Abstract
Objective: To investigate the clinical efficacy of cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) for treating malignant peritoneal mesothelioma (MPM) and to assess the impact of this approach on patient prognosis. Methods: A retrospective analysis of 44 patients with MPM was performed. The control group (CNG, N = 23) was treated with CRS combined with postoperative intraperitoneal (IP) chemotherapy, while the observation group (OG, N = 21) was treated with CRS combined with HIPEC. The treatment efficacy, volume of blood loss, operation time, postoperative length of stay, and 3-year survival rate (SR) were compared, and the factors affecting the prognosis of MPM patients were analyzed by multivariate analysis. Results: The OG showed decreased volume of blood loss and operation time, while also showing increased overall treatment efficacy compared with the CNG. The SR in the OG was 65.22% compared with a rate of 33.33% in the CNG, and the 3-year SR in the OG was significantly higher than that in the CNG. Multivariate analysis revealed that tumor-node-metastasis (TNM) stage, Eastern Cooperative Oncology Group (ECOG) score, and treatment modality were independent risk factors for the prognosis of MPM patients. Conclusion: CRS combined with HIPEC for MPM has a favorable treatment efficacy and prolongs the survival of MPM patients. Additionally, TNM stage, ECOG score, and treatment modality are independent risk factors for the prognosis of MPM patients.
Keywords: Cytoreductive surgery, efficacy, hyperthermic intraperitoneal chemotherapy, malignant peritoneal mesothelioma, prognosis
Introduction
Malignant peritoneal mesothelioma (MPM) is a malignant tumor originating from peritoneal mesothelial cells [1,2]. MPM accounts for approximately 10%-15% of all malignant mesotheliomas. Its main symptoms are abdominal pain, bloating, an abdominal mass, ascites, weight loss, fever, and vomiting. It is usually related to occupational or environmental inhalation of asbestos fibers and other elongated mineral particles [3-5]. The onset of MPM is insidious, and owing to the lack of specificity of clinical symptoms, it is easily missed, making early diagnosis and effective treatment difficult. The incubation period of MPM can be as long as 20-40 years, and the prognosis is extremely poor, with most patients dying within 1 year of diagnosis, making it a malignant tumor type with a high mortality rate [6,7].
The traditional treatment options for MPM include surgery, radiation therapy, and chemotherapy [8,9]. Patients with early stage MPM can achieve good outcomes with surgery, but if MPM involves the entire peritoneal cavity, achieving good outcomes with surgery may be difficult. Without adjuvant treatment, complete resection may not prolong patient survival, and this approach may lead to serious complications and high mortality rate [10-12].
Cytoreductive surgery (CRS) involves the removal of the tumor foci in the pelvic and abdominal cavities to the greatest possible extent such that the residual foci are ≤1 cm in diameter [7], making them invisible to the naked eye [13,14]. During hyperthermic intraperitoneal chemotherapy (HIPEC), the surgeon continuously circulates a heated, sterile chemotherapy solution throughout the peritoneal cavity, increasing the temperature of malignant tumor tissue to the effective temperature to induce apoptosis of tumor cells without damaging normal tissues. HIPEC also has immunostimulatory effects on primary tumors and metastases, and this has drawn attention in recent years [15,16]. Sugarbaker et al. [17] performed a pharmacokinetic study of HIPEC with adriamycin. CRS combined with HIPEC was performed in 145 colorectal cancer patients with peritoneal metastases. The concentration of adriamycin on the surface of the peritoneal cavity was found to be 78 times higher than that in serum. After 90 min of treatment, 12% of the agent was retained in the solution and 88% could be absorbed by the body. The extent of visceral resection and peritoneal resection increased the clearance of adriamycin in the peritoneal cavity. The largest study to date on this topic was conducted by Yonemura et al. [18], who showed that 83 patients with gastric cancer underwent CRS combined with HIPEC (mitomycin C [MMC], etoposide, and cisplatin [DDP]), and the 1-year and 5-year survival rates (SRs) were 43% and 11%, respectively.
CRS is usually performed for visible lesions [19], this study investigated the efficacies of different treatment modalities in 44 in-patients with MPM and analyzed the factors affecting the prognosis of patients with MPM.
Materials and methods
Baseline data
Forty-four MPM patients treated at our hospital between July 2014 and August 2015 were enrolled as study subjects and divided into an observation group (OG) (23 cases) and a control group (CNG) (21 patients). Patients in the CNG were treated with CRS combined with postoperative intraperitoneal (IP) chemotherapy, and those in the OG were treated with CRS combined with HIPEC. There were 15 men and 8 women in the OG aged 44-78 years, with a mean age of 59.58±7.63 years (21 cases, diffuse type MPM and 2 cases, limited type MPM). There were 12 men and 9 women in the CNG aged 42-73 years, with a mean age of 56.27±5.23 years (18 cases, diffuse type MPM and 3 cases, limited type MPM). The inclusion criteria were as follows: patients diagnosed through pathological examination and meeting the diagnostic criteria for MPM [20]; willingness to accept the treatment option provided; age 35-85 years; no history of radiotherapy or chemotherapy prior to treatment; no distant metastasis; and availability of complete clinicopathological and follow-up data. The following patients were excluded: those with malignant tumors in other organs, patients with missing clinical data, patients with hepatic and renal insufficiency, patients with poor cardiopulmonary function, patients expected to be intolerant to the involved agents and treatments, patients with severe inflammation, and pregnant or lactating women. This study was approved by the Ethics Committee of Xi’an No. 3 Hospital, the Affiliated Hospital of Northwest University (No. SYLL-2021-038). The research subjects and their families were informed and they signed a fully-informed consent form.
Treatment methods
Patients in the CNG were treated with CRS combined with postoperative IP chemotherapy, and those in the OG were treated with CRS combined with HIPEC. All patients underwent laparotomy under general anesthesia, and a median incision was made from below the xiphoid process (or 5 cm above the umbilicus) to the pubic symphysis. After the cavity was opened, the severity and range of tumor invasion from the diaphragm to the pelvic peritoneum, tumor size, tumor location, and the volume of ascites were recorded. The peritoneal cancer index (PCI) was calculated [21]. After performing CRS, patients in the OG underwent high-precision HIPEC using a therapy system (Guangzhou Borui Medical Technology Co., BRTRG-II). The following regimen was used: DDP 100 mg/m2, MMC 20 mg/m2, and loplatin 50 mg/m2. Two to three weeks after surgery, postoperative adjuvant chemotherapy was administered to patients with a stable condition, including systemic chemotherapy and/or IP chemotherapy and intravenous/peritoneal chemotherapy. In platinum-sensitive patients, platinum-based chemotherapy was continued postoperatively, and in platinum-resistant patients, platinum-free regimens were used for chemotherapy, such as regimens including paclitaxel (100 mg/m2). The following conditions were used: perfusion solution, 0.9% sodium chloride solution (12,000 ml); perfusion temperature, 43±0.5°C; and continuous perfusion time, 60-90 min.
Follow-up visits
Thirty-three patients were followed-up by telephone interviews and home visits at 3-month intervals for a duration of 3 years. The follow-up period ended in August 2018. All patients underwent complete follow-up.
Assessment of therapeutic efficacy
Patients’ clinical outcomes were evaluated after treatment [22], with outcomes categorized as complete response (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). CR was defined as the complete disappearance of the tumor with no new lesions; PR was defined as a tumor size reduction of more than 50%; SD was defined as an increase in tumor size of less than 25% with the appearance of no new lesions; and PD was defined as an increase in tumor diameter of more than 25% or the appearance of new lesions. The overall effectiveness rate was calculated as (CR + PR)/n.
Statistical analysis
SPSS 22 (IBM Corp, Armonk, NY, USA) was used for all analyses. GraphPad Prism 8.0 (GraphPad Software Inc., US) was used to illustrate the figures. The measurement data are expressed as the mean ± standard deviation (x ± sd). The independent sample t-test was used to make comparisons between the groups, while the paired t-test was used to make comparisons within each group. Count data are expressed as the percentage (%), and between-group comparisons were performed using the χ2 test. The Kaplan-Meier method was used to draw survival curves, and the log-rank test was used to compare survival curves. A Cox regression model was established to analyze the factors influencing prognosis. Differences were considered statistically significant at a p value <0.05.
Results
Baseline data
No significant differences in sex, age, smoking history, history of alcohol consumption, education level, place of residence, tumor-node-metastasis (TNM) stage, pathological classification, body mass index, aspartate aminotransferase (AST) levels, and alanine aminotransferase (ALT) levels between the two groups were noted (P>0.05, Table 1).
Table 1.
Clinical data of the patients (n [%]) (x ± sd)
| Grouping | Observation group (n = 23) | Control group (n = 21) | t/χ2 | P |
|---|---|---|---|---|
| Gender | 0.302 | 0.582 | ||
| Male | 15 (65.22) | 12 (57.14) | ||
| Female | 8 (34.78) | 9 (42.86) | ||
| Age (years) | 59.58±7.63 | 56.27±5.23 | 1.622 | 0.104 |
| History of smoking | 0.954 | 0.329 | ||
| Yes | 11 (47.83) | 7 (33.33) | ||
| No | 12 (52.17) | 14 (66.67) | ||
| History of drinking consumption | 0.302 | 0.583 | ||
| Yes | 15 (65.22) | 12 (57.14) | ||
| No | 8 (34.78) | 9 (42.86) | ||
| Education level | 2.277 | 0.131 | ||
| High school and below | 9 (39.13) | 13 (61.9) | ||
| College and university | 14 (60.87) | 8 (38.1) | ||
| Residence | 1.386 | 0.239 | ||
| Rural | 15 (65.22) | 10 (47.62) | ||
| Urban | 8 (34.78) | 11 (52.38) | ||
| TNM Staging | 0.192 | 0.661 | ||
| I + II | 13 (56.52) | 11 (52.38) | ||
| III + IV | 10 (43.48) | 10 (47.62) | ||
| Pathological typing | 0.341 | 0.560 | ||
| Diffuse | 21 (91.3) | 18 (85.71) | ||
| Limited | 2 (8.7) | 3 (14.29) | ||
| Body mass index (kg/m2) | 22.09±3.46 | 21.37±3.14 | 0.72 | 0.475 |
| AST (U/L) | 19.27±5.32 | 18.79±4.03 | 0.335 | 0.739 |
| ALT (U/L) | 21.58±6.31 | 22.19±6.75 | 1.601 | 0.117 |
The OG showed decreased volume of blood loss and operative time compared with the CNG (P<0.05) (Table 2; Figure 1).
Table 2.
Comparison of the volume of blood loss, operative time, and length of hospital stay between the two groups (x ± sd)
| Grouping | n | Volume of blood loss (mL) | Operating time (min) | Post-operative length of hospital stay (d) |
|---|---|---|---|---|
| Observation group | 23 | 511.48±127.32* | 683.82±205.64* | 15.24±6.23 |
| Control group | 21 | 727.317±138.59 | 346.18±217.245 | 14.37±3.24 |
| t | 10.45 | 6.486 | 0.573 | |
| P | <0.001 | <0.001 | 0.570 |
Notes: Compared to the control group;
P<0.05.
Figure 1.
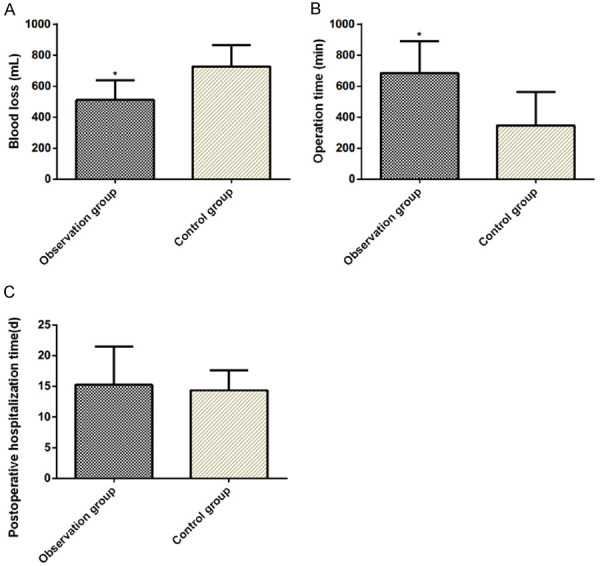
Comparison of the volume of blood loss, operative time, and length of hospital stay between two groups. Volume of blood loss (A); operative time (B); and length of hospital stay (C). *P<0.05.
Comparison of clinical efficacy
After treatment, 8 cases of CR (34.78%), 13 cases of PR (56.52%), and 2 cases of SD + PD (8.70%) were reported in the OG, with a total effective rate of 91.30%. Meanwhile, 6 cases of CR (28.57), 8 cases of PR (38.10%), and 7 cases of SD + PD (33.33%) were found in the CNG, with a total effective rate of 66.67%. The overall effectiveness of treatment was significantly higher in the OG than in the CNG (P<0.05) (Table 3).
Table 3.
Comparison of clinical efficacy between the two groups (n [%])
| Categories | Observation group (n = 23) | Control group (n = 21) | χ2 | P |
|---|---|---|---|---|
| CR | 8 (34.78) | 6 (28.57) | ||
| PR | 13 (56.52) | 8 (38.10) | ||
| SD + PD | 2 (8.70) | 7 (33.33) | ||
| Total efficiency | 21 (91.30) | 14 (66.67) | 4.095 | 0.043 |
All 44 patients were followed-up. Within 3 years, 22 patients died and 22 survived, with a SR of 50.00%. In the OG, 8 patients died and 15 survived, with a SR of 65.22%. In the CNG, 14 patients died and 7 survived, with a SR of 33.33%. The OG exhibited an increased 3-year SR compared with the CNG (P<0.05) (Figure 2).
Figure 2.
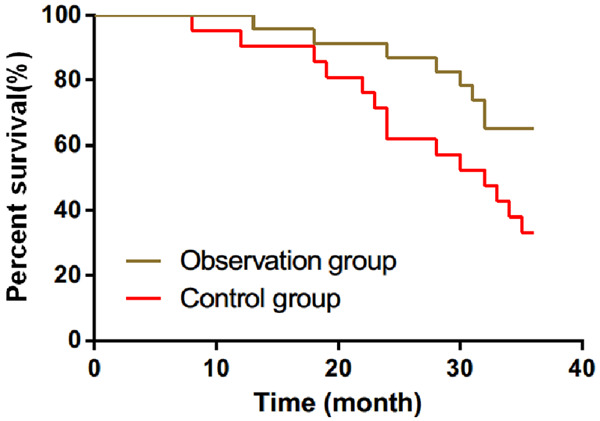
Three-year survival of patients in the two groups. The survival rates in the observation and control groups were 65.22% and 33.33%, respectively. The 3-year survival rate in the observation group was significantly higher than that in the control group (P<0.05). *P<0.05.
Univariate and multivariate analysis of the factors affecting prognosis in patients with MPM
There were no differences in age, sex, body mass index, history of alcohol consumption, smoking history, educational level, and place of residence between the two groups (P>0.05). However, there were differences in pathological classification, PCI, TNM stage, Eastern Cooperative Oncology Group (ECOG) score, treatment modality, and SR between the groups (P<0.05, Table 4).
Table 4.
Single-factor analysis of the factors influencing prognosis in patients with malignant peritoneal mesothelioma (x ± sd)
| Factors | Survival group (n = 22) | Death group (n = 22) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 3.300 | 0.069 | ||
| <60 | 15 (68.18) | 9 (40.91) | ||
| ≥60 | 7 (31.82) | 13 (59.09) | ||
| Gender | 2.277 | 0.131 | ||
| Male | 13 (59.09) | 14 (63.64) | ||
| Female | 9 (40.91) | 8 (36.36) | ||
| BMI | 23.09±5.17 | 23.41±4.26 | 0.224 | 0.824 |
| History of drinking consumption | 0.863 | 0.353 | ||
| Yes | 15 (68.18) | 12 (54.55) | ||
| No | 7 (31.82) | 10 (45.45) | ||
| History of smoking | 0.377 | 0.540 | ||
| Yes | 10 (45.45) | 8 (36.36) | ||
| No | 12 (54.55) | 14 (63.64) | ||
| Education level | 0.364 | 0.547 | ||
| High school and below | 10 (45.45) | 12 (54.55) | ||
| College and university | 12 (54.55) | 10 (45.45) | ||
| Residence | 0.834 | 0.361 | ||
| Rural | 11 (50) | 14 (63.64) | ||
| Urban | 11 (50) | 8 (36.36) | ||
| TNM Staging | 5.867 | 0.015 | ||
| I + II | 16 (72.73) | 8 (36.36) | ||
| III + IV | 6 (27.27) | 14 (63.64) | ||
| Pathological typing | 0.226 | 0.635 | ||
| Diffuse | 19 (86.36) | 20 (90.91) | ||
| Limited | 3 (13.64) | 2 (9.09) | ||
| ECOG score | 6.017 | 0.014 | ||
| <3 points | 17 (77.27) | 9 (40.91) | ||
| ≥3 points | 5 (22.73) | 13 (59.09) | ||
| Peritoneal carcinoma index | 3.300 | 0.069 | ||
| <4 | 15 (68.18) | 9 (40.91) | ||
| ≥4 | 7 (31.82) | 13 (59.09) | ||
| Treatment method | 13.880 | <0.001 | ||
| CRS + HIPEC | 17 (77.27) | 6 (27.27) | ||
| CRS + i.p. chemotherapy | 5 (22.73) | 16 (72.73) |
We included the indicators showing differences in univariate analysis (Table 5) in multivariate Cox regression analysis. The results showed that TNM stage, ECOG score, and treatment modality were independent risk factors affecting the prognosis of MPM patients (P<0.05, Table 6).
Table 5.
Assignment table
| Factors | Assignment |
|---|---|
| Pathological histology typing | 0 = epithelial, 1 = sarcomatous |
| PCI | 0 = ≤4, 1 = >4 |
| TNM Staging | 0 = l + ll, 1 = lll + lV |
| ECOG score | 0 = <3, 1 = ≥3 |
| Treatment modalities | 1 = CRS + HIPEC, 0 = CRS + i.p. chemotherapy |
| Survival | 0 = survival, 1 = death |
Table 6.
Multivariate analysis of the factors affecting prognosis in malignant peritoneal mesothelioma patients
| Variable | Β | SE | Wald χ2 | OR (95% CI) | P |
|---|---|---|---|---|---|
| TNM Staging | 0.801 | 0.329 | 5.531 | 2.142 (1.154-0.876) | 0.038 |
| ECOG score | 0.641 | 0.313 | 4.317 | 3.91 (1.035-3.572) | 0.017 |
| Treatment modality | -0.783 | 0.279 | 7.546 | 0.477 (0.253-0.732) | 0.025 |
Discussion
MPM is a rapidly progressing malignancy originating in the epithelial or mesothelial tissue of the serous cavity [23]. Approximately 10%-20% of MPMs originate from the peritoneum, and its incidence has increased in recent years [24,25]. Due to its insidious onset, the clinical manifestations of MPM are diverse and nonspecific, and early diagnosis is difficult, leading to poor treatment efficacy and prognosis [6,26,27]. Therefore, appropriate treatment modalities should be identified for patients with MPM to reduce their mortality.
Normal tissue cells can tolerate a higher temperature than tumor cells, and within a certain temperature range, the thermal effect can promote tumor apoptosis, enhance the efficacy of chemotherapeutic drugs, inhibit angiogenesis, and stimulate the immune system to kill tumor cells [28-30]. CRS alone can only deal with visible lesions, while HIPEC can reduce the multi-drug resistance of tumor cells to chemotherapy drugs, removing all visible lesions in the abdominal cavity to achieve “histological cure”, and also remove all microscopic lesions remaining in the body [31-34]. Park et al. [35] found that 18 patients treated with CRS + HIPEC showed a 2-year SR of 80% and a progression-free survival duration of 26 months. A study by Baratti et al. [36] found that patients who underwent repeated CRS and/or HIPEC had better prognoses than patients who received only systemic chemotherapy. The results of this study showed that the volume of bleeding and operation time were significantly lower in the OG than in the CNG, and the total effective rate of treatment was significantly higher in the OG than in the CNG. The study of 15 MPM patients by Brigand et al. [37] found that CRS combined with HIPEC had better clinical efficacy in the treatment of MPM, similar to our findings, suggesting that CRS coupled with HIPEC is a feasible treatment option for MPM.
We found that the SR of MPM patients was 50.00%. The SR in the OG was significantly higher than that in the CNG, suggesting that CRS in combination with HIPEC can improve the survival of MPM patients. The study by Sugarbaker et al. [30] showed that CRS/HIPEC could not only achieve a median survival of 3-5 years in MPM patients but also achieve long-term progression-free survival and symptom relief. The study by Salo et al. [38] also found that CRS plus HIPEC could improve the survival of MPM patients. Finally, we performed a Cox regression analysis and found that the TNM stage, ECOG score, and treatment modality were independent risk factors affecting the prognosis of MPM. Contrarily, Yan et al. [39] showed that HIPEC was an independent prognostic factor in MPM patients in a study of 405 patients with MPM. The study by Kaya et al. [40] showed that MPM patients with an ECOG score of >3 had a poor prognosis. These results are similar to those of our study, which suggests that the TNM stage, ECOG score, and treatment modality can be used as indicators for the clinical assessment of prognosis in MPM patients.
There were no significant differences between the two groups in terms of sex, age, smoking history, alcohol consumption history, education level, place of residence, and other baseline parameters, which ensured the rigor and reliability of the study. However, there are still some limitations. First, we did not discuss the correlation between other clinical indicators and MPM in depth, which needs to be evaluated in a follow-up study. Second, long-term survival was not assessed.
In summary, the CRS plus HIPEC regimen for MPM has a good therapeutic effect and can prolong the survival of patients, showing its potential for clinical application. Additionally, TNM stage, ECOG score, and treatment modality were independent risk factors affecting the prognosis of MPM patients.
Disclosure of conflict of interest
None.
References
- 1.Magge D, Zenati MS, Austin F, Mavanur A, Sathaiah M, Ramalingam L, Jones H, Zureikat AH, Holtzman M, Ahrendt S, Pingpank J, Zeh HJ, Bartlett DL, Choudry HA. Malignant peritoneal mesothelioma: prognostic factors and oncologic outcome analysis. Ann Surg Oncol. 2014;21:1159–1165. doi: 10.1245/s10434-013-3358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Shammaa HA, Li Y, Yonemura Y. Current status and future strategies of cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. World J Gastroenterol. 2008;14:1159–1166. doi: 10.3748/wjg.14.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma V, Sleightholm RL, Rusthoven CG, Koshy M, Sher DJ, Grover S, Simone CB 2nd. Malignant peritoneal mesothelioma: national practice patterns, outcomes, and predictors of survival. Ann Surg Oncol. 2018;25:2018–2026. doi: 10.1245/s10434-018-6499-1. [DOI] [PubMed] [Google Scholar]
- 4.Mazurek JM, Syamlal G, Wood JM, Hendricks SA, Weston A. Malignant mesothelioma mortality - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2017;66:214–218. doi: 10.15585/mmwr.mm6608a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soeberg MJ, Leigh J, Driscoll T, Armstrong B, Young JM, van Zandwijk N. Incidence and survival trends for malignant pleural and peritoneal mesothelioma, Australia, 1982-2009. Occup Environ Med. 2016;73:187–194. doi: 10.1136/oemed-2015-103309. [DOI] [PubMed] [Google Scholar]
- 6.Boffetta P, Donato F. Epidemiology of mesothelioma. 2020. pp. 379–391. [Google Scholar]
- 7.Jiang Z, Chen T, Chen J, Ying S, Gao Z, He X, Miao C, Yu M, Feng L, Xia H, Wu W, Chen R, Morinaga K, Lou J, Zhang X. Hand-spinning chrysotile exposure and risk of malignant mesothelioma: a case-control study in Southeastern China. Int J Cancer. 2018;142:514–523. doi: 10.1002/ijc.31077. [DOI] [PubMed] [Google Scholar]
- 8.Raza A, Huang WC, Takabe K. Advances in the management of peritoneal mesothelioma. World J Gastroenterol. 2014;20:11700–11712. doi: 10.3748/wjg.v20.i33.11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaster A, Wachsmann J. Serendipitous discovery of peritoneal mesothelioma. Proc (Bayl Univ Med Cent) 2016;29:185–187. doi: 10.1080/08998280.2016.11929410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee PS, Auyeung KM, King DA. Pitfalls in diagnosis of early stage malignant peritoneal mesothelioma: a case report. Clin Imaging. 2002;26:263–266. doi: 10.1016/s0899-7071(02)00424-2. [DOI] [PubMed] [Google Scholar]
- 11.Chua TC, Chong CH, Morris DL. Peritoneal mesothelioma: current status and future directions. Surg Oncol Clin N Am. 2012;21:635–643. doi: 10.1016/j.soc.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Shavelle R, Vavra-Musser K, Lee J, Brooks J. Life expectancy in pleural and peritoneal mesothelioma. Lung Cancer Int. 2017;2017:2782590. doi: 10.1155/2017/2782590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Jiang Z, Yan J, Ying S. HMGB1 as a potential biomarker and therapeutic target for malignant mesothelioma. Dis Markers. 2019;2019:4183157. doi: 10.1155/2019/4183157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Leeuwen BL, Graf W, Pahlman L, Mahteme H. Swedish experience with peritonectomy and HIPEC. HIPEC in peritoneal carcinomatosis. Ann Surg Oncol. 2008;15:745–753. doi: 10.1245/s10434-007-9700-5. [DOI] [PubMed] [Google Scholar]
- 15.Cui HB, Ge HE, Bai XY, Zhang W, Zhang YY, Wang J, Li X, Xing LP, Guo SH, Wang ZY. Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp Ther Med. 2014;7:1083–1088. doi: 10.3892/etm.2014.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Pan Q, Xiao S, Li L, Xue M. Docetaxel combined with intraperitoneal hyperthermic perfusion chemotherapy and hyperthermia in the treatment of advanced ovarian cancer. Oncol Lett. 2016;11:3287–3292. doi: 10.3892/ol.2016.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugarbaker PH, Van der Speeten K, Anthony Stuart O, Chang D. Impact of surgical and clinical factors on the pharmacology of intraperitoneal doxorubicin in 145 patients with peritoneal carcinomatosis. Eur J Surg Oncol. 2011;37:719–726. doi: 10.1016/j.ejso.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Yonemura Y, Fujimura T, Nishimura G, Falla R, Sawa T, Katayama K, Tsugawa K, Fushida S, Miyazaki I, Tanaka M, Endou Y, Sasaki T. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery. 1996;119:437–444. doi: 10.1016/s0039-6060(96)80145-0. [DOI] [PubMed] [Google Scholar]
- 19.Terracini B. Commentary: past and current asbestos exposure and future mesothelioma risks in Britain. Int J Epidemiol. 2018;47:1756–1757. doi: 10.1093/ije/dyy175. [DOI] [PubMed] [Google Scholar]
- 20.Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, Butnor KJ, Chirieac LR, Churg AM, Dacic S, Galateau-Sallé F, Gibbs A, Gown AM, Krausz T, Litzky LA, Marchevsky A, Nicholson AG, Roggli VL, Sharma AK, Travis WD, Walts AE, Wick MR. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018;142:89–108. doi: 10.5858/arpa.2017-0124-RA. [DOI] [PubMed] [Google Scholar]
- 21.Elias D, Mariani A, Cloutier AS, Blot F, Goéré D, Dumont F, Honoré C, Billard V, Dartigues P, Ducreux M. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eur J Surg Oncol. 2014;40:1467–1473. doi: 10.1016/j.ejso.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Armato SG 3rd, Nowak AK. Revised modified response evaluation criteria in solid tumors for assessment of response in malignant pleural mesothelioma (Version 1.1) J Thorac Oncol. 2018;13:1012–1021. doi: 10.1016/j.jtho.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Sépult C, Bellefroid M, Rocks N, Donati K, Gérard C, Gilles C, Ludwig A, Duysinx B, Noël A, Cataldo D. ADAM10 mediates malignant pleural mesothelioma invasiveness. Oncogene. 2019;38:3521–3534. doi: 10.1038/s41388-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan R, Morrow B, Thomas A, Walsh T, Lee MK, Gulsuner S, Gadiraju M, Panou V, Gao S, Mian I, Khan J, Raffeld M, Patel S, Xi L, Wei JS, Hesdorffer M, Zhang J, Calzone K, Desai A, Padiernos E, Alewine C, Schrump DS, Steinberg SM, Kindler HL, King MC, Churpek JE. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A. 2019;116:9008–9013. doi: 10.1073/pnas.1821510116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao S, Jin S, Cao J, Shen J, Hu J, Che D, Pan B, Zhang J, He X, Ding D, Gu F, Yu Y. Advances in malignant peritoneal mesothelioma. Int J Colorectal Dis. 2015;30:1–10. doi: 10.1007/s00384-014-2029-1. [DOI] [PubMed] [Google Scholar]
- 26.Attanoos RL, Churg A, Galateau-Salle F, Gibbs AR, Roggli VL. Malignant mesothelioma and its non-asbestos causes. Arch Pathol Lab Med. 2018;142:753–760. doi: 10.5858/arpa.2017-0365-RA. [DOI] [PubMed] [Google Scholar]
- 27.Beebe-Dimmer JL, Fryzek JP, Yee CL, Dalvi TB, Garabrant DH, Schwartz AG, Gadgeel S. Mesothelioma in the United States: a Surveillance, Epidemiology, and End Results (SEER)-Medicare investigation of treatment patterns and overall survival. Clin Epidemiol. 2016;8:743–750. doi: 10.2147/CLEP.S105396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Giorgio A, Naticchioni E, Biacchi D, Sibio S, Accarpio F, Rocco M, Tarquini S, Di Seri M, Ciardi A, Montruccoli D, Sammartino P. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer. 2008;113:315–325. doi: 10.1002/cncr.23553. [DOI] [PubMed] [Google Scholar]
- 29.Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692–698. doi: 10.1002/jso.22017. [DOI] [PubMed] [Google Scholar]
- 30.Sugarbaker PH, Turaga KK, Alexander HR Jr, Deraco M, Hesdorffer M. Management of malignant peritoneal mesothelioma using cytoreductive surgery and perioperative chemotherapy. J Oncol Pract. 2016;12:928–935. doi: 10.1200/JOP.2016.011908. [DOI] [PubMed] [Google Scholar]
- 31.Latin American Registry of Peritoneal Diseases - LARPD participants. Current practice of Latin American centers in the treatment of peritoneal diseases with cytoreductive surgery with HIPEC. Eur J Surg Oncol. 2018;44:1800–1804. doi: 10.1016/j.ejso.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Alley E, Tanvetyanon T, Jahan T, Gandhi L, Peikert T, Stevenson J, Schlienger K, Liu W, Nair N, Honarmand S, Kindler H. A phase II single-arm study of CRS-207 with pembrolizumab (pembro) in previously treated malignant pleural mesothelioma (MPM) J. Clin. Oncol. 2019;37:29–29. [Google Scholar]
- 33.Nonaka D, Kusamura S, Baratti D, Casali P, Cabras AD, Younan R, Rosai J, Deraco M. Diffuse malignant mesothelioma of the peritoneum: a clinicopathological study of 35 patients treated locoregionally at a single institution. Cancer. 2005;104:2181–2188. doi: 10.1002/cncr.21239. [DOI] [PubMed] [Google Scholar]
- 34.Le Roy F, Gelli M, Hollebecque A, Honoré C, Boige V, Dartigues P, Benhaim L, Malka D, Ducreux M, Elias D, Goéré D. Conversion to complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma after bidirectional chemotherapy. Ann Surg Oncol. 2017;24:3640–3646. doi: 10.1245/s10434-017-6033-x. [DOI] [PubMed] [Google Scholar]
- 35.Park BJ, Alexander HR, Libutti SK, Wu P, Royalty D, Kranda KC, Bartlett DL. Treatment of primary peritoneal mesothelioma by continuous hyperthermic peritoneal perfusion (CHPP) Ann Surg Oncol. 1999;6:582–590. doi: 10.1007/s10434-999-0582-6. [DOI] [PubMed] [Google Scholar]
- 36.Baratti D, Kusamura S, Cabras AD, Dileo P, Laterza B, Deraco M. Diffuse malignant peritoneal mesothelioma: failure analysis following cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2009;16:463–472. doi: 10.1245/s10434-008-0219-1. [DOI] [PubMed] [Google Scholar]
- 37.Brigand C, Monneuse O, Mohamed F, Sayag-Beaujard AC, Isaac S, Gilly FN, Glehen O. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol. 2006;13:405–412. doi: 10.1245/ASO.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 38.Salo SAS, Ilonen I, Laaksonen S, Myllärniemi M, Salo JA, Rantanen T. Malignant peritoneal mesothelioma: treatment options and survival. Anticancer Res. 2019;39:839–845. doi: 10.21873/anticanres.13183. [DOI] [PubMed] [Google Scholar]
- 39.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P, Mohamed F, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J. Clin. Oncol. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 40.Kaya H, Sezgı C, Tanrıkulu AC, Taylan M, Abakay O, Sen HS, Abakay A, Kucukoner M, Kapan M. Prognostic factors influencing survival in 35 patients with malignant peritoneal mesothelioma. Neoplasma. 2014;61:433–438. doi: 10.4149/neo_2014_053. [DOI] [PubMed] [Google Scholar]


