Abstract
Objective: To determine the expression of tyrosine protein kinase Met (c-MET), epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor 2 (HER-2) in cases with gastric adenocarcinoma (GA). Methods: The positive rates of the c-MET, EGFR, and HER-2 proteins between cancerous tissues and normal tissues sampled from 87 patients with GA were compared. The patients were assigned to different subgroups according to their clinicopathological characteristics and analyzed. Then the relationship between the above three indexes and the positive expression of Ki-67 were analyzed. In addition, the patients were assigned to positive and negative groups based on the situation of c-MET, EGFR and HER-2 proteins, and followed up for three years. These groups were compared in terms of recurrence-free survival, overall survival, and risks factors of prognosis. Results: The positive rates of c-MET, EGFR and HER-2 proteins in GA tissues were all higher than those in corresponding non-tumor tissues (all P<0.001), and the positive rates of them were greatly different in subgroups with different differentiation, invasion depth, TNM stage, lymph node metastasis (LNM), distant metastasis and presence of tumor thrombus (all P<0.05) and were positively correlated with the expression of Ki-67 protein (P<0.05). Moreover, the survival analysis results revealed lower recurrence-free survival and overall survival rates in groups with negative expression of c-MET, EGFR, and HER-2 than those in groups with positive expression of them (both P<0.001). Furthermore, the positive EGFR was an independent prognostic factor affecting the survival of patients with GA. Conclusion: The expression of c-MET, EGFR and HER-2 proteins is correlated with clinical characteristics of patients with GA, and patients with positive expression of them face a higher recurrence rate. Additionally, EGFR protein may affect patients’ survival.
Keywords: Gastric adenocarcinoma, tyrosine protein kinase Met, epidermal growth factor receptor, human epidermal growth factor receptor 2
Introduction
Gastric adenocarcinoma (GA) is a prevalent malignant tumor of the digestive tract, with morbidity and mortality ranking at the forefront of those of malignant tumors [1,2]. With the development of society, the incidence of GA is increasing year by year. Patients with GA have no obvious clinical manifestations in the early state, so more than half of them have already entered the later phase at the time of diagnosis, and have missed the optimal therapy time [3-6]. Over the past few years, as molecular biology advances rapidly, the roles of signal pathways in tumor development have gradually captured the attention of medical researchers and have provided new ideas for the treatment of GA [7,8]. Receptor tyrosine kinases (RTKs) include tyrosine protein kinase Met (c-MET), epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER-2). According to previous studies, RTKs pathway is closely related to the growth and reproduction of many kinds of tumor cells. c-MET is a hepatocyte growth factor receptor, with a main biological function of activating RTKs pathway, which plays an important role in cell proliferation and differentiation. HER-2, a member of the human epidermal growth factor receptor family, can lead to over-expression of corresponding proteins when it increases in large quantities and can thus promote cell proliferation and inhibit apoptosis, resulting in rapid growth of tumor cells. Studies have confirmed that c-MET and HER-2 in solid tumor tissue such as breast cancer tissues increase notably, and overexpression of the two can stimulate the activity of various signal pathways, thus accelerating the proliferation and infiltration of tumor cells [9,10]. EGFR is a specific protein capable of promoting vascular proliferation and increasing vascular permeability. It can regulate the vascular permeability of tumor tissues to a certain extent and its positive expression in tumors can promote angiogenesis in tumor tissues, and then regulate the growth of tumor tissues [11]. One earlier study has investigated the expression of RTKs family members in cases with GA, but the difference of their expression in patients with different clinical characteristics is still controversial [12]. Therefore, this study analyzed the expression of c-MET, EGFR and HER-2 in cases with GA and its correlation with clinicopathological features, and determined its clinical value in evaluating prognosis, with the purpose of providing relevant basis for diagnosis and prognosis evaluation of GA.
Materials and methods
General data
A total of 87 patients with GA who received radical gastrectomy in our hospital from January 2016 to January 2018 were enrolled, and their general data were collected. In our study, all enrolled participants voluntarily participated in the study after they and their families were informed of the study and signed informed consent forms. This study was approved by the ethics committee of our hospital. Cases enrolled in this study were evaluated according to the GC staging (8-th edition) formulated by the American Joint Committee on Cancer [13].
Inclusion and exclusion criteria
The inclusion criteria
Patients who were confirmed with GA and received surgical resection; patients ≥18 years old; patients who had not received treatments affecting our experimental results such as radiotherapy, chemotherapy, biological immunotherapy and targeted therapy before operation.
The exclusion criteria
Patients with other comorbid tumors; patients who had taken immunosuppressants and other drugs with possible impacts on the experimental results within the last 3 months; those without detailed pathological data.
Methods
Cancerous tissues and corresponding non-tumor tissues were sampled, paraffined, and cut into 4 μm sections. Then immunohistochemistry was adopted to determine the positive status of ki-67, c-MET, EGFR and HER-2 in them. Immunohistochemical antibodies and kits were all purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.
Result determination
The sections were scored according to the following criteria. 0 point: no observed cells were stained; 1 point: 1-25% observed cells were stained; 2 points: 26-50% observed cells were stained; 3 points: over 50% observed cells were stained. 0 point was adopted to indicate negative expression, while 1-3 points indicate positive expression [14-16].
Follow-up observation
The follow-up ended on January 2021. The postoperative recurrence time and survival time of patients were calculated from the time of the first surgical resection of GA in our hospital to the time of death or the last follow-up time, namely January 2021 (for patients who did not die).
Outcome measures
Primary outcome measures: The positive expression rates of c-MET, EGFR and HER-2 proteins in GA tissues from the 87 enrolled patients were compared with those in corresponding non-tumor tissues. The K-M survival curve was adopted to analyze the difference of recurrence-free survival (RFS) and overall survival (OS) rates between positive and negative groups, and the COX proportional hazard model (PHM) was adopted to conduct multivariate survival analysis.
Secondary outcome measures: The patients were assigned into different subgroups according to their gender, age, tumor location, tumor diameter, differentiation, invasion depth, TNM stage, lymph node metastasis (LNM), distant metastasis, and presence of tumor thrombus. The subgroups were compared in positive expression rates of c-MET, EGFR and HER-2 proteins. Additionally, Spearman’s rank correlation was adopted for correlation analysis between the positive expression of c-MET, EGFR and HER-2 proteins and that of Ki-67 protein.
Statistical analyses
SPSS22.0 was used for statistical analyses. The study also compared enumeration data, expressed as the number of cases and percentage, between groups via the χ2 test. Spearman’s rank correlation method was used for correlation of two indices, and COX proportional hazard model (PMH) for multivariate survival analysis. P<0.05 indicates a significant difference.
Results
Immunohistochemical results
The positive rates of c-MET, EGFR and HER-2 proteins in GA tissues were 42.53%, 37.93% and 44.83%, respectively, and those in corresponding non-tumor tissues were 11.49%, 10.34% and 11.49%, respectively. Therefore, the positive rates of them in the two different tissues were significantly different (P<0.001, Table 1 and Figure 1).
Table 1.
Immunohistochemical results of c-MET, EGFR and HER-2 proteins [n (%)]
| Parts | c-MET | EGFR | HER-2 |
|---|---|---|---|
| Gastric adenocarcinoma tissues (n=87) | 37 (42.53) | 33 (37.93) | 39 (44.83) |
| Paracancerous tissues (n=87) | 10 (11.49) | 9 (10.34) | 10 (11.49) |
| χ2 | 21.521 | 18.408 | 23.891 |
| P | <0.001 | <0.001 | <0.001 |
Note: c-MET: tyrosine protein kinase Met; EGFR: epidermal growth factor receptor; HER-2: human epidermal growth factor receptor 2.
Figure 1.
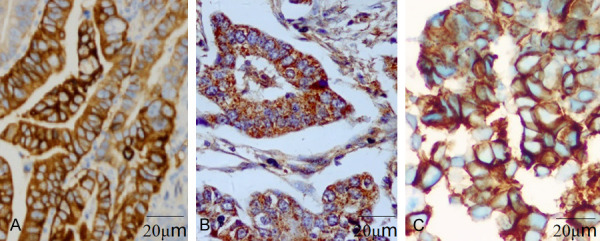
Immunohistochemical results of c-MET, EGFR and HER-2 proteins (×400). A. Gastric adenocarcinoma tissue is positive for c-MET protein. B. Bastric adenocarcinoma tissue is positive for EGFR protein. C. Gastric adenocarcinoma tissue is positive for HER-2 protein. c-MET: tyrosine protein kinase Met; EGFR: epidermal growth factor receptor; HER-2: human epidermal growth factor receptor 2.
Comparison of positive rates of c-MET, EGFR and HER-2 proteins in patients with different clinicopathological features
Among the 87 cases of GA tissues, patients with low differentiation showed higher positive rates of c-MET, EGFR and HER-2 proteins than those with moderate or high differentiation (all P<0.05); patients with T3 or T4 infiltration depth showed higher positive rates of them than those with T1 or T2 infiltration depth (all P<0.05); patients with higher TNM staging showed higher positive rates of them (all P<0.05); patients without LNM, distant metastasis or cancer thrombus showed higher positive rates of them than those with the condition (all P<0.05; Table 2).
Table 2.
Comparison of positive rates of c-MET, EGFR and HER-2 protein in patients with different clinicopathological characteristics
| Project | Numbers | c-MET expression | EGFR expression | HER-2 expression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Positive (%) | χ2 | P | Positive (%) | χ2 | P | Positive (%) | χ2 | P | ||
| Gender (n) | ||||||||||
| Male | 54 | 24 (44.44) | 0.214 | 0.644 | 18 (33.33) | 1.278 | 0.258 | 25 (46.30) | 0.124 | 0.725 |
| Female | 33 | 13 (39.39) | 15 (45.45) | 14 (42.42) | ||||||
| Age (years) | ||||||||||
| <55 | 30 | 12 (40.00) | 0.12 | 0.729 | 10 (33.33) | 0.411 | 0.521 | 13 (43.33) | 0.041 | 0.839 |
| ≥55 | 57 | 25 (43.86) | 23 (40.35) | 26 (45.61) | ||||||
| Tumor location (n) | ||||||||||
| Cardia | 26 | 12 (46.15) | 0.199 | 0.655 | 11 (42.31) | 0.302 | 0.583 | 12 (46.15) | 0.026 | 0.871 |
| Non cardia | 61 | 25 (40.98) | 22 (36.01) | 27 (44.26) | ||||||
| Tumor diameter (CM) | ||||||||||
| >5 | 51 | 23 (45.10) | 0.333 | 0.564 | 20 (39.22) | 0.086 | 0.769 | 25 (49.02) | 0.876 | 0.349 |
| ≤5 | 36 | 14 (38.89) | 13 (36.11) | 14 (38.89) | ||||||
| Differentiation degree (n) | ||||||||||
| Medium high differentiation | 35 | 9 (25.71) | 6.774 | 0.009 | 7 (20.00) | 7.997 | 0.005 | 9 (25.71) | 8.649 | 0.003 |
| Low differentiation | 52 | 28 (53.85) | 26 (50.00) | 30 (57.69) | ||||||
| Infiltration depth (n) | ||||||||||
| T1+t2 | 42 | 11 (26.19) | 8.868 | 0.003 | 10 (23.81) | 6.878 | 0.009 | 11 (26.19) | 11.404 | 0.001 |
| T3+t4 | 45 | 26 (57.78) | 23 (51.11) | 28 (62.22) | ||||||
| TNM staging (n) | ||||||||||
| Phase I | 13 | 2 (15.38) | 13.086 | 0.001 | 2 (15.38) | 10.133 | 0.006 | 3 (23.08) | 12.526 | 0.002 |
| Phase II | 32 | 9 (28.13) | 8 (25.00) | 9 (28.13) | ||||||
| Phase III | 42 | 26 (61.90) | 23 (54.76) | 27 (64.29) | ||||||
| Lymph node metastasis (n) | ||||||||||
| Yes | 50 | 27 (54.00) | 6.33 | 0.012 | 24 (48.00) | 5.063 | 0.024 | 28 (56.00) | 5.934 | 0.015 |
| No | 37 | 10 (27.03) | 9 (24.32) | 11 (29.73) | ||||||
| Distant metastasis (n) | ||||||||||
| Yes | 24 | 23 (95.83) | 38.529 | <0.001 | 22 (91.67) | 40.649 | <0.001 | 23 (95.83) | 34.863 | <0.001 |
| No | 63 | 14 (22.22) | 11 (17.46) | 16 (25.40) | ||||||
| Tumor thrombus (n) | ||||||||||
| Yes | 51 | 29 (56.86) | 10.361 | 0.001 | 26 (50.98) | 8.915 | 0.003 | 30 (58.83) | 9.762 | 0.002 |
| No | 36 | 8 (22.22) | 7 (19.44) | 9 (25.00) | ||||||
| Histological type (n) | ||||||||||
| Tubular adenocarcinoma | 67 | 29 (43.28) | 0.436 | 0.509 | 27 (40.30) | 0.694 | 0.405 | 31 (46.27) | 0.245 | 0.621 |
| Non tubular adenocarcinoma | 20 | 7 (35.00) | 6 (30.00) | 8 (40.00) | ||||||
Note: c-MET: tyrosine protein kinase Met; EGFR: epidermal growth factor receptor; HER-2: human epidermal growth factor receptor 2.
Correlation analysis between the positive expression of c-MET, EGFR and HER-2 proteins and that of Ki-67 protein
The positive expression of c-MET, EGFR and HER-2 proteins were positively correlated with that of Ki-67 protein (all P<0.05, Table 3).
Table 3.
Correlation results
| Statistics | c-MET | EGFR | HER-2 |
|---|---|---|---|
| r | 0.319 | 0.338 | 0.374 |
| P | 0.017 | 0.029 | 0.005 |
Note: c-MET: tyrosine protein kinase Met; EGFR: epidermal growth factor receptor; HER-2: human epidermal growth factor receptor 2.
Comparison of OS rate between positive and negative groups of c-MET, EGFR and HER-2 proteins
By the time of follow-up, the survival rate was 64.37%, with 31 deaths. The median survival time of positive groups of c-MET, EGFR, and HER-2 proteins was 19.95, 19.28 and 21.49 months, respectively, while that of negative groups was 33.84, 33.10, and 33.26 months, respectively. Therefore, the positive and negative groups were significantly different in OS (χ2=41.435, 38.314, 29.538; all P<0.001, Figure 2).
Figure 2.
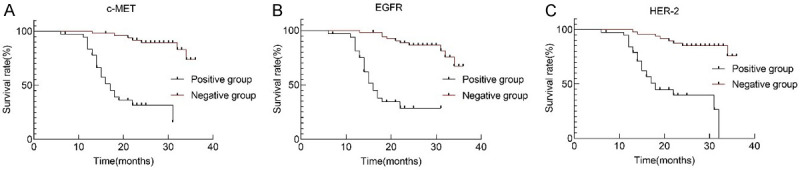
The overall survival curves of c-MET, EGFR and HER-2 protein positive and negative groups. A. c-MET. B. EGFR. C. HER-2. c-MET: tyrosine protein kinase Met; EGFR: epidermal growth factor receptor; HER-2: human epidermal growth factor receptor 2.
Comparison of RFS rate between positive and negative groups of c-MET, EGFR and HER-2 proteins
All patients were followed up for three years, and the RFS rate was 64.37%, with 35 patients suffering recurrence. The median RFS of positive groups of c-MET, EGFR and HER-2 proteins was 14.59, 13.84, and 15.35 months, respectively, while that of negative groups was 27.83, 27.37, and 27.57 months, respectively. Therefore, the positive and negative groups were greatly different in RFS (χ2=37.778, 39.991, 31.006; all P<0.001, Figure 3).
Figure 3.
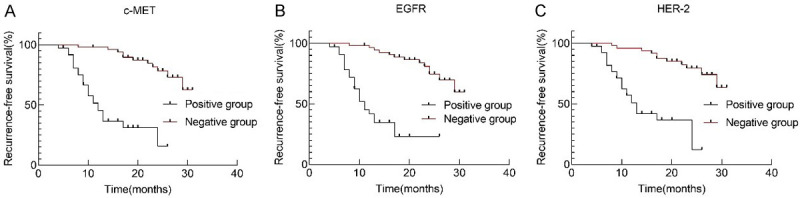
Recurrence-free survival curves of c-MET, EGFR and HER-2 protein positive and negative groups. A. c-MET. B. EGFR. C. HER-2. c-MET: tyrosine protein kinase Met; EGFR: epidermal growth factor receptor; HER-2: human epidermal growth factor receptor 2.
COX PMH analysis results
The results indicated that positive EGFR was an independent prognostic factor for the survival of patients with GA (P<0.001, Table 4).
Table 4.
COX proportional hazard model analysis results
| Index | EGFR positive |
|---|---|
| B | 1.453 |
| Se | 0.363 |
| Wald | 16.024 |
| P | <0.001 |
| Rr | 4.277 |
| 95ci | |
| Lower limit | 2.099 |
| Upper limit | 8.713 |
Note: EGFR: epidermal growth factor receptor.
Discussion
Currently, RTKs pathway has attracted enormous attention of medical researchers. Studies have pointed out the close association between RTKs overexpression and the development of many solid cancers such as colorectal cancer and glioma [9,10]. Therefore, the study adopted RTKs pathway-associated proteins as indices to explore their clinical value in evaluating prognosis.
C-MET, a member of the RTKs family, is expressed on various cell surfaces. One study has revealed that under normal physiological conditions, c-MET is able to accelerate growth and healing of damaged tissues [17]. However, under special pathological conditions, it can speed up the proliferation of tumor cells and suppress their apoptosis. According to a previous study, c-MET protein shows overexpression in solid cancers such as breast cancer and liver cancer [18]. In our study, among the enrolled 87 patients with GA, the positive rate of c-MET protein in cancerous tissues was 42.53%, significantly higher than that in normal tissues. However, in cases with low differentiation, high infiltration depth or TNM stage, LNM, distant metastasis or cancer thrombus, the positive expression rate of c-MET protein was higher, and the correlation analysis showed a positive association between positive rate of c-MET protein and that of ki-67. The results indicate the close association of its positive rate with the severity of disease, and its ability of indicating unfavorable prognosis. HER-2, a RTKS I-type receptor, is in an inactive state under normal physiological conditions, but once stimulated by external factors, it will be activated due to its abnormality in structure and function, and then it will enhance the activity of tumor transformation, thus leading to malignant transformation of cells [19]. In one earlier study, in cases with GC, positive rate of HER-2 protein was approximate 7.1-42.6% [10,20]. In our study, among the 87 patients with GA, the positive rate of HER-2 protein in GA tissues was significantly different from that in corresponding non-tumor tissues (44.83% vs. 11.49%, P<0.05). We also found that in cases with GA, the positive rate of HER-2 protein increased with the decrease of differentiation degree, increase of infiltration depth or TNM stage, or occurrence of LNM, distant metastasis or cancer thrombus. The results are in consistent with previous research results, which suggest that cases with positive HER-2 protein have stronger cancer cell invasion and metastasis, and thus have worse prognosis. EGFR also belongs to the RTKs family. Under normal physiological conditions, it binds to its ligand, which leads to dimerization of receptors, and thus participates in cell biological behaviors including cell proliferation, growth and differentiation [21]. One study has pointed out a significant increase in EGFR in cancerous tissues [22], and another study found that the expression rate of EGFR protein in cases with GA was over 40%, notably higher than that in normal gastric tissues [23]. In contrast, our study showed that the expression rate of EGFR protein in cases with GA was 37.93%, which was significantly higher than that in corresponding non-tumor tissues, but lower than that mentioned above. We believe that reasons for the difference are probably related to factors such as race and detection method. In patients with different clinicopathological features, similar to c-MET and HER-2, the positive rate of EGFR protein increased with the decrease of differentiation, increase of infiltration depth or TNM stage, or occurrence of LNM, distant metastasis or cancer thrombus. The correlation analysis showed a positive association between the positive rate of EGFR protein and that of ki-67. The results indicate an involvement of EGFR in the development of GA. We adopted K-M survival curve for analysis of RFS and OS rates of positive and negative groups, and found that negative groups showed higher RFS and OS rates and experienced longer median RFS time and OS time than those of positive groups. The data indicate that patients with positive expression of c-MET, EGFR and HER-2 proteins may have worse prognosis. Additionally, we adopted COX PMH for analysis, and found that only EGFR protein was an independent prognostic factor for the survival time of patients with GA. The result also suggests the relatively shorter survival of patients with positive EGFR protein, but the specific mechanism needs further study.
This study also has the following limitations: (1) All specimens were taken from a single center, so the sample size was small. (2) It was difficult to discuss the sequential order between the expression of c-MET, EGFR, HER-2 proteins and tumorigenesis, and clarify the specific mechanism between them.
To sum up, in cases with GA, the expression of c-MET, EGFR and HER-2 proteins is correlated with differentiation, TNM stage, LNM, distant metastasis, and presence of tumor thrombus, and patients with positive expression of them face a higher recurrence rate. Furthermore, EGFR protein is an independent prognostic factor for patients’ survival.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Cao W, Zheng C, Hu W, Liu C. Marital status and survival in patients with rectal cancer: an analysis of the surveillance, epidemiology and end results (SEER) database. Cancer Epidemiol. 2018;54:119–124. doi: 10.1016/j.canep.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Xu T, Yan FF, Zhang CH, Zhang YQ. Analysis of clinical pathology and prognosis in patients with bone metastases from cancer. J Pract Oncol. 2019;33:436–441. [Google Scholar]
- 4.Cui R, Cao Y. To explore the clinicopathological features and prognosis of gastric cancer patients in different age groups. Clin Med Res Pract. 2018;3:9–11. [Google Scholar]
- 5.Ma JJ, Chen YJ. Comparative observation of the clinical efficacy of laparoscopy and open surgery for early gastric cancer. Dig World Latest Med Inf. 2019;19:76–77. [Google Scholar]
- 6.Lu J, Zheng CH, Cao LL, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Lin M, Tu RH, Huang CM. Comparison of the 7th and 8th editions of the American joint committee on cancer TNM classification for patients with stage III gastric cancer. Oncotarget. 2017;8:83555–83562. doi: 10.18632/oncotarget.18375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilleron S, Sarfati D, Janssen-Heijnen M, Vignat J, Ferlay J, Bray F, Soerjomataram I. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int J Cancer. 2019;144:49–58. doi: 10.1002/ijc.31664. [DOI] [PubMed] [Google Scholar]
- 8.Rothlin CV, Ghosh S. Lifting the innate immune barriers to antitumor immunity. J Immunother Cancer. 2020;8:000695. doi: 10.1136/jitc-2020-000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, Wu Y, Li X, Li X, Li G, Zeng Z, Xiong W. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17:45. doi: 10.1186/s12943-018-0796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrelli F, Tomasello G, Barni S, Lonati V, Passalacqua R, Ghidini M. Clinical and pathological characterization of HER2 mutations in human breast cancer: a systematic review of the literature. Breast Cancer Res Treat. 2017;166:339–349. doi: 10.1007/s10549-017-4419-x. [DOI] [PubMed] [Google Scholar]
- 11.Miao F, Zhang M, Zhao Y, Li X, Yao R, Wu F, Huang R, Li K, Miao S, Ma C, Ju H, Song W, Wang L. RHBDD1 upregulates EGFR via the AP-1 pathway in colorectal cancer. Oncotarget. 2017;8:25251–25260. doi: 10.18632/oncotarget.15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh KJ, Sung JH, Kim JW, Han SH, Lee HS, Min A, Kang MH, Kim JE, Kim JW, Kim SH, Lee JO, Kim YJ, Lee KW, Kim JH, Bang SM, Im SA, Lee JS. EGFR or HER2 inhibition modulates the tumor microenvironment by suppression of PD-L1 and cytokines release. Oncotarget. 2017;8:63901–63910. doi: 10.18632/oncotarget.19194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. Organization of the AJCC Cancer Staging Manual 8th edition. In: Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, editors. New York: 2017. [Google Scholar]
- 14.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 15.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 17.Salgia R. MET in lung cancer: biomarker selection based on scientific rationale. Mol Cancer Ther. 2017;16:555–565. doi: 10.1158/1535-7163.MCT-16-0472. [DOI] [PubMed] [Google Scholar]
- 18.Tretiakova M, Salama AK, Karrison T, Ferguson MK, Husain AN, Vokes EE, Salgia R. MET and phosphorylated MET as potential biomarkers in lung cancer. J Environ Pathol Toxicol Oncol. 2011;30:341–354. doi: 10.1615/jenvironpatholtoxicoloncol.v30.i4.70. [DOI] [PubMed] [Google Scholar]
- 19.Solomon JP, Dell’Aquila M, Fadare O, Hasteh F. Her2/neu Status determination in breast cancer: a single institutional experience using a dual-testing approach with immunohistochemistry and fluorescence in situ hybridization. Am J Clin Pathol. 2017;147:432–437. doi: 10.1093/ajcp/aqw224. [DOI] [PubMed] [Google Scholar]
- 20.Shi P, Chen C, Yao Y. Correlation between HER-2 gene amplification or protein expression and clinical pathological features of breast cancer. Cancer Biother Radiopharm. 2019;34:42–46. doi: 10.1089/cbr.2018.2576. [DOI] [PubMed] [Google Scholar]
- 21.Cai S, Zhang YX, Han K, Ding YQ. Expressions and clinical significance of COX-2, VEGF-C, and EFGR in endometrial carcinoma. Arch Gynecol Obstet. 2017;296:93–98. doi: 10.1007/s00404-017-4386-9. [DOI] [PubMed] [Google Scholar]
- 22.Njoku K, Chiasserini D, Whetton AD, Crosbie EJ. Proteomic biomarkers for the detection of endometrial cancer. Cancers (Basel) 2019;11:1572. doi: 10.3390/cancers11101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, Ferry D, Mansoor W, Crosby T, Coxon F, Smith D, Waters J, Iveson T, Falk S, Slater S, Peckitt C, Barbachano Y. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


