Abstract
Endothelial cells sense changes in blood flow shear stress and affect the progression of atherosclerotic plaques. Pyroptosis is an inflammatory form of cell death and has been implicated in cardiovascular diseases. Melatonin and its nuclear receptor retinoid-related orphan receptor α (RORα) have protective effects on the development of atherosclerosis. To date, whether melatonin can prevent endothelial cell pyroptosis and dysfunction in pathological shear stress remains unclear. In the present study, human umbilical vein endothelial cells (ECs) were cultured under low shear stress conditions (5 dyne/cm2) for 24 h and treated with or without melatonin (2 µmol/l). The binding sites of the microRNA (miR)-223 promoter and RORα were predicted using the JASPAR website. Expression of pyroptosis-related proteins, including cleaved N-terminal gasdermin D, caspase-1, intercellular adhesion molecule 1 (ICAM-1) and nitric oxide (NO) were assessed. The results indicated that low shear stress increased pyroptosis and ICAM-1 expression, whereas it decreased NO levels. Melatonin alleviated pyroptosis and ICAM-1 expression and increased the production of NO in ECs. Further assessment revealed that low-level shear stress decreased RORα protein and mRNA expression, whereas melatonin would bind to RORα and thereby promoted miR-223 transcription in ECs. The present study also identified signal transducer and activator of transcription 3 (STAT-3) as a potential target gene of miR-223-3p. When transfected with miR-223 inhibitor, ECs up-regulated the expression of pyroptosis-related proteins and ICAM-1, and down-regulated NO levels. By contrast, silencing STAT-3 expression diminished the protective effect of miR-223. These results indicated that melatonin prevented ECs from undergoing pyroptosis and alleviated dysfunction via the RORα/miR-223/STAT-3 signalling pathway. This information could aid in the development of novel therapeutic approaches and provide new insights into atherosclerosis.
Keywords: melatonin, low shear stress, retinoic acid-related orphan receptor-α, endothelial dysfunction, pyroptosis
Introduction
Intensive care units typically treat numerous patients with cardiovascular and cerebral vascular diseases associated with atherosclerosis. In addition to traditional risk factors, mounting evidence indicates that shear stress is involved in the initiation and development of atherosclerosis (1). Endothelial cells (ECs) located in the innermost site of the vascular wall, sense changes in blood flow shear stress and affect the development of atherosclerotic plaques via intracellular signal regulatory factors, gene expression and specific transcription factors (2). Studies show that low-level shear stress (≤5 dynes/cm2) favours the occurrence of atherosclerosis and plaque growth (3), whereas high-level shear stress displays an anti-atherosclerotic effect (4). Therefore, it is important to demonstrate the underlying mechanism between low shear stress and the gene regulation network in atherosclerosis.
Melatonin is a notable endocrine hormone secreted by the pineal gland in a rhythmic manner. Melatonin exhibits diverse biological functions against the development of atherosclerosis, including antioxidant and anti-inflammatory functions (5). Multiple melatonin functions are mediated by membrane or nuclear receptors (6). Among them, retinoid-related orphan receptor α (RORα) is the nuclear receptor of melatonin (7). RORα regulates the expression of numerous genes at the transcriptional level and participates in a number of biological processes, including anti-inflammatory and anti-apoptotic processes (8). A previous study also revealed that microRNA (miRNA/miR)-223 inhibits signal transducer and activator of transcription 3 (STAT-3) signalling pathway activation and inhibits vascular calcification of smooth muscle cells (9). In addition, via bioinformatical analysis, the present study revealed that melatonin potentially binds to the miR-223 promoter and promotes miR-223 transcription.
To validate this hypothesis, the present study was designed to assess the biological effect of melatonin on EC pyroptosis and dysfunction induced by low shear stress and to demonstrate the notable role of the RORα-miR-223/STAT-3 signalling pathway.
Materials and methods
Cell culture and transfection
Human immortalized umbilical vein ECs, purchased from the China Infrastructure of Cell Line Resource, were cultured on rectangular glass slides (length, ~4x3-cm2) in Dulbecco's Modified Eagle's Medium supplemented with 10% foetal bovine serum (both Invitrogen; Thermo Fisher Scientific, Inc.) and maintained at 37˚C with 5% CO2. The cell line was certified by the supplier using the short tandem repeat method. When 70-80% confluence was achieved, ECs were transfected with pcDNA-RORα/pGL3-basic-miR-223 promoter plasmid (1 µg/µl), small interfering (si) RORα, miR-223 inhibitor, miR-223 mimics, siSTAT-3 or the siSTAT-3 scrambled control with each final concentration at 100 nM, which were synthesized by Sangon Biotech Co., Ltd. Empty plasmids were used as the control for plasmid transfection. For siRNAs transfection, the control was the siRNA scrambled group. The negative control with the mutant sequence of miR-223 was the control group for the miR transfection. All of the transfections were performed using Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. For the siRNAs and miR transfections, the sequences of all constructs were provided in Table SI and transfection was confirmed using PCR or western blotting (Fig. S1, Fig. S2 and Fig. S3).
ECs cultured with low shear stress
After 24 h transfection, the glass slides were placed into the plate flow chamber culture system (Shanghai Naturethink Life & Scientific Co., Ltd.) with or without low shear stress (5 dynes/cm2) treatment for 24 h. ECs were then further treated with or without melatonin (2 µmol/l; cat. no. M5250; Sigma-Aldrich; Merck KGaA) for another 24 h at 37˚C. In brief, the experimental groups were defined as follows: i) Static group; ii) low shear stress group; iii) static plus melatonin group; and iv) low shear stress plus melatonin group.
Bioinformatical analysis
JASPAR is an open-access database of curated, non-redundant transcription factor (TF) binding profiles stored as position frequency matrices and TF flexible models for TFs across multiple species. The candidate transcription factor of the target miRNA (NCBI gene ID: 407008) was predicted using the JAPAR website (v8; http://jaspar.genereg.net/) with-3000 to 100 bp of the transcription starting point. The possible binding sites with a score provided by the website of >0.9 were selected.
Dual-luciferase reporter assays
A dual-luciferase reporter assay was performed to further validate the results of the predicted binding site of RORα with the miR-223 promoter. Luciferase reporter plasmids (pGL3-basic; Sangon Biotech Co., Ltd.) were constructed with full-length or truncated promoters of miR-223. In addition, the full-length RORα gene was cloned into the pcDNA3.0 (Sangon Biotech Co., Ltd.) plasmid to overexpress RORα. The pcDNA3.0 plasmid cloned with the RORα gene was co-transfected with the pGL3-basic plasmid cloned with the miR-223 promoter and Renilla luciferase reporter plasmids (Promega Corporation) into 293 cells at 37˚C for 24 h using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). After another 24 h, firefly and Renilla luciferase activities were measured in 293 cells using the Dual-Luciferase Reporter Assay system (Dual-Glo®; cat. no. E2920; Promega Corporation). The sequences of the miRNA-223 mimics and inhibitor are presented in Table SI.
Western blotting
ECs were lysed in RIPA lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.), and the protein concentration of samples was detected using the BCA method. Protein samples (20 µg/well) were loaded onto a 10% SDS polyacrylamide gel and transferred onto a PVDF membrane. After blocking with 5% BSA for 2 h at room temperature, the membrane was incubated with primary antibodies overnight at 4˚C. The concentration of primary antibodies and manufacturers' information are provided as follows: Cleaved caspase-1 (1:1,000; cat. no. ab207802; Abcam), Cleaved N-terminal gasdermin D (GSDMD-N; 1:1,000; cat. no. ab215203; Abcam) and monoclonal STAT-3 (1:1,000; cat. no. #9139; Cell Signaling Technology, Inc.). Membranes were then washed in TBST (0.1% Tween-20) routinely and incubated with the horseradish peroxide-conjugated goat anti-mouse/rabbit IgG secondary antibody (1:10,000; cat. nos. 21010/21020, respectively; Abbkine Scientific Co., Ltd.) at room temperature for 1 h. GAPDH (1:10,000; cat. no. K200057M; Beijing Solarbio Science & Technology Co., Ltd.) served as the internal control. Western blotting bands were visualised using the ECL method with a TIANGEN Imaging system (Tiangen Biotech Co., Ltd.), and quantification analysis was performed using ImageJ software v1.46 (National Institutes of Health).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from ECs using the FastPure Cell/Tissue Total RNA Isolation kit (cat. no. RC112-01; Vazyme Biotech Co., Ltd.) according to the manufacturer's instructions. Total RNA was reverse transcribed using the HiScript II 1st Strand cDNA Synthesis kit (cat. no. R211-01/02; Vazyme Biotech Co., Ltd.) and the RT kit was used according to the manufacturer's protocol. Quantitative real-time PCR was performed using the ChamQ Universal SYBR® qPCR Master Mix (Q711-02/03; Vazyme Biotech Co., Ltd.) and GAPDH served as a housekeeping control. The primers are as follows: GAPDH Forward, 5'-CATACCAGGAAATGAGCTTG-3', and reverse, 5'-ATGACATCAAGAAGGTGGTG-3'; STAT-3 forward, 5'-CGGA GAAGCATCGTGAGTGAGC-3' and reverse, 5'-GTTGCCGCCTCTTCCAGTCAG-3'; miR-223 forward, 5'-GGCAGCACCCCATAAACTGTT-3', and reverse 5'-CAGTGCGTGTCGTGTCGTGGAG-3'; RORα forward 5'-GATCGCTCGTGGCTTCAGGAA-3', and reverse, 5'-TGGAGGAAAATGGAGTCGCACA-3'; GSDMD forward 5'-CCATCGGCCTTTGAGAAAGTG-3', and reverse, 5'-ACACATGAATAACGGGGTTTCC-3'; caspase-1 forward, 5'-GGTCCTGAAGGAGAAGAGAA-3' and reverse, 5'-AGGCCTGGATGATGATCACC-3'. The PCR parameters were 95˚C for 30 sec followed by 40 cycles of 95˚C for 10 sec and 60˚C for 30 sec. The results between different groups were calculated using the 2-ΔΔCq method (10).
Enzyme-linked immunosorbent assay (ELISA)
IL-1β and IL-18 expression levels were detected using ELISA. After treatment, 100 µl of the undiluted supernatants of HUVECs treated with static or low shear stress supplemented with or without melatonin were prepared for ELISA measurement. The human IL-1β (cat. no. 214025) and human IL-18 (cat. no. 215539) ELISA kits were purchased from Abcam. ELISA was performed according to the manufacturer's instructions.
Nitric oxide (NO) test
The concentration of NO was detected using a Griess Reagent assay. Briefly, the supernatants were collected from HUVECs treated with static or low shear stress supplemented with or without melatonin, and NO measurement was performed according to the instructions of the commercial NO kit (Griess Reagent kit; cat. no. S0021S; Beyotime Institute of Biotechnology). The NO concentration was calculated based on the optical density value of the supernatants detected by the microplate reader at a 550-nm wavelength.
Immunofluorescent analysis
After ECs were treated with low shear stress for 24 h, they were fixed with 4% formaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 5 min both at room temperature and then washed with PBS three times for 3 min each. Subsequently, the cells were blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 60 min. The samples were incubated with intercellular adhesion molecule 1 (ICAM-1; 1:1,000; cat. no. ab109361; Abcam) overnight at 4˚C. The cells were then washed thrice with PBS for 3 min each and incubated with a goat anti-rabbit IgG (H+L) Fluor647-conjugated secondary antibody (1:200; cat. no. S0013; Affinity Biosciences) for 60 min at room temperature. After washing with PBS three times for 3 min each time, the samples were covered with DAPI mounting fluid. The images were captured using a laser confocal microscope (A1R; Nikon Corporation), and ImageJ software was used to analyse the fluorescent density of the images.
Statistical analysis
Statistical analyses were performed using Excel 2007 (Microsoft Corporation) and GraphPad Prism software v7.0 (GraphPad Software, Inc.). Error bars are reported as the standard error of mean. Pairwise comparisons were performed using unpaired two tailed Student's t-test or one-way ANOVA followed by Tukey's post hoc test where appropriate. A total of three biologically independent experiments were performed for each quantified western blotting experiment.
Results
Melatonin suppresses pyroptosis in ECs
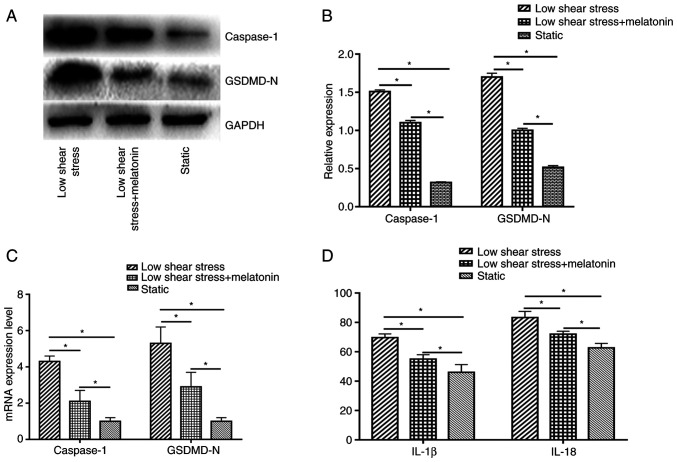
To study the effect of pyroptosis in ECs exposed to low shear stress and the protective effect of melatonin, the expression levels of pyroptosis-related proteins were measured, including caspase-1 and GSDMD-N. The results indicated that low shear stress significantly induced pyroptosis-related protein expression compared with the static group; whereas treatment with melatonin significantly decreased the expression of caspase-1 and GSDMD-N compared with the low shear stress group (Fig. 1A and B). In addition, Caspase-1 and GSDMD-N mRNA expression levels were significantly higher in ECs exposed to low-level shear stress compared with ECs exposed to static stress, and were significantly suppressed by melatonin in ECs exposed to low-level shear stress (Fig. 1C). The expression levels of cytokines were also detected, including IL-18 and IL-1β. Low-level shear stress significantly increased the expression levels of IL-18 and IL-1β compared with the static group, while melatonin suppressed the secretion of IL-18 and IL-1β compared with the low shear stress group (Fig. 1D).
Figure 1.
Melatonin suppresses pyroptosis in ECs. (A) Representative immunoblots and (B) quantitative analysis of caspase-1 and GSDMD-N of ECs. (C) Relative mRNA expression of caspase-1 and GSDMD-N of ECs. (D) Concentration of IL-1β and IL-18 in ECs. *P<0.05. GSDMD-N, gasdermin D N-terminal domain; ECs, endothelial cells.
Melatonin ameliorates EC dysfunction
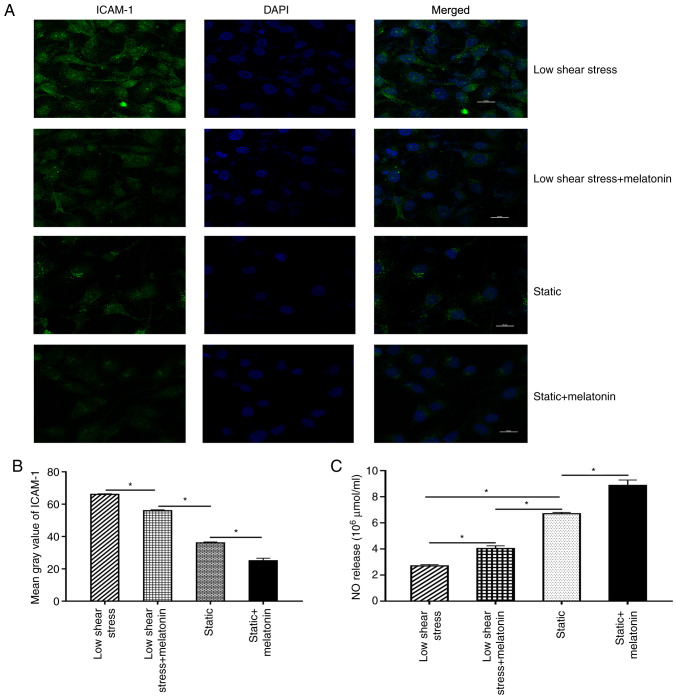
Next, EC dysfunction was further evaluated by measuring the expression levels of ICAM-1 and NO. Briefly, immunofluorescence staining was performed to evaluate the expression of ICAM-1 in ECs. The results revealed that the mean grey value of ICAM-1 in the low shear stress treatment group was significantly increased compared with that in the static group. After melatonin treatment, the mean grey value of ICAM-1 decreased significantly compared with that of the low shear stress group (Fig. 2A and B). In addition, the effect of melatonin on NO expression in ECs was validated. The results revealed that NO levels were significantly lower in ECs subject to low-level shear stress compared with the static group. Moreover, treatment with melatonin and low shear stress significantly increased NO expression compared with that in the low shear stress only group (Fig. 2C).
Figure 2.
Melatonin ameliorates the dysfunction of ECs. (A) Representative fluorescent images. (B) Mean grey value of ICAM-1 in ECs. (C) NO release of ECs. *P<0.05. ICAM-1, intercellular adhesion molecule 1; NO, nitric oxide; ECs, endothelial cells.
Melatonin suppresses pyroptosis through RORα
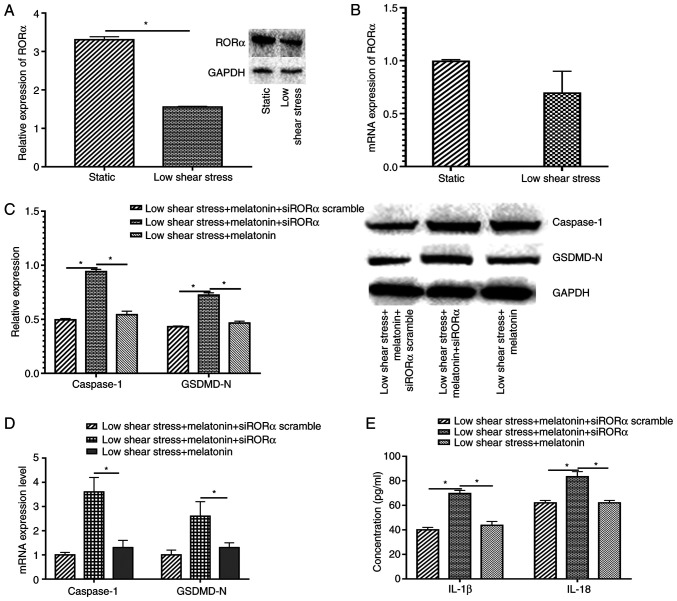
To explore the protective mechanism of melatonin in ECs exposed to low level shear stress, the relative RORα mRNA and protein expression levels were measured in ECs with or without low level shear stress treatment. The results indicated that low shear stress significantly decreased RORα protein expression and markedly decreased mRNA expression compared with the static group (Fig. 3A and B). Furthermore, when transfected with siRORα, the expression levels of pyroptosis-related proteins in melatonin treated ECs increased compared with those in the siRORα group (Fig. 3C). The expression of pyroptosis-related proteins at the mRNA level were also detected in ECs transfected with siRORα and treated with melatonin. The results demonstrated that caspase-1 and GSDMD-N expression significantly increased compared with that of the untransfected low shear stress and melatonin-treated group (Fig. 3D). The expression levels of IL-18 and IL-1β were detected, and the data revealed that ECs treated with siRORα exhibited significantly increased IL-18 and IL-1β secretion compared with the siRORα group or the scrambled group (Fig. 3E).
Figure 3.
Melatonin suppresses pyroptosis through RORα. Relative (A) protein and (B) mRNA expression levels of RORα in static and low shear stress groups. (C) Relative protein expression of caspase-1 and GSDMD-N of ECs. (D) Relative mRNA expression of caspase-1 and GSDMD-N of ECs. (E) Concentration of IL-1β and IL-18 of ECs. *P<0.05. GSDMD-N, gasdermin D N-terminal domain; RORα, retinoid-related orphan receptor α; ECs, endothelial cells; si, small interfering.
Melatonin ameliorates EC dysfunction through RORα
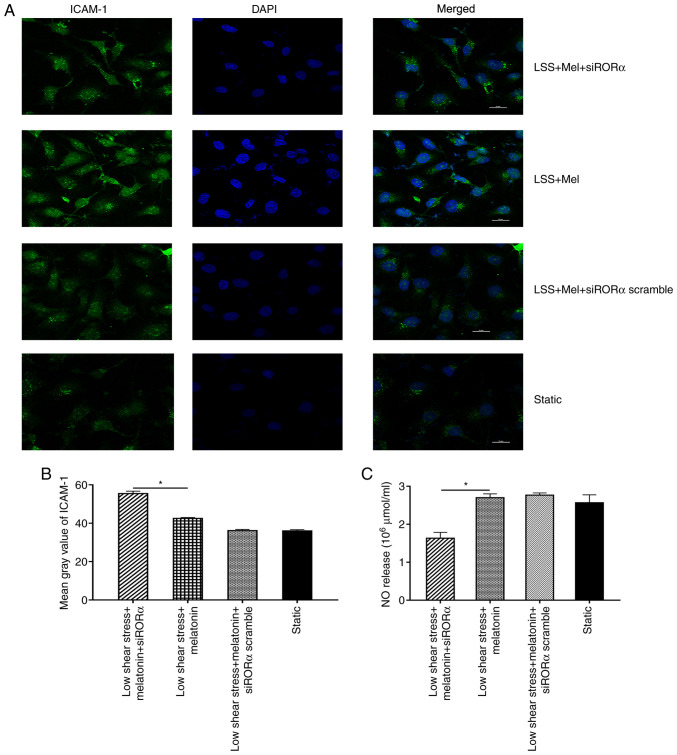
ICAM-1 and NO expression levels were analysed in ECs transfected with siRORα. The results demonstrated that the mean grey value of ICAM-1 in ECs transfected with siRORα was significantly increased compared with that in untransfected ECs treated with melatonin (Fig. 4A and B). In addition, NO expression was detected in ECs exposed to low shear stress. The results revealed that NO expression in ECs transfected with siRORα was significantly decreased compared with that of untransfected ECs treated with melatonin (Fig. 4C).
Figure 4.
Melatonin ameliorates the dysfunction of ECs through RORα. (A) Representative fluorescent images. (B) Mean grey value of ICAM-1 in ECs. (C) NO release of ECs. *P<0.05. LSS, low shear stress; Mel, melatonin; ICAM-1, intercellular adhesion molecule 1; ECs, endothelial cells; NO, nitric oxide; si, small interfering; RORα, retinoid-related orphan receptor α.
Melatonin regulates miR-223 expression via RORα
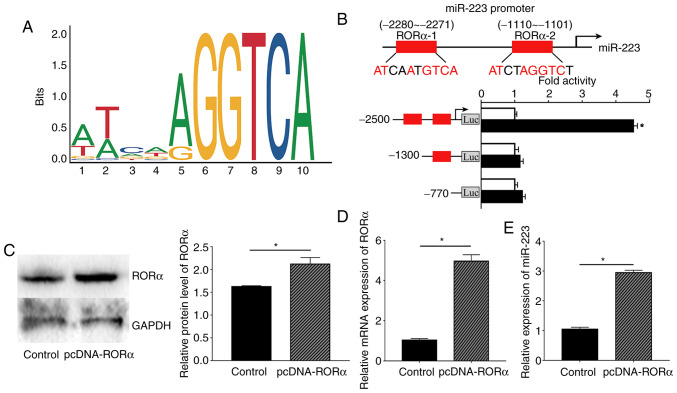
To further study the underlying mechanism by which melatonin affects miR-223 expression in ECs, the miR-223 promoter region was analysed and revealed to be a possible putative binding site of RORα using the JAPAR website. First, the oligonucleotide sequence of the transcription factor binding site of RORα with the miR-223 promoter region was predicted (Fig. 5A). Next, the possible binding sites with a score provided by the website of >0.9 were selected; therefore, two binding sites were selected as the candidate binding sites, -2280 to -2271 bp and -1110 to -1101 bp. According to the dual-luciferase results, the luciferase activity of RORα bound to the-2280 to -2271 site was considerably increased compared with that obtained from binding to the -1110 to -1101 site. These results indicated that RORα could bind to the promoter region of miR-223 at -2280 to -2271 bp, which also had the highest score according to the JASPAR website (Fig. 5B).
Figure 5.
RORα mediates the transcription of miR-223 in ECs. (A) Depiction of the oligonucleotides sequence of transcription factor binding site. The height measured by bits of each letter is proportional to the frequency of occurrence of the corresponding bases at this position. (B) Upper: Schematic representation of the RORα binding site in miR-223 promoter presented as red boxes. Putative RORα binding site located at -2280 to -2271 and -1110 to -1101 bp upstream of the transcription initiation site in the hsa-miR-223 promoter. Lower: Luciferase activity of RORα bound to -2280 to -2271 site was increased compared with that bound to the -1110 to -1101 site, indicating that RORα could bind to the promoter region of miR-223 at -2280 to -2271 bp. (C) RORα protein levels in ECs. (D) Relative mRNA expression level of RORα in ECs. (E) Relative expression of miR-223 in ECs. *P<0.05. pcDNA-RORα, pcDNA 3.0 plasmid expressing full length human RORα gene; RORα, retinoid-related orphan receptor α; ECs, endothelial cells; miR, microRNA; Luc, luciferase.
Based on previous results, RORα was confirmed to bind to the miR-223 promoter region. Moreover, the present study further investigated whether RORα could regulate the expression of miR-223 in ECs. Relative RORα protein and mRNA expression in ECs were measured to confirm that the pcDNA3.0-RORα plasmid was successfully transfected into ECs. As expected, both RORα protein and mRNA expression levels were significantly higher compared with those in the control group (Fig. 5C and D). Next, ECs transfected with the RORα plasmid exhibited increased expression of miR-223 compared with the control group (Fig. 5E).
Melatonin prevents pyroptosis and dysfunction through the RORα/miR-223/STAT-3 signalling pathway
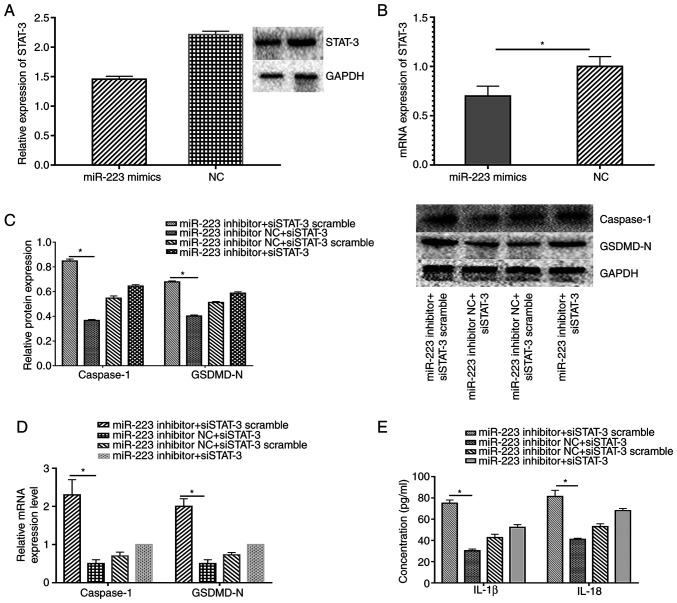
miR-223 regulates STAT-3 expression at the posttranscriptional level (9). In the present study, miR-223 and STAT-3 expression levels were altered to validate whether the RORα-miR-223/STAT-3 signalling pathway was involved in the regulation of melatonin in ECs exposed to low shear stress. As expected, miR-223 up-regulation decreased STAT-3 expression at the protein and mRNA level in ECs treated with low shear stress compared with the negative control (Fig. 6A and B). In addition, the silencing of STAT-3 down-regulated the expression levels of caspase-1 and GSDMD-N compared with the control group, whereas the miR-223 inhibitor partially counteracted the protective effect of silencing STAT-3 in ECs (Fig. 6C and D). IL-18 and IL-1β secretion displayed the same trend in ECs (Fig. 6E).
Figure 6.
Melatonin exhibits a protective effect via the miR-223/STAT-3 signalling pathway. Relative (A) protein and (B) mRNA expression of STAT-3 in ECs transfected with miR-223 mimics and NC. Relative (C) protein and (D) mRNA expression levels of caspase-1 and GSDMD-N of ECs co-transfected with the miR-223 inhibitor and siSTAT-3. (E) Concentration of IL-1β and IL-18 of ECs. *P<0.05. GSDMD-N, gasdermin D N-terminal domain; miR, microRNA; STAT-3, signal transducer and activator of transcription 3; ECs, endothelial cells; NC, negative control; si, small interfering.
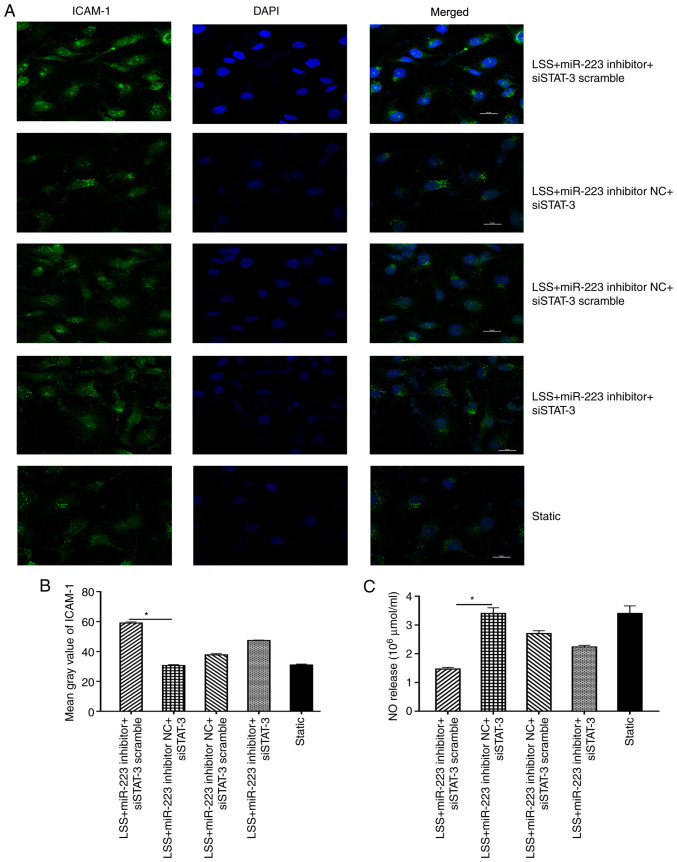
Furthermore, silencing of STAT-3 (miR-223 inhibitor NC + siSTAT-3) resulted in the decreased expression of ICAM-1 compared with the control (miR-223 inhibitor NC+siSTAT-3 scramble) group, while introduction of miR-223 inhibitor (miR-223 inhibitor+siSTAT-3) markedly counteracted this trend (Fig. 7A and B). The expression level of NO was also suppressed when ECs were transfected with miR-223 inhibitor compared with the inhibitor negative control, and this trend could be reversed by transfection with siSTAT-3 in ECs subjected to low shear stress treated with melatonin (Fig. 7C).
Figure 7.
Melatonin ameliorates the dysfunction of ECs via the miR-223/STAT-3 signalling pathway. (A) Representative fluorescent images. (B) Mean grey value of ICAM-1 in ECs co-transfected with STAT-3 and the miR-223 inhibitor. (C) NO release of ECs co-transfected with STAT-3 and the miR-223 inhibitor. *P<0.05. ICAM-1, intercellular adhesion molecule 1; miR, microRNA; NC, negative control; si, small interfering; STAT-3, signal transducer and activator of transcription 3; ECs, endothelial cells; LSS, low shear stress.
Discussion
EC pyroptosis and dysfunction are considered to be the major causes of the initiation and development of numerous atherosclerotic cardiovascular diseases (11). The present paper demonstrated that melatonin could induce miR-223 expression by binding to the promoter of miR-223. Furthermore, melatonin attenuated low-level shear stress-induced ECs pyroptosis and dysfunction via the ROR-α/miR-223/STAT-3 signalling pathway. The present paper demonstrated that low shear stress-induced EC dysfunction, and that melatonin prevented ECs pyroptosis and dysfunction. Moreover, the anti-atherosclerotic effect of melatonin was demonstrated to be associated with its nuclear receptor, RORα. Overall, the present study provided a new therapeutic approach for melatonin in cardiovascular disease.
The Intensive Care Unit Department in Second Affiliated Hospital of Dalian Medical University (Dalian, China) normally treats numerous patients with severe cardiovascular diseases, including acute myocardial infarction, stroke and aortic dissection. Vascular shear stress plays a notable role in the onset and development of these diseases (12). High physiological shear stress is hypothesised to be anti-atherosclerotic, whereas low shear stress is associated with pro-atherosclerosis effects (13). EC dysfunction is a notable contributor to the local and systemic manifestations of atherosclerotic cardiovascular disease (14). Therefore, it is of importance to inhibit endothelial dysfunction to prevent atherosclerosis induced by low shear stress. The present study revealed that low shear stress, which is associated with atherosclerosis, increased the incidence of pyroptosis and induced the expression of cell adhesion molecules. These results indicated that low shear stress could damage the physiological function of ECs and might be associated with atherosclerosis.
Melatonin regulates numerous biological functions, including antioxidant effects, anti-inflammatory processes, sleep regulation and immune regulation (15). Melatonin plays biological functions mainly via: i) Membrane receptors, such as high-affinity G protein-coupled receptors (MT)1 and MT2; ii) nuclear receptors, such as RORα (16); iii) interactions with cytoplasmic proteins, such as calmodulin and hydroquinone; and iv) receptor-independent actions, such as scavenging reactive oxygen species/reactive nitrogen species (17). A previous study indicated that melatonin ameliorates intraplaque inflammation in a rupture-prone vulnerable carotid plaque model induced by low shear stress in apolipoprotein E-/- mice in a RORα-dependent manner (18). Another previous study demonstrated that RORα may modulate pro-inflammatory gene expression in atherosclerosis (19). Researchers have also reported that RORα suppresses the expression of cyclooxygenase-2, IL-6 and IL-8 induced by TNF-α in vascular smooth muscle cells (20). In addition, RORα expression levels in atherosclerotic plaques are suppressed compared with those in healthy controls (21). Consistent with the aforementioned evidence, the present paper revealed that low shear stress could suppress the expression of RORα and that the STAT-3 signalling pathway may be associated with RORα. Both RORα and STAT-3 are notable molecules involved in the regulation of inflammatory processes in atherosclerosis. The present paper demonstrated that RORα negatively regulated STAT-3 and further decreased the expression levels of the target molecules of STAT-3.
The regulation of biological development involves the regulation of transcription factors, non-coding RNA, DNA modification and other multi-level regulation mechanisms, in which transcription factors and miRNAs are closely associated in the regulatory network (22). The expression levels of miRNAs are regulated by complex transcription factors, and the expression of transcription factors themselves are regulated by miRNAs (23). Previous research has mainly focused on the expression of miRNAs in the regulation of melatonin. For instance, Zhang et al (24) demonstrated that melatonin could suppress long non-coding RNA maternally expressed-3 expression, and by doing so, increase miR-223 expression to prevent pyroptosis in atherosclerosis via competing endogenous RNA theory. This study mainly explores the relationship between melatonin and non-coding RNA. However, a few associated studies have researched the effect of melatonin on upstream transcriptional regulation of miRNAs (25-28). The present study revealed a novel mechanism by which melatonin regulated miRNA transcription in cardiovascular disease, and this regulation was dependent on the RORα pathway. Overexpression of RORα in ECs induced the expression of miR-223. In previous years, research has indicated that the abnormal expression of RORα can cause atherosclerosis (29). Wang et al (30) demonstrated that 7-oxysterol, an inverse agonist of RORα, inhibits its transcriptional activity and reduces the anti-atherosclerotic effects of RORα. Together with these findings, the present study provided novel ideas about how to prevent atherosclerosis with melatonin.
Cell death and inflammation play a pivotal role in the occurrence and development of atherosclerosis (31). Pyroptosis is an inflammatory form of cell death and is thought to be associated with multiple cardiovascular diseases (32). Yin et al (33) demonstrated that hyperlipidaemia stimulates caspase-1 activation and pyroptosis occurrence in ECs; in addition to increasing adhesion molecule expression and triggering monocyte adhesion to ECs. The present study demonstrated that low shear stress also stimulated the development of pyroptosis in ECs, which was consistent with the hypothesis that low shear stress is pro-atherosclerotic in cardiovascular disease (34). A previous study has demonstrated that pyroptosis of ECs induced by low shear stress plays an important role in the initiation and progression of atherosclerosis (35). Furthermore, the present study revealed that down-regulating STAT-3 expression was associated with a reduction in the expression of pyroptosis-related proteins, whereas exposure to a miR-223 inhibitor counteracted these effects. These findings indicated that the miR-223/STAT-3 signalling pathway plays a notable role in the regulation of pyroptosis in ECs.
ICAM-1 is an important glycoprotein molecule. Injured ECs exhibit increased ICAM-1 expression compared with healthy ECs, and atherosclerotic lesion areas also exhibit increased ICAM-1 expression (36). Elevated levels of adhesion molecules are associated with the severity of acute coronary syndrome (37). A number of studies have also demonstrated that the type, exposure time and magnitude of shear stress affects ICAM-1 expression (38-40). Acute exercise may increase cardiac ICAM-1 expression accompanied by a significant increase in inflammatory mediators (41). Melatonin administration reverses this effect, suggesting its protective effect against cardiac damage induced by exercise (42). A number of molecules can regulate the expression of ICAM-1 at the transcriptional level (43). The present study also indicated that ICAM-1 expression was induced by low shear stress and that melatonin reversed this effect. A previous study indicated that the STAT-3 signalling pathway plays an important role in the regulation of ICAM-1(44). The results of the current paper further validated that silencing of STAT-3 expression suppressed the expression of ICAM-1, whereas exposure to amiR-223 inhibitor increased ICAM-1 expression. Therefore, the present study provided evidence that the miR-223/STAT-3 signalling pathway was involved in the regulation of ICAM-1 in ECs treated with melatonin.
There are also some limitations in the current study. First, the present paper did not include a high shear stress treatment group. According to the relevant references, low shear stress is pro-atherosclerotic factor, whereas high shear stress inhibits atherosclerosis (45-48). Future studies should include a high shear stress group to certify that melatonin could inhibit pyroptosis in ECs under multiple types of shear stress via a RORα-dependant manner. Secondly, the present study did not compare the effect of different concentration of melatonin on ECs dysfunction. In the future, the effect of different concentration of melatonin on pyroptosis in ECs treated with shear stress will be examined.
In conclusion, the results of the current study demonstrated that melatonin exerted its protective effect via RORα in ECs exposed to low shear stress. Melatonin decreased pyroptosis and ICAM-1expression and increased NO bioavailability. The present study also indicated that the RORα/miR-223/STAT-3 signalling pathway may represent a promising therapeutic target in atherosclerotic disease. However, considering that the pathophysiology of atherosclerosis and the effects of melatonin are extremely complicated, further studies are needed to demonstrate the mechanism of melatonin in atherosclerosis. The present paper provided a novel therapeutic approach and insights into atherosclerosis.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SY and YY confirm the authenticity of all the raw data. SY conceived the study, analysed the data and participated in writing, review and editing the manuscript. YY conceived the study, performed the methodology, data collection and formal analysis and wrote the original draft. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Collet C, Onuma Y, Sonck J, Asano T, Vandeloo B, Kornowski R, Tu S, Westra J, Holm NR, Xu B, et al. Diagnostic performance of angiography-derived fractional flow reserve: A systematic review and Bayesian meta-analysis. Eur Heart J. 2018;39:3314–3321. doi: 10.1093/eurheartj/ehy445. [DOI] [PubMed] [Google Scholar]
- 2.Roux E, Bougaran P, Dufourcq P, Couffinhal T. Fluid shear stress sensing by the endothelial layer. Front Physiol. 2020;11(861) doi: 10.3389/fphys.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morita T, Kurihara H, Maemura K, Yoshizumi M, Nagai R, Yazaki Y. Role of Ca2+ and protein kinase C in shear stress-induced actin depolymerization and endothelin 1 gene expression. Circ Res. 1994;75:630–636. doi: 10.1161/01.res.75.4.630. [DOI] [PubMed] [Google Scholar]
- 4.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 5.Sun H, Gusdon AM, Qu S. Effects of melatonin on cardiovascular diseases: Progress in the past year. Curr Opin Lipidol. 2016;27:408–413. doi: 10.1097/MOL.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pourhanifeh MH, Dehdashtian E, Hosseinzadeh A, Sezavar SH, Mehrzadi S. doi: 10.1007/s10557-020-07052-3. Clinical application of melatonin in the treatment of cardiovascular diseases: Current evidence and new insights into the cardioprotective and cardiotherapeutic properties. Cardiovasc Drugs Ther, Sep 14, 2020 (Epub ahead of print) doi: 10.1007/s10557-020-07052-3. [DOI] [PubMed] [Google Scholar]
- 7.Cook DN, Kang HS, Jetten AM. Retinoic acid-related orphan receptors (RORs): Regulatory functions in immunity, development, circadian rhythm, and metabolism. Nucl Receptor Res. 2015;2(101185) doi: 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao CN, Wang P, Mao YM, Dan YL, Wu Q, Li XM, Wang DG, Davis C, Hu W, Pan HF. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019;48:1–10. doi: 10.1016/j.cytogfr.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Han Y, Zhang J, Huang S, Cheng N, Zhang C, Li Y, Wang X, Liu J, You B, Du J. MicroRNA-223-3p inhibits vascular calcification and the osteogenic switch of vascular smooth muscle cells. J Biol Chem. 2021;296(100483) doi: 10.1016/j.jbc.2021.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Wang Z, Zhang L, Wang Y. Roles of cells from the arterial vessel wall in atherosclerosis. Mediators Inflamm. 2017;2017(8135934) doi: 10.1155/2017/8135934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E. Fluid shear stress and the vascular endothelium: For better and for worse. Prog Biophys Mol Biol. 2003;81:177–199. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 13.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99:315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeyens N. Fluid shear stress sensing in vascular homeostasis and remodeling: Towards the development of innovative pharmacological approaches to treat vascular dysfunction. Biochem Pharmacol. 2018;158:185–191. doi: 10.1016/j.bcp.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Claustrat B, Leston J. Melatonin: Physiological effects in humans. Neurochirurgie. 2015;61:77–84. doi: 10.1016/j.neuchi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: Critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- 17.Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ. Extrapineal melatonin: Sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding S, Lin N, Sheng X, Zhao Y, Su Y, Xu L, Tong R, Yan Y, Fu Y, He J, et al. Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORα-dependent manner. J Pineal Res. 2019;67(e12581) doi: 10.1111/jpi.12581. [DOI] [PubMed] [Google Scholar]
- 19.Migita H, Satozawa N, Lin JH, Morser J, Kawai K. RORalpha1 and RORalpha4 suppress TNF-alpha-induced VCAM-1 and ICAM-1 expression in human endothelial cells. FEBS Lett. 2004;557:269–274. doi: 10.1016/s0014-5793(03)01502-3. [DOI] [PubMed] [Google Scholar]
- 20.Delerive P, Monté D, Dubois G, Trottein F, Fruchart-Najib J, Mariani J, Fruchart JC, Staels B. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep. 2001;2:42–48. doi: 10.1093/embo-reports/kve007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besnard S, Heymes C, Merval R, Rodriguez M, Galizzi JP, Boutin JA, Mariani J, Tedgui A. Expression and regulation of the nuclear receptor RORalpha in human vascular cells. FEBS Lett. 2002;511:36–40. doi: 10.1016/s0014-5793(01)03275-6. [DOI] [PubMed] [Google Scholar]
- 22.Khachigian LM. Transcription factors targeted by miRNAs regulating smooth muscle cell growth and intimal thickening after vascular injury. Int J Mol Sci. 2019;20(5445) doi: 10.3390/ijms20215445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Gu C, Ye M, Zhang Z, Li L, Fan W, Meng Y. Integration analysis of microRNA and mRNA paired expression profiling identifies deregulated microRNA-transcription factor-gene regulatory networks in ovarian endometriosis. Reprod Biol Endocrinol. 2018;16(4) doi: 10.1186/s12958-017-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J, Li M, Zhao T, Yang H, Xu R, et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J Pineal Res. 2018;64 doi: 10.1111/jpi.12449. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Chen S, Jiang Y, Xu Y, Zhao Y, Chen L, Li C, Zhou X. Analysis of miRNA expression profiles in melatonin-exposed GC-1 spg cell line. Gene. 2018;642:513–521. doi: 10.1016/j.gene.2017.11.068. [DOI] [PubMed] [Google Scholar]
- 26.Hardeland R. Melatonin, noncoding RNAs, messenger RNA stability and epigenetics-evidence, hints, gaps and perspectives. Int J Mol Sci. 2014;15:18221–18252. doi: 10.3390/ijms151018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian Y, Gong Z, Zhao R, Zhu Y. Melatonin inhibits RANKL-induced osteoclastogenesis through the miR-882/Rev-erbα axis in Raw264.7 cells. Int J Mol Med. 2021;47:633–642. doi: 10.3892/ijmm.2020.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murodumi H, Shigeishi H, Kato H, Yokoyama S, Sakuma M, Tada M, Ono S, Rahman MZ, Ohta K, Takechi M. Melatonin-induced miR-181c-5p enhances osteogenic differentiation and mineralization of human jawbone-derived osteoblastic cells. Mol Med Rep. 2020;22:3549–3558. doi: 10.3892/mmr.2020.11401. [DOI] [PubMed] [Google Scholar]
- 29.Boukhtouche F, Mariani J, Tedgui A. The ‘CholesteROR’ protective pathway in the vascular system. Arterioscler Thromb Vasc Biol. 2004;24:637–643. doi: 10.1161/01.ATV.0000119355.56036.de. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem. 2010;285:15668–15673. doi: 10.1074/jbc.M110.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang W, Lin J, Dong J, Li D. Pyroptosis: An inflammatory cell death implicates in atherosclerosis. Med Hypotheses. 2013;81:484–486. doi: 10.1016/j.mehy.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Xu YJ, Zheng L, Hu YW, Wang Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2018;476:28–37. doi: 10.1016/j.cca.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Yin DL, Zhao XH, Zhou Y, Wang Y, Duan P, Li QX, Xiong Z, Zhang YY, Chen Y, He H, et al. Association between the ICAM-1 gene polymorphism and coronary heart disease risk: A meta-analysis. Biosci Rep. 2019;39(BSR20180923) doi: 10.1042/BSR20180923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Zhang J, Wu J, Zhang S, Liang Y, Zhou B, Wu P, Wei D. Low shear stress induced vascular endothelial cell pyroptosis by TET2/SDHB/ROS pathway. Free Radic Biol Med. 2021;162:582–591. doi: 10.1016/j.freeradbiomed.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J Cell Physiol. 2018;233:2116–2132. doi: 10.1002/jcp.25930. [DOI] [PubMed] [Google Scholar]
- 36.Raman K, Chong M, Akhtar-Danesh GG, D'Mello M, Hasso R, Ross S, Xu F, Paré G. Genetic markers of inflammation and their role in cardiovascular disease. Can J Cardiol. 2013;29:67–74. doi: 10.1016/j.cjca.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Mulvihill NT, Foley JB, Murphy RT, Pate G, Crean PA, Walsh M. Enhanced endothelial activation in diabetic patients with unstable angina and non-Q-wave myocardial infarction. Diabet Med. 2001;18:979–983. doi: 10.1046/j.1464-5491.2001.00605.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 39.Wei G, Zhu D, Sun Y, Zhang L, Liu X, Li M, Gu J. The protective effects of azilsartan against oscillatory shear stress-induced endothelial dysfunction and inflammation are mediated by KLF6. J Biochem Mol Toxicol. 2021;35:1–8. doi: 10.1002/jbt.22766. [DOI] [PubMed] [Google Scholar]
- 40.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: Low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–H374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Sun D, Zheng Y, Cheng Y. Swimming exercise activates aortic autophagy and limits atherosclerosis in ApoE-/- mice. Obes Res Clin Pract. 2020;14:264–270. doi: 10.1016/j.orcp.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Veneroso C, Tuñón MJ, González-Gallego J, Collado PS. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J Pineal Res. 2009;47:184–191. doi: 10.1111/j.1600-079X.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhong L, Simard MJ, Huot J. Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation. FASEB J. 2018;32:4070–4084. doi: 10.1096/fj.201701536R. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Wang Y, Chen H, Zhang J, Xu C, Li J, Li M. Enhancement of ICAM-1 via the JAK2/STAT3 signaling pathway in a rat model of severe acute pancreatitis-associated lung injury. Exp Ther Med. 2016;11:788–796. doi: 10.3892/etm.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peiffer V, Sherwin SJ, Weinberg PD. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res. 2013;99:242–250. doi: 10.1093/cvr/cvt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siasos G, Sara JD, Zaromytidou M, Park KH, Coskun AU, Lerman LO, Oikonomou E, Maynard CC, Fotiadis D, Stefanou K, et al. Local low shear stress and endothelial dysfunction in patients with nonobstructive coronary atherosclerosis. J Am Coll Cardiol. 2018;71:2092–2102. doi: 10.1016/j.jacc.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 47.Eshtehardi P, Teng Z. Protective or destructive: High wall shear stress and atherosclerosis. Atherosclerosis. 2016;251:501–503. doi: 10.1016/j.atherosclerosis.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 48.Rashad S, Han X, Saqr K, Tupin S, Ohta M, Niizuma K, Tominaga T. Epigenetic response of endothelial cells to different wall shear stress magnitudes: A report of new mechano-miRNAs. J Cell Physiol. 2020;235:7827–7839. doi: 10.1002/jcp.29436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.