Abstract
Context:
Xylanase constitutes 20% of world enzyme market. Significantly, they are used in poultry feed, paper pulp, bakery, and textile industries. In view of the increasing demand of the enzyme, it is vital to develop indigenous strains and scalable technologies for the production of industrial enzymes.
Aims:
The objective of the present paper was to isolate a high-yielding strain of thermo-alkali-stable xylanase-producing bacteria for potential application in paper and pulp and biofuel industry.
Methods:
Sampling for prospecting of suitable organism was carried out from the places with dead and decaying lignocellulosic waste, and then Congo red screening was employed for the primary isolation of xylanase producers.
Results:
We report the isolation of 18 different strains of xylanase producer bacteria from natural hot water geyser of Sohna, Haryana, India. Subsequently, two of these isolates were chosen for further studies based on xylanase yield and desirable properties such as thermostability and alkali stability of xylanase produced.
Conclusion:
Isolate B2 was later identified as Bacillus licheniformis, whereas isolate Y3 was identified as Brevibacillus borstelensis. This strain when cultured at 35°C for 72 h showed xylanase production at 128 U/ml. The molecular weight of xylanase was determined to be 25 kDa. The production was scaled up in a 5-L stirred-tank bioreactor which led to high xylanase concentration of 380 U/ml in the first 48 h of culture.
Keywords: Bacillus, industrial enzymes, lignocellulosic biomass, paper, thermostable enzymes, xylanase
INTRODUCTION
Xylanases are a class of enzymes that hydrolyze xylan into xylose. They find extensive applications in industries such as feed and food, paper and pulp, textile, and fine chemicals. The demand of xylanases has been increasing, and due to many potential applications, the demand is expected to rise further in future. Many types of microorganisms including bacteria, fungi, and yeast have been reported to produce xylanase.[1,2,3,4] The properties of xylanases obtained from different microorganisms differ in their activity, stability, and specificity. The ideal candidate for xylanase, for different applications, should be thermostable and alkali tolerant. Moreover, xylanases used for biobleaching of paper pulp should be free from cellulase activity; otherwise, it would damage the paper.[5] In the present paper, we report the isolation and characterization of a thermostable and alkali-stable bacterial xylanase which is an excellent candidate for application in industries such as feed and paper pulp.
METHODS
Isolation and screening of xylanolytic bacteria
Soil samples were collected from Shiv Kund (hot water geyser), Sohna, Haryana, India. The soil samples from a depth of 10–12 cm from surface were collected in clean sterile containers and transferred to the laboratory conditions. The enrichment of xylanolytic microbial population was carried out using specific enrichment medium (ammonium sulfate 0.1% w/v, magnesium sulfate 0.02%w/v, potassium dihydrogen phosphate 0.05%w/v, calcium chloride 0.01%w/v, yeast extract 0.2% w/v, and xylan 0.5% w/v and pH adjusted to 9.0) containing xylan as the sole carbon source. The enrichment was carried out in two different sets: one set was incubated at 35°C (for the enrichment of mesophilic bacteria) and another at 50°C (for the enrichment of thermophilic bacteria). After 7 days of enrichment, the bacteria were isolated using serial dilution technique on plates containing medium consisting of xylan 0.5% w/v, yeast extract 0.2% w/v, sodium chloride 0.25% w/v, potassium dihydrogen phosphate 1.5% w/v, sodium dihydrogen phosphate 3.0% w/v, ammonium chloride 0.5%w/v, magnesium sulfate heptahydrate 0.025% w/v, and agar 2% w/v adjusted to a pH of 9. Single isolated colonies were picked carefully, and pure cultures were isolated by repeated streak plate technique.
Screening of isolates
Initial screening of xylanase-producing bacteria was done by Congo red assay method, as described by Samanta et al.[6] Congo red dyes stain polysaccharides such as xylan and cellulose but not their hydrolysis products such as oligosaccharides and monosaccharides. Xylanase produced by microorganisms hydrolyzes the xylan present in the medium-producing xylooligosaccharides which are not stained by Congo red. This results in the formation of clear zones around the colonies of microorganisms that produce xylanase.
A 3-day-old separated and purified colony was selected and inoculated in the center of the culture media plate and incubated for 3 days at 37°C. After 3 days of incubation, the plates were flooded with the 0.01% w/v Congo red solution and incubated at room temperature for 15–20 min. After the incubation, destaining of the plates was done by flooding them with 1% w/v NaCl solution. Xylanase-producing bacteria were identified by the formation of clear zones around them. Colonies that showed clear zones having diameters >5 cm were selected and further screened in submerged fermentation.
Submerged fermentation medium was similar to the solid plate medium (given above) but without agar. It was inoculated with the pure culture of the isolates and incubated at 37°C with shaking at 120 rpm for 3 days. Samples were withdrawn at regular time intervals of 24 h. The samples were centrifuged at 10,000 rpm for 10 min. Supernatant was separated and used for assay of xylanase activity.
Assay of xylanase
Xylanase activity was determined by the determination of reducing sugars liberated from xylan by 3,5-dinitrosalicylic acid (DNSA) method.[7] In brief, the culture broth was first centrifuged at 10,000 rpm for 10 min at 10°C. The 1% w/v beechwood xylan (Himedia MB141-10G) prepared in 0.05M sodium citrate buffer pH 6.5 was used as substrate. One milliliter of the substrate was incubated with 500 μl of enzyme (supernatant obtained from centrifuged culture broth) at 50°C for 15 min. The reaction was terminated by the addition of 3 ml of DNSA reagent, and the mixture was boiled for 10 min in a water bath. The absorbance was measured at 540 nm after cooling the mixture. Xylose was used as a standard. One unit of xylanase activity (U) is defined as the amount of enzyme that liberates 1 μmol of reducing sugar-xylose per min under the standard assay conditions.

Characterization of xylanase-producing bacteria
Xylanase-producing bacteria were characterized by 16 S ribosomal RNA (rRNA) sequencing method. The analysis was carried out by Chromous Biotech Pvt. Ltd. (Bengaluru, Karnataka, India). The method involved amplification of 16 S-rDNA fragments. In the first step, genomic DNA was isolated. The ~1.3 kb/1.5 kb, 16 S-rDNA fragment was amplified using high-fidelity PCR polymerase. The product obtained was sequenced bidirectionally, and sequence data were then aligned and analyzed to identify the bacterium and its closest neighbors.
Molecular mass determination by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and zymogram analysis
The molecular weight of the xylanase was estimated by performing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as described by the method of Laemmli using 12% polyacrylamide gels.[8] The electrophoresis was run at 100 mA and 4°C. Coomassie Brilliant Blue R 250 was used to stain the gel and visualize the bands. Broad-range molecular weight marker (20 kDa–220 kDa) was purchased from Bangalore Genei Private Limited, Bengaluru, Karnataka, India, for the determination of the molecular weight of protein bands.
Zymogram analysis was performed with 0.1% beechwood xylan (w/v) incorporated into the polyacrylamide gel. After the gel run, the gel was washed 4–5 times with distilled water for 30 min at 4°C and incubated in PBS for 24 h at room temperature. The gel was then immersed in 0.1% Congo red solution. The stained gel was then washed with excess of 1M NaCl. The position of xylanase could be obtained as a clear band in the gel. Subsequently, the gel was treated with 0.5% v/v acetic acid to enhance the contrast of the clear band in the gel.
RESULTS AND DISCUSSION
The isolates positive for xylanase production were maintained and cultured on agar plates for further characterization. The typical morphological characteristics of the isolates are tabulated in Table 1.
Table 1.
Morphological characteristics of the isolates and xylanase production (original work)
| Isolates | Shape | Margin (cms) | Elevation | Opacity | Gram nature |
|---|---|---|---|---|---|
| A1 | Round | 1.6 | Flattened | + | + |
| A2 | Scattered | 1.4 | Flattened | + | + |
| A3 | Round | 1.4 | Elevated | + | + |
| B1 | Round | 1.5 | Flattened | + | - |
| B2 | Round | 2.3 | Elevated | + | + |
| B3 | Irregular | 2.3 | Flattened | - | - |
| C1 | Irregular | 2.1 | Flattened | + | - |
| C2 | Round | 2.3 | Elevated | + | + |
| C3 | Irregular | 1.8 | Flattened | + | + |
| X1 | Scattered | 1.8 | Flattened | + | + |
| X2 | Scattered | 2.1 | Elevated | + | + |
| X3 | Irregular | 1.9 | Flattened | - | - |
| Y1 | Round | 1.9 | Flattened | - | - |
| Y2 | Irregular | 2.4 | Elevated | + | + |
| Y3 | Scattered | 2.6 | Elevated | + | + |
| Z1 | Round | 2.1 | Flattened | + | - |
| Z2 | Round | 2.2 | Flattened | + | + |
| Z3 | Round | 1.9 | Elevated | - | + |
The isolates were inoculated in the xylanase production medium having xylan as the sole carbon source. The culture broth was harvested at the end of 7 days, and the supernatant was assayed for xylanase activity [Figure 1].
Figure 1.
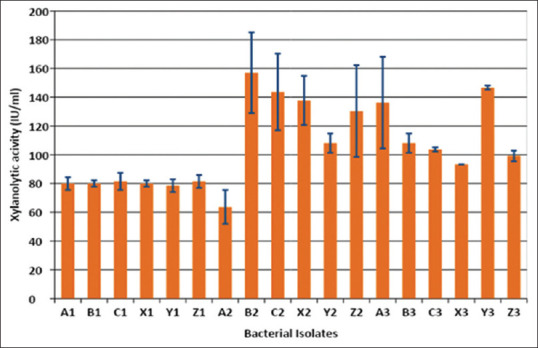
Xylanase activity of different isolates at the end of 7 days in culture (original work)
Out of these 18 strains, four were selected based on high xylanolytic potential production. Isolates B2 and C2 were mesophiles which grew best at 35°C, while two isolates X2 and Y3 were thermophiles that could be cultured at 45°C. Out of these four selected isolates, two major xylanase-producing isolates were selected: a mesophile and a thermophile, i.e., B2 and Y3, respectively, for further comparison of different parameters between the two types.
The selected B2 and Y3 isolates were cultured in xylanase production medium at temperatures of 35°C and 50°C, respectively, keeping all other parameters constant. The samples were withdrawn at the regular intervals of 24 h, and the growth, xylanase activity, and substrate consumption were determined. Biomass was determined by measuring the optical density at 620 nm in spectrophotometer. Xylanase activity and residual sugars were determined by the DNSA method as mentioned earlier.
Figure 2a shows the batch growth curve of isolates B2 and Y3, wherein isolate B2 clearly has a higher growth rate. The growth curve for both isolates B2 and Y3 illustrated the commencement of stationary phase at 72 h and maximum growth at 96 h. This also correlates with the xylanase production curve for isolate B2, where xylanase concentration increased with time and growth of the bacteria during exponential growth phase. The maximum xylanase concentration was achieved at 72 h [Figure 2c]. Xylanase concentration in the batch culture of isolate Y3 also increased with time; nevertheless, the concentration obtained remained lesser at all the times as compared to that obtained for isolate B2. A fall in xylanase concentration was observed if the culture was continued beyond 96 h. This is probably due to extracellular protease production by bacteria.[9] Therefore, industrial fermentation will warrant harvesting of xylanase before proteolysis occurs. Figure 2b shows the profile of reducing sugars in the culture broth. Reducing sugar concentrations were very small at the start of batch growth for both isolates as the culture medium did not contain readily available carbon sources (reducing sugars). However, production of xylanase is induced during the exponential growth phase which results in the breakdown of xylan, leading to an increase in reducing sugar concentrations. During the exponential growth phase, the concentration of reducing sugars remains low due to active metabolism by the bacteria. However, later, it increases and then remains more or less constant after the stationary phase sets in.
Figure 2.
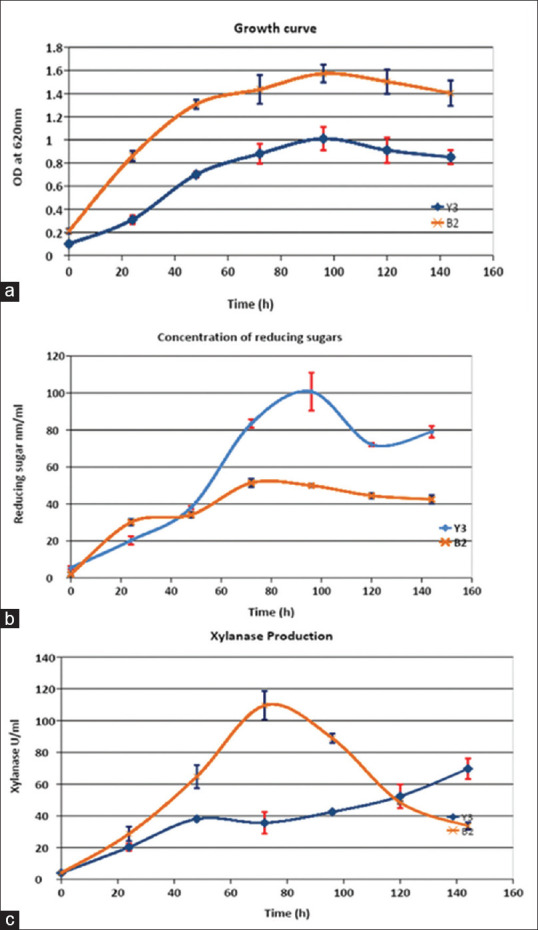
(a) Growth curves of isolates Y3 and B2, (b) concentration of residual reducing sugars, (c) time course of xylanase production by isolates Y3 and B2 (original work)
The thermostability of the crude xylanase obtained from the selected strains was determined by incubating the enzyme at different temperatures and then determining the residual activity [Figure 3]. Similarly, pH stability of the crude xylanase was obtained by incubating the enzyme in 0.05M buffer solutions in pH range 6.5–9.5 (citrate in the range of 4.5–6.5, phosphate in the range 7.5–8.5, and tris buffer in the 9.5 range), and the residual activity was measured [Figure 4].
Figure 3.
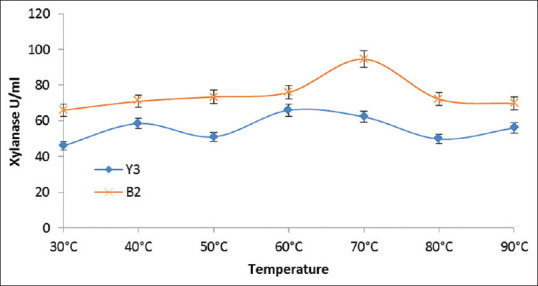
Temperature optima of xylanases from the Y3 and B2 isolates
Figure 4.
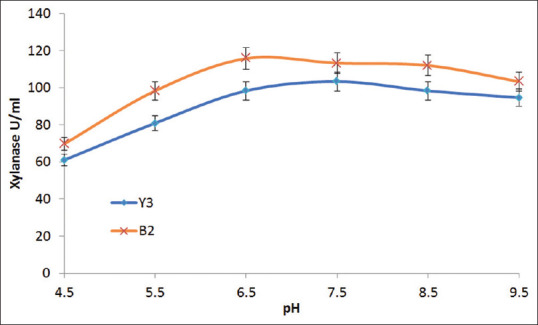
pH optima of xylanases from the Y3 and B2 isolates (original work)
The results indicate that xylanase produced by strain Y3 and B2 both are thermostable up to 90°C. Maximum xylanase produced by Y3 and B2 was 70 and 110 U/ml, respectively, at the respective optimal culture temperatures of 45°C and 35°C. Therefore, B2 strain showed higher xylanase production as compared to Y3. Xylanase from both the strains showed excellent pH stability in the range of pH 6.5–9.5.
Enzyme preparations were submitted to denaturing (SDS-PAGE) on 12% gel and zymogram analysis for xylanase. Figure 5 shows the SDS-PAGE and zymogram for the crude xylanase obtained from isolates B2 and Y3.
Figure 5.
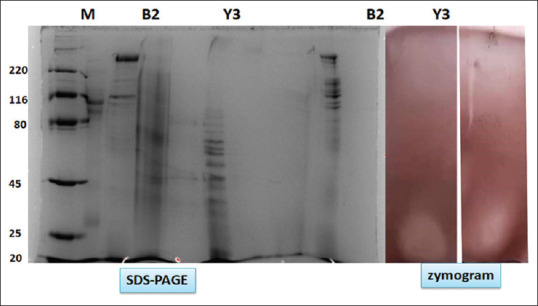
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and zymogram analysis showing xylanase active band at 23 kDa Lane M – marker, B2 – culture broth B2, Y3 – culture broth Y3, B2 – zymogram, Y3 – zymogram (original work)
The molecular weight of xylanase active band was confirmed by zymogram analysis in native PAGE. Xylanase from isolates B2 and Y3 had molecular weight around 25 kDa. Preliminary characterization of the culture was carried out by Gram staining. The culture was analyzed by 16 S rRNA sequencing method. Isolate B2 was found to be closest to bacterium Bacillus licheniformis strain FY-6. The thermophilic isolate Y3 was identified with Brevibacillus sp. B13.
Bioage 5-L stirred-tank bioreactor was used for scaling up production. The pH and DO probes were first calibrated according to the standard procedure given by the manufacturer. The bioreactor was operated at optimized process parameters. The formulation of the medium was carried out according to the optimized factor values obtained during the medium optimization experiment. The medium consisted of 2% wheat bran (w/v), 2.5% yeast extract (w/v), 0.25% NaCl (w/v), 0.5% NH4Cl (w/v), and 0.025% MgSO4·7H2O (w/v). The reactor was sterilized by autoclaving for 1 h. After sterilization, the reactor vessel was connected to the control unit and recirculating cooling water bath. The reactor was allowed to cool. After the reactor cooled to the process temperature, it was inoculated with 10% v/v of actively growing culture of isolate B2 that was grown in 500 ml shake flasks. Inoculation was carried out from the inoculation port using fire loop to maintain the aseptic conditions for inoculum transfer. The process parameters were controlled at optimized values of pH 6.0, temperature 35°C, rpm 125, and DO 60% [Figures 6 and 7].
Figure 6.

Scale-up of xylanase production in 5L Bioage stirred-tank bioreactor (original work)
Figure 7.
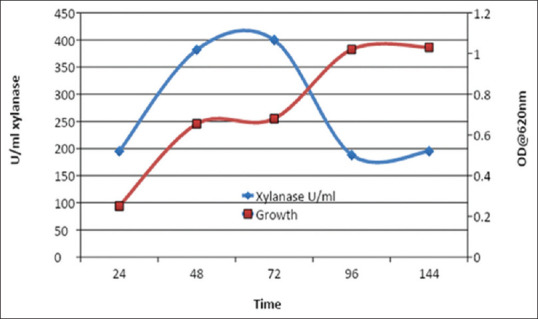
Xylanase production and growth obtained in stirred-tank reactor (original work)
It could be observed that peak xylanase activity of 380 U/ml was achieved in 48 h in the bioreactor, which is much faster as compared to shake-flask experiments where peak activity is obtained in 72 h. Moreover, the enzyme concentration obtained was much higher than that of the shake flask cultures. However, as observed in the batch culture, the xylanase activity decreased which could be due to proteolysis catalyzed by protease secreted during the culture. Interestingly, this is also showing diauxic growth as described by Schneider et al. where they have proposed that the bacteria first utilize nitrogen source for their growth successively; xylan is used as the source of carbon after depolymerization is carried out by xylanase produced by the bacteria.[10]
CONCLUSION
The present research work was successful in the isolation of at least 18 xylanase-producing bacteria from soil. Two of these strains were selected on the basis of high xylanase production. Isolate B2 was identified as closest to B. licheniformis. The xylanase isolated had high thermal and pH stability, whereas the mesophilic-isolated strain isolate B2 was identified as B. licheniformis. The production was scaled up to 2.5 L using batch stirred-tank bioreactor which was successful in achieving a high rate and yield of xylanase.
Financial support and sponsorship
This study was financially supported and sponsored by the Molecular Biosciences Research Cluster, Manav Rachna Educational Institutes.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge the facilities provided by the Molecular Biosciences Research Laboratory of Manav Rachna Research Innovation and Incubation Center, for making the present work successful.
REFERENCES
- 1.Morosoli R, Roy C, Yaguchi M. Isolation and partial primary sequence of a xylanase from the yeast Cryptococcus albidus. Biochim Biophys Acta Protein Struct Mol. 1986;870:473–8. [Google Scholar]
- 2.Chakdar H, Kumar M, Pandiyan K, Singh A, Nanjappan K, Kashyap PL, et al. Bacterial xylanases. Biology to biotechnology. 2016;6:150. doi: 10.1007/s13205-016-0457-z. 3 Biotech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polizeli MM, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: Properties and industrial applications. Appl Microbiol Biotechnol. 2005;67:577–91. doi: 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra G, Chapadgaonkar SS. Production and applications of xylanases – An overview. Biotechnologia. 2018;99:59–72. Doi: 10.5114/bta. 2018.73562. [Google Scholar]
- 5.Walia A, Guleria S, Mehta P, Chauhan A, Parkash J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech. 2017;7:11. doi: 10.1007/s13205-016-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samanta AK, Kolte AP, Senani S, Sridhar M, Jayapal N. A simple and efficient diffusion technique for assay of endo β-1,4-xylanase activity. Braz J Microbiol. 2011;42:1349–53. doi: 10.1590/S1517-838220110004000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–70. [Google Scholar]
- 8.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Sarker PK, Talukdar SA, Deb P, Sayem SA, Mohsina K. Optimization and partial characterization of culture conditions for the production of alkaline protease from Bacillus licheniformis P003. Springerplus. 2013;2:506. doi: 10.1186/2193-1801-2-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider G, Taillandier P, Strehaiano P. Stoichiometry of diauxic growth of a xylanase-producing Bacillus strain. Can J Microbiol. 2000;46:784–9. doi: 10.1139/w00-064. [DOI] [PubMed] [Google Scholar]


