Abstract
Objective: To evaluate the value of the non-predominant micropapillary and solid patterns in prognosis of lung adenocarcinoma. Methods: Totally 422 patients diagnosed with stage IA lung adenocarcinomas were included, and all their slides were reviewed. We compared clinicopathological characteristics and survival outcomes between MP- & SD- (both micropapillary and solid component were absent), MP+/SD+ (either micropapillary or solid component was present, but the single or combined percentage of the MP and SD was not greater than 50%) and MPp/SDp (either micropapillary or solid or the combined percentage of these two components was great than 50%). Results: Patients with MP- & SD- had smaller tumor size (P=0.012) and lower spread through air spaces rates (P<0.001). Patients with MP- & SD- had significantly better 5-year recurrence free survival than MP+/SD+ (91% versus 70%, P<0.001) and MPp/SDp (91% versus 56%, P<0.001). The difference of RFS between MP+/SD+ subgroup and MPp/SDp subgroup was not significant (P=0.177). In the multivariate analysis, patients with MP- & SD- had a better recurrence free survival than the other two groups (versus: MP+/SD+, HR, 3.198; 95% CI, 1.537-6.653; P=0.002; versus MPp/SDp: HR, 4.981; 95% CI, 2.266-10.950; P<0.001). Conclusions: The presence of micropapillary or solid patterns, even not predominant, was a risk factor for predicting poor recurrence free survival in very early stage lung adenocarcinoma.
Keywords: Lung adenocarcinoma, micropapillary pattern, solid pattern, prognosis
Introduction
Lung cancer remains the leading cause of global cancer incidence and mortality, and adenocarcinoma is the most common histologic type of nonsmall cell lung cancer (NSCLC) [1]. In 2011, a new lung adenocarcinoma classification was proposed by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) [2]. Since then, many studies have begun to evaluate this new classification system and its impact on tumor progression and mortality [3,4].
It is reported that micropapillary- and solid-predominant subtypes of lung adenocarcinoma are associated with poor prognoses [5,6]. However, heterogeneous histologic patterns are present in most (80%-90%) lung adenocarcinomas and are classified as mixed type according to the third edition (2004) of WHO classification [7]. The clinical relevance of minor components of these two unfavorable patterns has not been established yet. We thus aimed to provide evidence for the predictive and prognostic value of minor components of stage IA lung adenocarcinoma.
Materials and methods
We obtained the approval from the Shanghai Pulmonary Hospital institutional review broad (No. 2018(234)-235).
Patients and methods
Over a period of January 2011 and December 2012, 462 consecutive patients diagnosed as stage IA primary lung adenocarcinoma measuring ≤3 cm had complete resection at Shanghai Pulmonary Hospital. Written informed consent was given by participants and informants. No patient received induction chemotherapy or radiotherapy. Among them, 13 patients were excluded because of invasive mucinous adenocarcinoma and 19 were excluded because of unavailable or unsatisfactory tumor specimen. Inclusion criteria: Adenocarcinoma of lung satge I patients confirmed by histopathology; Men and women aged 18 to 75 (including 18 and 75 years old); The estimated survival period is not less than 1 year; The Eastern Cooperative Oncology Group (ECOG) physical status score is 0 to 1. Exclusion criteria: Accompanied by serious medical diseases, such as severe infections, uncontrollable diabetes, cardiovascular disease; Those who suffered from other malignant tumors; Major surgery (except for baseline tumor biopsy) or severe trauma occurred within 4 weeks before the start of study treatment; The researcher believes that it is not suitable to participate in the research due to other reasons.
All available slides were reviewed by two pathologists, and were re-evaluated by another pathologist independently according to the IASLC/ATS/ERS classification, without knowledge of any clinical data. Typical pathological images of micropapillary and solid patterns in stage IA lung adenocarcinoma were shown in Figure 1. An average of 3 tumor slides was processed per case. Each histologic component present was recorded in 5% increments.
Figure 1.
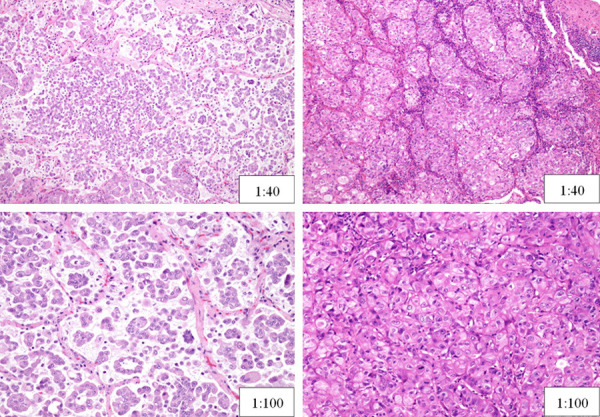
Typical pathological images of Micropapillary (left) and Solid Patterns (right) in Stage IA Lung Adenocarcinoma.
Eight cases were excluded because of the misdiagnosis of histology (seven cases are squamous cell carcinoma and one case is carcinoid tumor in re-evaluation). Thus, 422 patients were included finally. Based on micropapillary and solid component, patients were divided into three groups: MP- & SD- (both micropapillary and solid component were absent), MP+/SD+ (either micropapillary or solid component was present, but the single or combined percentage of the MP and SD was not greater than 50%) and MPp/SDp (either micropapillary or solid or the combined percentage of these two components was great than 50%). Tumor spread through air spaces (STAS) was defined as tumor cells observed within air spaces in the surrounding lung parenchyma beyond the edge of the main tumor. Lympho-vascular invasion (LVI) was also assessed in all patients.
Lobectomy was the first choice. If tumor nodule was small and located in peripheral lung, segmentectomy/wedge resection can be considered if R0 margin (free of disease margin) can be achieved. After surgery, patients received chest CT every 6 month for 2-3 year, then a low-dose non-contrast-enhanced chest CT annually.
Statistical analysis
The correlations between clinicopathologic and histologic patterns were analyzed using either chi-square test or the Fisher’s exact test. The Kaplan-Meier method was applied to analyze the overall survival (OS) and recurrence-free survival (RFS). The log-rank test was used to compare the survival curves estimated by the Kaplan-Meier method between histologic subtypes. Univariate and multivariate analyses were performed with a Cox proportional hazards model. All analyses were performed with SPSS 21.0 software (IBM Corporation, Armonk, NY), and the survival curves were plotted using GraphPad Prism 6.0 software (GraphPad Software, San Diego, CA). All tests were two sided, a P value of <0.05 was defined as statistical significance.
Results
Clinicopathological characteristics
Out of the 422 patients, 176 were men (41.7%) and 246 were women (58.3%). The median patient age was 60 (range, 24-81 years). The median tumor size was 1.5 cm (range, 0.4-3.0 cm). The percentage of T1a, T1b and T1c patients was 23.9%, 58.5% and 17.5% respectively. STAS was observed in 50 cases (11.8%), LVI was only observed in 6 cases (1.4%). In total, there were 390 patients had lobectomy (92.4%) and 32 patients underwent sublobar resection (segmentectomy or wedge resection, 7.6%). For the three sub-stage patients (T1a, T1b and T1c), the frequency of those who received lobectomy was 87.1%, 92.7% and 98.6% respectively. No postoperative mortality occurred in this study.
According to the IASLC/ATS/ERS classification, the most frequent subtype in our study was papillary predominant adenocarcinoma (50.5%, n=213), followed by acinar predominant adenocarcinoma (38.4%, n=161), lepidic predominant adenocarcinoma (5.0%, n=21), micropapillary predominant adenocarcinoma (3.6%, n=15) and solid predominant adenocarcinoma (2.6%, n=11). The recorded histologic subtype components were as follows: 330 patients (78.2%) had papillary component, 330 patients (78.2%) had acinar component, 163 patients (38.6%) had lepidic component, 77 patients (18.2%) had micropapillary component and 28 patients (6.6%) had solid component.
Most adenocarcinomas were composed of more than one growth pattern. Two hundred and fifty-nine tumors (61.4%) contained two growth patterns, 116 tumors (27.5%) presented three growth patterns, 5 tumors (1.2%) displayed four distinct growth patterns. Single pattern tumors were rare (42, 10.0%).
The relationship between micropapillary/solid component and clinicopathological characteristics is presented in Table 1. MP- & SD-, MP+/SD+ and MPp/SDp contained 324 (76.8%), 72 (17.1%) and 26 (6.2%) cases respectively. Patients with MPp/SDp had a tendency to be older, though not significant (P=0.064). The tumor size for MP- & SD- group was smaller than other two groups with micropapillary/solid component (P=0.012). MP- & SD- group had the lowest frequency of STAS+ (3.7%, 43.1% and 24.9% in three groups, respectively, P<0.001).
Table 1.
Association between micropapillary/solid component and clinicopathological characteristics of patients with stage IA lung adenocarcinoma
| Characteristics | Group | P | ||
|---|---|---|---|---|
|
| ||||
| MP- & SD- | MP+/SD+ | MPp/SDp | ||
| Age (%) | 0.064 | |||
| ≤60 | 168 (51.9) | 44 (61.1) | 9 (34.6) | |
| >60 | 156 (48.1) | 28 (38.0) | 17 (65.4) | |
| Sex | 0.205 | |||
| male | 128 (39.5) | 34 (47.2) | 14 (53.8) | |
| female | 196 (60.5) | 38 (52.8) | 12 (46.2) | |
| Tumor size | 0.012 | |||
| ≤2 cm | 276 (85.2) | 51 (70.8) | 20 (76.9) | |
| >2 cm | 48 (14.8) | 21 (29.2) | 6 (23.1) | |
| STAS | <0.001 | |||
| - | 312 (96.3) | 41 (56.9) | 19 (73.1) | |
| + | 12 (3.7) | 31 (43.1) | 7 (26.9) | |
| LVI | 0.160 | |||
| - | 321 (99.1) | 70 (97.2) | 25 (96.2) | |
| + | 3 (0.9) | 2 (2.8) | 1 (3.8) | |
| Predominant subtybe | <0.001 | |||
| lepidic | 21 (6.5) | 0 | 0 | |
| acinar | 129 (39.8) | 33 (45.8) | 0 | |
| papillary | 174 (53.7) | 39 (54.2) | 0 | |
| micropapillary | 0 | 0 | 15 (57.7) | |
| solid | 0 | 0 | 11 (42.3) | |
| Surgery | 0.167 | |||
| lobectomy | 295 (91.0) | 70 (97.2) | 25 (96.2) | |
| segmentectomy/wedge resection | 29 (9.0) | 2 (2.8) | 1 (4.0) | |
CI, confidence interval. HR, hazard ratio. MP- & SD- (both micropapillary and solid component were absent), MP+/SD+ (either micropapillary or solid component was present, but the single or combined percentage of the MP and SD was not greater than 50%), MPp/SDp (either micropapillary or solid or the combined percentage of these two components was great than 50%). STAS, spread through air space.
Survival analysis
Among the 422 patients, 354 patients have complete follow-up information. We did survival analysis for them. In general, forty-eight (13.6%) patients experienced recurrence, twenty patients (5.6%) died. Among the patients with recurrence, 11 were loco-regional (22.9%), 19 were distant (39.6%), and 18 were both sites (37.5%). The median time to follow up was 56.3 months (interquartile range, 51.7 to 63.1 months).
The survival curves are shown in Figure 2. The 5-year OS rates for the patients in MP- & SD-, MP+/SD+, MPp/SDp subgroups were 94%, 90%, 67%, and the 5-year RFS rates were 91%, 70%, 56%, respectively. The OS rate was only significantly different between MP- & SD- and MPp/SDp subgroup (P=0.023). While in term of RFS, patients without MP or SD component had a lower risk of recurrence than patients in MP+/SD+ subgroup (P<0.001) and patients in MPp/SDp subgroup (P<0.001). The difference of RFS between MP+/SD+ subgroup and MPp/SDp subgroup was not significant (P=0.177).
Figure 2.
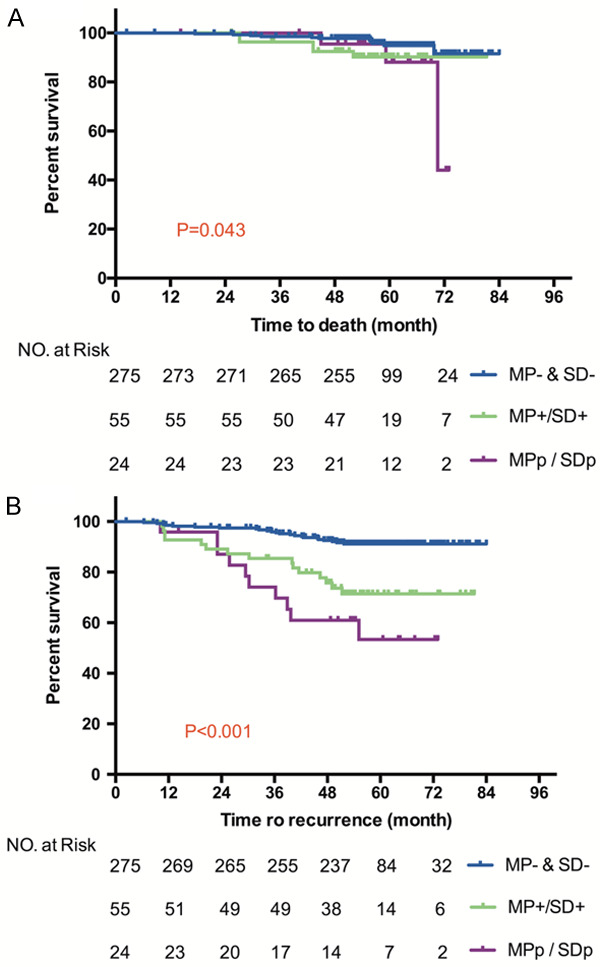
Kaplan-Meier curve of OS (A) and RFS (B) according to micropapillary and solid component in 354 stage IA lung adenocarcinoma patients.
The univariate and multivariate analysis results are summarized in Table 2. Sex, age, tumor size, STAS, LVI, micropapillary and solid component subgroup and surgery type were included. In the univariate analysis, smaller tumor size, STAS negative were predictors of decreased risk of death (P=0.001; P=0.009). Patients with MP- & SD- had a better RFS than those in the other two subgroups (vs. MP+/SD+, P<0.001; vs. MPp/SDp, P<0.001). The multivariate analysis demonstrated that only tumor size and micropapillary and solid component subgroups were two independent prognostic factors for RFS (HR, 1.977; 95% CI, 1.069-3.655; P=0.030; HR, 3.198; 95% CI, 1.537-6.653; P=0.002 for MP+/SD+; HR, 4.981; 95% CI, 2.266-10.950; P<0.001 for MPp/SDp).
Table 2.
Univariate and multivariate analysis for recurrence free survival
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Sex | 0.346 | 0.658 | ||||
| Male | reference | |||||
| Female | 0.761 | 0.431-1.343 | 0.878 | 0.492-1.564 | ||
| Age | 0.195 | 0.335 | ||||
| ≤60 | reference | |||||
| >60 | 1.458 | 0.824-2.578 | 1.353 | 0.732-2.500 | ||
| Size | 0.001 | 0.030 | ||||
| ≤2 cm | reference | |||||
| >2 cm | 2.645 | 1.464-4.780 | 1.977 | 1.069-3.655 | ||
| STAS | 0.009 | 0.794 | ||||
| - | reference | |||||
| + | 2.546 | 1.268-5.111 | 1.110 | 0.508-2.427 | ||
| LVI | 0.695 | 0.555 | ||||
| - | reference | |||||
| + | 1.486 | 0.205-10.772 | 1.857 | 0.238-14.510 | ||
| Group | ||||||
| MP- & SD- | reference | |||||
| MP+/SD+ | 3.639 | 1.898-6.975 | <0.001 | 3.198 | 1.537-6.653 | 0.002 |
| MPp/SDp | 6.305 | 2.996-13.265 | <0.001 | 4.981 | 2.266-10.950 | <0.001 |
| Surgery | 0.171 | 0.319 | ||||
| Lobectomy | reference | |||||
| Segmentectomy/wedge resection | 0.251 | 0.035-1.816 | 0.361 | 0.049-2.683 | ||
CI, confidence interval. HR, hazard ratio. LVI, lymphovascular invasion. MP- & SD- (both micropapillary and solid component were absent), MP+/SD+ (either micropapillary or solid component was present, but the single or combined percentage of the MP and SD was not greater than 50%), MPp/SDp (either micropapillary or solid or the combined percentage of these two components was great than 50%).
To investigate the effect of micropapillary and solid component on survival, we compared the 5-year OS and RFS between MP- and MP+, between SD- and SD+. As shown in Figure 3, the 5-year OS rates in patients with MP- and MP+ were 92% and 85%, respectively (P=0.868), in patients with SD- and SD+ were 94% and 57%, respectively (P<0.001). The 5-year RFS rates in patients with MP- and MP+ were 89% and 66%, respectively (P<0.001), in patients with SD- and SD+ were 87% and 56%, respectively (P<0.001).
Figure 3.
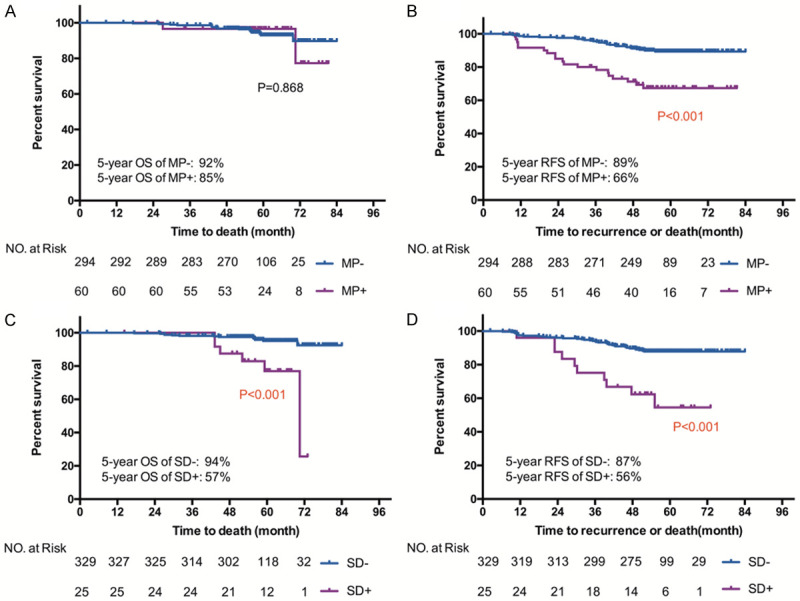
Kaplan-Meier curve of OS and RFS in stage IA patients with and without micropapillary component (A, B), in stage IA patients with and without solid component (C, D).
Pulmonary adenocarcinoma is a very heterogeneous tumor histologically. Due to the difficulty in defining the extent of every component, the clinical relevance of the heterogeneity has not been established [6-8]. In the present study, as shown in Figure 4, the number of different component had no influence on prognosis (P=0.204). However, there was a tendency for tumors with only one component to have a better survival than those contained more than one component.
Figure 4.
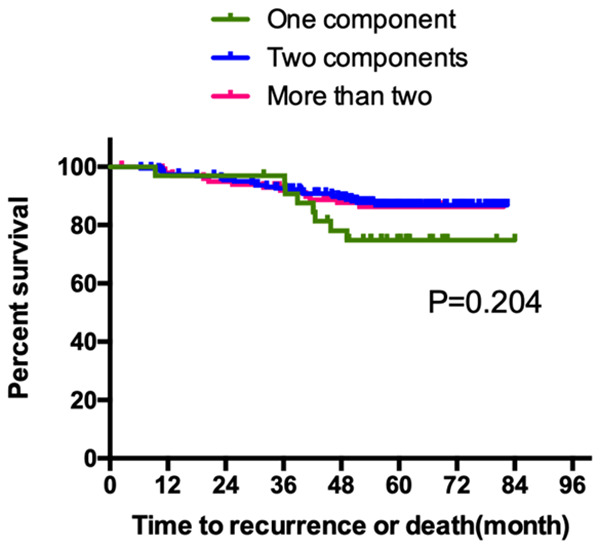
Kaplan-Meier curve of RFS according to the number of different component within the tumor.
Discussion
Our study demonstrates that micropapillary and solid histological patterns are associated with larger tumor size, a higher incidence of STAS positive status, and worse prognosis for stage IA lung adenocarcinoma. These two patterns have been considered a high-grade malignancy in lung adenocarcinoma [5-11].
Until now, it is still controversial whether the percentage of micropapillary or solid pattern is connected with poor prognosis. In this cohort, the difference between MP+/SD+ subgroup and MPp/SDp subgroup was not significant (P=0.177). Similarly, Sumiyoshi [12] reported that no marked difference was observed in mean percentage of micropapillary between the recurrence and nonrecurrence groups (20.4% and 18.3%, respectively, P=0.996). In contrast, Kamiya [13] et al. have divided 383 cases into four groups according the proportion of micropapillary pattern: none (0% of the tumor), focal (<10%), moderate (<50%), and extensive (≥50%), and their results showed that the prognosis was worse with the increase in proportion of micropapillary in tumors. Zhang [14] and his colleagues drew the same conclusion.
Tumors with micropapillary and/or solid component appear to behave more aggressively, manifest with a higher stage at presentation, and show more frequent nodal and lympho-vascular invasion [5-15]. We showed that the frequency of STAS+ patients is the highest in MP+/SD+ subgroup (43.7%), followed by MPp/SDp subgroup (24.0%). Univariate analysis identified it as being significantly associated with worse RFS. Extensive studies have revealed that the presence of STAS in lung adenocarcinoma has a negative impact on recurrence and survival [16,17]. Kadota et al. [18] pointed out that STAS was more frequent in patients with a solid/micropapillary pattern. According to Miyoshi [19], micropapillary pattern demonstrates disruption of the entire cadherin-catenin-actin network, which may be associated with adherins junction disassembly and the release of non-adhesive invasive and metastatic cancer cells. It is a logical assumption that micropapillary pattern acts as a basis for STAS. In the present study, the proportions of patients with micropapillary pattern in MP+/SD+ subgroup and MPp/SDp subgroup were 84.5% and 60% respectively. This may partly explain the higher frequency of STAS+ in the former subgroup. The incidence of STAS in our study is 11.8%, however it was 32%-38% as reported by other studies [18-20]. This could be a function of the small tumor size in our study as well as the low stage. Since many of the studies in the literature comment on STAS in Stages I-III. This is supported by Toyokawa et al. and de Margerie-Mellon C et al. who found that a larger radiologic tumor diameter was more predictive of STAS [21].
In the present study, there were 79 patients in MP+/SD+ and MPp/SDp subgroups, only 2 of them received sublobectomy. The median RFS time was 72 months and 60 months for lobectomy and sublobectomy group respectively. The presence of micropapillary or solid components was reported to be associated with significantly greater risk of recurrence after limited resection in early stage small lung tumors [7,8]. A micropapillary component of 5% or greater increased risk of recurrence was observed in the limited resection group but not in the lobectomy group [22]. Furthermore, despite the low sensitivity, the presence of micropapillary and solid patterns in frozen sections still correlated with loco-regional recurrence (P=0.028 for micropapillary pattern; P=0.003 for solid pattern) [23].
Several limitations are presented in this study. Firstly, this was a retrospective study and a small sample size was enrolled. We followed the 2015 WHO classification of lung cancer to record the proportion of each histological subtype in 5% increments [2] and proved that micropapillary and solid subtypes had a negative impact on prognosis. However, the optimal cutoff for the proportion of these two patterns cannot be determed. Additionally, the follow-up time was limited. The cause of death for the 16 patients could not be obtained. These defects might influence overall survival analysis. Third, some important clinical parameters like smoking history and EGFR status were not evaluated. Fourth, in patients with micropapillary or solid component, only two of them underwent segmentectomy/wedge resection. Whether the type of surgery is an independent predictor for recurrence cannot be evaluated here. Further study in this area is needed.
To sum up, correlation between micropapillary and/or solid patterns and clinicopathological background were evaluated. Micropapillary and solid patterns were associated with statistically significant increased risk of local recurrence, even if they were not the predominant pattern.
Disclosure of conflict of interest
None.
References
- 1.Chang C, Sun X, Zhao W, Wang R, Qian X, Lei B, Wang L, Liu L, Ruan M, Xie W, Shen J. Minor components of micropapillary and solid subtypes in lung invasive adenocarcinoma (≤3 cm): PET/CT findings and correlations with lymph node metastasis. Radiol Med. 2020;125:257–264. doi: 10.1007/s11547-019-01112-x. [DOI] [PubMed] [Google Scholar]
- 2.Kim M, Chung YS, Kim KA, Shim HS. Prognostic factors of acinar- or papillary-predominant adenocarcinoma of the lung. Lung Cancer. 2019;137:129–135. doi: 10.1016/j.lungcan.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Miyazawa T, Marushima H, Saji H, Kojima K, Hoshikawa M, Takagi M, Nakamura H. PD-L1 expression in non-small-cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surg. 2019;25:1–9. doi: 10.5761/atcs.oa.18-00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn E, Morbini P, Cancellieri A, Damiani S, Cavazza A, Comin CE. Adenocarcinoma classification: patterns and prognosis. Pathologica. 2018;110:5–11. [PubMed] [Google Scholar]
- 5.Zhao ZR, Lau RWH, Long H, Mok TSK, Chen GG, Underwood MJ, Ng CSH. Novel method for rapid identification of micropapillary or solid components in early-stage lung adenocarcinoma. J Thorac Cardiovasc Surg. 2018;156:2310–2318.e2. doi: 10.1016/j.jtcvs.2018.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Tsai PC, Yeh YC, Hsu PK, Chen CK, Chou TY, Wu YC. CT-guided core biopsy for peripheral sub-solid pulmonary nodules to predict predominant histological and aggressive subtypes of lung adenocarcinoma. Ann Surg Oncol. 2020;27:4405–4412. doi: 10.1245/s10434-020-08511-9. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Y, Ma G, Zhang Y, Chen H. Presence of micropapillary and solid patterns are associated with nodal upstaging and unfavorable prognosis among patient with cT1N0M0 lung adenocarcinoma: a large-scale analysis. J Cancer Res Clin Oncol. 2018;144:743–749. doi: 10.1007/s00432-017-2571-7. [DOI] [PubMed] [Google Scholar]
- 8.Choi SH, Jeong JY, Lee SY, Shin KM, Jeong SY, Park TI, Do YW, Lee EB, Seok Y, Lee WK, Park JE, Park S, Lee YH, Seo H, Yoo SS, Lee J, Cha SI, Kim CH, Park JY. Clinical implication of minimal presence of solid or micropapillary subtype in early-stage lung adenocarcinoma. Thorac Cancer. 2021;12:235–244. doi: 10.1111/1759-7714.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yagi Y, Aly RG, Tabata K, Barlas A, Rekhtman N, Eguchi T, Montecalvo J, Hameed M, Manova-Todorova K, Adusumilli PS, Travis WD. Three-dimensional histologic, immunohistochemical, and multiplex immunofluorescence analyses of dynamic vessel co-option of spread through air spaces in lung adenocarcinoma. J Thorac Oncol. 2020;15:589–600. doi: 10.1016/j.jtho.2019.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian F, Yang W, Wang R, Xu J, Wang S, Zhang Y, Jin B, Yu K, Han B. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J Thorac Cardiovasc Surg. 2018;155:1227–1235.e2. doi: 10.1016/j.jtcvs.2017.09.143. [DOI] [PubMed] [Google Scholar]
- 11.Gao S, Stein S, Petre EN, Shady W, Durack JC, Ridge C, Adusumilli P, Rekhtman N, Solomon SB, Ziv E. Micropapillary and/or solid histologic subtype based on pre-treatment biopsy predicts local recurrence after thermal ablation of lung adenocarcinoma. Cardiovasc Intervent Radiol. 2018;41:253–259. doi: 10.1007/s00270-017-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao ZR, To KF, Mok TS, Ng CS. Is there significance in identification of non-predominant micropapillary or solid components in early-stage lung adenocarcinoma? Interact Cardiovasc Thorac Surg. 2017;24:121–125. doi: 10.1093/icvts/ivw283. [DOI] [PubMed] [Google Scholar]
- 13.Cha MJ, Lee HY, Lee KS, Jeong JY, Han J, Shim YM, Hwang HS. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg. 2014;147:921–928.e2. doi: 10.1016/j.jtcvs.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida Y, Nitadori JI, Shinozaki-Ushiku A, Sato J, Miyaji T, Yamaguchi T, Fukayama M, Nakajima J. Micropapillary histological subtype in lung adenocarcinoma of 2 cm or less: impact on recurrence and clinical predictors. Gen Thorac Cardiovasc Surg. 2017;65:273–279. doi: 10.1007/s11748-017-0747-3. [DOI] [PubMed] [Google Scholar]
- 15.Emoto K, Eguchi T, Tan KS, Takahashi Y, Aly RG, Rekhtman N, Travis WD, Adusumilli PS. Expansion of the concept of micropapillary adenocarcinoma to include a newly recognized filigree pattern as well as the classical pattern based on 1468 stage i lung adenocarcinomas. J Thorac Oncol. 2019;14:1948–1961. doi: 10.1016/j.jtho.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butnor KJ. Controversies and challenges in the histologic subtyping of lung adenocarcinoma. Transl Lung Cancer Res. 2020;9:839–846. doi: 10.21037/tlcr.2019.12.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, Park BJ, Rusch VW, Travis WD. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Wang R, Shen X, Pan Y, Cheng C, Li Y, Shen L, Zhang Y, Li H, Zheng D, Ye T, Zheng S, Sun Y, Chen H. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23:2099–105. doi: 10.1245/s10434-015-5043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales-Oyarvide V, Mino-Kenudson M. High-grade lung adenocarcinomas with micropapillary and/or solid patterns: a review. Curr Opin Pulm Med. 2014;20:317–23. doi: 10.1097/MCP.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 20.Huang KY, Ko PZ, Yao CW, Hsu CN, Fang HY, Tu CY, Chen HJ. Inaccuracy of lung adenocarcinoma subtyping using preoperative biopsy specimens. J Thorac Cardiovasc Surg. 2017;154:332–339.e1. doi: 10.1016/j.jtcvs.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 21.Caso R, Sanchez-Vega F, Tan KS, Mastrogiacomo B, Zhou J, Jones GD, Nguyen B, Schultz N, Connolly JG, Brandt WS, Bott MJ, Rocco G, Molena D, Isbell JM, Liu Y, Mayo MW, Adusumilli PS, Travis WD, Jones DR. The underlying tumor genomics of predominant histologic subtypes in lung adenocarcinoma. J Thorac Oncol. 2020;15:1844–1856. doi: 10.1016/j.jtho.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh MS, Lin MW, Lee YH. Lung adenocarcinoma with sarcomatoid transformation after tyrosine kinase inhibitor treatment and chemotherapy. Lung Cancer. 2019;137:76–84. doi: 10.1016/j.lungcan.2019.08.029. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Zhu LZ, Jiang MJ, Yuan Y. Clinical impacts of a micropapillary pattern in lung adenocarcinoma: a review. Onco Targets Ther. 2015;9:149–58. doi: 10.2147/OTT.S94747. [DOI] [PMC free article] [PubMed] [Google Scholar]


