.03) and decreased if delivered in a high encoding state (p < 0.05). The difference between low and high stimulation was also significant (p < 0.003). Red bars show mean SE of the difference.
(D) Stimulation significantly increased classifier output when delivered at low encoding states (p < 0.01).
(E) Stimulation significantly decreased classifier output when delivered at high encoding states (p < 0.001).
See also Table S2.
Stimulation enhanced recall performance when delivered just after low encoding states (t(38) = 2.26, p < 0.03) but decreased performance when delivered just after high encoding states (t(38) = 2.09, p < 0.05; low-high difference t(38) = 3.32, p < 0.003; Figure 4C). We compared the classifier estimates of the brain’s encoding state post- and pre-stimulation, which showed that low-state stimulation increased evidence for good encoding (p < 0.02; Figure 4D) whereas high-state stimulation decreased evidence for good encoding (p < 0.001; Figure 4E). The data suggest that stimulation influences memory function by perturbing the brain’s encoding state relative to its status at the time of delivery.
Evoked Spectral Tilt after Poor Encoding States
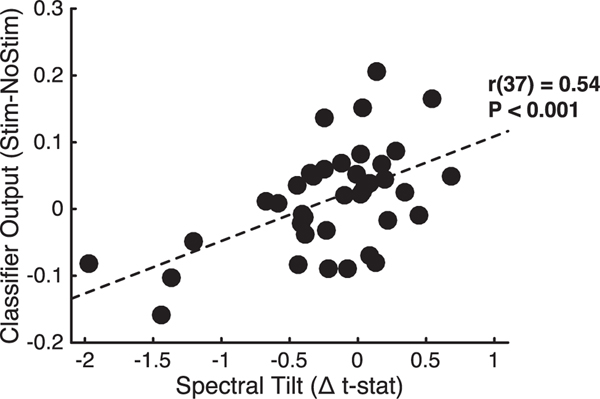
When stimulation was delivered in low states, both recall performance and classifier evidence increased. We next asked how increased classifier evidence relates to stimulation-evoked changes in neural activity across the brain. To measure stimulation’s effect on neural activity, we used an index of the spectral tilt, which is characterized by increased high-frequency power simultaneous with widespread decreases in low-frequency power. These spectral modulations are thought to reflect both local increases in multi-unit firing [17] and decreased long-range low-frequency synchrony [38]. Evidence for these patterns correlates with the fMRI blood-oxygen-level-dependent (BOLD) signal [39], predicts successful memory encoding [6, 40], and is related to core memory processes such as item-context binding [41]. We found that the change in classifier output after stimulation was related to how much stimulation evoked the tilt pattern (r(37) = 0.54, p < 0.001; Figure 5), suggesting that stimulation increased classifier evidence by modulating a neural marker that has been linked to contextual memory encoding.
Figure 5. Correlation between the Stimulation-Related Change in Classifier Output and the Spectral Tilt Effect: (High-Frequency Activity t Stat) –(Low-Frequency Activity t Stat).
DISCUSSION
We applied direct electrical stimulation to nodes of the memory network and found that stimulation reliably modulates memory in a way that depends on the brain’s encoding state. In showing that stimulation improves memory in low encoding states and disrupts memory in high encoding states, the findings suggest that stimulation alters the ongoing course of memory processing in the brain. By using brain-state-matched trials from non-stimulated lists, our data show that stimulation modulates neural activity beyond what might be expected by regression to the mean arising from temporal autocorrelation in the brain’s encoding state. Our data offer insight into the inconsistent effects that have been reported in studies of how brain stimulation modulates memory performance [27–34], and suggest that using brain-state decoding can improve the ability to influence memory outcomes with stimulation.
Our results are consistent with a model in which targeted stimulation leads to changes in network activity across brain areas that contribute to successful memory encoding. There is growing consensus that direct electrical stimulation is likely to influence physiology across a network connected to the targeted site [10, 12]. In using DBS for the treatment of Parkinson’s [42], for example, researchers have had success targeting multiple structures within the affected motor network [12], which suggests that it is more important to target the relevant functional network rather than individual structures within the network. In the case of episodic memory, it may therefore be possible to enhance the effectiveness of stimulation by using measures of connectivity to identify nodes that offer a high degree of controllability over the memory network [43]. Resting-state data could be leveraged to predict the stimulation targets that are most likely to modulate the core memory network [44].
Prior work has shown that stimulation’s effects on physiology depend not only on the excitability of the targeted neurons [26] but also on ongoing rhythms generated by synchronous activity in larger populations. Hippocampal stimulation, for example, alternately promotes long-term potentiation or long-term depression depending on whether theta phase is at the peak or trough at the time of stimulation delivery [24, 25]. Learning itself is also state dependent, as shown in classical conditioning experiments in which animals show faster learning when trials are triggered based on theta rhythm [16]. Our data confirm the role of pre-stimulus brain states for upcoming learning and show that these states can be directly modulated.
We applied classification to whole-brainiEEG to decode brain states that predict later recall. Multivariate decoding allowed us to overcome individual differences in neural connectivity, clinical etiology, and electrode placement that could increase variability in stimulation’s neural and behavioral effects. Decoding may also have provided a more sensitive index of the encoding state than would be possible if using a single feature to identify good and poor memory states [17]. We then related stimulation’s effect on physiology to its effect on memory, extending prior work that has used stimulation to map behavior. Using direct brain recordings most likely facilitated decoding, but future work should address the extent to which non-invasive decoding and stimulation methods [45] could be combined to modulate memory states.
We used the classifier trained on encoding data to decode pre-stimulation states. Our approach suggests that, at a broad level, similar whole-brain patterns of neural activity predict successful encoding during and prior to stimulus onset. In both training and testing our classifier, we averaged spectral power over a temporal interval of several hundred milliseconds, meaning our model was sensitive to consistent spectral power fluctuations over the pre- and post-stimulus intervals. There is evidence that assessing neural activity at a finer temporal scale can identify distinct patterns of pre- and post-stimulus activity that predict encoding. Increased pre-stimulus theta power recorded using non-invasive methods, for example, has been shown to predict successful memory [6, 46], and increased intracranial theta power has also been shown to predict memory pre-stimulus [5, 47], although not during free recall. Taken together with our data, these findings suggest that both tonic and phasic pre-stimulus signals are predictive of memory success. Algorithms to identify good encoding states could therefore be improved by incorporating time as a feature, which would allow both sustained and transient fluctuations in spectral power to influence estimates of the encoding state.
By testing classifier generalization across days and using the free recall task to measure memory performance, our data support the interpretation that the decoded brain states are stable in their neural representation over time and globally predict memory function. Free recall is a complex task that recruits multiple core episodic memory processes [20]. We show that stimulation increases encoding states by increasing high-frequency activity (HFA) power and decreasing low-frequency activity (LFA) power across the brain, a pattern that predicts behavioral measures of item-context encoding [41]. Although such encoding processes promote memory function, free recall is also known to depend heavily on retrieval processes, suggesting that future work may find success influencing memory function by applying stimulation during memory search.
We show an overall reduction in verbal memory performance when stimulating a large set of brain regions, including many outside of the MTL. This is broadly consistent with recent work focused on the hippocampus and entorhinal cortex that showed that stimulation tends to impair both verbal and spatial memory [31]. However, we further test the hypothesis that stimulation’s effects on memory depend on timing relative to the brain’s encoding state [48]. Our findings therefore extend prior studies of human intracranial brain stimulation in several ways. First, we use multivariate decoding of neural activity to separate pre-stimulation brain states, and show that stimulation counteracts low encoding states but disrupts high encoding states. Second, we show that stimulation at low and high encoding states differentially modulates neural activity in a manner consistent with the effect on memory. Third, we show that stimulation’s effect on the encoding state is correlated with the spectral tilt, a biomarker of successful memory encoding. Our work therefore identifies situations in which stimulation increases and decreases memory, and relates stimulation’s effects on behavior to its influence on neural activity through novel use of subject-specific multivariate classification.
By showing that stimulation is most likely to improve memory when encoding efficiency is low prior to stimulation delivery, our data provide the foundation for future work to apply stimulation when it is most likely to improve memory function. Non-invasive closed-loop approaches have improved attention through training using fMRI [49] and maximized the benefit of restudy opportunities using scalp EEG [50]. Closed-loop neural decoding could thus optimally target stimulation for treatment of memory disorders [48, 51].
EXPERIMENTAL PROCEDURES
Participants
One hundred and two patients undergoing iEEG monitoring as part of clinical treatment for drug-resistant epilepsy were recruited to participate in this study. Data were collected as part of a multi-center project designed to assess the effects of electrical stimulation on memory-related brain function. Data were collected at the following centers: Thomas Jefferson University Hospital, Mayo Clinic, Hospital of the University of Pennsylvania, Emory University Hospital, University of Texas Southwestern Medical Center, Dartmouth-Hitchcock Medical Center, Columbia University Medical Center, National Institutes of Health, and University of Washington Medical Center. The research protocol was approved by the institutional review board (IRB) at each hospital and informed consent was obtained from each participant. Electrophysiological data were collected from electrodes implanted subdurally on the cortical surface as well as deep within the brain parenchyma. In each case, the clinical team determined the placement of the electrodes so as to best localize epileptogenic regions. Subdural contacts were arranged in both strip and grid configurations with an inter-contact spacing of 10 mm. Most subjects (N = 83) also had temporal lobe depth electrodes with 5 mm inter-contact spacing.
Verbal Memory Task
Each subject participated in a delayed free recall task in which they were instructed to study lists of words for a later memory test; no encoding task was used. Lists were composed of 12 words chosen at random and without replacement from a pool of high-frequency nouns (either English or Spanish, depending on the participant’s native language; http://memory.psych.upenn.edu/Word_Pools). Each word remained on the screen for 1,600 ms, followed by a randomly jittered 750- to 1,000 ms blank inter-stimulus interval (ISI).
Immediately after the final word in each list, participants performed a distractor task (20 s) consisting of a series of arithmetic problems of the form A + B + C = ?, where A, B, and C were randomly chosen integers ranging from 1 to 9. After the distractor task, participants were given 30 s to verbally recall as many words as possible from the list in any order; vocal responses were digitally recorded and later manually scored for analysis. Each session consisted of 25 lists of this encoding-distractor-recall procedure. A subset of subjects completed additional sessions of the free recall task using categorized word lists, which were included in the electrophysiological analyses. The categorized recall task is identical to the free recall task, with the exception that the word pool was drawn from 25 semantic categories (e.g., fruit, furniture, office supplies). Each list of 12 items in the categorized version of the task consisted of four words drawn from each of three categories. In total, 41 patients completed at least one session of the categorized recall task.
Stimulation Methods
At the start of each session, we determined the safe amplitude for stimulation using a mapping procedure in which stimulation was applied at 0.5 mA while a neurologist monitored for after discharges. This procedure was repeated, incrementing the amplitude in steps of 0.5 mA, up to a maximum of 1.5 mA for depth contacts and 3.5 mA for cortical surface contacts. These maximum amplitudes were chosen to be well below accepted safety limits for charge density [52]. For each stimulation session, we passed electrical current through a single pair of adjacent electrode contacts. Because the electrode locations were determined strictly by the monitoring needs of the clinicians, we used a combination of anatomical and functional information to select stimulation sites. If available, we prioritized electrodes in the hippocampus, entorhinal cortex, perirhinal cortex, parahippocampal cortex, and dorsolateral prefrontal cortex. To choose among these regions in cases in which more than one was available, we selected the electrode demonstrating the largest subsequent memory effect (SME) in the high-frequency range (70–200 Hz) among these regions. In cases in which none of the aforementioned regions was available, we selected the contact with the largest SME. We used a mapping procedure at the start of each session to determine the safe amplitude for stimulation. Stimulation was delivered using charge-balanced biphasic rectangular pulses (pulse width = 300 μs) at 50 Hz frequency, and was applied continuously for 4.6 s while subjects encoded two consecutive words; stimulation was not applied for the following two words. This alternation of stimulated and non-stimulated word pairs continued until the end of the list. Stimulation onset was 200 ms prior to word presentation and lasted until 200–450 ms after the offset of the next word (the range is due to the variable ISI between words). Stimulation was applied in 20 of the 25 lists in a session, and each stimulation list was randomly chosen to begin with a stimulated or non-stimulated pair of words. We randomized the order of the 20 stimulation lists and the remaining five non-stimulation control lists within each session.
Statistics
Data are presented as mean ± SEM. Unless otherwise specified, all statistical comparisons were conducted as two-tailed tests. Data distributions were either visually inspected or assumed to be normal for parametric tests. For both the record-only and stimulation samples, we included any enrolled subject who completed at least one full session of the task. In both cases, the sample sizes were chosen to at least match or exceed the sample sizes reported in prior human intracranial record-only and stimulation studies. For stimulation analyses, we treated all sessions collected in a single patient at a single stimulated bipolar pair as our unit of observation.
All other methods are described in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Intracranial brain stimulation has variable effects on episodic memory performance
Stimulation increased memory performance when delivered in poor encoding states
Recall-related brain activity increased after stimulation of poor encoding states
Neural activity linked to contextual memory predicted encoding state modulation
ACKNOWLEDGMENTS
We dedicate this paper in loving memory of Anastasia Lyalenko, without whose contributions this work would not have been possible. This work was supported by the DARPA Restoring Active Memory (RAM) program (cooperative agreement N66001-14-2-4032). We thank Blackrock Microsystems for providing neural recording and stimulation equipment. We thank J.A. Wachter for suggesting the analysis in Figure 4. We are indebted to the patients and their families for their participation and support. The views, opinions, and/or findings contained in this material are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government. B.C.J. receives research funding from NeuroPace and Medtronic not relating to this research. M.J.K. and D.S.R. are in the process of organizing Nia Therapeutics, LLC (“Nia”), a company intended to develop and commercialize brain stimulation therapies for memory restoration. Currently, Nia has no assets and has not commenced operations. M.J.K. and D.S.R. each holds a greater than 5% equity interest in Nia.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, three figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2017.03.028.
Data and Software Availability
All deidentified raw data and analysis code may be downloaded at http://memory.psych.upenn.edu/Electrophysiological_Data.
REFERENCES
- 1.Paller KA, and Wagner AD (2002). Observing the transformation of experience into memory. Trends Cogn. Sci 6, 93–102. [DOI] [PubMed] [Google Scholar]
- 2.Davachi L, Mitchell JP, and Wagner AD (2003). Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. USA 100, 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uncapher MR, and Wagner AD (2009). Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol. Learn. Mem 91, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H. (2011). Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage 54, 2446–2461. [DOI] [PubMed] [Google Scholar]
- 5.Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE, and Axmacher N. (2011). Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG. J. Neurosci 31, 5392–5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guderian S, Schott BH, Richardson-Klavehn A, and Duzel E. (2009). Medial temporal theta state before an event predicts episodic encoding success in humans. Proc. Natl. Acad. Sci. USA 106, 5365–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park H, and Rugg MD (2010). Prestimulus hippocampal activity predicts later recollection. Hippocampus 20, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutishauser U, Ross IB, Mamelak AN, and Schuman EM (2010). Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907. [DOI] [PubMed] [Google Scholar]
- 9.Hanslmayr S, Matuschek J, and Fellner MC (2014). Entrainment of prefrontal beta oscillations induces an endogenous echo and impairs memory formation. Curr. Biol 24, 904–909. [DOI] [PubMed] [Google Scholar]
- 10.Kim K, Ekstrom AD, and Tandon N. (2016). . A network approach for modulating memory processes via direct and indirect brain stimulation: toward a causal approach for the neural basis of memory. Neurobiol. Learn. Mem 134, 162–177. [DOI] [PubMed] [Google Scholar]
- 11.Kringelbach ML, Jenkinson N, Owen SLF, and Aziz TZ (2007). Translational principles of deep brain stimulation. Nat. Rev. Neurosci 8, 623–635. [DOI] [PubMed] [Google Scholar]
- 12.McIntyre CC, and Hahn PJ (2010). Network perspectives on the mechanisms of deep brain stimulation. Neurobiol. Dis 38,329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matzen LE, Trumbo MC, Leach RC, and Leshikar ED (2015). Effects of non-invasive brain stimulation on associative memory. Brain Res.1624, 286–296. [DOI] [PubMed] [Google Scholar]
- 14.Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, et al. (2011). Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch. Neurol 68, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, and de Rougemont J. (1991). Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337, 403–406. [DOI] [PubMed] [Google Scholar]
- 16.Seager MA, Johnson LD, Chabot ES, Asaka Y, and Berry SD (2002). Oscillatory brain states and learning: impact of hippocampal theta-contingent training. Proc. Natl. Acad. Sci. USA 99, 1616–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke JF, Ramayya AG, and Kahana MJ (2015). Human intracranial high-frequency activity during memory processing: neural oscillations or stochastic volatility? Curr. Opin. Neurobiol 31, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grober E, Lipton RB, Hall C, and Crystal H. (2000). Memory impairment on free and cued selective reminding predicts dementia. Neurology 54, 827–832. [DOI] [PubMed] [Google Scholar]
- 19.Gershberg FB,andShimamura AP(1995).Impaireduseoforganizational strategies in free recall following frontal lobe damage. Neuropsychologia 33, 1305–1333. [DOI] [PubMed] [Google Scholar]
- 20.Lohnas LJ, Polyn SM, and Kahana MJ (2015). Expanding the scope of memory search: modelingintralist and interlist effects in free recall. Psychol. Rev 122, 337–363. [DOI] [PubMed] [Google Scholar]
- 21.Haglund MM, Ojemann GA, and Blasdel GG (1993). Optical imaging of bipolar cortical stimulation. J. Neurosurg 78, 785–793. [DOI] [PubMed] [Google Scholar]
- 22.Suh M, Bahar S, Mehta AD, and Schwartz TH (2006). Blood volume and hemoglobin oxygenation response following electrical stimulation of human cortex. Neuroimage 31, 66–75. [DOI] [PubMed] [Google Scholar]
- 23.Borchers S, Himmelbach M, Logothetis N, and Karnath HO (2011). Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nat. Rev. Neurosci 13, 63–70. [DOI] [PubMed] [Google Scholar]
- 24.Hyman JM, Wyble BP, Goyal V, Rossi CA, and Hasselmo ME (2003). Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J. Neurosci 23, 11725–11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlides C, Greenstein YJ, Grudman M, and Winson J. (1988). Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res.439, 383–387. [DOI] [PubMed] [Google Scholar]
- 26.Pollen DA (1977). Responses of single neurons to electrical stimulation of the surface of the visual cortex. BrainBehav. Evol 14, 67–86. [DOI] [PubMed] [Google Scholar]
- 27.Fell J, Staresina BP, Do Lam AT, Widman G, Helmstaedter C, Elger CE, and Axmacher N. (2013). Memory modulation by weak synchronous deep brain stimulation: a pilot study. Brain Stimulat.6, 270–273. [DOI] [PubMed] [Google Scholar]
- 28.Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, and Fried I. (2012). Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med 366, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JP, Sweet JA, Bailey CM, Munyon CN, Luders HO, and Fastenau PS (2015). Visual-spatial memory may be enhanced with theta burst deep brain stimulation of the fornix: a preliminary investigation with four cases. Brain 138, 1833–1842. [DOI] [PubMed] [Google Scholar]
- 30.Coleshill SG, Binnie CD, Morris RG, Alarcon G, van Emde Boas W, Velis DN, Simmons A, Polkey CE, van Veelen CWM, and van Rijen PC (2004). Material-specific recognition memory deficits elicited by unilateral hippocampal electrical stimulation. J. Neurosci 24, 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs J, Miller J, Lee SA, Coffey T, Watrous AJ, Sperling MR, Sharan A, Worrell G, Berry B, Lega B, et al. (2016). Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron 92, 983–990. [DOI] [PubMed] [Google Scholar]
- 32.Halgren E, and Wilson CL (1985). Recall deficits produced by after-discharges in the human hippocampal formation and amygdala. Electroencephalogr. Clin. Neurophysiol 61, 375–380. [DOI] [PubMed] [Google Scholar]
- 33.Lacruz ME, Valentin A, Seoane JJG, Morris RG, Selway RP, and Alarcón G. (2010). Single pulse electrical stimulation of the hippocampus is sufficient to impair human episodic memory. Neuroscience 170, 623–632. [DOI] [PubMed] [Google Scholar]
- 34.Perrine K, Devinsky O, Uysal S, Luciano DJ, and Dogali M. (1994). Left temporal neocortex mediation of verbal memory: evidence from functional mapping with cortical stimulation. Neurology 44, 1845–1850. [DOI] [PubMed] [Google Scholar]
- 35.Manning JR, Jacobs J, Fried I, and Kahana MJ (2009). Broadband shifts in local field potential power spectra are correlated with singleneuron spiking in humans. J. Neurosci 29, 13613–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haufe S, Meinecke F, Gorgen K,Da€hne S, Haynes JD, Blankertz B, and Bießmann F. (2014). On the interpretation of weight vectors of linear models in multivariate neuroimaging. Neuroimage 87, 96–110. [DOI] [PubMed] [Google Scholar]
- 37.Long NM, Burke JF, and Kahana MJ (2014). Subsequent memory effect in intracranial and scalp EEG. Neuroimage 84, 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Stein A, and Sarnthein J. (2000). Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol 38, 301–313. [DOI] [PubMed] [Google Scholar]
- 39.Winawer J, Kay KN, Foster BL, Rauschecker AM, Parvizi J, and Wandell BA (2013). Asynchronous broadband signals are the principal source of the BOLD response in human visual cortex. Curr. Biol 23, 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR, and Kahana MJ (2014). Human intracranial high-frequency activity maps episodic memory formation in space and time. Neuroimage 85, 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long NM, and Kahana MJ (2015). Successful memory formation is driven by contextual encoding in the core memory network. Neuroimage 119, 332–337. [DOI] [PubMed] [Google Scholar]
- 42.Benabid AL, Pollak P, Louveau A, Henry S, and de Rougemont J. (1987). Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Stereotact. Funct. Neurosurg 50, 344–346. [DOI] [PubMed] [Google Scholar]
- 43.Gu S, Pasqualetti F, Cieslak M, Telesford QK, Yu AB, Kahn AE, Medaglia JD, Vettel JM, Miller MB, Grafton ST, and Bassett DS (2015). Controllability of structural brain networks. Nat. Commun 6, 8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, and Pascual-Leone A. (2014). Resting-state networks link invasive and non-invasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci. USA 111, E4367–E4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, Hermiller MS, and Voss JL (2014). Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 345, 1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salari N, and Rose M. (2016). Dissociation of the functional relevance of different pre-stimulus oscillatory activity for memory formation. Neuroimage 125, 1013–1021. [DOI] [PubMed] [Google Scholar]
- 47.Merkow MB, Burke JF, Stein JM, and Kahana MJ (2014). Prestimulus theta in the human hippocampus predicts subsequent recognition but not recall. Hippocampus 24, 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young NP, and Deisseroth K. (2017). Cognitive neuroscience: in search of lost time. Nature 542, 173–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.deBettencourt MT, Cohen JD, Lee RF, Norman KA, and TurkBrowne NB (2015). Closed-loop training of attention with real-time brain imaging. Nat. Neurosci 18, 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukuda K, and Woodman GF (2015). Predicting and improving recognition memory using multiple electrophysiological signals in real time. Psychol. Sci 26, 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, and Kennedy SH (2008). Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 64, 461–467. [DOI] [PubMed] [Google Scholar]
- 52.Shannon RV (1992). A model of safe levels for electrical stimulation. IEEE Trans. Biomed. Eng 39, 424–426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.