Abstract
Objective: To determine the clinical efficacy and safety of transcatheter arterial chemoembolization (TACE) for patients with lung cancer (LC). Methods: A total of 513 inpatients with LC admitted to our hospital from January 2012 to January 2020 were analyzed retrospectively. Based on different treatment methods, they were assigned into a control group (CG; n=249) for traditional bronchial artery infusion (BAI) and an experimental group (EG; n=264) for TACE, with shared chemotherapy drugs and treatment courses. The two groups were compared with respect to clinical efficacy, pre- and post-treatment pulmonary function, adverse reactions, as well as negative emotions and quality of life (QoL) scores. Results: The curative effect in EG was far superior to CG (P<0.05). In comparison with CG, the pulmonary function in EG was better and the incidence of adverse reactions was lower after treatment (P<0.05). The negative emotions and the QoL were improved in both groups, with more distinct improvement in EG compared with CG (P<0.05). Conclusions: With higher safety and efficacy, TACE can improve the clinical efficacy and QoL of patients with LC while relieving bad mood and reducing adverse reactions.
Keywords: Lung cancer, transcatheter arterial chemoembolization, quality of life, adverse reaction
Introduction
With a 5-year survival rate as low as 15%, lung cancer (LC) remains the main cause of cancer-related death in humans [1]. In terms of morbidity and prevalence, LC is one of the most common cancers, with more than 80% of cases being non-small cell lung cancer (NSCLC) [2]. Smoking, environmental exposure, ionizing radiation, occupational diseases and heredity are all the influencing factors of the disease [3-5]. The main clinical presentations of LC are cough, chest tightness and hemoptysis [6].
It is difficult to detect LC in its early stage because the lesions are mostly deep in the thoracic cavity without typical clinical symptoms [7]. Given the current shortage of valid diagnostic means and biomarkers, LC is often diagnosed as advanced stage IIIB or IV, which is characterized by distant metastasis with unfavorable prognosis [8,9]. For patients with unresectable advanced LC who have missed the opportunity of surgical treatment, chemotherapy has been clinically proven to be an effective treatment, but the 2-year overall survival rate is lower than 20% [10]. At present, radiotherapy plus chemotherapy is considered as the standard therapy for advanced LC patients free of pleural and pericardial effusion [11]. Chemotherapy is a method of treating diseases with chemically synthesized drugs [12], usually administered by intravenous drip. Whereas, the lesion of advanced LC is usually large, which leads to blood circulation disorders in the tumor. As such, it is difficult to reach the tumor and the center of the tumor through intravenous drip, let alone reach the effective drug concentration, resulting in poor systemic efficacy and high toxicity [13,14]. Bronchial artery infusion (BAI) can be used since the treatment of LC is mainly supplied by bronchial artery, and on this basis, bronchial artery embolization (BAE) can further improve the curative effect of patients.
Through retrospective analysis, this study observed the efficacy of transcatheter arterial chemoembolization (TACE) for patients with lung cancer (LC) admitted to our hospital from 2012 to 2020, so as to find a more effective treatment scheme.
Materials and methods
Participants
This retrospective analysis included 513 LC patients admitted to our hospital from January 2012 to January 2020. Among them, 249 patients who received traditional bronchial artery infusion (BAI) were assigned into the control group (CG), and 264 patients who received TACE were included in experimental group (EG). To be included, patients had to fulfill the following criteria: confirmed diagnosis by imaging and cytology; single primary lesion; no history of radiotherapy; no history of surgical treatment of LC; Karnofsky Performance Status (KPS) score >70 points; life expectancy >6 months. Exclusion criteria: Patients with incomplete clinical data, mental disorders and allergies to drug therapy or interventional therapy were excluded, as well as those who do not cooperate with treatment. This study was approved by the Ethics Committee of the Second Hospital Affiliated to Harbin Medical University (No. 0415-26), and all patients signed an informed consent in accordance with the Declaration of Helsinki.
Treatment
CG: The Seldinger technique was used to perform pulmonary arteriography through puncture of one femoral artery, and the appropriate catheter was selected to enter the target artery for perfusion chemotherapy with 1.0 g/m2 gemcitabine (Qilu Pharmaceutical, Jinan, China, Lot No. H20113285) and 30 mg/m2 cisplatin (Qilu Pharmaceutical, Jinan, China, Lot No. H37021357). EG: On the basis of CG, gelatin sponge (Qingdao Dongfang Weier Medical Technology Co., Ltd., Qingdao, China, Lot No. (2014) 3771056) was injected from the catheter, and 0.9% NaCl solution (Anhui Double-crane Pharmaceutical Co. Ltd., Wuhu, China, Lot No. H20054037) was injected into the bronchial artery. After the procedure, the catheter was removed and the bleeding was stopped by compression. The procedure was repeated every 4-6 weeks. In case of nausea and vomiting during the treatment, the patient was given antiemetic treatment in time. After operation, the patient was ordered to rest in bed and given routine anti-infection treatment. In the meanwhile, hydration and diuresis were given to expel toxins, and nutritional support was strengthened. In addition, postoperative complications such as spinal cord injury, intercostal artery injury, and esophageal injury were observed.
Outcome measures
Curative effect evaluation employed the Response Evaluation Criteria In Solid Tumors (RECIST) [15]
Complete response (CR): complete disappearance of the mass without new lesions; Partial response (PR): mass reduction ≥50% without new lesions; Stable disease (SD): mass reduction <50% or increase <25%, with some new lesions; Progressive disease (PD): mass increase >25% with many new lesions. Total effective rate = (CR+PR) ×100%.
Pulmonary function [16]
Changes in pulmonary function indexes, including forced expiratory volume in one second (FEV1), forced vital capacity (FVC), maximum voluntary ventilation (MVV) and the diffusing capacity of the lung for carbon monoxide (DLCO), were evaluated before and after two cycles of treatment.
Evaluation of tumor markers [17]
Serum levels of carbohydrate antigen 125 (CA125), tumor-associated macrophages (TAM), as well as carcinoembryonic antigen (CEA) and NSCLC associated antigen cytokeratin 19 fragment antigen 21-1 (Cyfra21-1) were detected before and after two cycles of treatment.
Karnofsky Performance Status (KPS) score [18]
KPS scores before treatment, as well as 2 cycles, 3 and 6 months post treatment were observed.
Adverse reactions and complications
Adverse reactions and complications, covering nausea and vomiting, dizziness, abdominal pain, abdominal distension, allergy and renal injury, were recorded.
Negative emotion evaluation: Self-rating Anxiety/Depression Scale (SAS/SDS) [19] were used to evaluate patients’ anxiety and depression.
Quality of life (QoL) evaluation
Pre- and post-treatment QoL of patients employed The EORTC QLQ-C30 [20]. The scale covers physical, role, emotional and social functioning, with 100 points for each item. A higher score indicates a better QoL.
Statistical processing
Statistical processing and visualization of the collected data employed SPSS21.0 and GraphPad 8.0 respectively. Continuous and categorical variables were given mean ± standard deviation and percentage, and compared by t test and χ2 test, respectively. P<0.05 indicates a difference with statistical significance. In this study, 95% confidence interval was set, and all tests were double-tailed.
Results
Clinical data of patients in the two groups
Clinical data of patients in CG and EG were collected. The male to female ratio in CG was 134:115, and the mean age was 58.46±5.71 years old. There were 112 cases of IIIA, 87 of stage IIIB, and 50 of stage IV according to TNM staging; 125 cases of squamous cell carcinoma (SCC), 93 of adenocarcinoma (AC) and 31 of adenosquamous carcinoma (ASC) according to the pathological type; and 148 cases of central lung cancer and 101 of peripheral lung cancer according to the type of LC. The male to female ratio in EG was 152:112, and the mean age was 59.18±6.13 years old. There were 138 cases of stage IIIA, 71 of stage IIIB and 55 of stage IV; 143 cases of SCC, 84 of AC and 37 of ASC; and 151 cases of central lung cancer and 113 of peripheral lung cancer. There was no significant difference in age, gender, body mass index (BMI), TNM staging and tumor pathological type between the two groups (P>0.05; Table 1).
Table 1.
General data of patients with lung cancer
| Control group (n=249) | Experimental group (n=264) | χ2/t | P value | |
|---|---|---|---|---|
| Age (year) | 58.46±5.71 | 59.18±6.13 | 0.7328 | 0.4639 |
| Gender | 0.2610 | 0.6094 | ||
| Male | 134 | 152 | ||
| Female | 115 | 112 | ||
| Body mass index | 23.81±2.48 | 23.41±2.83 | 0.6800 | 0.4968 |
| TNM staging | 4.1272 | 0.1270 | ||
| Stage IIA | 112 | 138 | ||
| Stage IIIB | 87 | 71 | ||
| Stage IV | 50 | 55 | ||
| Pathological types of tumors | 1.7592 | 0.4150 | ||
| Squamous cell carcinoma | 125 | 143 | ||
| Adenocarcinoma | 93 | 84 | ||
| Adenosquamous carcinoma | 31 | 37 | ||
| KPS score | 74.15±2.56 | 73.79±2.92 | 1.4813 | 0.1391 |
| Types of lung cancer | 0.2646 | 0.6070 | ||
| Central lung cancer | 148 | 151 | ||
| Peripheral lung cancer | 101 | 113 | ||
| Clinical presentations | ||||
| Cough | 177 | 189 | 0.01608 | 0.8991 |
| Chest pain | 159 | 166 | 0.0526 | 0.8185 |
| Polypnea | 147 | 157 | 0.0099 | 0.9204 |
| Hemoptysis | 102 | 109 | 0.0674 | 0.7951 |
Clinical efficacy of two groups of patients
In this study, the curative effect was evaluated using the RECIST. In CG, CR was observed in 95 cases, PR in 81 cases, SD in 29 and PD in 44. In EG, CR was observed in 129 cases, PR in 83 cases, SD in 32 and PD in 20. The total effective rate was calculated when all the patients finished the treatment, and the results determined a higher total effective rate in EG compared with CG (80.30% vs. 70.68%; P<0.05; Table 2).
Table 2.
Clinical efficacy
| CR | PR | SD | PD | Total effective rate | |
|---|---|---|---|---|---|
| Control group (n=249) | 95 | 81 | 29 | 44 | 176 (70.68) |
| Experimental group (n=264) | 129 | 83 | 32 | 20 | 212 (80.30) |
| χ2 | 6.4351 | ||||
| P | 0.0112 |
Pulmonary function of two groups of patients
FVL, FEV1, MVV and TLCO levels increased in both groups after two cycles of treatment, with higher values in EG compared with CG (P<0.05; Table 3).
Table 3.
Pulmonary function of two groups of patients
| FVC (L) | FEV1 (L) | MVV (L) | TLCO [mmol/(kPa·min)] | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Before treatment | After 2 cycles of treatment | Before treatment | After 2 cycles of treatment | Before treatment | After 2 cycles of treatment | Before treatment | After 2 cycles of treatment | |
| Control group (n=249) | 2.08±0.32 | 2.39±0.21 | 1.71±0.22 | 1.98±0.19 | 66.81±7.15 | 74.94±7.84 | 3.12±0.68 | 5.77±1.26 |
| Experimental group (n=264) | 2.11±0.28 | 2.76±0.34 | 1.75±0.33 | 2.16±0.23 | 67.56±8.06 | 81.6±7.36 | 3.16±0.59 | 6.18±1.09 |
| t | 1.1317 | 14.7256 | 1.6055 | 9.6324 | 1.1124 | 9.9241 | 0.7127 | 3.9479 |
| P | 0.2583 | <0.0001 | 0.1089 | <0.0001 | 0.2665 | <0.0001 | 0.47563 | <0.0001 |
Levels of tumor markers in two groups
Differed insignificantly before treatment (P>0.05), the levels of tumor markers TAM, CEA, CA125 and Cyfra21-1 dropped in both groups after intervention and were evidently lower in EG compared with CG (P<0.05; Figure 1).
Figure 1.
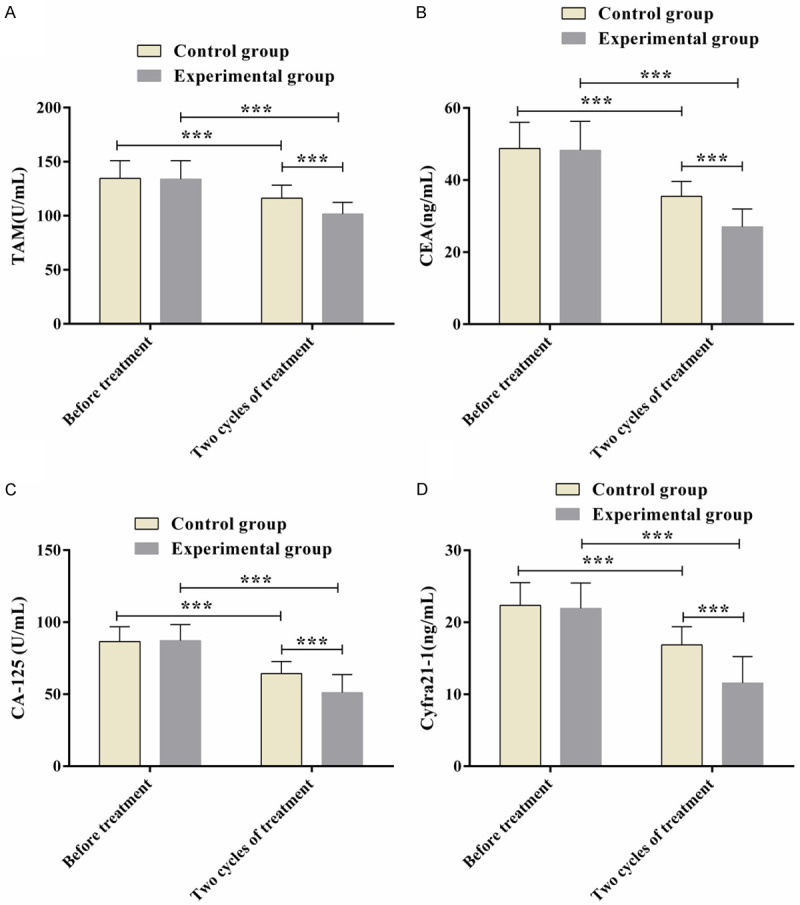
Levels of tumor markers in two groups. (A: TAM levels; B: CEA levels; C: CA125 levels; D: Cyfra21-1 levels; Note: ***P<0.001).
KPS scores in two groups
After two cycles of treatment, KPS scores increased in both groups, especially in EG. KPS scores increased continuously at 3 and 6 months after treatment, and the scores in EG were higher than those in CG at each time point (P<0.05; Table 4).
Table 4.
KPS scores
| Before treatment | After 2 cycles of treatment | 3 month after treatment | 6 month after treatment | |
|---|---|---|---|---|
| Control group (n=249) | 74.15±2.56 | 79.85±6.37 | 81.87±9.23 | 86.42±8.34 |
| Experimental group (n=264) | 73.79±2.92 | 82.21±7.72 | 85.21±8.84 | 88.13±8.93 |
| t | 0.7776 | 3.7643 | 4.1863 | 2.2382 |
| P | 0.4371 | 0.0001 | <0.0001 | 0.0256 |
Negative emotion scores in two groups
SAS and SDS scores differed insignificantly between the two groups before treatment (P>0.05), but decreased in both groups after treatment, with lower scores in EG than in CG (P<0.05; Table 5).
Table 5.
Negative emotion scores in two groups
| SAS | SDS | |||
|---|---|---|---|---|
|
|
|
|||
| Before treatment | After treatment | Before treatment | After treatment | |
| Control group (n=249) | 56.14±6.29 | 46.87±3.69 | 57.68±6.87 | 48.71±4.59 |
| Experimental group (n=264) | 55.68±5.97 | 34.15±3.77 | 57.15±6.44 | 37.12±4.05 |
| t | 0.8539 | 38.5886 | 0.9018 | 30.3663 |
| P | 0.3935 | <0.0001 | 0.3675 | <0.0001 |
Occurrence of adverse reactions
In CG, there were 23 cases of nausea and vomiting, 20 cases of dizziness, 11 cases of abdominal pain, 7 cases of abdominal distension, 5 cases of allergy and 3 cases of renal function injury. In EG, 13 patients developed nausea and vomiting, 11 developed dizziness, 7 developed abdominal pain, 5 developed abdominal distension, and 3 each developed allergy and renal function injury. The total incidence of adverse reactions in EG was 15.91%, a rate significantly lower than the 27.71% in CG (P<0.05; Table 6).
Table 6.
Incidence of adverse reactions
| Nausea and vomiting | Dizziness | Abdominal pain | Abdominal distension | Allergy | Renal function injury | Spinal cord injury | Total incidence | |
|---|---|---|---|---|---|---|---|---|
| Control group (n=249) | 23 (9.24) | 20 (8.03) | 11 (4.42) | 7 (2.81) | 5 (2.01) | 3 (1.20) | 0 (0) | 69 (27.71) |
| Experimental group (n=264) | 13 (4.92) | 11 (4.17) | 7 (2.65) | 5 (1.89) | 3 (1.14) | 3 (1.14) | 0 (0) | 42 (15.91) |
| χ2 | 3.6531 | 3.3721 | 1.1811 | 0.4720 | 0.6342 | 0.0052 | - | 10.5310 |
| P | 0.0560 | 0.0663 | 0.2772 | 0.4921 | 0.4258 | 0.9425 | - | 0.0012 |
QoL of patients in two groups
The patients’ QoL was scored from physical, role, emotional and social functioning. Differed insignificantly before treatment (P<0.05), these scores increased post treatment, with more distinct increases in EG as compared to CG (P<0.05; Table 7).
Table 7.
Quality of life of patients in two groups
| Physical functioning | Role functioning | Emotional functioning | Social Functioning | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Control group (n=249) | 35.07±4.26 | 46.97±4.62 | 50.56±5.16 | 57.49±4.26 | 55.87±6.02 | 61.84±7.15 | 43.97±5.03 | 52.06±5.63 |
| Experimental group (n=264) | 35.64±4.51 | 55.81±4.38 | 51.17±5.81 | 66.94±4.87 | 55.03±6.34 | 68.48±6.33 | 44.71±5.37 | 60.51±4.67 |
| χ2/t | 1.4696 | 22.2468 | 1.2545 | 23.3357 | 1.5369 | 11.1512 | 1.6085 | 18.5437 |
| P | 0.1423 | <0.0001 | 0.2102 | <0.0001 | 0.1249 | <0.0001 | 0.1083 | <0.0001 |
Discussion
Due to the hidden location of LC and the non-specific early symptoms, patients often lose the optimal timing for surgical treatment; hence, the incidence and mortality of LC remain high [21]. The classic treatment modes of LC are surgery, radiotherapy, chemotherapy, targeted therapy and immunotherapy, while for patients with advanced LC, chemotherapy is necessary [22,23]. The way of chemotherapy is systemic intravenous chemotherapy, but the therapeutic effect is limited due to the low local drug concentration of tumor, entailing a more efficient method. With the advances in medical technology, interventional therapy has become a commonly used minimally invasive treatment in clinical treatment of malignancies. It can directly act on lung tumor lesions through BAI of chemical drugs, with the drug concentration in tumor increased by 2-6 times compared with intravenous chemotherapy [24]. Moreover, arterial catheterization can reduce the binding of the drug to plasma protein, thus improving the bioavailability of the drug [25]. TACE is to inject embolization agent into the corresponding site on the basis of perfusion chemotherapy, which can control the blood supply to the tumor while increasing the residence time of high concentration chemoembolization drug in the tumor, so as to make the tumor ischemia and necrosis, and finally achieve the purpose of relieving airway obstruction.
In this study, 513 patients with LC were treated with BAI or TACE to observe and compare the efficacy. The results showed that the efficacy in the TACE group was significantly higher than that in the BAI group. Similarly, study of Bie et al. reported that for patients with NSCLC who did not meet the criteria or refuse to receive standard treatment, gemcitabine-eluting bead bronchial arterial chemoembolization was a feasible and well-tolerated treatment [26]. This may be because that embolization of tumor blood vessels can reduce the blood supply of the tumor and block tumor growth, thus achieving a better therapeutic effect. In this study, patients intervened by TACE also showed better pulmonary function than those treated with BAI. Due to the compression caused by lymph node and pleural metastasis in advanced LC, it is easy to cause bronchial stenosis or occlusion, complicated with dyspnea and other conditions, which seriously affects pulmonary function [27]. Gopal et al. showed in their study that lung function of patients with NSCLC would be significantly affected after radiotherapy and chemotherapy [28]. In our study, lung function was significantly improved after treatment and tumor marker levels were significantly reduced. This may be due to the fact of the gradual shrinkage and even necrosis of tumors after interventional therapy, which can relieve the dyspnea and venous return disorders caused by tumor invading and compressing bronchus, ameliorate the pulmonary function of patients, and lower the levels of tumor markers [29]. Further, it was found that the adverse mood was effectively relieved and the QoL of patients was significantly improved after TACE treatment. The reason behind the results may be that interventional therapy allows patients to recover some physical strength consumed by tumor and relieve tumor-induced pain. Also, with the recovery of physical strength and the relief of pain, patients’ negative mood is mitigated and the QoL is improved accordingly. Furthermore, we observed notably fewer adverse reactions in patients from TACE group. Due to the large blood flow and strong siphon action in vessels of advanced LC, severe complications related to interventional chemotherapy, such as spinal cord injury, are likely to occur [30]. However, no such severe complications were observed in this study, nor in any other scholars’ studies, [31], indicating the safety of TACE. Similarly, evidence has shown that TACE is beneficial to the implementation of other local sequential therapy after surgery and has a synergistic effect with postoperative radiotherapy [32,33].
This study is the first to demonstrate that TACE improves lung function, mood and quality of life in patients with LC. Nevertheless, Zhu et al. reported that TACE combined with apatinib for hepatocellular carcinoma had good clinical efficacy and can prolong the overall survival of patients [34]. While we were unable to observe the 5-year overall survival of patients due to the time frame of this study (January 2012-January 2020). In addition, further investigation is warranted to determine whether TACE is a factor of prognostic relevance. These deficiencies need to be further studied in the future.
To sum up, TACE is effective in the treatment of patients with LC; It can reduce adverse reactions, relieve patients’ bad mood and bolster their QoL with a high safety profile, which is an approach worthy of clinical promotion.
Disclosure of conflict of interest
None.
References
- 1.Zhao F, Han Y, Liu Z, Zhao Z, Li Z, Jia K. circFADS2 regulates lung cancer cells proliferation and invasion via acting as a sponge of miR-498. Biosci Rep. 2018;38:BSR20180570. doi: 10.1042/BSR20180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 3.Saito S, Espinoza-Mercado F, Liu H, Sata N, Cui X, Soukiasian HJ. Current status of research and treatment for non-small cell lung cancer in never-smoking females. Cancer Biol Ther. 2017;18:359–368. doi: 10.1080/15384047.2017.1323580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz AG, Cote ML. Epidemiology of lung cancer. Adv Exp Med Biol. 2016;893:21–41. doi: 10.1007/978-3-319-24223-1_2. [DOI] [PubMed] [Google Scholar]
- 5.Cheng YI, Gan YC, Liu D, Davies MPA, Li WM, Field JK. Potential genetic modifiers for somatic EGFR mutation in lung cancer: a meta-analysis and literature review. BMC Cancer. 2019;19:1068. doi: 10.1186/s12885-019-6317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latimer KM, Mott TF. Lung cancer: diagnosis, treatment principles, and screening. Am Fam Physician. 2015;91:250–256. [PubMed] [Google Scholar]
- 7.Inage T, Nakajima T, Yoshino I, Yasufuku K. Early lung cancer detection. Clin Chest Med. 2018;39:45–55. doi: 10.1016/j.ccm.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75. doi: 10.1007/978-3-319-40389-2_3. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current Diagnosis and management of small-cell lung cancer. Mayo Clin Proc. 2019;94:1599–1622. doi: 10.1016/j.mayocp.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Yang B, Ren H, Xiao T, Zhang L, Li L, Li M, Wang X, Zhou H, Zhang W. Hsa_circ_0002483 inhibited the progression and enhanced the Taxol sensitivity of non-small cell lung cancer by targeting miR-182-5p. Cell Death Dis. 2019;10:953. doi: 10.1038/s41419-019-2180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Men Y, Feng L, Kang J, Sun X, Yuan M, Jiang W, Hui Z. A current review of dose-escalated radiotherapy in locally advanced non-small cell lung cancer. Radiol Oncol. 2019;53:6–14. doi: 10.2478/raon-2019-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Khan OF, Cusano E, Raissouni S, Pabia M, Haeseker J, Bosma N, Ko JJ, Li H, Kumar A, Vickers MM, Tang PA. Immediate-term cognitive impairment following intravenous (IV) chemotherapy: a prospective pre-post design study. BMC Cancer. 2019;19:150. doi: 10.1186/s12885-019-5349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q, Zhang B, Dong Y, Mo X, Zhang L, Huang W, Jiang H, Xia J, Zhang S. Comparison between intravenous chemotherapy and intra-arterial chemotherapy for retinoblastoma: a meta-analysis. BMC Cancer. 2018;18:486. doi: 10.1186/s12885-018-4406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Q, Cheng X, Yang L, Zhang Q, Chen J, Li T, Shi H. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST) J Thorac Dis. 2014;6:677–683. doi: 10.3978/j.issn.2072-1439.2014.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai Y, Wang X, Zhou K, Su J, Che G. Impact of one-week preoperative physical training on clinical outcomes of surgical lung cancer patients with limited lung function: a randomized trial. Ann Transl Med. 2019;7:544. doi: 10.21037/atm.2019.09.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li CG, Huang XE, Xu L, Li Y, Lu YY. Clinical application of serum tumor associated material (TAM) from non-small cell lung cancer patients. Asian Pac J Cancer Prev. 2012;13:301–304. doi: 10.7314/apjcp.2012.13.1.301. [DOI] [PubMed] [Google Scholar]
- 18.Hung HY, Wu LM, Chen KP. Determinants of quality of life in lung cancer patients. J Nurs Scholarsh. 2018;50:257–264. doi: 10.1111/jnu.12376. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Sun LY, Peng KW, Luo MJ, Deng L, Tang T, You CX. Sleep quality, anxiety and depression in advanced lung cancer: patients and caregivers. BMJ Support Palliat Care. 2020 doi: 10.1136/bmjspcare-2018-001684. bmjspcare-2018-001684. [DOI] [PubMed] [Google Scholar]
- 20.Pompili C, Koller M, Velikova G, Franks K, Absolom K, Callister M, Robson J, Imperatori A, Brunelli A. EORTC QLQ-C30 summary score reliably detects changes in QoL three months after anatomic lung resection for Non-Small Cell Lung Cancer (NSCLC) Lung Cancer. 2018;123:149–154. doi: 10.1016/j.lungcan.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Schabath MB, Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:1563–1579. doi: 10.1158/1055-9965.EPI-19-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naylor EC, Desani JK, Chung PK. Targeted therapy and immunotherapy for lung cancer. Surg Oncol Clin N Am. 2016;25:601–609. doi: 10.1016/j.soc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin. 2020;13:17–33. doi: 10.1016/j.path.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhao JY, Zhang CY. The key points in intravenous chemotherapy and intra-arterial chemotherapy on retinoblastoma treatment. Zhonghua Yan Ke Za Zhi. 2017;53:566–569. doi: 10.3760/cma.j.issn.0412-4081.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan VM, Lang FF, Chen SR, Chen MM, Gumin J, Johnson J, Burkhardt JK, Kan P. Advances in endovascular neuro-oncology: endovascular selective intra-arterial (ESIA) infusion of targeted biologic therapy for brain tumors. J Neurointerv Surg. 2020;12:197–203. doi: 10.1136/neurintsurg-2019-015137. [DOI] [PubMed] [Google Scholar]
- 26.Bie Z, Li Y, Li B, Wang D, Li L, Li X. The efficacy of drug-eluting beads bronchial arterial chemoembolization loaded with gemcitabine for treatment of non-small cell lung cancer. Thorac Cancer. 2019;10:1770–1778. doi: 10.1111/1759-7714.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oki M, Saka H. Airway stenting for patients with airway stenosis because of small cell lung cancer. Clin Respir J. 2018;12:2257–2263. doi: 10.1111/crj.12901. [DOI] [PubMed] [Google Scholar]
- 28.Gopal R, Starkschall G, Tucker SL, Cox JD, Liao Z, Hanus M, Kelly JF, Stevens CW, Komaki R. Effects of radiotherapy and chemotherapy on lung function in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:114–120. doi: 10.1016/s0360-3016(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 29.Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the task force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26–33. doi: 10.1007/s10120-013-0259-5. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai H, Isaka M, Sugawara Y, Okada K, Imai K, Orihashi K, Sueda T. Intra-aortic injection of propofol prevents spinal cord injury during aortic surgery. Eur J Cardiothorac Surg. 2006;29:714–719. doi: 10.1016/j.ejcts.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 31.Shang B, Li J, Wang X, Li D, Liang B, Wang Y, Han X, Dou W, Chen G, Shang J, Jiang S. Clinical effect of bronchial arterial infusion chemotherapy and CalliSpheres drug-eluting beads in patients with stage II-IV lung cancer: a prospective cohort study. Thorac Cancer. 2020;11:2155–2162. doi: 10.1111/1759-7714.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015;1:756–765. doi: 10.1001/jamaoncol.2015.2189. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J, Zeng L, Wu Q, Wang L, Lei J, Luo H, Yi F, Wei Y, Yu J, Zhang W. Stereotactic body radiotherapy combined with transcatheter arterial chemoembolization versus stereotactic body radiotherapy alone as the first-line treatment for unresectable hepatocellular carcinoma: a meta-analysis and systematic review. Chemotherapy. 2019;64:248–258. doi: 10.1159/000505739. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y, Feng B, Mei L, Sun R, Guo C, Zhu J. Clinical efficacy of TACE combined with Apatinib in the treatment of advanced hepatocellular carcinoma. J BUON. 2019;24:608–614. [PubMed] [Google Scholar]


