Abstract
Background: Osteoarthritis (OA) is common in the elderly. Baicalin (BA) is a flavonoid monomer extracted from Scutellaria baicalensis Georgi, which has been reported to have anti-inflammatory, anti-deformation and anti-bacterial effects. Methods: Cultures of micromass and 3D alginate beads, Alcian blue and Safranin O (SO)/fast green staining were used to investigate chondrocyte viability and extracellular matrix (ECM) synthesis in chondrocytes of all groups. The expression of SOX9, Smad3, Aggrecan (ACAN), type II collagen (Col2α), matrix metallopetidase 9 (MMP9), MMP13 and ADAMTS5 in chondrocytes of all groups were detected by western blot or qRT-PCR. Results: The present study demonstrates that BA neutralized the IL-1β-induced downregulation of chondrocyte viability and ECM secretion, including ACAN and Col2α. The downregulation of SOX9, and the upregulation of MMP9, MMP13 and ADAMTS5 induced by IL-1β were reversed by BA treatment. Moreover, BA increased the nuclear translocation of Smad3 and SOX9 in chondrocytes cultured by micromass and 3D alginate beads. Interestingly, Smad3 inhibitor SIS3 reversed the promoting effect of BA on chondrocyte viability, ECM secretion, SOX9 and Smad3 nuclear translocation, and the inhibiting effect of BA on MMP9 and ADAMTS5 expressions. BA treatment also attenuated the decrease of Smad3 phosphorylation, SOX9 expression and the damage of cartilage integrity in mice which were induced by destabilization of the medial meniscus (DMM). Conclusion: BA promotes chondrocyte viability and the cell matrix synthesis through TGF-β/Smad3 pathway in IL-1β-treated chondrocytes and DMM treated mice. BA is a potential therapeutic target for OA.
Keywords: Baicalin (BA), Smad3, chondrocyte, extracellular matrix (ECM)
Introduction
Osteoarthritis (OA) is a common chronic joint disease in the elderly [1]. OA is characterized by joint pain, limb stiffness, as well as difficulty in movement which seriously affects the daily life of patients [2]. The high incidence of OA has become a great burden of individuals and society [3]. Currently, drug and surgical treatments are the main treatment options for OA [4]. Expensive surgical treatment is usually used to treat OA patients at the advanced stage [5], while the drug treatment of OA is to inhibit the inflammation. Although drugs may have an advantage in relieving inflammation and pain symptoms, they are not able to prevent or delay the progress of OA completely [6]. It is, therefore, still an urgent need to seek new drugs for OA treatments.
Baicalin (BA) is a flavonoid monomer extracted from Scutellaria baicalensis Georgi. BA has anti-inflammatory, anti-bacterial and diuretic effects [7]. It has been reported that BA can significantly inhibit the inflammatory response and the expression of IL-1β in chondrocytes [8]. IL-1β is a pro-inflammatory factor, which has been shown to inhibit SOX9 expression and extracellular matrix (ECM) synthesis and to induce the productions of MMPs and ADAMTS5 [9]. BA can transcriptionally activate SOX9, the synthesis of Aggrecan (ACAN) and type II collagen (Col2α), as well as two main components of cartilage ECM [10] while inhibit the expressions of matrix metalloproteinases (MMPs) [11]. SOX9 is a key transcription factor in the development and differentiation of chondrocytes. The decreased expression of SOX9 arrests differentiation of prechondrocytes and results in serious skeletal deformities [12]. MMPs are proteases and can degrade the ECM [13]. SOX9 is involved in several pathways including TGF-β/Smads pathway [14]. TGF-β/Smads pathway plays a key role in regulation of SOX9 expression during cartilage formation [15]. Recent studies have shown that TGF-β/Smads could activate SOX9 expression [16]. As a key component of TGF-β/Smads pathway, Smad3 is actively involved in chondrocyte differentiation during cartilage development [17]. The accelerated chondrocyte maturation induced by TGF-β/Smads signaling pathway can be inactivated by de phosphorylation of Smad3 [18]. BA is known to protect chondrocytes by up-regulating SOX9 expression, but it remains unclear whether BA protects chondrocytes through activation of TGF-β/Samd3 and its association with phosphorylation of Smad3. In this study, an in vitro chondrocyte inflammatory model was established by IL-1β stimulation. Cellular and molecular biology methods were used to investigate whether BA protects chondrocyte viability and ECM expression from inflammation through TGF-β/Smad3 pathway in vivo.
Materials and methods
Reagents
The main chemical component in BA is C33H40O15 (Figure 1A), and it was purchased from Shanghai PureOne Biotechnology Co., Ltd (#21967-41-9, Shanghai, China). BA was dissolved in dimethyl sulfoxide (DMSO) (#101590880, Sigma-Aldrich Co, St Louis, Mo, USA) and the solution (10-2 mol/L) was stored at -20°C. The final concentration of DMSO in the culture was 0.01% (v/v). IL-1β was purchased from PeproTech (#200-01B, Cranbury, NJ, USA). Smad3 inhibitor SIS3 was obtained from MedChemExpress Biological Technology Co., Ltd (#CS-7115. Shanghai, China).
Figure 1.
BA promoted the viability of ADTC5 chondrocytes. A. Structural formula of BA. B. The cell viabilities of ADTC5 chondrocytes cultured with increasing concentrations of BA (0, 10, 20 and 40 μmol/L) for 24 hours were checked by MTS analysis. C. The cell viabilities of ADTC5 chondrocytes cultured with increasing concentrations of IL-1β (1, 2, 5 and 10 ng/mL) for 24 hours were checked by MTS analysis. D. The cell viabilities of ADTC5 chondrocytes with various treatments for 24 hours were checked by MTS analysis. E. The cell morphologies of ADTC5 chondrocytes. *P<0.05 vs control group; #P<0.05 vs IL-1β group; ΔP<0.05 vs IL-1β + BA group, n=3.
Cell vitality assay
Mouse chondrocyte (ADTC5) was gifted by Dr Yang from Basic Medical College of Jinan University. All cells were grown in Dulbecco’s Modified Eagle medium (DMEM) (#C11965500137, Gibco, Grand Island, NY, USA) containing 10% (v/v) FBS (#10099-141, Gibco), 100 U/mL penicillin and 100 μg/mL streptomycin (#15-140-122, Gibco) in a 5% CO2 incubator at 37°C. The chondrocytes were harvested, resuspended and then seeded in 96-well plates at a density of 4×103 cells/well. Two hundred microliters of DMEM medium with different concentrations of BA (0, 10, 20 and 40 μmol/L) or IL-1β (0, 1, 2, 5 and 10 ng/mL) with or without SIS3 (10 μmol/L) were added to the plates respectively the next day. After culturing for 24 hours, cell viability was checked using the MTS method (Promega Corporation, WI, USA). The absorbance (OD value) at a wavelength of 490 nm was recorded using a microplate luminescence detector Promega GloMax 96 (Promega Corporation).
Micromass culture and Alcian blue staining
Twenty microliters of chondrocyte suspension (1×106 cells) were added to 12-well plates and incubated for 3 hours at 37°C. One milliliter of fresh DMEM medium containing various concentrations of drugs was added to each well. The experiments were divided into four groups, namely control group, IL-1β group, IL-1β + BA group and IL-1β + BA + SIS3 group. The medium was changed every three days. After 2 weeks, ACAN was checked by alcian blue staining.
The alcian blue staining procedure: the plate wells were washed once with PBS (phosphate buffer saline) and then fixed for 20 min with 1.0 mL of 4% (v/v) paraformaldehyde (PFA) (#158127, Sigma-Aldrich Co.). Subsequently, 0.5 ml of 1.0% (w/v) alcian blue solution (#A5268, Sigma-Aldrich Co.) (v/v) was added to each well and incubated for 20 min at room temperature. After 1× wash with 70% (v/v) ethanol and three times’ wash with PBS, photomicrographs of the stained cell mass were obtained by a scanner (EPSON, V550, Nagano, Japan). The intensity of the alcian blue staining was expressed as the integrated optical density (IOD) and analyzed by Image Pro Plus 6 software.
Three-dimensional (3D) alginate-chondrocyte beads culture
A concentration of 4×106 cells/mL chondrocyte suspension was obtained with 2% (w/v) alginate solution. 20 μL alginate/cell mixtures were dropped into sterile 102 mM Calcium Chloride solution, and the 3D alginate-chondrocytes beads were formed in the Calcium Chloride solution for three minutes. After the stabilization of 3D alginate-chondrocyte beads, the beads were transferred to a 6-well plate containing conditioned medium. The experiments were divided into the same four groups as previously, namely control group, IL-1β group, IL-1β + BA group and IL-1β + BA + SIS3 group. The 3D alginate-chondrocytes beads were cultured in the incubator at 37°C. The medium was changed every 3 days up to 21 days. The alginate/cell complexes were processed for mRNA expression, alcian blue staining and immunohistochemistry analysis.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Chondrocytes (2×105/mL) were cultured in 6-well plates at 37°C in an incubator containing 5% CO2. After 24 hours of culture, total RNA was extracted using TRIzol reagent (#15596018, Thermo Fisher, Carlsbad, CA, USA). cDNA was synthesized with a PrimeScriptTM Master Mix reagent kit (#RR036A, Takara Bio Inc., Japan). qRT-PCR was performed with SYBR Premix ExTaq (#RR420A, Takara Bio) using the qTOWER version 3.0 PCR system (Analytikjena, Jena, Germany). The mRNA relative expressions of all target genes were analyzed using a formula (2-ΔΔT). GAPDH was used as the internal control. The primer sequences of all genes are as listed in Table 1.
Table 1.
Sequences of primers used for gene amplification
| Genes | Forward | Reverse |
|---|---|---|
| GAPDH | 5’-ATTGTGCACCGCAAATGCTT-3’ | 5’-ACCACAGCACGATTGTCGAT-3’ |
| SOX9 | 5’-GTGCAAGCTGGCAAAGTTGA-3’ | 5’-TGCTCAGTTCACCGATGTCC-3’ |
| Col2α | 5’-GGTGAGCCATGATCCGCC-3’ | 5’-TGGCCCTAATTTTCGGGCATC-3’ |
| ACAN | 5’-CGTTGCAGACCAGGAGCAAT-3’ | 5’-CTCGGTCATGAAAGTGGCG-3’ |
| MMP9 | 5’-GTACTCGACCTGTACCAGCG-3’ | 5’-AGAAGCCCCACTTCTTGTCG-3’ |
| MMP13 | 5’-CTGGACCAAACTATGGTGGG-3’ | 5’-GGTCCTTGGAGTGATCCAGA-3’ |
| ADAMTS5 | 5’-AAGAGGAGGAGGAGGAGGAGGAG-3’ | 5’-AATGGTTGTGAGCTGCCGTATGG-3’ |
Immunofluorescence staining and chondrocyte morphology
Chondrocytes (1×105/mL) were seeded on the slides placed on 6-well plates. After 24 hours, the chondrocyte morphologies were captured using an inverted phase contrast microscope (#CKX41-A32PH, Olympus, Japan). For the immunofluorescence staining, the chondrocytes were fixed with PFA for 10 min at room temperature, and were then incubated with the primary antibody diluent of rabbit anti-p-Smad3 (#BM4033; 1:50; Boster, Wuhan, China) overnight at 4°C. The coverslips were covered with fluorescence secondary antibody (#A32723; 1:200; Thermo Fisher) against light for 30 min at room temperature. Mounting solution with DAPI (#C1005; Beyotime, Beijing, China) was used to detect the nuclei of chondrocytes. Fluorescence was detected by an inverted fluorescence microscope (CX31-32RFL, Olympus). The intensity of the immunofluorescence staining was expressed as the integrated optical density (IOD) and analyzed by Image Pro Plus 6 software.
Western blot
The chondrocytes (2×105/mL) were cultured in 6-well plates. The four experimental groups were: control group, IL-1β (10 ng/mL) group, IL-1β (10 ng/mL) + BA (20 μmol/L) group and IL-1β (10 ng/mL) + BA (20 μmol/L) + SIS3 blocker (10 μmol/L). The extracted protein was loaded, separated and transferred onto a polyvinylidene fluoride (PVDF) membrane (#ISEQ00010, Merck Millipore, Tullagreen, Germany) using semi-dry transfer method. The membranes blocked by 5% Bovine Serum Albumin (BSA) (#11021029; Thermo Fisher) were soaked overnight at 4°C with solutions of primary anti-SOX9 (#5173; 1:1000; Cell Signal Technology, Shanghai, China), anti-p-Smad3 (#BM4033, 1:50, Boster), Smad3 (#BA4559; 1:50; Boster) and anti-GAPDH (#51745; 1:1000; Cell Signal Technology). The next day, the membrane was incubated with secondary antibody (#ARG65351; 1:3000; Arigo Biolaboratories, Shanghai, China) for 1 hour at room temperature. Images of the stained-protein bands were recorded using an ECL Western Blotting Substrate (#32106; Thermo Fisher) kit and quantified using the Image Lab system (Bio-Rad Laboratories, Hercules, CA, USA).
Establishment of mouse OA model and treatment of OA by BA
We carried out all animal experiments following the ethical requirements of the Guangzhou Red Cross Hospital, Guangzhou, China. Thirty of 4-weeks old C57BL/6 male mice with weights of 15-20 g could freely access to water and food. Before destabilization of the medial meniscus (DMM) surgery, the mice were adjusted to the environment for five days. For establishment of mouse OA model, DMM surgery was implemented on the right knee joint after the mice were anesthetized using 5 mg/kg xylazine (#X1126, Sigma-Aldrich) and 40 mg/kg ketamine (#693561, Sigma-Aldrich). After DMM surgery, 10 mice were orally given corn oil only and were considered as OA group; other 10 mice were orally given 50 mg/kg/day of BA dissolved in corn oil and were considered as BA group [4]. Ten of mice without DMM surgery were used as control group. Six weeks later, the mice were euthanized by carbondioxide gas and the knee joints were harvested.
Histologic evaluation and immunohistochemical analysis
For 3D beads, alginate-chondrocyte complexes were fixed for 24 h with 4% (w/v) PFA after being rinsed in PBS. And then, the beads were soaked in a series of gradient sucrose, embedded in optimum cutting temperature (OCT) compound (#4583, Sakura USA Co., Torrance, CA, USA), and solidified by liquid nitrogen. Frozen sections were cut for standard alcian blue and immunohistochemical analysis.
For the histologic evaluation of the knee joints, the samples were fixed in 4% (w/v) PFA in PBS, decalcified, and embedded with paraffin. The sections were cut with a microtome at the thick-ness of 5 μm. The Serial sections were used for Hematoxylin & Eosin (HE), Safranin O/fast green (SO) and Smad3 immunohistochemical staining. Safranine O positive staining area was determined by Image Pro Plus 6 software.
For immunohistochemistry analysis, the slides were incubated with either Smad3 or SOX9 primary antibody overnight at 4°C, and then the slides were incubated with HRP-conjugated secondary antibody (#ARG65351, 1:3000, ArigoBiolaboratories, Shanghai, China) for 1 hour at room temperature. 3,3’-diaminobenzidine (DAB) (#ZLI-9018, Beijing Zhongshan Jinqiao Biotechnology) was used to visualize the color by reacting for less than 10 min at room temperature. Images were captured with an inverted phase contrast microscope (#CKX41-A32PH, Olympus). The repair degree of BA on DMM-induced knee damage was scored by three readers blindly according to the International Cartilage Repair Society (ICRS) II parameters. The matrix and cartilage surface integrity were adapted as the histological parameters for ICRS II [16].
Statistical analysis
All data were expressed as mean ± standard deviation and were calculated by SPSS 22.0 software. A student’s t-test was used to calculate the statistical differences between two groups. One-way ANOVA followed by a post hoc Bonferroni test was used for comparison among many groups respectively. A P-value <0.05 was considered statistically significant.
Results
BA promoted the viability of ADTC5 chondrocytes
In order to confirm the optimal concentration of BA on chondrocytes viability, the chondrocytes were cultured with various concentrations of BA (0, 10, 20 and 40 μmol/L) for 24 hours. The chondrocytes viability was analyzed using the MTS method. Various concentrations of BA (10-40 μmol/L) increased the cell viability (P<0.05) (Figure 1B). The most significant effect on chondrocyte activity was found at 20 μmol/L of BA. As shown in Figure 1C, cell viability was significantly decreased in 10 ng/mL of IL-1β group compared with the control group (P<0.05).
Combining MTS experimental results and literature [19], 20 μmol/L of BA, 10 ng/mL of IL-1β and 10 μmol/L of SIS3 were used in the subsequent experiments. The experimental groups were divided into 4 groups, namely control group (without any drugs), IL-1β group, IL-1β + BA group andIL-1β + BA + SIS3 group. After 24 hours of culture, cell viability was analyzed using the MTS method. As shown in Figure 1D, the downregulation of chondrocyte viability induced by IL-1β was significantly attenuated by BA treatment (P<0.05). The addition of SIS3 significantly inhibited the protective effect of BA on chondrocyte viability. Figure 1E indicated that the addition of IL-1β, IL-1β + BA or IL-1β + BA + SIS3 did not induce any change in chondrocyte morphology.
BA promoted ECM synthesis in ADTC5 chondrocytes
Alcian blue staining is a classic method to detect ECM key component ACAN [20]. As shown in Figure 2A, 2B, IL-1β treatment notably down-regulated the expression of ACAN in chondrocytes compared with the control group. On the contrary, the downregulation of ACAN induced by IL-β was obviously inhibited by BA. Moreover, the addition of SIS3 significantly reversed the protective effect of BA on the expression trend of ACAN. The staining results indicated that BA promoted the secretion of ACAN in chondrocytes and Smad3 inhibitor SIS3 decreased the secretion of ACAN stimulated by BA.
Figure 2.
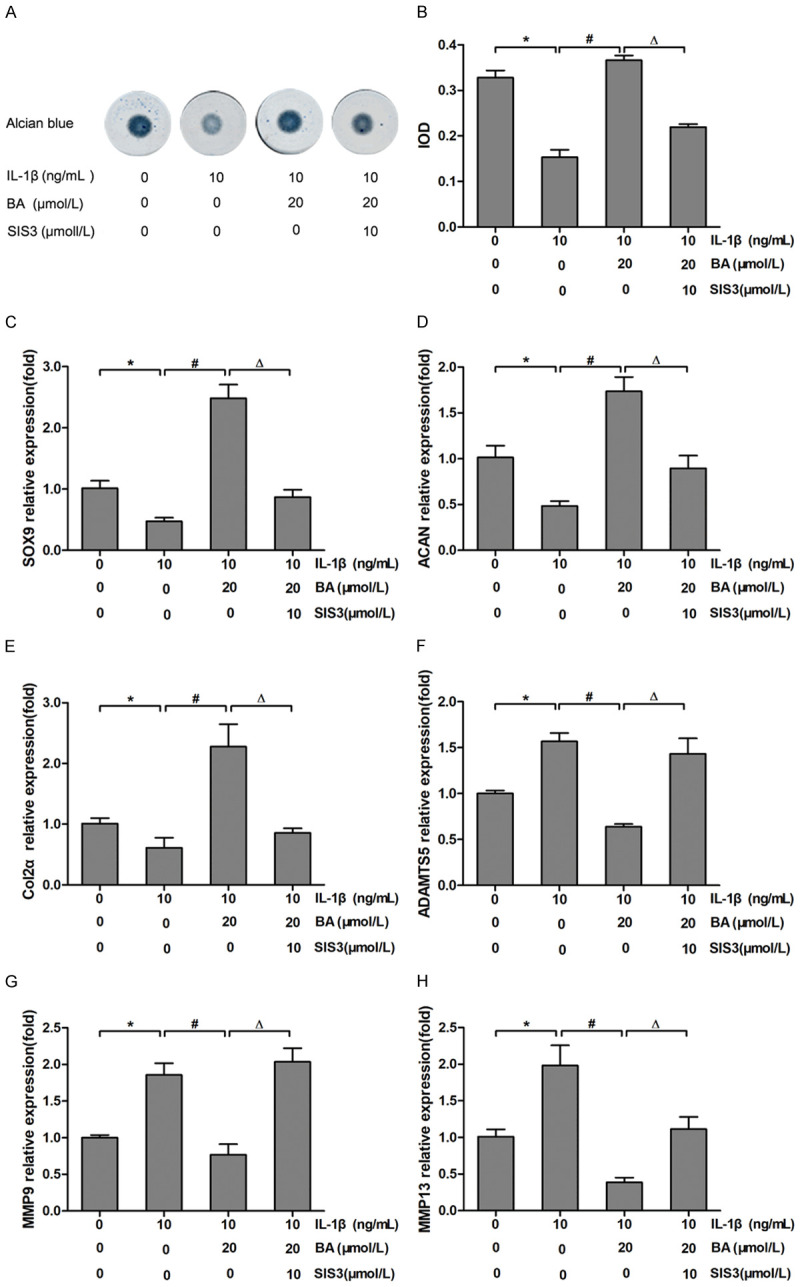
BA promoted ECM synthesis and adjusted the expressions of ECM regulating genes in ADTC5 chondrocytes. (A) The chondrocyte masses were stained by alcian blue staining. (B) Quantitative analysis of alcian blue staining in chondrocyte masses. (C-H) The relative expression of SOX9 (C), ACAN (D), Col2α (E), ADMTS5 (F), MMP9 (G) and MMP13 (H) were determined by qRT-PCR in chondrocytes with various treatments for 24 hours. *P<0.05 vs control group; #P<0.05 vs IL-1β group; ΔP<0.05 vs IL-1β + BA group, n=3.
By qRT-PCR, we confirmed that BA upregulated the expression of ACAN and Col2α in chondrocytes. As shown in Figure 2C-E, compared with the control group, the expressions of SOX9, ACAN and Col2α in IL-1β group were significantly down-regulated (P<0.05). Meanwhile, the expressions of ADAMTS5, MMP9 and MMP13 in IL-1β group were significantly increased (P<0.05) (Figure 2F-H). BA reduced the inhibition of IL-1β on SOX9, ACAN and Col2α expressions (P<0.05), as well as the enhancement of IL-1β on MMP9, MMP13 and ADAMTS5 expressions (P<0.05). The addition of SIS3 reversed the promotive effect of BA on IL-1β-induced down-regulation of SOX9, ACAN and Col2α (all P<0.05), and the inhibitory effect of BA on IL-1β-induced up-regulation of MMP9, MMP13 and ADAMTS5 (all P<0.05). These results indicated that BA promoted ECM secretion in chondrocytes and Smad3 inhibitor SIS3 decreased the promotion of BA on ECM secretion.
BA promoted ECM synthesis and adjusted the expressions of ECM regulating genes in ADTC5 chondrocytes cultured in 3D alginate beads
To further define the effect of BA on ECM synthesis of chondrocytes, an alginate-chondrocytes 3D culture system was employed, in which the 3D complexes served as a model for cartilage tissue engineering. Histological analysis by alcian blue staining indicated that BA increased the production of ACAN which was down-regulated by IL-1β treatment. Moreover, the addition of SIS3 obviously reversed the protective effect of BA on the expression trend of ACAN (Figure 3A, 3B). These results indicated that BA promoted the secretion of ACAN in alginate-chondrocyte beads and Smad3 inhibitor SIS3 decreased the promotion of BA on the secretion of ACAN. Immunohistochemical staining for SOX9 revealed that BA upregulated the expression of SOX9 in chondrocytes in the alginate-chondrocyte 3D beads. Compared with control groups, the expression of SOX9 in the IL-1β group was significantly down-regulated (P<0.05, Figure 3C, 3D). BA abolished the inhibition of IL-1β on protein expression of SOX9 (P<0.05). The addition of SIS3 reversed the promotive effect of BA on IL-1β-induced down-regulation in protein expression of SOX9 (P<0.05). As shown in Figure 3E-G, the mRNA expressions of SOX9, Col2α and ACAN in the IL-1β group were significantly down-regulated compared with those of the control group (P<0.05). However, the inhibition of IL-1β on the expressions of SOX9, Col2α and ACAN was abolished by BA treatment (P<0.05). The addition of SIS3 reversed the protective effect of BA on chondrocytes treated by IL-1β (P<0.05). These results suggest that BA promotes ECM synthesis and adjusts the expressions of ECM regulating genes in ADTC5 chondrocytes cultured in 3D alginate beads.
Figure 3.
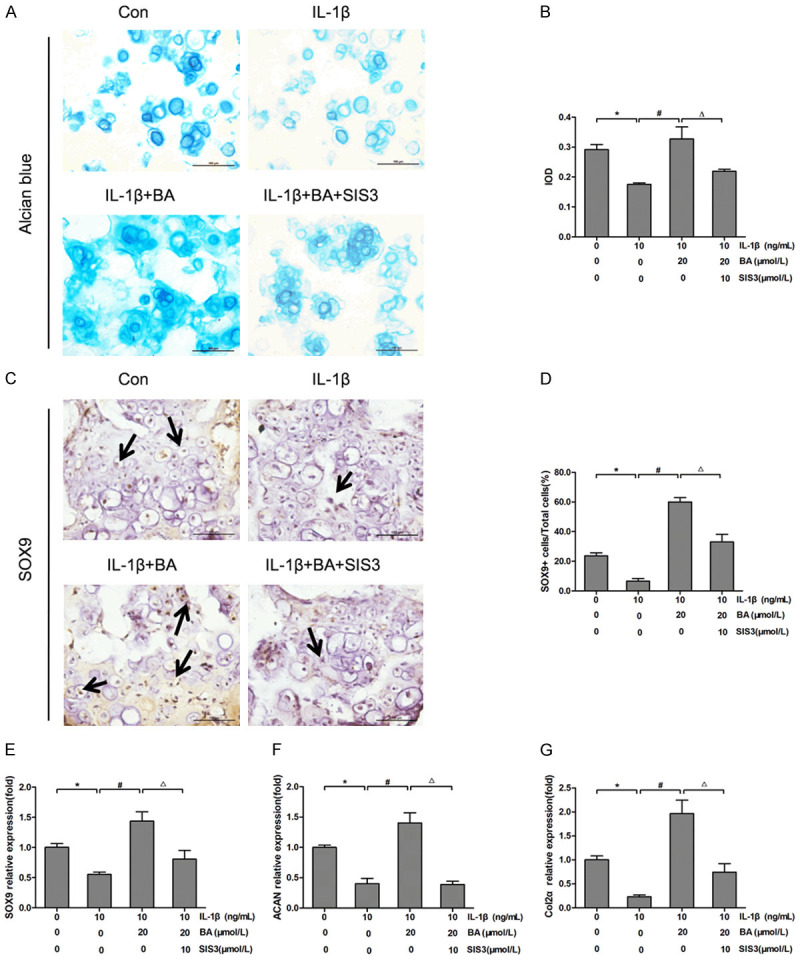
BA promoted ECM synthesis and adjusted the expressions of ECM regulating genes in alginate-chondrocyte beads. (A) The chondrocytes from the 3D alginate beads were stained by alcian blue staining. (B) Quantitative analysis of alcian blue staining in alginate-chondrocyte beads. (C, D) Immunohistochemical staining (C) and quantitative analysis (D) of SOX9 in chondrocytes from the 3D alginate beads with various treatments for 24 hours. (E-G) The relative expressions of SOX9 (E), ACAN (F) and Col2α (G) in chondrocytes from 3D alginate beads with various treatments for 24 hours by qRT-PCR. *P<0.05 vs control group; #P<0.05 vs IL-1β group; ΔP<0.05 vs IL-1β + BA group, n=3.
BA activated Smad3 in ADTC5 chondrocytes
The effect of BA on the activation of Smad3 and SOX9 in chondrocytes treated with IL-1β was detected by immunofluorescence and western blot. As shown in Figure 4A, 4B, 4E, 4F, Smad3 and SOX9 were expressed both in the cytoplasm and nucleus of chondrocytes in the control group, while Smad3 and SOX9 were mainly expressed in the cytoplasm of chondrocytes in the IL-1β group. The Smad3 and SOX9 in the nuclear of chondrocytes in IL-1β-treated group were obviously less than that of control and BA + IL-1β groups. Conversely, the addition of SIS3 obviously reversed the nuclear translocation trend of Smad3 and SOX9. Figure 4B, 4F showed the expressions of Smad3 and SOX9 by IOD, respectively. On the other side, as shown in Figure 4C, 4D, 4G, 4H, the ratios of p-Smad3/Smad3 and SOX9/GAPDH were down-regulated by IL-1β treatment (P<0.05). But BA treatment slowed down the decrease of p-Smad3/Smad3 and SOX9/GAPDH induced by IL-1β treatment (P<0.05). Additionally, in the presence of IL-1β and BA simultaneously, the addition of SIS3 obviously blocked the influence of BA on IL-1β-inhibited Smad3 expression, phosphorylation, nuclear localization and SOX9 expression. These results suggest that BA activates Smad3 and SOX9 in chondrocytes.
Figure 4.
BA activated the phosphorylation of Smad3 and the nuclear localization of SOX9 in ADTC5 chondrocytes. (A, B) Smad3 localization (A) and quantitative analysis of Smad3 expression (B) in chondrocytes with various treatments for 24 hours by immunohistochemistry staining analysis. (C, D) The expressions (C) and the relative expression (D) of Smad3 and phosphorylated Smad3 in chondrocytes with various treatments for 24 hours by western blot. (E, F) SOX9 localization (E) and quantitative analysis of SOX9 expression (F) in chondrocytes with various treatments for 24 hours by immunohistochemistry staining analysis. (G, H) The expressions (G) and the relative expression (H) of SOX9 in chondrocytes with various treatments for 24 hours by western blot. *P<0.05 vs control group; #P<0.05 vs IL-1β group; ΔP<0.05 vs IL-1β + BA group, n=3.
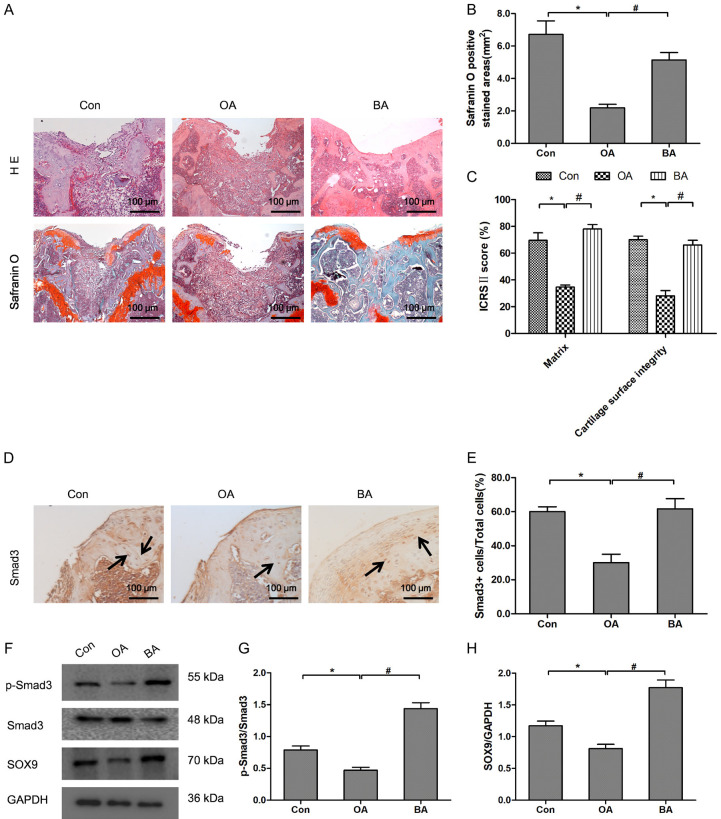
BA reduced the cartilage degradation in DMM-induced OA mice by increasing Smad3 phosphorylation
The in vivo effect of BA on the structural features of articular cartilage was examined using a DMM-induced OA mouse model, which showed reduced Safranin O staining and a rough articular surface in mice undergone DMM surgery and exhibited OA pathology [4]. As shown in Figure 5A, 5B, DMM surgery reduced the area of Safranin O staining. On the contrary, BA treatment recovered the reduced Safranin O staining induced by DMM-surgery. The ICRS II score of OA group including matrix staining and the articular surface integrity was significantly lower than that of control and BA groups. BA treatment significantly increased ICRS II score which was decreased by DMM surgery (Figure 5C). The result indicated that BA could inhibit the progress of OA by increasing matrix staining and articular surface integrity. Immunohistochemistry staining and quantitative analysis showed that the Smad3 positive cells in OA group mice were less than control and BA group mice (Figure 5D, 5E). Western blot and quantitative analysis revealed that the expressions of Smad3, p-Smad3, SOX9 and the ratio p-Smad3/Smad3 in OA group mice were less than control and BA group mice (Figure 5F-H). These results indicated that DMM treatment significantly reduced the Smad3 positive cells and BA treatment obviously recovered the DMM-induced reduction of Smad3 positive cells. This was consistent with the fact that BA resulted in increased Smad3 expression in chondrocytes. Taken together; these results suggested that BA treatment improved the integrity of the articular cartilage, partially by activating the TGF-β/Smad3 pathway in chondrocytes.
Figure 5.
BA reduced the cartilage degradation and increased the expression of Smad3 and SOX9 in OA mice. (A) HE and Safranin O staining of the articular cartilage from the control, the DMM-induced OA (OA) and the BA-treated OA (BA) mice 6 weeks after surgery. (B) Quantitative analysis of Safranin O positive area. (C) The ICRS II scores for articular cartilages 6 weeks after surgery. (D, E) Immunohistochemical analysis of Smad3 (D) and the ratio of Smad3 positive cells (E) in articular cartilage from the control, OA and BA mice 6 weeks after surgery. (F-H) The protein bands (F) and the relative protein expressions of p-Smad3 (G) and SOX9 (H) in articular cartilage from the control, OA and BA mice 6 weeks after surgery. *P<0.05 vs control group; #P<0.05 vs OA group, n=10.
Discussion
OA is a common chronic joint disease in the elderlies [1]. At present, drug and surgical treatments are the two main therapeutic intervention for OA worldwide [4]. Compared with the high cost of surgical treatment, drug treatment of OA is more acceptable and cost-effective [5], which could effectively relieve inflammation and pain symptoms. It is reported that BA has anti-inflammatory, anti-deformation, anti-bacterial and diuretic effects [7,8]. In this study, we demonstrated that BA increased the viability of chondrocytes, matrix expression and articular surface integrity. It is reasonable to postulate that BA protects cartilage from degradation induced by IL-1β in vitro or DMM surgery in vivo through, at least partially, the inhibition on TGF-β/Smad3 pathway.
IL-1β is an inflammatory factor and is related to the progress of OA [20]. It has been shown that IL-1β inhibited the secretion of Col2α and ACAN [21]. In addition, IL-1β stimulated the release of MMP9/13 and ADAMTS5 in chondrocytes which are responsible for the degradation of ECM [22]. IL-1β has also been shown to inhibit ECM synthesis through decreasing the expression of SOX9, one of ECM synthesis promoters [9]. In this study, IL-1β treatment reduced chondrocyte viability, the secretions of Col2α and ACAN and the expression of SOX9; in contrast, IL-1β treatment increased the expressions of MMP9, MMP13 and ADAMTS5. All these results are consistent with the previous reports. It is appreciated that we used IL-1β as an inducer to establish an inflammatory and injury model of chondrocytes.
BA is known to significantly inhibitthe inflammatory response and the expression of IL-1β in chondrocytes [8]. BA protects chondrocyte viability either by inhibiting IL-1β-induced apoptosis or by activating IL-1β-inhibited autophagy through mi-R-766-3p/AIFMI axis [23]. Different from previous studies, we demonstrated that BA protects chondrocyte viability by reversing IL-1β-inhibited the expressions of Col2α, ACAN and SOX9 and decreasing IL-1β-induced expression of MMP9, MMP13 and ADAMTS5. The promoting effect of BA on the expressions of SOX9 and ECM components is consistent with previous report [23].
The transcriptional activations of SOX9 and TGF-β are necessary for prechondrocyte differentiation [24,25]. It is reported that TGF-β regulates the transcription of SOX9 in human chondrocytes via TGF-β/Smad2/3 signaling pathway [26]. overexpression of Smad3 can significantly induce the formation of primary cartilage originated from human mesenchymal stem cell [27]. In addition, Smad3 enhances the transcriptional activity of SOX9 and Col2α expression, while Smad3 silencing inhibited the expression of SOX9 [28]. According to the literature [29], Smad2/3 is associated with SOX9 in TGF-β dependent manner and forms a transcription complex with SOX9 in the enhancer region of Col2α. The TGF-β/Smad3 pathway plays a key role in SOX9-dependent cartilage formation through up-regulating Smad3 phosphorylation [30]. In chondrocytes, we found that that IL-1β treatment inhibited Smad3 expression, phosphorylation and nuclear localization and BA treatment reversed these effects of IL-1β. Furthermore, the addition of Smad3 inhibitor SIS3 blocked BA effect and resurrected IL-1β-decreased Smad3 expression, phosphorylation and nuclear localization. Also, BA treatment reversed IL-1β-inhibited SOX9 expression, and Smad3 inhibitor SIS3 reactivated IL-1β function on Smad3 expression. In in vivo experiments, we demonstrated that BA suppressed DMM-induced OA development and recovered DMM-inhibited Smad3 expression, phosphorylation and SOX9 expression. These results indicate that the protective effect of BA on chondrocytes is, at least partially, through the regulation of TGF-β/Smad3 signal pathway. In order to make substantial conclusion, Smad3 gene knockdown, even knockout experiments are needed in our future study since only Smad3 inhibitor was used in current study.
In summary, this study demonstrates that BA promotes chondrocyte viability and the cell matrix synthesis through TGF-β/Smad3 pathway in IL-1β-treated chondrocytes and DMM treated mice. BA is a potential candidate drug for OA treatment. The main results are illustrated in Figure 6.
Figure 6.

Schematic diagram of research ideas.
Acknowledgements
This work was supported by the Medical and Health Science and Technology Project of Guangzhou (20191A011018 & 20192A010009, PW), the Medical Science and Technology Research Foundation of Guangdong (A2021335, PW), the National Natural Science Foundation of China (81770291, SZ; 81902802, XX), the Guangdong Provincial Natural Science Foundation of China (2018B030311011, SZ), Guangzhou Science and Technology Planning Project (202002030081, SZ), the Medical Science and Technology Research Foundation of Guangdong (B2016018 & A2018063, XX), the research grants of Guangdong Bureau of Traditional Chinese Medicine (20181206, ZL), Guangzhou Science and Technology Project (202102010060, ZL; 202102020121, JL), Guangdong Provincial Basic and Applied Basic Regional Joint Fund (2020A1515110009, PZ).
Disclosure of conflict of interest
None.
References
- 1.Van den Bosch MHJ. Osteoarthritis year in review 2020: biology. Osteoarthritis Cartilage. 2021;29:143–150. doi: 10.1016/j.joca.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104:293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Khlopas H, Khlopas A, Samuel LT, Ohliger E, Sultan AA, Chughtai M, Mont MA. Current concepts in osteoarthritis of the ankle: review. Surg Technol Int. 2019;35:280–294. [PubMed] [Google Scholar]
- 4.Pu Y, Zhang H, Wang P, Zhao Y, Li Q, Wei X, Cui Y, Sun J, Shang Q, Liu D, Zhu Z. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cell Physiol Biochem. 2013;32:1167–77. doi: 10.1159/000354516. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira de Meneses S, Rannou F, Hunter DJ. Osteoarthritis guidelines: barriers to implementation and solutions. Ann Phys Rehabil Med. 2016;59:170–173. doi: 10.1016/j.rehab.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Lin X, Chai L, Liu B, Chen H, Zheng L, Liu Q, Lin C. Synthesis, biological evaluation, and docking studies of a novel sulfonamido-based gallate as pro-chondrogenic agent for the treatment of cartilage. Molecules. 2016;22:3. doi: 10.3390/molecules22010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing D, Gao H, Liu Z, Zhao Y, Gong M. Baicalin inhibits inflammatory responses to interleukin-1β stimulation in human chondrocytes. J Interferon Cytokine Res. 2017;37:398–405. doi: 10.1089/jir.2017.0030. [DOI] [PubMed] [Google Scholar]
- 8.Chang CP, Huang WT, Cheng BC, Hsu CC, Lin MT. The flavonoid baicalin protects against cerebrovascular dysfunction and brain inflammation in experimental heatstroke. Neuropharmacology. 2007;52:1024–1033. doi: 10.1016/j.neuropharm.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Li R, Zhong Y, Zhang S, Zhou L, Shang S. Fuyuan decoction enhances SOX9 and COL2A1 expression and Smad2/3 phosphorylation in IL-1β-activated chondrocytes. Evid Based Complement Alternat Med. 2015;2015:821947–821947. doi: 10.1155/2015/821947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Wu H, Wang L, Zheng L, Zhao J. Protective effects of baicalin on rabbit articular chondrocytes in vitro. Exp Ther Med. 2017;13:1267–1274. doi: 10.3892/etm.2017.4116. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Xing D, Gao H, Liu Z, Zhao Y, Gong M. Baicalin inhibits inflammatory responses to interleukin-1β stimulation in human chondrocytes. J Interferon Cytokine Res. 2017;37:398–405. doi: 10.1089/jir.2017.0030. [DOI] [PubMed] [Google Scholar]
- 12.Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1-α regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 13.Robert S, Gicquel T, Victoni T, Valença S, Barreto E, Bailly-Maître B, Boichot E, Lagente V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci Rep. 2016;36:e00360. doi: 10.1042/BSR20160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez RD, Coricor G, Perez J, Seo HS, Serra R. SOX9 protein is stabilized by TGF-β and regulates PAPSS2 mRNA expression in chondrocytes. Osteoarthritis Cartilage. 2017;25:332–340. doi: 10.1016/j.joca.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furumatsu T, Ozaki T, Asahara H. Smad3 activates the Sox9-dependent transcription on chromatin. Int J Biochem Cell Biol. 2009;41:1198–1204. doi: 10.1016/j.biocel.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38:880–90. doi: 10.1177/0363546509359068. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zhu Y, Tao H, Jin C, Liu Y, Lu X, Hu X, Fan C. Smad2 and Smad3 regulate chondrocyte proliferation and differentiation in the growth plate. PLoS Genet. 2016;12:e1006352. doi: 10.1371/journal.pgen.1006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Zhu Y, Tao H, Jin C, Liu Y, Lu X, Hu X, Fan C. Interaction of ERK1/2 and Smad2/3 signaling pathways in TGF-β1-induced TIMP-3 expression in rat chondrocytes. Arch Biochem Biophys. 2014;564:229–36. doi: 10.1016/j.abb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Zhong H, Wei J, Lin S, Zong Z, Gong F, Huang X, Sun J, Li P, Lin H, Wei B, Chu J. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther. 2019;23:300. doi: 10.1186/s13075-019-2085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, Gao XJ, Zhao X. CDMP1 promotes type II collagen and ACANrecan synthesis of nucleus pulposus cell via the mediation of ALK6. Eur Rev Med Pharmacol Sci. 2020;24:10975–10983. doi: 10.26355/eurrev_202011_23581. [DOI] [PubMed] [Google Scholar]
- 21.Hu P, Du J, Zhang S, Wang T, Li J, Chen G, Zhou G. Oral administration of strontium gluconate effectively reduces articular cartilage degeneration through enhanced anabolic activity of chondrocytes and chondrogenetic differentiation of mesenchymal stromal cells. Biol Trace Elem Res. 2020;193:422–433. doi: 10.1007/s12011-019-01711-9. [DOI] [PubMed] [Google Scholar]
- 22.Xue M, McKelvey K, Shen K, Minhas N, March L, Park SY, Jackson CJ. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology (Oxford) 2014;12:2270–2279. doi: 10.1093/rheumatology/keu254. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Cheng J, Liu J. Baicalin protects human OA chondrocytes against IL-1β-induced apoptosis and ECM degradation by activating autophagy via MiR-766-3p/AIFM1 axis. Drug Des Devel Ther. 2020;14:2645–2655. doi: 10.2147/DDDT.S255823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heldin CH, Miyazono K, Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara N, Maeda S, Yahiro Y, Sakuma D, Matsuyama K, Imamura K, Kawamura I, Setoguchi T, Ishidou Y, Nagano S, Komiya S. TGF-β signalling and PEG10 are mutually exclusive and inhibitory in chondrosarcoma cells. Sci Rep. 2017;7:13494. doi: 10.1038/s41598-017-13994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahr H, Gunes S, Kuhn AR, Nebelung S, Pufe T. Bioreactor-controlled physoxia regulates TGF-β signaling to alter extracellular matrix synthesis by human chondrocytes. Int J Mol Sci. 2019;20:1715. doi: 10.3390/ijms20071715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M. SOX9 enhances ACANrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- 28.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280:8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 29.Yang H, Yuan C, Wu C, Qian J, Shi Q, Li X, Zhu X, Zou J. The role of TGF-β1/Smad2/3 pathway in platelet-rich plasma in retarding intervertebral disc degeneration. J Cell Mol Med. 2016;20:1542–1549. doi: 10.1111/jcmm.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coricor G, Serra R. TGF-β regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci Rep. 2016;6:38616. doi: 10.1038/srep38616. [DOI] [PMC free article] [PubMed] [Google Scholar]