Abstract
Ras and Rho family GTPases have been ascribed important roles in signalling pathways determining cellular morphology and growth. Here we investigated the roles of the GTPases Ras, Cdc42, Rac1, and Rho and that of phosphatidylinositol 3-kinase (PI 3-kinase) in the pathway leading from serum starvation to neurite outgrowth in N1E-115 neuroblastoma cells. Serum-starved cells grown on a laminin matrix exhibited integrin-dependent neurite outgrowth. Expression of dominant negative mutants of Ras, PI 3-kinase, Cdc42, or Rac1 all blocked this neurite outgrowth, while constitutively activated mutants of Ras, PI 3-kinase, or Cdc42 were each sufficient to promote outgrowth even in the presence of serum. A RasH40C;G12V double mutant which binds preferentially to PI 3-kinase also promoted neurite formation. Activated RasG12V-induced outgrowth required PI 3-kinase activity, but activated PI 3-kinase-induced outgrowth did not require Ras activity. Although activated Rac1 by itself did not induce neurites, neurite outgrowth induced by activated Cdc42G12V was Rac1 dependent. Cdc42G12V-induced neurites appeared to lose their normal polarization, almost doubling the average number of neurites produced by a single cell. Outgrowth induced by activated Ras or PI 3-kinase required both Cdc42 and Rac1 activity, but Cdc42G12V-induced outgrowth did not need Ras or PI 3-kinase activity. Active RhoG14V reduced outgrowth promoted by RasG12V. Finally, expression of dominant negative Jun N-terminal kinase or extracellular signal-regulated kinase did not inhibit outgrowth, suggesting these pathways are not essential for this process. Our results suggest a hierarchy of signalling where Ras signals through PI 3-kinase to Cdc42 and Rac1 activation (and Rho inactivation), culminating in neurite outgrowth. Thus, in the absence of serum factors, Ras may initiate cell cycle arrest and terminal differentiation in N1E-115 neuroblastoma cells.
The Ras p21 GTPases have been shown to play important roles in many aspects of cell growth, proliferation, and differentiation. Mutant constitutively activated versions of Ras have been shown to promote cell growth in some cell types, often resulting in a transformed phenotype, and such Ras mutations have been identified in many naturally occurring tumors (1, 4, 5). In some cases, however, activation of Ras causes cell cycle arrest rather than cell proliferation (29, 52, 56).
Mutants of Ras with preferential binding to selective effectors have been used in an attempt to determine which effectors are involved in a particular Ras-dependent response. Work with these Ras mutants has suggested that at least three pathways which are dependent on various Ras effectors act downstream from Ras to evoke cell transformation. The best characterized of these effectors are those belonging to the Raf family, where Ras causes translocation of Raf to the plasma membrane and the mitogen-activated protein kinase cascade is activated (39). The effector domain of Ras has also been found to interact with the Ral guanine nucleotide dissociation stimulator (RalGDS) in the yeast two-hybrid system (15), but the significance of this interaction is unclear. In addition, the Ras effector region binds to the catalytic p110α subunit of phosphatidylinositol 3-kinase (PI 3-kinase) (54). These three pathways have been shown to act together in the process of cell transformation (20, 54, 64).
Ras has also been shown to have clear effects on the actin cytoskeleton. When microinjected into fibroblasts, Ras stimulated membrane ruffling (3). This activity is Rac1 dependent (53) and could also be inhibited by a dominant negative mutant of the PI 3-kinase regulatory subunit, Δp85 (53). Active PI 3-kinase was also able to generate Rac1-dependent ruffling (50), indicating a hierarchy of activation from Ras to Rac1 through PI 3-kinase in these fibroblasts.
The Rho family GTPases, Cdc42, Rac1, and Rho, are also able to transform cells to various degrees. Results from the use of dominant negative mutants of these GTPases indicate that they could act downstream from Ras in transforming cells (25, 48–50). Cdc42 is likely to act downstream of Ras in two instances: in the yeast Saccharomyces cerevisiae, Ras2 acts upstream of Cdc42 in the initiation of filamentous growth (41); in the case of Ras-dependent cell transformation, Cdc42 plays a role in the development of anchorage-independent growth (50). However little else is known of the role that Cdc42 may play in other Ras-dependent biological events in higher eucaryotes.
Cdc42 has been shown to bind to the p85 regulatory subunit of PI 3-kinase (66) in a GTP-dependent manner (61), and Rac1 acts downstream from Ras and PI 3-kinase in the case of membrane ruffling (13). Despite this evidence, Rac1 has also been shown to have effects consistent with an action upstream of PI 3-kinase. For example, epithelial polarization was disrupted by activated mutants of Rac1, Cdc42, or PI 3-kinase, but the Rac1 or Cdc42 effects were blocked by the PI 3-kinase inhibitor wortmannin or LY294002 (24).
In contrast to the situation in nonneural cells, Ras has been shown to induce neurite outgrowth (2, 6, 44) as well as being involved in neural plasticity stimulated by muscarinic receptor activation (7). PI 3-kinase has also been implicated in neurite formation, as wortmannin or Δp85 could inhibit nerve growth factor (NGF)-induced neurite outgrowth (18, 26), and activated PI 3-kinase leads to neurite-like process formation in PC12 cells (27, 28). However, the evidence for PI 3-kinase involvement in neurite outgrowth is somewhat contradictory, as studies using receptor mutants of Trk suggest that PI 3-kinase is not crucial for neurite outgrowth in PC12 cells (45, 57). Recently it has been shown that expression of a dominant negative Jun N-terminal kinase (JNK) suppresses neurite-like process formation in PC12 cells induced by expression of an activated PI 3-kinase (27), implicating a requirement for JNK cascade in neurite outgrowth downstream of PI 3-kinase.
The Rho family GTPases have the ability to alter various actin-derived morphologies in a variety of cells and have been ascribed important roles in neuritogenesis. Rac1 is involved in axonal growth and guidance (22, 36) and dendritic formation (37, 59) and is required for neurite formation (32, 33, 63). Cdc42 is involved in filopodium formation and neurite outgrowth, and Rho is responsible for causing serum-dependent neurite retraction in N1E-115 cells (19, 32, 63). Cdc42 signals through Rac1 to produce membrane ruffling in both neuroblastoma cells and fibroblasts (30, 32, 43), and the activities of Rho appear to compete with Cdc42 and Rac1 in N1E-115 cells (32, 63).
We have examined the role of Ras in neuroblastoma cells and present evidence that Ras is important for integrin-dependent neurite outgrowth in neuroblastoma cells following serum deprivation; that PI 3-kinase is an important mediator for this response; that Ras and PI 3-kinase-induced outgrowth is mediated by both Cdc42 and Rac1 activity; that Cdc42 but not Rac1 is sufficient to induce outgrowth, although Rac1 activity is sufficient for cell flattening; and finally that active Rho competes with Ras to inhibit neurite outgrowth.
MATERIALS AND METHODS
Expression constructs and preparation of plasmid DNA.
Ras and Ras mutant cDNAs in pCDNA3, and p110α, p110CAAX, and RafCAAX in pSG5, were obtained from J. Downward (54); pEXV3-MEK-AL (dominant negative MEK) was obtained from C. Marshall; pXJ-HA-SEK-AL (dominant negative SEK1) was obtained from J. Woodgett (55); hemagglutinin epitope (HA)-tagged Rho family p21s and mutants were expressed in pXJ-HA vectors (38). Plasmids were expressed in Escherichia coli JM109 competent cells in selective Luria-Bertani medium grown to a density of approximately 109 cells/ml. They were then purified by passage through a Qiagen-tip anion-exchange column according to the manufacturer's protocol (Qiagen).
Cell culture and cell adhesion experiments.
N1E-115 cells were grown in Dulbecco's modified Eagle medium–10% fetal calf serum supplemented with penicillin, streptomycin, and amphotericin (all from Gibco) at 37°C in an atmosphere of humidified air and 5% CO2. Cells were seeded at a density of 4 × 105 per slide onto glass slides which had been previously coated with laminin (10 μg/ml; ICN) for 1 h at room temperature, washed twice with water, and left to air dry. For adhesion experiments, slides were coated with fibronectin (10 μg/ml; ICN) at 4°C overnight or poly-l-lysine (5 μg/ml; Sigma) for 5 min at room temperature. For adhesion following incubation with β-1 integrin antibody (ICN), cells were treated with increasing concentrations of β-1 integrin antibody for 5 min prior to plating and then seeded onto laminin-coated slides as described above.
Transient transfection.
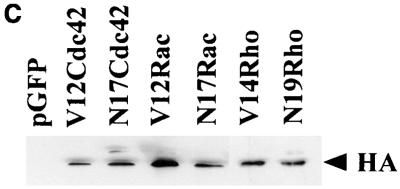
At 16 to 20 h following plating on laminin-coated slides, cells were transfected with the plasmid containing the cDNA of interest mixed in a 3:1 ratio with the plasmid encoding green fluorescent protein (GFP; gift from J. C. Martineau and A. Nichols). Transfection was carried out with Lipofectamine transfection reagent (GibcoBRL). Briefly, cells were incubated in serum-free medium for 1 h. During this time, the plasmids of interest were mixed with the Lipofectamine reagent and incubated for 45 min at room temperature, whereupon they were added to the serum-starved cells. Five percent serum was added to the cells 5 h after addition of the transfection mix; 20 h later, the cells were fixed in phosphate-buffered saline (PBS) containing 3% paraformaldehyde for 20 min. Cells which had been positively transfected with GFP were assumed to be expressing the protein of interest, as during transfection the concentration of GFP DNA was a third of that of the test DNA. We could not obtain antibodies that would reliably stain Ras or PI 3-kinase in situ; however, HA staining of the HA-tagged Rho family proteins confirmed that expression of GFP and the plasmid under investigation did indeed correspond. Additionally, it was found by immunoblotting that levels of protein expression were unchanged by cotransfection of more than one vector (see Fig. 3C). The various morphologies of the green cells were then assessed and scored. Cells were categorized as being rounded, flattened, or neurite bearing (cells with a process of at least the length of one cell body were considered neurite bearing). The percentage of the total number of GFP-expressing cells that was either flattened or neurite bearing was recorded. To test for the effects of anti-β-1 integrin antibody on transfected cells, cells were plated for 24 h, transfected, pipetted off, and mixed with the antibody for 5 min prior to replating.
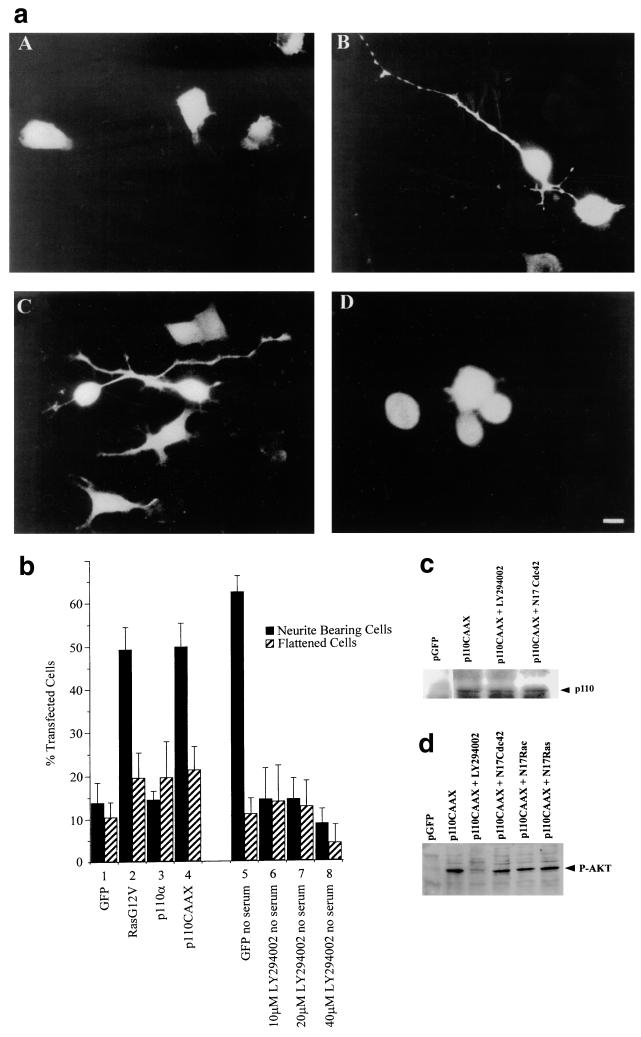
FIG. 3.
Involvement of PI 3-kinase in Ras-induced neurite outgrowth. (a) Cells were transiently cotransfected with constructs coding for GFP (A), RasG12V (B), p110CAAX (C), and p110α (D). Slides were fixed and mounted 20 h after transfection, and GFP-expressing cells were analyzed. Note the appearance of the relatively unbranched single neurites produced by RasG12V- and p110CAAX-expressing cells in panels B and C. (b) The morphology of transfected cells was scored as for Fig. 2; cells with a neurite longer than the length of one cell body were scored as neurite bearing, and large ruffled cells were scored as flattened. Numbers were expressed as a percentage of the total number of GFP-expressing cells. Transfection of cells in lanes 1 to 6 was carried out in the presence of serum; transfections in lanes 7 to 11 were performed in the absence of serum. LY294002 was added with the DNA under test, and cells were viewed in a fluorescence microscope. At least 20 randomly selected fields of view were assessed, and each experiment was repeated at least three times. Standard deviations are shown as vertical bars. (c) Expression of p110CAAX vector in N1E-115 cells. Following transfection, cell lysates were prepared, and equal quantities of proteins were electrophoresed on polyacrylamide gels, Western immunoblotted, and probed with anti-p110 antibodies. (d) Activation of Akt in N1E-115 cells by p110CAAX. Following transfection with expression vectors, cell lysates were prepared, and equal quantities of proteins were electrophoresed through polyacrylamide gels, Western immunoblotted, and probed with anti-phospho-Akt antibodies.
Western immunoblotting.
Following transfection with appropriate vectors, cell lysates were prepared, protein concentrations were determined by Bio-Rad protein assay, and equal amounts were loaded per lane. Polyacrylamide gels were run and blotted onto nitrocellulose filters as previously described (31). Antibodies used were phospho-JNK (G7) mouse monoclonal antibody and Ras 259 (sc-035) rat monoclonal antibody (Santa Cruz), phospho-Akt polyclonal antibody (New England Biolabs), pan-Ras OP41 antibody (Calbiochem), and HA monoclonal antibody (Boehringer). The p110 antibody was a gift from Stefan Weenstrom. Following incubations with primary and secondary peroxidase-linked antibodies (Dako) as previously described (31), the filters were incubated with enhanced chemiluminescence reagent (Amersham) and then exposed to Amersham Hyperfilm.
Immunofluorescence.
N1E-115 cells were grown on laminin-coated chamber slides as described above. Following transfection, cells were fixed in 3% paraformaldehyde for 20 min at room temperature, the paraformaldehyde was quenched with 100 mM glycine for 10 min, and the cells were then permeabilized with 0.2% Triton X-100 for 10 min. Slides were washed briefly and then blocked with 3% bovine serum albumin in PBS for 10 min. All antibody incubations were performed in PBS–1% bovine serum albumin and washed three times over 12 min between each incubation. Primary antibodies used were anti-HA (Boehringer) at 5 μg/ml to confirm positive transfection of Cdc42, Rac1, or RhoA mutants in addition to GFP expression. Monoclonal anti-neurofilament 200 clone NE14 (Sigma) was also used to confirm expression of this neural protein in neurite-bearing cells. Cells were incubated with primary antibodies for 1 h at 37°C in a humidified chamber, washed, and incubated for a further 1 h at 37°C with rhodamine-conjugated rabbit-anti mouse immunoglobulin G monoclonal second antibody (Dako). Rhodamine-conjugate phalloidin (1 μg/ml; Sigma) was occasionally used in the final incubation to stain F-actin. Slides were mounted in Mowiol–2.5% DABCO [1,4-diazabicyclo(2,2,2)octane] in a 2.5% (wt/vol) glycerol solution–0.1 M Tris (pH 8.5) (Aldrich) and viewed under a Zeiss Axioplan fluorescence microscope. Slides were photographed using Ektachrome 400 ASA color slide film (Kodak).
Other materials.
LY294002 was obtained from Sigma, and cells were incubated with various concentrations of this inhibitor overnight following transfection.
RESULTS
Neurite outgrowth following serum starvation on laminin requires the β-1 integrin subunit.
The mouse N1E-115 neuronal cell line exhibits neurite outgrowth in response to serum deprivation (19, 32). We carried out experiments to determine the signalling pathways involved in this phenomenon. In the absence of serum, rounded neuroblastoma cells initially became more flattened and then produced filopodia and lamellipodia around their circumference, which gradually polarized to one or two discrete regions (data not shown). The cells gradually extended neurites from these areas in the subsequent 16 to 24 h. The immature neurites continued to produce filopodia and lamellipodia along their lengths, presumably in response to various extracellular guidance cues, such as the extracellular matrix and various chemoattractants and repellents, as well as autocrine factors.
To investigate the effects of the extracellular matrix on neurite outgrowth, N1E-115 neuroblastoma cells were grown in serum-free conditions on glass slides that had been coated with either fibronectin, poly-l-lysine, or laminin. During the 24 h following plating, the adherent cells were counted and the morphologies of the cells were assessed. Within an hour of being plated on laminin, the cells had adhered to the slides singly and exhibited a flattened morphology; 24 h following plating, approximately 50% of the cells possessed a neurite (Fig. 1a, lane 4). A neurite was defined as a process that measured at least the length of one cell body and stained positively for neurofilament protein. Cells plated on fibronectin did not adhere quickly and often formed clumps in suspension, and although there were similar numbers of cells growing on the slide after 24 h compared to plating on laminin (Fig. 1b, lane 1), they were generally found in large groups with rounded morphologies, often with cells adhering to each other rather than the slide. None of the cells possessed neurites after 24 h of starvation (Fig. 1a, lane 1). Cells seeded onto poly-l-lysine-coated slides adhered more quickly than those seeded onto fibronectin but not as fast as cells plated onto laminin-coated slides. The number of adherent cells on poly-l-lysine after 24 h was equivalent to that seen with laminin (Fig. 1b, lane 2); however, very few of the cells possessed neurites after serum starvation (Fig. 1a, lane 2).
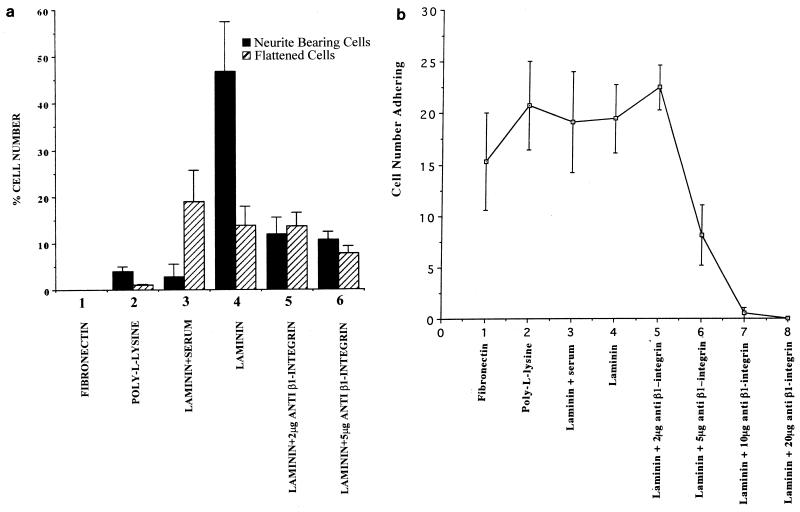
FIG. 1.
Integrin-dependent N1E-115 cell adherence and neurite outgrowth when cells are grown on laminin. (a) The number of cells possessing neurites of at least the length of one cell body or exhibiting a flattened morphology was assessed after plating on the different matrices or following pretreatment with anti-β-1 integrin antibody. Cells were stained with rhodamine-conjugated phalloidin. Numbers are shown as a percentage of each set of 50 cells counted possessing either a neurite or a spread cell body. At least 200 cells were randomly examined, and standard deviations are indicated as vertical bars. (b) The number of cells adhering to a glass chamber slide which had been coated with different extracellular matrices was analyzed by fixing the cells 24 h following plating and staining with rhodamine-conjugated phalloidin. The number of cells per randomly selected field of view was determined on a Zeiss Axioplan fluorescence microscope, and results were compared. At least 20 fields of view were counted for each slide, and the experiments were repeated three times. Cell numbers are shown as the mean number of cells per field of view plus standard deviations shown as vertical bars.
We briefly preincubated cells with increasing concentrations of an antibody directed against β-1 integrin and then plated them on laminin-coated glass slides in serum-free medium. As can be seen from Fig. 1b, lanes 6 to 8, higher concentrations of antibody effectively blocked adhesion of the cells to the substrate. Although plating the cells in lower concentrations of antibody (2 μg/ml) did not affect the number of cells that were present (Fig. 1b, compare lanes 4 and 5), it did lower the number of neurite bearing cells (Fig. 1a, compare lanes 4 and 5). Although it was hard to precisely measure the level of adherence of each cell, these results show that laminin is a particularly permissive substrate for neurite outgrowth in these cells and that β-1 integrin is involved both in adhesion of these cells to the substrate and in neuritogenesis.
Ras is required for neurite outgrowth in N1E-115 cells, and activated Ras can stimulate neurite outgrowth.
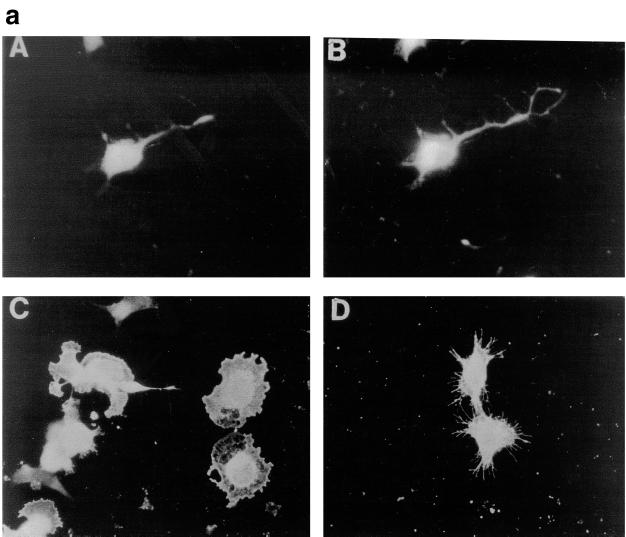
Studies have shown that oncogenic H-ras is involved in nerve cell differentiation (2, 6). To determine whether Ras is involved in N1E-115 neurite outgrowth on laminin, we transfected N1E-115 neuroblastoma cells that had been plated on laminin-coated glass slides with wild-type Ras and various Ras mutants. The ability of all these molecules to induce neurite outgrowth was measured by cotransfecting them with a GFP marker and comparing the morphologies of the Ras mutant-GFP cotransfected cells and cells transfected with GFP alone. Expression of Ras protein was detected by immunoblotting (Fig. 2c). Transfection of activated RasG12V was able to induce significant neurite outgrowth compared with controls (Fig. 2a, panel A; Fig. 2b, compare lanes 1 and 2), and this outgrowth could be inhibited by prior treatment with the antibody against β-1 integrin (data not shown) and was seen only on a laminin extracellular matrix. In addition, we tested three other mutants for their effects on neurite outgrowth: RasE37G;G12V, which is unable to bind to Raf1 or p110α, the catalytic region of PI 3-kinase, but can bind to RalGDS; RasT35S;G12V, which binds Raf1 but not RalGDS or p110α; and RasH40C;G12V, which can bind to p110α but not RalGDS or Raf1 (48). RasH40C;G12V induced an increase in outgrowth but to a lesser extent than RasG12V (Fig. 2a, panel F; Fig. 2b, lane 3). Wild-type Ras, RasE37G;G12V, and RasT35S;G12V induced no significant outgrowth (Fig. 2a, panels C to E; Fig. 2b, lanes 4 and 5), although RasT35S;G12V gave a slight increase but at a later time point (around 30 to 48 h following transfection [data not shown]). A combined transfection of all three mutants induced outgrowth to a level comparable to that seen following RasG12V transfection (Fig. 2b, compare lanes 2 and 6). Conversely, transfection of pairs of the mutants did not increase outgrowth to levels any higher than transfection of single mutants (Fig. 2b, lanes 7 and 8). Dominant negative RasT17N was able to inhibit the neurite outgrowth induced by the absence of serum following transfection (Fig. 2b, compare lanes 11 and 12). These results suggest a requirement for activated Ras in the induction of neurites caused by serum starvation and also show that activated Ras itself is sufficient to induce neurite outgrowth even in the presence of serum, but this is dependent on β-1 integrin function. Additionally, the results suggest that PI 3-kinase may be involved in this pathway, since expression of the Ras mutant RasH40C;G12V, which binds selectively to p110α, also produced the highest level of neurite outgrowth of the three Ras mutants. However, RasH40C;G12V did not give the same level of outgrowth as RasG12V, whereas cotransfection of the three mutants was able to induce similar levels, suggesting that all three pathways could play some role in outgrowth. There is also a basal level of neurite outgrowth shown in the control sample (Fig. 2b, lane 1) which is not completely abolished by transfection of RasT17N (Fig. 2b, lane 9), nor does the dominant negative Ras totally inhibit serum-starved neurite induction (Fig. 2b, compare lanes 11 and 12). This may be because levels of expression vary so that not all cells are producing enough protein to alter their response or that there is a population of cells which are independent of Ras with respect to neurite outgrowth. To investigate the possibility that other pathways known to be activated by Ras are involved in neurite outgrowth, in addition to the putative involvement of PI 3-kinase, cells were transfected with a Raf-CAAX expression vector. Outgrowth was not seen during the normal time course of 24 h; however, after 48 h there was some neurite formation, most likely indicating the stimulation of an indirect pathway (data not shown).
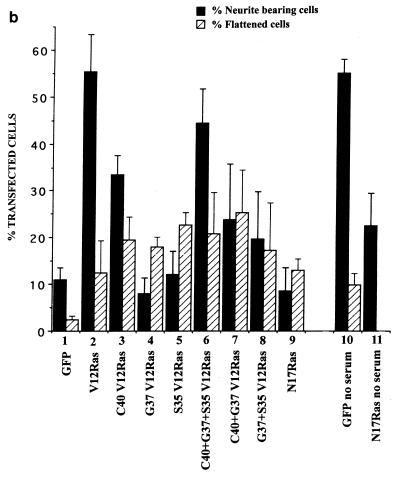
FIG. 2.
Effects of transfection of Ras and Ras mutants on neurite outgrowth. (a) Fluorescence photographs of cells transiently transfected with RasG12V (A and B), wild-type Ras (C), RasT35S;G12V (D), RasE37G;G12V (E), and RasH40C;G12V (F); 20 h following cotransfection with GFP, cells were fixed, stained, and mounted, and the cells expressing GFP were analyzed. Cells were also stained for the neural cell protein neurofilament, shown to be present in the RasG12V-expressing cell (B). (b) Quantification of morphological changes. The morphology of cells expressing GFP was assessed and scored as a percentage of the total number of transfected cells. Cells were designated as neurite bearing if they possessed a neurite at least the length of the cell body and as flattened if they had a large ruffled cell body. Transfections were carried out in the presence of 5% serum except for lanes 8 and 9, in which case transfections were performed in the absence of serum. At least 20 randomly selected fields of view were assessed, and each experiment was repeated at least three times. Standard deviations are shown as vertical bars. (c) Expression of Ras vector constructs in N1E-115 cells. Following transfection, cell lysates were prepared, and equal quantities of proteins were electrophoresed through polyacrylamide gels, Western immunoblotted, and probed with anti-Ras antibodies. wtRas, wild-type Ras.
Ras-induced outgrowth requires PI 3-kinase activity, and p110CAAX transfection results in neurite formation.
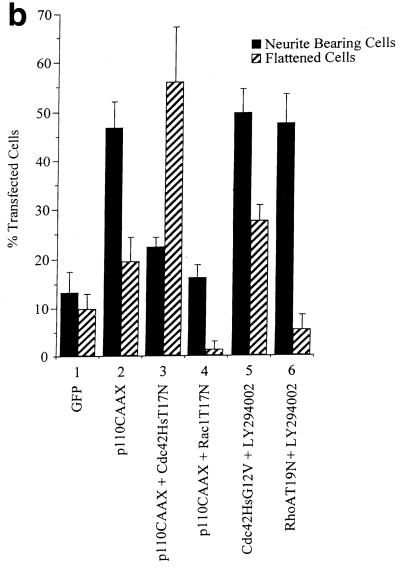
To investigate further the involvement of PI 3-kinase in neurite outgrowth induced by Ras, RasG12V-transfected N1E-115 cells were treated with the PI 3-kinase inhibitor LY294002. LY294002 treatment inhibited outgrowth (data not shown). To determine whether PI 3-kinase itself could induce neurite outgrowth, the cells were transfected with various PI 3-kinase mutants. Cells transfected with p110α, the catalytic subunit of PI 3-kinase, exhibited no increase in neurite outgrowth (Fig. 3a, panel D; Fig. 3b, lane 3). Cells transfected with an activated form of PI 3-kinase, p110CAAX (51), however, showed very rapid neurite outgrowth shortly after transfection, and 16 to 20 h following transfection a relatively high proportion of transfected cells possessed neurites (Fig. 3a, panel C; Fig. 3b, lane 4). p110CAAX-induced neurite outgrowth could also be blocked by pretreatment with anti β-1 integrin (data not shown). The morphologies of the RasG12V-transfected, p110CAAX-transfected, and serum-starved cells were similar, in that most of the cells were polarized. They possessed long, thin, and fairly straight, unbranched neurites, with most cells bearing one neurite only and some possessing two processes, but rarely more than two. Interestingly, 24 to 30 h after transfection, the p110CAAX-transfected cells had retracted their neurites, the cell bodies had condensed, and the cells had begun to detach from the substrate. The number of living cells over time was substantially less in this sample than that of the control cells (data not shown). Thus, p110CAAX seems to hasten both the differentiation process and the subsequent apoptotic cell death.
To investigate the role of PI 3-kinase in neurite outgrowth induced by serum withdrawal, cells were treated with the PI 3-kinase inhibitor LY294002. Cells were maintained in the absence of serum, and outgrowth was monitored. To confirm the efficacy of this inhibitor, Western immunoblotting to detect the active (phosphorylated) form of a known substrate of PI 3-kinase, Akt (protein kinase B), was carried out (Fig. 3d). This analysis showed that levels of an active, phosphorylated form of Akt decreased in cells in the presence of LY294002. Treatment with LY294002 did indeed block outgrowth caused by serum deprivation (Fig. 3b, lanes 5 to 8). These results show an involvement of PI 3-kinase in neurite outgrowth due to both serum starvation and RasG12V transfection and that membrane association of the catalytic subunit is presumably an important factor in this process. PI 3-kinase activity appears to be a requirement of Ras-induced outgrowth, as this type of neurite induction could be blocked by LY294002; conversely, neurite outgrowth stimulated by p110CAAX transfection was not affected by RasT17N transfection (data not shown); thus, PI 3-kinase appears to act downstream of Ras in this morphological pathway.
Cdc42G12V stimulates neurite outgrowth which requires Rac1 activity.
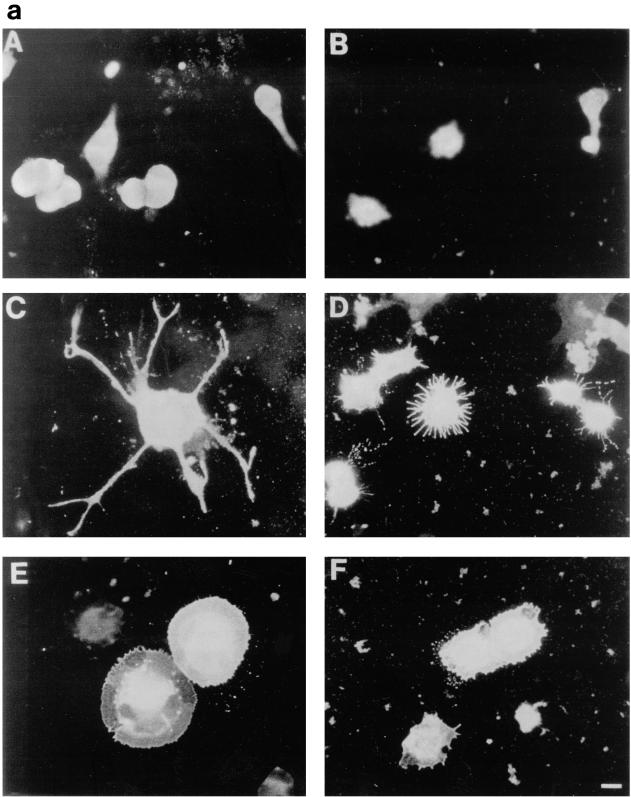
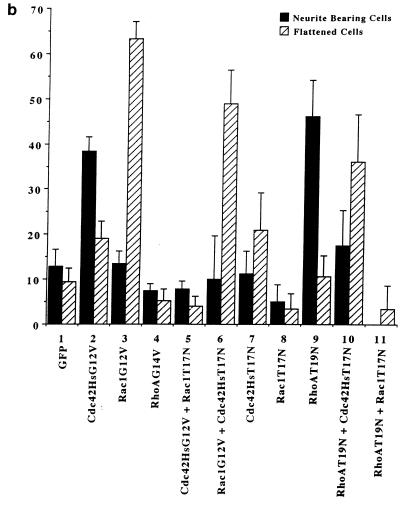
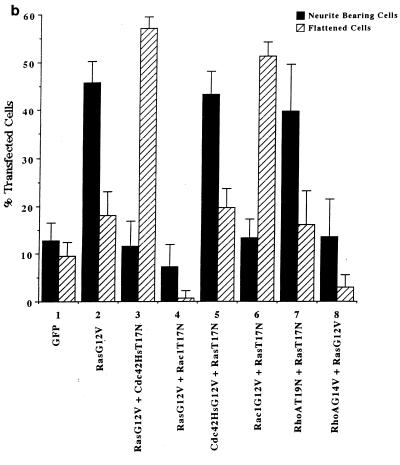
We have previously shown a requirement for Cdc42 and Rac1 in neurite outgrowth (32). In the present study, using DNA transfection, we were able to show that expression of Cdc42G12V induced neurite outgrowth and an increase in cell flattening in the presence of serum on laminin-coated glass slides but not on poly-l-lysine- or fibronectin-coated glass slides (Fig. 4b, lane 2). Furthermore, outgrowth could be inhibited by incubating the transfected cells with anti-β-1 integrin antibody prior to plating (data not shown). The appearance of the Cdc42G12V-induced neurites was different from that seen following serum starvation, RasG12V or p110CAAX transfection. As can be seen in Fig. 4a, panel C, Cdc42G12V-transfected cells possessed several short and branched neurites, an average of 3.8 neurites per cell, compared to an average of 2.0 neurites per cell for control-starved cells. RasG12V or p110CAAX transfection produced longer, straighter, and less branched neurites than those seen after Cdc42HsG12V transfection, with an average of 1.8 or 1.7 neurites per cell, respectively. Transfection of Rac1G12V did not produce neurites in serum-containing medium in this study (Fig. 4b, lane 3); instead, the cells became greatly flattened and spread, with a large actin ruffle all around the circumference of the cell, exhibiting a characteristic morphology rather resembling fried eggs (Fig. 4a, panel E). Western immunoblotting was used to confirm the expression of HA-tagged proteins (Fig. 4c). At later time points (up to 72 h [data not shown]) the cells showed no significant changes in morphology, indicating that effects of Cdc42G12V and Rac1G12V transfection on these cells are indeed different. Cotransfection of Cdc42G12V and Rac1G12V still produced cells with multiple neurites, showing that another signal must be required for normal production of polarized neurites (data not shown). In contrast, RhoG14V transfection caused the cell bodies to contract and become very small (Fig. 4a, panel B), with almost no outgrowth or flattening (Fig. 4b, lane 4). Cotransfection of Cdc42G12V with dominant negative Rac1T17N inhibited the Cdc42G12V-induced outgrowth (Fig. 4b, lane 5), but again the cells exhibited a characteristic morphology. The cell bodies remained rounded and small but possessed large numbers of very short spikes around the periphery, giving them a “hairy” appearance (Fig. 4a, panel D). Transfection of dominant negative mutant Cdc42T17N with dominant positive mutant Rac1G12V still produced a Rac1-type morphology, i.e., flattened, fried-egg-type cells (Fig. 4a, panel F; Fig. 4b, lane 6). These results indicate that Cdc42G12V can induce neurite outgrowth even though normal cell polarization appears to be affected. Rac1 is necessary for this outgrowth but is not sufficient to drive it. As might be expected from previous studies using C3 toxin, RhoT19N transfection induced neurite outgrowth in these cells (Fig. 4b, lane 9); this outgrowth was inhibited by Cdc42T17N cotransfection, giving an increase in flattened cells (Fig. 4b, lane 10), and was also inhibited by cotransfection of Rac1T17N, producing very spiky or hairy cells (Fig. 4b, lane 11).
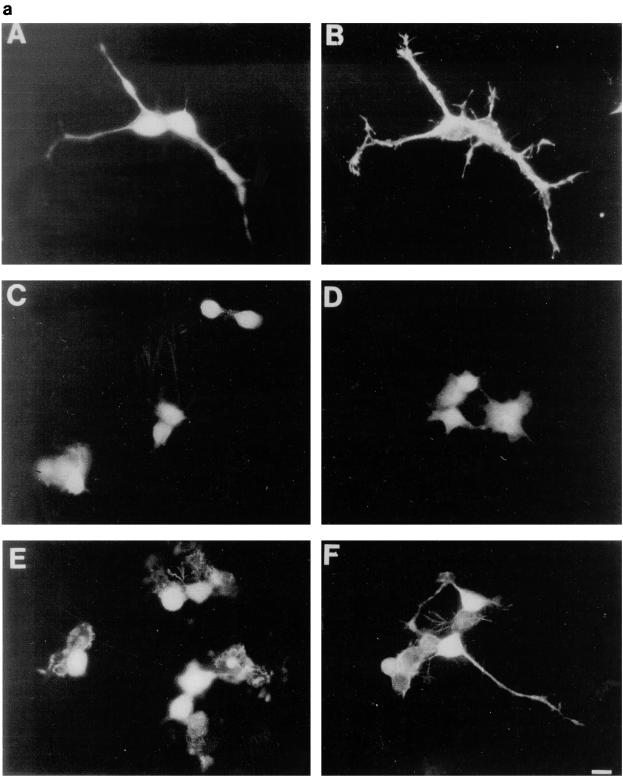
FIG. 4.
Cdc42G12V promotes neurite outgrowth dependent on Rac activity. (a) N1E-115 cells were transiently transfected with pGFP and plasmids expressing various HA-tagged p21 GTPase mutants and then stained with anti-HA as follows: (A) rounded GFP-expressing cells; (B) condensed densely stained RhoG14V-expressing cells; (C) Cdc42G12V-expressing cells with many branched neurites, unlike those seen on RasG12V or p110CAAX expression; (D) cells coexpressing Cdc42G12V and Rac1T17N, which are rounded, spiky, and hairy; (E) Rac1G12V-expressing flattened cells with extensive peripheral ruffling and a fried-egg appearance, which was not altered by cotransfection of Cdc42T17N (F). (b) Quantification of morphological changes produced by Rho family GTPase transfection. Cells were stained with anti-HA, and positively stained cells were scored as for Fig. 2 and 3. Expression of GFP and HA correlated in all cases. At least 20 randomly selected fields of view were assessed, and each experiment was repeated at least three times. Standard deviations are shown as vertical bars. (c) Expression of Rho family vector constructs in N1E-115 cells. Following transfection, cell lysates were prepared, and equal quantities of proteins were electrophoresed through polyacrylamide gels, Western immunoblotted, and probed with anti-HA antibodies.
Both Ras and PI 3-kinase-induced outgrowth require Cdc42 and Rac1 activity.
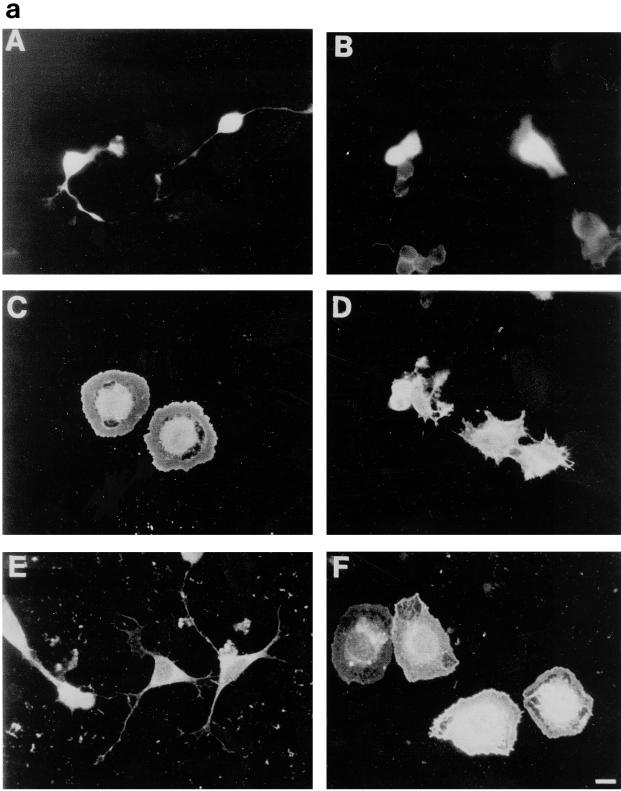
To explore further the roles of Cdc42 and Rac1 in neurite outgrowth with respect to Ras and PI 3-kinase, we cotransfected N1E-115 cells with RasG12V and dominant negative Cdc42T17N and Rac1T17N. Both Cdc42T17N and Rac1T17N were able to block the neurite outgrowth that occurred after RasG12V transfection (Fig. 5b, lanes 3 and 4). It did, however, appear that Ras can signal through Cdc42 and Rac independently. RasG12V and Cdc42T17N cotransfection still produced many of the spread, extensively ruffled fried-egg cells characteristic of Rac1 expression (Fig. 5a, panel C). Transfection of RasG12V and Rac1T17N gave hairy cells with short spikes around the periphery but few with long neurites (Fig. 5a, panel D), similar to the morphology caused by transfection of Cdc42G12V and Rac1T17N. Expression of RasT17N with Cdc42G12V or with Rac1G12V gave the same results as transfection with Cdc42G12V or Rac1G12V alone (Fig. 5a, panels E and F, respectively; Fig. 5b, lanes 5 and 6), indicating that Ras functions upstream of Cdc42 and Rac1 in neurite outgrowth. Cotransfection of RasG12V and Cdc42G12V generated the multiple neurite-bearing cells normally seen following Cdc42G12V transfection alone (data not shown), showing that overexpression of active Cdc42 seems to have a more dominant effect on polarity than active Ras. The significance of this finding is hard to assess, as it was not possible to compare quantitatively expression levels of the two proteins in individual cells, which may have an effect on the final morphological outcome of the transfection. Transfection of RhoAT19N with RasT17N still produced the neurite-bearing cells seen after transfection of RhoAT19N alone (Fig. 5b, lane 7), showing that Ras is not involved in the outgrowth seen following inhibition of Rho, even though the morphology of the RhoAT19N-induced neurites was more similar to that seen with RasG12V than that produced by Cdc42G12V transfection. Inhibition of Rho may be important in neurite outgrowth, as cotransfection of activated RasG12V and RhoG14V markedly reduced the number of neurite-bearing cells normally seen upon RasG12V transfection (Fig. 5b, lane 8), although the level of outgrowth was slightly greater than that seen with RhoG14V alone (compare with Fig. 4b, lane 4). There was a high proportion of the contracted, blebbing cell bodies usually seen following RhoG14V transfection (Fig. 4a, panel B) but also a small number of cells with truncated, variegated, very thin neurites.
FIG. 5.
RasG12V-induced neurite outgrowth requires both Cdc42 and Rac1 activity. (a) Neurite outgrowth normally induced by RasG12V transfection (A) is blocked by Cdc42T17N coexpression (C) and Rac1T17N coexpression (D). Cdc42G12V expression still gives neurite outgrowth when cotransfected with RasT17N (E). Rac1G12V cotransfection with RasT17N still gave the typical flattened Rac1-type cells (F) rather than rounded RasT17N cells as shown in panel B. Note the similarity in the appearance of the RasG12V-plus-Cdc42T17N-cotransfected cells (C) to the fried-egg cells seen following Rac1G12V transfection (Fig. 4a, panel E) and the hairy cells produced with RasG12V plus Rac1T17N coexpression, akin to the Cdc42G12V-plus-Rac1T17N-coexpressing cells shown previously (Fig. 4a, panel D). (b) Quantification of the above morphologies. Cells coexpressing GFP or stained with HA were scored as being neurite bearing, flattened, or rounded, and this was expressed as a percentage of the total number of positively expressing cells in a field of view. At least 20 randomly selected fields of view were assessed, and each experiment was repeated at least three times. Standard deviations are shown as vertical bars.
To investigate the link between PI 3-kinase and Cdc42Hs and Rac1, p110CAAX was cotransfected with either dominant negative Cdc42T17N or Rac1T17N. Cdc42T17N transfection blocked the neurite outgrowth seen after p110CAAX transfection. However, the proportion of flattened fried-egg cells was greatly increased, similar to that seen upon Rac1G12V transfection (Fig. 6a, panel C; Fig. 6b, lane 3). Following cotransfection with Rac1T17N, p110CAAX-transfected cells again did not possess neurites but instead had a spiky or hairy appearance resembling the cells produced when Rac1T17N was coexpressed with Cdc42G12V (Fig. 6a, panel D; Fig. 6b, lane 4). Additionally, the subsequent reduction in cell numbers associated with p110CAAX transfection was lessened after the Rac1T17N cotransfection (data not shown). Thus, it appears that PI 3-kinase is also able to signal through Cdc42 and Rac1 separately in inducing changes in morphology. Since Ras requires PI 3-kinase activity in this pathway, it is presumably PI 3-kinase acting downstream of Ras that signals through the two molecules independently rather than Ras itself. LY294002 did not block Cdc42G12V-induced neurite outgrowth (Fig. 6b, lane 5) although there was a concomitant increase in cell flattening. LY294002 also did not block RacG12V-induced cell flattening (results not shown) or outgrowth produced by dominant negative RhoT19N (Fig. 6b, lane 6).
FIG. 6.
p110CAAX-induced neurite outgrowth requires Cdc42Hs and Rac1 activity. (a) At 20 h after transfection, p110CAAX expression produced neurites (A) which also stained positively for neurofilament protein (B). Transient cotransfection of p110CAAX and Cdc42T17N gave flattened Rac1-type cells and no neurite outgrowth (C). Coexpression of p110CAAX and dominant negative Rac1T17N also inhibited PI 3-kinase-induced outgrowth, but cells exhibited the hairy cell morphology (D). (b) Quantification of transfected cell morphologies was carried out as described in legend to Fig. 5.
Neither the JNK nor ERK pathway is required for neurite outgrowth in N1E-115 cells.
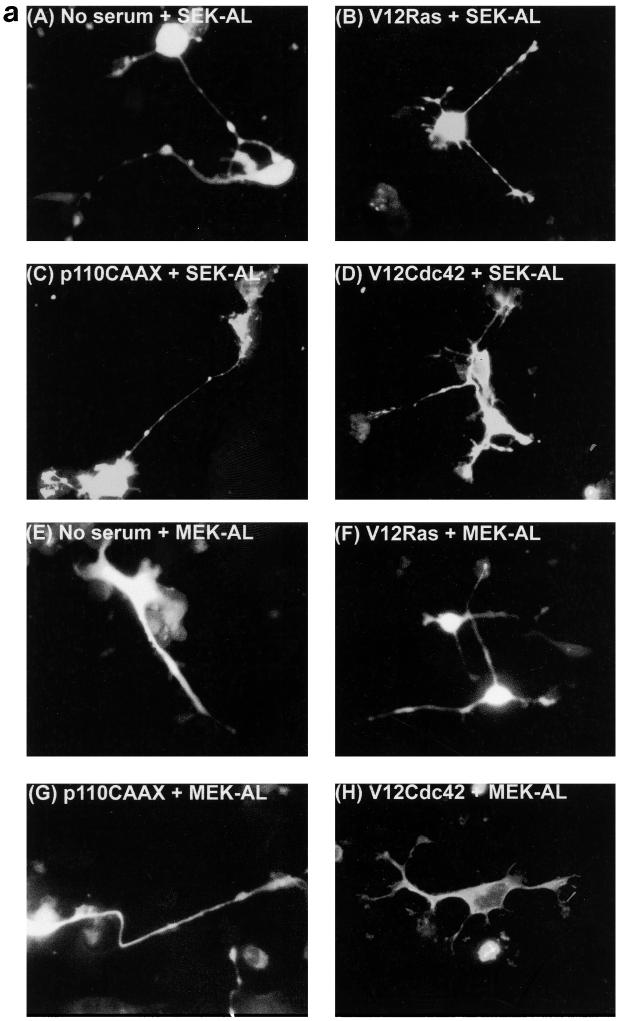
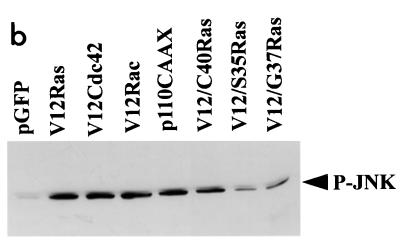
Previous studies have suggested that either JNK or the extracellular signal-regulated kinase (ERK) pathway may be required in NGF-induced neurite outgrowth in PC12 cells (9, 27, 34). We wanted to determine whether these pathways are required for outgrowth in N1E-115 cells induced by Ras. First, we examined the levels of phosphorylated, active JNK following transfection with various vectors. Expression of RasG12V, Cdc42G12V, Rac1G12V, and p110CAAX all resulted in an elevation of phospho-JNK levels (Fig. 7b). Furthermore, of the three Ras effector mutants used, RasG12V;H40C expression resulted in the greatest level of phospho-JNK. We also attempted to monitor levels of active ERK following transfection, but only low levels were detected (results not shown). To determine whether JNK or ERK activation was required for outgrowth, we examined whether dominant negative mutants for either the JNK pathway (SEK-AL) or the ERK pathway (MEK-AL) could block neurite formation. Following transfection with each construct, we monitored outgrowth in serum-starved N1E-115 cells (Fig. 7a). We then coexpressed dominant negative SEK-AL or MEK-AL with RasG12V, with p110CAAX, and with Cdc42G12V (Fig. 7a). Expression of neither SEK-AL nor MEK-AL in any of the conditions used inhibited neurite formation, suggesting that neither pathway is required for the process of outgrowth in N1E-115 cells.
FIG. 7.
Neurite outgrowth in N1E-115 cells does not require the JNK or ERK pathway. (a) Transfection of SEK-AL (A) or MEK-AL (E) dominant negative constructs in serum-starved conditions and cotransfections as indicated. At 20 h after transfection, cells were fixed and stained. (b) Activation of JNK in N1E-115 cells following transfection with expression vectors. Following transfection, cell lysates were prepared, and equal quantities of proteins were electrophoresed through polyacrylamide gels, Western immunoblotted, and probed with anti-phospho-JNK antibodies.
DISCUSSION
We have described elements of a signal transduction pathway involved with neurite outgrowth in neuroblastoma cells. It appears that this is an integrin-dependent pathway, as a laminin matrix was required for neurite outgrowth to be seen. In addition, a β-1 integrin antibody was able to reduce outgrowth in all cases. A requirement for integrin signalling has previously been described for neurite-like process formation induced by Cdc42 and the Rac1 exchange factor Tiam (64). Our results also implicate Ras and PI 3-kinase upstream of Cdc42 and Rac1 in neurite outgrowth in these cells. The involvement of Ras, PI 3-kinase, Cdc42, and Rac1 in integrin-dependent outgrowth is indicated by the inhibition of neurite formation following serum starvation on laminin by dominant negative versions of the GTPases RasT17N, Cdc42T17N, and Rac1T17N or an inhibitor of PI 3-kinase, LY294002. The mutants of Ras which display some selectivity in effector binding have been of limited use in our assay, and they can give only a partial indication of the relative roles of the different Ras effectors. These mutants display decreased interaction with all effectors. For instance, RasH40C;G12V still binds at a low basal level to Raf and RalGDS, and it also exhibits reduced (although relatively less) binding to PI 3-kinase. Thus, we cannot completely rule in or out a pathway by using such mutants alone, since they give only a limited view of what may be occurring in the neuroblastoma cells.
In contrast to Cdc42, although Rac1 appeared to be required for neurite outgrowth, we did not find activated Rac1G12V expression to be sufficient to induce outgrowth. Instead, expression of Rac1G12V resulted in the formation of large numbers of flattened cells containing extensive edge ruffling. This result is contrary to that reported by van Leeuwen et al. (63), who state that both Cdc42G12V expression and Rac1G12V expression are sufficient to induce neurite formation in N1E-115 cells. However, there are some differences between the two studies. First, their assay involves overnight incubation of transfected cells prior to replating on laminin for a further day before assaying. Second, the morphologies of their transfected cells are different from those we obtain; their cells transfected with either Cdc42G12V or Rac1G12V are very polarized and contain extensive membrane ruffling at ends of processes which we did not observe. Instead, we found Cdc42G12V expression to produce cells with several neurites and a loss of polarization, while Rac1G12V expression led to cells that were flattened but without significant neurite formation. Third, they report that the neurites obtained in their studies do not contain neurofilament, whereas we found that the neurite types formed in our studies stained positively for neurofilament. We conclude that there are differences in either the cell populations or the assays performed which may account for the difference in findings between the two studies. It appears that Cdc42 is intimately involved in the process of cell polarity. Neurites formed following serum starvation or transfection with RasG12V or p110CAAX were rather similar in appearance, each cell usually exhibiting one or two long processes with rather simple growth cones and relatively unbranched neurites, not possessing many actin microspikes along their length. In contrast, following transfection with activated Cdc42G12V, cells usually possessed several spiky, ruffled, and flattened neurites, suggesting that polarity had been lost following perturbations in Cdc42 activities. This notion of an involvement of Cdc42 in cell polarity is in agreement with previous studies using T cells (58). An involvement of Cdc42 and Rac1 in epithelial cell polarization has also been proposed (24); in that case PI 3-kinase appeared to play a role, as the effects of Cdc42 and Rac1 were blocked by PI 3-kinase inhibitors.
Interestingly, we have noticed that following transfection with p110CAAX the cells undergo a sequence of morphological events whereby neurite outgrowth is followed by cell death. The dying cells display all of the morphological hallmarks of cells undergoing apoptosis. This observation is surprising as PI 3-kinase activity has hitherto always been associated with protection from apoptosis and not promotion of apoptosis (12, 23, 47). However, serum-starved differentiated N1E-115 cells generally undergo cell death 48 to 72 h following withdrawal from serum (unpublished data). The cell death seen after p110CAAX transfection may be a natural consequence of the terminal differentiation of these cells, albeit somewhat hastened.
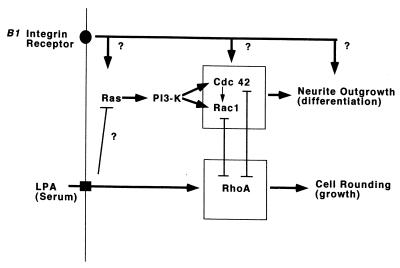
We have shown that RasG12V-induced neurite formation requires PI 3-kinase activity, being inhibited by LY294002, while p110CAAX-induced outgrowth was not affected by cotransfection with dominant negative RasT17N. Both RasG12V and p110CAAX-induced neurite formation was blocked by dominant negative Cdc42T17N and Rac1T17N. However activated Cdc42G12V-induced neurite outgrowth was not inhibited by dominant negative RasT17N or LY294002. Overall, our results suggest that following serum deprivation on laminin there is a hierarchy of activation states leading from Ras to PI 3-kinase and then to Cdc42 and Rac1, resulting in neurite outgrowth (Fig. 8).
FIG. 8.
Neurite outgrowth following serum deprivation signals through Ras, PI 3-kinase, and Cdc42 and Rac1. Activation of Ras, PI 3-kinase or Cdc42 (but not activation of Rac1 alone) is able to promote neurite outgrowth even in the presence of serum factors, but in each case it requires the presence of a laminin matrix which signals via integrin receptors. It is not known whether the GTPases signal upstream or downstream from the integrins or whether the integrin requirement is independent of these GTPases. Inhibition of Rho also results in neurite outgrowth, and Rho has been shown to compete with Cdc42 and Rac1 (32, 63). The mechanism responsible for the activation of Cdc42 and Rac1 by PI 3-kinase is not clear but probably involves phosphatidylinositol 3,4,5-triphosphate-dependent increased nucleotide exchange activity and/or inhibition of GTPase activity for the p21s (13).
We have not been able to bypass a requirement for a laminin matrix, suggesting that integrin signalling is also important in this process. Assembly of integrin adhesion complexes requires Rho family GTPases (16). However, it is not clear how, in N1E-115 cells, integrin signalling links with Ras, PI 3-kinase, Cdc42, or Rac1. Cdc42 and Rac are required for integrin-dependent ruffling in Rat1 cells (8), while integrin-dependent spreading in T cells requires Ras (11). Ras expression has also been shown to alter integrin receptor affinity (17, 65). Thus, evidence from other cell types indicates that signalling can occur both from integrins to GTPases and vice versa. This notion is consistent with our results: outgrowth induced by serum starvation, RasG12V, Cdc42G12V, or p110CAAX all required a laminin substrate; however, outgrowth induced on laminin by serum starvation was inhibited by dominant negative RasT17N, dominant negative Cdc42T17N, or LY294002 treatment. Together, these results suggest a role for integrins both upstream and downstream from these GTPases and PI 3-kinase (Fig. 8). Outgrowth is inhibited by RasT17N; as this dominant negative protein is thought to function by blocking exchange activity on Ras, it is possible that Ras is activated by serum withdrawal, and it would be interesting to directly determine the nucleotide-bound state of Ras under these conditions in these cells. PI 3-kinase seems to activate Cdc42 and Rac1 independently, but the mechanism is unclear. It could be due to either a direct phosphorylation event or the indirect increase in levels of phospholipids such as phosphatidylinositol 3,4-bisphosphate or phosphatidylinositol 3,4,5-triphosphate having effects either on the GTPases themselves or on exchange factors for these GTPases.
It has been proposed that for PC12 cells, both the ERK pathway (9, 34) and the JNK pathway (27, 34) are required for NGF-induced neurite outgrowth. However, it has also been noted that neurite outgrowth via stimulation of the ERK pathway may be indirect and partly due to the induced synthesis of c-Jun (9, 34). We find that for N1E-115 cells, neither of these pathways appears necessary for outgrowth. However, we cannot rule out the possibility that basal levels of activity remain in the cells even though they are expressing the dominant negative SEK-AL or MEK-AL protein. Our observation that transfection of Raf-CAAX in N1E-115 cells does not result in outgrowth, except after a lag period, is consistent with the notion that ERK-induced outgrowth is indirect.
Several potential effectors, including the kinases ACK, PAK, and MRCK and nonkinases WASP, n-chimaerin, POR1, and p67phox, have now been identified for Cdc42 and Rac1. The only effector ascribed a direct role in neurite outgrowth so far is PAK, for which expression of a membrane targeted form by C-terminal CAAX extension results in neurite outgrowth in PC12 cells (10). Microinjection of n-chimaerin into N1E-115 cells stimulates growth cone development (31), a morphological activity which has been linked to neurite outgrowth (32). As n-WASP and MRCK have both been proposed as effectors for eliciting Cdc42-dependent morphological activities in nonneural cells (35, 40), and POR1 has been proposed a similar role for Rac-dependent activities (62), all of these are candidates for roles in neurite outgrowth. In contrast to the activities of Cdc42 and Rac1, Rho induces neurite collapse (19, 32, 42, 60). In fact, Rho appears to compete with Cdc42 and Rac1 in this process (32, 63). Rho may stimulate growth cone and neurite collapse via p160ROCKα (14, 21). Here we have found that outgrowth promoted by RhoT19N was blocked by Cdc42T17N or by RacT17N, further suggesting antagonism between Cdc42/Rac1 and Rho signalling pathways. In addition, RasG12V-induced outgrowth was blocked by cotransfection with RhoG14V, indicating that Rho can also compete with Ras in the process of neurite outgrowth.
A recent study by Olsen et al. (46) using Swiss 3T3 cells showed that when signalling through Rho is inhibited, such as in serum-starved conditions, constitutively active Ras induces the cyclin-dependent kinase inhibitor p21Waf/Cip1. In these conditions, cell cycle arrest occurs and DNA synthesis is blocked. When Rho is active, for example, in the presence of serum, Ras induces DNA synthesis, not p21Waf/Cip1. It is possible that in these N1E-115 neuroblastoma cells, as in Swiss 3T3 cells, one function of Rho is to suppress p21Waf/Cip1 induction and allow cell proliferation; in the absence of serum, where Rho is inactive, Ras induces p21Waf/Cip1 and terminal differentiation occurs.
ACKNOWLEDGMENTS
We thank Julian Downward for the Ras and PI 3-kinase expression vectors, Chris Marshall for the MEK-AL vectors, James Woodgett for the SEK-AL vector, John-Claud Martinou and Antony Nichols for the GFP vectors, Ed Manser and Tom Leung for the HA-tagged Rho family vectors, Stefan Wennstrom for the p110 antibody, and Nansi Cann and Kate Marler for expert help.
We thank the Glaxo-Singapore Research Fund for support.
REFERENCES
- 1.Barbacid M. Ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Sagi D, Feramisco J R. Microinjection of the ras oncoprotein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Sagi D, Feramisco J R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 4.Bollag G, McCormick F. Regulators and effectors of Ras proteins. Annu Rev Cell Biol. 1991;7:601–632. doi: 10.1146/annurev.cb.07.110191.003125. [DOI] [PubMed] [Google Scholar]
- 5.Boguski M S, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 6.Borasio G D, John J, Wittinghofer A, Barde Y A, Sendtner M, Heumann R. Ras p21 protein promotes survival and fiber outgrowth of cultured embryonic neurones. Neuron. 1989;2:1087–1096. doi: 10.1016/0896-6273(89)90233-x. [DOI] [PubMed] [Google Scholar]
- 7.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance A J, Herron C E, Ramsey M, Wolfer D P, Cestari V, Rossi-Arnaud C, Grant S G N, Chapman P F, Lipp H-P, Sturani E, Klein R. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 8.Clark E A, King W G, Brugge J S, Symons M, Hynes R O. Integrin-mediated signals regulated by members of the Rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley S, Patterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 10.Daniels R H, Hall P S, Bokoch G M. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Souza-Schorey C, Boettner B, Van Aelst L. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol Cell Biol. 1998;18:3936–3946. doi: 10.1128/mcb.18.7.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins P T, Eguinoa A, Qui R-G, Stokoe D, Cooke F T, Walters R, Wennstrom S, Cleasson-Welsh L, Evans T, Symons M, Stephens L. PDGF stimulates an increase in GTP-Rac via activation of phosphoinisitide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 14.Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar W H, Matsumara F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodelling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer F, Fields S, Schneider C, Martin G S. Activated Ras interacts with the Ral guanine-nucleotide dissociation stimulator. Proc Natl Acad Sci USA. 1994;91:11089–11093. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchin N A, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes P E, Renshaw M W, Pfaff M, Forsyth J, Keivens J M, Schwartz M A, Ginsberg M H. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 18.Jackson T R, Blader I J, Hammonds-Odie L P, Burga C R, Cooke F, Hawkins P T, Wolf A G, Heldmann K A, Theibert A B. Initiation and maintenance of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. J Cell Sci. 1996;109:289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalink K, van Corven E J, Hengeveld T, Morii N, Narumiya S, Moolenaar W H. Inhibition of lysophosphatidate and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of Ras. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 21.Katoh H, Aoki J, Ichikawa A, Negishi M. P160 RhoA-binding kinase ROKα induces neurite retraction. J Biol Chem. 1998;273:2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann N, Wills Z P, Vactor D V. Drosophila Rac1 controls motor axon guidance. Development. 1998;125:453–461. doi: 10.1242/dev.125.3.453. [DOI] [PubMed] [Google Scholar]
- 23.Kauffmann-Zeh A, Rodrigues-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc induced apoptosis by Ras signalling through PI(3) K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 24.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 25.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi K, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- 27.Kita Y, Kimura K D, Kobayashi M, Ihara S, Kaibuchi K, Kuroda S, Ui M, Iba H, Konishi H, Kikkawa U, Nagata S, Fukui Y. Microinjection of activated phosphatidylinositol-3 kinase induces process outgrowth in rat PC12 cells through the Rac-JNK signal transduction pathway. J Cell Sci. 1998;111:907–915. doi: 10.1242/jcs.111.7.907. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M, Nagata S, Kita Y, Nakatsu N, Ihara S, Kaibuchi K, Kuroda S, Ui M, Iba H, Konishi H, Kikkawa U, Saitoh I, Fukui Y. Expression of a constitutively active phosphatidylinositol 3-kinase induces process formation in rat PC12 cells. J Biol Chem. 1997;272:16089–16092. doi: 10.1074/jbc.272.26.16089. [DOI] [PubMed] [Google Scholar]
- 29.Kohl N E, Ruley H E. Role of c-myc in the transformation of REF52 cells by viral and cellular oncogenes. Oncogene. 1987;2:41–48. [PubMed] [Google Scholar]
- 30.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozma R, Ahmed S, Best A, Lim L. The GTPase-activating protein n-chimaerin cooperates with Rac1 and Cdc42Hs to induce the formation of lamellipodia and filopodia. Mol Cell Biol. 1996;16:5069–5080. doi: 10.1128/mcb.16.9.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamoureux P, Altun-Gultekin Z F, Lin C, Wagner J A, Heidemann J A. Rac is required for growth cone function but not neurite assembly. J Cell Sci. 1997;110:635–641. doi: 10.1242/jcs.110.5.635. [DOI] [PubMed] [Google Scholar]
- 34.Leppa S, Saffrich R, Ansorg W, Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung T, Chen X-Q, Tan I, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo L, Liao Y J, Jan L Y, Jan Y N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac is involved on axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 37.Luo L, Hensch T K, Ackerman L, Barbel S, Jan L Y, Jan Y N. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 38.Manser E, Leung T, Salhuddin H, Zhao Z-S, Lim L. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall C J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 40.Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- 41.Mosch H-U, Roberts R L, Fink G R. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5352–5356. doi: 10.1073/pnas.93.11.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishiki T, Narumiya S, Morii N, Yamamoto M, Fujiwara M, Kamata Y, Sakaguchi G, Kozaki S. ADP-ribosylation of the rho/rac proteins induces growth inhibition, neurite outgrowth and acetylcholine esterase in cultured PC12 cells. Biochem Biophys Res Commun. 1990;167:265–272. doi: 10.1016/0006-291x(90)91760-p. [DOI] [PubMed] [Google Scholar]
- 43.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multi-molecular focal complexes associated with actin stress fibres, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 44.Noda M, Ko M, Ogura A, Lim D G, Amano T, Takano T, Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985;318:73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- 45.Obermeier A, Bradshaw R A, Seedorf K, Choidas A, Schlessinger J, Ullrich A. Neuronal differentiation signals are controlled by nerve growth factor receptor/Trk binding sites for SHC and PLC gamma. EMBO J. 1994;13:1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen M F, Paterson H F, Marshall C J. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 47.Philpott K L, McCarthy M J, Klippel A, Rubin L L. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139:809–815. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qui R-G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 49.Qui R-G, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qui R-G, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reif K, Nobes C D, Thomas G, Hall A, Cantrell D. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- 52.Ridley A J, Paterson H F, Noble M, Land H. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J. 1988;7:1635–1645. doi: 10.1002/j.1460-2075.1988.tb02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridley A J, Paterson H F, Johnson C, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigues-Viciana P, Warne P H, Khwaja A, Marte B, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role for phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–797. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 56.Serrano M, Lin A W, McMurrach M E, Beach D, Lowe S. Oncogenic Ras provokes premature cell senescence associated with accumulation of p53 and p161K41. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 57.Stephens R M, Loeb D M, Copeland T D, Pawson T, Grenne L A, Kaplan D R. Trk receptors use redundant signal transduction pathways involving SHC and PLC gamma to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 58.Stowers L, Yelon D, Berg L, Chant J. Regulation of the polarization of T-cells toward antigen presenting cells by the Ras-related GTPase Cdc42. Proc Natl Acad Sci USA. 1995;92:5027–5031. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodelling by Rho, Rac and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 60.Tigyi G, Fischer D J, Sebok A, Yang C, Dyer D L, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signalling and Rho. J Neurochem. 1996;66:537–547. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- 61.Tolias K F, Cantley L C, Carpenter C L. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 62.van Aelst L, Joneson T, Bar-Sagi D. Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO J. 1996;15:3778–3786. [PMC free article] [PubMed] [Google Scholar]
- 63.van Leeuwen F N, Kain H E T, van der Kammen R A, Michiels F, Kranenburg O W, Collard J G. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Vuori K, Wang H, Reed J C, Ruoslahati E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]
- 66.Zheng Y, Bagrodia S, Cerione R. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]