Abstract
An increasing epidemic of obesity has become a serious public health concern primarily because it contributes to pathogenesis of many chronic diseases including type 2 diabetes, cardiovascular disease, hepatobiliary disease, obstructive sleep apnea, kidney disease, some types of cancer, among others. Consumption of a variety of phytochemicals has emerged as a promising potential for combating obesity and its comorbidities. However, the generally low aqueous solubility, stability, bioavailability, and target specificity of phytochemicals, along with their side-effects and toxicity seen when used at high doses, have restricted their clinical applications. As a solution, phytochemicals can be encapsulated into nanoparticles to increase their stability and solubility, enhance their bioavailability, protect them from premature degradation in the body, prolong their circulation time, and thus enhance their antiobesity activity. In this perspective, we summarize the problems and limitations of the prominent phytochemicals (epigallocatechin gallate, trans-resveratrol, curcumin, and quercetin), the major biocompatible and biodegradable nanoparticles, and the efficacy of nanoencapsulated forms of these phytochemicals in combating obesity and its comorbidities.
Keywords: obesity, nanoparticles, epigallocatechin gallate, trans-resveratrol, curcumin, quercetin
INTRODUCTION
Obesity prevalence continues to increase steadily in the United States and worldwide. When energy intake exceeds expenditure, excess energy is stored in the form of fat in adipose tissue, increasing fat mass and body weight. Obesity often leads to a chronic low-grade inflammation, which contributes to pathogenesis of many chronic diseases and conditions including type 2 diabetes, insulin resistance, hypertension, hyperlipidemia, myocardial infarction, heart failure, stroke, gallbladder disease, nonalcoholic fatty liver disease, obstructive sleep apnea, kidney disease, some types of cancer, among others.1,2 Weight control is critical for preventing and alleviating these diseases.
Weight loss can be achieved by reducing energy intake and/or increasing energy expenditure. Current methods for weight loss include lifestyle intervention, pharmacotherapy, and surgery.3 The latter one has high efficacy but is highly invasive and expensive and is the last-line option for obesity. Pharmacotherapy utilizes orally administered drugs. Most Food and Drug Administration (FDA) approved drugs target energy intake by either suppressing appetite (phentermine) or decreasing nutrient absorption (orlistat). Orally administered drugs have the highest compliance but are beset with major problems such as a high level of hepatic metabolism (the first-pass effect) and low target specificity, leading to significant side effects and toxicity. Additionally, patients may have obesity relapse after stopping drug therapy. Lifestyle intervention is commonly recommended due to its noninvasiveness, low cost, convenience to practice, and low side effects. It includes medical nutrition therapy, exercise, and behavioral modification. Low-calorie diets and enhanced physical activity can result in short-term weight loss. Behavioral modification can enhance weight loss. However, long-term maintenance of weight loss is challenged by adhesion to the intervention programs and body offset response.3 Therefore, other approaches to combat obesity and its comorbidities are greatly needed.
Enhancing consumption of fruits and vegetables helps with weight control.4 This is directly related to the antiobesity functions of phytochemicals, the bioactive compounds which are abundant in these fruits and vegetables. Many cell-based and animal studies have demonstrated that phytochemicals have antiobesity activity via suppressing adipocyte proliferation, decreasing preadipocyte differentiation, promoting adipocyte apoptosis, inhibiting lipogenesis, enhancing lipolysis and fatty acid beta (β)-oxidation, and diminishing inflammation.4–6 Human studies have also indicated that phytochemicals can maintain metabolic health, but there is no consistent evidence supporting their antiobesity efficacy.7–9 The major factors that are commonly blamed include phytochemicals’ low aqueous solubility, poor stability, trivial bioavailability, and quick metabolism by enzymes in the gastrointestinal tract, liver, kidneys and other tissues.10
Nanoencapsulation opens up a new avenue in overcoming the aforementioned problems.2,5 Phytochemicals can be encapsulated into biocompatible and biodegradable nanoparticles to improve their bioavailability, aqueous solubility, and stability.11 Indeed, early results have suggested that enough doses of these phytochemicals, when encapsulated, could be delivered to the targeted organs and cells such that they could effectively promote fat loss and improve overall metabolism status.2 These early evidence, although preliminary, may herald a new strategy, which potentially is also highly compliant, in coping with the prevalence of obesity. To attract more research interests in this promising area, in this perspective, we will survey the biocompatible and biodegradable nanoparticles that are suitable for carrying phytochemicals and then review the available evidence on the efficacy of these phytochemical-loaded nanoparticles in combating obesity and its comorbidities. However, first we will give an overview on the great potentials of several phytochemicals and point out their practical problems.
PHYTOCHEMICALS: POTENTIALS AND PROBLEMS
Data from three prospective cohort studies, Nurses’ Health Studies, the Nurses’ Health Study II, and the Health Professional’s Follow-up Study, show that increased intakes of fruits and nonstarchy vegetables were negatively associated with weight change over a 24-year period.7 Additional human cohort studies also confirm this inverse association.12 Beneficial effects of these diets are attributed for the most part to the significant amounts of phytochemicals with recognized anti-inflammatory, antiproliferative, and antioxidant properties. These phytochemicals modulate signaling pathways including peroxisome proliferator activator receptor gamma (PPARγ), CCAAT/enhancer binding protein α (C/EBPα), AMP-activated protein kinase (AMPK), PPARγ activator 1-alpha (PGC-1α), sirtuin-1 (Sirt1), sterol regulatory element binding protein-1c (SREBP-1c), uncouple protein (UCP)-1 and UCP-2, and nuclear factor-kappa B(NF-κB), which further regulate adipogenesis, lipogenesis, browning and brown adipose tissue activity, and antioxidant and anti-inflammatory responses.4 Concomitantly, phytochemicals can reduce fat mass and body weight through decreasing adipocyte formation and lipid accumulation, increasing lipolysis, thermogenesis, and energy expenditure, and suppressing inflammation and oxidative stress.4,13,14
Although phytochemicals have great potential to address the obesity challenge, their low levels of bioavailability and high levels of metabolism in the body, however, restrain their antiobesity bioactivity. Phytochemical metabolism in the body involves absorption, metabolism, distribution, and excretion, and each of these processes may affect their biological activity.15 Initially, ingested phytochemicals need to be dissolved in gastrointestinal fluids and endure a wide range of pH throughout the gastrointestinal tract. Subsequently, they may be subjected to degradation and metabolism by intestinal enzymes like glycosidase, esterase, oxidases, and hydrolyases.16 Another absorption barrier is the intestinal epithelium. Enterocytes have membrane-bound ATP-binding cassette (ABC) transport proteins, which can facilitate phytochemical transportation in both directions, i.e., to the basolateral side (into blood) or to the apical side (back into the intestinal lumen).15 Genetic variations and polymorphisms in these transporter proteins may alter the absorption rate of phytochemicals.17 After free phytochemicals are absorbed into the circulation system, they further undergo extensive modifications through methylation, sulfation, and glucuronidation in the liver and other tissues, which further reduce blood concentrations of free phytochemicals.18 Moreover, phytochemicals themselves do not have target specificity and therefore cannot target adipose tissue. Enzymes in the liver and other tissues metabolize most of the circulating phytochemicals, which are then excreted in urine and bile.15,19
This perspective will focus on the four most promising phytochemicals, trans-resveratrol, curcumin, epigallocatechin gallate (EGCG), and quercetin (Figure 1). Not incidentally, these phytochemicals violate the “Lipinski’s rule of five” and thus have low bioavailability. Their low solubility and stability, together with the additional factors such as intestinal wall barrier, active efflux in enterocytes, and degradation and metabolism by microbiota, intestinal, and hepatic enzymes, make these phytochemicals much less bioavailable.10,19,20 Indeed, after oral ingestion of free trans-resveratrol, curcumin, EGCG, and quercetin, their concentrations in blood and adipose tissue are extremely low.21 They are eliminated into urine and bile as their metabolites.15,19 The low bioavailability and quick metabolism of these phytochemicals significantly limit their bioactivities in the body to trivial levels. Thus, solutions are in great need to circumvent these restrictions and boost their antiobesity efficacy to levels that meet clinical requirements.
Figure 1.
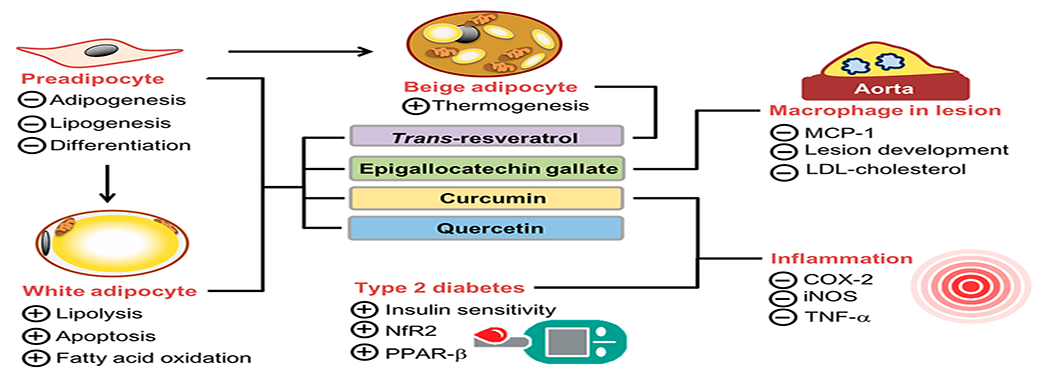
Potential actions of phytochemicals, particularly trans-resveratrol, EGCG, curcumin, and quercetin on obesity and its comorbidities. Abbreviations: EGCG, epigallocatechin gallate; MCP-1, monocyte chemotactic proteins-1; LDL, low-density lipoprotein; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; TNFα, Tumor Necrosis Factor; Nrf2, nuclear factor erythroid-2-related factor 2; PPAR-β, peroxisome proliferator-activated receptor-β. (+): Stimulate; (−): Inhibit.
BIOCOMPATIBLE AND BIODEGRADABLE NANOPARTICLES
Nanoparticles are particles having a size range of 1–100 nm in at least one dimension.2 The pathological expansion of adipose tissue in obesity states enhanced permeation and retention effect, by which these nanoparticles exhibit highly differential uptake efficiency in the expanded adipose tissue over other tissues. Nanoparticles can also increase antiobesity efficacy through preventing phytochemicals from prematurely interacting with the biological environment in the body and improving cellular uptake in the adipose tissue.2 Major nanoparticle characteristics include nanoparticle size, Zeta potential (surface charge), polydispersity index (size homogeneity), physical and chemical stabilities, encapsulation efficiency, and loading capacity. They are key factors to determine nanoparticles’ stability, bioavailability, biodistribution, and metabolism.22 In the broad fields of nanomedicine, many organic and inorganic materials have been investigated to construct nanoparticles with a variety of structures and morphologies and functions for diagnosis and treatment of malignant tumors and other fatal diseases. However, in combating chronic diseases such as obesity, the compositions of nanoparticles should be based on those biocompatible and biodegradable materials that are safe to humans with trivial side effects. Therefore, this perspective will only cover liposomes, lipid and polymeric nanoparticles, micelles, and nanoemulsions that can be synthesized from biocompatible and biodegradable materials (Figure 2).
Figure 2.
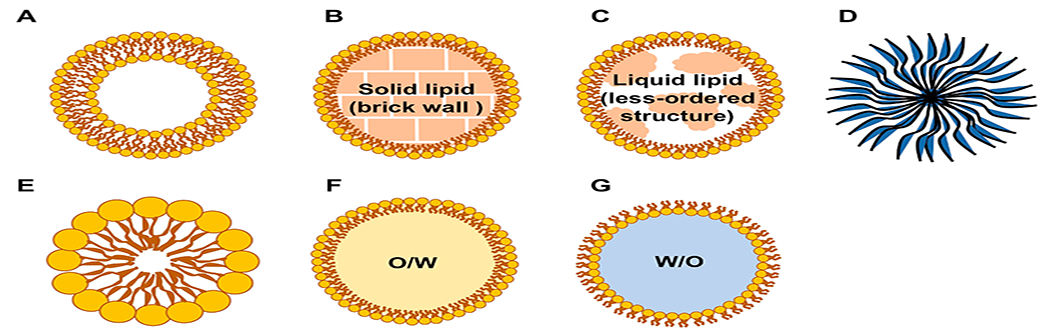
Schematic structure of biocompatible and biodegradable nanoparticles: (A) liposome, (B) solid lipid nanoparticle (SLN), (C) nanostructured lipid carriers (NLC), (D) polymeric nanoparticle, (E) micelle, (F) nanoemulsion (oil-in-water, O/W), and (G) nanoemulsion (water-in-oil, W/O).
Liposomes.
A liposome is a spherical vesicle consisting of one or multiple lipid bilayers. Most often, liposomes are composed of phospholipids, such as phosphatidylcholine. Phospholipids have a hydrophilic head and two hydrophobic fatty acid tails, which self-organize into the conventional lipid bilayer structure in an aqueous solution. The common liposome synthesis methods include extrusion, sonication, freeze–thawing, ether injection, microemulsification, and microfluidization.23–25 With its composition flexibility and various preparation methods, the liposome can be fabricated into different types such as the small unilamellar liposome vesicle, the large unilamellar vesicle, and the multilamellar vesicle, with sizes from the nanoscale to microscale.
Since a liposome has an aqueous solution core surrounded by its lipid bilayer, hydrophilic molecules can be dissolved in the core and cannot readily pass through the bilayer, unless the latter is fused with other bilayers such as a cell membrane, where the hydrophilic solute will then be delivered to this cell. Similarly, hydrophobic molecules can also be loaded into the lipid bilayer of the liposome for protective delivery. Due to this capability and its biosafety, the liposome has been used as a vehicle for administration of nutrients and medicines. In 1995, FDA approved liposome-based product Doxil drug for treating ovarian cancer. After that, many liposomal products have been approved by the FDA, including DaunoXome for advanced HIV-associated Kaposi’s sarcoma and Amphotec and Ambisome for the treatment of fungal infections. In the food industry, liposomes have also been used for carrying and delivering flavors, nutrients, and enzymes.26
With its versatility in loading both hydrophilic and hydrophobic molecules and its biocompatibility, the liposome is also a good vesicle to carry phytochemicals in combating chronicle diseases including obesity. Hydrophilic phytochemicals like EGCG can be encapsulated into the hydrophilic core of liposomes, while hydrophobic phytochemicals like trans-resveratrol, curcumin, and quercetin can be incorporated into the hydrophobic domain of their phospholipid tails.27 Encapsulation of these phytochemicals into liposomes will increase their stability, aqueous solubility, bioavailability, and antiobesity bioactivity.2,28,29 Importantly, multiple phytochemicals with synergistic effects can be encapsulated into the same liposome vesicle. Target ligands can be further conjugated on the surface of liposomes to enhance targeted delivery of phytochemicals to specific cells with improved therapeutic efficacy.30 Other functional modifications of the liposome structure can also be made. For example, polyethylene glycol (PEG) can be grafted on the liposome surface to prolong its circulation time in the blood.31
Instability might be a concern for liposomes in certain applications. They also suffer from a low loading efficiency for hydrophobic phytochemicals.32 However, the aforementioned many advantages render the liposome as a good vehicle for delivering drugs, phytochemicals, and other agents.33 We consider that the liposome is a very promising technique for phytochemical delivery in combating obesity and its comorbidities.
Lipid Nanoparticles.
Lipid nanoparticles can be categorized into solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). The difference lies at their core structure, which leads to different loading capacities. With a lipid core, lipid nanoparticles are suitable for encapsulating hydrophobic molecules.
As the first generation of lipid nanoparticles, SLNs are composed of a monolayer of phospholipids and a hydrophobic lipid core, which is solid at body temperature. Fatty acids, triglycerides, waxes, or mixtures of the above lipids have been used as solid lipids to make the hydrophobic core. One or more surfactants are usually used to stabilize the SLN structure.34 SLNs have many advantages including protection of encapsulated phytochemicals from enzymatic degradation and metabolism and improvement of their solubility, stability, bioavailability, and bioactivity. Nevertheless, SLNs suffer from their low drug loading capacity due to the “brick wall” structure in the hydrophobic core formed by a single solid lipid. To overcome the low loading issue, NLCs were developed. They have a mixture of solid and liquid lipids in their hydrophobic cores, which provide a less ordered crystalline structure, resulting in the high loading capacity of hydrophobic phytochemicals.35 The common preparation methods include hot/cold/high-pressure homogenization, ultrasonication, solvent evaporation/emulsification, and phase inversion.36
Even though NLCs have some issues related to their thermodynamical instability, aggregation, and sedimentation,32 their increased drug stability and loading capacity and reduced drug expulsion have attracted much interest in developing NLC delivery systems for various routes of administration.37 With increased number of patented NLC products and updated knowledge on NLC’s transport, biodistribution, and working mechanisms, the clinical application of NLCs in combating chronic diseases, including obesity and its comorbidities, is expected to speed up in the near future.
Polymeric Nanoparticles.
Polymeric nanoparticles are made of polymers. Commonly used biodegradable polymers include synthetic polymers [poly(lactide) (PLA), poly(ε-caprolactone) (PCL), poly(lactide-coglycolide) (PLGA), and poly(ethylene glycol) (PEG)], and natural polymers (chitosan and alginate). Phytochemicals can be conjugated to polymers or encapsulated into polymeric nanoparticles to increase their bioavailability and improve their sustained and controlled release manner.38 The common preparation methods for polymeric nanoparticles include emulsification–diffusion, nanoprecipitation, and solvent evaporation methods.2
trans-Resveratrol,39 EGCG,40 and curcumin41 have been encapsulated into PLGA nanoparticles for improving their characteristics and bioactivities. PLGA is a FDA-approved material for making medical devices.2 PLGA is synthesized via copolymerization of two monomers, glycolic acid and lactic acid. Different ratios of glycolic acid to lactic acid can produce different types of PLGA. It can be metabolized naturally to yield glycolic acid and lactic acid in the body.42 Another commonly used biocompatible polymer is PEG, which is highly hydrophilic, and has negligible immunogenicity. PEG has been widely used as the nanoparticle’s hydrophilic shell for prolonging their circulation time and increasing their stability. The unique surface chemistry of polymeric nanoparticles can adsorb, entrap, or covalently attach therapeutic phytochemicals to offer a significant delivery improvement.43 However, for treatment of chronicle diseases, the metabolism and long-term safety of polymeric nanoparticles require further investigation.
Micelles.
Micelles are a group of amphiphilic colloids made of amphiphilic monomers. The common amphiphilic monomers include phospholipids, PEG, and poly(N-vinyl-2-pyrrolidone) (PVP). The common preparation methods for micelles include solvent evaporation, oil in water emulsion, dialysis, filtration, and solid dispersion.44,45 Micelles have a hydrophobic core and many hydrophobic phytochemicals can be encapsulated into it. Specific targeting ligands can be grafted on the micelle surface to generate “targeting micelles” to disease cells and tissues.46 Micelles are easy to synthesize and have a long circulation time, high stability, and increased cellular uptake,32,47 but they suffer from a high surfactant content and low encapsulation efficiency and loading capacity.32
Nanoemulsions.
Nanoemulsions can be oil-in-water (O/W) or water-in-oil (W/O) dispersion of two immiscible liquids stabilized by surfactants and cosurfactants.48 O/W nanoemulsions are formed by dispersing oil into an aqueous phase. On the contrary, W/O nanoemulsions are formed by dispersing an aqueous solution into an oil phase.
O/W nanoemulsions are commonly used in nanomedicine research, which have a hydrophobic core, and many hydrophobic phytochemicals, like trans-resveratrol,49 quercetin,50 and curcumin,51 can be encapsulated into the core.52 In order to stabilize the emulsion structure, amphiphilic surfactants/emulsifiers are needed for synthesizing nanoemulsions.2 Homogenization and sonication are the common preparation methods for nanoemulsions in a laboratory scale. Industry uses high-pressure homogenization and microfluidization for producing a large amount of nanoemulsions.2 To improve the target specificity of nanoemulsions, many targeted nanoemulsions have been developed by incorporating targeting ligands including peptides53 and antibodies54 on the surface of nanoemulsions. Nanoemulsions are easy to prepare and have high loading capacity and tunable rheology, which render them as excellent delivery vesicles for phytochemicals.32,55 The disadvantages of nanoemulsions include thermodynamic instability and easy aggregation and creaming.32
NANOENCAPSULATED PHYTOCHEMICALS IN COMBATING OBESITY AND ITS COMORBIDITIES
Trans-resveratrol, curcumin, EGCG, and quercetin are the most extensively researched phytochemicals in their nanoformulations. Relevant researches were determined via a systematic search of MEDLINE (Pubmed) and Web of Science Core Collection databases. Search keywords included phytochemical classes in combination with different nanoencapsulation techniques and obesity and related comorbidities.
trans-Resveratrol.
trans-Resveratrol is a natural polyphenolic compound particularly enriched in various berries, red grapes, and other foods of plant origins.56,57 trans-Resveratrol is increasingly recognized to have beneficial effects against obesity and obesity-related insulin resistance, inflammation, and hyperlipidemia by inhibiting adipogenesis and lipogenesis,58 stimulating lipolysis, improving glucose uptake in adipocytes,59 and increasing fatty acid oxidation and thermogenesis60 in cell-based and animal studies. Emerging evidence suggests that trans-resveratrol may induce beige adipocyte formation in white adipose tissue that will further bring up subsequent beneficial activities by enhancing mitochondria biogenesis and uncoupling protein 1 expression by activating AMPK.61
Although a large body of in vitro and in vivo studies point to the antiobesity benefit of trans-resveratrol, evidence from human studies is limited and inconclusive.62 The primary factors for this limitation include its low aqueous solubility (<0.1 mg/mL), trivial bioavailability (peak plasma trans-resveratrol concentration <10 μM after high-dose oral administration), and lack of targeting specificity.63 Although the absorption of trans-resveratrol after oral consumption is nearly 75%, most of trans-resveratrol is trapped and metabolized in the liver and the major metabolites are glucuronides and sulfates of trans-resveratrol.64 One approach to overcoming the aforementioned restrictions is to encapsulate trans-resveratrol into biocompatible and biodegradable nanoparticles. Oral bioavailability of trans-resveratrol after nanoencapsulation showed an 8- to 19-fold increase in several studies.65,66 trans-Resveratrol-loaded liposomes and lipid nanoparticles have been investigated to enhance its solubility and antiobesity bioactivity.28 However, liposomes in circulation can be rapidly captured and removed by the mononuclear phagocyte system. To prolong its circulation and integrity, the liposome surface was modified by using PEG, which protected liposomes by its shielding effect against opsonin recognition and consequent liposome phagocytosis upon binding.67 Chitosan is another widely used polymer for modifying liposomes in order to bypass the acidic stomach environment and allow the release of trans-resveratrol in the intestines.68 trans-Resveratrol was also reported to be loaded into polymeric nanoparticles such as PEG—PLA nanoparticles,69 and PLGA nanoparticles.39 Polymeric nanoparticles increased oral delivery efficacy of trans-resveratrol due to their small particle size, controlled delivery, and high encapsulation efficiency.
Effects of nanoencapsulated trans-resveratrol on obesity, type 2 diabetes, and inflammation have been investigated (summarized in Table 1). In our study, we demonstrated that trans-resveratrol-encapsulated lipid nanoparticles and liposomes had the advantage over free trans-resveratrol in enhancing gene expression of UCP-1 and beige marker CD137 and decreasing white adipose tissue specific marker (insulin growth factor binding protein 3) gene expression in 3T3-L1 white adipocytes.28 We also developed ligand-coated trans-resveratrol-loaded nanoparticles that target adipose stromal cells (ASCs) via binding to their glycanation site-deficient decorin receptor. When administered intravenously to obese mice, nanoencapsulated trans-resveratrol significantly increased the targeted delivery of trans-resveratrol to ASCs in subcutaneous white adipose tissue and induced UCP-1 expression, resulting in enhanced white adipose tissue browning and fat loss. These results suggest that trans-resveratrol nanoencapsulation approach may have a promising clinical potential for combating obesity and improving metabolic health. Mechanistic studies have explored the involvement of gut microbiota in the antiobesity effect of trans-resveratrol. It was proposed that trans-resveratrol and its metabolites might promote white adipose tissue browning through inducing remodeling of gut microbiota and Sirt1 signaling appeared to play a key role in this “gut microbiota-adipose tissue” axis.70 In summary, trans-resveratrol has an antiobesity potential, but its clinical application is greatly limited by its low level of solubility, stability, and bioavailability as well as a high level of hepatic metabolism. Encapsulating trans-resveratrol into biocompatible and biodegradable nanoparticles represents a potentially effective approach for overcoming these limitations and thus improving its antiobesity bioactivity.
Table 1.
Characteristics and Bioactivities of Phytochemical-Encapsulated Nanoparticlesa
| phytochemicals |
nanoparticles |
experiments |
||||
|---|---|---|---|---|---|---|
| name | name/components | size (nm) | EE | model/treatments | main outcomes | refs |
| trans-resveratrol | solid lipid nanoparticles | 248 | 79.9 | model: Wistar male diabetic rats | ↓ fasting glucose sugar | 133 |
| treatment: oral administration of 10 mg/kg/day of resveratrol in SLN form or free form for 1 month | ↓ insulin resistance | |||||
| ↓ serum oxidative stress status | ||||||
| ↓ Snap23, Stx4 and Vamp2 level in muscle | ||||||
| PLGA nanoparticles | 176 | 97.2 | model: HepG2 hepatocytes | ↑ stability, water solubility and bioactivity of resveratrol | 134 | |
| treatment: 12.5, 25, 50, and 100 μM of resveratrol in PLGA form or free form for 24h. | ↓ lipogenesis | |||||
| ↓ lipolysis | ||||||
| ↓ hepatocellular proliferation and lipid accumulation | ||||||
| galactosylated PLGA nanoparticles | 108.4 | 97.2 | model: Caco-2 cells for uptake study | ↑ Caco-2 cellular uptake of resveratrol | 135 | |
| mouse macrophage cell line RAW 264.7 for anti-inflammation study | ↓ TNF-α and IL-6 expressions | |||||
| treatment: 20 μg/mL of resveratrol in PLGA form or free form. | ||||||
| EGCG | lipid nanoparticles; phosphatidylcholine, kolliphor HS15, a-tocopherol acetate, EGCG, and 1-(palmitoyl)-2-(5-keto-6-octene-dioyl) phosphatidylcholine (KOdiA-PC) | 108 | 96.0 | model: human monocytic | ↑ EGCG stability | 80 |
| THP-1 cells | ↑ sustained release of EGCG | |||||
| treatment: free or nano-EGCG at EGCG dose of 5, 10, 20, and 40 μg/mL in combination with 40 μg protein/mL of oxLDL for 18 h | ↑ binding affinity to and uptake by macrophages | |||||
| ↑ macrophage EGCG content | ||||||
| ↓ macrophage MCP-1 mRNA levels and protein secretion | ||||||
| chitosan–tripolyphosphate nanoparticles of EGCG (EGCG is encapsulated into self-assembled nanoparticles made of chitosan and aspartic acid) | 100 | 25.0 | model: male New Zealand white rabbits | ↓ TC | 84 | |
| treatment: free or nano-EGCG (7 mL of 0.5% aqueous acetic acid) at EGCG dose of 100 mg/day for 5 weeks. | ↓ TG | |||||
| ↓ HDL | ||||||
| ↓ LDL | ||||||
| soy lecithin, glyceryl tridecanoate, glyceryl tripalmitate, and Kolliphor HS15 | 45- 50 | 99.0 | model: THP-1-derived | ↑ EGCG stability | 81 | |
| macrophages | ↑ sustained release of EGCG | |||||
| treatment: free or nano-EGCG at EGCG dose of 5 μM, 10 μM and 20 μM were tested | ↑ cellular bioavailability of EGCG | |||||
| ↓ TC content in macrophages | ||||||
| ↓ MCP-1 expression in macrophages | ||||||
| soy PC, surfactant Kolliphor HS15, and (+)-α-tocopherol acetate | 104 | 95.0 | Model: male 6-week old LDLr−/− mice | ↓ secretion of inflammatory factors in mouse peritoneal macrophages | 77 | |
| treatment: free or nano-EGCG at EGCG dose of 25 mg/kg/body weight EGCG per week for 22 weeks | ↓ lesion surface areas of aortic arches | |||||
| curcumin | turmeric extract-loaded nanoemulsions | 136–138 | 88.0 | Model: Balb/c mice | ↓ SREBP-1 | 110 |
| treatment: oral administration of free or nanocurcumin at a curcumin dose of 300 mg/kg/day 3 times/week for 9 weeks | ↓ PPARγ2 | |||||
| ↓ Cleaved caspase-3 | ||||||
| ↓ PARP in the liver | ||||||
| polylactide-poly(ethylene glycol) (PLA–PEG) copolymer nanoparticles | 100–150 | 98.3 | model: albino rats (STZ-induced diabetic) | ↓ NF-κB activation | 113 | |
| treatment: oral administration of free or nanocurcumin at a curcumin dose of 20 mg/kg/day | ↓ COX-2 and TGF-β expression | |||||
| ↑ PPARγ expression | ||||||
| PLGA based nanoparticles | 237 ± 6 | 66.0 | model: Sprague–Dawley rats (STZ induced diabetic) | ↓ CRP, IL-6, TNF-α | 136 | |
| treatment: oral administration of nanocurcumin at a curcumin dose of 100 mg/kg/day for 15 days. | ↓ plasma TG and TC | |||||
| ↑ HDL-cholesterol | ||||||
| quercetin | succinylated chitosan-alginate core—shell-corona nanoparticles | 91.58 ± 1.14 | 95.0 | model: Male Wistar rats (STZ induced diabetic) | ↓ TG | 11 |
| treatment: oral administration of free or nanoquercetin at a quercetin dose of 100 mg/kg/day for 28 days | ↓ TC | |||||
| ↓ AST, ALT and ALP levels | ||||||
| PLGA nanoparticles | 179.9 ± 11.2 | 86.0 | model: Sprague–Dawley rats (STZ induced diabetic) | ↓ blood glucose levels (similar effect to free quercetin although with a lower dose and frequency) | 129 | |
| treatment: oral administration of free or nanoquercetin at a quercetin dose of 150 mg/kg every day for free group and every 5th day for NP group for 15 days | ||||||
Abbreviations: SLN, solid lipid nanoparticles; EE, encapsulation efficiency; EGCG, epigallocatechin gallate; oxLDL, oxidized low density lipoprotein; MCP-1, monocyte chemotactic proteins-1; NP, nanoparticle; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PC, phosphatidylcholine; Snap23, synaptosomal-associated protein 23; Stx4, Syntaxin4; Vamp2, vesicle-associated membrane protein 2; R, resveratrol; SREBP1c, sterol regulatory element-binding protein-1c; PPAR, peroxisome proliferator-activated receptors; PARP, poly(ADP-ribose) polymerase; STZ, streptozotocin; NF-κB, suppressing nuclear factor-kappa B; COX-2, cyclooxygenase-2; TGF, tumor growth factor; CRP, C-reactive protein; IL-6, interleukin-6; TNFα, Tumor Necrosis Factor; TG, triglycerides; TC, total cholesterol; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase.
EGCG.
EGCG, a polyphenol, is the most abundant green tea catechin. EGCG has anti-inflammatory, antioxidant, antibacterial, and antiviral properties.71 Studies have indicated that EGCG exerts its antiobesity and antidiabetic activities via inhibiting adipocyte proliferation, decreasing preadipocyte differentiation, promoting adipocyte apoptosis, reducing lipogenesis, enhancing lipolysis and β-oxidation, improving insulin sensitivity, and suppressing inflammation. 6,71,72 In preclinical studies with animal models, EGCG has been shown to be beneficial in managing obesity and improving its related metabolic diseases/disorders including insulin resistance, dyslipidemia, diabetes, and nonalcoholic fatty liver disease.73–76 In contrast, these results for the large part have not been reproduced in humans. The allowable doses of EGCG used in human studies are usually much lower than those in animal studies. EGCG has low stability, bioavailability, and target specificity and a high rate of enzymatic metabolization in tissues, primarily liver and kidneys. All these factors are blamed for its low effectiveness in antiobesity activity.77
EGCG bioavailability is relatively low. The peak plasma EGCG concentrations are in the range of high nanomolar to low micromolar levels after EGCG or green tea consumption in humans and animals.6 For example, in a human study, after oral administration of 3 g of decaffeinated green tea, the peak plasma concentrations of EGCG, epigallocatechin (EGC), and (—)-epicatechin (EC) were 0.57, 1.60, and 0.6 μM, respectively.78
Moreover, EGCG in water and physiological fluids is not stable;2 EGCG in the body can be quickly metabolized/transformed by enzymes in the liver, kidneys, and other tissues.2 The major EGCG metabolic transformations include glucuronidation, methylation, sulfation, and oxidative degradation.2 Encapsulating EGCG into biocompatible and biodegradable nanoparticles, such as liposomes, lipid nanoparticles, and chitosan-coated lipid nanoparticles, can solve these problems.71,79 Lipid, liposomes, and polymeric nanocarriers are the most commonly used nanoparticles for EGCG delivery.71,72 Its stability and bioactivity are significantly enhanced after encapsulated into liposomes or lipid nanoparticles.80–82 In one study, after EGCG-loaded nanoparticles were added to the simulated digestive fluids, the majority of EGCG (>60%) remained entrapped in the nanoparticles, so that EGCG absorption by the intestinal mucosa was enhanced.83
Nanoencapsulation can also improve EGCG’s bioactivity. Compared to free EGCG, nanoencapsulated EGCG increased EGCG stability, decreased macrophage inflammation and cholesterol accumulation, and prevented atherosclerosis development (Table 1).80,81 Ligand-coated EGCG lipid nanoparticles, which target intimal macrophages, were shown to increase macrophage EGCG content, which coincided with diminished macrophage monocyte chemoattractant protein (MCP)-1 mRNA expression and protein secretion, and reduced lesion areas of aortic arches in mice.77 In one study, rabbits were given oral administration of 100 mg/day EGCG in the free or the nanoparticulated form. It was found that EGCG nanoparticles relative to free EGCG significantly decreased blood concentrations of triglyceride, total cholesterol, and low density lipoprotein (LDL)-cholesterol and reduced atherosclerotic lesion development.84 Nanoencapsulation of EGCG into polymeric nanoparticles can enhance antiatherosclerosis and antitumor bioactivity.71,83 So far, no information is available regarding the application of EGCG nanoparticles in treating obesity or type 2 diabetes, but the gap is expected to be filled soon given the developing groundwork discussed above.
Curcumin.
Turmeric spice has been used for medicinal purposes for thousands of years in Asia, and curcumin is a primary bioactive ingredient in turmeric and gives it the yellow color.85 Curcumin is hydrophobic phenolic compound with antioxidant properties, and it was first extracted from Curcuma longa Linn. by Vogel et al.85 In recent decades, investigators have demonstrated that curcumin has a variety of pharmacological activities in preventing and treating chronic metabolic and inflammatory diseases such as cardiovascular diseases, type 2 diabetes, rheumatoid arthritis, Alzheimer’s disease, and multiple sclerosis as well as cancer.86–89 These effects of curcumin are well in line with, its anti-inflammatory, antitumorigenic, antiangiogenic, and antioxidant properties.90 Studies have also discovered that curcumin has antiobesity bioactivity via inhibiting preadipocyte differentiation and lipogenesis, increasing fatty acid β-oxidation, and suppressing inflammation in adipose tissue, while improving lipid metabolism and insulin sensitivity.2,91 It can further increase insulin sensitivity, and this effect may be related to activation of nuclear factor erythroid-2-related factor 2 (Nfr2) and PPAR-β and inhibition of NF-κB and Wnt/β-catenin pathways, plasminogen activator inhibitor-1 (PAI-1), and tumor necrosis factor (TNF)-α expressions.92–94 In addition, curcumin has been reported to suppress inflammation via inhibiting proinflammatory enzymes cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS).95 As with the cases of resveratrol and EGCG, the antiobesity activity of curcumin is largely observed in cell-based and animal studies,92–95 while the evidence from human studies is limited and inconsistent.96,97 Likewise, its low solubility and bioavailability as well as the complexity of human diets may be the factors responsible for the disappointing results in human trials. Furthermore, complex pharmacological features, pharmacokinetics, and possible side effects at high doses present challenges in clinical application of curcumin for obesity management.98
Curcumin has a very low aqueous solubility (0.6 μg/mL).99,100 Free curcumin undergoes rapid chemical degradation in alkaline aqueous conditions and crystallization in acidic aqueous conditions. Curcumin stability is further affected by pH alteration, that is the case when it travels through the gastrointestinal tract.101 Furthermore, curcumin is quickly metabolized by hepatic enzymes resulting in very low blood concentrations that may not be adequate to produce a therapeutic effect.102 In a study by Shoba et al., peak serum curcumin concentration was only 1.35 μg/mL at 1 h after oral administration of 2 g/kg curcumin to rats, and in contrast, extremely lower serum concentration (0.006 μg/mL) was found in healthy males (50–75 kg) after receiving a single dose of 2 g curcumin.103 Similarly, other studies reported that oral administration of 3.6–8 g of curcumin to humans resulted in the peak plasma curcumin concentrations at 0.004–0.644 μg/mL.104,105
Nanoparticles appear to be a promising solution for the poor bioavailability and retaining of curcumin in human body. Nanoencapsulation can increase curcumin’s solubility, stability, and bioavailability. It has been reported that encapsulating curcumin into micelles or PLGA nanoparticles increased both its aqueous solubility and stability.106,107 Nanoencapsulation of curcumin into SLNs, nanoemulsions, and PLGA nanoparticles enhanced its bioavailability by more than 2-fold.2,107–109
Accordingly, nanoencapsulation is shown to increase curcumin’s antiobesity bioactivity.2 In a study by Lee et al., high fat diet fed mice were given oral administration of free curcumin or curcumin-loaded nanoemulsions three times per week for 9 weeks.110 Even though curcumin-loaded nanoemulsions contained 5% of curcumin as in the free curcumin treatment, mice in two groups showed similar results in reducing the weight gain.110 Additionally, compared to mice fed free curcumin, those fed with curcumin-loaded nanoemulsions had lower lipogenesis and adipogenesis mediated through inhibition of SREBP-1, PPARγ, cleaved caspase-3, and poly(ADP-ribose) polymerase (PARP) in the liver.110 This study indicates that nanoemulsions can reduce the therapeutic dose of curcumin (Table 1).110 In another animal study, metabolic syndrome was induced in male Wistar rats using a high carbohydrate and high fat diet for 8 weeks.111 After that, four groups of rats were given oral administration of curcumin PLGA nanoparticles (5 mg/kg/day), blank nanoparticles, free low dose curcumin (5 mg/kg/day), and free high dose curcumin (100 mg/kg/day), respectively, for an additional 8 weeks. Even though the curcumin nanoparticle group had significantly higher food intake than the free curcumin group, no significant differences in body weight were found between these two groups.
The reported effects of nanoencapsulated curcumin on oxidation, inflammation, glucose homeostasis are inconsistent. Some studies demonstrated that curcumin nanoparticles compared to free curcumin, improved antioxidant, anti-inflammatory and antidiabetic bioactivities,112,113 but others did not find such difference.114–116 Other studies also indicated that nanoencapsulated compared to free curcumin improved blood lipid profiles and cardiovascular health. Considering the many health benefits of curcumin, more studies are warranted for definitive evaluation to ascertain whether nanoencapsulation can enhance its antiobesity and other bioactivities.
Quercetin.
Quercetin is a flavonoid compound found in various types of food such as onions, berries, citrus fruits, apples, brassica vegetables, nuts, seeds, and tea.2 In addition to widely reported anti-inflammatory, antiviral, and antioxidant properties,6 quercetin has been shown to have antiobesity activity.6,117,118 It increases fatty acid β-oxidation by stimulating mitochondrial biogenesis and promotes lipolysis by elevating cyclic adenosine monophosphate (cAMP) levels and hormone sensitive lipase (HSL) activity.119,120 Furthermore, quercetin suppresses lipogenesis by inhibiting fatty acid synthase and acetyl-CoA carboxylase and adipogenesis by inhibiting PPARγ and C/EBPα.98 Quercetin’s antiobesity bioactivity is largely based on results from cell-based and animal studies, but evidence in human studies is limited and inconclusive,6,121 which is partly attributed to its low solubility and bioavailability and high levels of metabolism and excretion.6,20 Quercetin is insoluble in cold water, poorly soluble in hot water, but quite soluble in alcohol and lipids.120,122 Quercetin’s solubility in water differs from 0.00215 g/L at 25 °C to 1.49 g/L at 140 °C in anhydrous or dihyrate forms. The solubility of quercetin in ethanol is approximately 2 g/L at 25 °C.122 Quercetin is an aglycone and undergoes the first-pass metabolism in the gastrointestinal tract and liver after oral administration. After a single dose of ingestion, bioavailability of quercetin is around 2%. In the glucoside form, the absorption rate was found between 3 and 17% with ingestion of 100 mg in healthy human subjects.120 Unabsorbed quercetin is rapidly metabolized by gut microbiota and excreted from the colon.120
Studies have demonstrated that nanoencapsulation can increase quercetin’s stability, aqueous solubility, bioavailability, and bioactivities.123–126 The aqueous solubility of quercetin can be increased by 110-fold using nanomicelles127 and 1000-fold using NLCs.122 NLCs can also increase quercetin’s stability and bioavailability.2,122 Furthermore, nanoencapsulation may prolong quercetin’s circulation time and prevent its degradation and metabolism in the body.2
Few studies have been published concerning the use of nanoencapsulated quercetin in obesity, type 2 diabetes, cardiovascular disease, and inflammation. In one study, diabetic rats were given 10 mg/kg free quercetin daily or 10 mg/kg nanoencapsulated quercetin every 5 days for 8 weeks via abdominal subcutaneous injections for 8 weeks, and it was found that nanoencapsulated compared to free quercetin had a better and prolonged glycemic control.128 Similarly, other animal studies demonstrated similar antidiabetic outcomes with different nanoformulations of quercetin (Table 1).11,129,130 Nanoencapsulation is also shown to improve anti-inflammatory properties of quercetin. After giving rats intravenous injection of free quercetin and quercetin-loaded liposomes at a dose of 5 mg/kg once a week for 4 weeks, quercetin-loaded liposomes compared to free quercetin significantly lowered the concentrations of inflammatory factors including TNF-α, IL-1β, and IL-6 in bronchoalveolar lavage fluid.131 Another study using quercetin-loaded polymeric nanoparticles demonstrated that nanoencapsulated compared to free quercetin increased mitochondrial integrity, size, and functions.132
Quercetin has a potential in suppressing lipogenesis, adipogenesis, and inflammation and therefore may help in obesity treatment; however, its rapid metabolism and low solubility, stability, and bioavailability diminish its efficacy in these effects. Biodegradable and biocompatible nanoparticles have emerged to be a potential solution to overcome these issues and increase the opportunity of its future clinical application in combating obesity and its comorbidities.
SUMMARY AND FUTURE PERSPECTIVES
Many phytochemicals are promising in the prevention and treatment of obesity and its comorbidities. However, their low stability, solubility, and bioavailability together with high metabolism rates prevent their application in these fields. This is true for EGCG, trans-resveratrol, curcumin, and quercetin, the most intensively studied phytochemicals with the best potentials in combating obesity and its comorbidities. A considerable amount of studies has demonstrated that biocompatible and biodegradable nanoparticles can effectively deliver phytochemicals, improve their bioavailability and bioactivities, and even increase synergistic effects by carrying multiple phytochemicals in one nanoparticle system.
There are several biocompatible nanoparticle systems that could be used to deliver these phytochemicals for combating obesity and other chronic diseases. Each nanoparticle has its own characteristics, loading capacity, and other pros and cons. Selection of nanoparticles for delivery of a particular phytochemical requires the match between the carrier and the load as well as consideration of effective doses, administration routes and frequency, their side effects, and toxicity among others.
Since most phytochemicals are administered via the oral route, a major challenge in designing biocompatible and biodegradable nanoparticles is how to minimize their digestion, absorption, and metabolism in the body before reaching the target tissues. Many biodegradable components in the nanoparticles can be digested by enzymes in the gastrointestinal tract. Even though nanoparticles, in their integral forms, can protect the encapsulated phytochemical cargo, gastrointestinal digestion may change the integrity, characteristics, and pharmacokinetics of nanoparticles themselves, thus diminishing their protection capability.2 Future studies should focus on developing nanoparticles which avoid gastrointestinal digestion and enter into the bloodstream in intact forms. Functionalizing the nanoparticle surface to extend their blood circulation duration for sustainable delivery and/or target delivery to the right tissues are some of the crucial research subjects.
Safety is a major concern for nanoparticle medical applications in general and for treatment of obesity and other chronic diseases in particular. The side effects of nanoparticles must be trivial when compared to its benefits. In order to produce commercially feasible nanoparticles, using generally recognized as safe (GRAS) food ingredients and good manufacturing practice is necessary.32 Target ligands, surfactants, cosurfactants, and emulsifiers in the nanoparticles may lead to hepatoxicity, nephrotoxicity, and immunotoxicity.2 The photochemical doses are also a major concern. Even though nanoencapsulation gives phytochemicals dose advantages via increasing the phytochemicals’ stability, bioavailability, target delivery, and bioactivities, the safe dose ranges of nanoencapsulated phytochemicals must be determined through rigorous studies.2 Additionally, some nanoparticles may have off-target effects, and nanoencapsulated and free phytochemicals may have completely different tissue biodistribution, pharmacokinetics, and metabolism. Both short- and long-term safety of nanoparticles and encapsulated phytochemicals need to be intensively evaluated. Currently, there are no standardized tests and guidelines available for determining side effects and toxicity of nanoparticles. Future research should focus on developing suitable in vitro and in vivo models, standardized safety tests, and guidelines.
Even though encouraging groundworks have demonstrated the potential of biocompatible and biodegradable nanoparticles in boosting the antiobesity activities of several phytochemicals, most of these studies have been conducted at the preclinical level with cells and animals as the tested subjects. We therefore recommend that future studies in this promising area might focus on (1) fundamental studies to understand the metabolism and antiobesity mechanisms and efficacy of nanoencapsulated phytochemicals and (2) the safety study of the phytochemical-loaded nanoparticle system. The fundamental understandings on these areas will lay a solid foundation to move the studies forward to clinical stages in near future, toward the ultimate goal of using natural phytochemicals in combating obesity and its comorbidities. Until efficacy and safety of any phytochemical-loaded nanoparticles are rigorously evaluated and approved by FDA, a diet rich in phytochemicals may still be helpful for combating obesity and its comorbidities.
Funding
Shu Wang was supported by the American Heart Association (Grant 19AIREA34480011) and the National Institute of Health (Grants 1R15AT008733 and 1R15AT010395). Zeynep Goktas was supported by The Scientific and Technological Research Council of Turkey (Grant 1059B191800262).
Footnotes
The authors declare no competing financial interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association, National Institutes of Health, and the Scientific and Technological Research Council of Turkey.
Contributor Information
Zeynep Goktas, Faculty of Health Sciences, Department of Nutrition and Dietetics, Hacettepe University, 06100 Ankara, Turkey.
Yujiao Zu, Department of Nutritional Sciences, Texas Tech University, Lubbock, Texas 79409, United States.
Mehrnaz Abbasi, Department of Nutritional Sciences, Texas Tech University, Lubbock, Texas 79409, United States.
Shannon Galyean, Department of Nutritional Sciences, Texas Tech University, Lubbock, Texas 79409, United States.
Dayong Wu, Nutrition Immunology Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, Massachusetts 02111, United States.
Zhaoyang Fan, Department of Electrical & Computer Engineering and Nano Tech Center, Texas Tech University, Lubbock, Texas 79409, United States.
Shu Wang, Department of Nutritional Sciences, Texas Tech University, Lubbock, Texas 79409, United States.
REFERENCES
- (1).Carrera-Quintanar L; Lopez Roa RI; Quintero-Fabian S; Sanchez-Sanchez MA; Vizmanos B; Ortuno-Sahagun D Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediators Inflammation 2018, 2018, 9734845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wang S; Su R; Nie S; Sun M; Zhang J; Wu D; Moustaid-Moussa N Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem 2014, 25 (4), 363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lagerros YT; Rossner S Obesity management: what brings success? Ther. Adv. Gastroenterol 2013, 6 (1), 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wang S; Moustaid-Moussa N; Chen L; Mo H; Shastri A; Su R; Bapat P; Kwun I; Shen CL Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem 2014, 25 (1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Labuschagne P Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int 2018, 107. 227–247. [DOI] [PubMed] [Google Scholar]
- (6).Wang S; Moustaid-Moussa N; Chen L; Mo H; Shastri A; Su R; Bapat P; Kwun I; Shen C-L Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem 2014, 25 (1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bertoia ML; Mukamal KJ; Cahill LE; Hou T; Ludwig DS; Mozaffarian D; Willett WC; Hu FB; Rimm EB Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLoS Med. 2015, 12 (9), No. e1001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gan Y; Tong X; Li L; Cao S; Yin X; Gao C; Herath C; Li W; Jin Z; Chen Y; Lu Z Consumption of fruit and vegetable and risk of coronary heart disease: a meta-analysis of prospective cohort studies. Int. J. Cardiol 2015, 183, 129–37. [DOI] [PubMed] [Google Scholar]
- (9).Mendonca RD; Pimenta AM; Gea A; de la Fuente-Arrillaga C; Martinez-Gonzalez MA; Lopes AC; Bes-Rastrollo M Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr 2016, 104 (5), 1433–1440. [DOI] [PubMed] [Google Scholar]
- (10).Aqil F; Munagala R; Jeyabalan J; Vadhanam MV Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334 (1), 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Mukhopadhyay P; Maity S; Mandal S; Chakraborti AS; Prajapati AK; Kundu PP Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym 2018, 182, 42–51. [DOI] [PubMed] [Google Scholar]
- (12).Nour M; Lutze SA; Grech A; Allman-Farinelli M The Relationship between Vegetable Intake and Weight Outcomes: A Systematic Review of Cohort Studies. Nutrients 2018, 10 (11), 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Howes MJ; Simmonds MS The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17 (6), 558–66. [DOI] [PubMed] [Google Scholar]
- (14).Holst B; Williamson G Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol 2008, 19 (2), 73–82. [DOI] [PubMed] [Google Scholar]
- (15).Lampe JW; Chang JL Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol 2007, 17 (5), 347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Sousa T; Paterson R; Moore V; Carlsson A; Abrahamsson B; Basit AW The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm 2008, 363 (1–2), 1–25. [DOI] [PubMed] [Google Scholar]
- (17).Kerb R Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006, 234 (1), 4–33. [DOI] [PubMed] [Google Scholar]
- (18).Selby-Pham SNB; Miller RB; Howell K; Dunshea F; Bennett LE Physicochemical properties of dietary phytochemicals can predict their passive absorption in the human small intestine. Sci. Rep 2017, 7 (1), 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Liu Z; Hu M Natural polyphenol disposition via coupled metabolic pathways. Expert Opin. Drug Metab. Toxicol 2007, 3 (3), 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Li C; Zhang J; Zu YJ; Nie SF; Cao J; Wang Q; Nie SP; Deng ZY; Xie MY; Wang S Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin J. Nat. Med 2015, 13 (9), 641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Niedzwiecki A; Roomi MW; Kalinovsky T; Rath M Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8 (9), 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Blanco E; Shen H; Ferrari M Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol 2015, 33 (9), 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Mozafari MR Nanoliposomes: preparation and analysis. Methods Mol. Biol 2010, 605, 29–50. [DOI] [PubMed] [Google Scholar]
- (24).Panahi Y; Farshbaf M; Mohammadhosseini M; Mirahadi M; Khalilov R; Saghfi S; Akbarzadeh A Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artif. Cells, Nanomed., Biotechnol 2017, 45 (4), 788–799. [DOI] [PubMed] [Google Scholar]
- (25).Akbarzadeh A; Rezaei-Sadabady R; Davaran S; Joo SW; Zarghami N; Hanifehpour Y; Samiei M; Kouhi M; Nejati-Koshki K Liposome: classification, preparation, and applications. Nanoscale Res. Lett 2013, 8 (1), 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Reza Mozafari M; Johnson C; Hatziantoniou S; Demetzos C Nanoliposomes and their applications in food nanotechnology. J. Liposome Res 2008, 18 (4), 309–327. [DOI] [PubMed] [Google Scholar]
- (27).Pattni BS; Chupin VV; Torchilin VP New developments in liposomal drug delivery. Chem. Rev 2015, 115 (19), 10938–10966. [DOI] [PubMed] [Google Scholar]
- (28).Zu Y; Overby H; Ren G; Fan Z; Zhao L; Wang S Resveratrol liposomes and lipid nanocarriers: comparison of characteristics and inducing browning of white adipocytes. Colloids Surf., B 2018, 164, 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Gao A; Hu X.-l.; Saeed M; Chen B.-f.; Li Y.-p.; Yu H.-j. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol. Sin 2019, 40, 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Accardo A; Morelli G Review peptide-targeted liposomes for selective drug delivery: Advantages and problematic issues. Biopolymers 2015, 104 (5), 462–479. [DOI] [PubMed] [Google Scholar]
- (31).Daraee H; Etemadi A; Kouhi M; Alimirzalu S; Akbarzadeh A Application of liposomes in medicine and drug delivery. Artif. Cells, Nanomed., Biotechnol 2016, 44 (1), 381–391. [DOI] [PubMed] [Google Scholar]
- (32).McClements DJ Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv 2020, 38, 107287. [DOI] [PubMed] [Google Scholar]
- (33).Lamichhane N; Udayakumar TS; D’Souza WD; Simone CB; Raghavan SR; Polf J; Mahmood J Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23 (2), 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Mehnert W; Mäder K Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Delivery Rev 2012, 64, 83–101. [DOI] [PubMed] [Google Scholar]
- (35).Beloqui A; Solinís MÁ; Rodríguez-Gascón A; Almeida AJ; Préat V Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12 (1), 143–161. [DOI] [PubMed] [Google Scholar]
- (36).Das S; Chaudhury A Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12 (1), 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Iqbal MA; Md S; Sahni JK; Baboota S; Dang S; Ali J Nanostructured lipid carriers system: recent advances in drug delivery. J. Drug Target 2012, 20 (10), 813–30. [DOI] [PubMed] [Google Scholar]
- (38).Banik BL; Fattahi P; Brown JL Polymeric nanoparticles: the future of nanomedicine. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2016, 8 (2), 271–299. [DOI] [PubMed] [Google Scholar]
- (39).Lozano O; Rodríguez-Varela A; García-Rivas G Optimization of PLGA-Resveratrol nanoparticle synthesis through combined response surface methodologies. Materials Today: Proceedings 2019, 13, 384–389. [Google Scholar]
- (40).Kuhne BA; Puig T; Ruiz-Martinez S; Crous-Maso J; Planas M; Feliu L; Cano A; Garcia ML; Fritsche E; Llobet JM; Gomez-Catalan J; Barenys M Comparison of migration disturbance potency of epigallocatechin gallate (EGCG) synthetic analogs and EGCG PEGylated PLGA nanoparticles in rat neurospheres. Food Chem. Toxicol 2019, 123, 195–204. [DOI] [PubMed] [Google Scholar]
- (41).Tabatabaei Mirakabad FS; Akbarzadeh A; Milani M; Zarghami N; Taheri-Anganeh M; Zeighamian V; Badrzadeh F; Rahmati-Yamchi M A Comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif. Cells, Nanomed., Biotechnol 2016, 44 (1), 423–430. [DOI] [PubMed] [Google Scholar]
- (42).Sharma S; Parmar A; Kori S; Sandhir R PLGA-based nanoparticles: a new paradigm in biomedical applications. TrAC, Trends Anal. Chem 2016, 80, 30–40. [Google Scholar]
- (43).Nigam K; Kaur A; Tyagi A; Nematullah M; Khan F; Gabrani R; Dang S Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Delivery Transl. Res 2019, 9 (5), 879–890. [DOI] [PubMed] [Google Scholar]
- (44).Kore G; Kolate A; Nej A; Misra A Polymeric micelle as multifunctional pharmaceutical carriers. J. Nanosci. Nanotechnol 2014, 14 (1), 288–307. [DOI] [PubMed] [Google Scholar]
- (45).Fritz HF; Ortiz AC; Velaga SP; Morales JO Preparation of a novel lipid-core micelle using a low-energy emulsification method. Drug Delivery Transl. Res 2018, 8 (6), 1807–1814. [DOI] [PubMed] [Google Scholar]
- (46).Torchilin VP Micellar nanocarriers: pharmaceutical perspectives. Pharm. Res 2006, 24 (1), 1. [DOI] [PubMed] [Google Scholar]
- (47).Kedar U; Phutane P; Shidhaye S; Kadam V Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine 2010, 6 (6), 714–729. [DOI] [PubMed] [Google Scholar]
- (48).Singh Y; Meher JG; Raval K; Khan FA; Chaurasia M; Jain NK; Chourasia MK Nanoemulsion: Concepts, development and applications in drug delivery. J. Controlled Release 2017, 252, 28–49. [DOI] [PubMed] [Google Scholar]
- (49).Herneisey M; Mejia G; Pradhan G; Dussor G; Price T; Janjic J Resveratrol nanoemulsions target inflammatory macrophages to prevent. J. Pain 2018, 19 (3), S75–S76. [Google Scholar]
- (50).Son H-Y; Lee M-S; Chang E; Kim S-Y; Kang B; Ko H; Kim I-H; Zhong Q; Jo Y-H; Kim C-T; Kim Y Formulation and Characterization of Quercetin-loaded Oil in Water Nanoemulsion and Evaluation of Hypocholesterolemic Activity in Rats. Nutrients 2019, 11 (2), 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Sari T; Mann B; Kumar R; Singh R; Sharma R; Bhardwaj M; Athira S Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocolloids 2015, 43, 540–546. [Google Scholar]
- (52).Hörmann K; Zimmer A Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Controlled Release 2016, 223, 85–98. [DOI] [PubMed] [Google Scholar]
- (53).Simion V; Stan D; Constantinescu CA; Deleanu M; Dragan E; Tucureanu MM; Gan A-M; Butoi E; Constantin A; Manduteanu I; Simionescu M; Calin M Conjugation of curcumin-loaded lipid nanoemulsions with cell-penetrating peptides increases their cellular uptake and enhances the anti-inflammatory effects in endothelial cells. J. Pharm. Pharmacol 2016, 68 (2), 195–207. [DOI] [PubMed] [Google Scholar]
- (54).Mahato R Nanoemulsion as targeted drug delivery system for cancer therapeutics. Journal of Pharmaceutical Sciences and Pharmacology 2017, 3 (2), 83–97. [Google Scholar]
- (55).Gupta A; Eral HB; Hatton TA; Doyle PS Nanoemulsions: formation, properties and applications. Soft Matter 2016, 12 (11), 2826–41. [DOI] [PubMed] [Google Scholar]
- (56).Burns J; Yokota T; Ashihara H; Lean ME; Crozier A Plant foods and herbal sources of resveratrol. J. Agric. Food Chem 2002, 50 (11), 3337–40. [DOI] [PubMed] [Google Scholar]
- (57).Hurst WJ; Glinski JA; Miller KB; Apgar J; Davey MH; Stuart DA Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J. Agric. Food Chem 2008, 56 (18), 8374–8. [DOI] [PubMed] [Google Scholar]
- (58).Li S; Bouzar C; Cottet-Rousselle C; Zagotta I; Lamarche F; Wabitsch M; Tokarska-Schlattner M; Fischer-Posovszky P; Schlattner U; Rousseau D Resveratrol inhibits lipogenesis of 3T3-L1 and SGBS cells by inhibition of insulin signaling and mitochondrial mass increase. Biochim. Biophys. Acta, Bioenerg 2016, 1857 (6), 643–652. [DOI] [PubMed] [Google Scholar]
- (59).Chen S; Zhao Z; Ke L; Li Z; Li W; Zhang Z; Zhou Y; Feng X; Zhu W Resveratrol improves glucose uptake in insulin-resistant adipocytes via Sirt1. J. Nutr. Biochem 2018, 55, 209–218. [DOI] [PubMed] [Google Scholar]
- (60).Andrade JMO; Barcala-Jorge AS; Batista-Jorge GC; Paraiso AF; Freitas K. M. d.; Lelis D. d. F.; Guimaraes ALS; de Paula AMB; Santos SHS Effect of resveratrol on expression of genes involved thermogenesis in mice and humans. Biomed. Pharmacother 2019, 112, 108634. [DOI] [PubMed] [Google Scholar]
- (61).Wang S; Liang X; Yang Q; Fu X; Rogers CJ; Zhu M; Rodgers B; Jiang Q; Dodson MV; Du M Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes 2015, 39 (6), 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Berman AY; Motechin RA; Wiesenfeld MY; Holz MK The therapeutic potential of resveratrol: a review of clinical trials. npj Precision Oncol 2017, 1, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Wenzel E; Somoza V Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res 2005, 49 (5), 472–481. [DOI] [PubMed] [Google Scholar]
- (64).Zhou L; Xiao X; Zhang Q; Zheng J; Deng M Deciphering the anti-obesity benefits of resveratrol: the ‘gut microbiota-adipose tissue’axis. Front. Endocrinol 2019, 10, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Penalva R; Esparza I; Larraneta E; Gonzalez-Navarro CJ; Gamazo C; Irache JM Zein-Based Nanoparticles Improve the Oral Bioavailability of Resveratrol and Its Anti-inflammatory Effects in a Mouse Model of Endotoxic Shock. J. Agric. Food Chem 2015, 63 (23), 5603–11. [DOI] [PubMed] [Google Scholar]
- (66).Singh G; Pai RS Optimized PLGA nanoparticle platform for orally dosed trans-resveratrol with enhanced bioavailability potential. Expert Opin. Drug Delivery 2014, 11 (5), 647–59. [DOI] [PubMed] [Google Scholar]
- (67).Caddeo C; Pucci L; Gabriele M; Carbone C; Fernàndez-Busquets X; Valenti D; Pons R; Vassallo A; Fadda AM; Manconi M Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int. J. Pharm 2018, 538 (1–2), 40–47. [DOI] [PubMed] [Google Scholar]
- (68).Caddeo C; Pons R; Carbone C; Fernàndez-Busquets X; Cardia MC; Maccioni AM; Fadda AM; Manconi M Physicochemical characterization of succinyl chitosan-stabilized liposomes for the oral co-delivery of quercetin and resveratrol. Carbohydr. Polym 2017, 157, 1853–1861. [DOI] [PubMed] [Google Scholar]
- (69).Jung K-H; Lee JH; Park JW; Quach CHT; Moon S-H; Cho YS; Lee K-H Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int. J. Pharm 2015, 478 (1), 251–257. [DOI] [PubMed] [Google Scholar]
- (70).Wang P; Li D; Ke W; Liang D; Hu X; Chen F Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes 2020, 44, 213. [DOI] [PubMed] [Google Scholar]
- (71).Granja A; Frias I; Neves AR; Pinheiro M; Reis S Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. BioMed Res. Int 2017, 2017, 5813793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Chen W; Zou M; Ma X; Lv R; Ding T; Liu D Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci 2018, 84 (1), 111–120. [DOI] [PubMed] [Google Scholar]
- (73).Feng Z; Hou X; Zhu C; Zhu J; Jiang C Epigallocatechin gallate ameliorates morphological changes of pancreatic islets in diabetic mice and downregulates blood sugar level by inhibiting the accumulation of AGE-RAGE. J. Cell. Biochem 2019, 120, 8510. [DOI] [PubMed] [Google Scholar]
- (74).Sampath C; Rashid MR; Sang S; Ahmedna M Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother 2017, 87, 73–81. [DOI] [PubMed] [Google Scholar]
- (75).Babu PVA; Liu D; Gilbert ER Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem 2013, 24 (11), 1777–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Casanova E; Salvadó J; Crescenti A; Gibert-Ramos A Epigallocatechin Gallate Modulates Muscle Homeostasis in Type 2 Diabetes and Obesity by Targeting Energetic and Redox Pathways: A Narrative Review. Int. J. Mol. Sci 2019, 20 (3), 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Zhang J; Nie S; Zu Y; Abbasi M; Cao J; Li C; Wu D; Labib S; Brackee G; Shen C-L; Wang S Anti-atherogenic effects of CD36-targeted epigallocatechin gallate-loaded nanoparticles. J. Controlled Release 2019, 303, 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Yang CS; Chen L; Lee MJ; Balentine D; Kuo MC; Schantz SP Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomarkers Prev 1998, 7 (4), 351–354. [PubMed] [Google Scholar]
- (79).Chen W; Zou M; Ma X; Lv R; Ding T; Liu D Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci 2019, 84 (1), 111–120. [DOI] [PubMed] [Google Scholar]
- (80).Zhang J; Nie S; Martinez-Zaguilan R; Sennoune SR; Wang S Formulation, characteristics and antiatherogenic bioactivities of CD36-targeted epigallocatechin gallate (EGCG)-loaded nanoparticles. J. Nutr. Biochem 2016, 30, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Zhang J; Nie S; Wang S Nanoencapsulation Enhances Epigallocatechin-3-gallate Stability and Its Antiatherogenic Bioactivities in Macrophages. J. Agric. Food Chem 2013, 61 (38), 9200–9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).de Pace RC; Liu X; Sun M; Nie S; Zhang J; Cai Q; Gao W; Pan X; Fan Z; Wang S Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res 2013, 23 (3), 187–96. [DOI] [PubMed] [Google Scholar]
- (83).Frias I; Neves AR; Pinheiro M; Reis S Design, development, and characterization of lipid nanocarriers-based epigallocatechin gallate delivery system for preventive and therapeutic supplementation. Drug Des. Dev. Ther 2016, 10, 3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Hong Z; Xu Y; Yin JF; Jin J; Jiang Y; Du Q Improving the effectiveness of (−)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J. Agric. Food Chem 2014, 62 (52), 12603–9. [DOI] [PubMed] [Google Scholar]
- (85).Naksuriya O; Okonogi S; Schiffelers RM; Hennink WE Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35 (10), 3365–83. [DOI] [PubMed] [Google Scholar]
- (86).Chuengsamarn S; Rattanamongkolgul S; Luechapudiporn R; Phisalaphong C; Jirawatnotai S Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35 (11), 2121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Devassy JG; Nwachukwu ID; Jones PJ Curcumin and cancer: barriers to obtaining a health claim. Nutr. Rev 2015, 73 (3), 155–65. [DOI] [PubMed] [Google Scholar]
- (88).Sundar Dhilip Kumar S; Houreld NN; Abrahamse H Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules 2018, 23 (4), 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Goozee KG; Shah TM; Sohrabi HR; Rainey-Smith SR; Brown B; Verdile G; Martins RN Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br. J. Nutr 2016, 115 (3), 449–65. [DOI] [PubMed] [Google Scholar]
- (90).Bradford PG Curcumin and obesity. Biofactors 2013, 39 (1), 78–87. [DOI] [PubMed] [Google Scholar]
- (91).Davatgaran Taghipour Y; Hajialyani M; Naseri R; Hesari M; Mohammadi P; Stefanucci A; Mollica A; Farzaei MH; Abdollahi M Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed 2019, 14, 5303–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Deck LM; Hunsaker LA; Vander Jagt TA; Whalen LJ; Royer RE; Vander Jagt DL Activation of anti-oxidant Nrf2 signaling by enone analogues of curcumin. Eur. J. Med. Chem 2018, 143, 854–865. [DOI] [PubMed] [Google Scholar]
- (93).He Y; Yue Y; Zheng X; Zhang K; Chen S; Du Z Curcumin, inflammation, and chronic diseases: how are they linked? Molecules 2015, 20 (5), 9183–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Ahn J; Lee H; Kim S; Ha T Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol Cell Physiol 2010, 298 (6), C1510–6. [DOI] [PubMed] [Google Scholar]
- (95).Jurenka JS Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev 2009, 14 (2), 141–153. [PubMed] [Google Scholar]
- (96).Ganjali S; Sahebkar A; Mahdipour E; Jamialahmadi K; Torabi S; Akhlaghi S; Ferns G; Parizadeh SM; Ghayour-Mobarhan M Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. Sci. World J 2014, 2014, 898361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Mohammadi A; Sahebkar A; Iranshahi M; Amini M; Khojasteh R; Ghayour-Mobarhan M; Ferns GA Effects of supplementation with curcuminoids on dyslipidemia in obese patients: a randomized crossover trial. Phytother. Res 2013, 27 (3), 374–9. [DOI] [PubMed] [Google Scholar]
- (98).Zhao Y; Chen B; Shen J; Wan L; Zhu Y; Yi T; Xiao Z The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxid. Med. Cell. Longevity 2017, 2017, 1459497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Ma Z; Wang N; He H; Tang X Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Controlled Release 2019, 316, 359–380. [DOI] [PubMed] [Google Scholar]
- (100).Liu W; Zhai Y; Heng X; Che FY; Chen W; Sun D; Zhai G Oral bioavailability of curcumin: problems and advancements. J. Drug Target 2016, 24 (8), 694–702. [DOI] [PubMed] [Google Scholar]
- (101).Kharat M; Du Z; Zhang G; McClements DJ Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem 2017, 65 (8), 1525–1532. [DOI] [PubMed] [Google Scholar]
- (102).Ganesan P; Ramalingam P; Karthivashan G; Ko YT; Choi DK Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed 2018, 13, 1569–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Shoba G; Joy D; Joseph T; Majeed M; Rajendran R; Srinivas PS Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64 (4), 353–6. [DOI] [PubMed] [Google Scholar]
- (104).Sharma RA; Euden SA; Platton SL; Cooke DN; Shafayat A; Hewitt HR; Marczylo TH; Morgan B; Hemingway D; Plummer SM; Pirmohamed M; Gescher AJ; Steward WP Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res 2004, 10 (20), 6847–54. [DOI] [PubMed] [Google Scholar]
- (105).Cheng AL; Hsu CH; Lin JK; Hsu MM; Ho YF; Shen TS; Ko JY; Lin JT; Lin BR; Ming-Shiang W; Yu HS; Jee SH; Chen GS; Chen TM; Chen CA; Lai MK; Pu YS; Pan MH; Wang YJ; Tsai CC; Hsieh CY Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21 (4B), 2895–2900. [PubMed] [Google Scholar]
- (106).Patil S; Choudhary B; Rathore A; Roy K; Mahadik K Enhanced oral bioavailability and anticancer activity of novel curcumin loaded mixed micelles in human lung cancer cells. Phytomedicine 2015, 22 (12), 1103–11. [DOI] [PubMed] [Google Scholar]
- (107).Xie X; Tao Q; Zou Y; Zhang F; Guo M; Wang Y; Wang H; Zhou Q; Yu S PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J. Agric. Food Chem 2011, 59 (17), 9280–9. [DOI] [PubMed] [Google Scholar]
- (108).Ji H; Tang J; Li M; Ren J; Zheng N; Wu L Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Delivery 2016, 23 (2), 459–70. [DOI] [PubMed] [Google Scholar]
- (109).Shaikh J; Ankola DD; Beniwal V; Singh D; Kumar MN Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci 2009, 37 (3–4), 223–30. [DOI] [PubMed] [Google Scholar]
- (110).Lee EJ; Hwang JS; Kang ES; Lee SB; Hur J; Lee WJ; Choi MJ; Kim JT; Seo HG Nanoemulsions improve the efficacy of turmeric in palmitate- and high fat diet-induced cellular and animal models. Biomed. Pharmacother 2019, 110, 181–189. [DOI] [PubMed] [Google Scholar]
- (111).du Preez R; Pahl J; Arora M; Ravi Kumar MNV; Brown L; Panchal SK Low-Dose Curcumin Nanoparticles Normalise Blood Pressure in Male Wistar Rats with Diet-Induced Metabolic Syndrome. Nutrients 2019, 11 (7), 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Boarescu PM; Boarescu I; Bocsan IC; Gheban D; Bulboaca AE; Nicula C; Pop RM; Rajnoveanu RM; Bolboaca SD Antioxidant and Anti-Inflammatory Effects of Curcumin Nanoparticles on Drug-Induced Acute Myocardial Infarction in Diabetic Rats. Antioxidants 2019, 8 (10), 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).El-Naggar ME; Al-Joufi F; Anwar M; Attia MF; El-Bana MA Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf., B 2019, 177, 389–398. [DOI] [PubMed] [Google Scholar]
- (114).Abdel-Mageid AD; Abou-Salem MES; Salaam N; El-Garhy HAS The potential effect of garlic extract and curcumin nanoparticles against complication accompanied with experimentally induced diabetes in rats. Phytomedicine 2018, 43, 126–134. [DOI] [PubMed] [Google Scholar]
- (115).Joshi RP; Negi G; Kumar A; Pawar YB; Munjal B; Bansal AK; Sharma SS SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: an insight into its mechanism for neuroprotection. Nanomedicine 2013, 9 (6), 776–85. [DOI] [PubMed] [Google Scholar]
- (116).Yekollu SK; Thomas R; O’Sullivan B Targeting curcusomes to inflammatory dendritic cells inhibits NF-kappaB and improves insulin resistance in obese mice. Diabetes 2011, 60 (11), 2928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Rivera L; Moron R; Sanchez M; Zarzuelo A; Galisteo M Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008, 16 (9), 2081–7. [DOI] [PubMed] [Google Scholar]
- (118).Stewart LK; Soileau JL; Ribnicky D; Wang ZQ; Raskin I; Poulev A; Majewski M; Cefalu WT; Gettys TW Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metab., Clin. Exp 2008, 57 (Suppl 1), S39–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Chen S; Jiang H; Wu X; Fang J Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediators Inflammation 2016, 2016, 9340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Li Y; Yao J; Han C; Yang J; Chaudhry MT; Wang S; Liu H; Yin Y Quercetin, Inflammation and Immunity. Nutrients 2016, 8 (3), 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Lee JS; Cha YJ; Lee KH; Yim JE Onion peel extract reduces the percentage of body fat in overweight and obese subjects: a 12-week, randomized, double-blind, placebo-controlled study. Nutr. Res. Pract 2016, 10 (2), 175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Sun M; Nie S; Pan X; Zhang R; Fan Z; Wang S Quercetin-nanostructured lipid carriers: characteristics and anti-breast cancer activities in vitro. Colloids Surf., B 2014, 113, 15–24. [DOI] [PubMed] [Google Scholar]
- (123).Li H; Zhao X; Ma Y; Zhai G; Li L; Lou H Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Controlled Release 2009, 133 (3), 238–44. [DOI] [PubMed] [Google Scholar]
- (124).Bose S; Michniak-Kohn B Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur. J. Pharm. Sci 2013, 48 (3), 442–52. [DOI] [PubMed] [Google Scholar]
- (125).Murota K; Terao J Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys 2003, 417 (1), 12–7. [DOI] [PubMed] [Google Scholar]
- (126).Rezaei-Sadabady R; Eidi A; Zarghami N; Barzegar A Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif. Cells, Nanomed., Biotechnol 2016, 44 (1), 128–34. [DOI] [PubMed] [Google Scholar]
- (127).Tan BJ; Liu Y; Chang KL; Lim BK; Chiu GN Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int. J. Nanomed 2012, 7, 651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Tong F; Liu S; Yan B; Li X; Ruan S; Yang S Quercetin nanoparticle complex attenuated diabetic nephropathy via regulating the expression level of ICAM-1 on endothelium. Int. J. Nanomed 2017, 12, 7799–7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Chitkara D; Nikalaje SK; Mittal A; Chand M; Kumar N Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Delivery Transl. Res 2012, 2 (2), 112–23. [DOI] [PubMed] [Google Scholar]
- (130).Ebrahimpour S; Esmaeili A; Beheshti S Effect of quercetin-conjugated superparamagnetic iron oxide nanoparticles on diabetes-induced learning and memory impairment in rats. Int. J. Nanomed 2018, 13, 6311–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Baowen Q; Yulin Z; Xin W; Wenjing X; Hao Z; Zhizhi C; Xingmei D; Xia Z; Yuquan W; Lijuan C A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur. J. Pharmacol 2010, 642 (1–3), 134–9. [DOI] [PubMed] [Google Scholar]
- (132).Chakraborty S; Stalin S; Das N; Thakur Choudhury S; Ghosh S; Swarnakar S The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials 2012, 33 (10), 2991–3001. [DOI] [PubMed] [Google Scholar]
- (133).Mohseni R; ArabSadeghabadi Z; Ziamajidi N; Abbasalipourkabir R; RezaeiFarimani A Oral Administration of Resveratrol-Loaded Solid Lipid Nanoparticle Improves Insulin Resistance Through Targeting Expression of SNARE Proteins in Adipose and Muscle Tissue in Rats with Type 2 Diabetes. Nanoscale Res. Lett 2019, 14 (1), 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Wan S; Zhang L; Quan Y; Wei K Resveratrol-loaded PLGA nanoparticles: enhanced stability, solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R. Soc. Open Sci 2018, 5 (11), 181457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Siu FY; Ye S; Lin H; Li S Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: enhanced bioavailability and in vitro anti-inflammatory activity. Int. J. Nanomed 2018, 13, 4133–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Devadasu VR; Wadsworth RM; Kumar MN Protective effects of nanoparticulate coenzyme Q10 and curcumin on inflammatory markers and lipid metabolism in streptozotocin-induced diabetic rats: a possible remedy to diabetic complications. Drug Delivery Transl. Res 2011, 1 (6), 448–55. [DOI] [PubMed] [Google Scholar]


