Abstract
Background
Inability to communicate in a manner that can be understood causes extreme distress for people requiring an artificial airway and has implications for care quality and patient safety. Options for aided communication include non‐vocal, speech‐generating, and voice‐enabling aids.
Objectives
To assess effectiveness of communication aids for people requiring an artificial airway (endotracheal or tracheostomy tube), defined as the proportion of people able to: use a non‐vocal communication aid to communicate at least one symptom, need, or preference; or use a voice‐enabling communication aid to phonate to produce at least one intelligible word.
To assess time to communication/phonation; perceptions of communication; communication quality/success; quality of life; psychological distress; length of stay and costs; and adverse events.
Search methods
We searched the Cochrane Library (Wiley version), MEDLINE (OvidSP), Embase (OvidSP), three other databases, and grey literature from inception to 30 July 2020.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs, cluster‐RCTs, controlled non‐randomised parallel group, and before‐after studies evaluating communication aids used in adults with an artificial airway.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. Two review authors independently performed data extraction and assessment of risk of bias.
Main results
We included 11 studies (1931 participants) conducted in intensive care units (ICUs). Eight evaluated non‐vocal communication aids and three voice‐enabling aids. Usual care was the comparator for all. For six studies, this comprised no aid; usual care in the remaining five studies comprised use of various communication aids.
Overall, our confidence in results regarding effectiveness of communication interventions was very low due to imprecision, measurement heterogeneity, inconsistency in results, and most studies at high or unclear risk of bias across multiple domains.
No non‐vocal aid studies reported our primary outcome. We are uncertain of the effects of early use of a voice‐enabling aid compared to routine use on ability to phonate at least one intelligible word (risk ratio (RR) 3.03, 95% confidence interval (CI) 0.18 to 50.08; 2 studies; very low‐certainty evidence).
Compared to usual care without aids, we are uncertain about effects of a non‐vocal aid (communication board) on patient satisfaction (standardised mean difference (SMD) 2.92, 95% CI 1.52 to 4.33; 4 studies; very low‐certainty evidence).
No studies of non‐vocal aids reported quality of life. Low‐certainty evidence from two studies suggests early use of a voice‐enabling aid may have no effect on quality of life (MD 2.27, 95% CI –7.21 to 11.75). Conceptual differences in measures of psychological distress precluded data pooling; however, intervention arm participants reported less distress suggesting there might be benefit, but our certainty in the evidence is very low.
Low‐certainty evidence suggest voice‐enabling aids have little or no effect on ICU length of stay; we were unable to determine effects of non‐vocal aids. Three studies reported different adverse events (physical restraint use, bleeding following tracheostomy, and respiratory parameters indicating respiratory decompensation). Adverse event rates were similar between arms in all three studies. However, uncertainty remains as to any harm associated with communication aids.
Authors' conclusions
Due to a lack of high‐quality studies, imprecision, inconsistency of results, and measurement heterogeneity, the evidence provides insufficient information to guide practice as to which communication aid is more appropriate and when to use them. Understanding effectiveness of communication aids would benefit from development of a core outcome measurement set.
Plain language summary
Strategies to help adults with a breathing tube to communicate
What is the issue?
Patients needing a machine to support breathing cannot speak due to a tube delivering gas to the lungs bypassing their voice box. Patients mouth words, gesture, and use facial expressions. However, these are very difficult to understand. Weakened muscles and difficulty concentrating, which are common in critical illness, makes using aids such as writing equipment or communication boards difficult. Consistent evidence on which communication aids are effective is lacking.
Why is this important?
Difficulty communicating places people at increased risk of harm, causes distress to patients and family, and causes stress for healthcare staff.
What evidence did we find?
We searched for studies (to 30 July 2020) exploring aids used to help people with a breathing tube to communicate. We found 11 studies involving 1931 participants admitted to intensive care units. We also looked for studies involving people needing a breathing tube and living at home or in long‐term care, but found none. Eight studies used communication boards or apps. Three studies used aids that help a patient to speak with the breathing tube in place. All studies compared the communication aid to routine communication practices. For six studies, routine practice did not include use of any type of communication aid. For the remaining five studies, usual care comprised a range of communication aids routinely used in the participating intensive care units including a communication board, paper notepad, and routine timing of the use of speech aids. We are unsure about whether the early use of aids to help with speaking may increase the number of people who can say words that can be understood or shorten the time to be able to speak. The evidence was of very low quality.
Similarly, compared to routine care in which an aid is not used, we are uncertain about the effects of communication boards on patient satisfaction. We are not sure about the effect on psychological distress and quality of life due to uncertainty in the evidence. Communication aids that help people to speak may have little or no effect on intensive care unit length of stay (low‐quality evidence). We are uncertain of possible harms with use of communication aids as only three studies reported this, and all measured different adverse events, and two were very small studies.
What does this mean?
We are unsure whether using speaking aids in intensive care might increase the number of people who can say words that can be understood. Use of communication boards may increase patient satisfaction, but we are not sure of these findings because of very low‐quality evidence. This means further studies are likely to change our understanding of the effects of communication aids. More studies are needed to understand the effects of communication aids, particularly effects on psychological well‐being and people's ability to communicate.
Summary of findings
Background
Description of the condition
Provision of interventions to enable patient communication is a fundamental patient right (Joint Commission 2010). For people requiring an artificial airway, establishing communication is particularly challenging. An artificial airway is established through endotracheal intubation (a tube inserted through the mouth or nose into the trachea) or a tracheostomy (a tube inserted into the trachea through a surgical opening in the neck). The trachea is the windpipe that conveys air from the larynx (the voice box that contains the vocal cords) to the lower airways of the lungs during breathing. People who require an artificial airway include those that require invasive mechanical ventilation (breathing support from a machine) in an intensive care unit (ICU), or another acute care location such as a specialised centre for mechanical ventilator weaning or step down/up or intermediate care unit. People with chronic respiratory failure (inability to breathe adequately for an extended period and without recovery of lung function) may require a tracheostomy and invasive mechanical ventilation in the long term in care locations such as a hospital ward, rehabilitation unit, long‐term care centre, or living in the home. This prolonged exposure to an artificial airway results in prolonged impairment of communication and reliance on communication aids (Huttmann 2018). An artificial airway without invasive mechanical ventilation may be required for secretion management or because their own airway is damaged or inflamed (swollen) after mechanical ventilation is discontinued.
To facilitate invasive mechanical ventilation, the endotracheal or tracheostomy tube has an inflatable cuff (balloon) that inflates into the trachea. When inflated, the cuff directs all gas (air plus an enhanced oxygen supply) to the patient's lungs via the endotracheal or tracheostomy tube. The cuff stops any airflow from the patient's lower airways reaching the larynx and the vocal cords during expiration (breathing out). This laryngeal airflow causes the vocal cords to vibrate which enables phonation (production of speech) (McGrath 2019), and is how voice is generated under normal conditions. For people experiencing inability to communicate with their own voice, alternative and augmentative communication methods are needed. Unaided communication relies on mouthing words, gestures, nodding, body language, and facial expressions. However, mouthing words is frequently difficult to understand and subject to misinterpretation (Carroll 2004). Reduced muscle strength and altered cognition (ability to think) also may make unaided communication methods difficult for people to use and difficult for communication partners to interpret. Options for aided communication include non‐vocal aids, that is visual‐based augmentative and alternative communication aids including writing equipment, communication boards, or digital apps that convey symptoms and basic needs without generating speech. Other non‐vocal sound‐based augmentative and alternative communication aids include speech‐generating aids that generate static and dynamic digitised sound such as voice output communication aids (VOCA), speech‐generating software, and eye gaze technology. Another speech‐generating option is the electrolarynx, a device that generates sound (not voice) via transmission of vibration through soft tissue, which is recognisable as speech with movement of the lips, tongue, and jaw (articulators) (Shimizu 2013).
For people with prolonged need for invasive mechanical ventilation and tracheostomy, vocal communication can be restored by voice‐enabling aids that re‐establish airflow through the larynx. Most voice‐enabling aids require deflation of the cuff of the artificial airway. Cuff deflation and the re‐establishment of voice can be considered part of the weaning process, that is the process that establishes unsupported breathing (Ambrosino 2018). However, the ability to tolerate cuff deflation depends on the person's cough strength enabling effective clearance of mucous, and bulbar (nerve) function enabling swallowing of saliva (Hunt 2015). Acquired swallowing disorders associated with artificial airways are common and, during cuff deflation, may cause saliva, liquids, food, or vomit to enter the lungs with devastating consequences such as pneumonia (lung infection), pneumonitis (lung inflammation), the need to reinsert an endotracheal or tracheostomy tube, prolonged ICU length of stay, and death (Macht 2013). In people in whom cuff deflation may be unsafe, certain voice‐enabling aids achieve vocal communication by delivery of a supply of air between the inflated cuff and the vocal cords. A glossary of terms can be found in Appendix 1.
Description of the intervention
For the purposes of this review, one or more of the following communication aids or techniques are interventions eligible for inclusion. We have grouped communication aids into the following categories (Figure 1):
1.
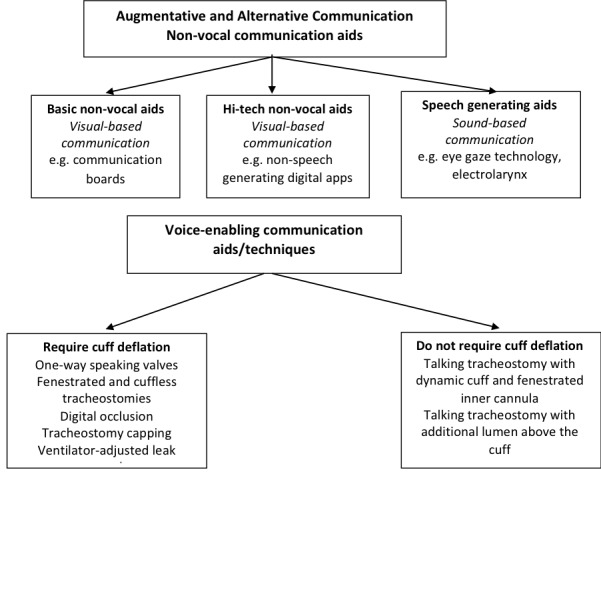
Communication Enabling Aids
non‐vocal aids, that is augmentative and alternative communication aids using visual‐based communication;
speech‐generating aids, that is augmentative and alternative communication aids using sound‐based communication that is not the patient's own voice;
voice‐enabling aids or techniques that require cuff deflation or a cuff‐less tube;
voice‐enabling aids that do not require cuff deflation.
Augmentative and alternative communication is an umbrella term that includes unaided and aided communication that supplements or replaces verbal communication. Voice‐enabling aids or techniques facilitate return of the patient's own voice and, therefore, do not fit under this umbrella term (ASHA 2019) (Figure 1).
Augmentative and alternative communication: non‐vocal aids
Basic non‐vocal visual augmentative and alternative communication aids include pen and paper or other writing equipment; communication board comprising letters, words, or pictures; or communication cards again comprising letters, words, or pictures. High‐tech non‐vocal visual augmentative and alternative communication aids include computer software programs and digital applications that do not generate speech such as the Society of Critical Care Medicine Patient Communicator app for use on tablet devices or smart phones (itunes.apple.com/us/app/patient-communicator/id732242570?mt=8). These visual communication aids require hand dexterity co‐ordination and muscle strength as well as intact cognitive abilities. These abilities may be compromised due to extreme physical stress and fatigue, muscle weakness, and emotional or psychological distress, all of which are common to people experiencing critical illness (Chlan 2015; Menzel 1998). Dexterity, muscle strength, and nerve function may also be compromised or absent in people with neuromuscular disorders (disorders that affect muscle and nerve function), or those with total loss of nerve innervation such as people requiring an artificial airway and breathing support due to high spinal cord injury. Other disadvantages of basic and high‐tech visual communication aids include being imprecise, cumbersome, costly, and prone to breakage (Hashmi 2010).
Augmentative and alternative communication: speech‐generating aids
Speech‐generating augmentative and alternative communication aids are those that use sound‐based communication that is not the person's own voice. Speech‐generating aids convert text to generate static and dynamic digitalised (uses human voice), synthesised (computer‐generated) speech (or a combination thereof), and include VOCAs, text to speech digital apps and software, and eye‐gaze technology. Eye‐gaze technology uses near infrared micro‐projectors, optical sensors, image processing, and mathematical models to determine eye position and gaze point (Garry 2016). By gaze dwelling on text or symbols, people can generate speech. Again, speech‐generating aids have significant limitations associated with cognitive and fine or gross motor capacity, time required to generate messages, and lack of device familiarity (Happ 2004).
The electrolarynx, or artificial larynx, is a distinct type of speech‐generating augmentative and alternative communication aid that enables phonation with movement of the articulators but does not restore the person's own voice. It also does not produce digitised speech and therefore is dissimilar to other speech‐generating aids. The electrolarynx transmits electronic sound source vibrations through soft tissue, at the neck, at the level of the glottis, or, less commonly, the cheek. Although phonation may be relatively easy to achieve with an electrolarynx, intelligibility of speech may be impaired in people who are dysarthric (have weakness or difficulty controlling the muscles used for speech) or those who have an endotracheal tube in place (Rose 2018). Other barriers to use of the electrolarynx include muscle strength and co‐ordination to enable appropriate device placement and to hold the device in place.
Voice‐enabling communication aids requiring cuff deflation or a cuff‐less tube
Voice‐enabling communication aids and techniques, that is those that aid return of patient voice, include those that require artificial airway cuff deflation and those that do not as they deliver a supply of air between an inflated cuff and the vocal cords. Voice‐enabling communication aids that require cuff deflation include one‐way speaking valves such as the Passy Muir or Montgomery speaking valves that open on inspiration allowing gas from the upper airway into the trachea and close on expiration thus diverting gas to the vocal cords. Other voice‐enabling communication aids requiring cuff deflation include speaking or fenestrated tracheostomy tubes. Fenestrated tracheostomies have an additional opening on the shaft of the tube that directs gas towards the vocal cords. Voice‐enabling communication techniques, that is, those that enable return of a person's voice and require cuff deflation but do not require an aid, include digital occlusion of the tracheostomy tube, tracheostomy capping, and ventilator‐adjusted leak speech. Digital occlusion involves covering of the opening of the tracheostomy tube with a gloved finger. With the cuff deflated, digital occlusion or placing a cap on the tracheostomy tube opening (capping) redirects the flow of gas through the vocal cords (Morris 2015). Finger occlusion and capping are generally not practiced on people who are receiving ventilation. Ventilator‐adjusted leak speech requires the ventilator (breathing machine) to be adjusted to give bigger breaths during inspiration to compensate for loss of gas due to the deflated cuff. As humans normally speak during expiration, patients need training to time speech with the inspiratory phase of gas delivery from the mechanical ventilator (Hoit 2003; Morris 2015). Another option is cuffless (a tube without a balloon) tracheostomy tubes that are used for people with prolonged need for a tracheostomy and ability to swallow their own saliva. Similar to a deflated cuff, the absence of the cuff means some of the airflow is directed to the larynx enabling speech.
Voice‐enabling communication aids without cuff deflation
Voice‐enabling communication aids that do not require cuff deflation include more recently developed talking tracheostomy designs such as the Blom tracheostomy system (Pulmodyne, Indianapolis, Indiana); the Portex Trach‐Talk Blue Line Tracheostomy Tube (Smiths Medical, Dublin, Ohio); and the Bivona Mid‐Range Aire‐Cuf and Fome‐Cuf Tracheostomy Tubes with Talk Attachment (Smiths Medical, Dublin, Ohio). The Blom tracheostomy system comprises a fenestrated, cuffed tracheostomy tube combined with a proprietary speech inner cannula (Adam 2015; Kunduk 2010). An inner cannula is an additional tube placed within the tracheostomy tube, which is more commonly used for enabling cleaning of the tracheal lumen to prevent mucous buildup. At the end of inspiration (breathing in), a flap valve closes the end of the tracheotomy tube. Increasing pressure forces a second bubble valve to collapse allowing gas to pass through the fenestrations to the vocal cards. The Portex Trach‐Talk Blue Line Tracheostomy Tube and the Bivona Mid‐Range Aire‐Cuf and Fome‐Cuf Tracheostomy Tubes with Talk Attachment have an additional lumen above the cuff through which gas is administered to facilitate phonation. However, a disadvantage of this additional lumen is that it quickly becomes encumbered by secretions that cannot easily be removed (Pandian 2014).
How the intervention might work
Non‐vocal, speech‐generating, and voice‐enabling communication aids or techniques help people with artificial airways to alert healthcare workers to troublesome and distressing symptoms, express needs and preferences, participate in decision‐making relating to care goals, and, in some cases, end‐of‐life, and to interact with family members and loved ones (Grossbach 2011). There is some evidence that communication aids influence patient satisfaction, increase communication frequency, and decrease difficulty associated with communication (Happ 2014). Identification of communication aids that effectively meet individual patient needs may relieve emotional and psychological distress including anxiety, agitation, frustration, and loneliness; and improve symptom identification, sleep, patient safety, outlook and sense of recovery, and quality of and satisfaction with life (Huttmann 2018; Ten Hoorn 2016).
Why it is important to do this review
Inability to communicate is one of the top stressors for people with an artificial airway (endotracheal or tracheostomy tube) in critical care, long‐term care, or home environments (Huttmann 2018; Johnston 1990; Rose 2014). Being unable to communicate when critically ill and requiring an artificial airway has negative outcomes that include: significant emotional distress (anxiety, panic, anger, agitation, loss of control); unrecognised pain and delirium; and sleeplessness (Breckenridge 2014; Khalaila 2011; Menzel 1998; Stein‐Parbury 2000). Qualitative studies characterise patient recall of inability to communicate during mechanical ventilation as frustrating, challenging, troublesome, and horrid (Flinterud 2015; Guttormson 2015). One qualitative study of communication for people receiving home ventilation described their experience in terms of a long and lonely struggle to find a voice (Carroll 2007; Laakso 2011). Other deleterious consequences in ICU settings due to agitation associated with an inability to communicate include increased use of physical restraints, and treatment interference such as patient removal of the endotracheal tube, intravenous lines, or nasogastric tubes (tube placed in the stomach) or catheters (tube placed in the bladder or other locations of the body). Other negative consequences of agitation arising from inability to communicate include injury to self and healthcare professionals (Bartlett 2008). Patient inability to communicate in a manner that can be understood also creates stress and frustration for family members (Broyles 2012) and healthcare professionals (Magnus 2006; Nilsen 2014), and limits patient ability to participate in care decisions. For people with chronic respiratory insufficiency requiring tracheostomy in long‐term care or home environments, inability or impaired ability to communicate negatively influences quality of life and life satisfaction (Huttmann 2018), psychological functioning, independence, and social interactions (Carroll 2007).
Communication impairment during hospitalisation has implications for the quality and safety of care and is a modifiable risk factor for adverse events (Bartlett 2008). The Joint Commission, a healthcare accreditation organisation in the USA, has produced standards that mandate identifying patients' oral and written communication needs and undertaking reasonable efforts to establish alternative communication strategies for people unable to speak (Joint Commission 2010). Therefore, healthcare organisations and providers are obliged to identify and use the most effective methods to augment patient communication and restore patient voice.
Despite the well‐recognised deleterious consequences of inability to communicate using other means, there is evidence of variable and, in some cases, limited adoption of communication aids and lack of prioritisation of communication by healthcare professionals (Happ 2011). One 2013 Canadian survey of 201 Canadian ICUs found only 11% used high‐tech visual‐ or sound‐based communication aids and 30% did not use one‐way speaking valves (Rose 2015). One 2016 systematic review of communication aids for mechanically ventilated people in the ICU unable to tolerate cuff deflation identified 29 studies including randomised, quasi‐randomised, and observational studies (Ten Hoorn 2016). All studies had small sample sizes, were judged low‐ to moderate‐quality, and only four had a comparator group. Importantly, the review excluded studies of voice‐enabling communication aids for people able to tolerate cuff deflation. These authors presented a narrative review identifying four communication types; low‐tech communication boards, speaking tracheostomy tubes used with an inflated cuff, the electrolarynx, and high‐tech sound‐generating aids, all of which improved communication ability. These authors used their data to suggest a communication algorithm and recommend multi‐component communication interventions be adopted in the ICU individualised to patient need (Ten Hoorn 2016).
Our systematic review aimed to summarise the evidence and assess the effectiveness of communication aids for people who require an artificial airway (endotracheal or tracheostomy tube) with or without cuff deflation irrespective of care location. It updates and extends previous systematic reviews that focused only on an ICU population, or that excluded communication aids for people able to tolerate cuff deflation. Our review aimed to address uncertainty in terms of which communication aids are most effective for the range of people requiring an artificial airway. This review will inform clinical practice enabling decisions about effective and individualised communication aids and techniques for this patient population. This review has relevance to patients; communication partners including family members, friends, caregivers, and healthcare professionals working with patients requiring an artificial airway; healthcare decision makers; and researchers working in this field. Through the conduct of this review, we have identified evidence gaps to inform future research related to communication aids for people requiring an artificial airway with or without cuff deflation.
Objectives
To assess effectiveness of communication aids for people requiring an artificial airway (endotracheal or tracheostomy tube), defined as the proportion of people able to: use a non‐vocal communication aid to communicate at least one symptom, need, or preference; or use a voice‐enabling communication aid to phonate to produce at least one intelligible word.
To assess time to communication/phonation; perceptions of communication; communication quality/success; quality of life; psychological distress; length of stay and costs; and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐RCTs (a trial in which randomisation is attempted but subject to potential manipulation, such as allocating participants by day of the week, date or birth, or sequence of entry into trial), and controlled parallel group trials without randomisation as we anticipated that few, if any, properly randomised controlled trials will have been conducted in the area of communication or speech aids for people requiring an artificial airway. We excluded randomised cross‐over trials. As we anticipated improving the ability to communicate using communication or speech aids for people requiring an artificial airway with or without mechanical ventilation may be considered a quality improvement imperative, we included controlled before‐after (CBA) studies.
We included CBA studies meeting the following criteria:
at least two intervention sites and two control sites;
the timing of study periods for control and intervention groups was comparable (i.e. pre‐ and postintervention periods of measurement are the same); and
intervention and control groups were comparable on key characteristics such as study population and intervention evaluated.
Types of participants
We included studies reporting on adults aged 16 years and over who required an artificial airway with or without invasive mechanical ventilation and their communication partners (family members, friends, caregivers, and healthcare professionals). Our inclusion criteria comprised adults receiving care in an ICU, specialised centre for mechanical ventilator weaning, step down/up or intermediate care unit, hospital ward, rehabilitation, long‐term care, or living in the home. We documented when available the reason for the artificial airway; type of artificial airway; length of time requiring an artificial airway prior to study enrolment; need for mechanical ventilation; and presence of pre‐existing conditions such as dementia, stroke, aphasia, dysarthria, dyspraxia, developmental disability, or other impairment of speech language or cognition.
We excluded studies of children under 16 years of age due to developmental issues associated with communication and ability to complete measures as well as the role parents assume in communication.
Types of interventions
We included studies that evaluated an intervention that comprised a non‐vocal (visual or speech‐generating) communication aid or a voice‐enabling communication aid used for people with an artificial airway (endotracheal or tracheostomy tube) with or without invasive mechanical ventilation (Figure 1).
We included the following as comparisons:
usual practice that did not include routine or standardised use of communication aids;
usual practice that included non‐vocal or voice‐enabling communication aids used as standard of care;
active comparator, that is, non‐vocal or voice‐enabling communication aids not used as standard care.
We excluded the following communication aids or techniques:
communication aids used during non‐invasive ventilation (i.e. ventilation delivered via a mask) for enhancing voice audibility as non‐invasive ventilation does not require an artificial airway (e.g. the Dolores One acoustic throat sensor);
communication aids used for enhancing voice audibility without any form of mechanical ventilation as these are used without an artificial airway; and
oesophageal and tracheoesophageal speech as these are techniques that cause mucosal vibration in the pharyngo‐oesophageal segment (nasal cavity to top of oesophagus) used in patients following laryngectomy (removal of the voice box) and do not require an artificial airway (Van Sluis 2018).
Types of outcome measures
We did not use reported outcomes as a criterion for including or excluding studies.
Primary outcomes
-
Depending on the nature of the intervention (non‐vocal or voice‐enabling aid) under investigation, our primary outcome was the proportion of participants able to:
use a non‐vocal communication aid to communicate at least one symptom, need, or preference; or
use a voice‐enabling communication aid to phonate to produce at least one intelligible word.
Secondary outcomes
Time to communication (non‐vocal aid) of a symptom, need, or preference or time to phonation of intelligible speech (voice‐enabling aid).
Patient or communication partner (family, friend, caregiver, or healthcare professional with whom a patient may interact) (or both) reported perceptions of communication including: ease/difficulty, satisfaction/frustration, aid/technique usability, and acceptability/unacceptability.
Communication frequency, quality, success, and efficiency.
Health‐related quality of life/satisfaction with life.
Emotional and psychological distress.
Length of stay and healthcare utilisation costs.
Adverse events including: respiratory instability (altered respiratory rate; oxygen desaturation); haemodynamic instability (tachy/bradycardia; hyper/hypotension); need for tracheostomy change due to secretion encumbrance; use of physical restraints; treatment interference.
Search methods for identification of studies
Electronic searches
We searched electronic databases from inception to 30 July 2020 including the Cochrane Library (Wiley version), MEDLINE (OvidSP), Embase (OvidSP), CINAHL (EBSCOhost), and ISI Web of Science. The Cochrane Library includes the Cochrane Database of Systematic Reviews (DSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), the Health Technology Assessment Database (HTA database), and the NHS Economic Evaluation Database (NHS EED).
We present the search strategy for MEDLINE (OvidSP) in Appendix 2. This search strategy was iteratively developed between the research team and an experienced information specialist. We tailored the search strategy to other databases (Appendix 3). Another senior information specialist reviewed the core search strategy prior to execution using the Peer Review for Electronic Search Strategies (PRESS) template (McGowan 2016). We applied a filter to remove animal‐only studies and opinion pieces (e.g. editorials, letters). We imposed no language or other restrictions. We applied the 2008 Cochrane Highly Sensitive Search Strategy filter for RCTs as well as a filter for non‐randomised intervention studies.
Searching other resources
We searched for systematic reviews using PROSPERO and the Joanna Briggs Institute EBP Database. We performed a grey literature search of relevant databases and websites using resources listed in Canadian Agency for Drugs and Technologies in Heath's (CADTH) Grey Matters (www.cadth.ca/en/resources/finding-evidence-is/grey-matters). We searched for unpublished studies and ongoing trials on the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch). We examined reference lists of relevant studies and reviews, and contacted corresponding authors of included studies for details of additional published or unpublished work and advice as to other relevant studies.
Data collection and analysis
Selection of studies
Two review authors (LR, CD) independently screened titles and abstracts of electronic and manual search results to identify citations possibly meeting eligibility criteria. We independently examined for eligibility the full‐text publications of all potentially relevant articles identified by either review author. We resolved any disagreements though discussion and when unable to achieve consensus, referred to an independent arbiter (AA). All potentially relevant papers excluded at this stage are listed as excluded studies, with reasons for exclusion provided in the Characteristics of excluded studies table. We provide citation details and available information on studies that are complete but not yet published in full in the Characteristics of studies awaiting classification table. We provide citation details and available information on eligible ongoing studies in the Characteristics of ongoing studies table. We collated and report details of duplicate publications, so that each study (rather than each report) was the unit of interest in the review. We reported the screening and study selection process in an adapted PRISMA flow chart (Liberati 2009).
Data extraction and management
Two review authors in pairs (A‐LS, AA; OS, LR) independently extracted data from eligible studies. We developed, piloted, and iteratively refined a data extraction form using a modified version of the Cochrane Consumers and Communication Group Data Extraction Template (available at cccrg.cochrane.org/author-resources). We extracted the study aim, study design, inclusion and exclusion criteria, participant characteristics, description of the intervention and comparison group, description of training of participants or communication partners (or both) in use of the non‐vocal communication or speech aid, study outcomes, study results including complications and adverse events, funding source, and study author declaration of interests. We resolved any discrepancies by discussion until consensus was reached, or through consultation with a third review author, where necessary. One review author (LR) entered extracted data into Review Manager 5 (Review Manager 2020), and a second review author working independently (A‐LS) checked for accuracy against the data extraction sheets. For CBA studies, we attempted to extract data on confounding factors, methods used to control confounding, and multiple effects estimates.
Assessment of risk of bias in included studies
We assessed and reported methodological risk of bias of included studies based on guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the Cochrane Consumers and Communication Review Group (Ryan 2013). For RCTs, two review authors in pairs (A‐LS, AA or OS, LR) independently assessed the risk of bias in the following domains: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data; selective outcome reporting; and other sources of bias including role of the study funder and investigator declaration of interest. We determined blinding separately for different outcomes as blinding has the potential to differently affect subjective versus objective outcome measures.
These two review authors independently judged each domain as high, low, or unclear risk of bias based on the criteria provided by Higgins 2011. We provided a quote from the study report that illustrated our assessment and a justification for our judgement for each item in the risk of bias table. We resolved disagreements on judgements relating to risk of bias by discussion to reach consensus, and referred to a third review author when consensus could not be reached. We contacted study authors for additional information enabling clarification of study methods to inform our assessment of risk of bias, as required.
Studies were at high risk of bias if they were scored at high or unclear risk of bias for either the sequence generation or allocation concealment domains, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011). We determined quasi‐RCTs as being at a high risk of bias for the random sequence generation domain of the risk of bias tool. We assessed CBA studies against the same criteria as RCTs but reported them as being at high risk of bias on both the random sequence generation and allocation sequence concealment items. We excluded CBA studies that were not reasonably comparable at baseline.
Measures of treatment effect
Given the expected methodological heterogeneity, where possible we presented individual study and pooled effect estimates separately for RCTs, quasi‐RCTs, and non‐randomised parallel group controlled trials, and for CBA studies. For dichotomous outcomes including proportion of participants able to phonate, produce intelligible speech, or communicate, and adverse events, we analysed data based on the number of events and the number of people assessed in both intervention and comparison groups. For each study, we calculated pooled risk ratios (RRs) with 95% confidence intervals (CIs) using a DerSimonian and Laird random‐effects model. For continuous outcomes including patient‐reported communication outcomes and length of stay, we calculated the study level mean difference (MD) and associated 95% CI. Pooled mean differences and 95% CIs were calculated using the inverse of the variance method for weighting. If more than one study measured the same outcome using different tools, we calculated the pooled standardised mean difference (SMD) and 95% CI weighted by using the inverse variance method in Review Manager 5 (Review Manager 2020). For CBAs, we calculated RR with 95% CIs for dichotomous outcomes and SMDs and 95% CIs for continuous outcomes.
Unit of analysis issues
For parallel group design trials, we used individual study participants as the unit of analysis. For multi‐armed studies, we combined groups to create a single pairwise comparison for the purposes of meta‐analysis, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For relevant cluster‐RCTs, we checked for unit‐of‐analysis errors and found none. If found, and sufficient information available, we had planned to re‐analyse the data using the appropriate unit of analysis, by considering the intracluster correlation (ICC). We planned to obtain estimates of the ICC by contacting authors of included studies, or impute them using estimates from external sources. If reanalysis of the data was not possible, we had planned to report effect estimates and annotate these as unit‐of‐analysis errors.
Dealing with missing data
We attempted to contact study authors (maximum of three emails) to obtain missing data (participant, outcome, or summary data). For participant data, we conducted analyses, when possible, on an intention‐to‐treat basis; otherwise, data were analysed as reported. We reported loss to follow‐up and assessed it as a source of potential bias.
Assessment of heterogeneity
Where studies were considered sufficiently similar (based on consideration of study populations and interventions) to allow pooling of data using meta‐analysis, we assessed the degree of clinical and methodological heterogeneity with visual inspection of forest plots of trial‐level effects and by examining the Chi² test for heterogeneity. We quantified heterogeneity using the I² statistic with I² > 50% representing substantial heterogeneity (Higgins 2011). However, we also interpreted this value considering the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi² test (P < 0.05 considered significant heterogeneity) (Higgins 2011).
Where we detected substantial clinical, methodological, or statistical heterogeneity across included studies, we did not pool results using meta‐analytic techniques but used a narrative approach to data synthesis. We had planned to explore possible clinical or methodological reasons for heterogeneity by grouping studies that were similar in terms of study population and intervention type and explored differences in intervention effects. However insufficient studies were found to meaningfully explore differences.
Assessment of reporting biases
We assessed reporting bias qualitatively based on the characteristics of the included studies as predominantly small studies that indicated positive findings were identified for inclusion. We obtain no indication of relevant unpublished studies from contacting experts and authors of studies. We planned to assess publication bias by constructing a funnel plot of the treatment effect for the primary outcome against trial precision (standard error) and formally test for funnel plot asymmetry (Eggers 1997; Peters 2006); however, we identified insufficient studies (only two studies provided data for the primary outcome).
Data synthesis
We provided a descriptive synthesis of the key demographic and clinical data from the identified studies. We meta‐analysed data when there were sufficient studies with interventions that were sufficiently similar in terms of participants, settings, intervention, comparison, and outcome measures to ensure meaningful conclusions from a statistically pooled result. We planned to analyse and present data from RCTs and quasi‐RCTs and non‐randomised parallel group trials, and from CBAs separately, but compare narratively. Due to the anticipated variability in the populations and interventions of included studies, for binary outcomes, including our primary outcome, we calculated pooled RRs and 95% CIs using a DerSimonian and Laird random‐effects model. For continuous outcomes, we calculated the study level MD and associated 95% CI. Pooled mean differences and 95% CIs were calculated using the inverse of the variance method for weighting. When more than one study measured the same outcome using different tools, we calculated the pooled SMD and 95% CI weighted by using the inverse variance method in Review Manager (Review Manager 2020).
If an outcome was reported within the same study using two types of measurement (e.g. self‐report of communication frequency versus independent observation), we planned to report both results narratively but to include only the measure at least risk of bias (i.e. independent observation, in this scenario) in analyses of treatment effect. If a study measured and reported multiple time points for the same outcome, we included the result reported most proximally to receiving the intervention. If there were multiple time points across studies, we planned to perform subgroup analyses of these time points if there were sufficient studies available. For studies enrolling participants in an ICU, we included ICU length of stay and ICU healthcare utilisation costs as opposed to those reported after ICU.
For outcomes for which we were unable to pool the data statistically using meta‐analysis, we provided a narrative synthesis of results. We presented the results pertaining to our review outcomes organised by intervention categories (e.g. non‐vocal aids versus voice‐enabling aids). We planned to also present results by study population (e.g. acute or critical care setting versus long‐term care or home setting); however, we did not identify studies conducted in a long‐term care or home setting.
Within the two intervention categories, we explored the following comparisons:
usual practice that did not include routine or standardised use of communication aids;
usual practice that included non‐vocal or voice‐enabling communication aid used as standard of care; and
active comparator, that is, non‐vocal or voice‐enabling communication aids not used as standard of care.
Subgroup analysis and investigation of heterogeneity
We had planned to perform statistical subgroup analyses using appropriate interaction tests by intervention categories (e.g. non‐vocal aids versus voice‐enabling aids); and by study population (e.g. acute or critical care setting versus long‐term care or home setting), however there were insufficient studies. We also had planned to perform statistical subgroup analyses within intervention categories, that is, comparing low‐ versus high‐tech non‐vocal aids and comparing voice‐enabling aids that required cuff deflation and those that did not. As there were too few studies to warrant statistical subgroup analyses, we narratively explored relationships in the data according to these subgroups.
Sensitivity analysis
If sufficient studies were identified, we had planned to conduct a sensitivity analysis for the primary outcome, excluding studies determined to be at high risk of bias. If randomised and quasi‐randomised trials were identified, we had planned to conduct a sensitivity analysis removing the quasi‐randomised trials.
Ensuring relevance to decisions in healthcare
This review was informed by consultation with key stakeholders with expertise and decision‐making authority in speech language pathology as well as two consumer referees (family caregiver for a person experiencing acute endotracheal intubation and subsequent prolonged ventilation requiring tracheostomy and use of communication aids). The review has received feedback from at least one consumer referee in addition to a health professional as part of the Cochrane Consumers and Communication Group's standard editorial process.
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables presenting the results of synthesis, informed by methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We presented the results of meta‐analysis or narrative synthesis for major comparisons and review outcomes, as outlined in the Types of outcome measures section. We provided a source and rationale for each assumed risk cited in the table, and used the GRADE system to rank the certainty of the evidence using GRADEpro software (GRADEpro GDT; Schünemann 2011).
We assessed and reported evidence certainty using GRADE for each outcome in the following domains: study limitations, consistency, imprecision, indirectness, and publication bias. Two review authors independently assessed evidence certainty as implemented and described in the GRADEpro software (GRADEpro GDT; Schünemann 2011).
We included the following outcomes in our summary of findings table.
Proportion of participants able to communicate a symptom, need, or preference; or phonate to produce intelligible speech.
Health‐related quality of life/satisfaction with life.
Emotional and psychological distress.
Length of stay and healthcare utilisation costs.
Adverse events including: respiratory instability (altered respiratory rate; oxygen desaturation); haemodynamic instability (tachy/bradycardia; hyper/hypotension); need for tracheostomy change due to secretion encumbrance; use of physical restraints; treatment interference.
Results
Description of studies
Results of the search
Our search identified 3207 citations: 2612 from electronic databases, 595 from grey literature, and three from reference and trial registration review. Contact with experts and authors of included studies provided no additional citations. After removing duplicates, and screening citation titles and abstracts, we retrieved 30 potentially eligible citations. Of these, 14 were full text, nine were abstracts, and seven were trial registrations. We excluded nine of these citations (see Figure 2). We included 11 studies, five are awaiting classification, and three are ongoing.
2.
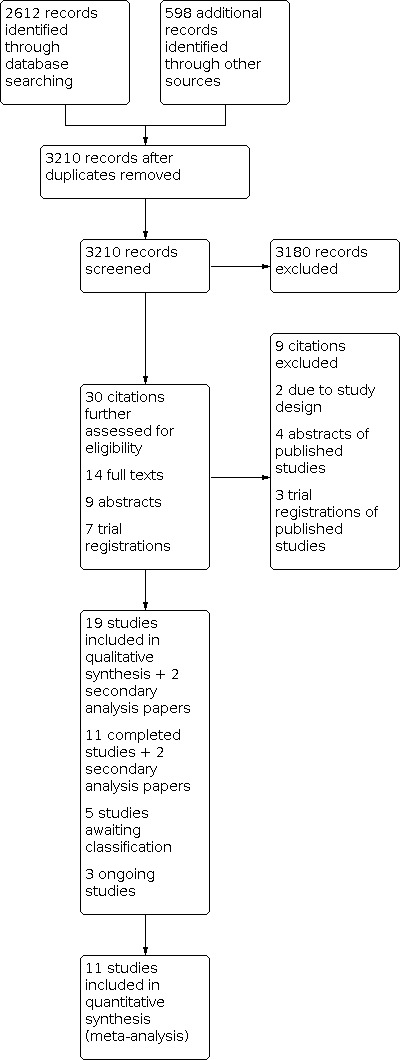
Study flow diagram.
Included studies
We included 11 completed studies plus two secondary analyses (Freeman‐Sanderson 2016a; Koszalinki 2020) (13 published papers in total) recruiting 1931 participants in total (Characteristics of included studies table). Study sample sizes ranged from 20 (Pandian 2020a) to 1440 (Happ 2015). We included six parallel group RCTs (El‐Soussi 2015; Farahani 2012; Freeman‐Sanderson 2016b; Kaur 2018; Pandian 2020a; Pandian 2020b), one stepped wedge cluster RCT (Happ 2015), one quasi‐RCT (Koszalinski 2020), two non‐randomised parallel group controlled trials (Hosseini 2018; Rathi 2015), and one CBA study (Rodriguez 2016).
Five studies were from the USA (Happ 2015; Koszalinski 2020; Pandian 2020a; Pandian 2020b; Rodriguez 2016), two from India (Kaur 2018; Rathi 2015), two from Iran (Farahani 2012; Hosseini 2018), one from Australia (Freeman‐Sanderson 2016b), and one from Egypt (El‐Soussi 2015).
We found studies that compared a non‐vocal communication aid to usual practice comprising either no aid or use of non‐vocal or voice‐enabling communication aids used as standard of care. We also found studies that compared a voice‐enabling communication aid to use of a non‐vocal or voice‐enabling communication aid as standard of care.
We found no studies that compared a voice‐enabling communication aid to usual care without an aid. We also found no studies that used an active comparator, that is, non‐vocal or voice‐enabling communication aids not used as standard care.
Study participants
All studies recruited conscious people requiring an artificial airway in an ICU setting. No studies were identified in long‐term care or home settings. Four studies recruited only participants with an endotracheal tube (Farahani 2012; Hosseini 2018; Rathi 2015; Rodriguez 2016), three studies recruited only participants with a tracheostomy tube (Freeman‐Sanderson 2016b; Pandian 2020a; Pandian 2020b); the remainder recruited participants using either artificial airway type (El‐Soussi 2015; Happ 2015; Kaur 2018; Koszalinski 2020) (see Characteristics of included studies table).
Study interventions
Eight studies evaluated non‐vocal communication aids compared to usual care. We included six studies that evaluated communication boards or charts comprising various combinations of pictures and descriptive words representing physical or emotional needs (El‐Soussi 2015; Farahani 2012; Happ 2015; Hosseini 2018; Kaur 2018; Rathi 2015). Four studies evaluated one type of communication board (El‐Soussi 2015; Hosseini 2018; Kaur 2018; Rathi 2015), whereas Farahani 2012 evaluated two types of communication board. In this study, Board A displayed the alphabet and words representing physical and mental needs; Board B displayed the alphabet and pictures of patient needs and potential demands. Happ 2015 evaluated a multi‐faceted intervention that included a communication board as well as provision of other communication supplies such as notebooks, felt‐tip pens, clipboards, and hearing aids as well as one hour of online training for nurses; and weekly training rounds by a speech language pathologist (SPEACS‐2).
Two of the six studies evaluating a communication board specifically stated no communication aids were used as usual care (El‐Soussi 2015; Happ 2015); the remainder provided no further description of what usual or routine care included and, therefore, we assumed no aids were in use.
Two studies evaluated communication apps provided on a tablet device (Koszalinski 2020; Rodriguez 2016). One quasi‐RCT evaluated the communication app Speak for Myself‐Voice compared to usual care comprising both non‐vocal or voice‐enabling communication aids (Koszalinski 2020). This app, developed by the authors, included an advanced care planning menu, a section for the patient to indicate pain, basic needs requests, and a free‐text section. The second study evaluated a speech‐generating app compared to usual care that comprised provision of a notepad to communicate needs and an urgent call button (Rodriguez 2016). The speech‐generating app comprised prerecorded spoken messages representing symptoms or basic needs as well as speech generation of handwritten (using a finger or stylus) or type‐written messages. The app was used in conjunction with an urgent call button that was also provided to the control group.
Three studies evaluated voice‐enabling communication aids compared with usual care that included routine use of communication aids (Freeman‐Sanderson 2016b; Pandian 2020a; Pandian 2020b). Two studies evaluated early versus routine use of cuff deflation and a one‐way speaking valve in people requiring a tracheostomy (Freeman‐Sanderson 2016b; Pandian 2020a). Freeman‐Sanderson 2016b used the Passy Muir 'Ventilator Speech and Swallowing Valve 007' and defined early use as with spontaneous breathing and pressure support during mechanical ventilation. In the control arm, participants received usual care during which they were not provided with a one‐way speaking valve (Portex orator speaking valve) until able to breathe without ventilator support (but with the tracheostomy tube still in situ and the patient using a Swedish nose (Themovent‐T) heat and moisture exchange filter. Pandian and colleagues defined early use of a one‐way speaking valve as within 12 to 24 hours of a percutaneous tracheostomy (Pandian 2020a). The control arm participants received usual care, which comprised evaluation by a speech language pathologist for use of a one‐way speaking valve 48 to 60 hours from a percutaneous tracheostomy. The third study of a voice‐enabling communication aid evaluated the Blue Line Ultra SuctionAid (BLUSA) talking tracheostomy (Pandian 2020b). This tracheostomy tube has an additional lumen (tube) that directs a flow of gas to the vocal cords sitting above the tracheostomy tube cuff enabling vocalisation. In this study, participants completed three treatment sessions with a speech language pathologist that focused on optimising voice while using the BLUSA tube. The control arm received usual care, which comprised assessment by a speech language pathologist and use of a one‐way speaking valve or non‐vocal communication aids such as a communication board/i‐Pad or writing materials (see Characteristics of included studies table).
Study outcomes
We found no studies that reported directly on our primary outcome of the proportion of participants able to communicate at least one symptom, need, or preference; or to phonate to produce at least one intelligible word. Two studies of a voice‐enabling aid reported on phonation ability or speech intelligibility that we considered to sufficiently address this outcome and therefore have reported in the Effects of interventions section (Freeman‐Sanderson 2016b; Pandian 2020b).
Excluded studies
We excluded two studies due to study design; both were single‐centre CBA studies (Otuzoğlu 2014; Stovsky 1988).
Studies awaiting classification
We found five studies reported in abstract or trial registration form, which are considered as awaiting classification (Characteristics of studies awaiting classification table).
Ongoing studies
Three studies are ongoing (Characteristics of ongoing studies table).
Risk of bias in included studies
We assessed the risk of bias for the 11 included studies, using Cochrane's domain‐based risk of bias tool (Higgins 2011). We provided our judgement of classification of bias in the Characteristics of included studies table, with a summary presented in Figure 3.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies used a computer‐generated randomisation table for random sequence generation (Freeman‐Sanderson 2016b; Happ 2015; Pandian 2020b). Two studies concealed allocation and were, therefore, at low risk of selection bias (Freeman‐Sanderson 2016b; Pandian 2020b). Happ 2015 used a stepped wedge cluster design. This poses challenges in terms of allocation concealment and needing to inform participating sites as to when they commence the intervention phase. Therefore, we rated this study at unclear risk in terms of allocation concealment. Four studies were at high risk of selection bias due to quasi‐randomisation (Koszalinski 2020), or no randomisation and no allocation concealment (Hosseini 2018; Rathi 2015; Rodriguez 2016). In Koszalinski 2020, study allocation was known to the study investigators. One study was at unclear risk due to the use of a non‐replacement lottery method and no description of allocation concealment (Kaur 2018). Three studies provided no description of randomisation or allocation concealment methods (El‐Soussi 2015; Farahani 2012; Pandian 2020a).
Blinding
Due to the nature of the intervention, and as anticipated, no study blinded personnel or participants to the intervention. This lack of blinding could have influenced participant self‐report measures such as satisfaction and frustration with communication. Only one study reported blinding of outcome assessors (Happ 2015). Seven studies reported inability to blind outcome assessors (El‐Soussi 2015; Farahani 2012; Freeman‐Sanderson 2016b; Hosseini 2018; Kaur 2018; Koszalinski 2020; Pandian 2020b). The remaining studies did not report blinding of outcome assessors with inability to gain clarification from the corresponding authors and therefore rated as unclear.
Incomplete outcome data
Risk of attrition bias was high in one study due to lack of reporting on missing data (Rodriguez 2016), and unclear for three studies (El‐Soussi 2015; Farahani 2012; Pandian 2020a); the remaining studies were assessed as at low risk of attrition bias.
Selective reporting
There was no evidence of selective reporting bias in the 11 included studies.
Other potential sources of bias
We found no evidence of other sources of bias in 9 of the included studies. We rated one study as unclear in this domain as the lead author held a creative commons license for the intervention that was under evaluation (Happ 2015). We rated El‐Soussi 2015 as unclear risk of bias as no conflict of interest or funding statement was provided.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Non‐vocal communication aid compared to usual care without an aid for adults requiring an artificial airway with or without mechanical ventilator support.
| Non‐vocal communication aid compared to usual care without an aid for adults requiring an artificial airway with or without mechanical ventilator support | ||||
| Patient or population: adults requiring an artificial airway with or without mechanical ventilator support Setting: – Intervention: non‐vocal communication aid Comparison: usual care without an aid | ||||
| Outcomes | № of participants (studies) | Impact | Certainty of the evidence (GRADE) | Comments |
| Proportion able to communicate or phonate | — | — | — | No study measured this outcome. |
| Health‐related quality of life | — | — | — | No study measured this outcome. |
| Emotional and psychological distress | 90 (2 RCTs) | 1 study of 60 participants reported 9 (30%) intervention participants using a communication board were quite to very distressed compared to 24 (80%) control participants. 1 study of 30 participants demonstrated a 15‐point reduction in HADS‐A (anxiety) (mean score 18.1 (SD 1.8) to 3.0 (SD 1.8) measured at baseline and after provision of a communication board for 48 hours compared to a 5‐point reduction in the control group (mean 16.9 (SD 2.4) to 12.0 (SD 4.3) measured at baseline and 48 hours. The HADS‐A is the anxiety subscale of the HADS. Scores range from 0 to 21 with higher scores indicating greater anxiety. |
⊕⊝⊝⊝ Very lowa,b | We downgraded 2 levels due to very serious risk of bias and 1 level due to imprecision. |
| ICU length of stay (days) | 1500 (2 RCTs) |
1 study of 1440 participants reported no difference in the unadjusted (0.20, 95% CI –1.18 to 1.59) or adjusted (–0.08, 95% CI –1.28 to 1.13) median ICU length of stay measured in days. 1 study of 60 participants reported a reduction in ICU length of stay (MD –0.21, 95% CI –0.29 to –0.13). |
⊕⊕⊝⊝ Lowc,d,e | Downgraded 1 level due to serious risk of bias and 1 level due to inconsistency of results. |
| Costs | 1440 (1 RCT) |
1 study reported cost‐adjusted charges from hospital administrative claims were slightly higher in the intervention group, but this difference was not statistically significant (unadjusted intervention effect USD 6380, 95% CI USD 579 to USD 13,339; P = 0.07; adjusted intervention effect USD 5797 (‐USD 936 to USD 12,529) (adjusting for participant age, sex, race, admission APACHE III, and neurological disorder as admitting diagnosis). | ⊕⊕⊝⊝ Lowc,f | Downgraded 1 level due to serious risk of bias, and 1 level due to imprecision. |
| Adverse events | 1440 (1 RCT) |
1 study of 1440 participants measured days of upper extremity restraint use and found no difference between intervention and control groups in number of ICU days physical restraint was used (50.1 (36.5%) ICU days with intervention vs 47.9 (36%) ICU days with control. | ⊕⊕⊝⊝ Lowg | Downgraded 1 level due to serious risk of bias and 1 level due to imprecision. |
| APACHE III: Acute Physiology, Age, Chronic Health Evaluation III; CI: confidence interval; HADS‐A: Hospital Anxiety and Depression Scale – Anxiety; ICU: intensive care unit; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation. | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aThe two studies contributing data to this outcome were assessed at high risk of selection, performance, and detection bias. bThe two studies contributing data to this outcome uses different measures of different emotions with different time‐points and were based on a small number of participants. cOne study was at high risk of performance bias; however, length of stay and costs are objective outcomes and outcome assessors were blinded. dOne study was at high risk of bias across multiple domains. eIncluded studies indicated either no effect or a small non‐clinically (i.e. 0.2 days) important reduction in ICU length of stay. fOne study reported this outcome with wide CIs. gOne study was at high risk of performance bias; however, duration of physical restraint use is an objective outcome and outcome assessors were blinded.
Summary of findings 2. Non‐vocal communication aid compared to usual care with an aid for adults requiring an artificial airway with or without mechanical ventilator support.
| Non‐vocal communication aid compared to usual care with an aid for adults requiring an artificial airway with or without mechanical ventilator support | ||||
| Patient or population: adults requiring an artificial airway with or without mechanical ventilator support Setting: – Intervention: non‐vocal communication aid Comparison: usual care with an aid | ||||
| Outcomes | № of participants (studies) | Impact | Certainty of the evidence (GRADE) | Comments |
| Proportion able to communicate or phonate | — | — | — | No study measured this outcome. |
| Health‐related quality of life | — | — | — | No study measured this outcome. |
| Emotional and psychological distress assessed with: HADS | 58 (1 RCT) | HADS depression subscale scores measured at baseline and on study completion favoured the intervention group (Speak for Myself‐Voice communication app) Intervention: mean baseline score 10.5, 95% CI 8.6 to 12.4 to mean postintervention score 8.0, 95% CI 5.8 to 10.2; Control: mean baseline score 6.4, 95% CI 3.9 to 8.9 to mean postintervention score 9.5, 95% CI 6.7 to 12.3, P = 0.006). The difference in the change in HADS anxiety subscale score did not reach statistical significance. Intervention: mean baseline score 12.6, 95% CI 10.5 to 14.6 to mean postintervention score 8.2, 95% CI 6.1 to 10.2; Control: mean baseline score 11.1, 95% CI 8.2 to 14.0 to mean postintervention score 10.3, 95% CI 7.4 to 13.2; P = 0.072). HADS depression and HADS anxiety subscale scores both range from 0 to 21 with higher scores indicating greater depression or anxiety. |
⊕⊝⊝⊝ Very lowa,b | Downgraded 2 levels due to very serious risk of bias and 1 level due to imprecision. |
| ICU length of stay and healthcare costs | — | — | — | No study measured these outcomes. |
| Adverse events | — | — | — | No study measured this outcome. |
| CI: confidence interval; HADS: Hospital Anxiety and Depression Scale; ICU: intensive care unit; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aThe one study contributing data to this outcome was at high risk of selection, performance, and detection bias. bOnly one study of 58 participants contributed to this outcome.
Summary of findings 3. Voice‐enabling communication aid compared to usual care with an aid for adults requiring an artificial airway with or without mechanical ventilator support.
| Voice‐enabling communication aid compared to usual care with an aid for adults requiring an artificial airway with or without mechanical ventilator support | ||||||
| Patient or population: adults requiring an artificial airway with or without mechanical ventilator support Setting: – Intervention: voice‐enabling communication aid Comparison: usual care with an aid | ||||||
| Outcomes | № of participants (studies) | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care using an aid | Risk with voice‐enabling communication aid | |||||
| Proportion able to communicate | 50 (2 RCTs) | — | — | RR 3.03 (0.18 to 50.08) | ⊕⊝⊝⊝ Very lowa,b,c | Downgraded 1 level for serious risk of bias, 1 level for imprecision, and 1 level for inconsistency. |
| Health‐related quality of life assessed with: QOL‐MV | 63 (2 RCTs) | — |
MD 2.27 higher
(95% CI 7.21 lower to 11.75 higher) The QOL‐MV is reported on a scale of 0–100 with lower scores indicating worse quality of life. Scores were assessed repeatedly over time from baseline and following treatment sessions. |
— | ⊕⊕⊝⊝ Lowa,b | Downgraded 1 level due to serious risk of bias and 1 level for imprecision. |
| Emotional and psychological distress | 80 (2 RCTs) |
1 study (30 participants) reported 7/8 domains of the VASES had mean between‐group differences that favoured the intervention group. VASES was measured at baseline, then daily on weekdays until return of voice. VASES is a 10‐item scale with items scored on a bipolar scale. 1 study (50 participants) reported intervention participants had lower mean scores (12.1 (SD 9.0)) (indicating more emotional distress) on the QOL‐MV emotional domain at treatment session end compared to control (13.5 (SD 6.6); and compared to their own baseline measurement (13.5 (SD 6.9)). Mean scores on the V‐RQOL emotional domain were higher (46.1 (SD 23.1) (indicating less emotional distress) compared to the control group (35.7 (SD 30.9). The QOL‐MV and V‐RQOL both comprise emotional and physical domains. Higher scores indicate less emotional distress. |
— | ⊕⊝⊝⊝ Very lowa,b,d | We downgraded 1 level due to serious risk of bias, 1 level for imprecision, and 1 level for inconsistency in results. | |
| ICU length of stay (days) and healthcare costs | 100 (3 RCTs) | — | ICU length of stay: MD 0.2 days longer (0.04 fewer to 0.44 longer) | — | ⊕⊕⊕⊝ Lowa,b | We downgraded 1 level due to serious risk of bias and 1 level for imprecision. No study measured healthcare costs. |
| Adverse events | 50 (2 RCTs) |
1 study of 30 participants reported 5 participants in both study arms experienced clinical events including oxygen desaturation, increased respiratory rate, increased upper respiratory tract secretions, excessive coughing, and hypertension. 1 study of 20 participants measured bleeding following percutaneous tracheostomy insertion with no participants in either study arm experiencing this adverse event outcome. |
— | ⊕⊕⊝⊝ Lowa,e | We downgraded 1 level due to serious risk of bias and 1 level due to imprecision. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; MD: mean difference; QOL‐MV: Quality of Life in Mechanically Ventilated Patients; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; VASES: Visual Analogue Self‐Esteem Scale; V‐RQOL: Voice‐Related Quality of Life. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe two included studies were at serious risk of performance and detection bias. bThe pooled estimate demonstrated wide confidence intervals and was based on a small number of participants. cI²statistic = 77%. dThe two included studies reporting conceptually different measures of emotional distress. One study reported results favouring the intervention whereas the second reported equivocal results. eThe results are based on two studies with a small number of participants.
See summary of findings for the main comparisons (Table 1; Table 2; Table 3). We presented the results organised in terms of the outcome listed in the Types of outcome measures section and present data grouped according to intervention category, that is, non‐vocal aids and voice‐enabling aids and grouped according to comparator, that is, usual care without use of aids and usual care with use of aids as standard of care (we identified no studies that used an active comparator). In the summary of findings tables, we alternatively presented three comparisons with outcomes listed within these comparisons.
Primary outcome
Proportion of participants able to use a non‐vocal communication aid to communicate at least one symptom, need, or preference; or use a voice‐enabling communication aid to phonate to produce at least one intelligible word
No studies reported directly on our primary outcome of the proportion of participants able to communicate at least one symptom, need, or preference; or to phonate to produce at least one intelligible word. Therefore, there was insufficient evidence to determine whether a non‐vocal communication aid increases the ability to communicate. We were able to indirectly determine this outcome for two of the three studies reporting on voice‐enabling communication aids enabling pooling of data.
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
No studies reported this outcome.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
See Analysis 3.1 and Figure 4.
3.1. Analysis.

Comparison 3: Voice‐enabling communication aid versus usual care with an aid, Outcome 1: Ability to phonate
4.

Forest plot of comparison: 1.1 Ability to phonate.
In one RCT (30 participants) of early versus routine use of a one‐way speaking valve for people requiring a tracheostomy, Freeman‐Sanderson 2016b found 15 (100%) of the intervention group versus 11 (73%) of the control group achieved phonation. Pandian 2020a (20 participants) measured speech intelligibility as an indication of ability to phonate in an RCT of early use of a one‐way speaking valve following percutaneous tracheostomy at three time points; 0 to 24 hours; 25 to 60 hours; and 61 hours and 21 days. At 0 to 24 hours, 5 (50%) of the intervention arm could phonate compared to 0 control arm participants; at 25 to 60 hours, 8 (80%) of the intervention group versus 7 (70%) of the control group; and at 61 hours and 21 days, 5 (71%) of the intervention group versus 4 (67%) of the control group.
We were uncertain about the effects of early use of a voice‐enabling aid compared to routine use of a voice‐enabling aid on ability to phonate to produce at least one intelligible word (RR 3.03, 95% CI 0.18 to 50.08; 2 studies, 50 participants; I² = 77%). We considered the certainty of evidence for this outcome very low, downgrading due to serious risk of bias, imprecision, and inconsistency. Interpretation of the size of effect was challenging given the wide CIs that included no effect as well as the possibility of a negative effect on this outcome.
Secondary outcomes
Time to communication or to phonation
Only one RCT (30 participants) of early use of a voice‐enabling communication aid (one‐way speaking valve) compared to routine use of a one‐way speaking valve reported on time from tracheostomy insertion to phonation (Freeman‐Sanderson 2016b). Therefore, pooling of data was not possible.
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
No studies reported this outcome.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
Freeman‐Sanderson 2016b reported the median time to return to phonation from tracheostomy insertion favoured the intervention group (7 days with intervention versus 18 days with usual care; median difference 11 days; hazard ratio 3.66, 95% CI 1.54 to 8.68). A median difference of 11 days can be considered a large effect size. We considered the certainty of evidence for this outcome very low, downgrading once due to serious risk of bias and twice due to imprecision with only one trial reporting this outcome.
Ease of communication
Two studies (145 participants) of non‐vocal communication aids, one evaluating a communication board (Hosseini 2018), and the other a speech‐generating communication app (Rodriguez 2016) used the Ease of Communication Scale developed by Menzel 1998, with Hosseini 2018 using an Iranian version. With this scale, scores range from 0 to 24 with lower scores indicative of greater communication ease.
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
One non‐randomised parallel‐group controlled trial (30 participants) measured ease of communication at two time‐points, that is, 24 hours after regaining consciousness and 48 hours after the first measurement (Hosseini 2018). Hosseini 2018 reported a reduction in the Ease of Communication Scale scores measured at baseline and 48 hours between participants using the communication board and the control group (MD –9.10, 95% CI –10.66 to –7.54; Analysis 1.1). Given scoring of the Ease of Communication Scale ranges from 24 (most difficult) to 0 (easiest communication), this can be considered a moderate effect size. We considered the certainty of evidence for this outcome very low, downgrading twice due to very serious risk of bias and once due to imprecision with only one trial reporting this outcome.
1.1. Analysis.

Comparison 1: Non‐vocal communication aid versus usual care without aids, Outcome 1: Ease of communication
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
One CBA study (115 participants) measured ease of communication at three time points, days two, four, and six (Rodriguez 2016). Rodriguez 2016 reported Ease of Communication Scale scores in the intervention arm on all three measurement time points (MD for day 6 scores –18.32, 95% CI –22.49 to –14.15; Analysis 2.1). Note we included day six scores in this analysis as we perceived this most representative and proximal to a continuously applied communication intervention and less confounded by residual sedation. Given scoring of the Ease of Communication Scale ranges from 24 (most difficult) to 0 (most easiest communication) this can be considered a large effect size. We considered the certainty of evidence for this outcome very low, downgrading twice due to very serious risk of bias and once due to imprecision with only one trial reporting this outcome.
2.1. Analysis.

Comparison 2: Non‐vocal communication aid versus usual care with an aid, Outcome 1: Ease of communication
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Satisfaction/frustration with communication
Six studies (375 participants) reported on patient satisfaction with communication (El‐Soussi 2015; Farahani 2012; Kaur 2018; Pandian 2020b; Rathi 2015; Rodriguez 2016). One stepped wedge cluster RCT reported on nurse satisfaction with communication (Happ 2015). Rodriguez 2016 also reported patient frustration with communication. Of the six studies measuring patient‐reported satisfaction, two used a 5‐point Likert scale (El‐Soussi 2015; Pandian 2020b), one a 10‐cm visual analogue scale (Farahani 2012), one the Patient Perception Scale for Satisfaction (26 items and four domains) (Kaur 2018), one a 20‐item, three‐domain questionnaire developed by the study authors (Rathi 2015), and one the Satisfaction with Communication Method Tool (nine items) adapted from The Quebec User Evaluation of Satisfaction with Assistive Technology (Rodriguez 2016).
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
See Analysis 1.2; Figure 5.
1.2. Analysis.

Comparison 1: Non‐vocal communication aid versus usual care without aids, Outcome 2: Satisfaction
5.

Forest plot of comparison: 1 Communication aid versus usual care without aids, outcome: 1.2 Satisfaction.
We are uncertain about the effects of a communication board, compared to no aids, on satisfaction (SMD 2.92, 95% CI 1.52 to 4.30; I² = 91%; 4 studies, 211 participants). We considered the certainty of evidence for this outcome very low, downgrading twice due to very serious risk of bias, and once due to imprecision.
Given the use of different measures of satisfaction, interpretation of the size of effect was challenging. El‐Soussi 2015 reported 83% (25) of participants in the intervention arm using a communication board were satisfied or very satisfied compared to 33% (10) of participants in the usual care (no communication aid) control arm. To be able to include these data in Analysis 1.2, we used the counts and proportions of the Likert scores (i.e. very dissatisfied = 1, dissatisfied = 2; satisfied = 4; very satisfied = 5) to calculate the mean (standard deviation (SD)).
Farahani 2012 reported mean visual analogue scale scores indicating greater satisfaction with either communication board (Board A 3.8 (SD 0.6); Board B 4.1 (SD 0.5) (combined mean 4.0 (SD 0.6)) compared to a usual care control group mean score of 2.6 (SD 0.5). Kaur 2018 reported higher mean gained scores on the Patient Perception Scale for Satisfaction from baseline to day 4 for participants using a communication board (gain score of 25.9) compared to usual care controls (gain score of 4.4). Mean scores on day 4 were 79.5 (SD 6.65) with intervention and 49.87 (7.02) with control favouring the intervention arm. Rathi 2015 reported higher mean scores on their patient‐reported satisfaction questionnaire for participants using a communication board compared to usual care controls (83.5 (SD 5.5) with intervention versus 65 (SD 3.6) with control; P < 0.001).
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
One CBA study (115 participants) reported greater satisfaction with use of the speech‐generating app (MD 0.59, 95% CI 0.27 to 0.91; P < 0.001) (Rodriguez 2016). Rodriguez 2016 also measured patient‐reported communication frustration using the Frustration with Communication Tool, a one‐item 5‐point Likert scale adapted from Patak's Frustration Survey. Participants using the speech‐generating app reported less frustration related to their ability to communicate needs than participants in the control group (MD –2.68, 95% CI –3.02 to –2.34; P < 0.001). We considered the certainty of evidence for this outcome very low, downgrading twice due to very serious risk of bias and once due to imprecision with only one trial reporting this outcome.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
Pandian 2020b measured patient satisfaction in the intervention arm (BLUSA tracheostomy tube) only. Of the 22 intervention arm participants reporting on satisfaction, 41% were somewhat or very satisfied; 36.4% were neutral; and 22.7% were somewhat or very dissatisfied. We considered the certainty of evidence for this outcome very low, downgrading twice due to very serious risk of bias and once due to imprecision with only one trial reporting this outcome.
Nurse satisfaction
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
Happ 2015 measured nurse satisfaction using the 16‐item Nurse Communication Survey developed by the study team and administered immediately before and three months after nurse training with the SPEACS‐2 intervention. Each item had a possible range of 1 to 5 indicating strength of agreement. The mean item scores increased from 3.21 to 3.43 from immediately before and three months after nurse training (P < 0.001). We considered the certainty of evidence for this outcome as low, downgrading due to serious risk of bias and once due to imprecision with only one trial reporting this outcome.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Usability of communication aid
Three studies (225 participants), two evaluating non‐vocal aids (El‐Soussi 2015; Rodriguez 2016), and one evaluating a voice‐generating communication aid (Pandian 2020b), reported on different aspects of usability of communication interventions.
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
El‐Soussi 2015 used a 5‐point Likert scale ranging from not helpful to extremely helpful. All 30 (100%) participants using the communication board rated it as mostly or extremely helpful compared to seven (23%) participants rating usual communication methods.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
Rodriguez 2016 measured independent use of the urgent call button by the entire study cohort at 136 data collection points and found 110/136 (81%) participants demonstrated independent ability to activate it. This study also measured unit clerks' abilities to understand messages generated by intervention participants when activating the urgent call button. Clerks understood the message 131/136 (96%) times these data were collected.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
Pandian 2020b used a 5‐point Likert scale to measure independence with the BLUSA tracheostomy tube in the intervention arm only. Ability to use the BLUSA with some level of independence was reported by 73% of the 22 participants reporting this outcome.
Communication frequency, quality, success, and efficiency
Two studies (70 participants) of voice‐enabling communication aids assessed speech intelligibility, a marker of communication quality or success (Pandian 2020a; Pandian 2020b).
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
No studies reported this outcome.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
Pandian 2020a measured speech intelligibility at three time points following percutaneous tracheostomy; 0 to 24 hours; 25 to 60 hours; and 61 hours and 21 days. Compared to usual care, participants who received early cuff deflation and a one‐way speaking valve had a higher mean % of intelligible words at 0 to 24 hours (25.7 (SD 33.4) with intervention versus 0 with usual care) and at 61 hours and 21 days (74.5 (SD 21) with intervention versus 36 (SD 40) with usual care), but not at 25 to 60 hours (13 (SD 26.5) with intervention versus 33.6 (SD 33.1) with usual care) (Analysis 3.2). Pandian 2020b measured speech intelligibility in the intervention arm only (53.1% (25.8%). Therefore, we were unable to pool data and there is insufficient evidence to determine if voice‐enabling aids influence communication quality or success.
3.2. Analysis.

Comparison 3: Voice‐enabling communication aid versus usual care with an aid, Outcome 2: Speech intelligibility
Health‐related quality of life/satisfaction with life
Three RCTs (100 participants) of voice‐enabling communication interventions reported on health‐related quality of life (Freeman‐Sanderson 2016b; Pandian 2020a; Pandian 2020b). Freeman‐Sanderson 2016b used the EuroQol‐5D questionnaire (EQ‐5D) administered at baseline and on return of voice. Pandian 2020b used two measurement tools, the Quality of Life in Mechanically Ventilated Patients (QOL‐MV) and the Voice‐Related Quality of Life (V‐RQOL), administered before (baseline) and on completion of speech language pathologist treatment sessions. Pandian 2020a used the QOL‐MV at three time points: up to 24 hours; 25 to 60 hours; and 61 hours to 21 days.
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
No studies reported this outcome.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
See Analysis 3.3.
3.3. Analysis.

Comparison 3: Voice‐enabling communication aid versus usual care with an aid, Outcome 3: Quality of life
Freeman‐Sanderson 2016b reported an MD of 2 points (95% CI –22 to 26) in the EQ‐5D favouring early use of a one‐way speaking valve. Pandian 2020b reported QOL‐MV and V‐RQOL scores measured two weeks after study enrolment were higher in the intervention group using a BLUSA tracheostomy compared to the usual care control (QOL‐MV: 50.2 (SD 22.5) with intervention versus 49.4 (SD 15.2) with usual care; V‐RQOL: 42.5 (SD 17.7) with intervention versus 32.3 (SD 24.9) with usual care). Pandian 2020a reported higher mean QOL‐MV scores at all three time points for participants in the intervention arm (up to 24 hours: 42.0 (SD 18.0) with intervention versus 41.7 (SD 13.4) with usual care; 25 to 60 hours: 53.1 (SD 24.8) with intervention versus 47.3 (SD 12.8) with usual care; 61 hours to 21 days: 55.0 (SD 22.0) with intervention versus 47.1 (SD 16.2) with usual care).
We pooled the QOL‐MV scores using the scores reported from the last time point, that is, 61 hours to 21 days in Pandian 2020a as this measurement time point was most similar to that used in Pandian 2020b. Low‐certainty evidence suggested early use of a voice‐enabling aid may have little or no effect on quality of life (MD 2.27, 95% CI –7.21 to 11.75; studies = 2, participants = 63; I² = 0%; Analysis 3.3). We downgraded the certainty due to once due to serious risk of bias and once due to imprecision.
Emotional and psychological distress
Five studies (206 participants), three evaluating non‐vocal communication aids (El‐Soussi 2015; Hosseini 2018; Koszalinski 2020), and two (Freeman‐Sanderson 2016b; Pandian 2020b) evaluating voice‐enabling communication aids, measured aspects of emotional and psychological distress. El‐Soussi 2015 used a 5‐point Likert scale with participant self‐report of distress from not at all to very much. Freeman‐Sanderson 2016b used the Visual Analogue Self‐Esteem Scale (VASES), administered at baseline and on return of voice. Hosseini 2018 and Koszalinski 2020 used the Hospital Anxiety and Depression Scale (HADS); however, Hosseini 2018 reported the anxiety subscale only. Pandian 2020b reported emotional components of the QOL‐MV and the V‐RQOL. Given conceptual differences in measures used to determine emotional and psychological distress, we did not pool data.
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
El‐Soussi 2015 reported nine (30%) intervention participants using a communication board were quite to very much distressed compared to 24 (80%) control participants. Hosseini 2018 demonstrated a 15‐point reduction in HADS‐A score (mean 18.1 (SD 1.8) to 3.0 (1.8)) measured at baseline and after provision of a communication board for 48 hours compared to a 5‐point reduction in the control group (16.9 (SD 2.4) to 12.0 (SD 4.3)). This effect size is large considering a minimally clinically important difference for the HADS‐A is estimated to range between –1.8 and –1.3 points (Smid 2017). We considered the certainty of evidence for this outcome very low, downgrading twice due to very serious risk of bias and once due to imprecision. This means we are uncertain about the effects of non‐vocal communication aid compared to usual care on emotional and psychological distress.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
Koszalinski 2020 reported HADS depression subscale scores measured at baseline and on study completion. Their results favoured the intervention group using the Speak for Myself‐Voice communication app (intervention: mean change from baseline score –2.5 versus control: mean change from baseline score +3.1; P = 0.006). This effect size (i.e. the difference in mean change from baseline score between intervention and control) was moderate considering a minimum clinically important difference for the HADS‐D is estimated to range between –1.7 and –1.5 points (Smid 2017).
Koszalinski 2020 also reported HADS anxiety subscale score measured at baseline and on study completion. This result did not reach statistical significance (mean change from baseline score: –4.4 with intervention versus –0.8 with control; P = 0.072). We considered the certainty of evidence for this outcome very low, downgrading twice due to very serious risk of bias and once due to imprecision.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
Freeman‐Sanderson 2016b reported seven of the eight domains of the VASES had mean between‐group differences that favoured the intervention group. Pandian 2020b reported equivocal results with participants receiving the BLUSA tracheostomy tube reporting lower mean scores (12.1 (SD 9.0)) (indicating more emotional distress) on the QOL‐MV emotional domain following treatment compared to the control group (13.5 (SD 6.6) and compared to their own baseline (13.5 (SD 6.9)). Conversely, mean scores provided for intervention participants on the emotional domain of the V‐RQOL were higher (46.1 (SD 23.1) compared to the control group (35.7 (SD 30.9). We considered the certainty of evidence for this outcome very low, downgrading due to serious risk of bias, imprecision, and inconsistency in results.
Length of stay and healthcare utilisation costs
Five studies (1600 participants), two evaluating non‐vocal communication aids (El‐Soussi 2015; Happ 2015), and three studies voice‐enabling communication aids (Freeman‐Sanderson 2016b; Pandian 2020a; Pandian 2020b) reported length of ICU stay; three studies also reported hospital length of stay (Happ 2015; Pandian 2020a; Pandian 2020b). One study evaluating non‐vocal communication aids reported on healthcare utilisation costs (Happ 2015).
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
We did not pool data on ICU length of stay as we were unable to obtain upper and lower quartiles or means and SDs from Happ 2015. In this study, there was no difference in the unadjusted or adjusted median ICU length of stay (unadjusted: median 0.20, 95% CI –1.18 to 1.59; adjusted: median –0.08, 95% CI –1.28 to 1.13). El‐Soussi 2015 reported a reduction in ICU length of stay (MD –0.21, 95% CI –0.29 to –0.13; Analysis 1.3). We considered the certainty of evidence for this outcome low, downgrading once due to serious risk of bias and once due to inconsistency of results.
1.3. Analysis.

Comparison 1: Non‐vocal communication aid versus usual care without aids, Outcome 3: Intensive care unit length of stay
Cost‐adjusted charges from hospital administrative claims were slightly higher in the intervention group but this difference was not statistically significant (unadjusted intervention effect: USD 6380, 95% CI ‐USD 579 to USD 13,339; P = 0.07; adjusted intervention effect: USD 5797, 95% CI ‐USD 936 to USD 12,529 (adjusting for participant age, sex, race, admission APACHE III, and neurological disorder as admitting diagnosis); P = 0.09) (Happ 2015). We considered the certainty of evidence for this outcome as low, downgrading due to serious risk of bias and imprecision.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
See Analysis 3.4.
3.4. Analysis.

Comparison 3: Voice‐enabling communication aid versus usual care with an aid, Outcome 4: Intensive care unit length of stay
Low‐certainty evidence suggested voice‐enabling aids may not influence ICU length of stay (MD 0.20, 95% CI –0.04 to 0.44; studies = 3, participants = 100; I² = 19%). We downgraded certainty due to serious risk of bias and imprecision.
Adverse events
Three studies reported adverse events including physical restraint use, respiratory parameters, and tracheostomy bleeding.
Non‐vocal communication aid compared to usual care that does not include routine or standardised use of communication aids
One study evaluating non‐vocal communication aids measured the ICU days with upper extremity physical restraint use and found there may be little or no difference between intervention and control groups (50.1 (36.5%) days with intervention versus 47.9 (36%) days with control) (Happ 2015). We considered the certainty of evidence for this outcome as low, downgrading due to serious risk of bias and imprecision.
Non‐vocal communication aid compared to usual care comprising of use of aids as standard of care
No studies reported this outcome.
Voice‐enabling aid compared to usual care comprising of use of aids as standard of care
Two studies evaluating voice‐enabling communication aids reported on adverse events (Freeman‐Sanderson 2016b; Pandian 2020a). Freeman‐Sanderson 2016b reported five participants in both study arms (comprising 15 participants each) experienced clinical events including oxygen desaturation, increased respiratory rate, increased upper respiratory tract secretions, excessive coughing, and hypertension. Pandian 2020a measured bleeding following percutaneous tracheostomy insertion with no participants in either study arm (comprising 10 participants each) experiencing this adverse event. We considered the certainty of evidence for this outcome as low, downgrading due to serious risk of bias and imprecision.
Discussion
Summary of main results
Our review aimed to evaluate the effectiveness of non‐vocal and voice‐enabling communication aids for adults requiring an artificial airway on a range of clinical and patient‐reported outcomes. We identified 11 studies meeting the eligibility criteria that recruited 1931 participants in an intensive care setting. We identified no studies evaluating communication aids used by participants in a long‐term or community setting.
No studies directly reported on our primary outcome, that is, the ability to communicate a symptom, preference, or need or to phonate to produce at least one intelligible word. This means there was no evidence to determine whether a non‐vocal communication aid increases the ability to communicate (Table 1). Using unpublished data from two studies conducted in tracheostomised participants, early use of a voice‐enabling aid compared to routine use of a voice‐enabling aid might increase ability to phonate to produce at least one intelligible word; however, we remained uncertain about this effect due to very low‐certainty evidence (Table 3). There was insufficient evidence to determine if communication aids improve the time to communication or phonation due to only one study reporting this outcome.
Low‐certainty evidence suggests voice‐enabling aids may have no effect on quality of life; there was no evidence to determine the effect of non‐vocal communication aids. Very low‐certainty evidence and inability to pool data due to conceptual differences and heterogeneity in measurement tools means it was difficult to discern the effect of communication aids on emotional and psychological distress.
Low‐certainty evidence suggests non‐vocal communication aids and voice‐enabling aids may not influence ICU length of stay. Insufficient and low‐quality evidence exists to determine the effect of communication aids on hospitalisation costs or adverse events such as respiratory decompensation or tracheostomy site bleeding.
Patient satisfaction with communication was the most commonly measured patient‐reported outcome with very low‐certainty evidence from four studies suggesting use of a communication board might increase patient satisfaction compared to usual care that does not routinely use communication aids. We found insufficient evidence to determine if voice‐enabling aids improve satisfaction with communication. Insufficient evidence also exists to determine if communication aids (non‐vocal or voice‐enabling) have any effect on communication ease, frequency, quality, success, or efficiency; or if certain communication aids have better usability.
Overall completeness and applicability of evidence
Heterogeneity of interventions, comparator groups, outcomes, and measures prevented pooling and meta‐analyses including all studies, all outcomes, or planned subgroup and sensitivity analyses. Results of meta‐analyses presented should be interpreted with caution due to primarily low or very low‐certainty evidence. Most included studies recruited small samples thereby limiting statistical power.
Importantly, no studies directly reported our primary outcome, that is, the ability to use a communication aid to communicate or a symptom, wish, or need or phonate an intelligible word. This means we are unable to make meaningful conclusions about the effectiveness of non‐vocal and voice‐enabling communication aids for establishing communication. These data are particularly important when considering which interventions to use in an ICU patient population as many patient‐related factors such as dexterity, muscle strength, and cognition may preclude use of non‐vocal communication aids in particular.
Only two studies evaluated a communication app. This is an important gap in the evidence given the increasing availability and number of apps to enable communication for critically ill people suddenly speechless due to an artificial airway. Only three studies evaluated a voice‐enabling communication aid, two of which introduced the same aid (one‐way speaking valve) earlier in the participant's clinical course than introduced as routine practice. This means measured outcomes pertained more to the timing of communication aid introduction than the effectiveness of the aid itself. Due to an absence of studies, we can draw no conclusions on the effectiveness of communication aids for people requiring an artificial airway in long‐term care or home settings.
Heterogeneity in the selection of patient‐reported outcomes and use of measurement tools with untested validity and reliability, and therefore unknown psychometric properties, contributes to uncertainty in the evidence related to patient perceived effects of communication aids. Only one study measured healthcare professional (nurse)‐reported communication outcomes, meaning we can draw no conclusions in terms of perceived effects. Given that communication is a partnership, this is another important evidence gap.
Conduct of research on the effect of communications is not without challenges. A patient's ability and need to communication can fluctuate considerable over a 24‐hour period due to changes in their sedation/conscious level, delirium presence, and recovery from their precipitating critical illness. This means no one communication aid will be sufficient to address all communication needs for all patients. Many patients even in the recovery phase experience cognitive and physical challenges to communication including fatigue. This presents challenges in terms of self‐reported patient outcomes. Furthermore, using an aid to communicate with a patient with an artificial airway is challenging and requires training, time for the communication encounter, and careful attention of the communication partner. Understanding of the effectiveness of individual communication aids may, therefore, be influenced by who is delivering the intervention, how it is delivered, and with what consistency. Future studies of communication aids should carefully report these details of intervention delivery as well as fidelity of delivery to the study protocol.
Quality of the evidence
We found the evidence to be at high risk of bias due to inadequate or unclear sequence generation and allocation concealment in several studies (selection bias), inability to blind participants and personnel in all studies and outcome assessors in most studies (performance bias and detection bias), and small sample sizes leading to imprecision. In terms of GRADE assessments, we rated the evidence for all outcomes for which there was evidence as low or very‐low certainty due to each individual study being rated at high risk of bias, imprecision of effect estimates, and evidence of inconsistency (i.e. high level of heterogeneity (I² > 60%) in pooled analyses.
Potential biases in the review process
We considered the potential for bias in our review process was low. We believe we have identified all relevant studies through use of a comprehensive search strategy informed by a senior information specialist; inclusion of studies in any language; review of trial databases, conference abstracts, and reference lists of relevant literature; and contact with experts.
We adhered to procedures outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and a priori described in our review protocol (Rose 2019). Two review authors independently identified studies for inclusion, extracted data, and assessed risk of bias. Additionally, we communicated with study authors whenever possible to clarify study methods. We made no assumptions about intensity of treatment that may have influenced findings, and made no decisions about analyses or investigation of heterogeneity after seeing the data.
Agreements and disagreements with other studies or reviews
Our review of communication aids for adults requiring artificial airways is the most comprehensive to‐date in terms of scope and rigorous in terms of review methods and evidence quality assessment. With the caveats of uncertainty and very low‐quality evidence, use of a non‐vocal communication aid likely has a positive influence on patient satisfaction. Early introduction of a voice‐enabling communication aid in tracheostomised patients might enable earlier return of ability to phonate intelligible words. Communication aids likely have no effect on quality of life or ICU length of stay. We are uncertain on the effect on emotional or psychological distress due to conceptual differences in measures used preventing pooling of data. Other reviews also identified challenges with evidence quality and certainty. Similar to our review, Carruthers 2017 concluded there was preliminary evidence suggesting communication aids are effective for improving patient satisfaction with communication and reducing communication difficulties. Other reviews with broad inclusion criteria in terms of the study designs including cohort, case series, and case reports suggest more general conclusions as to the effectiveness of communication aids such as improved ability to communicate or having utility, and being safe (Ten Hoorn 2016; Zaga 2019).
Authors' conclusions
Implications for practice.
Due to the absence of high‐quality studies, it is difficult to make recommendations for practice, particularly in terms of choice of communication aid. There is some evidence indicating provision of a communication board may improve patient satisfaction with communication compared to usual care without routine use of a communication aid. Early use of a voice‐enabling aid might improve the ability to phonate or reduce the time to phonate but has no effect on intensive care unit (ICU) length of stay. Effects on other outcomes are uncertain.
Implications for research.
Due to limited and uncertain evidence for understanding the effectiveness of non‐vocal and voice‐enabling communication aids, there is a need for high‐quality, adequately powered individual or cluster randomised controlled trials including participants from an ICU setting. Trials recruiting participants from long‐term or home settings are urgently needed. Due to increasing availability of communication apps, more trials are needed, particularly head‐to‐head comparisons of non‐vocal communication aids to understand which aids are most effective for establishing communication. However, before such trials are commenced, there is a need for consensus on a core outcome and measurement set. This set would include outcomes, measures, and measurement time points considered important to patients, their families, and clinical staff. Furthermore, development and rigorous psychometric evaluation of patient‐ and clinician‐reported measures that assess communication aid effectiveness is required. These may include a quality of life measure that is more sensitive to success with communication. Use of methods that objectively observe communication outcomes may also address the challenges of patient self‐report measures in critically ill people. Design of future trials will also need to consider confounding factors such as fluctuations in patient communication ability due to critical illness and intensive care treatments.
History
Protocol first published: Issue 7, 2019
Notes
The description of methods of this review was based on standard text and guidance provided by the Cochrane Consumers and Communication Group (CCCG 2013).
Acknowledgements
We thank the editors and staff of the Cochrane Consumers and Communication Group, particularly our contact editor Diane Lowe, for their input to this review. We thank Marina Robinson (consumer referee), Carla McClintock (consumer referee for the protocol; Rose 2019), Tanis Cameron, Amy Freeman‐Sanderson, Sue McGowan, and Sarah Wallace for their expert input to this review. We thank Becky Skidmore (Information Specialist Consultant) for her expertise and assistance in developing our search strategies. We thank Afifi Ghazale for assistance with translation of the study by Farahani 2012 from Fahsi into English.
Appendices
Appendix 1. Glossary of terms
Alternative and augmentative communication aids: basic and high‐tech aids that facilitate communication. These aids do not include aids or techniques that restore patient voice, that is, non‐vocal communication aids.
Articulators: lips, tongue, and jaw.
Artificial airway: tubes to assist breathing required to deliver breathing support from a machine.
Bradycardia: slow heart rate.
Bulbar function: function of the nerves that control swallowing.
Catheter: tube placed in the bladder or other locations of the body.
Chronic respiratory failure: inability to breathe adequately for an extended period and without recovery of lung function.
Cognition: ability to think.
Communication partner: family member, friend, carer, or healthcare professional with whom a patient may interact.
Cuffless tracheostomy tubes: a tracheostomy tube without a balloon that separates the airways from mouth/nose/voice box.
Digital occlusion: covering of the opening of the tracheostomy tube with a gloved finger to divert airflow to the vocal cords.
Digitised speech devices: devices that use recorded human speech.
Electrolarynx: a device that generates sound (not voice) via transmission of vibrations through soft tissue under the jaw or on the cheek, which is recognisable as speech with movement of the lips, tongue, and jaw.
Endotracheal intubation: a breathing tube inserted through the mouth or nose into the trachea.
Expiration: breathing out.
Eye‐gaze technology: users focus their eye gaze on words or phrases which a computer system generates into speech.
Fenestrated tracheostomy: tube with an additional opening on the shaft of the tube that directs gas towards the vocal cords.
Hyper/hypotension: high or low blood pressure.
Inflatable cuff: balloon towards the base of an endotracheal or tracheostomy tube that inflates into the trachea separating the airways from mouth/nose/voice box.
Inner cannula: additional tube placed within the tracheostomy tube which is more commonly used for enabling cleaning of the tracheal lumen to prevent mucous build up
Inspiration: breathing in.
Invasive ventilation: breathing support from a machine via an artificial airway.
Larynx: voice box that contains the vocal cords.
Mechanical ventilation: breathing support from a machine. Breathing support from a machine can be provided via an artificial airway and is referred to as invasive mechanical ventilation. Alternatively, breathing support from a machine can be provided via a mask and is referred to as non‐invasive ventilation.
Nasogastric tube: tube placed in the nose that runs all the way to the stomach.
Non‐invasive ventilation: breathing support from a machine provided via a mask.
Non‐vocal communication aids: communication aids that do not restore the patient's own voice.
Phonation: production of speech.
Pneumonia: infection of the lungs.
Pneumonitis: inflammation of the lungs.
Synthesised speech devices: devices that use computer‐generated speech.
Tachycardia: fast heart rate.
Trachea: windpipe.
Tracheostomy: a tube inserted into the trachea through a surgical opening in the neck.
Treatment interference: patient removal of the endotracheal tube, intravenous lines, nasogastric tubes (tube placed in the stomach), or catheters (tube placed in the bladder or other locations of the body).
Upper airway: the nose, nasal cavity, mouth, throat, and the part of the trachea above the voice box.
Voice‐enabling communication aids: communication aids that restore the patient's own voice.
Voice output communication aid: electronic speech‐generating device.
Weaning: the process that establishes breathing that is not supported by a breathing machine.
Appendix 2. MEDLINE search strategy
1 exp Respiration, Artificial/
2 exp Ventilators, Mechanical/
3 ((artificial* or mechanical*) adj3 (respirat* or ventilat*)).tw,kf.
4 artificial airway?.tw,kf.
5 (high‐frequency adj3 ventilat*).tw,kf.
6 ((assist* or support* or wean*) adj3 (respirat* or ventilat*)).tw,kf.
7 ((liquid or fluorocarbon or fluoro‐carbon) adj3 ventilat*).tw,kf.
8 (invasive* adj3 ventilat*).tw,kf.
9 controlled ventilation.tw,kf.
10 (airway pressure release adj3 ventilat*).tw,kf.
11 APRV.tw,kf.
12 IPPB.tw,kf.
13 Airway Extubation/
14 exp Intubation, Intratracheal/
15 (intubat* or extubat* or detubat*).tw,kf.
16 Tracheostomy/
17 tracheo?tom*.tw,kf.
18 (endotrachea* adj3 (tube? or tubat* or ventilat*)).tw,kf.
19 Ventilator Weaning/
20 (ventilat* adj3 (wean* or liberat*)).tw,kf.
21 (cuff? adj3 deflat*).tw,kf.
22 (cuff? adj3 inflat*).tw,kf.
23 or/1‐22 [INVASIVE MECHANICAL VENTILATION/TRACHEOSTOMY]
24 Communication/
25 exp Communication Barriers/
26 Communication Disorders/
27 exp Nonverbal Communication/
28 communicat*.tw,kf.
29 Phonation/
30 phonat*.tw,kf.
31 (utter* adj3 vocal*).tw,kf.
32 exp Speech/
33 exp Voice/
34 Communication Aids for Disabled/
35 ((nonvocal* or non‐vocal* or speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 (aid or aids or app or apps or application? or board? or device? or equipment or software or technolog* or tool?)).tw,kf.
36 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 (augment* or enabl* or empower* or emulat* or establish* or facilitat* or generat* or produce* or producing or production* or promot* or restor* or simulat*)).tw,kf.
37 (artificial larynx* or electrolarynx* or electro‐larynx*).tw,kf.
38 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 artificial*).tw,kf.
39 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 button?).tw,kf.
40 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 computer*).tw,kf.
41 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 (digital* or digiti*)).tw,kf.
42 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 electronic*).tw,kf.
43 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 processor?).tw,kf.
44 ((speech or speak* or talk* or tracheo?esophageal or tracheo‐esophageal or tracheo‐oesophageal or verbal* or voice? or vocal* or vox) adj3 prosthes#s).tw,kf.
45 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 synthesi*).tw,kf.
46 ((fenestrat* or speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 tracheo?tom*).tw,kf.
47 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 leak*).tw,kf.
48 (speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 tube?).tw,kf.
49 ((speech or speak* or talk* or tracheo?esophageal or tracheo‐esophageal or tracheo‐oesophageal or verbal* or voice? or vocal* or vox) adj3 valve?).tw,kf.
50 ((esophageal or oesophageal or tracheo?esophageal or tracheo‐esophageal or tracheo‐oesophageal) adj3 (speech or speak* or talk* or verbal* or voice? or vocal* or vox)).tw,kf.
51 Blom‐Singer$2.tw,kf.
52 Passy Muir$2.tw,kf.
53 (Montgomery$2 adj3 valve?).tw,kf.
54 Phonax$2.tw,kf.
55 Provox$2.tw,kf.
56 (VOCA or VOCAs).tw,kf.
57 VoiceMaster$2.tw,kf.
58 Blom$2 Tracheostomy.tw,kf.
59 (CommuniTrach$2 or Communi‐Trach$2).tw,kf.
60 (Portex$2 BLUSA$2 or Portex$2 Talk$2 or Trachtalk$2 or Trach Talk$2).tw,kf.
61 (Bivona$2 adj3 (Aire‐Cuf$2 or Fome‐Cuf$2)).tw,kf.
62 LifeVoice$2.tw,kf.
63 ((eye or eyes) adj2 (gaze? or gazing) adj3 (aid? or app or apps or application* or board? or device? or digital* or software or technolog* or tool?)).tw,kf.
64 (gaze‐control* adj3 (aid? or app or apps or application* or board? or computer* or device? or digital* or equipment or software or technolog* or tool?)).tw,kf.
65 ((gaze? or gazing or scan* or track*) adj3 (text? or symbol?)).tw,kf.
66 ((gaze? or gazing) adj3 dwell*).tw,kf.
67 AAC.tw,kf. [Augmentative and Alternative Communication]
68 ((alphabet* or icon? or letter? or phrase? or picture? or sentence? or symbol? or word? or writ*) adj3 (aid or aids or app or apps or application? or board? or card or cards or computer* or device? or equipment or guide or guides or software or technol* or tool?)).tw,kf.
69 ((alphabet* or icon? or letter? or phrase? or picture? or sentence? or symbol? or word? or writ*) adj3 magnet*).tw,kf.
70 ((keyboard? or key board?) adj3 (emulat* or simulat*)).tw,kf.
71 (pen or pens or paper or papers or paper‐based).tw,kf.
72 pictogra*.tw,kf.
73 or/24‐72 [COMMUNICATION/BARRIERS/DEVICES]
74 23 and 73 [COMMUNICATION/BARRIERS/DEVICES ‐ INVASIVE MECHANICAL VENTILATION/TRACHEOSTOMY]
75 exp Child/ not (exp Adult/ or Adolescent/)
76 exp Infant/ not (exp Adult/ or Adolescent/)
77 40 not (41 or 42) [CHILD‐/INFANT‐ONLY REMOVED]
78 (controlled clinical trial or randomized controlled trial).pt.
79 clinical trials as topic.sh.
80 exp Randomized Controlled Trials as Topic/
81 (randomi#ed or randomi#ation? or randomly or RCT? or placebo*).tw,kf.
82 ((singl* or doubl* or trebl* or tripl*) adj (mask* or blind* or dumm*)).tw,kf.
83 trial.ti.
84 or/78‐83 [RCTS]
85 controlled clinical trial.pt.
86 Controlled Clinical Trial/ or Controlled Clinical Trials as Topic/
87 (control* adj2 trial*).tw,kf.
88 Non‐Randomized Controlled Trials as Topic/
89 (nonrandom* or non‐random* or quasi‐random* or quasi‐experiment*).tw,kf.
90 (nRCT or nRCTs or non‐RCT?).tw,kf.
91 Controlled Before‐After Studies/
92 (control* adj3 ("before and after" or "before after")).tw,kf.
93 Interrupted Time Series Analysis/
94 time series.tw,kf.
95 or/85‐94 [QUASI‐RANDOMIZED, CBA]
96 77 and 84 [RCTS]
97 77 and 95 [QUASI‐RANDOMIZED, CBA]
98 96 or 97 [RCTS, QUASI‐RANDOMIZED, CBA]
99 exp Animals/ not (exp Animals/ and Humans/)
100 98 not 99 [ANIMAL‐ONLY REMOVED]
101 (comment or editorial or news or newspaper article).pt.
102 (letter not (controlled clinical trial or randomized controlled trial)).pt.
103 100 not (101 or 102) [OPINION PIECES REMOVED]
Appendix 3. Other Database Search Strategies
The Cochrane Library search
1 [mh "Respiration, Artificial"]
2 [mh "Ventilators, Mechanical"]
3 ((artificial* or mechanical*) NEAR/3 (respirat* or ventilat*)):ti,ab,kw
4 artificial NEXT airway*:ti,ab,kw
5 "high‐frequency" NEAR/3 ventilat*:ti,ab,kw
6 ((assist* or depend* or support* or wean*) NEAR/3 (respirat* or ventilat*)):ti,ab,kw
7 ((liquid or fluorocarbon or "fluoro‐carbon") NEAR/3 ventilat*):ti,ab,kw
8 ((invasive* or noninvasiv* or (non NEXT invasiv*)) NEAR/3 ventilat*):ti,ab,kw
9 "controlled ventilation":ti,ab,kw
10 "airway pressure release" NEAR/3 ventilat*:ti,ab,kw
11 APRV:ti,ab,kw
12 IPPB:ti,ab,kw
13 [mh "Airway Extubation"]
14 [mh "Intubation, Intratracheal"]
15 (intubat* or extubat* or detubat*):ti,ab,kw
16 [mh Tracheostomy]
17 (tracheotom* or tracheostom*):ti,ab,kw
18 ((endotrachea* or (endo NEXT trachea*)) NEAR/3 (tube or tubes or tubat* or ventilat*)):ti,ab,kw
19 [mh "Ventilator Weaning"]
20 ventilat* NEAR/3 (wean* or liberat*):ti,ab,kw
21 ((cuff or cuffs) NEAR/3 deflat*):ti,ab,kw
22 ((cuff or cuffs) NEAR/3 inflat*):ti,ab,kw
23 {or #1‐#22}
24 [mh ^Communication]
25 [mh "Communication Barriers"]
26 [mh ^"Communication Disorders"]
27 [mh "Nonverbal Communication"]
28 communicat*:ti,ab,kw
29 [mh Phonation]
30 phonat*:ti,ab,kw
31 (utter* NEAR/3 vocal*):ti,ab,kw
32 [mh Speech]
33 [mh Voice]
34 [mh "Communication Aids for Disabled"]
35 ((nonvocal* or (non NEXT vocal*) or speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 (aid or aids or app or apps or application* or board or boards or device or devices or equipment or software or technolog* or tool or tools)):ti,ab,kw
36 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 (augment* or enabl* or empower* or emulat* or establish* or facilitat* or generat* or produce* or producing or production* or promot* or restor* or simulat*)):ti,ab,kw
37 ((artificial NEXT larynx*) or electrolarynx* or (electro NEXT larynx*)):ti,ab,kw
38 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 artificial*):ti,ab,kw
39 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 button*):ti,ab,kw
40 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 computer*):ti,ab,kw
41 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 (digital* or digiti*)):ti,ab,kw
42 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 electronic*):ti,ab,kw
43 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 processor*):ti,ab,kw
44 ((speech or speak* or talk* or tracheoesophageal or tracheooesophageal or tracheo‐esophageal or tracheo‐oesophageal or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 prosthes*):ti,ab,kw
45 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 synthesi*):ti,ab,kw
46 ((fenestrat* or speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 (tracheotom* or tracheostom*)):ti,ab,kw
47 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 leak*):ti,ab,kw
48 ((speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 (tube or tubes)):ti,ab,kw
49 ((speech or speak* or talk* or tracheoesophageal or tracheooesophageal or tracheo‐esophageal or tracheo‐oesophageal or verbal* or voice or voices or voiceless or vocal* or vox) NEAR/3 (valve or valves)):ti,ab,kw
50 ((esophageal or oesophageal or tracheoesophageal or tracheooesophageal or tracheo‐esophageal or tracheo‐oesophageal) NEAR/3 (speech or speak* or talk* or verbal* or voice or voices or voiceless or vocal* or vox)):ti,ab,kw
51 ("Blom‐Singer" or "Passy Muir" or (Montgomery NEAR/3 valve*) or Phonax or Provox or VOCA or VOCAs or VoiceMaster or "Blom Tracheostomy" or CommuniTrach or "Communi‐Trach" or "Portex BLUSA" or "Portex Talk" or Trachtalk or "Trach Talk" or (Bivona NEAR/3 ("Aire‐Cuf" or "Fome‐Cuf")) or LifeVoice):ti,ab,kw
52 ((eye or eyes) NEAR/2 (gaze or gazed or gazes or gazing or scan* or track*) NEAR/3 (aid or aids or app or apps or application* or board or boards or computer* or device or devices or digital* or equipment or software or technolog* or tool or tools)):ti,ab,kw
53 ((gaze NEXT control*) NEAR/3 (aid or aids or app or apps or application* or board or boards or computer* or device or devices or digital* or equipment or software or technolog* or tool or tools)):ti,ab,kw
54 ((gaze or gazed or gazes or gazing or scan* or track*) NEAR/3 (text or texts or symbol or symbols)):ti,ab,kw
55 ((gaze or gazed or gazes or gazing) NEAR/3 dwell*):ti,ab,kw
56 AAC:ti,ab,kw
57 ((alphabet* or icon or icons or letter or letters or phrase or phrases or picture or pictures or sentence or sentences or symbol or symbols or word or words or writ*) NEAR/3 (aid or aids or app or apps or application* or board or boards or card or cards or computer* or device or devices or equipment or guide or guides or software or technol* or tool or tools)):ti,ab,kw
58 ((alphabet* or icon or icons or letter or letters or phrase or phrases or picture or pictures or sentence or sentences or symbol or symbols or word or words or writ*) NEAR/3 magnet*):ti,ab,kw
59 ((keyboard* or (key NEXT board*)) NEAR/3 (emulat* or simulat*)):ti,ab,kw
60 (pen or pens or paper or papers or "paper‐based"):ti,ab,kw
61 pictogra*:ti,ab,kw
62 {or #24‐#61}
63 #23 AND #62
64 [mh Child] not ([mh Adult] or [mh Adolescent])
65 [mh Infant] not ([mh Adult] or [mh Adolescent])
66 #63 NOT (#64 or #65)
Embase search
1 exp artificial ventilation/
2 mechanical ventilator/
3 ((artificial* or mechanical*) adj3 (respirat* or ventilat*)).tw,kw.
4 artificial airway?.tw,kw.
5 (high‐frequency adj3 ventilat*).tw,kw.
6 ((assist* or depend* or support* or wean*) adj3 (respirat* or ventilat*)).tw,kw.
7 ((liquid or fluorocarbon or fluoro‐carbon) adj3 ventilat*).tw,kw.
8 ((invasive* or noninvasiv* or non‐invasiv*) adj3 ventilat*).tw,kw.
9 controlled ventilation.tw,kw.
10 (airway pressure release adj3 ventilat*).tw,kw.
11 APRV.tw,kw.
12 IPPB.tw,kw.
13 extubation/
14 endotracheal intubation/
15 (intubat* or extubat* or detubat*).tw,kw.
16 tracheostomy/
17 tracheo?tom*.tw,kw.
18 ((endotrachea* or endo‐trachea*) adj3 (tube? or tubat* or ventilat*)).tw,kw.
19 ventilator weaning/
20 (ventilat* adj3 (wean* or liberat*)).tw,kw.
21 pneumatic cuff/
22 (cuff? adj3 deflat*).tw,kw.
23 (cuff? adj3 inflat*).tw,kw
24 or/105‐127 [INVASIVE MECHANICAL VENTILATION/TRACHEOSTOMY]
25 interpersonal communication/
26 communication barrier/
27 communication disorder/
28 exp nonverbal communication/
29 exp verbal communication/
30 communicat*.tw,kw
31 phonat*.tw,kw.
32 (utter* adj3 vocal*).tw,kw.
33 exp speech/
34 voice/
35 communication aid/
36 ((nonvocal* or non‐vocal* or speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 (aid or aids or app or apps or application? or board? or device? or equipment or software or technolog* or tool?)).tw,kw.
37 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 (augment* or enabl* or empower* or emulat* or establish* or facilitat* or generat* or produce* or producing or production* or promot* or restor* or simulat*)).tw,kw.
38 larynx prosthesis/
39 (artificial larynx* or electrolarynx* or electro‐larynx*).tw,kw.
40 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 artificial*).tw,kw.
41 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 button?).tw,kw.
42 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 computer*).tw,kw.
43 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 (digital* or digiti*)).tw,kw.
44 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 electronic*).tw,kw.
45 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 processor?).tw,kw.
46 ((speech or speak* or talk* or tracheo?esophageal or tracheo‐esophageal or tracheo‐oesophageal or verbal* or voice? or vocal* or vox) adj3 prosthes#s).tw,kw.
47 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 synthesi*).tw,kw.
48 ((fenestrat* or speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 tracheo?tom*).tw,kw.
49 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 leak*).tw,kw.
50 ((speech or speak* or talk* or verbal* or voice? or vocal* or vox) adj3 tube?).tw,kw.
51 ((speech or speak* or talk* or tracheo?esophageal or tracheo‐esophageal or tracheo‐oesophageal or verbal* or voice? or vocal* or vox) adj3 valve?).tw,kw.
52 ((esophageal or oesophageal or tracheo?esophageal or tracheo‐esophageal or tracheo‐oesophageal) adj3 (speech or speak* or talk* or verbal* or voice? or vocal* or vox)).tw,kw.
53 Blom‐Singer$2.dv,my,tw,kw.
54 Passy Muir$2.dv,my,tw,kw.
55 (Montgomery$2 adj3 valve?).dv,my,tw,kw.
56 Phonax$2.dv,my,tw,kw.
56 Provox$2.dv,my,tw,kw.
57 (VOCA or VOCAs).dv,my,tw,kw.
58 VoiceMaster$2.dv,my,tw,kw.
59 Blom$2 Tracheostomy.dv,my,tw,kw.
60 (CommuniTrach$2 or Communi‐Trach$2).dv,my,tw,kw.
61 (Portex$2 BLUSA$2 or Portex$2 Talk$2 or Trachtalk$2 or Trach Talk$2).dv,my,tw,kw.
62 (Bivona$2 adj3 (Aire‐Cuf$2 or Fome‐Cuf$2)).dv,my,tw,kw.
63 LifeVoice$2.dv,my,tw,kw.
64 ((eye or eyes) adj2 (gaze? or gazing or scan* or track*) adj3 (aid? or app or apps or application* or board? or computer* or device? or digital* or equipment or software or technolog* or tool?)).tw,kw.
65 (gaze‐control* adj3 (aid? or app or apps or application* or board? or computer* or device? or digital* or equipment or software or technolog* or tool?)).tw,kw.
66 ((gaze? or gazing or scan* or track*) adj3 (text? or symbol?)).tw,kw.
67 ((gaze? or gazing) adj3 dwell*).tw,kw.
68 AAC.tw,kw. [Augmentative and Alternative Communication]
69 ((alphabet* or icon? or letter? or phrase? or picture? or sentence? or symbol? or word? or writ*) adj3 (aid or aids or app or apps or application? or board? or card or cards or computer* or device? or equipment or guide or guides or software or technol* or tool?)).tw,kw.
70 ((alphabet* or icon? or letter? or phrase? or picture? or sentence? or symbol? or word? or writ*) adj3 magnet*).tw,kw.
71 ((keyboard? or key board?) adj3 (emulat* or simulat*)).tw,kw.
72 (pen or pens or paper or papers or paper‐based).tw,kw.
73 pictogra*.tw,kw.
74 or/129‐178 [COMMUNICATION/BARRIERS/DEVICES]
75 128 and 179 [COMMUNICATION/BARRIERS/DEVICES ‐ INVASIVE MECHANICAL VENTILATION/TRACHEOSTOMY]
76 fetus/ not (adolescent/ or exp adult/)
77 exp child/ not (adolescent/ or exp adult/)
78 180 not (181 or 182) [CHILD‐/INFANT‐ONLY REMOVED]
79 exp randomized controlled trial/ or controlled clinical trial/
80 clinical trial/
81 exp "controlled clinical trial (topic)"/
82 (randomi#ed or randomi#ation? or randomly or RCT or placebo*).tw,kw. (2393454)
83 ((singl* or doubl* or trebl* or tripl*) adj (mask* or blind* or dumm*)).tw,kw. (419669)
84 trial.ti.
85 or/184‐189 [RCTS]
86 controlled clinical trial/
87 "controlled clinical trial (topic)"/
88 (control* adj2 trial*).tw,kw.
89 (nonrandom* or non‐random* or quasi‐random* or quasi‐experiment*).tw,kw.
90 (nRCT or nRCT or non‐RCT).tw,kw.
91 (control* adj3 ("before and after" or "before after")).tw,kw.
92 time series analysis/ (
93 time series.tw,kw. (67607)
94 or/191‐198 [QUASI‐RANDOMIZED, CBA, ITS] (1324883)
95 183 and 190 [RCTS] (1899)
96 183 and 199 [QUASI‐RANDOMIZED, CBA, ITS] (868)
97 200 or 201 [RCTS, QUASI‐RANDOMIZED, CBA, ITS] (1995)
98 exp animal/ or exp animal experimentation/ or exp animal model/ or exp animal experiment/ or nonhuman/ or exp vertebrate/ (53128961)
99 exp human/ or exp human experimentation/ or exp human experiment/ (41104036)
100 202 not 203 (118)
101 202 not 205 [ANIMAL‐ONLY REMOVED] (1877)
102 editorial.pt. (1196588)
103 letter.pt. not ((controlled clinical trial/ or exp randomized controlled trial/) and letter.pt.)
104 206 not (207 or 208) [OPINION PIECES REMOVED]
CINAHL search
1 TI ( (artificial* or mechanical*) N3 (respirat* or ventilat*) ) OR AB ( (artificial* or mechanical*) N3 (respirat* or ventilat*) )
2 TI artificial W0 airway* OR AB artificial W0 airway*
3 TI "high‐frequency" N3 ventilat* OR AB "high‐frequency" N3 ventilat*
4 TI ( (assist* or depend* or support* or wean*) N3 (respirat* or ventilat*) ) OR AB ( (assist* or depend* or support* or wean*) N3 (respirat* or ventilat*) )
5 TI ( (liquid or fluorocarbon or "fluoro‐carbon") N3 ventilat* ) OR AB ( (liquid or fluorocarbon or "fluoro‐carbon") N3 ventilat* )
6 TI ( (invasive* or noninvasiv* or non‐invasiv*) N3 ventilat* ) ) OR AB ( (invasive* or noninvasiv* or non‐invasiv*) N3 ventilat* ) )
7 TI "controlled ventilation" OR AB "controlled ventilation"
8 TI "airway pressure release" N3 ventilat* OR AB "airway pressure release" N3 ventilat*
9 TI ( APRV or IPPB or intubat* or extubat* or detubat* or tracheotom* or tracheostom* ) OR AB ( APRV or IPPB or intubat* or extubat* or detubat* or tracheotom* or tracheostom* )
10 TI ( (endotrachea* or endo‐trachea*) N3 (tube or tubes or tubat* or ventilat*) ) OR AB ( (endotrachea* or endo‐trachea*) N3 (tube or tubes or tubat* or ventilat*) )
11 TI ( ventilat* N3 (wean* or liberat*) ) OR AB ( ventilat* N3 (wean* or liberat*) )
12 TI ( (cuff or cuffs) N3 (deflat* or inflat*) ) OR AB ( (cuff or cuffs) N3 (deflat* or inflat*) )
13 (MH "Respiration, Artificial+")
14 (MH "Ventilators, Mechanical")
15 (MH "Ventilator Patients")
16 (MH "Extubation")
17 (MH "Tracheostomy")
18 (MH "Tracheostomy Tube") OR (MH "T‐Piece")
19 (MH "Ventilator Weaning")
20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
21 (MH "Communication")
22 (MH "Communication Barriers")
23 (MH "Communicative Disorders") OR (MH "Voice Disorders")
24 (MH "Nonverbal Communication+")
25 TI communicat* OR AB communicat*
26 (MH "Phonation+")
27 TI phonat* OR AB phonat*
28 TI utter* N3 vocal* OR AB utter* N3 vocal*
29 (MH "Speech+")
30 MH "Voice+")
31 (MH "Communication Aids for Disabled")
32 (MH "Tracheostomy and Ventilator Swallowing and Speaking Valve")
33 TI ( (nonvocal* or (non W0 vocal*) or speech or speak* or talk* or verbal* or voice* or vocal* or vox) N3 (aid or aids or app or apps or application* or board or boards or device or devices or equipment or software or technolog* or tool or tools) ) OR AB ( (nonvocal* or (non W0 vocal*) or speech or speak* or talk* or verbal* or voice* or vocal* or vox) N3 (aid or aids or app or apps or application* or board or boards or device or devices or equipment or software or technolog* or tool or tools) )
34 TI ( (speech or speak* or talk* or verbal* or voice* or vocal* or vox) N3 (augment* or enabl* or empower* or emulat* or establish* or facilitat* or generat* or produce* or producing or production* or promot* or restor* or simulat*) ) OR AB ( (speech or speak* or talk* or verbal* or voice* or vocal* or vox) N3 (augment* or enabl* or empower* or emulat* or establish* or facilitat* or generat* or produce* or producing or production* or promot* or restor* or simulat*) )
35 TI ( (artificial W0 larynx*) or electrolarynx* or (electro W0 larynx*) ) OR AB ( (artificial W0 larynx*) or electrolarynx* or (electro W0 larynx*) )
36 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 artificial* ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 artificial* )
37 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 button# ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 button# )
38 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 computer* ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 computer* )
39 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 (digital* or digiti*) ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 (digital* or digiti*) )
40 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 electronic* ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 electronic* )
41 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 processor# ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 processor# )
42 TI ( (speech or speak* or talk* or tracheo#esophageal or "tracheo‐esophageal" or "tracheo‐oesophageal" or verbal* or voice# or vocal* or vox) N3 prosthes?s ) OR AB ( (speech or speak* or talk* or tracheo#esophageal or "tracheo‐esophageal" or "tracheo‐oesophageal" or verbal* or voice# or vocal* or vox) N3 prosthes?s )
43 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 synthesi* ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 synthesi* )
44 TI ( (fenestrat* or speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 tracheo#tom* ) OR AB ( (fenestrat* or speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 tracheo#tom* )
45 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 leak* ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 leak* )
46 TI ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 tube# ) OR AB ( (speech or speak* or talk* or verbal* or voice# or vocal* or vox) N3 tube# )
47 TI ( (speech or speak* or talk* or tracheo#esophageal or "tracheo‐esophageal" or "tracheo‐oesophageal" or verbal* or voice# or vocal* or vox) N3 valve# ) OR AB ( (speech or speak* or talk* or tracheo#esophageal or "tracheo‐esophageal" or "tracheo‐oesophageal" or verbal* or voice# or vocal* or vox) N3 valve# )
48 TI ( (esophageal or oesophageal or tracheo#esophageal or "tracheo‐esophageal" or "tracheo‐oesophageal") N3 (speech or speak* or talk* or verbal* or voice# or vocal* or vox) ) OR AB ( (esophageal or oesophageal or tracheo#esophageal or "tracheo‐esophageal" or "tracheo‐oesophageal") N3 (speech or speak* or talk* or verbal* or voice# or vocal* or vox) )
49 TI ( "Blom‐Singer" or "Passy Muir" or (Montgomery* N3 valve*) or Phonax or Provox or VOCA or VOCAs or VoiceMaster* or "Blom Tracheostomy" or CommuniTrach or "Communi‐Trach" or "Portex BLUSA" or "Portex Talk" or Trachtalk or "Trach Talk" or (Bivona N3 ("Aire‐Cuf" or "Fome‐Cuf")) or LifeVoice ) OR AB ( "Blom‐Singer" or "Passy Muir" or (Montgomery* N3 valve*) or Phonax or Provox or VOCA or VOCAs or VoiceMaster* or "Blom Tracheostomy" or CommuniTrach or "Communi‐Trach" or "Portex BLUSA" or "Portex Talk" or Trachtalk or "Trach Talk" or (Bivona N3 ("Aire‐Cuf" or "Fome‐Cuf")) or LifeVoice )
50 TI ( (eye or eyes) N2 (gaze or gazed or gazes or gazing or scan* or track*) N3 (aid or aids or app or apps or application* or board or boards or computer* or device or devices or digital* or equipment or software or technolog* or tool or tools) ) OR AB ( (eye or eyes) N2 (gaze or gazed or gazes or gazing or scan* or track*) N3 (aid or aids or app or apps or application* or board or boards or computer* or device or devices or digital* or equipment or software or technolog* or tool or tools) )
51 TI ( (gaze W0 control*) N3 (aid or aids or app or apps or application* or board# or computer* or device# or digital* or equipment or software or technolog* or tool#) ) OR AB ( (gaze W0 control*) N3 (aid or aids or app or apps or application* or board# or computer* or device# or digital* or equipment or software or technolog* or tool#) )
52 TI ( (gaze# or gazing or scan* or track*) N3 (text# or symbol#) ) OR AB ( (gaze# or gazing or scan* or track*) N3 (text# or symbol#) )
53 TI ( (gaze# or gazing) N3 dwell* ) OR AB ( (gaze# or gazing) N3 dwell* )
54 TI AAC OR AB AAC
55 TI ( (alphabet* or icon# or letter# or phrase# or picture# or sentence# or symbol# or word# or writ*) N3 (aid or aids or app or apps or application# or board# or card or cards or computer* or device# or equipment or guide or guides or software or technol* or tool#) ) OR AB ( (alphabet* or icon# or letter# or phrase# or picture# or sentence# or symbol# or word# or writ*) N3 (aid or aids or app or apps or application# or board# or card or cards or computer* or device# or equipment or guide or guides or software or technol* or tool#) )
56 TI ( (alphabet* or icon# or letter# or phrase# or picture# or sentence# or symbol# or word# or writ*) N3 magnet* ) OR AB ( (alphabet* or icon# or letter# or phrase# or picture# or sentence# or symbol# or word# or writ*) N3 magnet* )
57 TI ( (keyboard# or (key W0 board#)) N3 (emulat* or simulat*) ) OR AB ( (keyboard# or (key W0 board#)) N3 (emulat* or simulat*) )
58 TI ( pen or pens or paper or papers or "paper‐based" ) OR AB ( pen or pens or paper or papers or "paper‐based" )
59 TI pictogra* OR AB pictogra*
60 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59
61 S20 AND S60
62 MH "Randomized Controlled Trials+")
63 TI ( randomi?ed or randomi?ation# or randomly or RCT or placebo* ) OR AB ( randomi?ed or randomi?ation# or randomly or RCT or placebo* )
64 TI ( (singl* or doubl* or trebl* or tripl*) W0 (mask* or blind* or dumm*) ) OR AB ( (singl* or doubl* or trebl* or tripl*) W0 (mask* or blind* or dumm*) )
65 (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies")
66 TI trial
67 S62 OR S63 OR S64 OR S65 OR S66
68 (MH "Clinical Trials")
69 TI control* N2 trial# OR AB control* N2 trial#
70 TI ( nonrandom* or (non W0 random*) or (quasi W0 random*) or (quasi W0 experiment*) ) OR AB ( nonrandom* or (non W0 random*) or (quasi W0 random*) or (quasi W0 experiment*) )
71 TI ( nRCT or nRCT or "non‐RCT" ) OR AB ( nRCT or nRCT or "non‐RCT" )
72 MH "Nonrandomized Trials")
73 (MH "Controlled Before‐After Studies")
74 TI ( control* N3 ("before and after" or "before after") ) OR AB ( control* N3 ("before and after" or "before after") )
75 MH "Interrupted Time Series Analysis")
76 TI "time series" OR AB "time series"
77 S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76
78 S61 AND S67
79 S61 AND S77
80 S78 OR S79
Web of Science search
1 ((artificial* or mechanical*) NEAR/3 (respirat* or ventilat*) )
2 (artificial NEAR/0 airway*)
3 ("high‐frequency" NEAR/3 ventilat*)
4 ((assist* or depend* or support* or wean*) NEAR/3 (respirat* or ventilat*) )
5 ((liquid or fluorocarbon or "fluoro‐carbon") NEAR/3 ventilat*)
6 ((invasive* or noninvasiv* or non‐invasiv*) NEAR/3 ventilat*)
7 ("controlled ventilation")
8 ("airway pressure release" NEAR/3 ventilat*)
9 (APRV or IPPB or intubat* or extubat* or detubat* or tracheotom* or tracheostom*)
10 ((endotrachea* or endo‐trachea*) NEAR/3 (tube or tubes or tubat* or ventilat*) )
11 (ventilat* NEAR/3 (wean* or liberat*) )
12 ((cuff or cuffs) NEAR/3 (deflat* or inflat*) )
13 #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
14 (communicat* or phonat* or (utter* adj3 vocal*) )
15 ((nonvocal* or (non NEAR/0 vocal*) or speech or speak* or talk* or verbal* or voice* or vocal* or vox) NEAR/3 (aid or aids or app or apps or application* or board or boards or device or devices or equipment or software or technolog* or tool or tools) )
16 ((speech or speak* or talk* or verbal* or voice* or vocal* or vox) NEAR/3 (augment* or enabl* or empower* or emulat* or establish* or facilitat* or generat* or produce* or producing or production* or promot* or restor* or simulat*) )
17 (artificial larynx* or electrolarynx* or (electro NEAR/0 larynx*) )
18 ((speech or speak* or talk* or verbal* or voice* or vocal* or vox) NEAR/3 (artificial* or button or buttons or computer* or digital* or digiti* or electronic* or processor*) )
19 ((speech or speak* or talk* or tracheoesophageal or tracheooesophageal or tracheo‐esophageal or tracheo‐oesophageal or verbal* or voice* or vocal* or vox) NEAR/3 prosthes*)
20 ((speech or speak* or talk* or verbal* or voice* or vocal* or vox or fenestrat* ) NEAR/3 (synthesi* or tracheotom* or tracheostom* or leak* or tube or tubes) )
21 ((speech or speak* or talk* or tracheoesophageal or tracheooesophageal or tracheo‐esophageal or tracheo‐oesophageal or verbal* or voice* or vocal* or vox) NEAR/3 (valve or valves) )
22 ((esophageal or oesophageal or tracheoesophageal or tracheooesophageal or tracheo‐esophageal or tracheo‐oesophageal) NEAR/3 (speech or speak* or talk* or verbal* or voice* or vocal* or vox) )
23 ("Blom‐Singer" or "Passy Muir" or (Montgomery* NEAR/3 valve*) or Phonax or Provox or VOCA or VOCAs or VoiceMaster* or "Blom Tracheostomy" or CommuniTrach or "Communi‐Trach" or "Portex BLUSA" or "Portex Talk" or Trachtalk or "Trach Talk" or (Bivona NEAR/3 ("Aire‐Cuf" or "Fome‐Cuf") ) or LifeVoice)
24 ((eye or eyes) NEAR/2 (gaze or gazed or gazes or gazing or scan* or track*) NEAR/3 (aid or aids or app or apps or application* or board or boards or computer* or device or devices or digital* or equipment or software or technolog* or tool or tools) )
25 ((gaze NEAR/0 control*) NEAR/3 (aid or aids or app or apps or application* or board or boards or computer* or device or devices or digital* or equipment or software or technolog* or tool or tools) )
26 ((gaze or gazed or gazes or gazing or scan* or track*) NEAR/3 (text or texts or symbol or symbols or dwell*) )
27 ((alphabet* or icon or icons or letter* or phrase* or picture* or sentence* or symbol or symbols or word or words or writ*) NEAR/3 (aid or aids or app or apps or application* or board or boards or card or cards or computer* or device or devices or equipment or guide or guides or software or technol* or tool or tools or magnet*) )
28 ((keyboard* or (key NEAR/0 board*) ) NEAR/3 (emulat* or simulat*) )
29 (pen or pens or paper or papers or "paper‐based" or pictogra* or AAC)
30 #29 OR #28 OR #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14
31 #30 AND #13
32 TOPIC: (randomised or randomized or randomisation* or randomization* or randomly or RCT or placebo*) ORTOPIC: ((singl* or doubl* or trebl* or tripl*) NEAR/0 (mask* or blind* or dumm*) ) ORTITLE: (trial)
33 TOPIC: (control* NEAR/2 trial*) ORTOPIC: (nonrandom* or (non NEAR/0 random*) or (quasi NEAR/0 random*) or (quasi NEAR/0 experiment*) ) ORTOPIC: (nRCT or nRCT or non‐RCT) ORTOPIC: (control* NEAR/3 ("before and after" or "before after") ) ORTOPIC: ("time series")
34 #32 AND #31
35 #33 AND #31
36 #35 OR #34
Data and analyses
Comparison 1. Non‐vocal communication aid versus usual care without aids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Ease of communication | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐9.10 [‐10.66, ‐7.54] |
| 1.2 Satisfaction | 4 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 2.94 [1.59, 4.30] |
| 1.3 Intensive care unit length of stay | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.29, ‐0.13] |
Comparison 2. Non‐vocal communication aid versus usual care with an aid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Ease of communication | 1 | 73 | Mean Difference (IV, Random, 95% CI) | ‐18.32 [‐22.49, ‐14.15] |
Comparison 3. Voice‐enabling communication aid versus usual care with an aid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Ability to phonate | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 3.03 [0.18, 50.08] |
| 3.2 Speech intelligibility | 1 | 20 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.3 Quality of life | 2 | 63 | Mean Difference (IV, Random, 95% CI) | 2.27 [‐7.21, 11.75] |
| 3.4 Intensive care unit length of stay | 3 | 100 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.04, 0.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
El‐Soussi 2015.
| Study characteristics | ||
| Methods | Parallel‐group randomised controlled trial | |
| Participants | 60 participants Intubated men with COPD admitted to a pulmonary critical care unit (Egypt). Exclusion criteria: unconscious people and people with visual or hearing (or both) impairment. |
|
| Interventions |
Intervention A communication board that was modified from the Othman board (2008) and EZ Boards translated into Arabic language. The board contained pictures and wording headings such as 'I am' and 'I want' with descriptive words listed under each picture. It also contained the Arabic alphabet and numbers 0–9 and included 2 drawings, 1 anterior view and 1 posterior view of the human body with a box entitled pain chart which contained descriptive expressions of pain. It also included a vertical pain scale from 0 to 10. The communication board was printed on A3 paper, stuck to rigid cardboard (49 cm height and 32 cm width), and covered with transparent plastic layer to easily disinfect. The participant kept the board at all times. The researchers trained nurses to use the board when the research team was not available. Control Usual care. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation. Further information sought but not obtained. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment. Further information sought but not obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded as members of research team delivering the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not report on communication barriers that are mentioned as part of the Patient Communication Tool. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | No evidence of other bias. Funding source: none stated. Author conflict of interest: none stated. |
Farahani 2012.
| Study characteristics | ||
| Methods | Parallel‐group randomised controlled trial; 3‐arm study | |
| Participants | 60 participants Inclusion criteria: tracheally intubated and mechanically ventilated for ≥ 72 hours; not sedated; no hearing or visual impairment (glasses and hearing aids provided); able to speak Persian; no mental illness or cognitive problems. Exclusion criteria: people with probable or definitive diagnosis of brain injury (concussion, cerebral haemorrhage). |
|
| Interventions |
Intervention 2 types of communication board. Board A comprised alphabet and words representing 2 categories of physical and mental needs. Board B comprised alphabet and pictures with various images of patient needs and potential demands. Control Usual care. |
|
| Outcomes |
|
|
| Notes | Article in Farsi and translated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Further information sought but not obtained. |
| Allocation concealment (selection bias) | Unclear risk | No description. Further information sought but not obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were collected by the researcher who would have been aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only satisfaction scale reported. |
| Selective reporting (reporting bias) | Low risk | No obvious indication for concern. No trial registration. |
| Other bias | Low risk | No evidence of other bias. Funding source: none stated. Author conflict of interest: none stated. |
Freeman‐Sanderson 2016b.
| Study characteristics | ||
| Methods | Parallel‐group randomised controlled trial with longitudinal follow‐up | |
| Participants | 30 participants Inclusion criteria: aged > 18 years; formation and placement of a tracheostomy tube > 48 hours; air‐filled cuffed tracheostomy tube in‐situ; actively mechanically ventilated with PEEP ≤ 10 cmH2O; FiO2 ≤ 40%, spontaneously breathing; triggering ventilatory support; voiceless ≥ 48 hours; awake; able to obey verbal commands. Exclusion criteria: people with hearing impairment. |
|
| Interventions |
Intervention Early use of speaking valve defined as cuff deflation and use of an in‐line Passy‐Muir ventilator speech and swallowing valve during pressure support ventilation via the tracheostomy tube. Control Usual care, i.e. cuff deflation and use of a speaking valve when a patient was able to self‐ventilate. |
|
| Outcomes |
|
|
| Notes | Quality of life data reported in second publication (Freeman‐Sanderson 2016a). Trial registration and 2 abstracts identified in screening. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation using computer‐generated, permuted‐block randomisation. |
| Allocation concealment (selection bias) | Low risk | Concealed allocation via sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or therapists who administered therapy. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of the primary outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No indication of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting (trial registration checked). |
| Other bias | Low risk | No evidence of other bias. Funding source: none stated. Author conflict of interest: none stated. |
Happ 2015.
| Study characteristics | ||
| Methods | Randomised cluster step‐wedged trial. Each if the 6 ICUs were randomised to a 3‐month intervention across 18 months | |
| Participants | 1440 participants (814 intervention phase, 626 control phase) Inclusion criteria: aged ≥ 18 years; first ICU admission during the hospital stay; mechanically ventilated for ≥ 2 days via an endotracheal or tracheostomy tube; awake, alert, and responsive to verbal communication from the clinicians. Exclusion criteria: people requiring brief intubation (< 2 days) in which they were extubated shortly after awakening. |
|
| Interventions |
Intervention SPEACS‐2 consisting of:
Control
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of the units to intervention period was conducted by the statistician using computer‐generated random ordering. Random selection of electronic charts within quarters and within units used a computer‐generated random number table for chart selection by unit within each quarter until 30 participants meeting criteria were reached. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment of randomisation of ICUs to stepped wedge is challenging due to the need to notify units to prepare for practice change. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants and personnel given the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained staff, blinded to intervention assignment abstracted clinical data from EMR. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | Funding: not considered source of bias: Robert Wood Johnson Foundation Interdisciplinary Nursing
Quality Research Initiative grant #66633. Author declared potential conflict of interest: the SPEACS‐2 programme is accessible online at go.osu.edu/speacs2. Dr Happ holds the Creative Commons copyright. |
Hosseini 2018.
| Study characteristics | ||
| Methods | Controlled parallel‐group trial without randomisation | |
| Participants | 30 participants Inclusion criteria: aged 18–65 years; oriented to person, place, and date (GSC > 13); intubated for > 24 hours; literate at least at the primary school level; no previous history of ICU stay; no hearing/vision difficulties and mental illness. Exclusion criteria: none reported. |
|
| Interventions |
Intervention A communication board partly derived from the Vidatak EZ Board (US 1999). Participant needs were illustrated on 1 side of the board using related images and written words. The other side of the board comprised 2 parts including a schematic picture of the body to determine pain locations and a whiteboard enabling the participant to write/draw. The research team taught clinical staff how to use the board. Control Usual care. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were divided into experimental and control groups by the research team. |
| Allocation concealment (selection bias) | High risk | As the researchers selected the allocation group, there was no allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were unable to be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Questionnaires collected by the researcher. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported on all participants. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. Financial support and sponsorship: Urmia University of Medical Sciences, Urmia, Iran. Author conflict of interest: none stated. |
Kaur 2018.
| Study characteristics | ||
| Methods | Parallel‐group randomised controlled trial | |
| Participants | 60 participants Inclusion criteria: conscious people intubated for ≤ 3 days but requiring a further 4 days of intubation. Exclusion criteria: haemodynamically unstable people. |
|
| Interventions |
Intervention A communication chart focused on physiological, emergency, and psychological needs of an intubated patient, with needs represented by pictures. Control Usual care. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Non‐replacement lottery method to allocate 30 participants into control and intervention groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Further information sought but not obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Principal investigator collected outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants or incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No sign of selective reporting although only 1 outcome of satisfaction. |
| Other bias | Low risk | No evidence of other bias. Funding source: none stated. Author conflict of interest: none stated. |
Koszalinski 2020.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled trial | |
| Participants | 36 participants Inclusion criteria: hospitalisation on any of the participating units for any length of time; RASS score between −1 and +1 (awake, aware, and not agitated); able to use Speak For Myself‐Voice for 48 hours; able to manipulate a computer tablet; ability to read and write English. Exclusion criteria: hospitalised on units other than those participating in the study; RASS score less than −1 or exceeding +1; unwilling to use Speak For Myself‐Voice for 48 hours; unable to manipulate the computer tablet; unable to read and write English. |
|
| Interventions |
Intervention Participants provided the Speak for Myself‐Voice communication tablet app provided on an i‐Pad. This included an advanced care planning menu, pain indication, basic needs requests, a free‐text section app was used on i‐Pads. Participants were provided the Speak for Myself‐Voice app for 48 hours or until they no longer needed communication assistance. Control Alphabet and picture communication board as usual care. |
|
| Outcomes |
|
|
| Notes | Secondary analyses paper reported each of the HADS items in detail (Koszalinki 2020). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assignment to groups was not entirely random, the randomisation schedule was described as beginning with first participant assigned to the intervention group, second to control, third to intervention, etc.). |
| Allocation concealment (selection bias) | High risk | Allocation was known to the research team but concealed from the clinical team and participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not performed (information provided by corresponding author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No indication of incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. Partially funded by the University of Tennessee, Center for Health Sciences Research – not considered a source of bias. Author conflict of interest: none stated. |
Pandian 2020a.
| Study characteristics | ||
| Methods | Parallel‐group randomised controlled trial | |
| Participants | 20 participants Inclusion criteria: received a percutaneous tracheostomy; GCS score ≥ 9; CAM‐ICU negative; RASS: –1 to +1; able to understand English. Exclusion criteria: open tracheostomy; laryngectomy; recently using 1‐way speaking valve or capped trach; foam‐filled cuffed tracheostomy tube; presence of known severe airway obstruction; presence of postoperative bleeding requiring transfusion or packing; presence of air‐leak around the cuff resulting in respiratory decompensation. |
|
| Interventions |
Intervention Early 1‐way speaking valve assessment by SLP following 12–24 hours after percutaneous tracheostomy procedure. Second 1‐way speaking valve evaluation with SLP following 48–60 hours from initial percutaneous tracheostomy procedure. Third 1‐way speaking valve evaluation with SLP following first tracheostomy tube change. Participants were allowed additional SLP sessions between second and third sessions per standard of care. Control Standard 1‐way speaking valve evaluation with SLP following 48–60 hours from initial percutaneous tracheostomy procedure. Second 1‐way speaking valve evaluation with SLP following first tracheostomy tube change. Participants were allowed additional SLP sessions between first and second sessions per standard of care. |
|
| Outcomes |
|
|
| Notes | Results taken from those posted on ClinicalTrials.gov and communication with the study lead – publication anticipated in 2021. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. Information sought but not obtained. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. Information sought but not obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described. Information sought but not obtained. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/20 (35%) participants did not complete third speech intelligibility test (primary outcome) but completed first and second test. |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias. |
| Other bias | Low risk | No evidence of other bias. Funding: none stated in results posted on ClinicalTrials.gov. Author conflict of interest: none stated. |
Pandian 2020b.
| Study characteristics | ||
| Methods | Parallel‐group randomised controlled trial | |
| Participants | 50 participants Inclusion criteria: mechanically ventilated adults in ICU who were awake, alert, and attempting to communicate; English‐speaking; and unable to tolerate a 1‐way speaking valve on initial screening. Exclusion criteria: people who were delirious, had a tracheostomy within previous 48 hours, or a laryngectomy. |
|
| Interventions |
Intervention BLUSA Talking tracheostomy that has an additional above cuff lumen. Participants received 3 treatment sessions from an SLP focusing on optimising voice – optimal airflow required communicate. Control Assessment by an SLP and provision of communication boards/i‐Pads. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Implemented a computerised randomisation procedure using Excel 2016 (Microsoft Corp., Redmond, WA) by the primary investigator. Research ID numbers for 50 potential patients were randomly allocated to the control or intervention arm. |
| Allocation concealment (selection bias) | Low risk | Upon obtaining consent, based on the research identification number, the SLP was notified of the allocated arm for each patient by the primary investigator via e‐mail to ensure concealed allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unable to blind outcome assessors as could visualise the intervention when collecting outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias (trial registration checked). |
| Other bias | Low risk | No evidence of other bias. Funding: Smiths Medical Research Grant, Society of Otorhinolaryngology and Head‐Neck Nurses Research Grant, and the Johns Hopkins Shirley Sohmer Research Grant (not considered source of bias). Author conflict of interest: none stated. |
Rathi 2015.
| Study characteristics | ||
| Methods | Controlled, parallel‐group, non‐randomised study | |
| Participants | 30 participants Inclusion criteria: mechanically ventilated in medical ICU Exclusion criteria: none reported |
|
| Interventions |
Intervention Communication board consisting of pictures related to physiological needs such as physical needs (pain, orientation, hygiene, suctioning, hunger, thirst, sleep and, comfort), discomfort (sick, dizziness, heat and cold, breathing difficulty, and vomiting), psychological needs (emotions, recreation, privacy, environment, and prayer/chaplain), and social needs (paper and pencil, meeting health team members and family). Control Routine care (no further description). |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation performed. |
| Allocation concealment (selection bias) | High risk | No allocation concealment performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. Funding: none stated. Author conflict of interest: none stated. |
Rodriguez 2016.
| Study characteristics | ||
| Methods | 2‐centre, controlled before‐after study | |
| Participants | 115 participants Inclusion criteria: intubated airway, surgery, or other event causing sudden speechlessness lasting for ≥ 8 hours; aged ≥ 21 years; able to read English or Spanish; ability to see and have use of ≥ 1 arm; no permanent speech disability and already using an adaptive speech device; RASS scores within acceptable range of +1 to –1; absence of delirium measured by CAM‐ICU. Exclusion criteria: people who had participated in a previous study cohort or had an admitting diagnosis of a DSM‐IV major mental illness documented in the medical record. |
|
| Interventions |
Intervention Received a speech‐generating app incorporated in a tablet device with 3 communication functions that included:
Also provided with a freestanding urgent button (a push button that announced "I need help" when activated). Control Usual practice that included access to a call light and provision of pen and paper to write messages. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequential sampling used. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants or personnel due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data were not addressed. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. Funding: NINR SBIR grants 1R43NR01084201 and 9R44DC01227502A1. This work was supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida KL2 TR000065 and UL1 TR000064 (not considered source of bias). Author conflict of interest: none stated. |
CAM‐ICU: Confusion Assessment Method – ICU; COPD: chronic obstructive pulmonary disease; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition; EMR: electronic medical record; FiO2: fraction of inspired oxygen; GCS: Glasgow Coma Scale; HADS: Hospital Anxiety Depression Scale; ICU: intensive care unit; PEEP: positive end expiratory pressure; RASS: Richmond Agitation Sedation Scale; SLP: speech language pathologist.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Otuzoğlu 2014 | Single‐centre controlled before‐after study. |
| Stovsky 1988 | Single‐centre controlled before‐after study. |
Characteristics of studies awaiting classification [ordered by study ID]
Blumenfeld 2012.
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria: people with a tracheostomy, naive to a speaking valve, and with dysphagia. |
| Interventions |
Intervention Received a speaking valve used for 45 minutes/day and during therapy. Control Received an inner cannula only. |
| Outcomes |
|
| Notes | Unable to identify published study. |
Cohn 2016.
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria: age > 18 years; intubation for > 24 hours; absence of sedation needs or neurological condition; able to follow commands and use hands/arms; visual acuity; language literacy. Exclusion criteria: none listed. |
| Interventions |
Intervention Used SVCCM ICU patient communicator app for iPad. Control Usual care. |
| Outcomes |
|
| Notes | Unable to identify published study |
Fernández Carmona 2015.
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria: people in the mechanical ventilation weaning‐decannulation phase; aged > 18 years; tracheostomised in the ICU; diagnosis of dysphagia secondary to an artificial airway. Exclusion criteria: people with neurological conditions. |
| Interventions |
Intervention Received a speaking valve. Control No speaking valve. |
| Outcomes |
|
| Notes | 2 abstracts published in 2015 and 2016. Unable to identify published study. |
Kolcac 2020.
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria: undergone cardiac surgery; receiving mechanical ventilation; aged ≥ 18 years; –2 to +2 on RASS; agree to participate. Exclusion criteria: not understanding Turkish; vision and hearing loss; cognitive or psychological problem that prevents communication; prior intubation experience; bleeding, etc. in the early postoperative period; other complication such as undergoing revision surgery or needing additional sedation. |
| Interventions |
Intervention Received illustrated communication material. Control No intervention. |
| Outcomes |
|
| Notes | Trial registered 3 March 2020; however, study completion date listed as 15 June 2017. |
Pouladi 2016.
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria: understood Persian; age > 18 years; having an artificial airway for ≥ 18 hours; ability to understand and work orders, i.e. people with GCS score 11; no audio imperfections. Exclusion criteria: reluctance to participate. |
| Interventions |
Intervention Received a communication board including numbers, letters, words, and images used for 12 hours. Nurses show the communication board every 4 hours. Control Usual care comprising non‐verbal communication way, such as lip‐reading, pen and paper. |
| Outcomes |
|
| Notes | Trial registration states study is complete but unable to locate published study and no response from study contact. |
CAM‐ICU: Confusion Assessment Method – ICU; GCS: Glasgow Coma Score; ICU: intensive care unit; RASS: Richmond Agitation Sedation Scale; SVCCM: Society of Critical Care Medicine.
Characteristics of ongoing studies [ordered by study ID]
Divani 2019.
| Study name | The effect of using a communication board on physiological parameters and perceived quality of care in patients with artificial airways |
| Methods | Parallel‐group randomised controlled trial without blinding |
| Participants | Inclusion criteria: age 18–60 years; artificial airway; full consciousness and no use of sedative drugs; minimum literacy; speaking and understanding Persia; no hearing, sight, or cognitive problems; no history of having artificial airway; no use of drugs affecting cortisol levels; no disease and medications that affect haemodynamic parameters. Exclusion criteria: psychotic and depressive disorder; definitive or probable diagnosis of brain injury; no willingness to participate in research. |
| Interventions |
Intervention Use of a communication board in the morning. Control Usual care including nurse frequent questioning and patient non‐verbal response. |
| Outcomes |
|
| Starting date | 21 October 2019 |
| Contact information | Investigator: Anahita Divani; adivani@razi.tums.ac.ir |
| Notes |
Happ 2016.
| Study name | Improving outcomes for mechanically ventilated patients with the Digital EZ Board |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria: aged ≥18 years; able to communicate in English; awake alert, responding appropriately to commands; normal (aided or unaided) hearing and vision; able to control head, arm, and hand movements; physiologically stable and in no acute distress (per nurse report); intubated via oral endotracheal or tracheal tube without speaking valve, received mechanical ventilation during past 48 hours. Exclusion criteria: pre‐existing communication impairments; diagnosis of severe dementia or brain injury; CAM‐ICU positive for delirium; non‐responsiveness or inattention. |
| Interventions |
Intervention Provided with an Android device with the VidaTalk app and protocolised instruction on its use. Control Provided with a bedside Android device without the VidaTalk app, focusing instead on a common tablet device app. |
| Outcomes |
|
| Starting date | 19 February 2018 |
| Contact information | Mary Beth Happ; happ.3@osu.edu |
| Notes | Abstracts published |
Nanchal 2017.
| Study name | A randomized controlled trial of an iPad for patient communication during mechanical ventilation |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria: sufficient motor and visual function to allow use of touch screen; mechanically ventilated; awake and able to participate in informed consent discussion. Exclusion criteria: non‐English speaking; receiving ventilator support prior to admission; delirium present in the last 24 hours; tracheostomy; structural neurological injury (such as stroke or traumatic brain injury); coma; deep sedation (RASS > –2). |
| Interventions |
Intervention App Proloquo2Go modified for the ICU setting on an iPad device. Control Usual care. |
| Outcomes |
|
| Starting date | 23 February 2017 |
| Contact information | Rahul Nanchal Jeanette Graf; jgraf@mcw.edu |
| Notes |
CAM‐ICU: Confusion Assessment Method – ICU; ICU: intensive care unit; RASS: Richmond Agitation Sedation Scale.
Differences between protocol and review
There are no differences between the protocol (Rose 2019) and review to report.
Contributions of authors
Conceiving the review: LR.
Co‐ordinating the review: LR.
Undertaking manual searches: LR.
Screening search results: LR, CD.
Data management for the review: LR.
Entering data into Review Manager 5 (Review Manager 2020): LR.
Review Manager 5 statistical data: LR, DF.
Other statistical analysis not using Review Manager 5: DF.
Interpretation of data: all authors.
Statistical inferences: all authors.
Writing the review: all authors.
Securing funding for the review: not applicable.
Performing previous work that was the foundation of the present study: not applicable.
Guarantor for the review (one author): LR.
Person responsible for reading and checking review before submission: LR.
Sources of support
Internal sources
-
TD Nurse Professorship in Critical Care Nursing held by Dr Louise Rose, Canada
Discretionary professorship funds
External sources
-
None, Other
No external sources of support were received for this study
Declarations of interest
LR: none.
AS: none.
AA: none.
DF: none.
OS: none.
CD: none.
New
References
References to studies included in this review
El‐Soussi 2015 {published data only}
- El-Soussi AH, Elshafey MM, Othman SY, Abd-Elkader FA. Augmented alternative communication methods in intubated COPD patients: does it make difference. Egyptian Journal of Chest Diseases and Tuberculosis 2015;64:21-8. [DOI: 10.1016/j.ejcdt.2014.07.006] [DOI] [Google Scholar]
Farahani 2012 {published data only}
- Farahani Borzabadi Z, Joodaki M, Ashktorab T, Zaeri F, Atashzadeh Shurideh F. Comparison of mechanically ventilated patients with three types of communication. Quarterly Advances in Nursing and Midwifery 2012;22:46-51. [Google Scholar]
Freeman‐Sanderson 2016b {published data only (unpublished sought but not used)}
- Freeman-Sanderson AL, Togher L, Elkins MR, Phipps PR. Quality of life improves with return of voice in tracheostomy patients in intensive care: an observational study. Journal of Critical Care 2016;33:186-91. [PMID: PMID: 26971032] [DOI] [PubMed] [Google Scholar]
- Freeman-Sanderson AL, Togher L, Elkins MR, Phipps PR. Return of voice for ventilated tracheostomy patients in ICU: a randomized controlled trial. Critical Care Medicine 2016;44:1075-81. [PMID: PMID 26855430] [DOI] [PubMed] [Google Scholar]
Happ 2015 {published data only}
- Happ MB, Sereika SM, Houze MP, Seaman JB, Tate JA, Nilsen ML, et al. Quality of care and resource use among mechanically ventilated patients before and after an intervention to assist nurse-nonvocal patient communication. Heart Lung 2015;44:408-15.e2. [PMID: PMID: 26354859] [DOI] [PubMed] [Google Scholar]
Hosseini 2018 {published data only}
- Hosseini SR, Valizad-Hasanloei MA, Feizi A. The effect of using communication boards on ease of communication and anxiety in mechanically ventilated conscious patients admitted to intensive care units. Iranian Journal of Nursing and Midwifery Research 2018;23:358-62. [DOI: 10.4103/ijnmr.IJNMR_68_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 2018 {published data only}
- Kaur S, Agnihotri M, Dhandapani M, Gopichandran L, Mukherjee KK, Dhandapani S. Effectiveness of communication chart on patient satisfaction among conscious intubated patients: a randomized controlled trial. Journal of Nursing Science & Practice 2018;8:15-23. [ISSN: 2249-4758] [Google Scholar]
Koszalinski 2020 {published data only}
- Koszalinski RS, Heidel RE, Hutson SP, Li X, Palmer TG, McCarthy J, et al. The use of communication technology to affect patient outcomes in the intensive care unit. Computers, Informatics, Nursing 2020;38:183-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Koszalinski RS, Heidel RE, McCarthy J. Difficulty envisioning a positive future: secondary analyses in patients in intensive care who are communication vulnerable. Nursing Health Science 2020;22:374-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pandian 2020a {published data only}
- Pandian V, Cole T, Kilonsky D, Holden K, Feller-Kopman DJ, Brower R, et al. Early speech with one-way speaking valve in tracheostomy patients. clinicaltrials.gov/ct2/show/NCT03008174 (first received 2 January 2017). [NCT03008174]
Pandian 2020b {published data only}
- Pandian V. Voice-related quality of life increases with a talking tracheostomy tube: a randomized controlled trial. Laryngoscope 2020;130:1249-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rathi 2015 {published data only}
- Rathi R, Baskaran M. Communication board satisfaction among clients on mechanical ventilator. International Journal of Nursing Education 2015;7:216-21. [DOI: 10.5958/0974-9357.2015.00168.3] [DOI] [Google Scholar]
Rodriguez 2016 {published data only}
- Rodriguez CS, Rowe M, Thomas L, Shuster J, Koeppel B, Cairns P. Enhancing the communication of suddenly speechless critical care patients. American Journal of Critical Care 2016;25:e40-e47. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Otuzoğlu 2014 {published data only}
- Otuzoğlu M, Karahan A. Determining the effectiveness of illustrated communication material for communication with intubated patients at an intensive care unit. International Journal of Nursing Practice 2014;20:490-8. [DOI: 10.1111/ijn.12190] [DOI] [PubMed] [Google Scholar]
Stovsky 1988 {published data only}
- Stovsky B, Rudy E, Dragonette P. Comparison of two types of communication methods used after cardiac surgery with patients with endotracheal tubes. Heart Lung 1988;17:281-9. [PMID: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Blumenfeld 2012 {published data only}
- Blumenfeld L. The effect of tracheostomy speaking valve use on disordered swallowing. Dysphagia 2012;27:579. [Google Scholar]
Cohn 2016 {published data only}
- Cohn J, Rizvi A, Deira Lorenzo J. Tablet-based communication for the mechanically ventilated patient: a pilot study. Critical Care Medicine 2016;12(Suppl 1):283. [Google Scholar]
Fernández Carmona 2015 {published data only (unpublished sought but not used)}
- Fernández Carmona A, Arias Díaz M, Aguilar Alonso E, Macías Guarasa I, Martínez López P, Díaz Castellanos MA, et al. Use of speaking valve on preventing respiratory infections, in critical traqueostomized patients diagnosed of dysphagia secondary to artificial airway. edisval study. Intensive Care Medicine Experimental 2015;3:A936. [PMID: ] [Google Scholar]
- Fernández-Carmona A, Arias-Díaz M, Aguilar-Alonso E, Macías-Guarasa I, Martínez-López P, Diaz-Castellanos MA. Use of speaking valve on preventing respiratory infections, in critical traqueostomized patients diagnosed of dysphagia secondary to artificial airway. Edisval study first 12 months. Intensive Care Medicine Experimental 2016;4(Suppl 1):A659. [DOI: 10.1186/s40635-016-0098-x] [DOI] [Google Scholar]
Kolcac 2020 {published data only (unpublished sought but not used)}
- Kolcac B. The effect of using illustrated communication material on anxiety and comfort in communication with patients receiving mechanical ventilator: randomised controlled clinical trial. clinicaltrials.gov/ct2/show/NCT04293913 (first received 3 March 2020). [NCT04293913]
Pouladi 2016 {published data only (unpublished sought but not used)}
- Pouladi S. Assessment of the Influence of nonverbal communication method on satisfaction of patients with artificial airway in medical centers in Shiraz in 1394. en.irct.ir/trial/19360 (first received 27 August 2016). [IRCT2015112122466N4]
References to ongoing studies
Divani 2019 {published data only}
- Divani A. The effect of using communication board on physiological parameters and perceived quality of care in patients with artificial airways. en.irct.ir/trial/42173 (first received 31 October 2019). [IRCT20190914044768N1]
Happ 2016 {published data only}
- Happ MB. Vidatalk communication application: usability, acceptability and efficacy study. clinicaltrials.gov/ct2/show/NCT02921776 (first received 3 October 2016). [NCT02921776]
Nanchal 2017 {published data only}
- Nanchal R. CTSI-iPad for Vented Patient Communication (iPad). www.clinicaltrials.gov/ct2/show/NCT03163823 (first received 23 May 2017). [NCT03163823]
Additional references
Adam 2015
- Adam SI, Srinet P, Aronberg RM, Rosenberg G, Leder SB. Verbal communication with the Blom low profile and Passy-Muir one-way tracheotomy tube speaking valves. Journal of Communication Disorders 2015;56:40-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ambrosino 2018
- Ambrosino N, Vitacca M. The patient needing prolonged mechanical ventilation: a narrative review. Multidisciplinary Respiratory Medicine 2018;13:6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASHA 2019
- American Speech-Language-Hearing Association (ASHA). Augmentative and alternative communication (AAC). www.asha.org/public/speech/disorders/AAC (accessed 23 April 2019).
Bartlett 2008
- Bartlett G, Blais R, Tamblyn R, Clermont R, MacGibbon B. Impact of patient communication problems on the risk of preventable adverse events in acute care settings. Canadian Medical Association Journal 2008;178(12):1555-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Breckenridge 2014
- Breckenridge SJ, Chlan L, Savik K. Impact of tracheostomy placement on anxiety in mechanically ventilated adult ICU patients. Heart and Lung 2014;43(5):392-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Broyles 2012
- Broyles L, Tate J, Happ M. Use of augmentative and alternative communication strategies by family members in the intensive care unit. American Journal of Critical Care 2012;21(2):e21-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2004
- Carroll SM. Nonvocal ventilated patients' perceptions of being understood. Western Journal of Nursing Research 2004;26(1):85-103. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carroll 2007
- Carroll SM. Silent, slow lifeworld: the communication experience of nonvocal ventilated patients. Qualitative Health Research 2007;17(9):1165-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carruthers 2017
- Carruthers H, Astin F, Munro W. Which alternative communication methods are effective for voiceless patients in Intensive Care Units? A systematic review. Intensive & Critical Care Nursing 2017;42:88-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
CCCG 2013
- Cochrane Consumers and Communication Group. Standard protocol text and additional guidance for review authors (2013). cccrg.cochrane.org/author-resources (accessed prior to 24 July 2018).
Chlan 2015
- Chlan LL, Tracy MF, Guttormson J, Savik K. Peripheral muscle strength and correlates of muscle weakness in patients receiving mechanical ventilation. American Journal of Critical Care 2015;24(6):e91-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eggers 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flinterud 2015
- Flinterud S, Andershed B. Transitions in the communication experiences of tracheostomised patients in intensive care: a qualitative descriptive study. Journal of Clinical Nursing 2015;24(15-16):2295-304. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freeman‐Sanderson 2016a
- Freeman-Sanderson AL, Togher L, Elkins MR, Phipps PR. Quality of life improves with return of voice in tracheostomy patients in intensive care: an observational study. Journal of Critical Care 2016;33:186-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garry 2016
- Garry J, Casey K, Cole TK, Regensburg A, McElroy C, Schneider E, et al. A pilot study of eye-tracking devices in intensive care. Surgery 2016;159(3):938-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 21 Oct 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grossbach 2011
- Grossbach I, Stranberg S, Chlan L. Promoting effective communication for patients receiving mechanical ventilation. Critical Care Nurse 2011;31(3):46-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guttormson 2015
- Guttormson J, Bremer K, Jones R. "Not being able to talk was horrid": a descriptive, correlational study of communication during mechanical ventilation. Intensive and Critical Care Nursing 2015;31(3):179-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Happ 2004
- Happ M, Roesch T, Garrett K. Electronic voice-output communication aids for temporarily nonspeaking patients in a medical intensive care unit: a feasibility study. Heart and Lung 2004;33(2):92-101. [PMID: ] [DOI] [PubMed] [Google Scholar]
Happ 2011
- Happ MB, Garrett K, Thomas DD, Tate J, George E, Houze M, et al. Nurse-patient communication interactions in the intensive care unit. American Journal of Critical Care 2011;20(2):e28-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Happ 2014
- Happ M, Garrett KL, Tate JA, DiVirgilio D, Houze MP, Demirci JR, et al. Effect of a multi-level intervention on nurse-patient communication in the intensive care unit: results of the SPEACS trial. Heart and Lung 2014;43(2):89-98. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashmi 2010
- Hashmi NK, Ransom E, Nardone H, Redding N, Mirza N. Quality of life and self-image in patients undergoing tracheostomy. Laryngoscope 2010;120(Suppl 4):S196. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hoit 2003
- Hoit JD, Banzett RB, Lohmeier HL, Hixon TJ, Brown R. Clinical ventilator adjustments that improve speech. Chest 2003;124(4):1512-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hunt 2015
- Hunt K, McGowan S. Tracheostomy management. British Journal of Anaesthesia: Education 2015;15(5):149-53. [Google Scholar]
Huttmann 2018
- Huttmann SE, Magnet FS, Karagiannidis C, Storre JH, Windisch W. Quality of life and life satisfaction are severely impaired in patients with long‑term invasive ventilation following ICU treatment and unsuccessful weaning. Annals of Intensive Care 2018;8(1):38. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 1990
- Johnson M, Sexton D. Distress during mechanical ventilation: patients' perceptions. Critical Care Nurse 1990;10(8):48-57. [PubMed] [Google Scholar]
Joint Commission 2010
- The Joint Commission. Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care: a Roadmap for Hospitals. Oakbrook Terrace (IL): The Joint Commission, 2010. [Google Scholar]
Khalaila 2011
- Khalaila R, Zbidat W, Anwar K, Bayya A, Linton D, Sviri S. Communication difficulties and psychoemotional distress in patients receiving mechanical ventilation. American Journal of Critical Care 2011;20(6):470-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Koszalinki 2020
- Koszalinski RS, Heidel RE, McCarthy J. Difficulty envisioning a positive future: secondary analyses in patients in intensive care who are communication vulnerable. Nursing Health Science 2020;22(2):374-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kunduk 2010
- Kunduk M, Appel K, Tunc M, Alanoglu Z, Alkis N, Dursun G, et al. Preliminary report of laryngeal phonation during mechanical ventilation via a new cuffed tracheostomy tube. Respiratory Care 2010;55(12):1661-70. [PMID: ] [PubMed] [Google Scholar]
Laakso 2011
- Laakso K, Markström A, Idvall M, Havstam C, Hartelius L. Communication experience of individuals treated with home mechanical ventilation. International Journal of Language and Communication Disorders 2011;46(6):686-99. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macht 2013
- Macht M, King C, Wimbish T, Clark B, Benson A, Burnham E, et al. Post-extubation dysphagia is associated with longer hospitalization in survivors of critical illness with neurologic impairment. Critical Care 2013;17(3):R119. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Magnus 2006
- Magnus V, Turkington L. Communication interaction in ICU: patient and staff experiences and perceptions. Intensive and Critical Care Nursing 2006;22(3):167-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
McGowan 2016
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of Clinical Epidemiology 2016;75:40-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
McGrath 2019
- McGrath B, Wallace S, Wilson M, Nicholson L, Felton T, Bowyer C, et al. Safety and feasibility of above cuff vocalisation for ventilator-dependant patients with tracheostomies. Journal of the Intensive Care Society 2019;20(1):59-65. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menzel 1998
- Menzel L. Factors related to the emotional responses of intubated patients to being unable to speak. Heart and Lung 1998;27(4):245-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morris 2015
- Morris LL, Bedon AM, McIntosh E, Whitmer A. Restoring speech to tracheostomy patients. Critical Care Nurse 2015;35(6):13-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nilsen 2014
- Nilsen M, Sereika S, Hoffman L, Barnato A, Donovan H, Happ M. Nurse and patient interaction behaviors' effects on nursing care quality for mechanically ventilated older adults in the ICU. Research in Gerontological Nursing 2014;7(3):113-25. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandian 2014
- Pandian V, Smith CP, Cole TK, Bhatti NI, Mirski MA, Yarmus LB, et al. Optimizing communication in mechanically ventilated patients. Journal of Medical Speech-Language Pathology 2014;21(4):309-18. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Peters 2006
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295(6):676-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rose 2014
- Rose L, Nonoyama M, Rezaie S, Fraser I. Psychological wellbeing, health related quality of life and memories of intensive care and a specialised weaning centre reported by survivors of prolonged mechanical ventilation. Intensive and Critical Care Nursing 2014;30(3):145-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rose 2015
- Rose L, Fowler RA, Fan E, Fraser I, Leasa D, Mawdsley C, et al, CANuVENT group. Prolonged mechanical ventilation in Canadian intensive care units: a national survey. Journal of Critical Care 2015;30(1):25-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rose 2018
Ryan 2013
- Ryan R, Hill S, Prictor M, McKenzie J, Cochrane Consumers and Communication Group. Study quality guide (2013). cccrg.cochrane.org/author-resources (accessed 18 June 2018).
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Shimizu 2013
- Shimizu K, Ogura H, Irisawa T, Nakagawa Y, Kuwagata Y, Shimazu T. Communicating by electrolarynx with a blind tetraplegic spinal cord injury patient on mechanical ventilation in the ICU. Spinal Cord 2013;51(4):341-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Smid 2017
- Smid DE, Franssen FM, Houben-Wilke S, Vanfleteren LE, Janssen DJ, Wouters EF, et al. Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. Journal of the American Medical Directors Association 2017;18(1):53-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stein‐Parbury 2000
- Stein-Parbury J, McKinley S. Patients' experiences of being in an intensive care unit: a select literature review. American Journal of Critical Care 2000;9(1):20-7. [PMID: ] [PubMed] [Google Scholar]
Ten Hoorn 2016
- Ten Hoorn S, Elbers PW, Girbes AR, Tuinman PR. Communicating with conscious and mechanically ventilated critically ill patients: a systematic review. Critical Care 2016;20(1):333. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Sluis 2018
- Van Sluis KE, Van der Molen L, Van Son RJ, Hilgers FJ, Bhairosing PA, Van den Brekel M. Objective and subjective voice outcomes after total laryngectomy: a systematic review. European Archives of Otorhinolaryngology 2018;275(1):11-26. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaga 2019
- Zaga CJ, Berney S, Vogel AP. The feasibility, utility, and safety of communication interventions with mechanically ventilated intensive care unit patients: a systematic review. American Journal of Speech Language Pathology 2019;28(3):1335-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rose 2019
- Rose L, Sutt AL, Amaral AC, Fergusson DA, Hart N, Smith OM, et al. Interventions to enable communication for adult patients requiring an artificial airway with or without mechanical ventilator support. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD013379. [DOI: 10.1002/14651858.CD013379] [DOI] [PMC free article] [PubMed] [Google Scholar]


