Abstract
Objective: This study set out to clarify the distribution and drug resistance of pathogens causing urinary tract infection (UTI) in patients with urinary calculi. Methods: Pathogens were isolated from urine samples of patients with urinary calculi also complicated with UTIs, during the period from 2015 to 2019, and the samples were cultured for drug sensitivity testing to study the drug resistance of pathogens. The results were analyzed by SPSS 22.0 software. Results: Gram-negative bacteria were the main pathogens found in patients with urinary calculi complicated with UTI (84.52%). Escherichia coli, Enterococcus faecalis and Monilia albicans were the most common Gram-negative bacteria (48.84%), Gram-positive bacteria (34.78%) and fungus (29.41%), respectively. The UTI rates were higher in female patients than in male patients, and were higher in patients ≥ 60 years old compared with those < 60 years old. Escherichia coli and Klebsiella pneumoniae had the highest resistance to ampicillin and the lowest resistance to imipenem. Enterococcus faecalis Enterococcus Faecium had the highest resistance to penicillin and ampicillin, but the lowest resistance to vancomycin and linezolid. Conclusion: The present study found that the pathogenic bacteria found in patients with urinary calculi complicated with UTI are mainly Gram-negative bacteria; and Escherichia coli is the main pathogenic bacteria causing the infection. Gender and age may be risk factors for urinary calculi complicated with UTI. Antibiotics should be selected reasonably according to the drug resistance pattern of pathogenic bacteria in clinical anti-infection management.
Keywords: Urinary calculi, urinary tract infection, distribution and drug resistance of pathogenic bacteria
Introduction
Urinary tract infection (UTI) is a common type of bacterial infection [1]. A growing number of studies [2,3] have shown that pathogenic Escherichia coli is the primary pathogen of UTIs. Escherichia coli belongs to the Enterobacteriaceae family, most of which are harmless to the human body, while some others that can cause infection or diarrhea are called pathogenic Escherichia coli [4,5]. In addition, the incidence of UTI in women is much higher than that in men [6].
UTI is prevalent in patients with urinary calculi [7], and the two interact with each other. On the one hand, UTI can induce urinary calculi by promoting the deposition of calcium oxalate and struvite [8,9], and on the other hand, urinary calculi and related treatments can easily lead to bacterial infection [10]. These two combined may give rise to acute renal damage [11]. Therefore, effective intervention or treatment of UTIs is essential in the treatment of urinary calculi.
Antibiotics are the routine treatment for UTIs [12], which can not only effectively inhibit the duration of infection symptoms, but also prevent the occurrence of repeated infections [13]. The antibacterial spectrum of antibiotics and the drug resistance pattern of pathogenic bacteria should be fully considered in clinical treatment. Therefore, understanding the common pathogens in UTIs and their drug susceptibility is an essential requirement for clinical anti-infective drug use [14,15].
Distribution and resistance patterns of pathogenic bacteria in UTIs are temporal and regional in nature [16]. Here, we performed a statistical analysis on the distribution of pathogenic bacteria in patients with urinary calculi collected from 2015 to 2019, and studied the drug resistance of the main pathogenic bacteria. The fundamental purpose of this study is to understand the common pathogenic bacteria of UTIs in patients complicated with urinary calculi and their drug sensitivity, so as to provide reliable medication basis for doctors to intervene and prevent UTIs, thus promoting patient recovery.
Methods
General data
From 2015 to 2019, 689 patients (male to female ratio 281:408) with urinary calculi were found to have a UTI after urine test, with 317 cases ≥ 60 years old and 372 cases < 60 years old. In terms of disease types, there were 248 cases of kidney calculi, 236 cases of ureteral alculus, 120 cases of kidney stones complicated with ureteral calculus, and 85 cases of bladder calculi. In terms of stone location, there were 392 cases with calculi on the left side and 297 cases on the right side, with the calculi diameter of 15.88±3.24 mm (Table 1). All participants signed an informed consent in accordance with the Declaration of Helsinki and this study was approved by our Hospital Ethics Committee (2015-03-1416).
Table 1.
General data
| Material | Data |
|---|---|
| Overall number of people | 689 |
| Gender | |
| Male | 281 (40.78) |
| Female | 408 (59.22) |
| Age | |
| < 60 | 372 (53.99) |
| ≥ 60 | 317 (46.01) |
| Smoking history | |
| Present | 286 (41.51) |
| Absent | 403 (58.49) |
| Alcohol abuse history | |
| Present | 236 (34.25) |
| Absent | 453 (65.75) |
| Type of calculi | |
| Kidney calculi | 248 (35.99) |
| Ureteral calculus | 236 (34.25) |
| Kidney calculi complicated with ureteral calculus | 120 (17.42) |
| Bladder calculi | 85 (12.34) |
| Stone diameter | 15.88±3.24 mm |
| Stone location | |
| Left | 392 (56.89) |
| Right | 297 (43.11) |
Research methods
Urine samples were collected from patients in sterile tubes for immediate quantitative microbiological culture. Specifically, the urine samples suspected of bacterial infection were inoculated on the culture plate and cultivated at 37°C for 24 h, and those suspected of fungal infection were inoculated on the culture plate and cultivated at 28°C for 7 days. The stability and morphology of colonies were observed under the microscope, the pathogenic bacteria were identified with a fully automated bacterial analyzer, and the resistance of pathogenic bacteria to antibacterial drugs was determined with the disk diffusion method. The drug resistance of pathogenic bacteria was quantitatively analyzed according to the performance standards for susceptibility testing.
The quality control strains were Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 25923) and Enterococcus faecalis (ATCC 25922).
Statistical analysis
The measurement data were expressed as mean ± standard deviation, and counting data were expressed as number of cases (percentage). All the data were statistically analyzed by SPSS 22.0 software. Chi-square test was utilized to compare the differences between the two groups. COX proportional hazard regression analysis was used to discuss the role of gender and age in UTI. P < 0.05 indicated a statistically significant difference.
Results
Distribution of pathogens causing UTIs in patients with urinary calculi
The distribution of pathogenic bacteria in patients with urinary calculi complicated with UTI in 5 years was counted, and 407 strains of pathogenic bacteria were isolated. As shown in Figure 1, the total detection rate of pathogenic bacteria peaked in 2017 and then returned to previous levels. In this study, 344 Gram-negative, 46 Gram-positive, and 17 fungus strains were isolated, and Gram-negative bacteria were the main pathogens (84.52%) of urinary calculi complicated with UTI.
Figure 1.
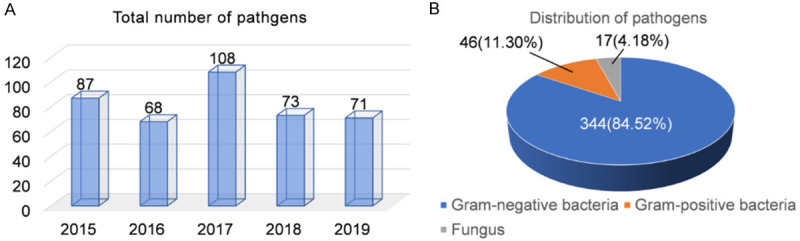
Distribution of pathogens causing urinary tract infection in patients with urinary calculi. A. The number of pathogenic bacteria detected from 2015 to 2019. The total detection rate of pathogenic bacteria peaked in 2017 and then returned to previous levels. B. Distribution of pathogenic bacteria within 5 years. A total of 344 Gram-negative, 46 Gram-positive, and 17 fungus strains were isolated, and Gram-negative bacteria were the main pathogens (84.52%) of urinary calculi complicated with urinary tract infection.
Escherichia coli is the most common gram-negative bacteria causing UTI (48.84%), followed by Klebsiella pneumoniae (6.10%), Pseudomonas aeruginosa (5.81), Proteus mirabilis (5.52%) and Enterobacter cloacae (4.94%). Enterococcus faecalis is the most common Gram-positive bacteria (34.78%), followed by Enterococcus Faecium (21.74%); and Monilia albicans is the most common UTI fungus (29.41%) Table 2.
Table 2.
Pathogens causing urinary tract infection in patients with urinary calculi
| Pathogens | Number of Pathogens | Proportion of GN/GP/Fungus (%) | |
|---|---|---|---|
| Gram-negative bacteria | Escherichia coli | 168 | 48.84 |
| Klebsiella pneumoniae | 21 | 6.10 | |
| Pseudomonas aeruginosa | 20 | 5.81 | |
| Proteus mirabilis | 19 | 5.52 | |
| Enterobacter cloacae | 17 | 4.94 | |
| Acinetobacter baumannii | 17 | 4.94 | |
| Stenotrophomonas maltophilia | 14 | 4.07 | |
| Aeromonas caviae | 13 | 3.78 | |
| Flavobacterium | 11 | 3.20 | |
| Citrobacter kreitsoni | 10 | 2.91 | |
| Aeromonas hydrophila | 9 | 2.62 | |
| Sphingomonas paucimobilis | 6 | 1.74 | |
| Serratia fonticola | 5 | 1.45 | |
| Citrobacter braakii | 4 | 1.16 | |
| Citrobacter freundii | 4 | 1.16 | |
| Morganella morganii | 3 | 0.87 | |
| ABurkholderia cepacia | 2 | 0.58 | |
| Acinetobacter haemolyticus | 1 | 0.29 | |
| Gram-positive bacteria | Enterococcus faecalis | 16 | 34.78 |
| Enterococcus Faecium | 10 | 21.74 | |
| Staphylococcus epidermidis | 8 | 17.39 | |
| Staphylococcus hominis | 8 | 17.39 | |
| Streptococcus oralis | 4 | 8.70 | |
| Fungus | Monilia albicans | 5 | 29.41 |
| Candida glabrata | 4 | 23.53 | |
| Candida tropicalis | 4 | 23.53 | |
| Candida parapsilosis | 2 | 11.76 | |
| Meyerozyma guilliermondii | 2 | 11.76 |
GN, Gram-negative bacteria. GP, Gram-positive bacteria.
Figure 2 exhibits the changes of detection rates of major Gram negative bacteria, major Gram positive bacteria and fungus from 2015 to 2019. Generally speaking, the lowest detection rates of Escherichia coli, Klebsiella pneumoniae, and major Gram-positive bacteria other than Streptococcus oralis were found in 2016, 2018, and 2018, respectively. Besides, there was no considerable change in the fungal detection rate over the 5 year period.
Figure 2.
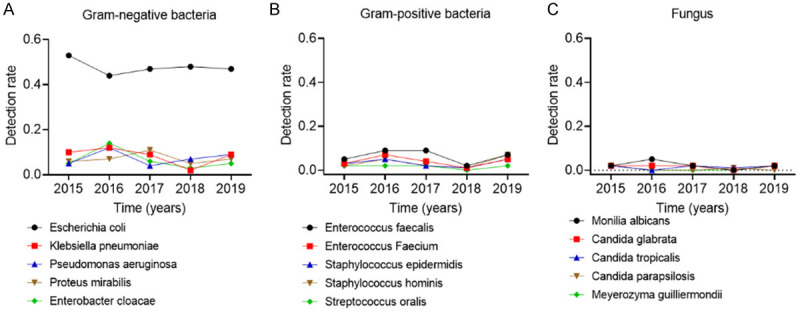
5-year change in the detection rate of pathogenic bacteria of urinary tract infection. A. 5-year change in the detection rate of Gram-negative bacteria. B. 5-year change in the detection rate of Gram-positive bacteria. C. 5-year change in the detection rate of Fungus.
Distribution of pathogenic bacteria of UTIs in gender and age stratification
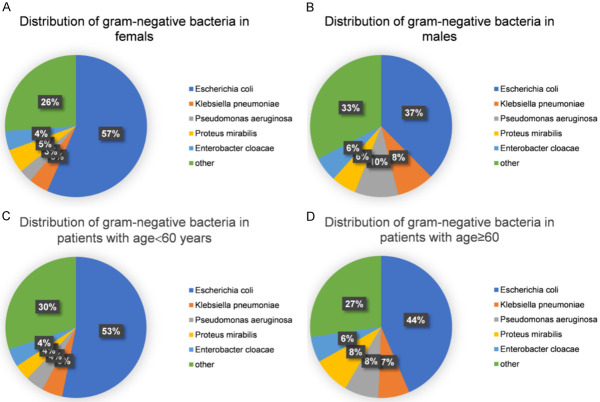
Age and gender may be risk factors for UTI, so we analyzed the distribution of pathogenic bacteria in men and women with different ages. As shown in Figures 3 and 4, the UTI rate was higher in female patients than in male patients, and was higher in people ≥ 60 years old compared with those < 60 years old, with statistically significant differences. The proportion of Escherichia coli in women (57%) was higher than that in men (37%), while the proportion of Klebsiella pneumoniae and Pseudomonas aeruginosa in females (both 5%) was lower than that in males (10% and 8%, respectively). The proportion of Escherichia coli in patients aged ≥ 60 (53%) was lower than that in patients aged < 60 (44%). COX proportional hazard regression analysis (Tables 3 and 4) showed that gender and age were potential risk factors for UTI. Therefore, the influence of gender and age should be considered in anti-infection management.
Figure 3.
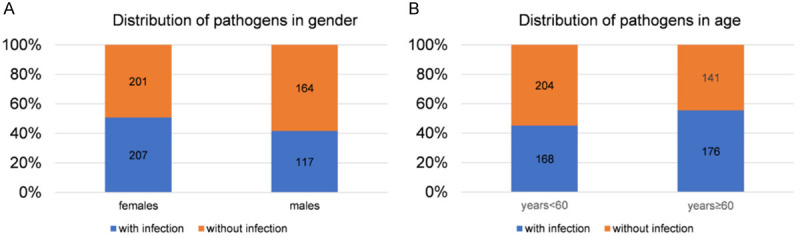
Distribution of pathogenic bacteria of urinary tract infection in gender and age stratification. A. Seeing from the angle of gender stratification in urinary calculi complicated with urinary calculi, the urinary tract infection rate in females is higher than males. B. Seeing from the angle of age group in the occurrence of urinary calculi, the urinary tract infection rate of patients equal or over 60 years old is higher than that of patients under 60 years old.
Figure 4.
Distribution of pathogenic bacteria in gender and age stratification. A. The distribution of gram-negative bacteria in female patients. B. The distribution of gram-negative bacteria in male patients. C. The distribution of gram-negative bacteria in patents with age < 60 years old. D. The distribution of gram-negative bacteria in patents with age ≥ 60 years old.
Table 3.
Univariate logistic regression analysis
| HR | P | 95% CI | SE | |
|---|---|---|---|---|
| Gender (male vs female) | 1.767 | 0.024 | 0.639 to 0.931 | 0.227 |
| Age (< 60 vs ≥ 60) | 1.579 | 0.042 | 0.506 to 0.913 | 0.163 |
| Stone location (left vs right) | 1.344 | 0.055 | 0.535 to 0.947 | 0.178 |
| Smoking history (yes vs no) | 1.352 | 0.073 | 0.453 to 0.854 | 0.142 |
| Drinking history (yes vs no) | 1.431 | 0.086 | 0.612 to 0.953 | 0.241 |
Table 4.
Multivariate logistic regression analysis
| HR | P | 95% CI | SE | |
|---|---|---|---|---|
| Gender (male vs female) | 1.821 | 0.021 | 0.609 to 0.923 | 0.235 |
| Age (< 60 vs ≥ 60) | 1.746 | 0.039 | 0.592 to 0.927 | 0.184 |
Drug resistance of main pathogenic bacteria causing UTI
Here, two main Gram-negative bacteria (Escherichia coli and Klebsiella pneumoniae) and the two main Gram-positive bacteria (Enterococcus faecalis and Enterococcus Faecium) which were selected as drug resistant types, and the results are shown in Tables 5 and 6.
Table 5.
Drug resistance rate of main gram-negative bacteria
| Escherichia coli n=168 | Klebsiella pneumoniae n=21 | |
|---|---|---|
| Ampicillin | 157 (93.45) | 16 (76.19) |
| Piperacillin | 114 (67.86) | 11 (52.38) |
| Piperacillin-tazobactam | 19 (11.31) | 2 (9.52) |
| Amoxicillin/clavulanic acid | 74 (44.05) | 9 (42.86) |
| Imipenem | 12 (7.14) | 1 (4.76) |
| Cefazolin | 143 (85.12) | 12 (57.14) |
| Cefuroxime | 131 (77.98) | 13 (61.90) |
| Ceftriaxone | 81 (48.21) | 11 (52.38) |
| Ceftazidime | 87 (51.79) | 13 (61.90) |
| Cefotaxime | 92 (54.76) | 8 (38.10) |
| Cefepime | 24 (14.29) | 12 (57.14) |
| Cefperazone-sulbactam | 26 (15.48) | 9 (42.86) |
| Levofloxacin | 89 (52.98) | 10 (47.62) |
| Gentamicin | 57 (33.93) | 6 (28.57) |
| Ciprofloxacin | 67 (39.88) | 2 (9.52) |
| Amikacin | 18 (10.71) | 3 (14.29) |
Table 6.
Comparison of drug resistance rates of main Gram-positive bacteria
| Enterococcus faecalis n=16 | Enterococcus Faecium n=10 | |
|---|---|---|
| Penicillin | 13 (81.25) | 9 (90.00) |
| Ampicillin | 14 (87.50) | 7 (70.00) |
| Gentamicin | 11 (68.75) | 8 (80.00) |
| Levofloxacin | 11 (68.75) | 4 (40.00) |
| Ofloxacin | 12 (75.00) | 7 (70.00) |
| Ciprofloxacin | 9 (56.25) | 7 (70.00) |
| Erythromycin | 7 (43.75) | 6 (60.00) |
| Vancomycin | 0 (0.00) | 0 (0.00) |
| Linezolid | 0 (0.00) | 0 (0.00) |
Escherichia coli has the highest resistance to ampicillin (93.45%) and the lowest resistance to imipenem (7.14%), with resistance to piperacillin, cefazolin, cefuroxime, ceftazidime, cefotaxime and levofloxacin over 50%, and ciprofloxacin, gentamicin, amoxicillin/clavulanic acid and ceftriaxone over 30%, and cefepime and cefoperazone-sulbactam over 10%.
Klebsiella pneumoniae has the highest resistance to ampicillin (76.19%) and the lowest resistance rate to imipenem, ciprofloxacin and piperacillin-tazobactam (< 10%), with resistance to piperacillin, cefazolin, cefuroxime, ceftriaxone, ceftazidime and cefepime over 50%, with amoxicillin/clavulanic acid, cefotaxime, cefoperazone-sulbactam and levofloxacin over 30%, and amikacin and gentamicin over 10%.
Enterococcus faecalis has the highest resistance to ampicillin (87.50%) and the lowest resistance to vancomycin and linezolid (0.00%). Enterococcus faecium has the highest resistance to penicillin (90.00%) and the lowest resistance to vancomycin and linezolid (0.00%).
Discussion
In this study, a total of 407 strains of pathogenic bacteria were isolated from patients with urinary calculi complicated with UTI, and the number of pathogenic bacteria detected between 2015 and 2019 peaked in 2017. Among the 407 pathogenic strains, there were 344 strains of Gram-negative bacteria (among which Escherichia coli was the most, accounting for 48.84%), 46 of the Gram-positive bacteria (among which Enterococcus faecalis was the most, accounting for 34.78%) and 17 types of fungi. The team of Rafalskiy [17] supported the idea that Gram-negative bacteria were the main pathogens inducing UTI, and the distribution ratio of Escherichia coli is the first among them. Gajdács et al. [16] believed that Gram-positive bacteria were important pathogens of UTI, among which Enterococcus faecalis accounted for the largest proportion. Therefore, Escherchia coli is the most common Gram-negative bacterium among the pathogens of urinary calculi complicated with UTI, and Enterococcus faecalis is the most common Gram-positive bacterium.
UTI may exhibit different pathogen distribution depending on gender and age [18]. In this paper, the UTI rate and the distribution of Gram-negative bacteria in male and female patients with urinary calculi were counted. In terms of infection, the UTI rate was higher in female patients than in male patients. Wang et al. [19] showed that among patients with urinary calculi, women were more prone to develop UTIs than men, which may be due to anatomical differences between men and women. As far as the distribution of Gram-negative bacteria is concerned, the proportion of Escherichia coli in females (57%) was higher than that of males (37%), while the proportion of Klebsiella pneumoniae and Pseudomonas aeruginosa in females (both 5%) was lower than that of males (10% and 8%, respectively). Similar results have been mentioned in the research of Iqbal et al. [20]. Unfortunately, the distribution ratio of Gram-negative bacteria in females and males is not statistically significant. Hence, more samples are needed to obtain more accurate conclusions. In addition, the UTI rate of patients aged ≥ 60 was higher than that of patients aged < 60, and the proportion of escherichia coli (53%) in patients ≥ 60 years old was lower than that in patients < 60 years old (44%). In the research of De Lorenzis et al. [21], the distribution of pathogenic bacteria showed age differences. Magliano et al. [22] reported that Escherichia coli was less present in people aged 60 or above than those aged under 60. The immune function of elderly patients gradually weakens with age, and there may be a decrease in physical activity, so the elderly patients are more likely to develop UTIs than those aged < 60 years. In this study, COX proportional hazard regression analysis was used to discuss the potential risk factors of developing UTIs. The results showed that age and gender are risk factors for UTIs, which may be the reason for the different distribution of urinary pathogens in different ages or genders. Hence, gender and age may be risk factors for UTIs, but a larger sample size is still needed for confirmation.
Escherichia coli is the most common pathogen in UTI and is highly resistant to first-line antibiotics [23,24]. According to the results in Table 2, two main Gram-negative bacteria (Escherichia coli and Klebsiella pneumoniae) and two main Gram-positive bacteria (Enterococcus faecalis and Enterococcus Faecium) were selected as the drug resistance models for study. Escherichia coli and Klebsiella pneumoniae were found to have the highest resistance to ampicillin and the lowest resistance to imipenem. Enterococcus faecalis and Enterococcus Faecium had the highest resistance to penicillin and ampicillin, but the lowest resistance to vancomycin and linezolid. Therefore, ampicillin is not recommended as the first choice for clinical anti-urinary infection. Of course, this study still has some shortcomings. First, although age and gender are found to be risk factors for UTIs in this study, a larger sample size is still needed for confirmation. Second, this study discussed the distribution and drug resistance of pathogenic bacteria for UTIs from 2015 to 2019, but with limited sample size. In the future, we will further expand the range of years and the number of samples for further study. Third, further investigation is warranted to explore the specific mechanism of distribution specificity and drug resistance of pathogenic bacteria for UTIs.
To sum up, this study found that the pathogens of urinary tract calculi complicated with UTI were mainly Gram-negative bacteria, and Escherichia coli was the main pathogen causing infection. Gender and age might be risk factors for urinary calculi complicated with UTI. Moreover, antibiotics should be selected reasonably according to the drug resistance pattern of pathogenic bacteria in clinical anti-infection management.
Acknowledgements
This study is financially supported by the funding for the analysis of bacterial distribution and drug resistance of urinary calculi.
Disclosure of conflict of interest
None.
References
- 1.Wagenlehner FME, Bjerklund Johansen TE, Cai T, Koves B, Kranz J, Pilatz A, Tandogdu Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020;17:586–600. doi: 10.1038/s41585-020-0362-4. [DOI] [PubMed] [Google Scholar]
- 2.Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G, Choroszy-Krol I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 2019;11:10. doi: 10.1186/s13099-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh KJ, Mundy L, Holter JJ, Johnson JR. Analysis of urine-specific antibiograms from veterans to guide empiric therapy for suspected urinary tract infection. Diagn Microbiol Infect Dis. 2019;95:114874. doi: 10.1016/j.diagmicrobio.2019.114874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staji H, Rassouli M, Jourablou S. Comparative virulotyping and phylogenomics of Escherichia coli isolates from urine samples of men and women suffering urinary tract infections. Iran J Basic Med Sci. 2019;22:211–214. doi: 10.22038/ijbms.2018.28360.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein RD, Hultgren SJ. Urinary tract infections: microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat Rev Microbiol. 2020;18:211–226. doi: 10.1038/s41579-020-0324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geerlings SE. Clinical presentations and epidemiology of urinary tract infections. Microbiol Spectr. 2016;4:5. doi: 10.1128/microbiolspec.UTI-0002-2012. [DOI] [PubMed] [Google Scholar]
- 7.Yongzhi L, Shi Y, Jia L, Yili L, Xingwang Z, Xue G. Risk factors for urinary tract infection in patients with urolithiasis-primary report of a single center cohort. BMC Urol. 2018;18:45. doi: 10.1186/s12894-018-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherng JH, Hsu YJ, Liu CC, Tang SH, Sartika D, Chang SJ, Fan GY, Wu ST, Meng E. Activities of Ca(2+)-related ion channels during the formation of kidney stones in an infection-induced urolithiasis rat model. Am J Physiol Renal Physiol. 2019;317:F1342–F1349. doi: 10.1152/ajprenal.00199.2019. [DOI] [PubMed] [Google Scholar]
- 9.Barr-Beare E, Saxena V, Hilt EE, Thomas-White K, Schober M, Li B, Becknell B, Hains DS, Wolfe AJ, Schwaderer AL. The Interaction between Enterobacteriaceae and Calcium Oxalate Deposits. PLoS One. 2015;10:e0139575. doi: 10.1371/journal.pone.0139575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanetti G, Paparella S, Trinchieri A, Prezioso D, Rocco F, Naber KG. Infections and urolithiasis: current clinical evidence in prophylaxis and antibiotic therapy. Arch Ital Urol Androl. 2008;80:5–12. [PubMed] [Google Scholar]
- 11.Hsiao CY, Chen TH, Lee YC, Hsiao MC, Hung PH, Chen YY, Wang MC. Urolithiasis is a risk factor for uroseptic shock and acute kidney injury in patients with urinary tract infection. Front Med (Lausanne) 2019;6:288. doi: 10.3389/fmed.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asadi Karam MR, Habibi M, Bouzari S. Urinary tract infection: pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol Immunol. 2019;108:56–67. doi: 10.1016/j.molimm.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Holm A, Cordoba G, Aabenhus R. Prescription of antibiotics for urinary tract infection in general practice in Denmark. Scand J Prim Health Care. 2019;37:83–89. doi: 10.1080/02813432.2019.1569425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai Y, Liu Q, Gu J, Zhang X, Hu S. Analysis of urinary pathogen cultures and drug sensitivity in patients with urinary stones for five consecutive years in Xiangya Hospital, China. Infect Drug Resist. 2020;13:1357–1363. doi: 10.2147/IDR.S241036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubbs SB, Sommerkamp SK. Evaluation and management of urinary tract infection in the emergency department. Emerg Med Clin North Am. 2019;37:707–723. doi: 10.1016/j.emc.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Gajdacs M, Abrok M, Lazar A, Burian K. Increasing relevance of Gram-positive cocci in urinary tract infections: a 10-year analysis of their prevalence and resistance trends. Sci Rep. 2020;10:17658. doi: 10.1038/s41598-020-74834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafalskiy V, Pushkar D, Yakovlev S, Epstein O, Putilovskiy M, Tarasov S, Glazunov A, Korenev S, Moiseeva E, Gorelysheva N. Distribution and antibiotic resistance profile of key Gram-negative bacteria that cause community-onset urinary tract infections in the Russian Federation: RESOURCE multicentre surveillance 2017 study. J Glob Antimicrob Resist. 2020;21:188–194. doi: 10.1016/j.jgar.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Hossain A, Hossain SA, Fatema AN, Wahab A, Alam MM, Islam MN, Hossain MZ, Ahsan GU. Age and gender-specific antibiotic resistance patterns among Bangladeshi patients with urinary tract infection caused by Escherichia coli. Heliyon. 2020;6:e04161. doi: 10.1016/j.heliyon.2020.e04161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Zhang Y, Zhang X, Tang Y, Li J. Upper urinary tract stone compositions: the role of age and gender. International Braz J Urol. 2020;46:70–80. doi: 10.1590/S1677-5538.IBJU.2019.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal M, Manzoor A, Hussain M. Gender differences in the microbiology of urinary tract infections in urolithiasis patients. Pak J Surg. 2017;33:269–272. [Google Scholar]
- 21.De Lorenzis E, Alba AB, Cepeda M, Galan JA, Geavlete P, Giannakopoulos S, Saltirov I, Sarica K, Skolarikos A, Stavridis S, Yuruk E, Geavlete B, Garcia C, Hristoforov S, Karagoz MA, Nassos N, Jurado GO, Paslanmaz F, Poza M, Saidi S, Tzelves L, Trinchieri A. Bacterial spectrum and antibiotic resistance of urinary tract infections in patients treated for upper urinary tract calculi: a multicenter analysis. Eur J Clin Microbiol Infect Dis. 2020;39:1971–1981. doi: 10.1007/s10096-020-03947-z. [DOI] [PubMed] [Google Scholar]
- 22.Magliano E, Grazioli V, Deflorio L, Leuci AI, Mattina R, Romano P, Cocuzza CE. Gender and age-dependent etiology of community-acquired urinary tract infections. ScientificWorldJournal. 2012;2012:349597. doi: 10.1100/2012/349597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeed A, Hamid SA, Bayoumi M, Shanan S, Alouffi S, Alharbi SA, Alshammari FD, Abd H. Elevated antibiotic resistance of Sudanese urinary tract infection bacteria. EXCLI J. 2017;16:1073–1080. doi: 10.17179/excli2017-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolle LE. Resistant pathogens in urinary tract infections. J Am Geriatr Soc. 2002;50:S230–235. doi: 10.1046/j.1532-5415.50.7s.3.x. [DOI] [PubMed] [Google Scholar]