Abstract
We have previously described the use of homologous recombination and CRE-loxP-mediated marker recycling to generate mouse embryonic stem (ES) cell lines homozygous for mutations at the Msh3, Msh2, and both Msh3 and Msh2 loci (2). In this study, we describe the analysis of these ES cells with respect to processes known to be affected by DNA mismatch repair. ES cells homozygous for the Msh2 mutation displayed increased resistance to killing by the cytotoxic drug 6-thioguanine (6TG), indicating that the 6TG cytotoxic mechanism is mediated by Msh2. The mutation rate of the herpes simplex virus thymidine kinase 1 (HSV-tk1) gene was unchanged in Msh3-deficient ES cell lines but markedly elevated in Msh2-deficient and Msh3 Msh2 double-mutant cells. Notably, the HSV-tk1 mutation rate was 11-fold higher, on average, than that of the hypoxanthine-guanine phosphoribosyl transferase (Hprt) locus in Msh2-deficient cells. Sequence analysis of HSV-tk1 mutants from these cells indicated the presence of a frameshift hotspot within the HSV-tk1 coding region. Msh3-deficient cells displayed a modest (16-fold) elevation in the instability of a dinucleotide repeat, whereas Msh2-deficient and Msh2 Msh3 double-mutant cells displayed markedly increased levels of repeat instability. Targeting frequencies of nonisogenic vectors were elevated in Msh2-deficient ES cell lines, confirming the role of Msh2 in blocking recombination between diverged sequences (homeologous recombination) in mammalian cells. These results are consistent with accumulating data from other laboratories and support the current model of DNA mismatch repair in mammalian cells.
Msh2, Msh3, and Msh6 are mammalian homologues of the bacterial DNA mismatch repair (DMR) mutS gene involved in the repair of base pair mismatches and insertion-deletion (I/D) heterologies (15). These DNA lesions arise in the genome through a number of mechanisms, including replication errors and recombination between diverged sequences (homeologous recombination) (16).
An increasing volume of data supports a model of action of these three mammalian MutS homologues. In humans, the MSH2 protein forms heterodimeric complexes with both MSH6 and MSH3. These heterodimers are known as MutSalpha (MSH2-MSH6) and MutSbeta (MSH2-MSH3) (13, 25). In combination with other DMR proteins, MutSalpha functions in the repair of single base mismatches and small I/D heterologies, whereas MutSbeta repairs larger I/D heterologies. Thus, MSH2 is critical for the repair of all mismatched lesions in mammals, whereas MSH6 and MSH3 modulate the function of MSH2 by providing specificity for different lesion types (44).
Mutations in these DMR genes lead to genomic instability in bacteria, yeast, and mammals by affecting a number of cellular processes. Msh2 and Msh6 mutations lead to an increase in the accumulation of spontaneous mutations, also known as a mutator phenotype, due to the lack of repair of mismatches and I/D heterologies that arise as errors during replication (5, 12, 18). Overexpression of MSH3 also leads to a mutator phenotype in mammalian cells due to the sequestration of the available MSH2 into MutSbeta heterodimers, leading to a functional loss of MutSalpha activity (9, 22).
Mutations in Msh2, Msh6, and Msh3 also result in instability of the size of simple sequence repeats or microsatellites (24, 26, 37, 41). This instability is thought to occur due to the lack of repair of DNA polymerase slippage products that arise during the replication of these highly repetitive sequences. Microsatellite instability in certain human tumors was a critical clue to the identification of the involvement of DMR genes in cancer and has since been used to demonstrate their involvement in familial cancer syndromes as well as in sporadic tumors (1, 12, 18, 28, 37, 43).
DMR acts as a block to mitotic homeologous recombination, presumably by recognizing and binding mismatches and I/D heterologies that arise in the heteroduplex intermediates of recombination between diverged substrates. Escherichia coli mutS mutants lack the barrier to cross-species recombination with Salmonella typhimurium displayed by the wild-type strains (34) and show increased recombination rates between homeologous loci, leading to duplications of large portions of their chromosome (29). Thus, the loss of this block to homeologous recombination adds an additional level of genetic instability in DMR mutants. Saccharomyces cerevisiae Msh2 and Msh3 mutants also display increased recombination rates between diverged tandem repeats in their chromosomes (40). In the S. cerevisiae system, two independent pathways involving MSH2 and MSH3 act as blocks to homeologous recombination (39, 40). In mammals, Msh2 mutations are known to affect the recombination rates between diverged substrates. Mouse embryonic stem (ES) cell lines homozygous for an msh2 mutation show increased targeting frequencies with constructs derived from nonisogenic sources (6).
A link between DMR deficiency and the sensitivity to killing by the cytotoxic drug 6-thioguanine (6TG) has been demonstrated (42). Mammalian cell lines deficient in DMR biochemical activity show increased resistance to killing by 6TG. This indicates that 6TG cytotoxicity is mediated by the DMR system in mammals. Human MutSalpha binds to DNA containing 6TG-induced lesions (46), and human MSH2 expression has been shown to correlate with the degree of resistance to methylating agents (7).
We have previously described the generation of mouse ES cells bearing single and compound mutations in Msh2 and Msh3. In this study, we have examined the roles of Msh3 and Msh2 in the four cellular processes described above which are known to be affected by DMR mutations in E. coli, S. cerevisiae, and mammalian cells: (i) sensitivity to 6TG cytotoxicity, (ii) mutation rate, (iii) microsatellite stability, and (iv) mitotic recombination.
MATERIALS AND METHODS
Msh2- and Msh3-deficient cell lines.
msh3Brdm1/msh3Brdm1 mutant (abbreviated as msh3−/−), msh2Brdm1/msh2Brdm1 mutant (abbreviated as msh2−/−), and msh3Brdm1/msh3Brdm1 msh2Brdm1/msh2Brdm1 double mutant (abbreviated as msh2,3−/−) ES cells have been described previously (2).
Reverse transcriptase PCR (RT-PCR) analysis of Msh3 and Msh2 transcripts.
Two oligonucleotide primers (5′-TCCACGGAGCCAGGAGAGA-3′ and 5′-GGGTGGTGAGATGCTACTGAGAT-3′) complementary to sequences in the Msh3 cDNA flanking the insertional mutation site in exon 2 (2) were used in PCRs to examine wild-type and mutants Msh3 mRNA transcripts. Control oligonucleotide primers (5′-CAGGCTAATCAGAAAGAC-3′ and 5′-ACGACACCAACGGAAAGG-3′) complementary to sequences downstream of exon 2 in the Msh3 cDNA were also utilized. For the analysis of Msh2 mRNA transcripts, two oligonucleotides (5′-TGGCCTGGAGAAGAAGATGC-3′ and 5′-ACGTGCCTCGGGAAGTTAGC-3′) were used as primers in PCR. These primers are complementary to sequences flanking the predicted deletion mutation in the Msh2 cDNA (2). In all reactions, 1 μg of reverse-transcribed (Superscript RT II first-strand cDNA synthesis kit; Gibco BRL) RNA from the appropriate ES cell lines was used as a template. PCR conditions were as follows: initial denaturation at 94°C for 3 min, followed by 37 cycles of 94°C for 1 min, annealing at 62°C for 1 min, and elongation at 72°C for 1 min. For all reactions, the sequences complementary to experimental and control primers are present in different exons of the Msh3 and Msh2 genomic loci in order to avoid the amplification of DNA fragments of the predicted size from genomic DNA contaminants in the template mixture. Amplification products were electrophoresed through 3% agarose gels containing 100 ng of ethidium bromide per ml and visualized under UV light.
To confirm the nature of amplified products, Southern blots of the appropriate gels were hybridized with oligonucleotide probes radiolabeled by standard methods (38). For Msh3, an oligonucleotide (5′-AAGGTGACAGCAGGAAGAGGTCACTGGGAA-3′) complementary to sequence external to the mutation in the Msh3 cDNA and an oligonucleotide (5′-CGATAAGCTTGATATCGAATTCGAGCTCGC-3′) specific for the insertional mutation were used. For Msh2, two oligonucleotides were used (5′-GTGAA TTATCCTCTTTAAATGAAGAATATA-3′ and 5′-GTCAGCAACCAAAGACTCCTTAATAATCAT-3′) complementary to the Msh2 cDNA outside and inside, respectively, of the deletion predicted by the targeted mutation.
Growth rate.
Wild-type, msh3−/−, msh2−/−, and msh2,3−/− ES cell lines were plated onto four six-well SNL76/7 feeder plates containing M15 (31). Then, 105 cells were plated onto each 36-mm-diameter well, and the M15 medium was changed as required at the same time for all four plates. Starting 72 h postplating and (continuing every 24 h thereafter, one well from each of the six-well plates was trypsinized and the total number of cells in the well was counted by using a Coulter cell counter.
Low-density colony forming ability.
Five hundred or 1,000 wild-type, msh3−/−, msh2−/−, and msh2,3−/− ES cells were plated onto 90-mm-diameter SNL 76/7 feeder plates and allowed to grow for 12 days. Plates were then washed in phosphate-buffered saline (PBS) and stained with 10% methylene blue in 70% ethanol, and the colonies were counted. Plating efficiencies were calculated by dividing the number of colonies obtained by the total number of cells plated.
Transformation efficiency.
The β-galactosidase reporter construct pPGKβgal was prepared by alkaline lysis and purified from a CsCl gradient. Then, 20 μg was electroporated into wild-type, msh3−/−, msh2−/−, and msh2,3−/− ES cells, and 107 cells for each class were plated onto one 90-mm-diameter SNL76/7 feeder plate containing M15. All 48 h after plating, cells were washed in PBS, trypsinized, and fixed by incubating for 10 min in PBS containing 0.5% glutaraldehyde (pH 7.2). After being fixed, cells were washed in PBS and stained overnight in the dark at room temperature on a shaking platform. The staining solution consisted of 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, and 1 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). After the staining, the cell solution was diluted and a sample counted by using a hemocytometer. Transformation efficiencies were calculated by dividing the number of blue-staining ES cells by the total number of ES cells counted.
6TG kill curves.
One thousand wild-type, msh3−/−, msh2−/−, and msh2,3−/− ES cells were plated onto 90-mm-diameter SNL76/7 feeder plates containing M15 and various concentrations (0, 1.9, 3.8, and 7.5 μM) of 6TG (2-amino-6-mercaptopurine; Sigma) and allowed to grow for 12 days. The medium was changed every 2 days. Plates were then washed with PBS and stained with 10% methylene blue in 70% ethanol and the colonies were counted.
Mutation rates. (i) Vector construction.
pMUT is composed of the laxP-PGK neocbpA-HSV-tk1-laxP cassette from pNTL (2) cloned into RIV6.0I, a targeting vector containing 6 kb of the mouse genomic Hprt locus, including exons 2 and 3. To generate pMUT, a double-stranded oligonucleotide containing KpnI, SalI, and NotI sites was introduced into the XhoI site in exon 3 of the Hprt fragment in RIV6.0I. A partial KpnI fragment containing the pNTL cassette was subsequently cloned into the KpnI site of the modified version of RIV6.0I.
(ii) Targeting pMUT to the mouse Hprt locus.
pMUT was prepared by alkaline lysis and purified from a CsCl gradient. NheI was used to cut pMUT within the region of homology to the mouse Hprt locus in order to generate an insertion targeting vector. Ten micrograms of NheI-digested pMUT was electroporated into wild-type, msh3−/−, msh2−/−, and msh3−/− ES cells and 2.5 × 106 cells were plated onto 90-mm-diameter SNL76/7 feeder plates containing M15. At 24 h after electroporation, G418 (Gibco-BRL; 180 μg of active ingredient per ml) selection was initiated. Five days after electroporation, 6TG selection was started at concentrations of 10 μM for wild-type and msh3−/− plates and 100 μM for msh2−/− and msh2,3−/− plates. The drug-containing M15 medium was changed as needed for 14 days, after which the colonies were picked and expanded in 96-well plates. Upon passaging, half of the cells in each well were frozen and the other half were plated onto a gelatinized 96-well plate for Southern blot analysis as described earlier (32). To identify clones in which a single copy of pMUT had targeted the Hprt locus, Southern blots of BamHI-digested genomic DNA from G418/6TGr clones were hybridized to a radiolabeled BamHI-XmnI DNA probe internal to the region of homology in pMUT.
(iii) Fluctuation tests.
For HSV-tk1 mutation rates, 8 to 10 individual G418/6TGr clones in each class identified by Southern analysis as being targeted at the Hprt locus with a single copy of pMUT were expanded in the presence of G418. Individual clones were then trypsinized and plated (3 × 106 to 4 × 106 cells for wild-type and msh3−/− cells, 2 × 105 cells for msh2−/− and msh2,3−/− cells) onto 90-mm-diameter SNL76/7 feeder plates containing G418. At 24 h after the plating, 1-(2-deoxy-2-fluoro-1-β-d-arabinofuranosyl)-5-iodouracil (FIAU) selection was applied (0.2 μM). Cells were grown in G418-FIAU selection for 12 days, after which single colonies were picked from independent plates for PCR amplification of the HSV-tk1 cassette. The plates were then stained with methylene blue, and the colonies were counted. Mutation rates were calculated according to the method of Luria and Delbruck (20). In calculating all mutation rates, the number of drug-resistant clones was not adjusted for the plating efficiency of the particular cell line (see results of low-density plating efficiency below). The plating efficiency of ES cells at low density cannot reliably be extrapolated to that at high density. Plating efficiency at high density is greater than at low density (unpublished observations). Thus, the purpose of the low-density plating efficiency measurements was to determine whether there were any gross differences from clone to clone. Although not adjusting for plating efficiency will lead to a slight underestimate of the mutation rates, we feel it is appropriate since the purpose of the study is to compare the relative mutation rates between the different lines rather than the absolute rate in each line.
For Hprt rates, msh2−/− and msh2,3−/− ES cells were plated at clonal density in M15 to obtain single colonies. Eight to ten individual colonies were picked and expanded in M15, and 105 cells were plated onto 90-mm-diameter SNL76-7 feeder plates containing M15. At 24 h after plating, 6TG (100 μM) was added to the medium, which was changed every 48 h. After 14 days the plates were washed in PBS and stained, and the colonies were counted. Mutation rates were calculated as described above (20).
(iv) Amplification and sequencing of the HSV-tk1 cassette.
Individual G418/FIAUr colonies were expanded, and genomic DNA was extracted as described earlier (32). Two oligonucleotide primers (5′-GGGGAGGCTGGGAGTTCACA-3′ and 5′-CGTCATAGCGCGGGTTCCTT-3′) external to the HSV-tk1 coding region in pMUT were used for PCR amplification (94°C for 3 min followed by 38 cycles of 93°C for 40, 62°C for 1 min, and 72°C for 2 min, followed by 20 min at 72°C). Amplification products were gel purified and cloned into pGEM-T (Promega). Forward and reverse primers, along with six primers internal to HSV-tk1, were used in sequencing reactions to obtain the full sequence of the HSV-tk1 coding region. The internal HSV-tk1 primers were 5′-GCGCGACGATATCGTCTACG-3′, 5′-GGGGAGGCTGGGAGTTCACA-3′, 5′-GCACAGGAGGGCGGCGATGG-3′, 5′-CAGCTTTCGGGGACGGCCGT-3′, and 5′-ACGGCCGTCCCCGAAAGCTG-3′. Sequence analysis was carried out in an ABI377 automated DNA sequencer (Applied Biosystems).
Microsatellite stability. (i) Vector construction.
pCAN is composed of the (CA)17 stability assay cassette from pRTM2 (10) and the PGKpurobpA puromycin resistance cassette (30) inserted into exon 3 of the mouse Hprt gene fragment in the targeting vector RIV6.0I. To generate pCAN, the pRTM2 and PGKpurobpA cassettes were initially cloned into the pBluescript (Stratagene) polylinker. A NotI-KpnI partial digestion fragment containing both cassettes was then cloned into the NotI-KpnI sites of the modified version of RIV6.0I described above.
(ii)Targeting pCAN to the mouse Hprt locus.
pCAN was prepared by alkaline lysis and purified from a CsC1 gradient. NheI was used to cut pCAN within the Hprt homology region in order to generate an insertion targeting vector. First, 10 μg of NheI-digested pCAN was electroporated into wild-type, msh3−/−, msh2−/−, and msh2,3−/− ES cells, and then 107 cells were plated onto 90-mm-diameter plates containing puromycin-resistant feeders in M15. At 24 h after electroporation, puromycin (Sigma; 3-μg/ml working concentration) selection was applied. Five days after electroporation, 6TG selection was started. After 14 days the colonies were picked, expanded, and screened by Southern analysis with an internal probe to obtain clones in which a single copy of pCAN had targeted the Hprt locus as described above for pMUT.
(iii)Fluctuation tests.
To determine the mutation rate of the (CA)17 repeat, individual puromycin-6TGr clones identified by Southern analysis as being targeted at the Hprt locus with a single copy of pCAN were expanded in the presence of puromycin. Individual clones were then trypsinized and plated (2 × 106 to 4 × 106 cells for wild-type and msh3−/− cells, 1 × 105 to 2 × 105 cells for msh2−/− and msh2,3−/− cells) onto 90-mm-diameter SNL76/7 feeder plates containing M15. At 24 h after plating, G418 selection was started (360 μg of active ingredient per ml). Cells were grown under G418 selection for 12 days. The plates were then washed and stained, and the colonies were counted. Mutation rates were calculated according to the method of Luria and Delbruck (20).
Mitotic recombination: targeting frequency at the Hprt locus.
RIV6.9I and RIV6.9NI are targeting constructs bearing 6.9 kb of homology to the mouse Hprt locus. The homology region in RIV6.9NI was obtained from a BALB/c mouse, while the ES cells used in the study were derived from 129Sv/Ev/Brd mice. The vectors were prepared by alkaline lysis, purified from CsCl gradients, and linearized with SalI outside the region of homology to generate replacement vectors. A total of 10 μg of linearized plasmid was then electroporated into wild-type, msh3−/−, msh2−/−, and msh2,3−/− ES cells, and 2.5 × 106 cells were plated onto 90-mm-diameter SNL76/7 feeder plates in M15. At 24 h after electroporation, G418 selection was applied. For a subset of plates, 6TG selection was initiated 5 days after electroporation. Drug-containing medium was changed as needed. After 14 days the plates were washed and stained, and the colonies counted. Targeting frequencies were estimated by dividing the average number of G418/6TGr colonies on the plates under double selection by the average number of G418r colonies on the plates under single selection.
RESULTS
General characterization.
RT-PCR analysis of Msh3 and Msh2 transcripts was carried out in order to confirm the expression of these genes in ES cells and to verify the predicted structures of wild-type and mutant mRNAs from the various ES cell lines generated.
Msh3.
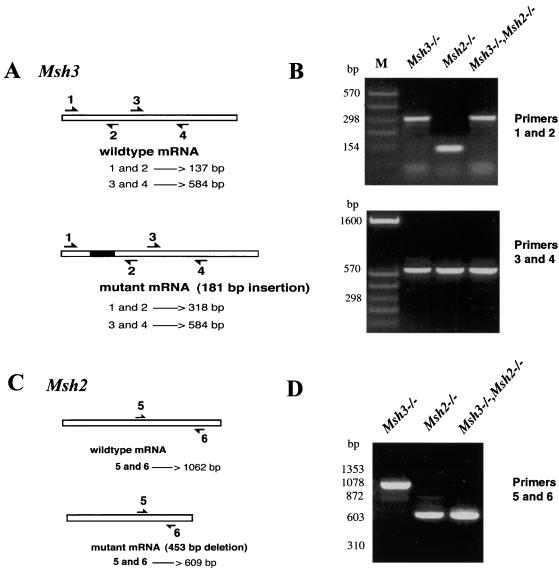
A control oligonucleotide primer pair was designed to amplify a 584-bp fragment of the Msh3 cDNA downstream from the predicted insertional mutation (2) in the Msh3 mRNA. An amplification product consistent with the predicted size was obtained from msh3−/−, msh2−/−, and msh2,3−/− ES cell lines, confirming the expression of Msh3 in mouse ES cells (Fig. 1B). A second primer pair was used that flanks the predicted insertion site in the mutant Msh3 mRNA (Fig. 1A). This primer pair amplified a fragment consistent with the predicted wild-type size (137 bp) from msh2 ES cell lines, confirming the wild-type structure of their Msh3 mRNA. For msh3−/− and msh2,3−/− ES cell lines, however, this primer pair amplified a larger fragment (318 bp) as predicted for the mutant Msh3 mRNA containing the 181-bp insertion (loxP site, polylinker and stop codons) (Fig. 1B). Southern blotting and hybridization with a radiolabeled oligonucleotide internal to the primer pair used for RT-PCR and specific to the Msh3 cDNA confirmed the nature of the amplified products. An oligonucleotide probe complementary to the insertional mutation hybridized only to the larger amplification products obtained from msh3−/− and msh2,3−/− ES cell lines (data not shown).
FIG. 1.
RT-PCR analysis of Msh3 and Msh2 transcripts. (A) Diagram of wild-type and mutant Msh3 transcripts and the position of PCR primers. Predicted sizes of amplification products are given in base pairs. (B) Agarose gel electrophoresis of amplification products from msh3−/−, msh2−/−, and msh2,3−/− ES cells. M, DNA size standard. (C) Diagram of wild-type and mutant Msh2 transcripts and the position of PCR primers. Predicted sizes of amplification products are given in base pairs. (D) Agarose gel electrophoresis of amplification products from msh3−/−, msh2−/−, and msh2,3−/− ES cells.
Msh2.
Two oligonucleotide primers complementary to sequences in the Msh2 cDNA flanking the predicted deletion mutation (2) were used in RT-PCR reactions (Fig. 1C). This primer pair amplified a fragment consistent with the predicted wildtype Msh2 mRNA (1,062 bp) from msh3−/− ES cell lines and a smaller (609-bp) fragment from msh2−/− and msh2,3−/− ES cell lines as predicted for a mutated Msh2 mRNA produced from the deleted genomic locus (Fig. 1D). Southern blotting and hybridization by using an Msh2 probe internal to the primer pair used in RT-PCR and specific to sequences outside the deletion confirmed the nature of the amplified products. A probe specific to the deleted portion of the Msh2 mRNA hybridized only to fragments amplified from msh3−/− ES cell lines (data not shown).
Control assays were performed in order to ascertain any possible effects of the Msh3 and Msh2 mutations on the general growth, plating, and transfection characteristics of the various mutant ES cell lines generated. The low-density colony-forming ability of the different cell lines was calculated by plating ES cells onto 90-mm-diameter SNL76/7 feeder plates in M15 and dividing the number of resulting colonies by the number of ES cells plated. The results were as follows: wild type, mean = 40% (range 42 to 37%); msh3−/− mean = 35% (range, 39 to 30%); msh2−/−, mean = 34% (range, 40 to 27%); and msh2,3−/−, mean = 44% (range, 47 to 42%). ES cell lines carrying single and compound mutations displayed growth rates and transformation efficiencies comparable to those of wild-type ES cells (data not shown).
Msh2-deficient ES cells are resistant to 6TG killing.
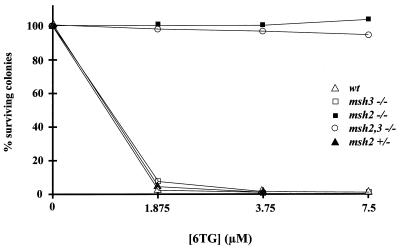
It has been discovered that the cytotoxic effects of 6TG are mediated by the DMR system in mammalian cells (7, 42, 46). In order to assess the effects of the Msh3 and Msh2 mutations on 6TG cytotoxicity, a survival curve for wild-type and mutant ES cell lines was determined with a range of 6TG concentrations. msh2−/− and msh2,3−/− ES cell lines are fully resistant to killing by 6TG at concentrations incompatible with the survival of wild-type, +/msh2Brdm1, and msh3−/− ES cell lines (Fig. 2). +/msh2Brdm1 cells were included in the study to assess whether the mutant Msh2 mRNA with the 453-bp in-frame deletion (2) gave rise to a protein product with dominant-negative effects.
FIG. 2.
Cytotoxicity of 6TG in mouse ES cell lines. Survival curves of wild-type and mutant ES cells in the presence of various concentrations of 6TG are shown. Datum points represent the average of two experiments. For details, see Materials and Methods.
Msh2 deficiency leads to a mutator phenotype.
DMR mutations lead to increased spontaneous mutation rates in bacteria and yeast (15). We assessed the effects of the Msh3 and Msh2 mutations on the mutation rates of two loci: HSV-tk1 and Hprt. The loss of function of these two expression cassettes can be selected in FIAU and 6TG, respectively, allowing for comparisons of their mutation rates in different genetic backgrounds.
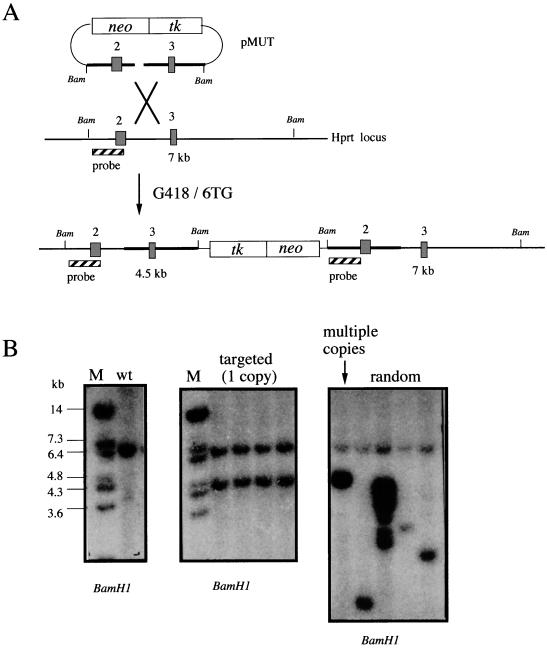
In order to control for the chromosomal position and copy number, a single copy of the HSV-tk1 cassette was targeted to the same chromosomal location in all ES cell lines. The HSV-tk1 cassette was cloned into an Hprt targeting construct along with the neo cassette for positive selection. Targeting of the HSV-tk1 cassette by using an Hprt insertion targeting construct allows for the selection of independent clones containing single targeted copies of the cassette. Successful targeting by the insertion vector leads to the duplication of the target sequences at the Hprt locus on the X chromosome. Southern analysis with an internal probe was used to screen 6TG-resistant clones for targeted clones in which the endogenous and novel bands were of equal intensity, indicating the integration of a single copy of the vector (Fig. 3). Targeting a single copy of the vector to each mutant background standardizes the assay conditions for all clones. Moreover, it is necessary to use clones containing only a single copy of the HSV-tk1 cassette, since FIAU-negative selection will be used to assay the mutation rates. To obtain FIAUr clones from cell lines with multiple copies, all HSV-tk1 cassettes would have to be inactivated. This would be an unlikely event. Furthermore, multiple tandem copies of the cassette could lead to marker loss through homologous recombination.
FIG. 3.
Targeting pMUT to the mouse Hprt locus. (A) Schematic diagram of pMUT targeting. Linearization within the Hprt homology region gives rise to an insertion vector. After double selection in G418 and 6TG, a proportion of the clones were targeted with a single copy of pMUT, resulting in the duplication of the target sequences. Hprt exons are represented by numbered boxes. BamHI restriction sites are indicated as Bam. The internal hybridization probe is shown as the striped box. BamHI size fragments are indicated in kilobases. (B) Southern blot analysis of BamHI-digested genomic DNA from wild-type (wt) and G418/6TGr clones by using the internal pMUT probe. Correctly targeted clones containing a single copy of pMUT display a novel band of the predicted size equal in intensity to the wild-type band. Clones displaying hybridization patterns consistent with multicopy and random integration are included for comparison. M, DNA size marker.
Independent ES clones containing a single copy of the HSV-tk1 cassette at the Hprt locus were used in fluctuation assays to measure the mutation rate as described in Materials and Methods. HSV-tk1 mutation rates were unchanged in msh3−/− cells compared to wild-type cells. msh2−/− cells displayed a 48-fold increase in HSV-tk1 mutation rates over wild-type cells. msh2,3−/− ES cells displayed 337- and 6.8-fold increases in the HSV-tk1 mutation rate compared to wild-type and msh2−/− cells, respectively (Tables 1 and 2). To confirm these results with independently generated msh2−/− and msh2,3−/− ES cell lines, fluctuation tests for HSV-tk1 mutation rates were carried out with independent clones in which a single copy of the HSV-tk1 cassette was targeted at the Msh2 locus. These clones were obtained independently as intermediates in the generation of the original msh2−/− and msh2,3−/− ES cell lines. Fluctuation tests with these cell lines revealed a 2.4-fold increase in HSV-tk1 mutation rate in the msh2,3−/− ES cells relative to the msh2−/− cells (Tables 1 and 2). To determine the nature of the mutations at the HSV-tk1 locus, the coding region of HSV-tk1 cassettes from FIAUr msh2−/− and msh2,3−/− ES clones was amplified by PCR, cloned, and sequenced. The results of sequence analysis of six FIAUr clones for each mutant class are summarized in Table 3. Seventy-five percent of the msh2−/− FIAUr clones and 50% of the msh2,3−/− FIAUr clones analyzed harbored the same frameshift mutation in a stretch of 7 guanines at position 430 of the HSV-tk1 coding region.
TABLE 1.
HSV-tk1 and Hprt mutation ratesa
| Locus assessed and genotype | Locus | No. of mutations/generation/locus |
|---|---|---|
| HSV-tk1 | ||
| Wild type | Hprt | 7.1 × 10−7 |
| msh3−/− | Hprt | 8.3 × 10−7 |
| msh2−/− (clone 1) | Hprt | 3.5 × 10−5 |
| msh2−/− (clone 2) | Msh2 | 1.1 × 10−4 |
| msh2,3−/− (clone 1) | Hprt | 2.4 × 10−4 |
| msh2,3−/− (clone 2) | Msh2 | 2.6 × 10−4 |
| Hprt | ||
| msh2−/− (clone 1) | 4.1 × 10−6 | |
| msh2−/− (clone 2) | 2.8 × 10−5 | |
| msh2,3−/− (clone 1) | 8.3 × 10−6 | |
| msh2,3−/− (clone 2) | 4.2 × 10−5 |
Mutation rates of the HSV-tk1 and Hprt loci in the different genetic backgrounds. Clones 1 and 2 contain a single copy of the HSV-tk1 cassette at the Hprt and Msh2 loci, respectively.
TABLE 2.
Relative HSV-tk1 and Hprt mutation rates
| Clone | Relative mutation rate
|
|
|---|---|---|
| Hprt | HSV-tk1 | |
| msh2−/− (clone 1) | 1 | 8.4 |
| msh2−/− (clone 2) | 1 | 3.9 |
| msh2,3−/− (clone 1) | 1 | 28.4 |
| msh2,3−/− (clone 2) | 1 | 6.3 |
| Avg | 1 | 11.7 |
TABLE 3.
Sequence analysis of HSV-tk1 mutations in FIAUr clones
| Clone | Mutation position | Result |
|---|---|---|
| msh2−/− | ||
| 1 | 151 | Arg to Trp |
| 2 | 430 | +1 frameshift |
| 3 | 430 | −1 frameshift |
| 4 | 430 | −1 frameshift |
| 5 | 430 | −1 frameshift |
| 6 | 430 | −1 frameshift |
| msh2,3−/− | ||
| 1 | 430 | −1 frameshift |
| 2 | ND | Promoter, other |
| 3 | 332 and 734 | Glu to Gly and Thr to Met |
| 4 | 430 | −1 frameshift |
| 5 | 430 | −1 frameshift |
| 6 | 860 | Thr to Met |
The HSV-tk1 sequence was obtained from six FIAUr clones derived from msh2−/− clone 1 and msh2,3−/− clone.
Hprt mutation rates were measured from msh2−/− and msh2,3−/− ES cell lines by using fluctuation tests as described in Materials and Methods. Hprt mutation rates in the two independently derived sets of mutant ES cell lines are shown in Tables 1 and 2. A high concentration (100 μM) of 6TG was used in order to bypass the resistance induced by the Msh2 mutation. msh2−/− cells which are wild type for Hprt do not survive this 6TG concentration (data not shown). Fluctuation tests were not performed for wild-type and msh3−/− cell lines due to practical limitations of the experimental requirements to plate no more than 105 cells per 90-mm-diameter dish when applying 6TG selection alone. A mutation frequency was determined, however, for wild-type and msh3−/− cells. A total of 106 cells were plated onto 10 90-mm-diameter feeder plates (105 cells/plate) and selected in 6TG for 12 days. Zero and one 6TGr colonies were observed in plates seeded with wild-type and msh3−/− cells, respectively.
msh3−/− and msh2−/− ES cells show mild and severe dinucleotide repeat instabilities, respectively.
DMR mutations in bacteria, yeast, and humans result in the instability of simple sequence repeats or microsatellites (15). pRTM2 is an expression vector designed to measure the mutation rate of a dinucleotide repeat in mammalian cells (10). pRTM2 contains a herpes simplex virus thymidine kinase-neomycin resistance (HSV-tk1/neo) fusion construct under the transcriptional control of the cytomegalovirus promoter. A (CA)17 repeat was inserted in this construct directly upstream of the neo sequences, placing them out of frame with respect to HSV-tk1 sequences. Thus, the fusion protein produced from pRTM2 does not confer resistance to G418 due to the inappropriate translation of the neo sequences in the fusion mRNA. If during the growth of the host cell, however, a change in the size of the repeat occurs so as to place the neo sequences back in frame within the fusion construct, a functional fusion protein will be produced and the host cell will acquire resistance to G418. The pRTM2 assay system can therefore be used to estimate the rates of instability of the (CA)17 microsatellite in mammalian cell lines of different genetic backgrounds by measuring the number of G418r colonies obtained.
In order to measure the stability of the (CA)17 microsatellite in the various DMR genetic backgrounds, the pRTM2 assay cassette was introduced into wild-type, msh3−/−, msh2−/−, and msh2,3−/− ES cell lines by using an Hprt targeting construct. As mentioned above for pMUT targeting the pRTM2 cassette to the Hprt locus allows for the selection of independent clones containing single copies of the assay cassette at the same chromosomal location. Fluctuation tests were carried out as described in Materials and Methods. The rates of conversion to G418 resistance in the different genetic backgrounds are shown in Table 4. msh3−/− ES cells displayed a modest (16-fold) increase in the stability of the pRTM2 repeat, whereas msh2−/− and msh2,3−/− ES cells displayed greatly elevated (1.6 × 104- and 3.3 × 104-fold, respectively) levels of instability of the pRTM2 microsatellite.
TABLE 4.
pRTM2 mutation rates in wild-type and mutant ES cellsa
| Variable | Wild-type cells (1.5 × 107 cells plated) | msh3−/− cells (5 × 106 cells plated) | msh2−/− cells (105 cells plated) | Double-mutant cells (104 cells plated) |
|---|---|---|---|---|
| Exp | ||||
| 1 | 0 | 0 | 748 | 69 |
| 2 | 0 | 12 | 924 | 188 |
| 3 | 0 | 5 | 848 | 99 |
| 4 | 1 | 8 | 1068 | 160 |
| 5 | 0 | 0 | 1512 | 103 |
| 6 | 2 | 1 | 1328 | 152 |
| 7 | 0 | 6 | 1080 | 150 |
| 8 | 1 | 4 | 1144 | 156 |
| 9 | 1 | 0 | 1464 | 128 |
| 10 | 0 | 22 | 1144 | 129 |
| Rate (mut/gen/locus) | 2.5 × 10−8 | 3.9 × 10−7 | 4.1 × 10−4 | 8.3 × 10−4 |
| Relative rate | 1 | 15 | 1.6 × 104 | 3.3 × 104 |
Fluctuation analysis of CA instability at the Hprt locus in wild-type, msh2−/−, msh3−/−, and double-mutant clones. For each cell line the number of cells plated and the number of G418-resistant clones is indicated. The calculated number of mutations/locus/generation (mut/gen/locus) is indicated, as well as the rate relative to the wild-type control.
Msh2 blocks homologous recombination in mouse ES cells.
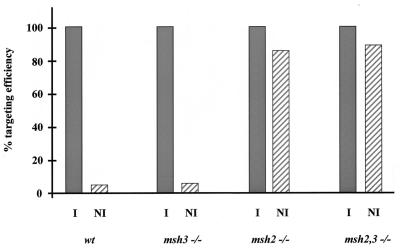
Loss of DMR in E. coli and S. cerevisiae and humans leads to an increase in the mitotic recombination rates between diverged DNA sequences, also known as homeologous recombination (6, 39, 40). In order to assess the effects of Msh2 and Msh3 mutations on the recombination rates of substrates derived from different genetic backgrounds, the targeting frequencies of constructs bearing homology to the Hprt locus were measured as described in Materials and Methods. The frequency of Hprt targeting by RIV6.0NI was approximately 5% of that by RIV6.0I in wild-type and msh3−/− ES cells. In msh2−/− and msh2,3−/− ES cells, however, the targeting frequency by RIV6.9NI was elevated to levels comparable (85%) to that of RIV6.9I (Fig. 4).
FIG. 4.
Targeting frequencies of isogenic and nonisogenic Hprt constructs. Isogenic (I) and nonisogenic (NI) Hprt constructs were electroporated into wild-type, msh3−/−, msh2−/−, and msh2,3−/− ES cells, and their targeting frequencies were calculated as described in Materials and Methods. Targeting frequencies with isogenic constructs are normalized to 100%.
DISCUSSION
Msh2 mediates 6TG cytotoxicity in mouse ES cells.
Mouse ES cell lines homozygous for a mutation at the Msh2 locus were resistant to the cytotoxic effects of 6TG relative to wild-type and msh3−/− ES cells (Fig. 2). The result shows that Msh2 is responsible for 6TG-mediated killing. DMR is known to be involved in mediating killing of mammalian cells by 6TG and several other drugs (11). Human MutSalpha binds to DNA containing methylated 6TG (46), and human MSH2 expression has been shown to correlate with the degree of resistance to methylating agents (7).
Upon modification by the HPRT enzyme, 6TG is incorporated into DNA during replication and subsequently methylated (42). DNA polymerase misincorporates thymidine across from this methylated 6TG, leading to the formation of a me6TG/T mismatch. DMR-mediated killing of wild-type cells is then thought to occur through one of two processes: (i) “runaway repair”, or sequential rounds of excision, resynthesis, and misincorporation leading to the formation of a double-strand break upon replication, or (ii) the formation of a double-strand break due to the excision of opposite strands from adjacent mismatches.
Resistance to cytotoxic agents such as 6TG by Msh2-deficient cells may be clinically important. A large proportion of transplant patients are treated with the immunosuppressant drug azathioprine, which is converted in vivo to thioguanine nucleotides (19). It has been proposed (42) that 6TG may be incorporated into the DNA of azathioprine-treated patients and result in the selection of DMR-deficient cells. The survival of Msh2-deficient cells after azathioprine treatment may be responsible for the increased incidence of cancer in long-term organ transplant survivors (3).
As mentioned above, +/msh2Brdm1 cells were included in the study to assess whether the mutant Msh2 mRNA with the 453-bp in-frame deletion (2) gave rise to a protein product with dominant-negative effects. +/msh2Brdm1 cells displayed sensitivity to 6TG indistinguishable from that of wild-type cells, indicating the absence of dominant-negative effects and the recessive nature of the msh2Brdm1 mutation. The lack of an effect of the Msh3 mutation on 6TG sensitivity supports the model in which MutSalpha, the MSH2-MSH6 heterodimer, repairs single base mismatches during DMR.
Msh2 deficiency leads to mutator phenotype.
The rate of mutation at the HSV-tk1 locus was not affected in msh3−/− ES cells relative to wild-type ES cells. msh2−/− and msh2,3−/− ES cells, however, displayed an elevated HSV-tk1 mutation rate. For ES cell clones in which the HSV-tk1 cassette was targeted to the Hprt locus, there was a significant difference in the rate of HSV-tk1 mutation between msh2−/− and msh2,3−/− cells. In independently derived clones carrying a single copy of the HSV-tk1 cassette at the Msh2 locus, however, the difference in HSV-tk1 mutation rates between msh2−/− and msh2,3−/− cells was not significant (0.1 < P < 0.2). The Hprt mutation rate of msh2−/− and msh2,3−/− cells was not significantly different (Tables 1 and 2).
Msh2 deficiency led to the instability of a mononucleotide run in the HSV-tk1 gene. The sequence analysis of mutant HSV-tk1 cassettes from independently arisen FIAUr clones revealed the presence of a frameshift mutation hotspot in the HSV-tk1 coding region. A total of 66% of the sequenced clones had a frameshift mutation (12.5%, + 1; 87.5%, −1) at a single stretch of seven guanines in the HSV-tk1 coding region (Table 3). Comparison of the HSV-tk1 and Hprt mutations rates within individual Msh2-deficient ES cell lines shows that HSV-tk1 mutates, on average, at an 11-fold-higher rate than Hprt in the same genetic background. Interestingly, the mouse Hprt coding region contains a stretch of six guanines which has been shown to be a frameshift hotspot (35% of mutations) in transfection studies in human DMR-deficient cells (4). It is possible that the difference in HSV-tk1 and Hprt mutation rates is due to the larger size of the mononucleotide stretch in the former (seven guanines in HSV-tk1 versus six guanines in Hprt). Notably the HSV-tk1 coding sequence also contains a mononucleotide stretch of six cytosines which was not found mutated in any of the cassettes sequenced. It will be interesting to determine the correlation between the length and mutation rate of a mononucleotide repeat in mammalian cells.
Such frameshift hotspots have been discovered for other genes in a DMR-deficient background (14, 21, 33) and have been proposed to play an important role in the etiology of tumorigenesis in a DMR-deficient background. The model proposes that tumor suppressors and other genes containing such frameshift hotspots would become primary targets for mutagenesis once a cell had lost a functional DMR system. Examples of such genes include Apc (14), transforming growth factor beta type II receptor (TGFβ-IIR), and Bax (33). Although many genes containing such frameshift hotspots are likely to be mutated in an Msh2-deficient cell, it will be important to determine which mutations are directly involved in tumor progression. In the case of human TGFβ-IIR, there is a high rate of mutation of a mononucleotide run of 10 adenines in the coding region (27). Furthermore, restoration of TGFβ-IIR activity in DMR-deficient colon carcinoma cells leads to reversion of their malignancy (45), suggesting that TGFβ-IIR mutations contribute to the malignant phenotype. Notably, Msh2-deficient mice develop intestinal carcinomas (35), despite the fact that the longest mononucleotide run in the mouse TGFβ-IIR coding region is just five bases long (17).
Instability of a dinucleotide repeat in msh3−/− and msh2−/− ES cells.
The stability of a dinucleotide microsatellite was measured in the different genetic backgrounds generated. Microsatellite instability is a hallmark of DMR deficiency in bacteria, yeasts, and humans (15). DMR mutants are unable to repair the mismatches and heterologies that arise, most likely through DNA polymerase slippage, during the replication of these repeats, leading to the enlargement or shortening of the overall repeat size upon subsequent replication.
As mentioned above, a simple repeat sequence in the HSV-tk1 gene was found to be mutated in a high percentage of the FIAUr msh2−/− and msh2,3−/− clones sequenced. It has been proposed that the instability of simple repeats within the coding regions of tumor suppressors and oncogenes plays an important role in tumor progression in a DMR-deficient background.
In S. cerevisiae, mutations in Msh3 and Msh6 lead to mild microsatellite instability that varies with the size of the repeat unit. msh3 msh6 strains display a microsatellite instability phenotype indistinguishable from the msh2 strains, supporting the model for functionally overlapping MSH2-MSH6 (MutSalpha) and MSH2-MSH3 (MutSbeta) complexes during yeast DMR. In humans, this functional overlap between MSH3 and MSH6 is supported by chromosome transfer studies in cells mutant for both genes (44).
To measure the stability of a dinucleotide repeat in different genetic backgrounds, we used the pRTM2 assay system described previously (10). pRTM2 allows for the selection of ES cell clones in which a (CA)17 repeat has changed in size, resulting in the in-frame translation of the selectable marker for neomycin resistance. In order to control for the chromosomal location and copy number of the pRTM2 assay system, we used a targeting construct as a vehicle to introduce a single copy of the pRTM2 cassette into the Hprt locus. The rate of conversion to G418 resistance was elevated 16-fold in msh3−/− ES cells relative to wild-type ES cells. msh2−/− and msh2,3−/− ES cell lines displayed an increase in the rate of reversion 3 orders of magnitude larger than that of msh3−/− cells (Table 4). These results are consistent with the yeast data in that the microsatellite instability effect of the MSH3 mutation is modest relative to that of the MSH2 mutation.
Interestingly, the mutation rate of the pRTM2 cassette in wild-type mouse ES cells is 800-fold lower than the published pRTM2 mutation rate in immortalized mouse CAK fibroblast cells (10), which are nontumorigenic and do not display a mutator phenotype. As discussed in Materials and Methods, we did not adjust our mutation rates for the plating efficiency of each cell line because low-density plating efficiencies in ES cells cannot be reliably extrapolated to the high-density platings used in our fluctuation experiments. Plating efficiencies of ES cells at high density are greater than those of low density (unpublished observations). Although this will result in a slight underestimate of mutation rates, it is not sufficient to explain the very large difference observed here.
It is possible that the lower mutation rate of the pRTM2 repeat in mouse ES cells is due to increased activity of the DMR system relative to that in fibroblasts. Alternatively, it is possible that CAK fibroblasts have other mutations which affect the mutation rate of the pRTM2 cassette.
Msh2 blocks homeologous recombination in mouse ES cells.
DMR acts as a block to homeologous recombination in E. coli, S. cerevisiae, and humans (6, 29, 34, 39, 40). DMR mutants are presumably unable to recognize and bind mismatches and other heterologies that arise in heteroduplex intermediates of recombination. In E. coli, mutS mutants display elevated levels of large duplications derived from the recombination between tandem diverged copies of genes (29). In mammals, a similar effect of DMR loss has the potential to induce increased recombination rates between homeologous sequences interspersed throughout the genome. Such recombination events could lead to chromosomal translocations, deletion, or inversions with deleterious consequences in the adult or during development. Hematological malignancies often arise through chromosomal rearrangements (8). Interestingly, Msh2-deficient mice display high incidence of lymphoma (36), suggesting that the loss of a block to homeologous recombination may contribute to tumor formation through increased rates of chromosomal rearrangements.
In contrast to their roles in mismatch repair, S. cerevisiae MSH2 and MSH3 appear to act independently in parallel pathways to block homeologous recombination between repeated chromosomal sequences (39, 40). We assessed homologous recombination rates in the different ES cell lines by measuring the targeting frequencies with Hprt vectors derived from different genetic backgrounds. ES cell lines carrying a homozygous Msh2 mutation showed increased targeting frequencies with nonisogenic Hprt constructs, to a level comparable to that of isogenic vectors. msh3−/− ES cells displayed no increase in the targeting frequency of these nonisogenic Hprt constructs (Fig. 4). Our results differ from those obtained in S. cerevisiae, in which MSH2 and MSH3 act independently in parallel pathways to block homeologous recombination (39, 40). This may be explained, however, by the relatively high degree (70%) of divergence between the DNA sequences used in the yeast study. We have limited data to suggest that our Hprt targeting constructs are not as diverged as the tested yeast sequences. The yeast results suggest that MSH3 acts independently of MSH2 during homeologous recombination blockage, perhaps by dimerizing with itself or some other mutS homologue. If its role during this process is also to recognize larger I/D heterologies, the fact that Msh3 does not block homeologous recombination with our nonisogenic Hprt vectors may reflect the absence of multiple base I/D heterologies in the intermediates of recombination with the chromosomal target sequences.
ACKNOWLEDGMENTS
We thank Sandra Rivera and Sukeshi Vaishnav for tissue culture support and Sylvia Perez for help in preparing the manuscript. We thank Mike Liskay for providing the pRTM2 plasmid and a computer program for fluctuation analysis and Tom Prolla for helpful discussions.
A.B. acknowledges support from the National Cancer Institute. A.B. is an Investigator with the Howard Hughes Medical Institute.
REFERENCES
- 1.Aaltonen L A, Peltomaki P, Leach F, Sistonen P, Pylkkanen S M, Mecklin J-P, Jarvinen H, Powell S, Jen J, Hamilton S R, Petersen G M, Kinzler K W, Vogelstein B, de la Chapelle A. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 2.Abuin A, Bradley A. Recycling selectable markers in mouse embryonic stem cells. Mol Cell Biol. 1996;16:1851–1856. doi: 10.1128/mcb.16.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett W L, First M R, Aron B S, Penn I. Clinical course of malignancies in renal transplant recipients. Cancer. 1993;72:2186–2189. doi: 10.1002/1097-0142(19931001)72:7<2186::aid-cncr2820720720>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya N P, Ganesh A, Phear G, Richards B, Skandalis A, Meuth M. Molecular analysis of mutations in mutator colorectal carcinoma cell lines. Hum Mol Genet. 1995;4:2057–2064. doi: 10.1093/hmg/4.11.2057. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya N P, Skandalis A, Ganesh A, Groden J, Meuth M. Mutator phenotypes in human colorectal carcinoma cell lines. Proc Natl Acad Sci USA. 1994;91:6319–6323. doi: 10.1073/pnas.91.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wind N, Dekker M, Bern A, Radman M, te Reile H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyper recombination, and predisposition to cancer. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 7.Dosch J, Christmann M, Kaina B. Mismatch G-T binding activity and MSH2 expression is quantitatively related to sensitivity of cells to methylating agents. Carcinogenesis. 1998;19:567–573. doi: 10.1093/carcin/19.4.567. [DOI] [PubMed] [Google Scholar]
- 8.Drexler H G, MacLeod R A, Borkhardt A, Janssen J W. Recurrent chromosomal translocations and fusion genes in leukemia-lymphoma cell lines. Leukemia. 1995;9:480–500. [PubMed] [Google Scholar]
- 9.Drummond J T, Genschel J, Wolf E, Modrich P. DHFR/MSH3 amplification in methotrexate-resistant cells alters the hMutSalpha/hMutSbeta ratio and reduces the efficiency of base-base mismatch repair. Proc Natl Acad Sci USA. 1997;94:10144–10149. doi: 10.1073/pnas.94.19.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farber R A, Petes T D, Dominska M, Hudgens S S, Liskay R M. Instability of simple sequence repeats in a mammalian cell line. Hum Mol Genet. 1994;3:253–256. doi: 10.1093/hmg/3.2.253. [DOI] [PubMed] [Google Scholar]
- 11.Fink D, Aebi S, Howell S B. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6. [PubMed] [Google Scholar]
- 12.Fishel R A, Lescoe M K, Rao M R S, Copeland N, Jenkins N, Garber J, Kane M, Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 13.Genschel J, Littman S J, Drummond J T, Modrich P. Isolation of MutSbeta from human cells and comparison of the mismatch repair specificities of MutSbeta and MutSalpha. J Biol Chem. 1998;273:19895–19901. doi: 10.1074/jbc.273.31.19895. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Papadopoulos N, McKinley A J, Farrington S M, Curtis L J, Wyllie A, Zheng S, Willson K V, Markowitz S D, Morin P, Kinzler K W, Vogelstein B, Dunlop M G. APC mutations in colorectal tumors with mismatch repair deficiency. Proc Natl Acad Sci USA. 1996;93:9049–9054. doi: 10.1073/pnas.93.17.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolodner R. Biochemistry and genetics of eucaryotic mismatch repair. Genes Dev. 1996;10:1433–1442. doi: 10.1101/gad.10.12.1433. [DOI] [PubMed] [Google Scholar]
- 16.Kolodner R D, Alani E. Mismatch repair and cancer susceptibility. Curr Opin Biotechnol. 1994;5:585–594. doi: 10.1016/0958-1669(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 17.Lawler S, Candia A F, Ebner R, Shum L, Lopez A R, Wright C V, Derynck R. The murine type II TGF-beta receptor has a coincident expression and binding preference for TGF-beta 1. Development. 1994;120:165–175. doi: 10.1242/dev.120.1.165. [DOI] [PubMed] [Google Scholar]
- 18.Leach F S, Nicolaides N C, Papadopoulos N, Liu B, Jen J, Parsons R, Peltomaki R, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 19.Lennard L, Thomas S, Harrington C I, Maddocks J L. Skin cancer in renal transplant recipients is associated with increased concentrations of 6-thioguanine nucleotide in red blood cells. Br J Dermatol. 1985;113:723–729. doi: 10.1111/j.1365-2133.1985.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 20.Luria S E, Delbruck M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan R S, Zborowska E, Kinzler K W, Vogelstein B, Brattain M, Willson K V. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 22.Marra G, Iaccarano I, Lettieri T, Roscilli G, Delmastro P, Jiricny J. Mismatch repair deficiency associated with overexpression of the MSH3 gene. Proc Natl Acad Sci USA. 1998;95:8568–8573. doi: 10.1073/pnas.95.15.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon A, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 24.Orth K, Hung J, Gazdar A, Bowcodk A, Mathis J M, Sambrook J. Genetic instability in human ovarian cancer cell lines. Proc Natl Acad Sci USA. 1994;91:9495–9499. doi: 10.1073/pnas.91.20.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palombo F, Gallinari P, Iaccarino I, Lettieri T, Hughes M, D'Arrigo A, Truong O, Hsuan J J, Jiricny J. GTBP, a 160-kilodalton protein essential for mismatch-binding activity in human cells. Science. 1995;268:1912–1914. doi: 10.1126/science.7604265. [DOI] [PubMed] [Google Scholar]
- 26.Papadopoulos N, Nicolaides N C, Liu B, Parsons R, Lengauer C, Palombo F, D'Arrigo A, Markowitz S, Willson J K V, Kinzler K W, Jiricny J, Vogelstein B. Mutations of GTBP in genetically unstable cells. Science. 1995;268:1915–1917. doi: 10.1126/science.7604266. [DOI] [PubMed] [Google Scholar]
- 27.Parsons R, Myeroff L L, Liu B, Willson J K, Markowitz S D, Kinzler K W, Vogelstein B. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res. 1995;55:5548–5550. [PubMed] [Google Scholar]
- 28.Peltomaki P, Lothe R A, Aaltonen L A, Pylkkanen L, Nystrom-Lahti M, Seruca R, David L, Holm R, Ryberg D, Haugen A, Brogger A, Borresen A-L, de la Chapelle A. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993;53:5853–5855. [PubMed] [Google Scholar]
- 29.Petit M A, Dimpfl J, Radman M, Echols H. Control of large chromosomal duplications in Escherichia coli by the mismatch repair system. Genetics. 1991;129:327–332. doi: 10.1093/genetics/129.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez-Solis R, Liu P, Bradley A. Chromosome engineering in mice. Nature. 1995;378:720–724. doi: 10.1038/378720a0. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez-Solis R, Davis A C, Bradley A. Gene targeting in mouse embryonic stem cells. Methods Enzymol. 1993;225:855–877. doi: 10.1016/0076-6879(93)25054-6. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez-Solis R, Rivera-Perez J, Wallace J D, Wims M, Zheng H, Bradley A. Genomic DNA microextraction: a method to screen numerous samples. Anal Biochem. 1992;201:331–335. doi: 10.1016/0003-2697(92)90347-a. [DOI] [PubMed] [Google Scholar]
- 33.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed J C, Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 34.Rayssiguier C, Thaler D S, Radman M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature. 1989;342:396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- 35.Reitmair A H, Redston M, Cai J C, Chuang T C, Bjerknes M, Cheng H, Hay K, Gallinger S, Bapat B, Mak T W. Spontaneous intestinal carcinomas and skin neoplasms in Msh2-deficient mice. Cancer Res. 1996;56:3842–3849. [PubMed] [Google Scholar]
- 36.Reitmair A H, Schmits R, Ewel A, Bapat B, Redston M, Mitri A, Waterhouse P, Mittrucker H-W, Wakeham A, Liu B, Thomason A, Griesser H, Gallinger S, Ballhausen W G, Fishel R, Mak T W. Msh2 deficient mice are viable and susceptible to lymphoid tumors. Nat Genet. 1995;11:64–70. doi: 10.1038/ng0995-64. [DOI] [PubMed] [Google Scholar]
- 37.Risinger J I, Umar A, Boyd J, Berchuck A, Kunkel T A, Barrett J C. Mutation of MSH3 in endometrial cancer and evidence for its functional role in heteroduplex repair. Nat Genet. 1996;14:102–105. doi: 10.1038/ng0996-102. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 39.Selva E M, Maderazo A B, Lahue R S. Differential effects of the mismatch repair genes MSH2 and MSH3 on homeologous recombination in Saccharomyces cerevisiae. Mol Gen Genet. 1997;257:71–82. doi: 10.1007/pl00008619. [DOI] [PubMed] [Google Scholar]
- 40.Selva E M, New L, Crouse G F, Lahue R S. Mismatch correction acts as a barrier to homeologous recombination in Saccharomyces cerevisiae. Genetics. 1995;139:1175–1188. doi: 10.1093/genetics/139.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strand M, Prolla T A, Liskay R M, Petes T D. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993;365:274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- 42.Swann P F, Waters T R, Moulton D C, Xu Y-Z, Zheng Q, Edwards M, Mace R. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996;273:1109–1111. doi: 10.1126/science.273.5278.1109. [DOI] [PubMed] [Google Scholar]
- 43.Thibodeau S N, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 44.Umar A, Risinger J I, Glaab W E, Tindall K R, Barrett J C, Kunkel T A. Functional overlap in mismatch repair by human MSH3 and MSH6. Genetics. 1998;148:1637–1646. doi: 10.1093/genetics/148.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Sun L, Myeroff L, Wang X, Gentry L E, Yang J, Liang J, Zborowska E, Markowitz S, Willson J K, et al. Demonstration that mutation of the type II transforming growth factor beta receptor inactivates its tumor suppressor activity in replication error-prone colon carcinoma cells. J Biol Chem. 1995;270:22044–22049. doi: 10.1074/jbc.270.37.22044. [DOI] [PubMed] [Google Scholar]
- 46.Waters T R, Swann P F. Cytotoxic mechanism of 6-thioguanine: hMutSalpha, the human mismatch binding heterodimer, binds to DNA containing S6-methylthioguanine. Biochemistry. 1997;36:2501–2506. doi: 10.1021/bi9621573. [DOI] [PubMed] [Google Scholar]