Abstract
Objective: This research aimed to probe into the effect of miR-145-5p in psoriasis by regulating Wnt/β-catenin. Methods: A total of 45 psoriasis patients treated in our hospital were enrolled into an observation group (OG), and other 40 healthy individuals in physical examination over the same period were enrolled into a control group (CG). miR-145-5p in both groups was quantified, and its value in diagnosis and recurrence prediction of psoriasis was analyzed. Additionally, miR-145-5p was transfected into HaCat cells to analyze the biological behaviors of transfected cells, and factors for Wnt/β-catenin pathway inhibition were injected into cells to detect its protein expression in the cells, so as to verify the regulation of miR-145-5p on the Wnt/β-catenin pathway. Results: with low expression in the serum of psoriasis patients (P<0.05), miR-145-5p was of great application value for diagnosis and recurrence prediction. In the inhibition group, miR-145-5p increased (P<0.05), while Wnt/β-catenin pathway-related proteins decreased (P<0.05). Compared with untreated HaCat cells, the protein expression in HaCat cells treated with XAV-939 decreased (P<0.05). There was no notable difference between the miR-145-5p-mimics+XAV-939 group and the empty vector group in cell proliferation, apoptosis rate, and expression of Wnt3a, Wnt5a, and β-catenin proteins (all P>0.05); but compared with both groups, the miR-145-5p-mimics group showed lower proliferation activity, higher apoptosis rate, and higher expression of Wnt3a, Wnt5a, and β-catenin proteins (all P<0.05). Conclusion: Up-regulating miR-145-5p can activate the Wnt β-catenin signal pathway, thus inhibiting psoriasis progression.
Keywords: MiR-145-5p, Wnt, β-catenin, psoriasis
Introduction
Psoriasis, also named psora, is an extremely common type of chronic inflammation [1]. Characterized by a long course of disease and a high recurrence rate, it can be hard to cure completely [2]. According to statistics, psoriasis has a global incidence second only to diabetes mellitus and enterogastritis in chronic inflammatory diseases, and is mainly seen in young adults [3]. The occurrence of psoriasis varies greatly in different regions and seasons, and the disease may attack any part of the body [4]. Therefore, a deep understanding of the pathogenesis of psoriasis is of great significance for future clinical prevention and treatment. Recently, some studies have pointed out that the development of psoriasis may be relevant to cellular physiological activities in the body such as heredity, infection and immune abnormality [5], but the specific mechanism has not been effectively confirmed.
Currently, microRNA is a hot spot in clinical research, and its regulatory effect on cells has been unanimously recognized clinically, especially in tumor diseases [6]. A growing number of studies have pointed out that microRNA may be relevant to the development of psoriasis [7]. For example, Soonthornchai W et al. [8] suggested that miR-155 could mitigate the inflammatory response of psoriasis, and Ghafouri-Fard S et al. [9] found that miR-876-5p could inhibit angiogenesis in cases with psoriasis. miR-145-5p is a kind of microRNA that is extremely sensitive to the inflammatory response, and its role in inflammatory diseases such as melanoma has been verified [10,11]. According to screening for epigenetic genes of psoriasis by Shao et al. [12], miR-145-5p was abnormally expressed. Therefore, we suspected that miR-145-5p might also have a crucial role in the development of psoriasis. One study by Yan et al. [13] has confirmed our conjecture. Research has revealed that miR-145-5p can promote the inflammatory response of skin of psoriasis patients, but it has not uncovered its exact action pathway. According to relevant references, the activation of the Wnt/β-catenin pathway is directly related to the inflammatory response [14], and there are also studies showing that the pathway is of great significance in cases with psoriasis [15]. Moreover, miR-145-5p may play a role in the development of rheumatoid arthritis through the Wnt/β-catenin signal pathway [16]. Hence, we suspected that the function of miR-145-5p in patients with psoriasis is also related to the Wnt/β-catenin pathway. In order to verify our conjecture and further confirm the mechanism of miR-145-5p in psoriasis, we carried out an experimental analysis, thereby providing a reliable theoretical basis for future clinical diagnosis and treatment of psoriasis.
Materials and methods
Collection of case data
A total of 85 individuals enrolled in our study from the First Affiliated Hospital of Zhejiang Chinese Medical University were grouped. Thereinto, 45 patients who had been diagnosed with psoriasis were assigned to an observation group (OG), while another 40 healthy individuals who had received a comprehensive physical examination in our hospital and whose examined items were all normal were assigned to a control group (CG). All the above enrolled participants were informed and they provided written informed consents. This experiment was been approved by the ethics committee of our hospital.
Exclusion and inclusion criteria
Inclusion criteria were as follows: all patients in the OG were treated in our hospital for the first time, and all of them were diagnosed by the laboratory and dermatologists in our hospital and were required to receive subsequent treatment. In addition, all participants in had complete case data and agreed to participate in this research. All indexes of physical examination of individuals in the CG were normal.
Exclusion criteria were as follows: patients with other inflammatory diseases, immunological diseases, tumor diseases, organ dysfunction, drug allergy, or other adverse reactions; those with low treatment compliance.
Determination methods
qRT-PCR assay
Fasting venous blood (4 mL) was sampled from each participant in both groups in the morning, let stand at room temperature for 30 min. Then, it was centrifugated for 10 min at 400×g to obtain the upper serum for later analyses. The serum was added with 1 mL Trizol (TRIzolTM LS Reagent, Invitrogen, USA) to extract total RNA. Subsequently, the total RNA was reversely transcribed into cDNA under the instructions of the reverse transcription kit (PrimeScriptTM RT reagent Kit, TaKaRa, Japan). The reaction conditions were as follows: 25°C for 10 min, 48°C for 30 min, 95°C for 5 min. After reverse transcription, amplification was carried out under the instructions of the amplification test kit: pre-denaturation for 15 min, followed by 40 cycles of denaturation at 95°C for 15 sec, annealing at 56°C for 15 sec, extension at 68°C for 30 sec. Three duplicate wells were set for each reaction. The primer sequences were designed by American Invitrogen (Table 1).
Table 1.
Primer sequences
| Forward (5’-3’) | Reverse (5’-3’) | |
|---|---|---|
| miR-145-5p | CCTTGTCCTCACGGTCCAGT | AAC-CATGACCTCAAGAACAGTATTT |
| U6 | GCT TCGGCAGCACATATACTAAAAT | CGCTTCA CGAATTTGCGTGTCAT |
Cell Data
Immortalized human keratinocytes (HaCat) were purchased from BeNa Culture Collection, a Chinese agent of American Type Culture Collection (ATCC). They were incubated in Dulbecco’s modified eagle (DMEM) (10 U/mL penicillin, 10 μg/mL streptomycin) under 5% CO2 at 37°C.
Cell transfection
HaCat cells were seeded into a 6-well plate at 1×105 cell/well, followed by incubation at 37°C under 5% CO2 overnight. When the cells grew to reach confluency of 60%-80%, miR-145-5p mimics, miR-145-5p-inhibition, or miR-NC were transfected into the cells.
Western blotting assay
Transfected HaCat cells were lysed with Radio Immunoprecipitation Assay (RIPA) buffer (Thermo Scientific). The protein concentration was adjusted to 4 μg/μL. Subsequently, the protein was isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to a PVDF membrane. The membrane was immersed for 1 h in 5% skim milk, and the Polyvinylidene Fluoride (PVDF) membrane containing the protein was added with primary antibody (1:1000), slightly shaken, and then incubated at 4°C over night. Afterwards, the membrane was cleaned with tris-buffered saline with tween 20 (TBST) three times, and then added with HRP-labeled goat anti-rabbit IgG secondary antibody (1:4000), followed by 2 h-incubation at room temperature. The membrane was then taken out, and cleaned with TBST three times again, 5 min/time. Finally, the membrane was developed in the dark, blotted with a filter paper to eliminate excess liquid, and visualized with luminescence via ECL and was developed.
Cell counting kit-8 (CCK8)
After transfection, the cells were seeded into a 96-well plate (2×103 cells/well), 200 µL cells in each well. After 24, 48, 72, and 96 h of incubation, each well was given 20 µL CCK-8 solution (Beyotime Corporation), followed by 1 h-incubation. In the end, the optical density at 450 nm was measured via a microplate reader.
Flow cytometry
The cells were trypsinized by 0.25% trypsin, and then cleaned with PBS twice, and mixed with 100 μL binding buffer to prepare 1×106 cells/mL suspension. AnnexinV-FITC and PI were added into the suspension successively, and the suspension was then incubated in the dark at room temperature for 5 min, and detected via the FC500MCL flow cytometry. Each sample was measured three times and the data were averaged as results.
Statistical analyses
In our research, data were statistically processed using SPSS 22.0. The calculated average values of experimental results were expressed as the mean ± standard deviation. The inter-group comparison was analyzed via the independent-samples t test, and comparison between multiple groups was assessed via the one-way ANOVA and LSD post-hoc test. Data at multiple time points were compared by repeated measures analysis of variance/Bonferroni post-hoc test. Receiver operating characteristic (ROC) curves were adopted for analyzing predictive value. P<0.05 suggests a significant difference.
Results
Clinical significance of miR-145-5p for psoriasis
Quantification of miR-145-5p in the peripheral blood of the OG and the CG revealed that the miR-145-5p expression in the former was lower than that in the latter (P<0.05) (Figure 1A). Additionally, analysis of the ROC curve for miR-145-5p in diagnosing psoriasis revealed that when the cut-off was 0.445, the area-under-the-curve (AUC), sensitivity, and specificity of miR-145-5p were 0.876, 75.00%, and 91.11%, respectively (all P<0.001) (Figure 1B; Table 2).
Figure 1.
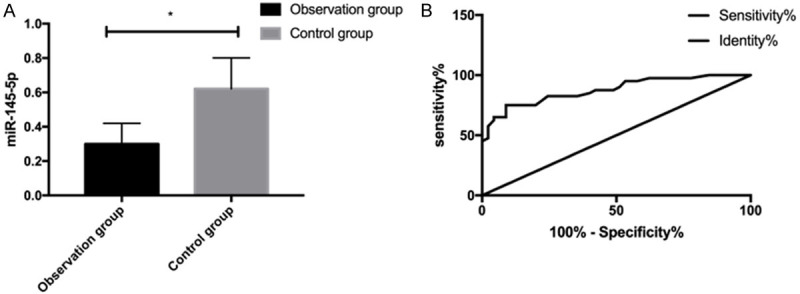
Clinical significance of miR-145-5p for psoriasis. A. Expression of miR-145-5p in peripheral blood of both groups. B. ROC curve of peripheral blood miR-145-5p for diagnosing psoriasis. * indicates P<0.05.
Table 2.
Diagnosis efficiency of miR-145-5p for psoriasis
| miR-145-5p | |
|---|---|
| AUC | 0.876 |
| Std. Error | 0.038 |
| 95% CI | 0.801-0.950 |
| Cut-off | >0.445 |
| Sensitivity (%) | 75.00 |
| Specificity (%) | 91.11 |
| Youden index (%) | 76.11 |
| P-value | <0.001 |
Predictive value of miR-145-5p for recurrence of psoriasis
We followed up patients in the OG for 3 months, and the follow-up rate was 100%. It was found that 17 patients suffered from mild or severe recurrence, and the other 28 did not. We also determined the changes of miR-145-5p in patients, finding that the miR-145-5p expression in patients with recurrence was lower than that in those without recurrence (P<0.05) (Figure 2A). The predictive value of miR-145-5p in diagnosing psoriasis recurrence was analyzed through ROC curves. It came out that when the cut-off value was 0.445, the AUC, sensitivity, and specificity of miR-145-5p for predicting psoriasis were 0.783, 60.71%, and 94.12%, respectively (all P<0.001) (Figure 2B; Table 3).
Figure 2.
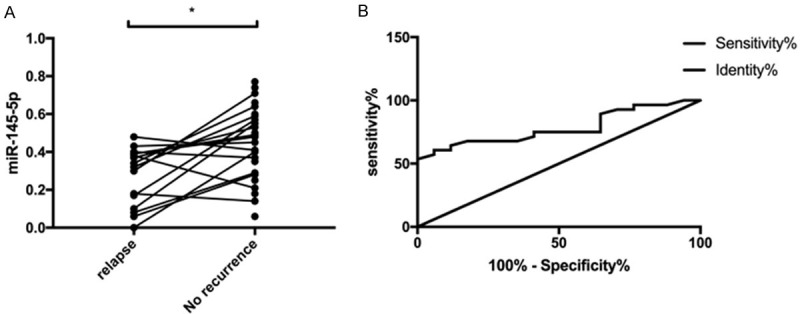
Predictive value of miR-145-5p for recurrence of psoriasis. A. Changes of miR-145-5p expression in patients with recurrence and those without recurrence. B. ROC curve of miR-145-5p for predicting recurrence of psoriasis. * indicates P<0.05.
Table 3.
Predictive value of miR-145-5p for recurrence of psoriasis
| miR-145-5p | |
|---|---|
| AUC | 0.783 |
| Std. Error | 0.068 |
| 95% CI | 0.650-0.915 |
| cut-off | >0.440 |
| Sensitivity (%) | 60.71 |
| Specificity (%) | 94.12 |
| Youden index (%) | 54.83 |
| P-value | <0.001 |
Influence of miR-145-5p on HaCat cells
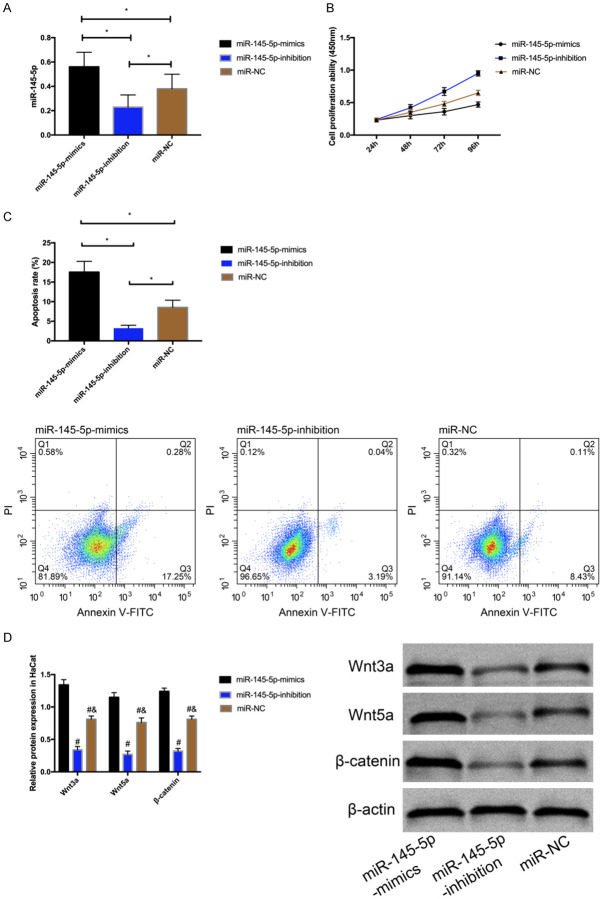
The miR-145-5p expression in cells was detected after transfection. It was found that miR-145-5p in the over-expression group increased, and decreased in the inhibition group (P<0.05) (Figure 3A). According to the determination of the biological behaviors of cells, proliferation ability of miR-145-5p in the inhibition group increased (P<0.05) (Figure 3B), and Wnt/β-catenin pathway-related proteins (P<0.05) (Figure 3D) and apoptosis (P<0.05) (Figure 3C) decreased. Compared with inhibition group and blank group, the proliferation ability of miR-145-5p in the over-expression group decreased (P<0.05), and Wnt/β-catenin pathway-related proteins (P<0.05) and apoptosis increased (P<0.05).
Figure 3.
Effect of miR-145-5p on HaCat cells. A. Expression of miR-145-5p after transfection. B. Proliferation of HaCat cells. C. Apoptosis of HaCat cells. D. Expression of Wnt/β-catenin pathway-related proteins in HaCat cells. * indicates P<0.05. # indicates P<0.05 vs. the miR-145-5p-mimics group; & indicates P<0.05 vs. the miR-145-5p-inhibition group.
Expression of Wnt3a, Wnt5a, and β-catenin proteins in HaCat cells treated with XAV-939
Ten μL XAV-939 (Wntβ-catenin signal pathway selective inhibitor) was used to treat the untreated HaCat cells for 48 h, and then Wnt3a, Wnt5a, and β-catenin proteins in the treated cells were quantified. It was found that HaCat cells treated with XAV-939 showed lower expression of Wnt3a, Wnt5a, and β-catenin proteins than untreated HaCat cells (all P<0.05) (Figure 4A-C).
Figure 4.
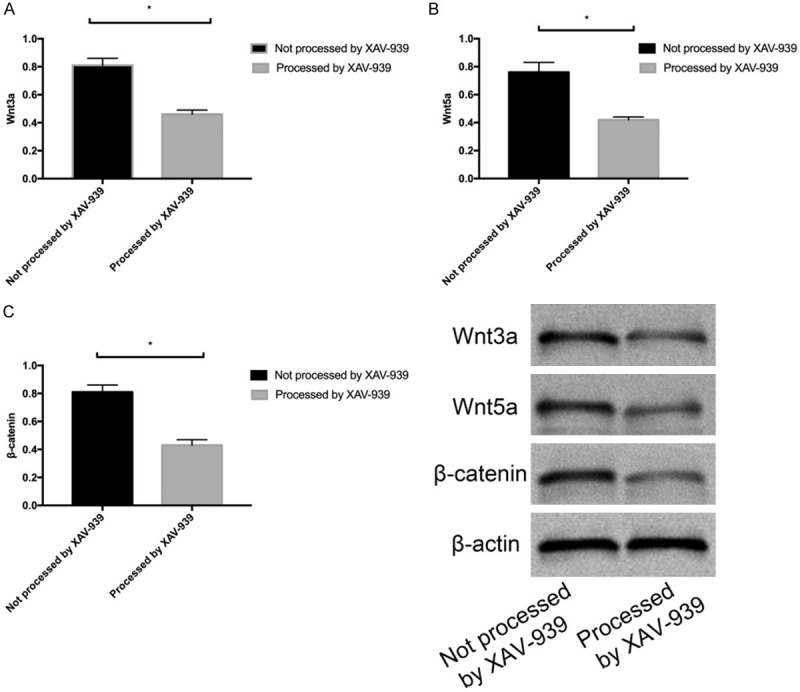
Expression of Wnt3a, Wnt5a, and β-catenin proteins in HaCat cells treated with XAV-939. A. Expression of Wnt3a in HaCat cells treated with XAV-939. B. Expression of Wnt5a in HaCat cells treated with XAV-939. C. Expression of β-catenin in HaCat cells treated with XAV-939. * indicates P<0.05.
Influence of miR-145-5p on HaCat cells by Wntβ-catenin signal pathway
We transfected miR-145-5p-mimics+XAV-939, miR-145-5p-mimics, or NC into HaCat cells and determined the biological behaviors of transfected cells. It came out that there was no marked difference between the miR-145-5p-mimics+XAV-939 group and the empty vector group in cell proliferation, apoptosis rate, and expression of Wnt3a, Wnt5a, and β-catenin proteins (all P>0.05) (Figure 5A-C); but compared with both groups, the miR-145-5p-mimics group showed lower proliferation activity, higher apoptosis rate, and higher expression of Wnt3a, Wnt5a, and β-catenin proteins (all P<0.05).
Figure 5.
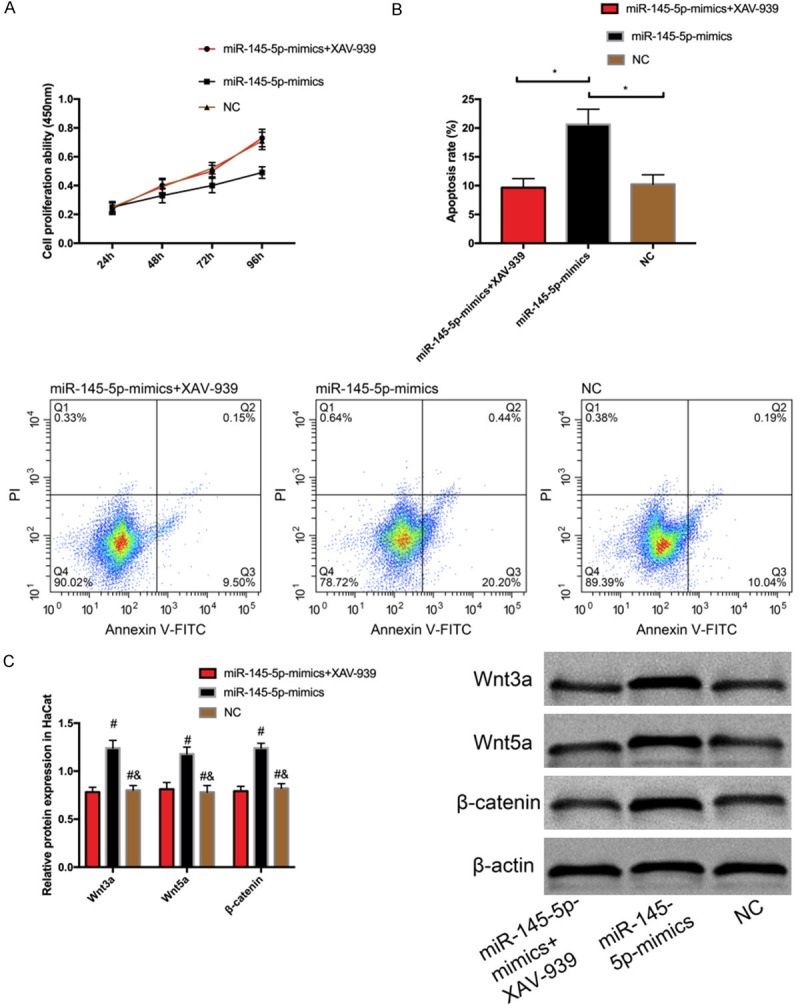
Effect of miR-145-5p on HaCat cells by Wntβ-catenin signal pathway. A. Cell proliferation. B. Cell apoptosis. C. Expression of Wnt3a, Wnt5a, and β-catenin proteins. * indicates P<0.05; # indicate P<0.05 vs. miR-145-5p-mimics+XAV-939 group; & indicates P<0.05 vs. miR-145-5p-inhibition group.
Discussion
Psoriasis is a common chronic inflammatory malady of the skin, increasingly considered as a systemic inflammatory disease [17]. Experts point out that psoriasis can be complicated with many diseases, among which psoriatic arthritis is a well-known complication [18]. A large number of references suggest that under continuous change of the environment and other factors, psoriasis has an influence on metabolic and cardiac diseases, gastrointestinal diseases, renal diseases, and malignant tumors [19], threatening the life and health of patients. However, the pathogenesis of the disease is still under investigation; so, fully understanding its pathogenesis is the key to clinical prevention and treatment of psoriasis and its complications in the future. We carried out the following research, and the results are as follows:
Firstly, we quantified miR-145-5p in the peripheral blood of the OG and the CG, and found that the former showed lower miR-145-5p expression than the latter. The results not only verified the related research of factors in chronic inflammatory diseases mentioned in the introduction, but also confirmed that abnormally expressed factors were involved in the development of inflammatory disease. According to ROC curve analysis, when the cut-off value was 0.445, the AUC, sensitivity and specificity of miR-145-5p for diagnosing psoriasis were 0.876, 75.00% and 91.11%, respectively. This suggests that miR-145-5p has a good diagnostic value for psoriasis and can be used as a serum marker to assist future clinical diagnosis. Then, we followed up patients with psoriasis for 3 months, and found that 17 patients suffered from recurrence, and the other 28 did not. We also quantified miR-145-5p, finding that patients with recurrence showed down-regulated miR-145-5p than those without it. The results further verify abnormal miR-145-5p in cases with psoriasis. Furthermore, we adopted ROC curves to analyze the predictive value of miR-145-5p in psoriasis recurrence, finding that miR-145-5p also had certain influence on prognosis; this indicated that it could not only be used as a future clinical screening index, but also be a possible therapeutic target for the disease. Therefore, miR-145-5p is quite marked for prevention and treatment of psoriasis. Psoriasis is a chronic inflammatory disease, with high recurrence rate, which can trouble patients all year round [20]. It not only attacks local skin, but also often causes systemic skin lesions. In severe cases, it can even induce various complications, seriously threatening the health of patients [21]. In recent years, research has confirmed that many endogenous small non-coding RNA (miRNA) are abnormally expressed in cases with chronic inflammatory diseases [22]. miR-145-5p has been found to be a crucial part in inflammatory diseases such as rheumatoid arthritis and melanoma [23]. Therefore, in order to more deeply explore the influence of miR-145-5p on psoriasis, we determined the biological behaviors of HaCat cells after transfecting them with miR-145-5p. It came out that inhibiting miR-145-5p expression enhanced the proliferation activity of HaCat cells, but notably decreased their apoptosis. According to the above experimental results, it can be inferred that miR-145-5p is an inhibitory part in psoriasis. It is also in line with the experimental conclusion drawn by Ye D et al. [24] that microRNA-145 inhibits the growth of laryngeal squamous cell carcinoma through targeting PI3K/Akt signal pathway, and the conclusion can support our results. It also suggests that miR-145-5p may be a potential therapeutic target for psoriasis, but our research has not carried out any further analysis, which will be the focus of our future research. Moreover, we quantified Wntβ-catenin pathway-related proteins (Wnt3a, Wnt5a, and β-catenin), finding that the miR-145-5p over-expression group showed up-regulated Wntβ-catenin signal pathway-related proteins, while the miR-145-5p inhibition group showed down-regulated proteins. The Wntβ-catenin signal pathway plays a crucial role in maintaining tissue balance, regeneration, and repair [25]. Previous research has also confirmed that the Wnt/β-catenin signal pathway is of great significance in psoriasis [26]. According to our research results, we preliminarily believed that the decrease of miR-145-5p might reduce the activation of Wntβ-catenin signal pathway, thus inducing the progression of psoriasis. To confirm our above conjecture, we first injected a Wntβ-catenin selective inhibitor into untransfected HaCat cells, and then detected the relative expression of Wnt3a, Wnt5a and β-catenin proteins in the HaCat cells. It came out that HaCat cells treated with XAV-939 showed lower expression of Wnt3a, Wnt5a, and β-catenin proteins than untreated HaCat cells. We further transfected miR-145-5p-mimics+XAV-939, miR-145-5p-mimics, or NC into HaCat cells, and determined the biological behaviors of transfected cells. It came out that there was no obvious difference between the miR-145-5p-mimics+XAV-939 group and the empty vector group in cell proliferation, apoptosis rate, and expression of Wnt3a, Wnt5a, and β-catenin proteins (all P>0.05), but compared with both groups, the miR-145-5p-mimics group showed lower proliferation activity, higher apoptosis rate, and higher expression of Wnt3a, Wnt5a, and β-catenin proteins. The results implied that the influence of transfecting miR-145-5p-mimics on biological behaviors of HaCat cells and protein expression were completely reversed by injection of XAV-939, which verified that miR-145-5p can regulate the Wntβ-catenin signal pathway, but how this pathway is regulated remains to be further investigated.
To sum up, we have come to the conclusion that up-regulating miR-145-5p can activate the Wntβ-catenin signal pathway and thus inhibit psoriasis progression.
However, this research still has some limitations that need to be addressed by improved experiments. For example, only a small number of patients are enrolled, so we need to include more case data for analysis in the follow-up study. Additionally, due to the short experimental period, we are unable to judge the influence of miR-145-5p on the long-term prognosis of psoriasis patients. We will conduct corresponding experimental analysis on the above limitations as soon as possible to obtain more comprehensive and better experimental results for clinical reference.
Disclosure of conflict of interest
None.
References
- 1.Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, Gelfand JM. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20:1475. doi: 10.3390/ijms20061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278–285. [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27–40. doi: 10.1016/j.jaad.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 5.Conrad C, Gilliet M. Psoriasis: from pathogenesis to targeted therapies. Clin Rev Allergy Immunol. 2018;54:102–113. doi: 10.1007/s12016-018-8668-1. [DOI] [PubMed] [Google Scholar]
- 6.Wu R, Zeng J, Yuan J, Deng X, Huang Y, Chen L, Zhang P, Feng H, Liu Z, Wang Z, Gao X, Wu H, Wang H, Su Y, Zhao M, Lu Q. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest. 2018;128:2551–2568. doi: 10.1172/JCI97426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava A, Nikamo P, Lohcharoenkal W, Li D, Meisgen F, Xu Landen N, Stahle M, Pivarcsi A, Sonkoly E. MicroRNA-146a suppresses IL-17-mediated skin inflammation and is genetically associated with psoriasis. J Allergy Clin Immunol. 2017;139:550–561. doi: 10.1016/j.jaci.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Soonthornchai W, Tangtanatakul P, Meephansan J, Ruchusatsawat K, Reantragoon R, Hirankarn N, Wongpiyabovorn J. Down-regulation of miR-155 after treatment with narrow-band UVB and methotrexate associates with apoptosis of keratinocytes in psoriasis. Asian Pac J Allergy Immunol. 2019 doi: 10.12932/AP-031218-0451. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Ghafouri-Fard S, Eghtedarian R, Taheri M, Rakhshan A. The eminent roles of ncRNAs in the pathogenesis of psoriasis. Noncoding RNA Res. 2020;5:99–108. doi: 10.1016/j.ncrna.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan M, Zhang L, You F, Zhou J, Ma Y, Yang F, Tao L. MiR-145-5p regulates hypoxia-induced inflammatory response and apoptosis in cardiomyocytes by targeting CD40. Mol Cell Biochem. 2017;431:123–131. doi: 10.1007/s11010-017-2982-4. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Gao G, Yan D, Chen X, Yao X, Guo S, Li G, Zhao Y. Effects of miR-145-5p through NRAS on the cell proliferation, apoptosis, migration, and invasion in melanoma by inhibiting MAPK and PI3K/AKT pathways. Cancer Med. 2017;6:819–833. doi: 10.1002/cam4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Liu R, Wang Q, Chang W, Zhou L, Li J, Zhang K. Characterisation of the circular RNA landscape in mesenchymal stem cells from psoriatic skin lesions. Eur J Dermatol. 2019;29:29–38. doi: 10.1684/ejd.2018.3483. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Yu Q, Gong Y, Liu Z, Xu H, Wang Y, Shi Y. Construction of a lncRNA-miRNA-mRNA network to determine the regulatory roles of lncRNAs in psoriasis. Exp Ther Med. 2019;18:4011–4021. doi: 10.3892/etm.2019.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Smits R, Hao H, He C. Wnt/beta-catenin signaling in liver cancers. Cancers (Basel) 2019;11:926. doi: 10.3390/cancers11070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X, Yan N, Li Z, Hua Y, Chen W. FGF19 sustains the high proliferative ability of keratinocytes in psoriasis through the regulation of Wnt/GSK-3beta/beta-catenin signalling via FGFR4. Clin Exp Pharmacol Physiol. 2019;46:761–769. doi: 10.1111/1440-1681.13103. [DOI] [PubMed] [Google Scholar]
- 16.Dinesh P, Kalaiselvan S, Sujitha S, Rasool M. MiR-145-5p mitigates dysregulated Wnt1/beta-catenin signaling pathway in rheumatoid arthritis. Int Immunopharmacol. 2020;82:106328. doi: 10.1016/j.intimp.2020.106328. [DOI] [PubMed] [Google Scholar]
- 17.Gisondi P, Fostini AC, Fossa I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol. 2018;36:21–28. doi: 10.1016/j.clindermatol.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Kridin K, Shani M, Schonmann Y, Fisher S, Shalom G, Comaneshter D, Batat E, Cohen AD. Psoriasis and hidradenitis suppurativa: a large-scale population-based study. J Am Acad Dermatol. 2018 doi: 10.1016/j.jaad.2018.11.036. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 2017;49:1–8. doi: 10.1016/j.coi.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the Immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19:179. doi: 10.3390/ijms19010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benhadou F, Mintoff D, Schnebert B, Thio HB. Psoriasis and microbiota: a systematic review. Diseases. 2018;6:47. doi: 10.3390/diseases6020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiaoyan W, Pais EM, Lan L, Jingrui C, Lin M, Fordjour PA, Guanwei F. MicroRNA-155: a novel armamentarium against inflammatory diseases. Inflammation. 2017;40:708–716. doi: 10.1007/s10753-016-0488-y. [DOI] [PubMed] [Google Scholar]
- 23.Di Munno O, Ferro F. The effect of biologic agents on bone homeostasis in chronic inflammatory rheumatic diseases. Clin Exp Rheumatol. 2019;37:502–507. [PubMed] [Google Scholar]
- 24.Ye D, Zhou C, Deng H, Lin L, Zhou S. MicroRNA-145 inhibits growth of laryngeal squamous cell carcinoma by targeting the PI3K/Akt signaling pathway. Cancer Manag Res. 2019;11:3801–3812. doi: 10.2147/CMAR.S199291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang P, Yan R, Zhang X, Wang L, Ke X, Qu Y. Activating Wnt/beta-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol Ther. 2019;196:79–90. doi: 10.1016/j.pharmthera.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Tian F, Mauro TM, Li Z. The pathological role of Wnt5a in psoriasis and psoriatic arthritis. J Cell Mol Med. 2019;23:5876–5883. doi: 10.1111/jcmm.14531. [DOI] [PMC free article] [PubMed] [Google Scholar]