Abstract
Background and aims
Although adjuvant transcatheter arterial chemoembolization (TACE) for resected hepatocellular carcinoma (HCC) may improve survival for some patients, identifying which patients can benefit remains challenging. The present study aimed to construct a survival prediction calculator for individualized estimating the net survival benefit of adjuvant TACE for patients with resected HCC.
Methods
From a multicenter database, consecutive patients undergoing curative resection for HCC were enrolled and divided into the developing and validation cohorts. Using the independent survival predictors in the developing cohort, two nomogram models were constructed for patients with and without adjuvant TACE, respectively, which predictive performance was validated internally and externally by measuring concordance index (C-index) and calibration. The difference between two estimates of the prediction models was the expected survival benefit of adjuvant TACE.
Results
A total of 2514 patients met the inclusion criteria for the study. The nomogram prediction models for patients with and without adjuvant TACE were, respectively, built by incorporating the same eight independent survival predictors, including portal hypertension, Child–Pugh score, alpha-fetoprotein level, tumor size and number, macrovascular and microvascular invasion, and resection margin. These two prediction models demonstrated good calibration and discrimination, with all the C-indexes of greater than 0.75 in the developing and validation cohorts. A browser-based calculator was generated for individualized estimating the net survival benefit of adjuvant TACE.
Conclusions
Based on large-scale real-world data, an easy-to-use online calculator can be adopted as a decision aid to predict which patients with resected HCC can benefit from adjuvant TACE.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-021-01180-5.
Keywords: Hepatocellular carcinoma, Hepatectomy, Transcatheter arterial chemoembolization, Adjuvant therapy, Survival
To the editor,
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related mortality worldwide [1]. Surgical resection represents a common approach to treat HCC and provides the possibility of cure [2]. Long-term prognosis after HCC resection is, however, still poor due to the high incidence of recurrence [3–5]. Transcatheter arterial chemoembolization (TACE) has been used in the postoperative setting as a means to decrease risk of recurrence and improve survival [6–8]. Whereas, in clinical practice, controversy persists relative to the role of adjuvant TACE for resected HCC [9–11]. The reasons for these disparate results are undoubtedly multifactorial, yet may relate to patient selection. Specifically, only certain high-risk patients with resected HCC may benefit from adjuvant TACE [12]. The objective of the current study was to construct a decision aid using a large multicenter database to predict which patient with resected HCC had a survival benefit from adjuvant TACE. In addition, we sought to estimate the magnitude of the survival benefit for given individual patients. A web-based decision tool was provided for clinicians and patients to aid in the decision-making process regarding adjuvant TACE after HCC resection. Patients and methods for this study are described in detail in Additional file 1.
Overall survival
All 2514 patients with HCC underwent curative liver resection were included (Additional file 2: Figure S1). Among them, 1755 and 759 patients were randomly segregated to the development and validation cohort, respectively (Table 1). Compared with patients who did not receive adjuvant TACE, patients who had adjuvant TACE had a longer survival in both the development and validation cohorts (all P < 0.001) (Additional file 3: Figure S2).
Table 1.
Baseline characteristics of patients with and without adjuvant TACE in the developing and validation cohorts
| Variables | The developing cohort (N = 1755) | The validation cohort (N = 759) | ||
|---|---|---|---|---|
| With adjuvant TACE (n = 533) | Without adjuvant TACE (n = 1222) | With adjuvant TACE (n = 224) | Without adjuvant TACE (n = 535) | |
| Preoperative variables | ||||
| Male sex | 476 (89.3) | 1080 (88.4) | 198 (88.4) | 468 (87.5) |
| Age > 65 years | 89 (16.7) | 260 (21.3) | 29 (12.9) | 115 (21.5) |
| Co-morbid illness | 111 (20.9) | 228 (18.7) | 72 (32.1) | 160 (29.9) |
| PS, 1–2/0 | 161/372 (30.2/69.8) | 345/877 (28.2/71.8) | 35/189 (15.7/84.3) | 82/453 (15.3/84.7) |
| ASA score > 2 | 56 (10.5) | 182 (14.9) | 19 (8.5) | 81 (15.1) |
| Etiology of liver disease, HBV/HCV/HBV + HCV/other | 446/26/15/46 (83.7/4.9/2.8/8.6) | 1010/73/27/112 (82.7/6.0/2.2/9.2) | 193/9/4/18 (86.2/4.0/1.8/8.0) | 446/32/9/48 (83.4/6.0/1.7/9.0) |
| Cirrhosis | 391 (73.4) | 958 (78.4) | 157 (70.1) | 421 (78.7) |
| Portal hypertension | 115 (21.6) | 329 (26.9) | 37 (16.5) | 129 (24.1) |
| Child–Pugh grade, A/B | 489/44 (91.7/8.3) | 1075/147 (88.0) | 207/17 (92.4/7.6) | 467/68 (87.3/12.7) |
| Preoperative ALT level > 40 U/L | 277 (52.0) | 683 (55.9) | 111 (49.6) | 289 (54.0) |
| Preoperative AST level > 40 U/L | 284 (53.3) | 703 (57.5) | 141 (62.9) | 314 (58.7) |
| Preoperative AFP level > 400 ug/L | 206 (38.6) | 489 (40.0) | 87 (38.8) | 198 (37.0) |
| Maximum tumor size, ≥ 10.0/5.0–9.9/< 5.0 cm | 128/212/193 (24.0/39.8/36.2) | 204/443/575 (16.7/36.3/47.1) | 45/96/83 (20.1/42.9/37.1) | 77/200/258 (14.4/37.4/48.2) |
| Tumor number, ≥ 3/2/1 | 91/82/360 (17.1/15.4/67.5) | 159/121/942 (13.0/9.9/77.1) | 33/35/156 (14.7/15.6/69.6) | 70/50/415 (13.1/9.3/77.6) |
| Macrovascular invasion | 67 (12.6) | 104 (8.5) | 35 (15.6) | 50 (9.3) |
| Intraoperative variables | ||||
| Intraoperative blood loss > 600 mL | 117 (22.0) | 263 (21.5) | 42 (18.8) | 104 (19.4) |
| Intraoperative blood transfusion | 145 (27.2) | 307 (25.1) | 45 (20.1) | 132 (24.7) |
| Operation time > 180 min | 86 (16.1) | 214 (17.5) | 30 (13.4) | 84 (15.7) |
| Anatomical resection | 145 (27.2) | 331 (27.1) | 63 (28.1) | 129 (24.1) |
| Major hepatectomy | 157 (29.5) | 318 (26.0) | 56 (25.0) | 132 (24.7) |
| Postoperative pathological variables | ||||
| Microvascular invasion | 306 (57.4) | 619 (50.7) | 134 (59.8) | 270 (50.5) |
| Poor tumor differentiation | 397 (74.5) | 918 (75.1) | 175 (78.1) | 415 (77.6) |
| Incomplete tumor encapsulation | 334 (62.7) | 785 (64.2) | 155 (69.2) | 344 (64.3) |
| Resection margin < 1 cm | 179 (33.6) | 386 (31.6) | 76 (33.9) | 162 (30.3) |
AFP alpha-fetoprotein, ALT alanine aminotransferase, ASA American Society of Anesthesiologists, AST aspartate transaminase, HBV hepatitis B virus, HCV hepatitis C virus, PS performance status, TACE transcatheter arterial chemoembolization
Independent predictors of survival
Univariable and multivariable Cox regression analyses of the development cohort demonstrated that independent predictors associated with overall survival after HCC resection among patients treated with and without adjuvant TACE included portal hypertension, Child–Pugh grade, preoperative AFP level, tumor size, tumor number, macrovascular invasion, microvascular invasion, and resection margin (all P < 0.05). (Additional file 4: Table S1 and Additional file 5: Table S2).
Development of the prediction models
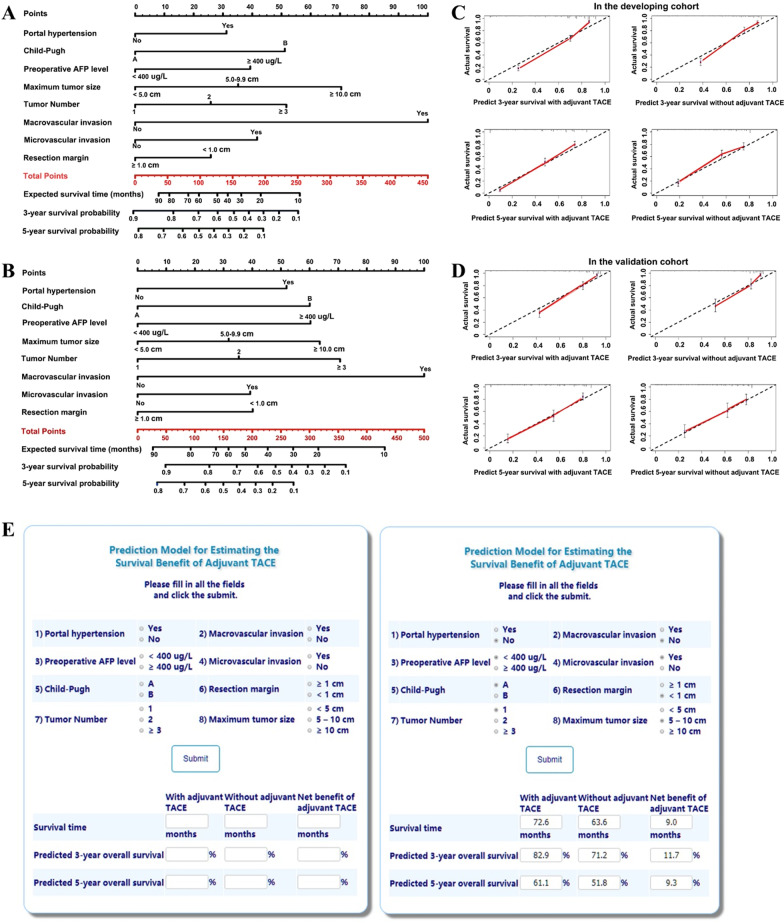
Two different nomogram models that integrated independent factors associated with overall survival were constructed to predict outcomes among patients who did and did not receive adjuvant TACE (Fig. 1a, b). To estimate the net survival benefit from adjuvant TACE, these two nomograms were compared and the difference between the two estimates was the expected net survival benefit from the addition of adjuvant TACE.
Fig. 1.
Nomograms to calculate the expected survival time, and 3- and 5-year survival probabilities for a patients who attempt to undergo adjuvant TACE and b patients who attempt not to undergo adjuvant TACE. Thus, the difference of the expected values between the two estimates is the expected net survival benefit of adjuvant TACE. Calibration plots of the models for predicting 3-year and 5-year survival for patients with and without adjuvant TACE c in the developing cohort and d in the validation cohort, respectively. e Screenshots of the web-based calculator for individualized estimates of the expected net survival benefit of adjuvant TACE for patients with resected hepatocellular carcinoma. Website: http://www.asapcalculate.top/Cal5_en.html. For example, suppose there is a male patient without portal hypertension (Child–Pugh A) who have underwent curative resection for a single HCC tumor (tumor size: 8.0 cm, without macrovascular invasion but with microvascular invasion). His preoperative AFP level was 718 ug/L (≥ 400 ug/L), and the tumor resection margin was 0.8 cm (< 1 cm). After putting these data into these specific parameters, we can get the expected net survival time benefit of adjuvant TACE was 9.0 months, and the net survival benefits of 3-year and 5-year survival rates are 11.7% and 9.3%, respectively
Validation of the prediction models
Bootstrapping with 400 resamples in the development cohort demonstrated good predictive performance, with the C-indexes of 0.791 (95% CI 0.742–0.840) and 0.810 (95% CI 0.779–0.841) for patients with and without adjuvant TACE, respectively. Accordingly, the C-indices were 0.756 (95% CI 0.678–0.834) and 0.765 (95% CI 0.720–0.810) in the validation cohort. There was also good calibration curve to predict 3- and 5-year survival probabilities among patients treated with and without adjuvant TACE, respectively (all P > 0.05) (Fig. 1c, d).
Construction of the online calculator
Based on the formula of the nomogram prediction models, an Internet browser-based software tool was constructed to predict the net survival benefit of adjuvant TACE for an individual patient, including the expected net survival time, and the increased 3- and 5-year survival probabilities (Fig. 1e). The corresponding score and the formula to calculate survival probability were provided (Additional file 6: Table S3). The online calculator is available for free use at: http://asapcalculate.top/Cal5_en.html. After the user inputs all the requested information relative to the prognostic factors, the predicted survival improvement associated with the addition of adjuvant TACE, including the expected survival time and the 3- and 5-year survival probabilities, is generated and displayed.
In summary, a survival prediction model that incorporated eight independent variables associated with survival was constructed to derive an individualized estimate of the net survival benefit of adding adjuvant TACE to a patient’s post-resection HCC treatment plan. The nomograms and online calculator had good predictive accuracy, and discrimination was validated. The calculator may help provide an estimate of the net survival benefit associated with adjuvant TACE for an individual patient following HCC resection. This tool may assist clinicians and patients in quantifying the benefit of adjuvant TACE after HCC resection and inform real-word discussions on this topic.
Supplementary Information
Additional file 1. Patients and methods.
Additional file 2: Figure S1. Flow chart of patient inclusion.
Additional file 2: Figure S2. (A) Kaplan-Meier curves of overall survival between patients in the development and validation cohorts (log-rank test, P = 0.594); (B) Kaplan-Meier curves of overall survival between patients with and without adjuvant TACE in the developing cohort (log-rank test, P < 0.001); and (C) Kaplan-Meier curves of overall survival between patients with and without adjuvant TACE in the validation cohort (log-rank test, P < 0.001). Number of patients at risk and censoring were list in the box at the bottom of each plot.
Additional file 3: Table S1. Univariable and multivariable Cox-regression analyses of predicting overall survival for patients with resected hepatocellular carcinoma who underwent adjuvant TACE in the developing cohort.
Additional file 2: Table S2. Univariable and multivariable Cox-regression analyses of predicting overall survival for patients with resected hepatocellular carcinoma who did not undergo adjuvant TACE in the developing cohort.
Additional file 2: Table S3. The corresponding score and the formula of our nomogram models.
Abbreviations
- HCC
Hepatocellular carcinoma
- TACE
Transcatheter arterial chemoembolization
- RCT
Randomized controlled trial
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- PS
Performance status
- ECOG
Eastern cooperativse oncology group
- ASA
American society of anesthesiologists
- HBV
Hepatitis B virus; HCV, hepatitis C virus
- ALT
Alanine aminotransferase
- AST
Aspartate transaminase
- AFP
Alpha-fetoprotein
- C-index
Concordance index
- CI
Confidence interval
- HR
Hazard ratio
Authors' contributions
LL, CL, M-DW, and HW contributed equally to this work. Dr TY and D-SH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: LL, WYL, FS, D-SH, TY. Acquisition, analysis, or interpretation of data: LL, CL, HW, Y-HZ, Y-YZ, W-GZ, T-HC, N-YW, JL, Y-MZ, YW, W-MG, HX, Y-KD, L-QY, CY, X-MT, C-WZ. Drafting of the manuscript: LL, CL, TY. Critical revision of the manuscript for important intellectual content: TMP, WYL, FS, TY. Statistical analysis: LL, HX, TY. Obtained funding: TY. Administrative, technical, or material support: HW, Y-HZ, Y-YZ, W-GZ, T-HC, N-YW, JL, Y-MZ, YW, W-MG, HX, Y-KD, C-WZ, FS, TY. Study supervision: FS, D-SH, TY. Thanks Xiao-Li Yin (Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital) for providing calculator web design and technical support. Thanks Lan-Qing Yao (Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital), Chen Yuan (Department of Hematology, Zhejiang Provincial People's Hospital) and Xiang-Min Tong (Department of Hematology, Zhejiang Provincial People's Hospital) for providing technical or material support. All authors read and approved the final manuscript.
Funding
Funding for the study was provided by the National Natural Science Foundation of China (Nos. 81672699 and 81972726, Dr Yang). Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies of all participating hospitals.
Informed consent
The study protocol was approved by the Institutional Review Board of all participating hospitals, and informed consent from the patients was waived. Written, informed consent for the data to be used for clinical researches was obtained from all enrolled patients.
Congress publication
The abstract of this study has been presented as an oral presentation in the Congress of “the 11th Asia-Pacific Primary Liver Cancer Expert, APPLE 2021” (August 13–15, 2021, Korea), and has been also presented as an oral presentation in the Congress of “the LIVER WEEK 2021” (May 13–15, 2021, Korea) and won a “Foreign Investigator Award”.
Competing interests
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 2020;72(2):262–276. doi: 10.1016/j.jhep.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 5.Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi: 10.1001/jamasurg.2018.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang L, Li C, Diao YK, Jia HD, Xing H, Pawlik TM, et al. Survival benefits from adjuvant transcatheter arterial chemoembolization in patients undergoing liver resection for hepatocellular carcinoma: a systematic review and meta-analysis. Ther Adv Gastroenterol. 2020;13:1756284820977693. doi: 10.1177/1756284820977693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng BG, He Q, Li JP, Zhou F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198(3):313–318. doi: 10.1016/j.amjsurg.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol. 2016;23(4):1344–1351. doi: 10.1245/s10434-015-5008-z. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3(10):593–603. doi: 10.1016/S1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249(2):195–202. doi: 10.1097/SLA.0b013e3181961c16. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(9):2074–2081. doi: 10.1158/1078-0432.CCR-17-2899. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Ke Q, Lin N, Zeng Y, Liu J. Does postoperative adjuvant transarterial chemoembolization benefit for all patients with hepatocellular carcinoma combined with microvascular invasion: a meta-analysis. Scand J Gastroenterol. 2019;54(5):528–537. doi: 10.1080/00365521.2019.1610794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Patients and methods.
Additional file 2: Figure S1. Flow chart of patient inclusion.
Additional file 2: Figure S2. (A) Kaplan-Meier curves of overall survival between patients in the development and validation cohorts (log-rank test, P = 0.594); (B) Kaplan-Meier curves of overall survival between patients with and without adjuvant TACE in the developing cohort (log-rank test, P < 0.001); and (C) Kaplan-Meier curves of overall survival between patients with and without adjuvant TACE in the validation cohort (log-rank test, P < 0.001). Number of patients at risk and censoring were list in the box at the bottom of each plot.
Additional file 3: Table S1. Univariable and multivariable Cox-regression analyses of predicting overall survival for patients with resected hepatocellular carcinoma who underwent adjuvant TACE in the developing cohort.
Additional file 2: Table S2. Univariable and multivariable Cox-regression analyses of predicting overall survival for patients with resected hepatocellular carcinoma who did not undergo adjuvant TACE in the developing cohort.
Additional file 2: Table S3. The corresponding score and the formula of our nomogram models.