Abstract
This study explored the effects of coenzyme Q10 (CoQ10) on the testicular functions of male mice exposed to cigarette smoke. Eight-week-old BALB/c male mice were divided into the following groups: the AV group (air with a vehicle), the AQ group (air with CoQ10), the SV group (smoke with a vehicle), and the SQ group (smoke with CoQ10). The results showed that the CoQ10 concentrations in the sera and testes were decreased in the groups subjected to smoke but they were improved after the administration of CoQ10. Neither smoke nor CoQ10 supplementation affected the serum or testis testosterone concentrations. Regarding the antioxidant system in the testis, the exposure to smoke induced malondialdehyde and hydrogen peroxide production and decreased the catalase and glutathione peroxidase activities. Oral CoQ10 administration reversed the oxidative damage. In apoptosis, the cytochrome c, c-caspase 9, and c-caspase 3 proteins were increased in the groups exposed to smoke but they were decreased after the CoQ10 administration. In mitochondrial biogenesis, smoke exposure led to decreases in the PGC1-α, NRF1, and NRF2 levels, but CoQ10 increased the expressions of these proteins. Additionally, oral CoQ10 administration improved the mitochondrial copy numbers that were reduced following the exposure to smoke. In summary, CoQ10 administration reduces smoke-induced testicular damage by regulating the antioxidant capacity, the cell apoptosis, the mitochondrial biogenesis, and the copy numbers in the testes.
Keywords: Smoke, coenzyme Q10, oxidative stress, spermatogenesis, male infertility
Introduction
According to the World Health Organization (WHO), the prevalence of cigarette smoking was 36.1% in males and 6.8% in females worldwide among persons over 15 years old in 2015 [1]. A report from the American Medical Association indicated that East Asia is a region with a high consumption and a high prevalence of smoking [2]. In Taiwan, the prevalence of cigarette smoking was reported to be 30% in males, most of whom were between 26 and 45 years old. A statistical report from the Department of Household Registration in Taiwan showed that the average age at which males had a child was 34.5 years old in 2018, and this increased yearly [3,4]. Some studies have found that male smokers have a poorer sperm quality than non-smokers. Approximately 34.4% of male smokers suffer from infertility [5,6]. According to data from the Ministry of Health and Welfare in Taiwan, 10-15% of people are infertile, and in approximately 40% of cases, infertility in couples is due to factors related to the male gender [7].
Cigarettes contain a variety of toxic chemicals. While they burn, nearly 4,000 compounds are released, including nicotine, cotinine, radioactive materials, benzopyrene, polycyclic aromatic hydrocarbons (PAHs), and heavy metals. These compounds may decrease the antioxidant levels and increase the reactive oxygen species levels (ROS) in the body [8]. Studies have shown that 30-80% of male infertility is caused by ROS-mediated damage to sperm [9]. Recent research suggests that smoking-induced toxic-compound ingestion may lead to a decreased antioxidant capacity, apoptosis and DNA damage, and may affect cell function, resulting in sperm and testicular damage [10].
Coenzyme Q10 (CoQ10), which mainly acts on mitochondria, is an electron carrier and can decrease oxidative stress [11]. Mitochondria have many functions, including the synthesis of steroid hormones, energy generation, and the regulation of calcium signaling and cell apoptosis [12]. Mitochondrial biogenesis is the process of growth and the division of pre-existing mitochondria [13]. The measurement of the mitochondria DNA (mtDNA) copy number is a method of evaluating overall mitochondrial health [14]. Studies have indicated that changes in mitochondrial function and integrity may affect the mitochondrial structure, DNA, transcription and proteins, potentially leading to sperm damage [15].
A recent study that evaluated male smokers showed that they had lower concentrations of CoQ10 than non-smokers [16]. Other studies have shown that CoQ10 supplementation can improve the sperm quality in infertile men by regulating the antioxidant capacity [17-19]. Therefore, the aim of this study was to explore the effects of CoQ10 on testicular antioxidant capacity, cell apoptosis, inflammation, and spermatogenesis in male mice exposed to cigarette smoke.
Materials and methods
Animal groups and the experimental design
The entire study was approved by the Institutional Animal Care and Use Committee of the Taipei Medical University (IACUC; ethical code number: LAC-2018-0005), and all the experiments were performed in accordance with the Taipei Medical University guidelines. Fortyeight 8-week-old male BALB/c mice were divided into four groups: the AV group (air with a vehicle), the AQ group (air with CoQ10), the SV group (smoke with a vehicle), and the SQ group (smoke with CoQ10). In the groups exposed to smoke, the animals were exposed to 4 cigarettes per day per six mice. One cigarette (Marlboro) contains 10 mg of tar and 0.8 mg of nicotine. The smoke exposure method was carried out as described in a previous study [20]. Corn oil and CoQ10 (oxidative form, dissolved in corn oil, 10 mg/kg/day) were administered once daily by gavage. Both the smoke and CoQ10 were administered five days per week. After eight weeks, the mice were sacrificed. Serum samples were obtained for the biochemical analysis. Their testes were collected for an analysis of their spermatogenesis, sex hormone concentrations, antioxidant capacities, cell apoptosis, and inflammation. Their epididymides were weighed, and their vas deferens were also weighed to assess their sperm quality.
Sperm analysis
Semen was collected from the vas deferens and diluted with 1X phosphate-buffered saline (PBS) to assess the sperm motility, count, and morphology. Briefly, the sperm motility was determined by calculating the ratio of motile and nonmotile spermatozoa in 4 fields of a Neubauer hemocytometer under a microscope. The sperm count was calculated using a TC20 Automated Cell Counter (Bio-Rad). To assess the sperm morphology, sperm smears were stained with hematoxylin and eosin, and after the slides had been air-dried, they were examined under a microscope. The sperm morphology was evaluated by calculating the percentage of normal sperm.
Hematoxylin and eosin staining
After the mice were sacrificed, the testis tissue was placed in 10% formaldehyde for two days, then stained with hematoxylin and eosin at the Department of Pathology, Cardinal Tien Hospital. The maturity of the testicular cells was determined from the mean seminiferous tubule diameter (MSTD) and Johnsen’s mean testicular biopsy score (MTBS) [21,22].
Western blot analysis
After the mice were sacrificed, their testes were collected. Approximately 50 mg of testis tissue was homogenized using a RIPA buffer (Thermo, #89900, USA), a protease inhibitor, and a phosphatase inhibitor, and the lysate was centrifuged at 14,000 rpm for 15 minutes. The supernatant was collected to serve as a protein sample. The samples were then electrophoresed and transferred onto a PVDF membrane (Millipore, USA). The protein expressions on the membranes were determined using ECL solution (Bio-Rad, USA). The expressions of the testosterone biogenesis-related proteins (CYP11A1, CYP17A1, 3β-HSD, 17β-HSD, and StAR), the apoptosis-related proteins (caspase 8, caspase 9, caspase 3, Bax, Bcl-xL, cytochrome c, and PARP), the inflammation-related proteins (TNF-α, NFκB, PPARγ, and IL-6), and the mitochondrial biogenesis-related proteins (PGC1-α, NRF1, NRF2, PPARα, and TFAM) were measured.
Antioxidant capacity
We analyzed the superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), malondialdehyde (MDA), and hydrogen peroxide (H2O2) levels to determine the antioxidant capacity in the testes. The SOD, CAT, and GPx concentration s were measured using a superoxide dismutase assay kit (Cayman, #706002, USA), a catalase assay kit (Cayman, #707002, USA), and a glutathione peroxidase assay kit (Cayman, #703102, USA), respectively. We used a TBARS assay kit (Cayman, #10009055, USA) to measure the MDA concentration, a marker of lipid peroxidation. With regards to the free radicals, the H2O2 concentration was determined using a hydrogen peroxide colorimetric/fluorometric assay kit (MyBioSource, MBS841818, UK).
The coenzyme Q10 concentration
To assess whether CoQ10 was consumed within the body, we employed a mouse CoQ10 Elisa kit (MyBioSource, MBS9346778, UK) to analyze the CoQ10 levels in the sera and testes.
The sex hormone concentrations
The testosterone levels in the sera and testes were measured using a testosterone Elisa kit (Cayman, #582701, USA). The follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in the testes were estimated using a mouse FSH Elisa kit (MyBioSource, MBS2507988, British) and a mouse LH Elisa kit (MyBioSource, MBS2514287, U.K.), respectively.
MtDNA copy number analysis
The mitochondrial DNA was extracted from the testes using a Mitochondrial DNA Isolation Kit (BioVision, #K280-50, USA), and the relative testis mtDNA copy numbers were determined using quantitative polymerase chain reactions (qPCR). The mtDNA levels were evaluated using a probe against mitochondrial gene ND2. GAPDH served as the loading control. The primer sequences for the mitochondrial ND2 gene were: 5’-TCC TAT CAC CCT TGC CAT CA-3’ (forward); 5’-GCT GTT GCT TGT GTG ACG AA-3’ (reverse).
Statistical analysis
Values reported are the means ± standard deviation. The differences among the groups were analyzed using two-way analyses of variance (ANOVA) followed by Duncan’s new multiple range post hoc tests. The data were analyzed using SAS 9.4, and the values were considered significant at P<0.05.
Results
The body, testis, epididymis, and vas deferens weights
The body weights were significantly lower in the mice exposed to cigarette smoke. There were no statistically significant differences in the weight changes of the testes, epididymides, or vas deferens between the air- and smoke-exposed groups. Moreover, the body, testis, epididymis, and vas deferens weights were not significantly different between the vehicle and the CoQ10 groups (Figure 1).
Figure 1.
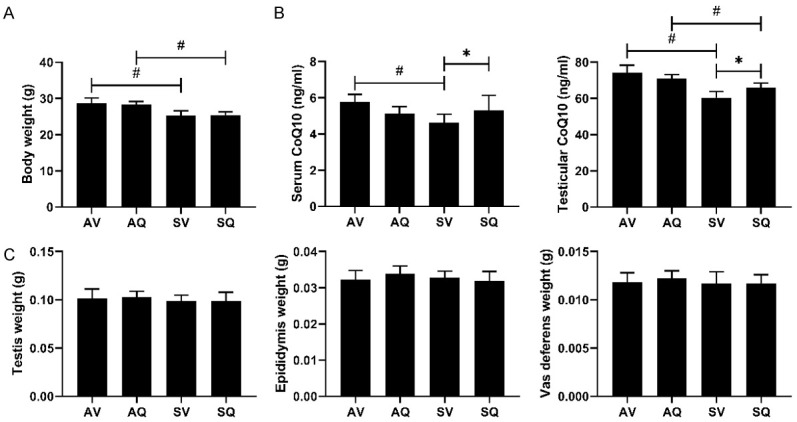
The effects of coenzyme Q10 supplementation on (A) body weight, (B) the serum and testicular coenzyme Q10 concentrations, and (C) the weights of the testes, the epididymides, and the vas deferens in the male mice exposed to cigarette smoke. Values are presented as the means ± SD. (A, C: n=10-12/group; B: n=6/group.) # indicates a significant difference between the air- and smoke-exposed groups (AV vs. SV and AQ vs. SQ); *Indicates a significant difference between the vehicle and coenzyme Q10 supplementation groups (AV vs. AQ and SV vs. SQ).
Concentration of coenzyme Q10
The concentrations of CoQ10 in the sera and testes were lower in the groups exposed to smoke. After we administered the CoQ10 supplementation, in the smoke-exposed groups, the concentrations of CoQ10 in the sera and testes were both improved.
Hematoxylin and eosin staining
There were no significant differences in the MSTD among the groups. The MTBS in the smoke-exposed group was significantly lower than it was in the non-exposed group, indicating that smoke may cause immaturity in testicular cells. The smoke-induced immaturity was ameliorated by the administration of oral CoQ10 supplementation. However, in the two non-smoke-exposed groups, the group with CoQ10 supplementation had a lower MTBS, indicating that additional CoQ10 supplementation may influence the histology of the testes in healthy animals (Figure 2).
Figure 2.
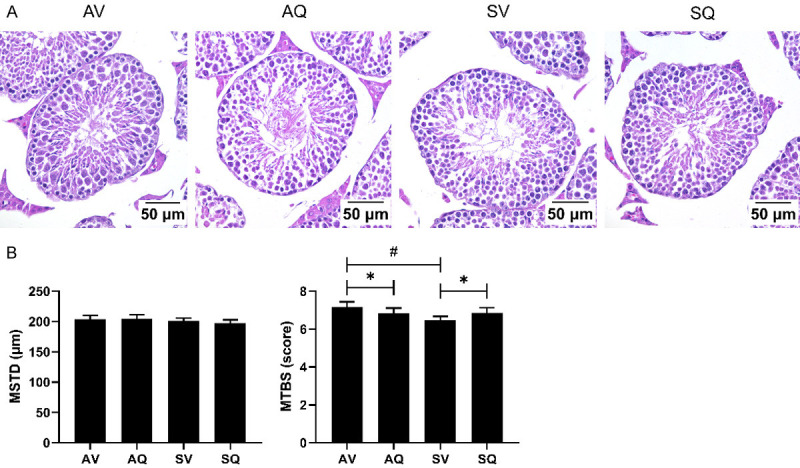
(A) Histology, (B) mean seminiferous tubule diameter (MSTD) and mean testicular biopsy score (MTBS) of the testis in the groups of male mice exposed to smoke and administered coenzyme Q10 supplementation. Values are presented as the means ± SD (n=6/group). #Indicates a significant difference between the air- and smoke-exposed groups (AV vs. SV and AQ vs. SQ); *Indicates a significant difference between the vehicle and coenzyme Q10 supplementation groups (AV vs. AQ and SV vs. SQ). MSTD: mean seminiferous tubule diameter. MTBS: mean testicular biopsy score.
Sperm quality
Figure 3A shows that the standard sperm motility and the normal morphology were significantly lower in the smoke-exposed group. In the group with CoQ10 supplementation, the sperm count, the motility, and the normal morphology were significantly improved. However, in the non-smoke-exposed group, the percentage of sperm of the normal morphology decreased after the administration of the CoQ10 supplementation.
Figure 3.
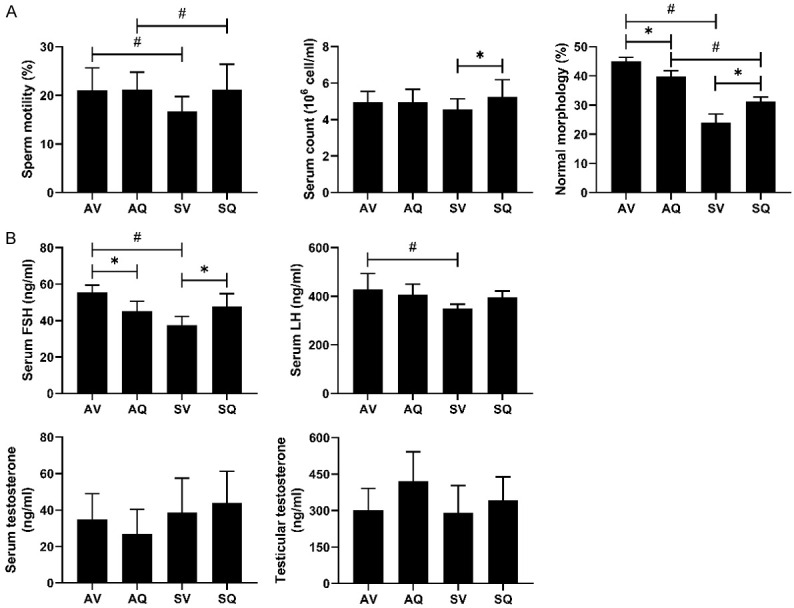
The effects of coenzyme Q10 supplementation on (A) semen quality and (B) hormone levels (serum FSH, LH, serum and testicular testosterone concentrations) in male mice exposed to cigarette smoke. Values are presented as the means ± SD. (A: n=6-12/group; B: n=6/group). #Indicates a significant difference between the air- and smoke-exposed groups (AV vs. SV and AQ vs. SQ); *Indicates a significant difference between the vehicle and coenzyme Q10 supplementation groups (AV vs. AQ and SV vs. SQ).
Sex hormone concentrations
The serum FSH concentrations were lower in the smoke-exposed group than they were in the air group, and the oral CoQ10 supplementation ameliorated the smoke-induced lower serum FSH. Otherwise, the CoQ10 supplementation decreased the concentrations of serum FSH under normal conditions. The serum LH concentration was significantly lower in the smoke-exposed group, but it did not improve after the CoQ10 supplementation. There were no significant differences in the serum and testis testosterone concentrations among the groups (Figure 3B).
Testosterone biosynthesis regulation
The protein expressions of StAR and CYP11A1 were lower in the smoke-exposed group, and did not improve following the CoQ10 supplementation. There were no significant differences in the 3β-HSD, CYP17A1, or 17β-HSD protein expressions among the groups (Figure 4).
Figure 4.
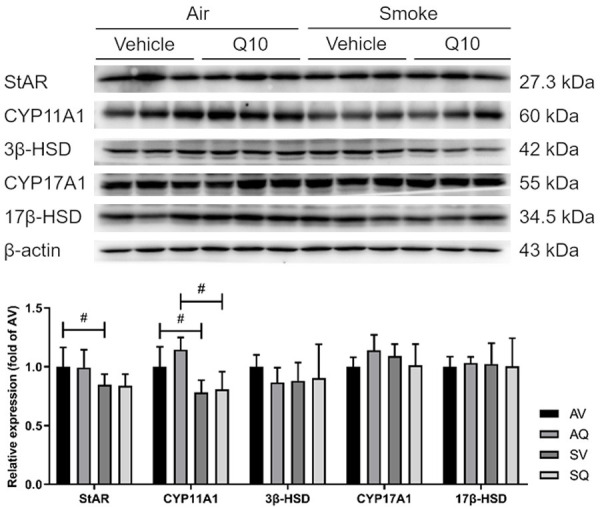
The effects of coenzyme Q10 supplementation on the testicular testosterone biogenesis protein expressions in male mice exposed to cigarette smoke. Values are presented as the means ± SD. (n=6/group). #Indicates a significant difference between the air- and smoke-exposed groups (AV vs. SV and AQ vs. SQ); *Indicates a significant difference between the vehicle and coenzyme Q10 supplementation groups (AV vs. AQ and SV vs. SQ).
Antioxidant capacity, lipid peroxidation and free radicals
With regards to antioxidant enzymes in the testes, the SOD concentrations were higher in the smoke-exposed group, and they increased after the administration of the CoQ10 supplementation. The catalase activity was significantly lower in the smoke-exposed group than it was the non-exposed group, and the smoke-induced lower catalase activity was not reversed by the CoQ10 supplementation. However, in the non-smoke-exposed group, the catalase activity was decreased after the CoQ10 supplementation. The GPx activity was also significantly lower in the smoke-exposed group, and the CoQ10 supplementation resulted in an improvement. In terms of the lipid peroxidation and the free radicals in the testis, the MDA and H2O2 levels were both significantly higher in the smoke-exposed group. The CoQ10 supplementation ameliorated the higher content of the MDA induced by exposure to the smoke, but not that of H2O2 (Figure 5).
Figure 5.
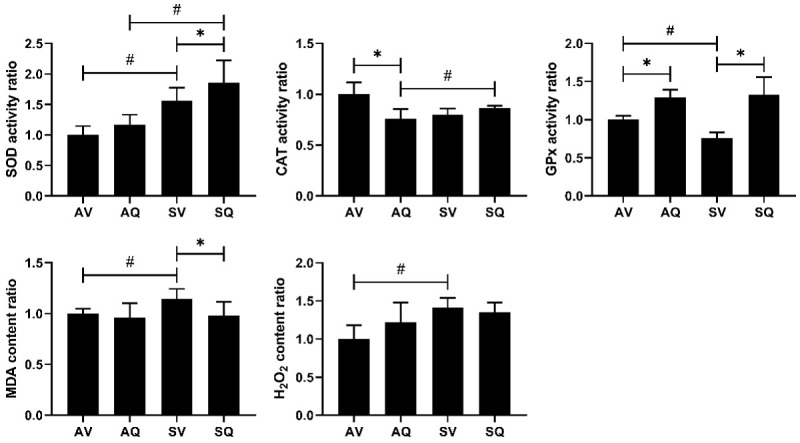
The effects of coenzyme Q10 supplementation on the testicular antioxidant enzyme, lipid peroxidation, and free radical levels in male mice exposed to cigarette smoke. The values are presented as the means ± SD. (n=6/group). #Indicates a significant difference between the air- and smoke-exposed groups (AV vs. SV and AQ vs. SQ); *Indicates a significant difference between the vehicle and coenzyme Q10 supplementation groups (AV vs. AQ and SV vs. SQ).
The extrinsic and intrinsic apoptotic pathways
Examining the apoptotic pathways, the caspase 8, c-caspase 9, cytochrome c, c-caspase 3, and c-PARP protein expressions were significantly higher in the smoke-exposed group. After administering the CoQ10 supplementation, the cytochrome c and c-caspase 3 protein expressions were improved. Although there were no significant differences in the c-PARP levels, there was a decreasing trend in the c-PARP protein expressions in the mice exposed to cigarette smoke and administered CoQ10 supplementation (Figure 6).
Figure 6.
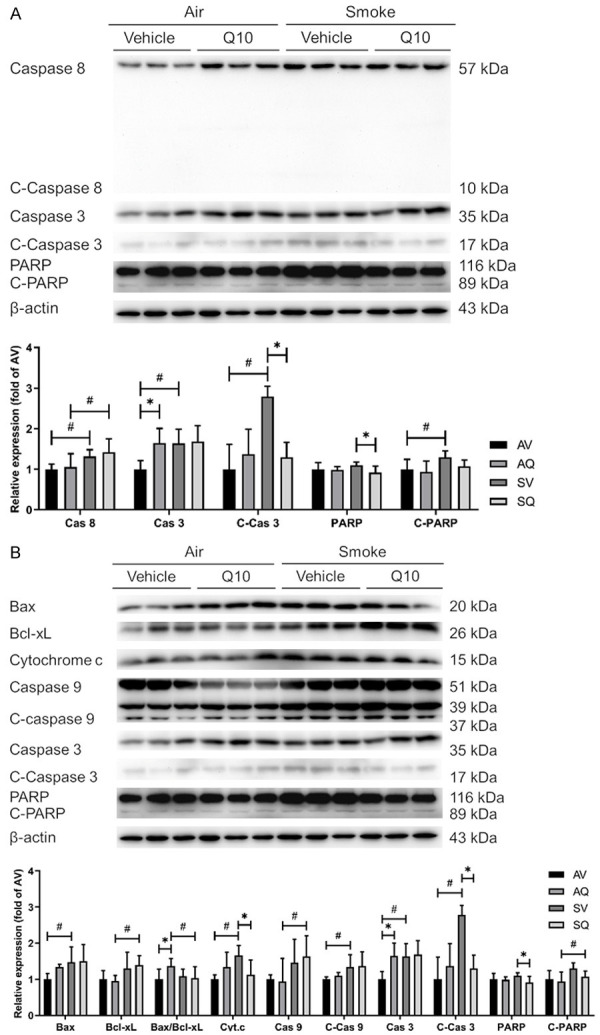
The effects of coenzyme Q10 supplementation on the protein expressions of testicular (A) extrinsic and (B) intrinsic apoptosis pathway proteins in male mice exposed to cigarette smoke. The caspase 3, c-caspase 3, PARP, and actin bands are from the same representative experiment. The values are presented as the means ± SD. (n=6/group). #Indicates a significant difference between the air- and smoke-exposed groups (AV vs. SV and AQ vs. SQ); *Indicates a significant difference between the vehicle and coenzyme Q10 supplementation groups (AV vs. AQ and SV vs. SQ).
Inflammatory pathway regulators
In the inflammatory pathway, the TNF-α and NFκB protein expressions increased, and the protein PPARγ protein expression decreased in the smoke-exposed group. After the CoQ10 supplementation, the TNF-α expression was improved. However, there were no significant differences between the groups in terms of their IL-6 expressions (Figure 7).
Figure 7.
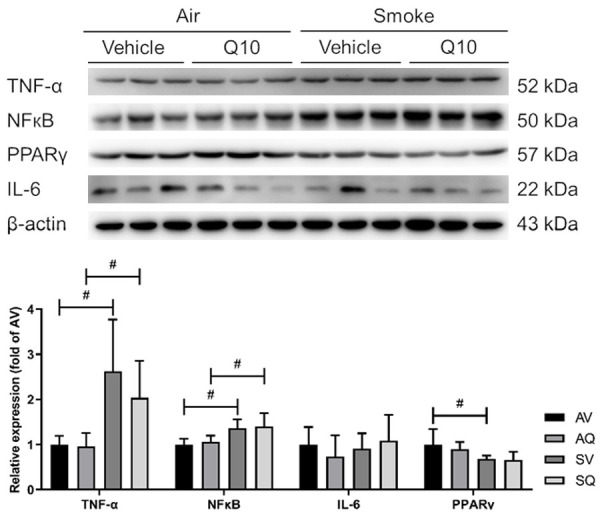
The effects of coenzyme Q10 supplementation on the expressions of the proteins in the testicular inflammation pathway in male mice exposed to cigarette smoke. #Indicates a significant difference between the air- and smoke-exposed groups (AV vs. SV and AQ vs. SQ); *Indicates a significant difference between the vehicle and coenzyme Q10 supplementation groups (AV vs. AQ and SV vs. SQ).
Mitochondrial biogenesis pathway and mtDNA copy number
With regards to the mitochondrial biogenesis pathway, the PGC-1α, NRF1, and NRF2 protein expressions were decreased in the smoke-exposed group. The Oral CoQ10 supplementation increased the PPARα, NRF1, and NRF2 levels. There were no significant differences between the groups in terms of their TFAM expressions. Regarding the mtDNA copy numbers in the testes, the results showed that the mtDNA copy numbers were lower in the smoke-exposed group, and this decrease was reversed after the CoQ10 supplementation (Figure 8).
Figure 8.
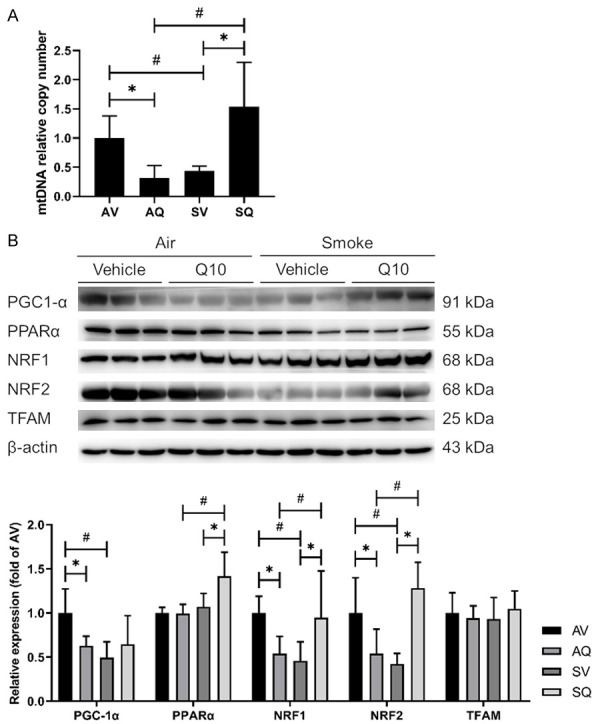
The effects of coenzyme Q10 supplementation on (A) the mitochondrial copy number and (B) the expressions of the proteins involved in testicular mitochondrial biogenesis in male mice exposed to cigarette smoke. The values are presented as the mean ± SD. (n=6/group). #Indicates a significant difference between the air- and smoke-exposed groups (AV vs. SV and AQ vs. SQ); *Indicates a significant difference between the vehicle and coenzyme Q10 supplementation groups (AV vs. AQ and SV vs. SQ).
Discussion
In a previous study, 8-week-old BALB/c mice were exposed to cigarette smoke, and the results showed that the mice’s body weights and food intake were lower in the smoke-exposed group. A possible reason was that the uncoupling protein 3 (UCP3) mRNA expression in the brown adipose tissue and skeletal muscle was increased. The main functions of UCP3 are related to mitochondrial fatty acid transport and basal metabolic rate regulation. When the UCP3 expression increases, it may result in weight loss [23].
Neuropeptide Y (NPY), synthesized by the arcuate nucleus of the hypothalamus, is mainly related to appetite and energy consumption. When one experiences hunger or a lack of energy, the production of NPY increases [24]. It has been confirmed that the nicotine receptor is located in the appetite-regulating area of the hypothalamus. Smoking might affect the orexigenic peptides (such as NPY and melanin) or the anorexigenic peptides to maintain an energy balance [25]. In an animal study, male Holtzman rats were administered a low dose of nicotine (4 mg/kg/day) for 14 days, and the nicotine administration resulted in a 19.5% reduced average food intake compared with the control group [26]. Therefore, we speculated that the exposure of the mice to cigarette smoke may influence appetite and energy consumption by increasing the UCP3 mRNA and NPY expressions and decreasing the body weight. However, the oral CoQ10 supplementation did not improve these conditions.
Under normal conditions, humans synthesize more than 90% of their CoQ10 themselves. When the concentration of CoQ10 is too high, it is discharged through the feces [11,27,28]. In a human study, 55 smokers and 51 non-smokers were recruited, and it was found that the serum concentrations of CoQ10 were lower in smokers [16]. The same result was observed in our study. We surmised that an increase in the smoke-induced ROS may reduce the levels of antioxidants such as CoQ10. During smoking, oral CoQ10 supplementation can increase the concentration of CoQ10 in the sera and testes; however, in the non-smoke-exposed group, the oral CoQ10 supplementation did not affect the level of CoQ10. It was considered that the CoQ10 in the body was sufficient for use, so the excess CoQ10 was discharged.
Our study results are consistent with a meta-analysis indicating that smoke exposure reduced the sperm parameters [5]. Toxic compounds in cigarettes such as nicotine, benzopyrene, and PAHs may damage testicular tissue and influence spermatogenesis.
CoQ10, an electron carrier, can maintain mitochondrial function and reduce oxidative stress. In a previous study, the antioxidant capacity and sperm parameters of infertile men were improved after the administration of the CoQ10 supplementation [17-19]. We speculated that CoQ10 can ameliorate sperm quality by regulating smoke-induced oxidative stress, cell apoptosis, and mitochondrial function.
The testosterone and spermatogenesis levels are mainly regulated by the hypothalamus pituitary gonadal axis. Our study results indicated that smoke exposure reduced the FSH and LH levels, a finding consistent with the animal study of Jana et al., in which a nicotine-treated group of Wistar rats was given IP injections of nicotine (0.6 mg/kg/day). The study results indicated that the nicotine-treated group had lower FSH and LH levels but increased corticosterone levels [29]. A possible explanation was that nicotine may affect the hypothalamus pituitary gonadal axis by stimulating the release of corticosterone. The sensitivity of gonadotropins to gonadotropin-releasing hormone reduced and inhibited the release of FSH and LH [30]. In this study, the lower FSH level induced by smoke exposure was reversed after the CoQ10 supplementation, which may improve the smoke-induce spermatogenesis damage.
Previous studies have shown inconsistent results with regards to the variations in testosterone levels after smoke exposure. In an animal study by Park et al., smoke exposure reduced the serum testosterone levels [31]. In a human study, Wang et al. demonstrated that the total testosterone and free testosterone levels in smokers were lower than in non-smokers, with no significant difference in the sex hormone binding globulin (SHBG) levels [32]. Otherwise, Halmenschlager et al. demonstrated no significant differences in the total testosterone, free testosterone, or SHBG levels between smokers and non-smokers [33], which is consistent with our study results. In our study, neither the smoke-exposed group nor the CoQ10 supplementation group showed changes in the testosterone levels in their sera or testes. It was hypothesized that nicotine and androgen metabolism requires UDP-glucuronosyltransferase (UGT) enzymes; therefore, nicotine competitively inhibits androgen metabolism, rendering the concentration of testosterone unchanged, or even increased [34,35].
Supriya et al. performed a study of 945 people, and the results showed that oxidative stress in smokers was higher than in non-smokers [36]. Cigarette smoke is a complex mixture of many compounds. It is carcinogenic and contains many free radicals, which increases oxidative stress and destroys cellular function [37]. Our study indicated that cigarette smoke results in decreases in antioxidant enzymes (CAT and GPx) and increases in lipid peroxidation (MDA) and H2O2. We also found that cigarette smoke increased the testicular SOD levels, in which SOD can transform O2 - into H2O2. According to the study of Jana et al, the H2O2 level increased and the GPx level decreased in the nicotine-treatment group [29]. Therefore, we surmised that the phenomenon of an increased SOD concentration is manifested in order to metabolize smoke-produced free radicals. Owing to decreased CAT and GPx concentrations, excess H2O2 cannot be metabolized, eventually leading to increased oxidative stress.
CoQ10 is an antioxidant and can protect against free radical-induced oxidative damage [38]. In a randomized double-blind study including 60 oligoasthenoteratozoospermia (OAT) patients, it was reported that the sperm parameters and the antioxidant enzyme activities increased in the CoQ10 supplementation group [39]. Our study also showed that the antioxidant enzyme activities (SOD and GPx) increased and the MDA activities decreased in the CoQ10 supplementation group, which may eliminate smoke-induced oxidative stress.
Our study showed that cigarette smoke mainly affects the protein expressions in the upstream intrinsic and downstream apoptotic pathways. It increases the protein expressions of cytochrome c and caspase 9 in the upstream intrinsic apoptotic pathway, and it activates caspase 3 and PARP in the downstream apoptotic pathway, a process that results in cell apoptosis. In the animal study of Mosadegh et al., Wistar rats were given an intraperitoneal injection of nicotine, and it was reported that the nicotine decreased the Bcl-2 protein expressions and increased the caspase 3 level [40]. We conjectured that the course of the ROS-induced oxidative stress changed the mitochondrial membrane potential and promoted cytochrome c release from the mitochondria. Therefore, the downstream markers were activated, finally leading to cell apoptosis.
CoQ10 mainly acts on mitochondria and can scavenge free radicals in the body. The permeability transition pore (PTP) on the mitochondria opening decreases the mitochondrial membrane potential, which facilitates cytochrome c release from mitochondria and induces cell apoptosis. CoQ10 can inhibit mitochondrial membrane potential change by regulating PTP, therein reducing cell apoptosis [41]. Our study results, which show that smoke-induced higher cytochrome c and caspase 3 levels were ameliorated by CoQ10, verifies the previous hypothesis.
Our study results indicate that the protein expressions of NFκB, TNF-α, and PPARγ were increased in the smoke-exposed group, which is consistent with previous research [42]. However, the CoQ10 administration did not diminish the expressions of the inflammatory pathway-related proteins in our study. Otherwise, a meta-analysis study illustrated that metabolic syndrome patients can have reduced TNF-α levels after the administration of CoQ10 [43], with a CoQ10 dosage greater than 100 mg/day. The dose of CoQ10 was 10 mg/kg/day in our animal study, and the conversion ratio of mice to humans is 12.3 [44], which is equal to 48.7 mg/day in humans. Therefore, in order to reduce the smoke-induced related inflammatory process, the CoQ10 should be administered at a higher dosage in future studies.
Exposure to cigarette smoke and air pollution may cause oxidative stress and cell apoptosis, and it may influence the mtDNA copy number and structure [45], and the mtDNA copy number is an indicator of the overall health of mitochondria. In a human study by Wu et al., it was found that smoke enhanced the oxidative stress levels and damaged the mitochondria, thereby reducing their quantity [46]. An animal study of male mice with an infertile phenotype illustrated that infertility decreased the mtDNA copy number and induced sperm mtDNA mutations. If the mtDNA copy number could be promoted, this could improve the testicular histology and spermatogenesis [47]. In our study, the reduced mtDNA copy number in the smoke-exposed group was reversed by the CoQ10 supplementation. However, in the non-smoke-exposed group, the mice that were administered oral CoQ10 supplementation showed decreased mtDNA copy numbers.
In a study by Hoffmann et al., cultured bronchial epithelial cells isolated from chronic obstructive pulmonary disease (COPD) patients indicated that cigarette smoke extract decreased the gene expressions of PGC-1α and TFAM in the mitochondrial biogenesis pathway, which may also alter the mitochondrial morphology and the electron transport chain [48]. In our study, we found that the protein expressions of PGC-1α, NRF1 and NRF2 were decreased in the smoke-exposed group. When the PGC-1α expression is higher, it may promote the transcription of NRF1 and NRF2, which are related to the electron transport chain [49]. Our results demonstrated that the oral CoQ10 supplementation ameliorated the smoke-induced lower protein expressions of NRF1 and NRF2. Therefore, the function of the electron transport chain might be improved.
A limitation of this study was that the mice’s respiratory function was not measured before the mice were sacrificed, and the process may cause stress and slight hemolysis. As for our analysis of the oxidative stress, we could consider measuring the total antioxidant capacity and the levels of the other free radicals in the testes to assess the overall testicular oxidative stress levels. Regarding the evaluation of the mitochondrial function, a mitochondrial membrane potential analysis can be performed to examine whether CoQ10 improved function by regulating the electron transport chain.
In this study, we found that oral CoQ10 supplementation ameliorated smoke-induced testicular damage by regulating oxidative stress, cell apoptosis, and mitochondrial function in the testes.
Acknowledgements
This study was supported by the National Defense Medical Center and Taipei Medical University.
Disclosure of conflict of interest
None.
References
- 1.WHO. Prevalence of tobacoo smoking. 2016 [Google Scholar]
- 2.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 3.Health Promotion Administration Ministry of Health and Welfare. Survey of smoking behavior in adults in 2016. Taipei City, Taiwan: 2016. [Google Scholar]
- 4.Dept. of Household Registration MOI. Demographic data. 2018 [Google Scholar]
- 5.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the examination of human semen. Eur Urol. 2016;70:635–645. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Mahboubi M, Foroughi F, Ghahramani F, Shahandeh H, Moradi S, Shirzadian T. A case-control study of the factors affecting male infertility. Turk J Med Sci. 2014;44:862–865. doi: 10.3906/sag-1304-35. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richthoff J, Elzanaty S, Rylander L, Hagmar L, Giwercman A. Association between tobacco exposure and reproductive parameters in adolescent males. Int J Androl. 2008;31:31–39. doi: 10.1111/j.1365-2605.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 9.Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 10.Dai JB, Wang ZX, Qiao ZD. The hazardous effects of tobacco smoking on male fertility. Asian J Androl. 2015;17:954–960. doi: 10.4103/1008-682X.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. 2012;26:711–723. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rooney JP, Ryde IT, Sanders LH, Howlett EH, Colton MD, Germ KE, Mayer GD, Greenamyre JT, Meyer JN. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol Biol. 2015;1241:23–38. doi: 10.1007/978-1-4939-1875-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilieva I, Sainova I, Zvetkova E. Sperm mitochondria-associated male infertility: sperm quality defects and mitochondria (mtDNA) anomalies: review. Acta morphologica et anthropologica. 2017;24:114–122. [Google Scholar]
- 16.Al-Bazi MM, Elshal MF, Khoja SM. Reduced coenzyme Q(10) in female smokers and its association with lipid profile in a young healthy adult population. Arch Med Sci. 2011;7:948–954. doi: 10.5114/aoms.2011.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafuente R, Gonzalez-Comadran M, Sola I, Lopez G, Brassesco M, Carreras R, Checa MA. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet. 2013;30:1147–1156. doi: 10.1007/s10815-013-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol. 2012;188:526–531. doi: 10.1016/j.juro.2012.03.131. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters_an evidence based review. Int J Reprod Biomed. 2016;14:729–736. [PMC free article] [PubMed] [Google Scholar]
- 20.Tang GJ, Wang HY, Wang JY, Lee CC, Tseng HW, Wu YL, Shyue SK, Lee TS, Kou YR. Novel role of AMP-activated protein kinase signaling in cigarette smoke induction of IL-8 in human lung epithelial cells and lung inflammation in mice. Free Radic Biol Med. 2011;50:1492–1502. doi: 10.1016/j.freeradbiomed.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 22.Osinubi AA, Noronha CC, Okanlawon AO. Morphometric and stereological assessment of the effects of long-term administration of quinine on the morphology of rat testis. West Afr J Med. 2005;24:200–205. doi: 10.4314/wajm.v24i3.28198. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Vlahos R, Bozinovski S, Jones J, Anderson GP, Morris MJ. Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology. 2005;30:713–719. doi: 10.1038/sj.npp.1300597. [DOI] [PubMed] [Google Scholar]
- 24.Beck B, Jhanwar-Uniyal M, Burlet A, Chapleur-Chateau M, Leibowitz SF, Burlet C. Rapid and localized alterations of neuropeptide Y in discrete hypothalamic nuclei with feeding status. Brain Res. 1990;528:245–249. doi: 10.1016/0006-8993(90)91664-3. [DOI] [PubMed] [Google Scholar]
- 25.Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li MD, Kane JK, Parker SL, McAllen K, Matta SG, Sharp BM. Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res. 2000;867:157–164. doi: 10.1016/s0006-8993(00)02283-6. [DOI] [PubMed] [Google Scholar]
- 27.Pravst I, Zmitek K, Zmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr. 2010;50:269–280. doi: 10.1080/10408390902773037. [DOI] [PubMed] [Google Scholar]
- 28.Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 29.Jana K, Samanta PK, De DK. Nicotine diminishes testicular gametogenesis, steroidogenesis, and steroidogenic acute regulatory protein expression in adult albino rats: possible influence on pituitary gonadotropins and alteration of testicular antioxidant status. Toxicol Sci. 2010;116:647–659. doi: 10.1093/toxsci/kfq149. [DOI] [PubMed] [Google Scholar]
- 30.Kamel F, Kubajak CL. Modulation of gonadotropin secretion by corticosterone: interaction with gonadal steroids and mechanism of action. Endocrinology. 1987;121:561–568. doi: 10.1210/endo-121-2-561. [DOI] [PubMed] [Google Scholar]
- 31.Park MG, Ko KW, Oh MM, Bae JH, Kim JJ, Moon du G. Effects of smoking on plasma testosterone level and erectile function in rats. J Sex Med. 2012;9:472–481. doi: 10.1111/j.1743-6109.2011.02555.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang MJ, Ou JX, Chen GW, Wu JP, Shi HJ, O WS, Martin-DeLeon PA, Chen H. Does prohibitin expression regulate sperm mitochondrial membrane potential, sperm motility, and male fertility? Antioxid Redox Signal. 2012;17:513–519. doi: 10.1089/ars.2012.4514. [DOI] [PubMed] [Google Scholar]
- 33.Halmenschlager G, Rossetto S, Lara GM, Rhoden EL. Evaluation of the effects of cigarette smoking on testosterone levels in adult men. J Sex Med. 2009;6:1763–1772. doi: 10.1111/j.1743-6109.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 34.Jahangir E, Lipworth L, Edwards TL, Kabagambe EK, Mumma MT, Mensah GA, Fazio S, Blot WJ, Sampson UK. Smoking, sex, risk factors and abdominal aortic aneurysms: a prospective study of 18 782 persons aged above 65 years in the Southern Community Cohort Study. J Epidemiol Community Health. 2015;69:481–488. doi: 10.1136/jech-2014-204920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chouinard S, Yueh MF, Tukey RH, Giton F, Fiet J, Pelletier G, Barbier O, Belanger A. Inactivation by UDP-glucuronosyltransferase enzymes: the end of androgen signaling. J Steroid Biochem Mol Biol. 2008;109:247–253. doi: 10.1016/j.jsbmb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Supriya K, Chaudhary J, Shamal SN. Oxidative stress in cigarette smoker and smokeless tobacco user among ethnic group north-eastern population of Uttar Pradesh, India. Int J Res Med Sci. 2017;5:1439. [Google Scholar]
- 37.Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health. 2009;6:445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee BJ, Lin YC, Huang YC, Ko YW, Hsia S, Lin PT. The relationship between coenzyme Q10, oxidative stress, and antioxidant enzymes activities and coronary artery disease. ScientificWorldJournal. 2012;2012:792756. doi: 10.1100/2012/792756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, Nazeri Kakhki SA, Akhondi MM, Sadeghi MR. Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double-blind randomised clinical trial. Andrologia. 2014;46:177–183. doi: 10.1111/and.12062. [DOI] [PubMed] [Google Scholar]
- 40.Mosadegh M, Hasanzadeh S, Razi M. Nicotine-induced damages in testicular tissue of rats; evidences for bcl-2, p53 and caspase-3 expression. Iran J Basic Med Sci. 2017;20:199–208. doi: 10.22038/ijbms.2017.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G, Brancato R, Capaccioli S. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220–28228. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 42.Dey T, Dutta P, Manna P, Kalita J, Boruah HPD, Buragohain AK, Unni B, Ozah D, Kumar Goswami M, Kotokey RK. Cigarette smoke compounds induce cellular redox imbalance, activate NF-kappaB, and increase TNF-alpha/CRP secretion: a possible pathway in the pathogenesis of COPD. Toxicol Res (Camb) 2016;5:895–904. doi: 10.1039/c5tx00477b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhai J, Bo Y, Lu Y, Liu C, Zhang L. Effects of Coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. PLoS One. 2017;12:e0170172. doi: 10.1371/journal.pone.0170172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair A, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fetterman JL, Sammy MJ, Ballinger SW. Mitochondrial toxicity of tobacco smoke and air pollution. Toxicology. 2017;391:18–33. doi: 10.1016/j.tox.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu S, Li X, Meng S, Fung T, Chan AT, Liang G, Giovannucci E, De Vivo I, Lee JH, Nan H. Fruit and vegetable consumption, cigarette smoke, and leukocyte mitochondrial DNA copy number. Am J Clin Nutr. 2019;109:424–432. doi: 10.1093/ajcn/nqy286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang M, Kauppila TES, Motori E, Li X, Atanassov I, Folz-Donahue K, Bonekamp NA, Albarran-Gutierrez S, Stewart JB, Larsson NG. Increased total mtDNA copy number cures male infertility despite unaltered mtDNA mutation load. Cell Metab. 2017;26:429–436. e424. doi: 10.1016/j.cmet.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann RF, Zarrintan S, Brandenburg SM, Kol A, de Bruin HG, Jafari S, Dijk F, Kalicharan D, Kelders M, Gosker HR, Ten Hacken NH, van der Want JJ, van Oosterhout AJ, Heijink IH. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res. 2013;14:97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamora M, Villena J. Targeting mitochondrial biogenesis to treat insulin resistance. Curr Pharm Des. 2014;20:5527–5557. doi: 10.2174/1381612820666140306102514. [DOI] [PubMed] [Google Scholar]


