Abstract
Background
Arterial vascular access is a frequently performed procedure, with a high possibility for adverse events (e.g. pneumothorax, haemothorax, haematoma, amputation, death), and additional techniques such as ultrasound may be useful for improving outcomes. However, ultrasound guidance for arterial access in adults is still under debate.
Objectives
To assess the effects of ultrasound guidance for arterial (other than femoral) catheterisation in adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS, and CINAHL on 21 May 2021. We also searched IBECS, WHO ICTRP, and ClinicalTrials.gov on 16 June 2021, and we checked the reference lists of retrieved articles.
Selection criteria
Randomised controlled trials (RCTs), including cross‐over trials and cluster‐RCTs, comparing ultrasound guidance, alone or associated with other forms of guidance, versus other interventions or palpation and landmarks for arterial (other than femoral) guidance in adults.
Data collection and analysis
Two review authors independently performed study selection, extracted data, assessed risk of bias, and assessed the certainty of evidence using GRADE.
Main results
We included 48 studies (7997 participants) that tested palpation and landmarks, Doppler auditory ultrasound assistance (DUA), direct ultrasound guidance with B‐mode, or any other modified ultrasound technique for arterial (axillary, dorsalis pedis, and radial) catheterisation in adults.
Radial artery
Real‐time B‐mode ultrasound versus palpation and landmarks
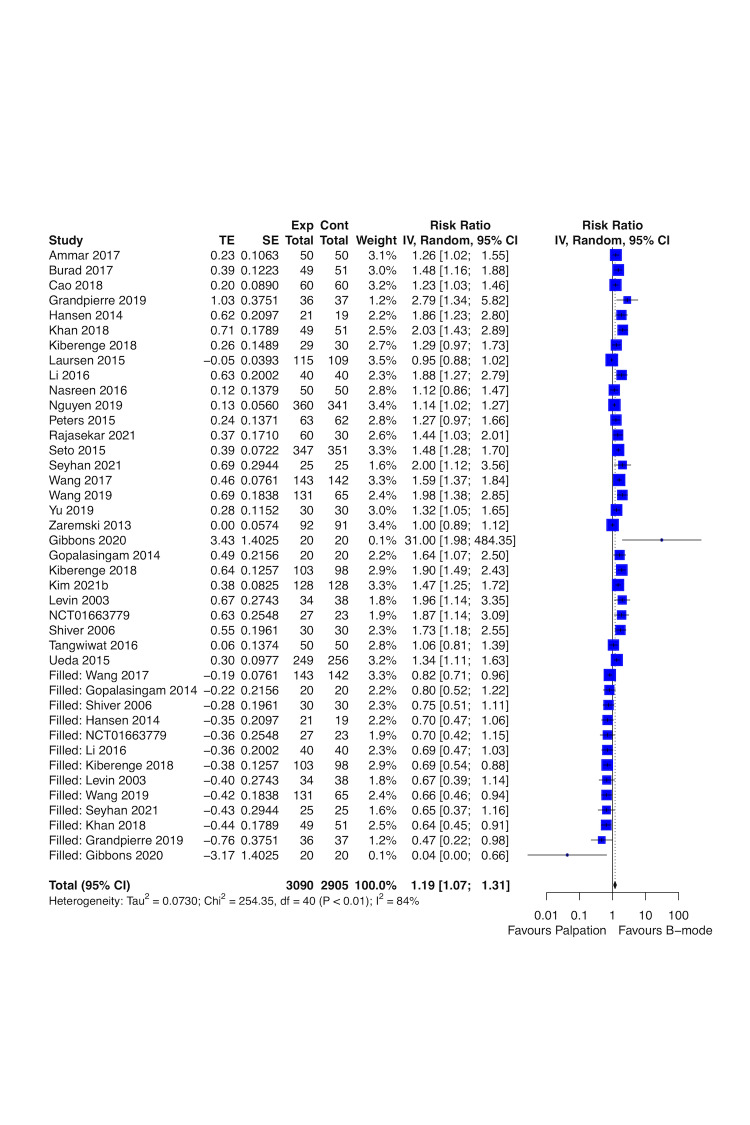
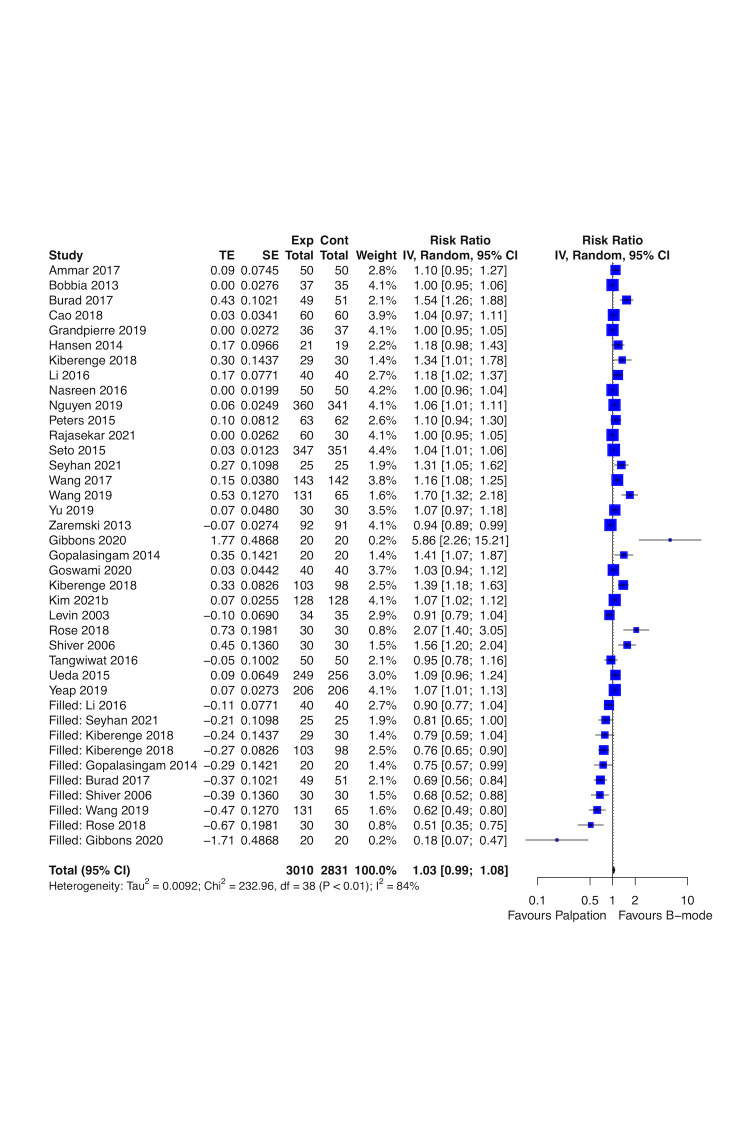
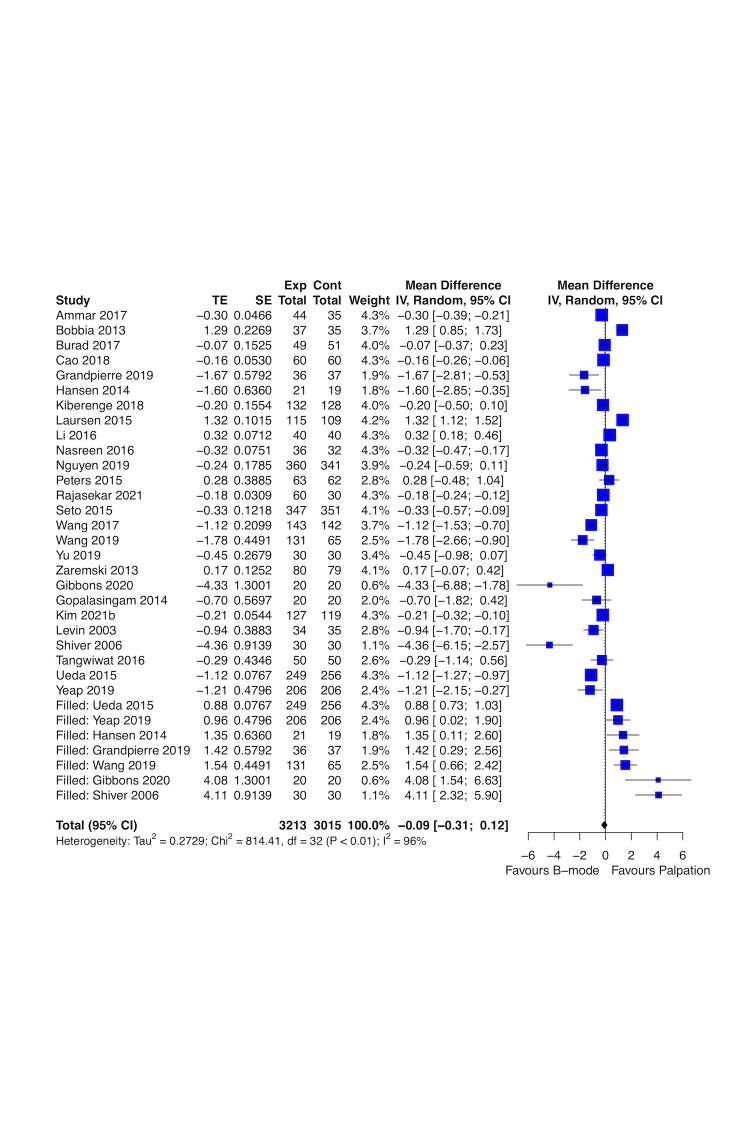
Real‐time B‐mode ultrasound guidance may improve first attempt success rate (risk ratio (RR) 1.44, 95% confidence interval (CI) 1.29 to 1.61; 4708 participants, 27 studies; low‐certainty evidence) and overall success rate (RR 1.11, 95% CI 1.06 to 1.16; 4955 participants, 28 studies; low‐certainty evidence), and may decrease time needed for a successful procedure (mean difference (MD) ‐0.33 minutes, 95% CI ‐0.54 to ‐0.13; 4902 participants, 26 studies; low‐certainty evidence) up to one hour compared to palpation and landmarks. Real‐time B‐mode ultrasound guidance probably decreases major haematomas (RR 0.35, 95% CI 0.23 to 0.56; 2504 participants, 16 studies; moderate‐certainty evidence). It is uncertain whether real‐time B‐mode ultrasound guidance has any effect on pseudoaneurysm, pain, and quality of life (QoL) compared to palpation and landmarks (very low‐certainty evidence).
Real‐time B‐mode ultrasound versus DUA
One study (493 participants) showed that real‐time B‐mode ultrasound guidance probably improves first attempt success rate (RR 1.35, 95% CI 1.11 to 1.64; moderate‐certainty evidence) and time needed for a successful procedure (MD ‐1.57 minutes, 95% CI ‐1.78 to ‐1.36; moderate‐certainty evidence) up to 72 hours compared to DUA. Real‐time B‐mode ultrasound guidance may improve overall success rate (RR 1.13, 95% CI 0.99 to 1.29; low‐certainty evidence) up to 72 hours compared to DUA. Pseudoaneurysm, major haematomas, pain, and QoL were not reported.
Real‐time B‐mode ultrasound versus modified real‐time B‐mode ultrasound
Real‐time B‐mode ultrasound guidance may decrease first attempt success rate (RR 0.68, 95% CI 0.55 to 0.84; 153 participants, 2 studies; low‐certainty evidence), may decrease overall success rate (RR 0.93, 95% CI 0.86 to 1.01; 153 participants, 2 studies; low‐certainty evidence), and may lead to no difference in time needed for a successful procedure (MD 0.04 minutes, 95% CI ‐0.01 to 0.09; 153 participants, 2 studies; low‐certainty evidence) up to one hour compared to modified real‐time B‐mode ultrasound guidance. It is uncertain whether real‐time B‐mode ultrasound guidance has any effect on major haematomas compared to modified real‐time B‐mode ultrasound (very low‐certainty evidence). Pseudoaneurysm, pain, and QoL were not reported.
In‐plane versus out‐of‐plane B‐mode ultrasound
In‐plane real‐time B‐mode ultrasound guidance may lead to no difference in overall success rate (RR 1.00, 95% CI 0.96 to 1.05; 1051 participants, 8 studies; low‐certainty evidence) and in time needed for a successful procedure (MD ‐0.06 minutes, 95% CI ‐0.16 to 0.05; 1134 participants, 9 studies; low‐certainty evidence) compared to out‐of‐plane B‐mode ultrasound up to one hour. It is uncertain whether in‐plane real‐time B‐mode ultrasound guidance has any effect on first attempt success rate or major haematomas compared to out‐of‐plane B‐mode ultrasound (very low‐certainty evidence). Pseudoaneurysm, pain, and QoL were not reported.
DUA versus palpation and landmarks
DUA may lead to no difference in first attempt success rate (RR 1.01, 95% CI 0.90 to 1.14; 666 participants, 2 studies; low‐certainty evidence) or overall success rate (RR 0.99, 95% CI 0.92 to 1.07; 666 participants, 2 studies; low‐certainty evidence) and probably increases time needed for a successful procedure (MD 0.45 minutes, 95% CI 0.20 to 0.70; 500 participants, 1 study; moderate‐certainty evidence) up to 72 hours compared to palpation and landmarks. Pseudoaneurysm, major haematomas, pain, and QoL were not reported.
Oblique‐axis versus long‐axis in‐plane B‐mode ultrasound
Oblique‐axis in‐plane B‐mode ultrasound guidance may increase overall success rate (RR 1.27, 95% CI 1.05 to 1.53; 215 participants, 2 studies; low‐certainty evidence) up to 72 hours compared to long‐axis in‐plane B‐mode ultrasound. It is uncertain whether oblique‐axis in‐plane B‐mode ultrasound guidance has any effect on first attempt success rate, time needed for a successful procedure, and major haematomas compared to long‐axis in‐plane B‐mode ultrasound. Pseudoaneurysm, pain, and QoL were not reported.
We are uncertain about effects in the following comparisons due to very low‐certainty evidence and unreported outcomes: real‐time B‐mode ultrasound versus palpation and landmarks (axillary and dorsalis pedis arteries), real‐time B‐mode ultrasound versus near‐infrared laser (radial artery), and dynamic versus static out‐of‐plane B‐mode ultrasound (radial artery).
Authors' conclusions
Real‐time B‐mode ultrasound guidance may improve first attempt success rate, overall success rate, and time needed for a successful procedure for radial artery catheterisation compared to palpation, or DUA. In addition, real‐time B‐mode ultrasound guidance probably decreases major haematomas compared to palpation. However, we are uncertain about the evidence on major haematomas and pain for other comparisons due to very low‐certainty evidence and unreported outcomes. We are also uncertain about the effects on pseudoaneurysm and QoL for axillary and dorsalis pedis arteries catheterisation. Given that first attempt success rate and pseudoaneurysm are the most relevant outcomes for people who underwent arterial catheterisation, future studies must measure both. Future trials must be large enough to detect effects, use validated scales, and report longer‐term follow‐up.
Plain language summary
Ultrasound to guide arterial (other than femoral) punctures and cannulation in adults
Research question
What is the effectiveness and safety of ultrasound technologies to guide arterial (other than femoral) punctures and cannulation in adults?
Background
Despite the availability of devices that help health professionals to access arteries, unwanted events such as pneumothorax (air outside the lung and inside the thorax), haemothorax (blood outside the lung and inside the thorax), haematoma (blooding in skin and other tissues), amputation, and death may happen. Additional techniques such as ultrasound may be useful for improving these results, but their effects for arterial access in adults remain under debate.
Study characteristics
Review authors identified 48 studies that evaluated the effects of different types of ultrasound guidance for adults who underwent arterial puncture or cannulation. Studies were conducted in hospitals and mainly for diagnostic purposes (smaller devices). Review authors identified the studies included in this review through electronic literature searches conducted up to May 2021.
Key results
Real‐time visual ultrasound guidance improved first attempt success rate, overall success rate, and time needed for a successful procedure for up to one month, mainly in radial artery, compared to palpation or non‐visual ultrasound guidance. In addition, real‐time visual ultrasound guidance probably decreased major haematomas compared to palpation. However, we are uncertain about the effects on major haematomas and on pain for other comparisons due to very low‐certainty evidence and unreported outcomes. We are also uncertain about the effects on pseudoaneurysm and QoL for axillary and dorsalis pedis arteries catheterisation.
Quality of evidence
We found very low‐ to moderate‐certainty evidence comparing real‐time visual ultrasound guidance versus palpation, and comparing one ultrasound guidance type versus another.
Summary of findings
Summary of findings 1. [Axillary] B‐mode ultrasound guidance compared to palpation and landmarks for arterial (other than femoral) catheterisation in adults.
| [Axillary] B‐mode ultrasound guidance compared to palpation and landmarks for axillary artery catheterisation in adults | |||||
| Patient or population: adults requiring axillary artery catheterisation Setting: ICU Intervention: B‐mode ultrasound guidance Comparison: palpation and landmarks | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with palpation and landmarks | Risk difference with [axillary] B‐mode ultrasound guidance | ||||
| First‐attempt success rate | not reported | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) |
33 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 1.35 (0.99 to 1.86) | study population | |
| 733 per 1000 | 257 more per 1000 (7 fewer to 631 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) |
33 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | mean time needed for a successful procedure was 9.288 minutes | MD 2.27 lower (7.36 lower to 2.82 higher) |
| Major haematoma Follow‐up: end of the procedure (< 1 hour) |
33 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 0.83 (0.06 to 12.22) | study population | |
| 67 per 1000 | 11 fewer per 1000 (63 fewer to 748 more) | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICU: intensive care unit; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of high risk of performance bias.
bDowngraded two levels due to imprecision: few participants, few studies, and 95% CI consistent with possible benefit and possible harm.
cDowngraded one level due to indirectness: few participants are not representative of the overall relevant population
Summary of findings 2. [Dorsalis pedis] B‐mode ultrasound guidance compared to palpation and landmarks for arterial (other than femoral) catheterisation in adults.
| [Dorsalis pedis] B‐mode ultrasound guidance compared to palpation and landmarks for dorsalis pedis artery catheterisation in adults | |||||
| Patient or population: adults requiring dorsalis pedis artery catheterisation Setting: operating room Intervention: B‐mode ultrasound guidance Comparison: palpation and landmarks | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with palpation and landmarks | Risk difference with [dorsalis pedis] B‐mode ultrasound guidance | ||||
| First‐attempt success rate Follow‐up: end of the procedure (< 1 hour) |
60 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 1.28 (0.90 to 1.82) | study population | |
| 600 per 1000 | 168 more per 1000 (60 fewer to 492 more) | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) |
60 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d | RR 1.00 (0.91 to 1.10) | study population | |
| 967 per 1000 | 0 fewer per 1000 (87 fewer to 97 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) |
60 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | mean time needed for a successful procedure was 0.58 minutes | MD 0.04 lower (0.16 lower to 0.08 higher) |
| Major haematoma | not reported | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of high risk of performance bias.
bDowngraded two levels due to imprecision: few participants, few studies, and 95% CI consistent with possible benefit and possible harm.
cDowngraded one level due to indirectness: few participants are not representative of the overall relevant population.
dDowngraded one level due to imprecision: few participants and few studies.
Summary of findings 3. [Radial] B‐mode ultrasound guidance compared to palpation and landmarks for arterial (other than femoral) catheterisation in adults.
| [Radial] B‐mode ultrasound guidance compared to palpation and landmarks for radial artery catheterisation in adults | |||||
| Patient or population: adults requiring radial artery catheterisation Setting: hospital Intervention: B‐mode ultrasound guidance Comparison: palpation and landmarks | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with palpation and landmarks | Risk difference with [radial] B‐mode ultrasound guidance | ||||
| First‐attempt success rate Follow‐up: from end of the procedure (< 1 hour) to 1 day |
4708 (27 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | RR 1.44 (1.29 to 1.61) | study population | |
| 542 per 1000 | 239 more per 1000 (157 more to 331 more) | ||||
| Pseudomaneurysm Follow‐up: up to 1 month |
679 (1 RCT) | ⊕⊝⊝⊝ VERY LOWd,e,f | RR 2.89 (0.12 to 70.63) | study population | |
| 0 per 333 | 1 per 346 (absolute risk with B‐mode ultrasound guidance) | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) to 1 day |
4955 (28 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | RR 1.11 (1.06 to 1.16) | study population | |
| 833 per 1000 | 92 more per 1000 (50 more to 133 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) to 1 day |
4902 (26 RCTs) | ⊕⊕⊝⊝ LOWa,b,g | ‐ | mean time needed for a successful procedure was 2.302 minutes | MD 0.33 lower (0.54 lower to 0.13 lower) |
| Major haematoma Follow‐up: end of the procedure (< 1 hour) to 1 month |
2504 (16 RCTs) | ⊕⊕⊕⊝ MODERATEh | RR 0.35 (0.23 to 0.56) | study population | |
| 122 per 1000 | 79 fewer per 1000 (94 fewer to 54 fewer) | ||||
| Adverse events (pain) Assessed with: VAS Scale from 0 to 10 Follow‐up: end of the procedure (< 1 hour) to 24 hours |
883 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWb,d,f,i | ‐ | mean number of adverse events (pain) was 1.849 | MD 0.81 higher (0.66 lower to 2.28 higher) |
| Quality of life (satisfaction) Assessed with: VAS Scale from 0 to 10 Follow‐up: end of the procedure (< 1 hour) |
72 (1 RCT) | ⊕⊝⊝⊝ VERY LOWd,f,j | ‐ | mean quality of life (satisfaction) was 7 | MD 0 (1.07 lower to 1.07 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of selection, performance, detection, attrition, reporting, and other bias.
bDowngraded half a level due to inconsistency: unexplained substantial heterogeneity.
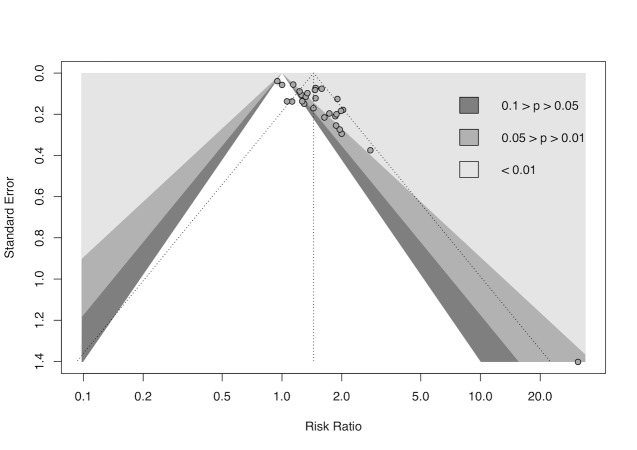
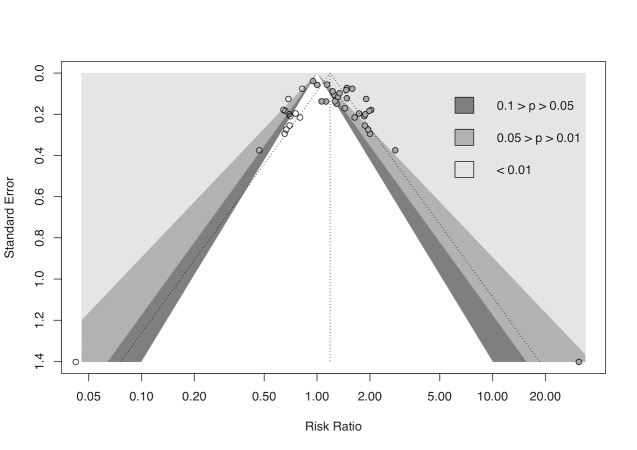
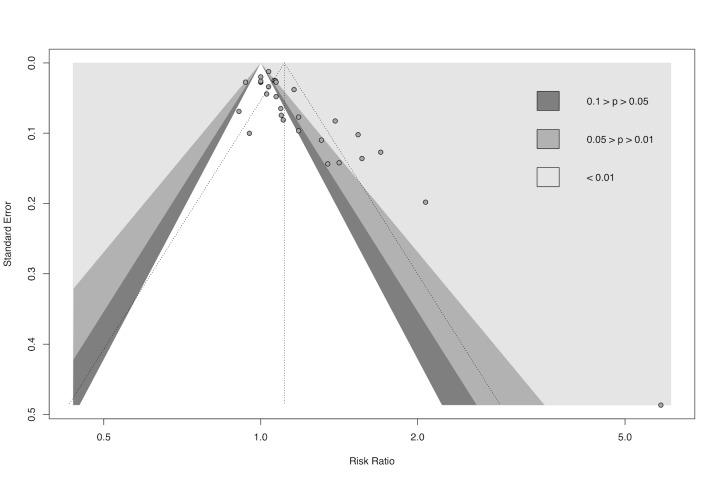
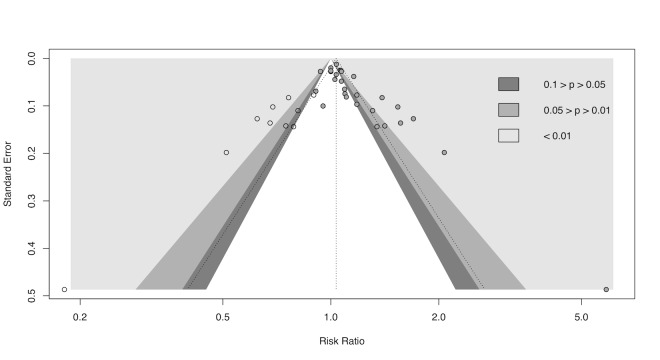
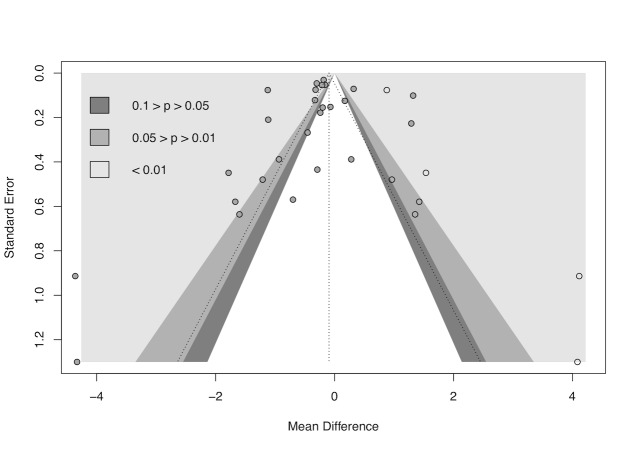
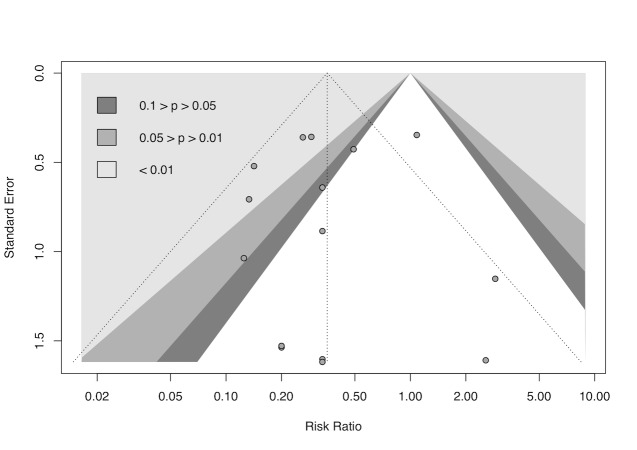
cDowngraded half a level due to suspected publication bias: funnel plot asymmetrical and statistical tests compatible with impaired effect size after correction by publication bias.
dDowngraded two levels due to imprecision: wide 95% CI consistent with possible benefit and possible harm.
eDowngraded one level due to high risk of performance bias.
fDowngraded half a level due to suspected publication bias: a large number of included trials did not contribute to this outcome.
gDowngraded half a level due to suspected publication bias: funnel plot symmetrical, but statistical tests compatible with impaired effect size after correction by publication bias.
hDowngraded one level due to high risk of performance, detection, attrition, reporting, and other bias.
iDowngraded one level due to high risk of attrition and reporting bias.
jDowngraded one level due to indirectness: few participants are not representative of the overall relevant population.
Summary of findings 4. [Radial] B‐mode ultrasound compared to Doppler assistance for arterial (other than femoral) catheterisation in adults.
| [Radial] B‐mode ultrasound compared to Doppler assistance for radial artery catheterisation in adults | |||||
| Patient or population: adults requiring radial artery catheterisation Setting: hospital Intervention: B‐mode ultrasound Comparison: Doppler assistance | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Doppler assistance | Risk difference with [radial] B‐mode ultrasound | ||||
| First‐attempt success rate Follow‐up: end of the procedure (< 1 hour) |
493 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | RR 1.35 (1.11 to 1.64) | study population | |
| 393 per 1000 | 138 more per 1000 (43 more to 252 more) | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) |
493 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | RR 1.13 (0.99 to 1.29) | study population | |
| 602 per 1000 | 78 more per 1000 (6 fewer to 175 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) |
493 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ‐ | mean time needed for a successful procedure was 2.138 minutes | MD 1.57 lower (1.78 lower to 1.36 lower) |
| Major haematoma | not reported | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of reporting and other bias.
bDowngraded one level due to imprecision: 95% CI consistent with possible benefit and possible harm.
Summary of findings 5. [Radial] B‐mode ultrasound compared to near‐infrared laser guidance for arterial (other than femoral) catheterisation in adults.
| [Radial] B‐mode ultrasound compared to near‐infrared laser guidance for radial artery catheterisation in adults | |||||
| Patient or population: adults requiring radial artery catheterisation Setting: hospital Intervention: B‐mode ultrasound Comparison: near‐infrared laser guidance | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with near‐infrared laser guidance | Risk difference with [radial] B‐mode ultrasound | ||||
| First‐attempt success rate Follow‐up: end of the procedure (< 1 hour) |
72 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 1.33 (0.82 to 2.16) | study population | |
| 583 per 1000 | 193 more per 1000 (105 fewer to 677 more) | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) |
72 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 1.50 (0.27 to 8.45) | study population | |
| 944 per 1000 | 472 more per 1000 (689 fewer to 7.036 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) |
72 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,c,d | ‐ | mean time needed for a successful procedure was 0.189 minutes | MD 0.2 higher (0.09 higher to 0.31 higher) |
| Major haematoma | not reported | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to imprecision: few participants and 95% CI consistent with possible benefit and possible harm.
bDowngraded one level due to indirectness: few participants are not representative of the overall relevant population.
cDowngraded one level due to risk of high risk of performance bias.
dDowngraded one level due to imprecision: few participants.
Summary of findings 6. [Radial] B‐mode ultrasound compared to modified B‐mode ultrasound for arterial (other than femoral) catheterisation in adults.
| [Radial] B‐mode ultrasound compared to modified B‐mode ultrasound for radial artery catheterisation in adults | |||||
| Patient or population: adults requiring radial artery catheterisation Setting: hospital Intervention: B‐mode ultrasound Comparison: modified B‐mode ultrasound | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with modified B‐mode ultrasound | Risk difference with [radial] B‐mode ultrasound | ||||
| First‐attempt success rate Follow‐up: end of the procedure (< 1 hour) to 1 day |
153 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | RR 0.68 (0.55 to 0.84) | study population | |
| 831 per 1000 | 266 fewer per 1000 (374 fewer to 133 fewer) | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) to 1 day |
153 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | RR 0.93 (0.86 to 1.01) | study population | |
| 974 per 1000 | 68 fewer per 1000 (136 fewer to 10 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) to 1 day |
153 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | ‐ | mean time needed for a successful procedure was 0.384 minutes | MD 0.04 higher (0.01 lower to 0.09 higher) |
| Major haematoma Follow‐up: end of the procedure (< 1 hour) to 1 day |
153 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,d | RR 3.23 (1.37 to 7.60) | study population | |
| 78 per 1000 | 174 more per 1000 (29 more to 514 more) | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of high risk of performance bias.
bDowngraded half a level due to inconsistency: unexplained substantial heterogeneity.
cDowngraded half a level due to imprecision: few participants.
dDowngraded two levels due to imprecision: few participants and 95% CI consistent with possible benefit and possible harm.
Summary of findings 7. [Radial] In‐plane B‐mode ultrasound compared to out‐of‐plane B‐mode ultrasound for arterial (other than femoral) catheterisation in adults.
| [Radial] In‐plane B‐mode ultrasound compared to out‐of‐plane B‐mode ultrasound for radial artery catheterisation in adults | |||||
| Patient or population: adults requiring radial artery catheterisation Setting: hospital Intervention: in‐plane B‐mode ultrasound Comparison: out‐of‐plane B‐mode ultrasound | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with out‐of‐plane B‐mode ultrasound | Risk difference with [radial] In‐plane B‐mode ultrasound | ||||
| First‐attempt success rate Follow‐up: end of the procedure (< 1 hour) |
1051 (8 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 0.85 (0.65 to 1.12) | study population | |
| 743 per 1000 | 111 fewer per 1000 (260 fewer to 89 more) | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) |
1051 (8 RCTs) | ⊕⊕⊝⊝ LOWa,b | RR 1.00 (0.96 to 1.05) | study population | |
| 880 per 1000 | 0 fewer per 1000 (35 fewer to 44 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) |
1134 (9 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ | mean time needed for a successful procedure was 0.771 minutes | MD 0.06 lower (0.16 lower to 0.05 higher) |
| Major haematoma Follow‐up: end of the procedure (< 1 hour) |
1159 (9 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 0.49 (0.22 to 1.08) | study population | |
| 144 per 1000 | 73 fewer per 1000 (112 fewer to 11 more) | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of performance, detection, attrition, reporting, and other bias.
bDowngraded one level due to inconsistency: unexplained substantial heterogeneity.
cDowngraded one level due to imprecision: 95% CI consistent with possible benefit and possible harm.
Summary of findings 8. [Radial] Doppler assistance compared to palpation and landmarks for arterial (other than femoral) catheterisation in adults.
| [Radial] Doppler assistance compared to palpation and landmarks for radial artery catheterisation in adults | |||||
| Patient or population: adults requiring radial artery catheterisation Setting: hospital Intervention: Doppler ultrasound assistance Comparison: palpation and landmarks | |||||
| Outcomes | №, of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with palpation and landmarks | Risk difference with [radial] Doppler assistance | ||||
| First‐attempt success rate Follow‐up: end of the procedure (< 1 hour) |
666 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | RR 1.01 (0.90 to 1.14) | study population | |
| 509 per 1000 | 5 more per 1000 (51 fewer to 71 more) | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) |
666 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | RR 0.99 (0.92 to 1.07) | study population | |
| 723 per 1000 | 7 fewer per 1000 (58 fewer to 51 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) |
500 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ‐ | mean time needed for a successful procedure was 1.688 minutes | MD 0.45 higher (0.2 higher to 0.7 higher) |
| Major haematoma | not reported | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of reporting and other bias.
bDowngraded one level due to imprecision: 95% CI consistent with possible benefit and possible harm.
Summary of findings 9. [Radial] Dynamic out‐of‐plane B‐mode ultrasound compared to static out‐of‐plane B‐mode ultrasound for arterial (other than femoral) catheterisation in adults.
| [Radial] Dynamic out‐of‐plane B‐mode ultrasound compared to static out‐of‐plane B‐mode ultrasound for radial artery catheterisation in adults | |||||
| Patient or population: adults requiring radial artery catheterisation Setting: hospital Intervention: dynamic out‐of‐plane B‐mode ultrasound Comparison: static out‐of‐plane B‐mode ultrasound | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with static out‐of‐plane B‐mode ultrasound | Risk difference with [radial] dynamic out‐of‐plane B‐mode ultrasound | ||||
| First‐attempt success rate Follow‐up: end of the procedure (< 1 hour) |
131 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 0.91 (0.67 to 1.23) | study population | |
| 591 per 1000 | 53 fewer per 1000 (195 fewer to 136 more) | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: end of the procedure (< 1 hour) |
131 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d | RR 1.07 (0.92 to 1.25) | study population | |
| 803 per 1000 | 56 more per 1000 (64 fewer to 201 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) |
131 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d | ‐ | mean time needed for a successful procedure was 0.981 minutes | MD 0.37 higher (0.07 higher to 0.66 higher) |
| Major haematoma | not reported | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of high risk of performance bias.
bDowngraded two levels due to imprecision: few participants, few studies, and 95% CI consistent with possible benefit and possible harm.
cDowngraded one level due to indirectness: few participants are not representative of the overall relevant population.
dDowngraded one level due to imprecision: few participants.
Summary of findings 10. [Radial] Oblique‐axis in‐plane B‐mode ultrasound compared to long‐axis in‐plane B‐mode ultrasound for arterial (other than femoral) catheterisation in adults.
| [Radial] Oblique‐axis in‐plane B‐mode ultrasound compared to long‐axis in‐plane B‐mode ultrasound for radial artery catheterisation in adults | |||||
| Patient or population: adults requiring radial artery catheterisation Setting: hospital Intervention: oblique‐axis in‐plane B‐mode ultrasound Comparison: long‐axis in‐plane B‐mode ultrasound | |||||
| Outcomes | №. of participants (studies) Follow‐up | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with long‐axis in‐plane B‐mode ultrasound | Risk difference with [radial] oblique‐axis in‐plane B‐mode ultrasound | ||||
| First‐attempt success rate Follow‐up: end of the procedure (< 1 hour) to 72 hours |
275 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | RR 1.11 (0.44 to 2.79) | study population | |
| 326 per 1000 | 36 more per 1000 (183 fewer to 583 more) | ||||
| Pseudoaneurysm | not reported | ||||
| Overall success rate Follow‐up: up to 72 hours |
215 (2 RCTs) | ⊕⊕⊝⊝ LOWd,e | RR 1.27 (1.05 to 1.53) | study population | |
| 571 per 1000 | 154 more per 1000 (29 more to 303 more) | ||||
| Time needed for a successful procedure Follow‐up: end of the procedure (< 1 hour) to 72 hours |
275 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,c,e | ‐ | mean time needed for a successful procedure was 0.634 minutes | MD 0.35 lower (0.95 lower to 0.25 higher) |
| Major haematoma Follow‐up: up to 72 hours |
215 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWb,d | RR 0.68 (0.32 to 1.47) | Study population | |
| 133 per 1000 | 43 fewer per 1000 (91 fewer to 63 more) | ||||
| Adverse events (pain) | not reported | ||||
| Quality of life | not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of high risk of performance, detection, attrition, and reporting bias.
bDowngraded two levels due to imprecision: few participants, few studies, and 95% CI consistent with possible benefit and possible harm.
cDowngraded one level due to inconsistency: unexplained substantial heterogeneity.
dDowngraded one level due to risk of high risk of performance, detection, and attrition bias.
eDowngraded one level due to imprecision: few participants.
Background
See Appendix 1 for a glossary of terms.
Description of the condition
In all medical specialties that encounter critically ill patients requiring invasive blood pressure measurements ‐ for instance, in the intensive care unit (ICU), emergency room, or surgical theatre; and for some diagnostic and therapeutic procedures, such as arterial catheterisation for cardiac angiography or percutaneous coronary intervention (PCI) ‐ arterial cannulation is the primary pathway for intravascular access. Arterial access for cardiovascular procedures, such as arterial blood sampling and blood pressure monitoring, may be performed using almost all peripheral arteries. The puncture site is commonly selected based on the diameter of devices to be used in the procedure and characteristics of the patient’s body (e.g. obesity, previous surgery, arterial stenosis, occlusion). In the vascular catheterisation setting, clinicians constantly seek to attain lower puncture injury rates and to reduce any other possible setbacks for better safety rates in diagnostic angiography, percutaneous intervention, or arterial monitoring (Sandoval 2017).
The transfemoral approach can be used for artery access, mainly when the devices used present wider diameters, such as for transcatheter aortic valve implantation (Pascual 2017). A randomised controlled trial (RCT) showed that ultrasound‐guided cannulation of the femoral artery improved access in patients with a weak arterial pulse and a hostile groin (Dudeck 2004). Investigators in the FAUST trial achieved better results with ultrasound‐guided femoral artery access in comparison with fluoroscopic access (Seto 2010). The same result was found when devices with wider diameters were necessary for endovascular placement of stent grafts (20 French or wider) (Arthurs 2008). There is a Cochrane registered title that aims to study the differences between ultrasound‐guided femoral artery access and landmark access (Strauss 2021).
The femoral artery approach presents complications such as bleeding, pseudoaneurysm, and dissection, among others (Flumignan 2018). These complications contribute to high costs and significant morbidity and mortality. A Cochrane Review compared transfemoral and transradial approaches for the diagnosis and treatment of cardiac disease. Kolkailah 2018 found that transradial access, although associated with increased radiation exposure and technique difficulties, demonstrated fewer access complications and less bleeding and death in the first 30 days.
Femoral access is still used for arterial access procedures because it allows for the use of devices of all sizes and it is an easily accessible site (one of the first sites used for arterial procedures such as angioplasties). Non‐femoral vascular access ‐ such as via the radial artery ‐ is common for PCI, however, and may be related to lower adverse event rates (Aboyans 2018; Attie 2019; Feldman 2013).
The radial artery is the most‐used site of arterial access for invasive blood pressure monitoring and for arterial blood gas sampling. Transradial access is preferable for patients with peripheral artery disease (PAD) in the lower limbs, as this access route appears to be safer for this patient population, with lower rates of vascular complications, including significant bleeding; it also allows patients to be mobile immediately following the procedure in contrast to transfemoral access (Aboyans 2018; Brueck 2009; Jolly 2009). Regarding cardiac procedures in the USA, however, it has been shown that less than 1.5% of PCIs were performed by the transradial access route between 2004 and 2007 (Rao 2008), with a slight increase between 2007 and 2012, and one PCI via radial access was used for every six procedures performed (Feldman 2013). Data are available regarding the feasibility of this access for non‐cardiac procedures, such as endovascular treatment of carotid disease (Jaroenngarmsamer 2019). Some factors have influenced study results for radial access. The permeability of the radioulnar arch, for instance, is still open to debate, mainly after release of results of the RADAR study (predictive value of Allen's test result in elective patients undergoing coronary catheterisation by radial approach), which reported no major ischaemic complications for patients with an incomplete palmar arch (Valgimigli 2014). Seto 2015 reported that ultrasound guidance may be better than palpation alone for radial artery cannulation in adults, but this is still under debate. Aouad‐Maroun 2016 found "moderate‐quality evidence suggesting that ultrasound guidance for radial artery cannulation improves first and second attempt success rates and decreases the rate of complications as compared with palpation or Doppler auditory assistance" in paediatric patients, but no similarly robust evidence is available for adults.
Current data show that brachial access is uncommon for arterial procedures. Parviz 2015 reported that in the UK, only 0.44% of all 26,602 procedures between 2005 and 2014 were performed via brachial access. Brachial access is an effective artery‐access option, primarily for treatment of lower‐limb PAD when femoral access cannot be used (e.g. graft or occlusion in a femoral path) because it is a more favourable entry route for procedures in caudal‐oriented visceral arteries, and because the brachial artery allows the use of larger‐diameter devices than can be used for the radial artery. The complication rate associated with brachial access is similar to that associated with femoral access and may be minimised via ultrasound‐guided puncture (Franz 2017; Lee 2015).
Percutaneous access through the axillary artery is a strategy used for more difficult endovascular interventions or in the absence of other feasible arterial access, mainly due to the particular location of this artery. As this artery is in close proximity to local nerves and the axillary vein, and has a relatively deep location, the use of ultrasonography to aid its catheterisation may be beneficial, reducing the risk of local iatrogenic lesions (Harris 2018).
Other less common sites ‐ direct aortic, carotid, or subclavian accesses ‐ for transcatheter aortic valve implantation (TAVI) and for popliteal or tibial arteries, mainly in critical arterial lesions of the lower limbs, also have utility. However, available evidence on the benefits and the best ways to perform these punctures remains under debate (Aboyans 2018; Conte 2019).
Description of the intervention
To cannulate an artery, healthcare providers primarily use the Seldinger technique, which consists of puncturing the anterior artery wall, passing a guidewire, removing the needle, and finally cannulating the artery through this guidewire with any medical device. Use of the anterior arterial wall puncture seems to be a good choice compared to total arterial transfixation because many patients who undergo an arterial puncture have critical illnesses, such as coagulopathy, or have been advised to use anticoagulants or antiplatelet agents (or both). These illnesses or medicines may cause any puncture to become a site of possible complications, such as bleeding or a pseudoaneurysm. Transfixation of the target artery can add some risk of inadvertent bleeding or even puncture of other nearby structures. Moreover, some 'catheter‐over‐needle' devices allow introduction of the guidewire initially through a catheter instead of initially through a needle (original Seldinger technique), and this may provide some advantages in simplifying the process, mainly in reduced calibre vessels. All subsequent interventions will be added to the Seldinger technique for a complete arterial catheterisation (Aboyans 2018; Flumignan 2018; Gopalasingam 2017; Hansen 2014; Higgs 2005; Kendall 2014; Seldinger 1953; Song 2016; Song 2018).
Palpation and landmarks
Anatomical marks are used as a guide for catheterisation, with or without a scope, in most procedures performed. To identify a reference point, pulse palpation is the most commonly used approach for insertion of an arterial catheter. The artery is localised using palpation for the subsequent puncture and cannulation attempt. However, haemodynamic instability, hypotension, or other shock‐causing conditions may hamper the palpation technique, as these make the pulse weaker and more difficult to find. Also, because of underlying diseases such as atherosclerosis, the pulse may not be present in a determined region, making it impossible to use this technique for puncture. Palpation of deeper arteries (e.g. axillary artery) and pulse palpation in patients with a higher body mass index (BMI) can be other challenges, mainly during the learning curve of the practitioner who will perform the procedure (AIUM 2013; Soverow 2016).
Doppler auditory ultrasound assistance
Doppler auditory ultrasound assistance (DUA) has been described as an alternative to the traditional palpation technique for arterial catheter insertion. A change in the Doppler tone to a higher tone suggests that the target artery has been located. Loss of Doppler sound is expected during the procedure, when the artery is compressed by the puncture (Ueda 2013).
Indirect ultrasound guidance
Indirect ultrasound guidance (IUG), or ultrasound‐assisted arterial cannulation, is defined as vessel imaging used to confirm location and patency, followed by arterial cannulation, without real‐time needle guidance. Commonly, IUG is performed by looking for the vessel using B‐mode ultrasound and marking the puncture site on the skin. Subsequently, the healthcare provider punctures the artery and performs the catheterisation without any sonographic guidance. IUG is coupled with arterial cannulation to facilitate locating the arteries and nearby structures (e.g. nerves, veins) and to help make the procedure safer, faster, as complication‐free as possible, and successful more often (Attie 2019; Lamperti 2012).
Direct ultrasound guidance
Direct ultrasound guidance (DUG), or ultrasound‐guided arterial cannulation, is defined as real‐time needle guidance via B‐mode ultrasound for vessel puncture and cannulation (Lamperti 2012). DUG is performed by a sterile technique, with the ultrasound probe inside a sterile cover, and is aided by sterile ultrasound gel (AIUM 2013).
During passage of the needle into the vessel, the artery can be seen by a transverse (short artery axis) view or a longitudinal (long artery axis) view. Benefits of the transverse artery view include a shorter learning curve and easier visualisation of small vessels. However, the transverse approach allows only a cross‐section of the artery to be visualised by DUG and may lead to errors in direction perception of the needle. Regarding needle visualisation, vessel access via DUG can be performed through two different techniques: in‐plane puncture technique; and out‐of‐plane technique. For the in‐plane puncture technique, the ultrasound plane and the longitudinal needle axis are in the same virtual plane. The in‐plane puncture allows continuous visualisation of the needle along its trajectory until it reaches the vessel. In contrast, for the out‐of‐plane technique, the ultrasound plane and the longitudinal needle axis are not in the same virtual plane (Lamperti 2012). The American College of Emergency Physicians has recommended the longitudinal needle view (i.e. in‐plane approach) because it permits the operator to trace the entire path and angle of the needle starting from the entry site at the skin (Kendall 2014).
How the intervention might work
When used for arterial puncture, the different ultrasound modes (i.e. DUA, IUG, and DUG) aim to improve correct identification of the target vessel location; to identify possible cannulation obstacles (e.g. artery obstruction or occlusion); and to avoid adverse events (e.g. inadvertent vein puncture, nerve lesion, blood leaks by multiple unnecessary artery punctures). Additional resources for better artery localisation may be beneficial in some special situations, such as for patients with a high body mass index (BMI), anatomical variations, or arterial obstruction or occlusion; or for critically ill patients and those in need of multiple punctures when palpation and landmarks are insufficient (Kendall 2014; Lamperti 2012). Without direct visualisation, the risk of complications is increased: these complications include bleeding, inadvertent nerve or venous injury, and pseudoaneurysm and puncture failure, among others (AIUM 2013; Soverow 2016).
Regarding first‐attempt punctures for radial artery access in adults, low‐certainty evidence suggests there is no difference between DUA and palpation; however, success rates of 46% were achieved in small children using this type of access (Gu 2016; Ueda 2013). IUG is coupled to arterial catheterisation to facilitate locating arteries and nearby structures (e.g. nerves, veins). IUG can result in procedures that are safer and faster, have fewer complications, and are more often successful. Then again, because the ultrasound evaluation is not conducted at the same time as the puncture, its real benefits are not clear (Attie 2019; Lamperti 2012).
For arterial access, DUG can reduce possible bleeding and other complications associated with this procedure. For radial and femoral arterial access, Nguyen 2019 reported that DUG increased success rates with shorter procedure times and a reduced number of puncture attempts, and it allowed for fewer difficult accesses or inadvertent venipunctures. However, the clinical effects regarding DUG for all arterial access procedures are still under discussion (Attie 2019; Gu 2016; Lamperti 2012). Although it is more challenging, Lamperti 2012 supports the in‐plane technique for all DUG procedures because it is related to higher precision and fewer complications. The longitudinal artery view allows better visualisation of the advancing needle tip, which may reduce perforation of the posterior vessel wall (Kendall 2014). It is recommended that the external diameter of the catheter should not exceed one‐third the internal diameter of the vein to avoid the risk of thrombosis, but similar evidence for arterial access is sparse. Additional benefits of DUG include the ability to change position before puncture and to measure the diameter of the artery during the procedure (Lamperti 2012).
Why it is important to do this review
Vascular access is a frequently performed procedure, with a high possibility of adverse events (e.g. pneumothorax, haemothorax, haematoma, amputation, death), and additional techniques such as ultrasound may be useful for improving outcomes. Arterial catheterisation is an intervention that is commonly performed in several settings, including major surgeries, emergency units, catheterisation laboratories, and ICUs for continuous blood pressure monitoring and arterial blood sampling. Moreover, arterial catheterisation is the main access for endovascular procedures, such as angioplasty and stenting (Sandoval 2017).
Given that the numbers of cardiovascular procedures and intensive antithrombotic therapies are on the rise, complications among patients undergoing endovascular procedures should not be underestimated. Paganin 2018 described a complication rate of almost 9% after puncture, which included minor and major haematomas, as well as stable and unstable bleeding. Therefore, the approaches used to reduce complications at arterial puncture sites may modify the clinical effects. The American Heart Association proposes the radial‐first strategy in the USA for patients with acute coronary syndromes and suggests ultrasound guidance, particularly in challenging cases (Mason 2018). For management of coronary disease, various European guidelines recommend radial access as the first choice compared to femoral access but do not mention ultrasound‐guided arterial access (Aboyans 2018; Ibanez 2018; NICE 2013). Other recent guidelines regarding management of PAD only superficially address the ultrasound‐guided resource for retrograde artery access or do not mention it at all (Conte 2019; NICE 2014; NICE 2018).
Ultrasonography is a widely available method; its use for arterial access guidance seems to improve the first‐attempt success rate while reducing the numbers of skin perforations, catheters used, and attempts targeting the vessels (Hansen 2014). However, DUG still is not used frequently (AIUM 2013; Soverow 2016). Soverow 2016 showed that only 13% of interventional cardiologists routinely used ultrasound for arterial access. Although other Cochrane Reviews have shown the benefits of performing venous access in patients of all ages and arterial access in paediatric patients, ultrasound guidance for arterial access in adults remains under debate (Attie 2019). In this setting, a high‐quality systematic review is mandatory to provide robust evidence.
Objectives
To assess the effects of ultrasound guidance for arterial (other than femoral) catheterisation in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) of parallel (e.g. cluster, individual) or cross‐over design. We used only data from the first phase of cross‐over studies to avoid the risk of carry‐over effects, as described in Section 23.2.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We included studies reported as full text, those published as abstract only, and unpublished data. We did not consider quasi‐randomised trials (i.e. studies in which participants are allocated to intervention groups based on methods that are not truly random, such as hospital number or date of birth).
Types of participants
We included adults (people ≥ 18 years of age) of either gender who require any form of arterial access (other than femoral) for diagnostic or therapeutic purposes. We considered all related arterial procedures such as arterial catheterisation for cardiac angiography, percutaneous coronary intervention (PCI), arterial blood sampling, or blood pressure monitoring. Paediatric patients and adults who underwent femoral arterial puncture are not relevant for our review, and we did not include them, to avoid overlap with other Cochrane Reviews: "Ultrasound‐guided versus anatomic landmark‐guided percutaneous femoral artery access" and "Ultrasound‐guided arterial cannulation for paediatrics" (Aouad‐Maroun 2016; Strauss 2021).
If we found studies with mixed populations, and only a subset of participants met our inclusion criteria, we attempted to obtain data for the subgroup of interest from the trialists so we could include the study. For studies with mixed populations for which we cannot get data for the subgroup of interest but at least 50% of the study population is of interest, we planned to include all participants in our analysis. Moreover, we planned to explore the effect of this decision in a sensitivity analysis. Studies in which less than 50% of the population is of interest and data for the subgroup of interest are not available were excluded.
Types of interventions
We considered all types of Seldinger techniques for artery access, such as anterior wall puncture, artery transfixation, 'catheter‐over‐needle', and other special devices, as the baseline eligible technique for arterial catheterisation. We evaluated possible clinical implications of these differences in the Subgroup analysis and investigation of heterogeneity section.
Many techniques for arterial cannulation guidance in adults have been described, such as palpation and landmarks, two‐dimensional ultrasound guidance, and Doppler ultrasound. We considered two‐dimensional ultrasound guidance as our intervention of interest. We therefore included trials comparing ultrasound guidance, B‐mode, in‐plane, or out‐of‐plane, with vessels accessed in a longitudinal or transversal way versus any other techniques for arterial puncture.
The most commonly accessed site for arterial cannulation in adults within our inclusion criteria is the radial artery, but we considered all other possible sites, such as axillary, brachial, and tibial arteries, each in a separate comparison. We did not include studies regarding femoral access to avoid overlap with the Cochrane Review entitled "Ultrasound‐guided versus anatomic landmark‐guided percutaneous femoral artery access" (Strauss 2021).
Possible comparisons are as follows.
B‐mode ultrasound guidance versus palpation and landmarks.
B‐mode ultrasound guidance versus Doppler auditory ultrasound assistance.
Direct ultrasound guidance (real‐time) versus indirect ultrasound guidance.
B‐mode ultrasound versus near‐infrared laser guidance.
B‐mode ultrasound versus modified B‐mode ultrasound.
In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound.
Doppler auditory ultrasound assistance versus palpation and landmarks.
Dynamic out‐of‐plane B‐mode ultrasound versus static out‐of‐plane B‐mode ultrasound.
Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound.
Any combination of the above treatments versus any other combination, with or without placebo (sham procedure).
Types of outcome measures
Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion for this review. When a published report did not appear to report one of these outcomes, we accessed the trial protocol and contacted the trial authors to ascertain whether outcomes were measured but not reported. We included in the review, as part of the narrative, relevant trials that measured these outcomes but did not report the data at all, or did not report them in a usable format.
Economic costs were evaluated indirectly by outcomes such as 'First‐attempt success rate' and 'Time needed for successful procedure'. Because this is not a cost‐effectiveness review, we planned to treat data regarding direct costs in the Discussion section in a narrative form, if these data were available.
We presented outcomes at two different time points following the start of the intervention, if data were available. Our time point of primary interest is early; we therefore intend to produce related 'Summary of findings' tables only for this time point, but we reported long‐term outcomes at the longest possible time of follow‐up.
Early outcomes (within 30 days after intervention).
Long‐term outcomes (more than 30 days after intervention).
Primary outcomes
Primary outcomes include the following, ordered according to priority.
First‐attempt success rate (i.e. number of participants for whom the proposed method of catheterisation was successful on the first attempt).
Pseudoaneurysm: total number of perioperative and postoperative pseudoaneurysms.
Secondary outcomes
Secondary outcomes include the following, ordered according to priority.
Overall success rate (i.e. number of participants for whom the proposed method of catheterisation was successful).
Time, in minutes, needed for a successful procedure. We will consider the successful procedure as complete catheter placement or complete blood sample collection.
Major haematoma, defined as that requiring an intervention (e.g. open surgical or percutaneous drainage) or prolonging duration of hospital stay. We will consider the total number of perioperative and postoperative major haematomas.
Adverse events. We will consider all possible adverse events separately, as individual outcomes, such as minor haematoma formation defined as neither requiring an intervention (e.g. open surgical or percutaneous drainage) nor prolonging duration of hospital stay; pain; local infection; events requiring prolonged hospitalisation such as artery thrombosis, artery embolism, nerve injury, and amputation; life‐threatening events; fatal events.
Quality of life (QoL): participants' subjective perception of improvement (yes or no) as reported by study authors, or using any validated scoring system such as the Short Form‐36 Health Survey (SF‐36) (Ware 1992).
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases on 21 May 2021.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 4 of 12), in the Cochrane Library.
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 20 May 2021).
Embase (Ovid, 1980 to 2021 week 19).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOHost, 1937 to 21 May 2021).
Latin American and Caribbean Health Sciences Literature database (LILACS) (Bireme, 1982 to 21 May 2021).
Indice Bibliográfico Español de Ciencias de la Salud (IBECS, via Virtual Health Library; 2011 to 16 June 2021) (searched 16 June 2021).
We adapted the preliminary search strategy for MEDLINE (Ovid) (Appendix 2) for use in the other databases. We applied the Cochrane sensitivity‐maximising RCT filter to MEDLINE (Ovid) and adaptations of it to the other databases, except CENTRAL (Lefebvre 2019).
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (ictrptest.azurewebsites.net/Default.aspx) for ongoing or unpublished trials on 16 June 2021.
We searched all databases from their inception to the present, and we imposed no restriction on language of publication nor on publication status. We considered adverse effects described in included studies only.
Searching other resources
We checked reference lists of all included studies and any identified relevant systematic reviews for additional references to trials. We examined any relevant retraction statements and errata for included studies. We contacted the authors of included trials for any possible unpublished data. Furthermore, we contacted field specialists and searched medical ultrasound company websites (Canon, Fujifilm, GE Healthcare, Mindray, Mobissom, Philips, Samsung, Siemens) to enquire about relevant ongoing and unpublished studies (16 June 2021).
Data collection and analysis
Selection of studies
Two review authors (RLGF, CDQF) independently screened titles and abstracts of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve', using the Covidence tool. If there were any disagreements, we asked a third review author to arbitrate (LCUN). We retrieved the full‐text study reports/publications, and two review authors (RLGF, CDQF) independently screened the full text, identified studies for inclusion, and identified ineligible studies and recorded reasons for their exclusion. We resolved any disagreement through discussion, or, if required, we consulted a third person (LCUN). We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Liberati 2009).
Data extraction and management
We used a data collection form, which has been piloted on at least one study in the review, to record study characteristics and outcome data. One review author (RLGF) extracted the following study characteristics from included studies.
Methods: study design, total duration of study, number of study centres and locations, study setting, and date of study.
Participants: N randomised, N lost to follow‐up/withdrawn, N analysed, N of interest, mean age, age range, gender, severity of condition, comorbidities, body mass index (BMI), artery of interest characteristics (e.g. access site, diameter), inclusion criteria, and exclusion criteria.
Interventions: intervention and comparison characteristics, level of experience of the person carrying out the procedure, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (RLGF, CDQF) independently extracted outcome data from included studies. We resolved disagreements by reaching consensus or by involving a third person (LCUN). One review author (RLGF) transferred data into the Review Manager 5 (RevMan 5) file (Review Manager 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review with data recorded on the data extraction form. A second review author (CDQF) spot‐checked study characteristics for accuracy against the trial report.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to obtain further details. When data were reported only in graphs, we extracted data of interest such as mean, standard deviation (SD), or standard error (SE) using software such as graphreader.com and RevMan. We identified translators for all foreign languages with which we were unfamiliar (e.g. Chinese, Japanese).
Assessment of risk of bias in included studies
Two review authors (RLGF, CDQF) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion or by consultation with another review author (LCUN). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
For cluster‐randomised trials, we planned to consider particular biases as recommended in Section 8.15.1.1 of the Cochrane Handbook for Systematic Reviews of Interventions: (1) recruitment bias; (2) baseline imbalance; (3) loss of clusters; (4) incorrect analysis; and (5) comparability with individually randomised trials (Higgins 2017). We graded each potential source of bias as high, low, or unclear and provided a quote from the study report, together with a justification for our judgement, in 'Risk of bias' tables, in the Characteristics of included studies section. We summarised risk of bias judgements across different studies for each of the domains listed. When information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account risk of bias for studies that contributed to that outcome. When the protocol text or the trial registry entry was not available, we judged the 'selective outcome reporting' domain by comparing outcomes planned in the methods section (specified) with those described in the results section (collected) of the available report (Higgins 2017).
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and report any deviations from it in the Differences between protocol and review section of the systematic review (Flumignan 2020).
Measures of treatment effect
We analysed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs), and continuous data as mean differences (MD) with the same scale, and as standardised mean differences (SMDs) with different scales, with 95% CIs. We entered data presented as a scale with a consistent direction of effect.
We estimated the MD using the method reported by Wan 2014 to convert median and interquartile range (IQR) into MD and CI. When this was not possible, we narratively described skewed data reported as medians and interquartile ranges.
We calculated the number needed to treat (NNT) for outcomes with direct implications for practice using RevMan 5 software (Review Manager 2014). As recommended by the Cochrane Handbook for Systematic Reviews of Interventions, we expressed the NNT as ‘number needed to treat for an additional beneficial outcome’ (NNTB) and as ‘number needed to treat for an additional harmful outcome’ (NNTH) to indicate direction of effect (Schünemann 2019).
Unit of analysis issues
Individuals were our unit of analysis. If trials included multi‐arm interventions, we considered only the arms relevant to the scope of our review.
Cross‐over trials
When we identified any cross‐over RCTs, we used only data from the first phase of these studies to avoid the risk of carry‐over effects, as described in Section 23.2.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually randomised trials. We planned to adjust their sample sizes using the methods described in Section 23.1.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), using an estimate of the intra‐cluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we had used ICCs from other sources, we planned to report this and to conduct sensitivity analyses to investigate effects of variations in the ICC. If we had identified both cluster‐randomised trials and individually randomised trials, we planned to synthesise the relevant information. We planned to consider it reasonable to combine the results from both types of trials if we noted little heterogeneity between study designs, and if we considered interaction between effects of intervention and choice of randomisation unit to be unlikely. We also planned to acknowledge heterogeneity in the randomisation unit and to perform a sensitivity analysis to investigate effects of the randomisation unit.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When possible, we used the RevMan 5 calculator to calculate missing standard deviations using other data from the trial, such as CIs. We estimated the MD using the method reported by Wan 2014 to convert median and IQR into MD and CI. When data were reported only in graphs, we extracted data of interest such as mean, standard deviation (SD), or standard error (SE) using software such as graphreader.com and RevMan. We identified translators for all foreign languages with which we were unfamiliar (e.g. Chinese, Japanese). When this was not possible, and missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by performing a sensitivity analysis. For all outcomes, we followed intention‐to‐treat (ITT) principles to the greatest degree possible, that is, we analysed participants in their randomised group regardless of what intervention they actually received. We used available case data for the denominator if ITT data were not available.
Assessment of heterogeneity
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between confidence intervals. We used the I² statistic to measure heterogeneity among the trials in each analysis, but we acknowledge that there was substantial uncertainty in the value of I² when only a small number of studies were included; we therefore also considered the P value from the Chi² test. When we identified substantial heterogeneity, we reported this and explored possible causes by conducting prespecified subgroup analysis.
As strict thresholds for interpretation of I² are not recommended, we followed the rough guide to interpretation provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
When I² lies in an area of overlap between two categories (e.g. between 50% and 60%), we considered differences in participants and interventions among trials contributing data to the analysis (Deeks 2019).
Assessment of reporting biases
When we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible small‐study biases for all available outcomes.
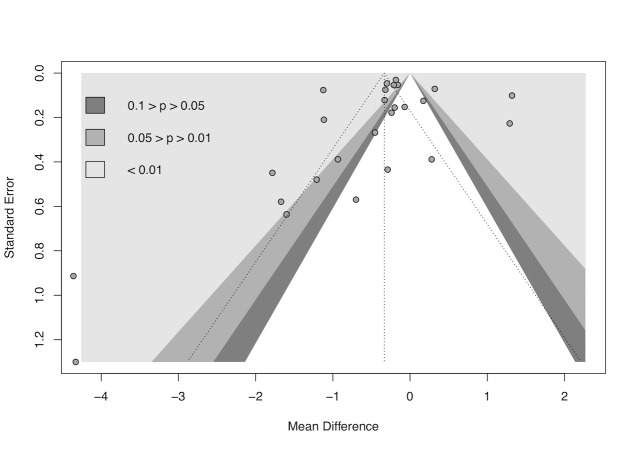
We used R Studio software, version 1.4.1106, for additional tests when we suspected reporting bias (R Studio). First, we re‐created all meta‐analyses with 10 or more included studies using the package 'meta' version 4.18‐0 with the 'metabin' function for dichotomous data and the 'metacont' function for continuous data. Next, we generated funnel plots with the 'funnel' function and used Egger's test to test graph asymmetry with the 'metabias' function, considering P < 0.05 a statistically significant value (Egger 1997; Page 2021). Finally, we used the trim‐and‐fill method with the 'trimfill' function to estimate and adjust for numbers and outcomes of missing studies in funnel graphs that showed asymmetry (Duval 2000; Page 2021).
Data synthesis
We synthesised data using Review Manager 5 (Review Manager 2014). We reported data narratively if it was not appropriate to combine them in a meta‐analysis. We undertook meta‐analyses only when this was meaningful (i.e. when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
We used a fixed‐effect model for meta‐analysis when included studies were homogenous (considering populations, interventions, comparators, and outcomes characteristics). We used a random‐effects model when we identified at least substantial heterogeneity, or when we noted significant clinical differences among included trials regarding patients and interventions (Deeks 2019).
We addressed all outcomes in order as listed in the Types of outcome measures section in the Results portion of the review under the heading Effects of interventions. In addition, we included a summary of main outcomes in the 'Summary of findings' table. We included results of individual studies and any statistical summary of these in Data and analyses tables.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Intervention characteristics
Experienced versus inexperienced (including residents and fellows) operators
Additional ultrasound technique (e.g. colour mode, Power Doppler, contrast‐enhanced ultrasound)
Seldinger technique or any possible variation (e.g. anterior wall puncture, artery transfixation, 'catheter‐over‐needle' puncture, hollow needle puncture)
Diameter of used devices (e.g. ≤ 20 French versus > 20 French)
Diagnostic versus therapeutics purpose
Anterograde versus retrograde access
Participant characteristics
Age (i.e. young adults (18 years to 24 years), adults (25 years to 64 years), and seniors (65 years and over))
Gender
Race
Comorbidities (e.g. critically ill subjects, vasopressors required, elective procedures)
Vessel diameter
1. Classification of adults according to BMI.
| Classification | BMI valuesa,b |
| Underweight | < 18.5 |
| Normal range | 18.5 to 24.99 |
| Overweight: | |
| Pre‐obese | 25.00 to 29.99 |
| Obese class I | 30.00 to 34.99 |
| Obese class II | 35.00 to 39.99 |
| Obese class Ill | ≥ 40 |
BMI: body mass index.
aThese BMI values are age‐independent and are the same for both sexes.
bBody mass divided by the square of body height, universally expressed in units of kg/m².
We considered type of vessels in different comparisons. We did not, therefore, consider the different vessels (e.g. radial arteries, brachial arteries) in subgroup analysis.
Because we planned to explore possible causes of substantial heterogeneity through subgroup analysis (Assessment of heterogeneity), we used all outcomes in subgroup analyses if at least 10 included studies contributed to the meta‐analysis. A unique possible subgroup analysis was planned to explore the operator's experience (experienced versus inexperienced) for one of the included comparisons (B‐mode ultrasound guidance versus palpation and landmarks in the radial artery). When trial authors did not report the level of experience of operators, we were conservative and included this study in the inexperienced operators' subgroup (e.g. Goswami 2020).
We used the formal test for subgroup differences in Review Manager 5 (Review Manager 2014), and we based our interpretation on this.
Sensitivity analysis
We planned to carry out the following sensitivity analyses, to test whether key methodological factors or decisions have affected the main result. These were grouped according to study design (individual, cross‐over, or cluster).
We planned to include only studies with low risk of bias. We considered the overall risk of bias of an included study as low if we noted no high‐risk judgement in all four main domains (i.e. random sequence, allocation concealment, incomplete outcome data, and selective reporting).
We planned to examine both fixed‐effect model and random‐effects model meta‐analyses, and we planned to explore differences between the two estimates. However, when we grouped two or more trials, a random‐effects analysis was more suitable due to participant differences.
We planned to explore the decision to include all participants when at least 50% were of interest in a trial with a mixed population. However, all subgroup of interest participants for our analysis were available, and we did not include any mixed population study in our review.
We planned to explore the impact of missing data. When we identified studies with missing data that are unobtainable, we repeated analyses by excluding these studies to determine their impact on the primary analyses. However, this was not necessary with the available data.
We also planned to carry out sensitivity analyses while considering cross‐over and cluster‐RCTs. We planned to investigate effects of variation in the ICC, and we planned to acknowledge heterogeneity in the randomisation unit and to perform a sensitivity analysis to investigate effects of the randomisation unit. We presented these results and compared them with the overall findings.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table for the early time point using the following outcomes: (1) first‐attempt success rate; (2) pseudoaneurysm; (3) overall success rate; (4) time needed for a successful procedure; (5) major haematoma; (6) pain; and (7) QoL. We used the five GRADE considerations (study limitations; consistency of effect; imprecision; indirectness; and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019), along with GRADEpro software (GRADEpro GDT 2015). Each comparison (e.g. B‐mode ultrasound guidance versus landmarks; B‐mode ultrasound guidance versus palpation; B‐mode ultrasound guidance versus Doppler ultrasound guidance; direct ultrasound guidance (real‐time) versus indirect ultrasound guidance; any combination of the above treatments versus any other combination, with or without placebo) had a separate 'Summary of findings' table. We justified all decisions to downgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review when necessary.
Judgements about evidence quality were made by two review authors (RLGF, CDQF) working independently, with disagreements resolved by discussion or by consultation with a third review author (LCUN). We justified, documented, and incorporated judgements into reporting of results for each outcome.
We extracted study data, formatted our comparisons in data tables, and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
We have presented the details of studies included in this review in the Characteristics of included studies table, as well as reasons for exclusion in the Characteristics of excluded studies table. We have detailed the status of ongoing trials in the Characteristics of ongoing studies section.
Results of the search
We completed the search in June 2021. We retrieved a total of 12,819 records from electronic databases and identified 197 additional records through other sources. After we excluded 1157 duplicate records, we screened 11,859 unique records. We considered a total of 11,667 records not relevant at this stage and we selected 192 records (147 studies) for full‐text reading. We included 48 studies (92 records). Two studies that were included had the same clinical trial registration number but different characteristics (Wang 2017Wang 2019). Participants were enrolled in separate periods, without any overlap: 1 June 2017 to 27 October 2017 (Wang 2017), and 1 July 2018 to 24 November 2018 (Wang 2019), which avoided double‐counting. Besides participants, interventions and comparisons between Wang 2017 and Wang 2019 are significantly different. Therefore, we treated them as separate studies. We excluded 14 studies (15 records) with reasons and assessed another 64 as not relevant at this stage (see Characteristics of excluded studies). Twenty trials are ongoing (see Characteristics of ongoing studies), and one study was tagged as 'awaiting classification' due to the fact that essential information about the artery of interest was lacking and our attempts to contact trial authors were unsuccessful (Flores‐Arévalo 2016). The flowchart for results of the search is presented in Figure 1.
1.
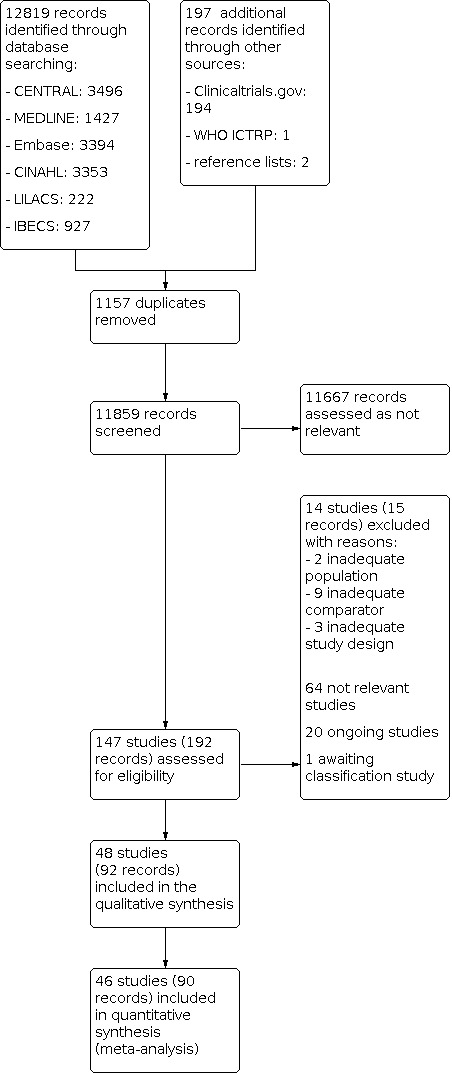
Study flow diagram.
Included studies
The 48 studies (7997 participants) tested at least one of the following interventions: (1) palpation and landmarks; (2) Doppler auditory ultrasound assistance (DUA); or (3) direct ultrasound guidance (DUG) with B‐mode or any other modified ultrasound technique for arterial (other than femoral) catheterisation in adults. These studies analysed ultrasound guidance in three different arteries (axillary, dorsalis pedis, and radial) and provided data for ten different comparisons.
Two trials did not report any data that we could use in our analysis (Edanaga 2012; Fujita 2012). Fujita 2012 is an abstract of events and provided no data for numerical analysis. Edanaga 2012 was evaluated in full text after translation from Japanese; investigators did not evaluate any outcomes of interest for our review.
For details of the included studies, see the Characteristics of included studies table.
Design
We classified all 48 included studies as randomised trials, but 21 of them did not provide details of the method used for randomisation (Abdalla 2017; Ammar 2017; Berk 2013; Burad 2017; Cao 2018; Edanaga 2012; Fujita 2012; Killu 2011; Laursen 2015; Nasreen 2016; NCT01663779; Nguyen 2019; Osuda 2020; Quan 2014; Rose 2018; Seyhan 2021; Seto 2015; Tada 2003; Yu 2019; Zaremski 2013; Zhefeng 2019).
Hansen 2014 and Gopalasingam 2014 are cross‐over RCTs; all of the other 46 included studies are individual parallel RCTs. We did not identify any cluster‐RCT.
No study was triple‐blinded because the nature of the intervention did not allow blinding of personnel. Eight were single‐blinded because the outcome assessment was blinded (Bai 2020; Cao 2020; Gibbons 2020; Kiberenge 2018; Kim 2021b; Nam 2020; Nguyen 2019; Ueda 2015), 17 studies were not blinded (Abdalla 2017; Anand 2019; Bobbia 2013; Burad 2017; Gopalasingam 2014; Grandpierre 2019; Khan 2018; Killu 2011; Li 2016; NCT01663779; Peters 2015; Quan 2014; Rajasekar 2021; Seto 2015; Seyhan 2021; Shiver 2006; Zhefeng 2019), and all of the other 25 studies were unclear about blinding.
Settings
Forty‐seven included studies were conducted in 17 different countries: 10 in China (Bai 2020; Cao 2018; Cao 2020; Li 2016; Quan 2014; Wang 2017; Wang 2019; Yu 2019; Zeng 2020; Zhefeng 2019), 8 in the USA (Gibbons 2020; Kiberenge 2018; Killu 2011; NCT01663779; Seto 2015; Shiver 2006; Ueda 2015; Yeap 2019), 4 in Japan (Edanaga 2012; Fujita 2012; Osuda 2020; Tada 2003), 3 in Denmark (Gopalasingam 2014; Hansen 2014; Laursen 2015), 5 in India (Anand 2019; Goswami 2020; Khan 2018; Rajasekar 2021; Sethi 2017), 2 in Korea (Kim 2021a; Kim 2021b), 2 in France (Bobbia 2013; Grandpierre 2019), 2 in Pakistan (Ammar 2017; Nasreen 2016), 2 in Turkey (Berk 2013; Seyhan 2021), 2 in Oman (Arora 2021; Burad 2017), and 1 each in Australia (Nguyen 2019), Canada (Peters 2015), Egypt (Abdalla 2017), Israel (Levin 2003), Korea (Nam 2020), Thailand (Tangwiwat 2016), and Switzerland (Zaremski 2013). All these 47 included studies were conducted in hospital settings. Rose 2018 did not provide data on setting and country in which it was carried out.
Participants
The mean age of participants included in the 48 trials ranged from 41 to 73 years. Some trials did not report the age of participants. All included studies considered both men and women for enrolment.
Sample size
The number of participants included in each of the 48 studies ranged from 33 in Killu 2011 to 749 in Ueda 2015. However, most of the studies had small sample sizes.
Funding
Twenty‐one trials did not report their funding sources (Ammar 2017; Arora 2021; Berk 2013; Bobbia 2013; Burad 2017; Edanaga 2012; Fujita 2012; Goswami 2020; Khan 2018; Killu 2011; Levin 2003; Nam 2020; Nasreen 2016; NCT01663779; Nguyen 2019; Osuda 2020; Rajasekar 2021; Rose 2018; Sethi 2017; Shiver 2006; Tada 2003). Twelve trials reported that they had no funding sources (Abdalla 2017; Anand 2019; Bai 2020; Cao 2020; Gibbons 2020; Kim 2021b; Quan 2014; Seto 2015; Seyhan 2021; Ueda 2015; Yeap 2019; Zeng 2020). The remaining fifteen trials were self‐funded or were funded by government grants or host hospitals and universities.
Conflicts of interest
Most of the trials stated they had no conflicts of interest (Abdalla 2017; Anand 2019; Bai 2020; Berk 2013; Burad 2017; Cao 2020; Edanaga 2012; Gopalasingam 2014; Hansen 2014; Kiberenge 2018; Killu 2011; Kim 2021a; Kim 2021b; Li 2016; Nam 2020; Nguyen 2019; Osuda 2020; Peters 2015; Quan 2014; Rajasekar 2021; Seyhan 2021; Tangwiwat 2016; Ueda 2015; Wang 2017; Wang 2019; Yeap 2019; Zeng 2020; Zhefeng 2019); 3 studies declared a potential conflict of interest (Grandpierre 2019; Seto 2015; Zaremski 2013); and the remaining trials did not mention conflicts of interest (Ammar 2017; Arora 2021; Bobbia 2013; Cao 2018; Fujita 2012; Gibbons 2020; Goswami 2020; Khan 2018; Laursen 2015; Levin 2003; Nasreen 2016; NCT01663779; Rose 2018; Sethi 2017; Shiver 2006; Tada 2003; Yu 2019).
Interventions
Thirty‐seven included studies tested two different types of ultrasound guidance for arterial (other than femoral) catheterisation: DUA, or DUG with B‐mode or any other modified ultrasound technique.
DUA
Ueda 2015 randomised participants for one of these three interventions for radial artery catheterisation: DUA (n = 244), B‐mode ultrasound guidance (n = 249), or palpation and landmarks (n = 256). Therefore, Ueda 2015 provided data for three different comparisons, and interventions were performed by inexperienced investigators for all methods. Tada 2003 compared DUA versus palpation in 166 participants. None of the other included studies provided data about DUA.
DUG
Forty‐six included studies provided data comparing B‐mode ultrasound guidance versus palpation and landmarks, DUA, modified B‐mode ultrasound guidance, or near‐infrared laser guidance for axillary, dorsalis pedis, or radial artery catheterisation.
Killu 2011 compared B‐mode ultrasound versus palpation and landmarks for axillary artery catheterisation, and interventions were performed by inexperienced (medical residents) and experienced (fellow) personnel.
Anand 2019 compared B‐mode ultrasound versus palpation and landmarks for dorsalis pedis artery catheterisation, and interventions were performed by a single experienced investigator.
Twenty‐eight included studies compared B‐mode ultrasound versus palpation and landmarks for radial artery catheterisation. Only experienced investigators performed the interventions in 18 studies (Ammar 2017; Burad 2017; Cao 2018; Grandpierre 2019; Hansen 2014; Khan 2018; Laursen 2015; Li 2016; Nasreen 2016; Nguyen 2019; Peters 2015; Rajasekar 2021; Seto 2015; Seyhan 2021; Wang 2017; Wang 2019; Yu 2019; Zaremski 2013), and inexperienced investigators performed the interventions in another 10 studies (Gibbons 2020; Gopalasingam 2014; Goswami 2020; Kim 2021b; Levin 2003; Rose 2018; Shiver 2006; Tangwiwat 2016; Ueda 2015; Yeap 2019). Kiberenge 2018 provided data about experienced and inexperienced operators.
Ueda 2015 also provided data about the comparisons 'B‐mode ultrasound versus DUA' and 'DUA versus palpation and landmarks' for radial artery catheterisation, and interventions were performed by inexperienced investigators for all methods. Tada 2003 compared DUA versus palpation and landmarks for radial artery catheterisation, and interventions were performed by an inexperienced investigator for the DUA method.
Osuda 2020 compared B‐mode ultrasound versus near‐infrared laser guidance for radial artery catheterisation, and interventions were performed by inexperienced investigators for both methods.
Zhefeng 2019 compared B‐mode ultrasound versus modified B‐mode ultrasound (i.e. with addition of a developing line for radial artery catheterisation and interventions performed by inexperienced investigators for both methods). Kim 2021a compared B‐mode ultrasound versus modified B‐mode ultrasound (i.e. with addition of electromagnetic guidance for radial artery catheterisation and interventions performed solely by a unique experienced investigator).
Nine studies compared in‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound for radial artery catheterisation. Interventions were performed by experienced investigators for both methods in seven studies (Arora 2021; Berk 2013; Nam 2020; Quan 2014; Rajasekar 2021; Sethi 2017; Wang 2019), and by an inexperienced operator in Cao 2020. Abdalla 2017 did not provide details about the level of experience of operators.
All included studies assessed data from arterial catheterisation for diagnostic purposes mainly (pressure monitoring or completion of blood tests). Only three included studies included participants for therapeutic purposes in the radial artery catheterisation with different prevalences (Nguyen 2019 = 22.5% for PCI, Seto 2015 = 19.4% for PCI, Zaremski 2013 = PCI % not detailed).
Outcomes
In most of the studies included in this review, outcomes were similar, and study authors provided data for all relevant outcomes for this review. The main outcome measures were overall success rate, first‐attempt success rate, and time needed for a successful procedure. These outcomes were assessed at different time periods, ranging from 5 minutes to 30 days after the start of the intervention, and major included studies evaluated data up to the end of the procedure. Therefore, all included studies reported data only for the early time point (at 30 days or less after intervention).
Primary outcomes
Forty studies reported our primary outcome 'first‐attempt success rate' (Abdalla 2017; Ammar 2017; Anand 2019; Arora 2021; Bai 2020; Burad 2017; Cao 2018; Cao 2020; Gibbons 2020; Gopalasingam 2014; Grandpierre 2019; Hansen 2014; Khan 2018; Kiberenge 2018; Kim 2021a; Kim 2021b; Laursen 2015; Levin 2003; Li 2016; Nam 2020; Nasreen 2016; NCT01663779; Nguyen 2019; Osuda 2020; Peters 2015; Quan 2014; Rajasekar 2021; Sethi 2017; Seto 2015; Seyhan 2021; Shiver 2006; Tada 2003; Tangwiwat 2016; Ueda 2015; Wang 2017; Wang 2019; Yu 2019; Zaremski 2013; Zeng 2020; Zhefeng 2019).
Only Nguyen 2019 reported pseudoaneurysm.
Secondary outcomes
Forty‐one studies reported overall success rate (Abdalla 2017; Ammar 2017; Anand 2019; Arora 2021; Bai 2020; Bobbia 2013; Burad 2017; Cao 2018; Cao 2020; Gibbons 2020; Gopalasingam 2014; Goswami 2020; Grandpierre 2019; Hansen 2014; Kiberenge 2018; Killu 2011; Kim 2021a; Kim 2021b; Levin 2003; Li 2016; Nam 2020; Nasreen 2016; Nguyen 2019; Osuda 2020; Peters 2015; Quan 2014; Rajasekar 2021; Rose 2018; Sethi 2017; Seto 2015; Seyhan 2021; Shiver 2006; Tada 2003; Tangwiwat 2016; Ueda 2015; Wang 2017; Wang 2019; Yeap 2019; Yu 2019; Zaremski 2013; Zhefeng 2019).
Forty studies reported time needed for a successful procedure (Abdalla 2017; Ammar 2017; Anand 2019; Arora 2021; Bai 2020; Berk 2013; Bobbia 2013; Burad 2017; Cao 2018; Cao 2020; Gibbons 2020; Gopalasingam 2014; Grandpierre 2019; Hansen 2014; Kiberenge 2018; Killu 2011; Kim 2021a; Kim 2021b; Laursen 2015; Levin 2003; Li 2016; Nam 2020; Nasreen 2016; Nguyen 2019; Osuda 2020; Peters 2015; Quan 2014; Rajasekar 2021; Sethi 2017; Seto 2015; Shiver 2006; Tangwiwat 2016; Ueda 2015; Wang 2017; Wang 2019; Yeap 2019; Yu 2019; Zaremski 2013; Zeng 2020; Zhefeng 2019).
Twenty‐six studies reported major haematomas (Abdalla 2017; Arora 2021; Berk 2013; Cao 2018; Cao 2020; Gibbons 2020; Goswami 2020; Killu 2011; Kim 2021a; Kim 2021b; Li 2016; Nam 2020; Nasreen 2016; NCT01663779; Nguyen 2019; Peters 2015; Quan 2014; Rajasekar 2021; Sethi 2017; Shiver 2006; Tangwiwat 2016; Wang 2017; Wang 2019; Yu 2019; Zaremski 2013; Zhefeng 2019).
Thirty studies reported adverse events (Abdalla 2017; Arora 2021; Bai 2020; Berk 2013; Bobbia 2013; Cao 2018; Cao 2020; Gibbons 2020; Goswami 2020; Grandpierre 2019; Hansen 2014; Khan 2018; Killu 2011; Kim 2021a; Kim 2021b; Li 2016; Nam 2020; NCT01663779; Nguyen 2019; Quan 2014; Rajasekar 2021; Sethi 2017; Seto 2015; Ueda 2015; Wang 2017; Wang 2019; Yu 2019; Zaremski 2013; Zeng 2020; Zhefeng 2019).
Only Bobbia 2013 reported quality of life (QoL) by a patient satisfaction scale.
Excluded studies
We excluded 14 studies for at least one reason (Characteristics of excluded studies). Two studies evaluated an inadequate population (i.e. paediatric participants) or femoral access (Anantasit 2017; NCT03537118). Nine studies used inadequate comparators (i.e. ultrasound guidance was used without differences for all participants, or differences between groups did not relate to ultrasound guidance) (Cronin 1986; CTRI/2018/11/016257; Dahl 1992; Elmahdy 2018; Kucuk 2014; Min 2016; NCT04001764; NCT04077762; Yao 2018). We excluded 3 other studies because they were not randomised (Mori 2020; Vaquerizo 2014; Wilson 2020). We considered Wilson 2020 a quasi‐RCT because participants were randomised by the last digit of their medical record number. Although the Vaquerizo 2014 publication stated that it was an RCT, in personal communication, trial authors confirmed that it was not a truly an RCT because participants were enrolled according to availability of staff to carry out the intervention. Vaquerizo 2014 did not use any additional method for randomisation. Mori 2020 compared two interventions of interest but without randomisation. In addition, they performed the two interventions in different periods.
Awaiting studies
Flores‐Arévalo 2016 compared B‐mode ultrasound guidance versus palpation for arterial catheterisation in adults, but trial authors did not clarify the artery of interest; we, therefore, had to maintain the study in the 'awaiting classification' section. We did not use these data for any analysis.
Ongoing studies
We identified 20 ongoing studies evaluating at least one of the following interventions for arterial (other than femoral) catheterisation in adults.
Palpation and landmarks (ChiCTR1800016772; ChiCTR‐IOR‐16009966; CTRI/2020/01/022989; CTRI/2020/06/025543; CTRI/2020/08/027199; CTRI/2020/12/029455; CTRI/2021/02/031051; NCT01189188; NCT01561196; NCT03144895; NCT03995264; NCT04318990; NCT04617106; NTR6107; TCTR20210202004).
DUG with B‐mode or any other modified ultrasound technique (ChiCTR1800016772; ChiCTR‐IOR‐16009966; CTRI/2020/01/022989; CTRI/2020/06/025543; CTRI/2020/08/027199; CTRI/2020/09/028136; CTRI/2020/12/029455; CTRI/2021/02/031051; KCT0004903; NCT01189188; NCT01561196; NCT02584673; NCT03144895; NCT03995264; NCT04318990; NCT04617106; NCT04806932; NTR6107; TCTR20210202004; UMIN000020698).
Eleven ongoing studies plan to report data on first‐attempt success rate (ChiCTR‐IOR‐16009966; CTRI/2020/01/022989; CTRI/2020/06/025543; CTRI/2020/08/027199; CTRI/2020/12/029455; CTRI/2021/02/031051; KCT0004903; NCT01189188; NCT04617106; NCT04806932; TCTR20210202004). Twelve ongoing studies plan to report data on overall success rate (ChiCTR1800016772; CTRI/2020/06/025543; CTRI/2020/08/027199; CTRI/2020/09/028136; CTRI/2020/12/029455; KCT0004903; NCT03144895; NCT03995264; NCT04617106; NCT04806932; NTR6107; TCTR20210202004). Fifteen ongoing studies plan to report data on time needed for a successful procedure (ChiCTR‐IOR‐16009966; CTRI/2020/01/022989; CTRI/2020/06/025543; CTRI/2020/08/027199; CTRI/2020/09/028136; CTRI/2020/12/029455; CTRI/2021/02/031051; KCT0004903; NCT01561196; NCT02584673; NCT04617106; NCT04806932; NTR6107; TCTR20210202004; UMIN000020698). NCT01189188 and NCT04806932 plan to report major haematoma data. Fourteen studies plan to report data on adverse events (ChiCTR‐IOR‐16009966; CTRI/2020/01/022989; CTRI/2020/06/025543; CTRI/2020/08/027199; CTRI/2020/09/028136; CTRI/2020/12/029455; CTRI/2021/02/031051; KCT0004903; NCT01189188; NCT01561196; NCT04318990; NCT04617106; NCT04806932; TCTR20210202004). No ongoing studies plan to report data on pseudoaneurysm or QoL.
We tried to contact trial authors; we also searched by trial number of registration and by title of the study on all databases of interest for this review. However, we found no additional data for these ongoing studies.
Risk of bias in included studies
Risk of bias varied considerably across the included studies, and insufficient detail was provided to inform judgement in several cases. Figure 2 and Figure 3 summarise risk of bias in the included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We judged the overall risk of bias in 15 included studies as high (Arora 2021; Cao 2020; Goswami 2020; Grandpierre 2019; Khan 2018; Laursen 2015; Rose 2018; Sethi 2017; Seto 2015; Shiver 2006; Tangwiwat 2016; Ueda 2015; Yeap 2019; Zaremski 2013; Zeng 2020). We judged all 33 other included studies as having low risk of bias.
Allocation
Twenty‐seven of the 48 studies had low risk of bias for random sequence generation (Anand 2019; Arora 2021; Bai 2020; Bobbia 2013; Cao 2020; Gibbons 2020; Gopalasingam 2014; Goswami 2020; Grandpierre 2019; Hansen 2014; Khan 2018; Kiberenge 2018; Kim 2021a; Kim 2021b; Levin 2003; Li 2016; Nam 2020; Peters 2015; Rajasekar 2021; Sethi 2017; Shiver 2006; Tangwiwat 2016; Ueda 2015; Wang 2017; Wang 2019; Yeap 2019; Zeng 2020); all others had unclear risk of bias in this domain.
Twenty studies had low risk of bias for allocation concealment (Anand 2019; Bai 2020; Cao 2020; Gibbons 2020; Kiberenge 2018; Killu 2011; Kim 2021a; Kim 2021b; Nam 2020; Nguyen 2019; Peters 2015; Quan 2014; Rajasekar 2021; Sethi 2017; Seto 2015; Shiver 2006; Ueda 2015; Wang 2017; Wang 2019; Yu 2019); Khan 2018 was at high risk of bias for this domain. All others had unclear risk of bias in this domain.
Blinding
All included studies had high risk of bias for blinding of participants and personnel due to the nature of the interventions.
We assessed eight studies to be at low risk of bias for blinding of outcome assessment (Bai 2020; Cao 2020; Gibbons 2020; Kiberenge 2018; Kim 2021b; Nam 2020; Nguyen 2019; Ueda 2015), and 17 to be at high risk of bias for this domain (Abdalla 2017; Anand 2019; Bobbia 2013; Burad 2017; Gopalasingam 2014; Grandpierre 2019; Khan 2018; Killu 2011; Li 2016; NCT01663779; Peters 2015; Quan 2014; Rajasekar 2021; Seto 2015; Seyhan 2021; Shiver 2006; Zhefeng 2019). The other studies had unclear risk of bias in this domain.
Incomplete outcome data
Eight studies had high risk of bias for incomplete outcome reporting (Cao 2020; Laursen 2015; Seto 2015; Shiver 2006; Tangwiwat 2016; Ueda 2015; Yeap 2019; Zaremski 2013). Hansen 2014 and Khan 2018 had unclear risk of bias, and the other studies had low risk of bias for this domain.
Selective reporting
Eleven studies were at high risk of bias for selective reporting (Arora 2021; Goswami 2020; Grandpierre 2019; Khan 2018; Laursen 2015; Rose 2018; Sethi 2017; Shiver 2006; Tangwiwat 2016; Ueda 2015; Yeap 2019; Zeng 2020); none was at unclear risk of bias for this domain. All other included studies (37/48) had low risk of bias for this domain.
Other potential sources of bias
We assessed 8 studies as having high risk for other potential sources of bias (Edanaga 2012; Gopalasingam 2014; Levin 2003; Rajasekar 2021; Tada 2003; Ueda 2015; Wang 2017; Wang 2019); all other studies were at low risk of bias for this domain.
Wang 2017 and Wang 2019 received the same trial registry number (ChiCTR‐IOR‐17011474) for their publications, but participants were enrolled in separate periods, without any overlap: 1 June 2017 to 27 October 2017 (Wang 2017), and 1 July 2018 to 24 November 2018 (Wang 2019). Characteristics of participants, interventions, and comparisons are different between studies. We therefore considered that no double‐counting of the same participants occurred, and we analysed the data as from two different studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
1. Axillary artery
1.1. B‐mode ultrasound guidance versus palpation and landmarks
See Table 1. Killu 2011 compared real‐time B‐mode ultrasound guidance to palpation and landmarks for axillary artery catheterisation and reported all outcomes up to one hour after the intervention. We judged the overall risk of bias for Killu 2011 as low and did not perform any sensitivity analysis in this comparison.
Primary outcomes
First‐attempt success rate
No data are available for this outcome.
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on overall success rate when compared to palpation and landmarks up to one hour (risk ratio (RR) 1.35, 95% confidence interval (CI) 0.99 to 1.86; 33 participants, 1 study; very low‐certainty evidence) (Analysis 1.1).
1.1. Analysis.

Comparison 1: [Axillary] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 1: Overall success rate
Time (minutes) needed for successful procedure
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on time needed for a successful procedure when compared to palpation and landmarks up to one hour (mean difference (MD) ‐2.27 minutes, 95% CI ‐7.36 to 2.82; 33 participants, 1 study; very low‐certainty evidence) (Analysis 1.2).
1.2. Analysis.

Comparison 1: [Axillary] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 2: Time needed for a successful procedure
Major haematoma
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on major haematomas when compared to palpation and landmarks up to one hour (RR 0.83, 95% CI 0.06 to 12.22; 33 participants, 1 study; very low‐certainty evidence) (Analysis 1.3).
1.3. Analysis.

Comparison 1: [Axillary] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 3: Major haematoma
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on venous puncture when compared to palpation and landmarks up to one hour (RR 0.83, 95% CI 0.20 to 3.54; 33 participants, 1 study; very low‐certainty evidence) (Analysis 1.4). We downgraded the certainty of evidence one level due to high risk of performance bias, and we downgraded two levels due to imprecision: few participants, few studies, and 95% CI consistent with possible benefit and possible harm. Killu 2011 also reported no events of nerve injury in both groups.
1.4. Analysis.

Comparison 1: [Axillary] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 4: Adverse events (venous puncture)
No data are available for pain or other adverse events.
Quality of life
No data are available for this outcome.
2. Dorsalis pedis artery
2.1. B‐mode ultrasound guidance versus palpation and landmarks
See Table 2. Anand 2019 compared real‐time B‐mode ultrasound guidance to palpation and landmarks for dorsalis pedis artery catheterisation and reported all outcomes up to one hour after the intervention. We judged the overall risk of bias for Anand 2019 as low and did not perform any sensitivity analysis in this comparison.
Primary outcomes
First‐attempt success rate
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on first‐attempt success rate when compared to palpation and landmarks up to one hour (RR 1.28, 95% CI 0.90 to 1.82; 60 participants, 1 study; very low‐certainty evidence) (Analysis 2.1).
2.1. Analysis.

Comparison 2: [Dorsalis pedis] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 1: First‐attempt success rate
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on overall success rate when compared to palpation and landmarks up to one hour (RR 1.00, 95% CI 0.91 to 1.10; 60 participants, 1 study; very low‐certainty evidence) (Analysis 2.2).
2.2. Analysis.

Comparison 2: [Dorsalis pedis] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 2: Overall success rate
Time (minutes) needed for successful procedure
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on time needed for a successful procedure when compared to palpation and landmarks up to one hour (MD ‐0.04 minutes, 95% CI ‐0.16 to 0.08; 60 participants, 1 study; very low‐certainty evidence) (Analysis 2.3).
2.3. Analysis.

Comparison 2: [Dorsalis pedis] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 3: Time needed for a successful procedure
Major haematoma
Anand 2019 reported that "complications were not noticed in any of the two groups", but they did not detail what complications were assessed.
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
Anand 2019 reported that "complications were not noticed in any of the two groups", but they did not detail what complications were assessed.
Quality of life
No data are available for this outcome.
3. Radial artery
3.1. B‐mode ultrasound guidance versus palpation and landmarks
See Table 3.
Two trials included in this comparison did not report any data that we could use in our analysis (Edanaga 2012; Fujita 2012). Fujita 2012 is an abstract of the event and provides no data for numerical analysis. Edanaga 2012 was evaluated in full text after translation from Japanese; investigators did not evaluate any outcomes of interest for our review.
Hansen 2014 and Gopalasingam 2014 are cross‐over studies, and data from the first phase were shared by trial authors in personal communication.
We judged the overall risk of bias as high for 11 studies (Goswami 2020; Grandpierre 2019; Khan 2018; Laursen 2015; Rose 2018; Seto 2015; Shiver 2006; Tangwiwat 2016; Ueda 2015; Yeap 2019; Zaremski 2013), and as low for all other studies in this comparison. We performed a sensitivity analysis while excluding trials with high overall risk of bias and a cross‐over design.
Primary outcomes
First‐attempt success rate
Twenty‐seven included studies evaluated the first‐attempt success rate (Ammar 2017; Burad 2017; Cao 2018; Gibbons 2020; Gopalasingam 2014; Grandpierre 2019; Hansen 2014; Khan 2018; Kiberenge 2018; Kim 2021b; Laursen 2015; Levin 2003; Li 2016; Nasreen 2016; NCT01663779; Nguyen 2019; Peters 2015; Rajasekar 2021; Seto 2015; Seyhan 2021; Shiver 2006; Tangwiwat 2016; Ueda 2015; Wang 2017; Wang 2019; Yu 2019; Zaremski 2013; Zeng 2020). Kiberenge 2018 reported outcomes data separately by experienced and inexperienced operators; therefore, we included this study in both subgroups.
B‐mode ultrasound guidance may improve the first‐attempt success rate compared with palpation and landmarks up to one hour (RR 1.44, 95% CI 1.29 to 1.61; 4708 participants, 27 studies; I² = 85%; low‐certainty evidence) (Analysis 3.1). The test for subgroup differences suggests that the experience of operators has not a modifying effect on the first‐attempt success rate (Chi² = 0.98, df = 1 (P = 0.32), I² = 0%) (Analysis 3.1).
3.1. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 1: First‐attempt success rate
Sensitivity analyses including only trials at low risk of bias (RR 1.46, 95% CI 1.33 to 1.60) (Analysis 3.2) and including only individual parallel‐design studies (RR 1.42, 95% CI 1.27 to 1.59) (Analysis 3.3) did not change the effect estimate substantially.
3.2. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 2: First‐attempt success rate ‐ trials at low risk of bias
3.3. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 3: First‐attempt success rate ‐ trials with individual parallel design
The related funnel plot was asymmetrical, and Egger's test was significant (t = 5.80, df = 26, P < 0.0001), suggesting that small studies in favour of palpation intervention may not have been published (Figure 4). When a trim‐and‐fill method was applied, the adjusted effect estimate changed substantially (RR 1.19, 95% CI 1.07 to 1.31) (Figure 5). The adjusted funnel plot is presented in Figure 6.
4.
Funnel plot without adjustment of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.1 First‐attempt success rate.
5.
Forest plot with adjustment (trim and fill method) of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.1 First‐attempt success rate.
Filled studies: imputed studies
6.
Funnel plot with adjustment (trim and fill method) of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.1 First‐attempt success rate.
Empty circles: imputed studies; Filled circles: original studies
Pseudoaneurysm
Nguyen 2019 showed uncertainty about the effects of real‐time B‐mode ultrasound guidance on pseudoaneurysm when compared to palpation and landmarks up to one month (RR 2.89, 95% CI 0.12 to 70.63; 679 participants, 1 study; very low‐certainty evidence) (Analysis 3.4).
3.4. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 4: Pseudomaneurysm
Secondary outcomes
Overall success rate
Twenty‐eight included studies evaluated the overall success rate (Ammar 2017; Bobbia 2013; Burad 2017; Cao 2018; Gibbons 2020; Gopalasingam 2014; Goswami 2020; Grandpierre 2019; Hansen 2014; Khan 2018; Kiberenge 2018; Kim 2021b; Laursen 2015; Levin 2003; Li 2016; Nasreen 2016; NCT01663779; Nguyen 2019; Peters 2015; Rajasekar 2021; Seto 2015; Seyhan 2021; Shiver 2006; Tangwiwat 2016; Ueda 2015; Wang 2017; Wang 2019; Yu 2019; Zaremski 2013). Kiberenge 2018 reported outcomes data separately by experienced and inexperienced operators; therefore, we included this study in both subgroups.
B‐mode ultrasound guidance may slightly improve the overall success rate compared with palpation and landmarks up to one hour (RR 1.11, 95% CI 1.06 to 1.16; 4955 participants, 28 studies; I² = 88%; low‐certainty evidence) (Analysis 3.5). The test for subgroup differences suggests that the experience of operators does not have a modifying effect on overall success rate (Chi² = 1.54, df = 1 (P = 0.21), I² = 35.0%) (Analysis 3.5).
3.5. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 5: Overall success rate
Sensitivity analyses including only trials at low risk of bias (RR 1.14, 95% CI 1.07 to 1.22) (Analysis 3.6) and including only individual parallel‐design studies (RR 1.10, 95% CI 1.05 to 1.15) (Analysis 3.7) did not change the effect estimate substantially.
3.6. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 6: Overall success rate ‐ trials at low risk of bias
3.7. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 7: Overall success rate ‐ trials with individual parallel design
The related funnel plot was asymmetrical, and Egger's test was significant (t = 4.56, df = 27, P = 0.0001), suggesting that small studies in favour of palpation intervention may not have been published (Figure 7). When a trim‐and‐fill method was applied, the adjusted effect estimate changed substantially (RR 1.03, 95% CI 0.99 to 1.08) (Figure 8). The adjusted funnel plot is presented in Figure 9.
7.
Funnel plot without adjustment of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.5 Overall success rate.
8.
Funnel plot with adjustment (trim‐and‐fill method) of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.5 Overall success rate.
Filled studies: imputed studies
9.
Funnel plot with adjustment (trim‐and‐fill method) of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.5 Overall success rate.
Empty circles: imputed studies; Filled circles: original studies
Time (minutes) needed for a successful procedure
Twenty‐six included studies evaluated the time needed for a successful procedure (Ammar 2017; Bobbia 2013; Burad 2017; Cao 2018; Gibbons 2020; Gopalasingam 2014; Grandpierre 2019; Hansen 2014; Kiberenge 2018; Kim 2021b; Laursen 2015; Levin 2003; Li 2016; Nasreen 2016; Nguyen 2019; Peters 2015; Rajasekar 2021; Seto 2015; Shiver 2006; Tangwiwat 2016; Ueda 2015; Wang 2017; Wang 2019; Yeap 2019; Yu 2019; Zaremski 2013).
B‐mode ultrasound guidance may slightly decrease the time needed for a successful procedure compared with palpation and landmarks up to one hour (MD ‐0.33 minutes, 95% CI ‐0.54 to ‐0.13; 4902 participants, 26 studies; I² = 96%; low‐certainty evidence) (Analysis 3.8). The test for subgroup differences suggests that the experience of operators may have a modifying effect on the time needed for a successful procedure (Chi² = 9.96, df = 1 (P = 0.002), I² = 90.0%) (Analysis 3.8).
3.8. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 8: Time needed for a successful procedure
Both sensitivity analyses including only trials at low risk of bias (MD ‐0.23 minutes, 95% CI ‐0.38 to ‐0.08) (Analysis 3.9) and including only individual parallel‐design studies (MD ‐0.30 minutes, 95% CI ‐0.51 to ‐0.09) (Analysis 3.10) did not change the effect estimate substantially.
3.9. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 9: Time needed for successful procedure ‐ trials at low risk of bias
3.10. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 10: Time needed for a successful procedure ‐ trials with individual parallel design
Although Egger's test was not significant (t = ‐0.67, df = 24, P = 0.5066), the related funnel plot was asymmetrical, suggesting that small studies in favour of palpation intervention may not have been published (Figure 10). When a trim‐and‐fill method was applied, the adjusted effect estimate changed substantially (MD ‐0.09, 95% CI ‐0.31 to 0.12) (Figure 11). The adjusted funnel plot is presented in Figure 12.
10.
Funnel plot without adjustment of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.8 Time needed for a successful procedure [minutes].
11.
Funnel plot with adjustment (trim‐and‐fill method) of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.8 Time needed for a successful procedure [minutes].
Filled studies: imputed studies
12.
Funnel plot with adjustment (trim‐and‐fill method) of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.8 Time needed for a successful procedure [minutes].
Empty circles: imputed studies; Filled circles: original studies
We could not use Khan 2018 data on this outcome for meta‐analysis because investigators reported these incompletely.
Major haematoma
Sixteen included studies evaluated major haematoma (Cao 2018; Gibbons 2020; Goswami 2020; Kim 2021b; Li 2016; Nasreen 2016; NCT01663779; Nguyen 2019; Peters 2015; Rajasekar 2021; Shiver 2006; Tangwiwat 2016; Wang 2017; Wang 2019; Yu 2019; Zaremski 2013). Cao 2018 did not differentiate haematomas in major and minor. Therefore, we dealt conservatively and considered all haematomas as major.
B‐mode ultrasound guidance probably decreases major haematoma when compared to palpation and landmarks up to one month (RR 0.35, 95% CI 0.23 to 0.56; 2504 participants, 16 studies; I² = 40%; moderate‐certainty evidence) (Analysis 3.11). The test for subgroup differences suggests that the experience of operators does not have a modifying effect on time needed for a successful procedure (Chi² = 0.56, df = 1 (P = 0.45), I² = 0%) (Analysis 3.11).
3.11. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 11: Major haematoma
Sensitivity analyses including only trials at low risk of bias (RR 0.30, 95% CI 0.21 to 0.43) (Analysis 3.12) did not change the effect estimate substantially. All trials that reported this outcome had individual parallel design; therefore, sensitivity analysis of trials with individual parallel design was not possible.
3.12. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 12: Major haematoma ‐ trials at low risk of bias
The related funnel plot was symmetrical and Egger's test was not significant (t = ‐0.30, df = 13, P = 0.7714), suggesting there is no suspicion that small studies in favour of palpation intervention may not have been published (Figure 13). Therefore, we did not perform a trim‐and‐fill method to adjust the effect estimate.
13.
Funnel plot without adjustment of comparison: 3 [Radial] B‐mode ultrasound guidance versus palpation and landmarks, outcome: 3.11 Major haematoma.
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
Four studies provided data for pain (Bobbia 2013; Grandpierre 2019; Hansen 2014; Seto 2015). We are uncertain about the effect of B‐mode ultrasound guidance on pain when compared to palpation and landmarks up to 24 hours (MD 0.81 visual analogue scale (VAS, 0 to 10), 95% CI ‐0.66 to 2.28; 883 participants, 4 studies; I² = 91%; very low‐certainty evidence) (Analysis 3.13). Both sensitivity analyses including only trials at low risk of bias (MD ‐0.10, 95% CI ‐0.95 to 0.75) (Analysis 3.14) and including only individual parallel‐design studies (MD 1.22, 95% CI ‐1.19 to 3.64) (Analysis 3.15) did not change the effect estimate substantially.
3.13. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 13: Adverse events (pain)
3.14. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 14: Adverse events (pain) ‐ trials at low risk of bias
3.15. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 15: Adverse events (pain) ‐ trials with individual parallel design
Khan 2018, Seto 2015, and Ueda 2015 reported bleeding, haematoma, ischaemia, or spasm as a combination of two or three outcomes. B‐mode ultrasound guidance may lead to no difference in bleeding, haematoma, ischaemia, or spasm compared with palpation and landmarks up to three days (RR 0.86, 95% CI 0.49 to 1.52; 1303 participants, 3 studies; I² = 15%; low‐certainty evidence) (Analysis 3.16). We downgraded the certainty of evidence one level due to high risk of selection, performance, detection, reporting, and other bias, and another level due to imprecision: 95% CI is consistent with possible benefit and possible harm. We did not perform any sensitivity analysis here because we judged Khan 2018, Seto 2015, and Ueda 2015 as having high overall risk of bias, and they had an individual parallel design.
3.16. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 16: Adverse events (bleeding, haematoma, ischaemia, or spasm)
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on local infection when compared to palpation and landmarks up to two days (RR 0.33, 95% CI 0.04 to 3.15; 260 participants, 3 studies; I² = 0%; very low‐certainty evidence) (Cao 2018; Goswami 2020; Yu 2019; Analysis 3.17). We downgraded the certainty of evidence one level due to high risk of performance bias, and two levels due to imprecision: wide 95% CI is consistent with possible benefit and possible harm. The sensitivity analysis including only trials at low risk of bias (RR 0.33, 95% CI 0.01 to 8.02) (Analysis 3.18) did not change the effect estimate substantially. All trials that reported this outcome had an individual parallel design; therefore, the sensitivity analysis of trials with individual parallel design was not possible.
3.17. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 17: Adverse events (local infection)
3.18. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 18: Adverse events (local infection) ‐ trials at low risk of bias
Li 2016 and Wang 2019 showed real‐time B‐mode ultrasound guidance may slightly decrease oedema when compared to palpation and landmarks up to three days (RR 0.15, 95% CI 0.04 to 0.64; 365 participants, 2 studies; I² = 0%; low‐certainty evidence) (Analysis 3.19). We downgraded the certainty of evidence one level due to high risk of performance, detection, and other bias, and another level due to imprecision: 95% CI is consistent with possible benefit and possible harm. We did not perform any sensitivity analysis here because we judged Li 2016 and Wang 2019 as having low overall risk of bias, and they had an individual parallel design.
3.19. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 19: Adverse events (oedema)
Goswami 2020, Kim 2021b, Nguyen 2019, Wang 2017, and Wang 2019 reported arterial thrombosis. We are uncertain about the effect of B‐mode ultrasound guidance on arterial thrombosis compared with palpation and landmarks up to one month (RR 0.71, 95% CI 0.14 to 3.54; 1496 participants, 5 studies; I² = 0%; very low‐certainty evidence) (Analysis 3.20). We downgraded the certainty of evidence one level due to high risk of performance and other bias, and two levels due to imprecision: wide 95% CI is consistent with possible benefit and possible harm. The sensitivity analysis including only trials at low risk of bias (RR 0.33, 95% CI 0.03 to 3.13) (Analysis 3.21) did not change the effect estimate substantially. All trials that reported this outcome had an individual parallel design; therefore, the sensitivity analysis of trials with an individual parallel design was not possible.
3.20. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 20: Adverse events (arterial thrombosis)
3.21. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 21: Adverse events (arterial thrombosis) ‐ trials at low risk of bias
Nguyen 2019 reported death. We are uncertain about the effect of real‐time B‐mode ultrasound guidance on death when compared to palpation and landmarks up to one month (RR 0.32, 95% CI 0.01 to 7.85; 679 participants, 1 study; very low‐certainty evidence) (Analysis 3.22). We downgraded the certainty of evidence one level due to high risk of performance bias, and two levels due to imprecision: 95% CI is wide.
3.22. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 22: Adverse events (death)
Kim 2021b, Rajasekar 2021, Seto 2015, Wang 2017, and Wang 2019 reported spasm. B‐mode ultrasound guidance may lead to no difference in spasm compared with palpation and landmarks up to three days (RR 1.11, 95% CI 0.62 to 1.97; 1525 participants, 5 studies; I² = 0%; low‐certainty evidence) (Analysis 3.23). We downgraded the certainty of evidence one level due to high risk of performance, detection, attrition, and other bias, and another level due to imprecision: 95% CI is consistent with possible benefit and possible harm. The sensitivity analysis including only trials at low risk of bias (RR 0.90, 95% CI 0.30 to 2.64) (Analysis 3.24) did not change the effect estimate substantially. All trials that reported this outcome had an individual parallel design; therefore, the sensitivity analysis of trials with individual parallel design was not possible.
3.23. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 23: Adverse events (spasm)
3.24. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 24: Adverse events (spasm) ‐ trials at low risk of bias
Wang 2019 showed real‐time B‐mode ultrasound guidance may slightly decrease posterior wall puncture when compared to palpation and landmarks up to three days (RR 0.41, 95% CI 0.28 to 0.61; 196 participants, 1 study; low‐certainty evidence) (Analysis 3.25). We downgraded the certainty of evidence one level due to high risk of performance and other bias, and another level due to imprecision: 95% CI is consistent with possible benefit and possible harm.
3.25. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 25: Adverse events (posterior wall puncture)
Kim 2021b reported no events of ischaemia in both groups up to the end of the procedure (not detailed); therefore, we could not estimate the effects of interventions for this outcome.
Zaremski 2013 reported no arterial dissection in both groups for up to two days; therefore, we could not estimate the effects of interventions for this outcome.
Quality of life
We are uncertain about the effect of B‐mode ultrasound guidance on QoL compared with palpation and landmarks up to the end of the procedure (MD 0.00, 95% CI ‐1.07 to 1.07; 72 participants, 1 study; very low‐certainty evidence) (Analysis 3.26). Bobbia 2013 used a VAS to report patient satisfaction (0 to 10 scale); higher values mean better QoL, and a 30% change in the relative effect estimate means a minimally important effect.
3.26. Analysis.

Comparison 3: [Radial] B‐mode ultrasound guidance versus palpation and landmarks, Outcome 26: Quality of life (satisfaction)
3.2. B‐mode ultrasound guidance versus Doppler auditory ultrasound assistance
See Table 4.
Primary outcomes
First‐attempt success rate
Ueda 2015 showed real‐time B‐mode ultrasound guidance probably improves first‐attempt success rate when compared to DUA up to 72 hours (RR 1.35, 95% CI 1.11 to 1.64; 493 participants, 1 study; moderate‐certainty evidence) (Analysis 4.1).
4.1. Analysis.

Comparison 4: [Radial] B‐mode ultrasound versus Doppler assistance, Outcome 1: First‐attempt success rate
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
Ueda 2015 showed real‐time B‐mode ultrasound guidance may increase overall success rate when compared to DUA up to 72 hours (RR 1.13, 95% CI 0.99 to 1.29; 493 participants, 1 study; low‐certainty evidence) (Analysis 4.2).
4.2. Analysis.

Comparison 4: [Radial] B‐mode ultrasound versus Doppler assistance, Outcome 2: Overall success rate
Time (minutes) needed for a successful procedure
Ueda 2015 showed real‐time B‐mode ultrasound guidance probably improves time needed for a successful procedure when compared to DUA up to 72 hours (MD ‐1.57 minutes, 95% CI ‐1.78 to ‐1.36; 493 participants, 1 study; moderate‐certainty evidence) (Analysis 4.3).
4.3. Analysis.

Comparison 4: [Radial] B‐mode ultrasound versus Doppler assistance, Outcome 3: Time needed for a successful procedure
Major haematoma
No data are available for this outcome.
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
Ueda 2015 showed real‐time B‐mode ultrasound guidance may lead to no difference in haematoma or ischaemia when compared to DUA up to 72 hours (RR 1.20, 95% CI 0.70 to 2.05; 493 participants, 1 study; low‐certainty evidence) (Analysis 4.4). We downgraded the certainty of evidence one level due to high risk of performance, reporting, and other bias, and another level due to imprecision: 95% CI is consistent with possible benefit and possible harm.
4.4. Analysis.

Comparison 4: [Radial] B‐mode ultrasound versus Doppler assistance, Outcome 4: Adverse events (haematoma or ischaemia)
No data are available for pain or other adverse events.
Quality of life
No data are available for this outcome.
3.3. B‐mode ultrasound guidance versus near‐infrared laser guidance
See Table 5.
Primary outcomes
First‐attempt success rate
Osuda 2020 showed that the effect of real‐time B‐mode ultrasound guidance is uncertain for first‐attempt success rate when compared to near‐infrared laser guidance (ILG) up to the end of the procedure (less than one hour) (RR 0.76, 95% CI 0.48 to 1.20; 72 participants, 1 study; very low‐certainty evidence) (Analysis 5.1).
5.1. Analysis.

Comparison 5: [Radial] B‐mode ultrasound versus near‐infrared laser guidance, Outcome 1: First‐attempt success rate
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
Osuda 2020 showed that the effect of real‐time B‐mode ultrasound guidance on overall success rate is uncertain when compared to ILG up to the end of the procedure (less than one hour) (RR 0.97, 95% CI 0.86 to 1.10; 72 participants, 1 study; very low‐certainty evidence) (Analysis 5.2).
5.2. Analysis.

Comparison 5: [Radial] B‐mode ultrasound versus near‐infrared laser guidance, Outcome 2: Overall success rate
Time (minutes) needed for a successful procedure
Osuda 2020 showed that the effect of real‐time B‐mode ultrasound guidance on time needed for a successful procedure is uncertain when compared to ILG up to the end of the procedure (less than one hour) (MD 0.20 minutes, 95% CI 0.09 to 0.31; 72 participants, 1 study; very low‐certainty evidence) (Analysis 5.3).
5.3. Analysis.

Comparison 5: [Radial] B‐mode ultrasound versus near‐infrared laser guidance, Outcome 3: Time needed for a successful procedure
Major haematoma
No data are available for this outcome.
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
No data are available for this outcome.
Quality of life
No data are available for this outcome.
3.4. B‐mode ultrasound guidance versus modified B‐mode ultrasound guidance
See Table 6.
Zhefeng 2019 compared traditional real‐time B‐mode ultrasound guidance versus real‐time B‐mode ultrasound guidance with the addition of a developing line in the transducer. This line produced an acoustic shadow that could guide radial artery catheterisation. Kim 2021a compared traditional real‐time B‐mode ultrasound guidance versus real‐time B‐mode ultrasound guidance with the addition of electromagnetic guidance. We did not perform any sensitivity analysis here because we judged both studies as having low overall risk of bias, and they had an individual parallel design.
Primary outcomes
First‐attempt success rate
Real‐time B‐mode ultrasound guidance may decrease first‐attempt success rate when compared to modified B‐mode ultrasound guidance up to one hour (RR 0.68, 95% CI 0.55 to 0.84; 153 participants, 2 studies; I² = 86%; low‐certainty evidence) (Analysis 6.1).
6.1. Analysis.

Comparison 6: [Radial] B‐mode ultrasound versus modified B‐mode ultrasound, Outcome 1: First‐attempt success rate
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
Real‐time B‐mode ultrasound guidance may reduce overall success rate when compared to modified B‐mode ultrasound guidance up to one hour (RR 0.93, 95% CI 0.86 to 1.01; 153 participants, 2 studies; I² = 88%; low‐certainty evidence) (Analysis 6.2).
6.2. Analysis.

Comparison 6: [Radial] B‐mode ultrasound versus modified B‐mode ultrasound, Outcome 2: Overall success rate
Time (minutes) needed for a successful procedure
Real‐time B‐mode ultrasound guidance may lead to no difference in time needed for a successful procedure when compared to modified B‐mode ultrasound guidance up to one hour (MD 0.04 minutes, 95% CI ‐0.01 to 0.09; 153 participants, 2 studies; I² = 70%; low‐certainty evidence) (Analysis 6.3).
6.3. Analysis.

Comparison 6: [Radial] B‐mode ultrasound versus modified B‐mode ultrasound, Outcome 3: Time needed for a successful procedure
Major haematoma
We are uncertain about the effects of real‐time B‐mode ultrasound guidance on major haematoma when compared to modified B‐mode ultrasound guidance up to 48 hours (RR 3.23, 95% CI 1.37 to 7.60; 153 participants, 2 studies; I² = 23%; very low‐certainty evidence) (Analysis 6.4).
6.4. Analysis.

Comparison 6: [Radial] B‐mode ultrasound versus modified B‐mode ultrasound, Outcome 4: Major haematoma
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
Zhefeng 2019 showed real‐time B‐mode ultrasound guidance may lead to no difference in spasm when compared to modified B‐mode ultrasound guidance up to 48 hours (RR 1.39, 95% CI 0.89 to 2.16; 77 participants, 1 study; low‐certainty evidence) (Analysis 6.5). We downgraded the certainty of evidence one level due to high risk of performance and detection bias, and another level due to imprecision: 95% CI is consistent with possible benefit and possible harm.
6.5. Analysis.

Comparison 6: [Radial] B‐mode ultrasound versus modified B‐mode ultrasound, Outcome 5: Adverse events (spasm)
We could not estimate the effects of arterial thrombosis because Zhefeng 2019 and Kim 2021a reported no events in both groups.
It is uncertain whether real‐time B‐mode ultrasound guidance leads to no difference in posterior wall puncture when compared to modified B‐mode ultrasound guidance up to 24 hours (RR 8.00, 95% CI 1.05 to 60.89; 76 participants, 1 study; low‐certainty evidence) (Analysis 6.6; Kim 2021a). We downgraded the certainty of evidence one level due to high risk of performance and detection bias, and two levels due to imprecision: 95% CI is wide.
6.6. Analysis.

Comparison 6: [Radial] B‐mode ultrasound versus modified B‐mode ultrasound, Outcome 6: Adverse events (posterior wall puncture)
No data are available for pain or other adverse events.
Quality of life
No data are available for this outcome.
3.5. In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound
See Table 7. We judged the overall risk of bias as high for Arora 2021, Cao 2020, and Sethi 2017, and as low for all of the six other studies in this comparison. We performed sensitivity analyses while excluding the trial with high overall risk of bias and did not perform a sensitivity analysis for the study design because all studies had an individual parallel design.
Primary outcomes
First‐attempt success rate
Eight included studies evaluated first‐attempt success rate (Abdalla 2017; Arora 2021; Cao 2020; Nam 2020; Quan 2014; Rajasekar 2021; Sethi 2017; Wang 2019).
We are uncertain about the effect of in‐plane B‐mode ultrasound guidance on first‐attempt success rate compared with out‐of‐plane B‐mode ultrasound guidance up to one hour (RR 0.85, 95% CI 0.65 to 1.12; 1051 participants, 8 studies; I² = 91%; very low‐certainty evidence) (Analysis 7.1). The sensitivity analysis including only trials at low risk of bias (RR 0.92, 95% CI 0.73 to 1.17) (Analysis 7.2) did not change the effect estimate substantially.
7.1. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 1: First‐attempt success rate
7.2. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 2: First‐attempt success rate ‐ trials at low risk of bias
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
Eight included studies evaluated the overall success rate (Abdalla 2017; Arora 2021; Cao 2020; Nam 2020; Quan 2014; Rajasekar 2021; Sethi 2017; Wang 2019).
In‐plane B‐mode ultrasound guidance may lead to no difference in the overall success rate compared with out‐of‐plane B‐mode ultrasound guidance up to one hour (RR 1.00, 95% CI 0.96 to 1.05; 1051 participants, 8 studies; I² = 64%; low‐certainty evidence) (Analysis 7.3). The sensitivity analysis including only trials at low risk of bias (RR 1.05, 95% CI 0.95 to 1.16) (Analysis 7.4) did not change the effect estimate substantially.
7.3. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 3: Overall success rate
7.4. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 4: Overall success rate ‐ trials at low risk of bias
Time (minutes) needed for a successful procedure
Nine included studies evaluated the overall success rate (Abdalla 2017; Arora 2021; Berk 2013; Cao 2020; Nam 2020; Quan 2014; Rajasekar 2021; Sethi 2017; Wang 2019).
In‐plane B‐mode ultrasound guidance may lead to no difference in time needed for a successful procedure compared with out‐of‐plane B‐mode ultrasound guidance up to one hour (MD ‐0.06 minutes, 95% CI ‐0.16 to 0.05; 1134 participants, 9 studies; I² = 88%; low‐certainty evidence) (Analysis 7.5). The sensitivity analysis including only trials at low risk of bias (MD ‐0.05 minutes, 95% CI ‐0.23 to 0.12) (Analysis 7.6) did not change the effect estimate substantially.
7.5. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 5: Time needed for a successful procedure
7.6. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 6: Time needed for a successful procedure ‐ trials at low risk of bias
Major haematoma
Nine included studies evaluated major haematoma (Abdalla 2017; Arora 2021; Berk 2013; Cao 2020; Nam 2020; Quan 2014; Rajasekar 2021; Sethi 2017; Wang 2019).
We are uncertain about the effect of in‐plane B‐mode ultrasound guidance on major haematoma compared with out‐of‐plane B‐mode ultrasound guidance up to 72 hours (RR 0.49, 95% CI 0.22 to 1.08; 1159 participants, 9 studies; I² = 67%; very low‐certainty evidence) (Analysis 7.7). The sensitivity analysis including only trials at low risk of bias (RR 0.59, 95% CI 0.23 to 1.54) (Analysis 7.8) did not change the effect estimate substantially.
7.7. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 7: Major haematoma
7.8. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 8: Major haematoma ‐ trials at low risk of bias
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
Abdalla 2017 reported no local infection events in both groups; therefore we are unable to estimate related effects.
Berk 2013, Quan 2014, Sethi 2017, and Wang 2019 reported no thrombosis events in both groups. Nam 2020 reported one event of thrombosis; therefore, we are uncertain about the effects of in‐plane B‐mode ultrasound on thrombosis compared with out‐of‐plane B‐mode ultrasound guidance up to 72 hours (RR 3.18, 95% CI 0.13 to 76.69; 136 participants, 1 study; very low‐certainty evidence) (Analysis 7.9). We downgraded the certainty of evidence one level due to high risk of performance bias, and two levels due to imprecision: wide 95% CI is consistent with possible benefit and possible harm. The sensitivity analysis including only trials at low risk of bias (RR 3.18, 95% CI 0.13 to 76.69) (Analysis 7.10) did not change the effect estimate.
7.9. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 9: Adverse events (thrombosis)
7.10. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 10: Adverse events (thrombosis) ‐ trials at low risk of bias
Quan 2014 and Sethi 2017 reported no oedema events in both groups. Berk 2013 reported one event of oedema. It is uncertain if in‐plane B‐mode ultrasound guidance leads to no difference in oedema compared with out‐of‐plane B‐mode ultrasound guidance up to 72 hours (RR 0.07, 95% CI 0.00 to 1.14; 108 participants, 1 study; very low‐certainty evidence) (Analysis 7.11). We downgraded the certainty of evidence one level due to high risk of performance bias, and two levels due to imprecision: wide 95% CI is consistent with possible benefit and possible harm. The sensitivity analysis including only trials at low risk of bias (RR 0.07, 95% CI 0.00 to 1.14) (Analysis 7.12) did not change the effect estimate.
7.11. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 11: Adverse events (oedema)
7.12. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 12: Adverse events (oedema) ‐ trials at low risk of bias
Quan 2014 reported no vasospasm in both groups. Berk 2013, Nam 2020, Rajasekar 2021, Sethi 2017, and Wang 2019 reported vasospasm events, but we are uncertain about the effect of in‐plane B‐mode ultrasound guidance on vasospasm compared with out‐of‐plane B‐mode ultrasound guidance up to 72 hours (RR 0.80, 95% CI 0.24 to 2.69; 748 participants, 6 studies; I² = 53%; very low‐certainty evidence) (Analysis 7.13). We downgraded the certainty of evidence one level due to high risk of performance, reporting, and other bias, one level due to inconsistency (unexplained substantial heterogeneity), and two levels due to imprecision: wide 95% CI is consistent with possible benefit and possible harm. The sensitivity analysis including only trials at low risk of bias (RR 0.95, 95% CI 0.25 to 3.54) (Analysis 7.14) did not change the effect estimate.
7.13. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 13: Adverse events (vasospasm)
7.14. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 14: Adverse events (vasospasm) ‐ trials at low risk of bias
Berk 2013, Nam 2020, and Wang 2019 evaluated posterior wall damage, but we are uncertain about the effect of in‐plane B‐mode ultrasound guidance on posterior wall damage compared with out‐of‐plane B‐mode ultrasound guidance up to 72 hours (RR 0.45, 95% CI 0.10 to 1.97; 375 participants, 3 studies; I² = 86%; very low‐certainty evidence) (Analysis 7.15). We downgraded the certainty of evidence one level due to high risk of performance and other bias, one level due to inconsistency (unexplained substantial heterogeneity), and one level due to imprecision: 95% CI is consistent with possible benefit and possible harm. We did not perform any sensitivity analysis because we judged all three studies as having low risk of bias, and they had a parallel design.
7.15. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 15: Adverse events (posterior wall damage)
No data are available for pain or other adverse events.
Quality of life
No data are available for this outcome.
3.6. Doppler auditory ultrasound assistance versus palpation and landmarks
See Table 8. We judged the overall risk of bias as high for Ueda 2015 and as low for Tada 2003. We performed sensitivity analyses while excluding the trial with high overall risk of bias and did not perform a sensitivity analysis for study design because studies had an individual parallel design.
Primary outcomes
First‐attempt success rate
Ueda 2015 and Tada 2003 showed DUA may lead to no difference in first‐attempt success rate compared to palpation and landmarks up to one hour (RR 1.01, 95% CI 0.90 to 1.14; 2 studies, 666 participants; low‐certainty evidence) (Analysis 8.1). The sensitivity analysis including only trials at low risk of bias (RR 1.02, 95% CI 0.88 to 1.17) (Analysis 8.2) did not change the effect estimate.
8.1. Analysis.

Comparison 8: [Radial] Doppler assistance versus palpation and landmarks, Outcome 1: First‐attempt success rate
8.2. Analysis.

Comparison 8: [Radial] Doppler assistance versus palpation and landmarks, Outcome 2: First‐attempt success rate ‐ trials at low risk of bias
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
Ueda 2015 and Tada 2003 showed DUA may lead to no difference in overall success rate when compared to palpation and landmarks up to one hour (RR 0.99, 95% CI 0.92 to 1.07; 666 participants, 2 studies; low‐certainty evidence) (Analysis 8.3). The sensitivity analysis including only trials at low risk of bias (RR 1.00, 95% CI 0.96 to 1.03) (Analysis 8.4) did not change the effect estimate.
8.3. Analysis.

Comparison 8: [Radial] Doppler assistance versus palpation and landmarks, Outcome 3: Overall success rate
8.4. Analysis.

Comparison 8: [Radial] Doppler assistance versus palpation and landmarks, Outcome 4: Overall success rate ‐ trials at low risk of bias
Time (minutes) needed for a successful procedure
Ueda 2015 showed DUA probably increases time needed for a successful procedure when compared to palpation and landmarks up to one hour (MD 0.45 minutes, 95% CI 0.20 to 0.70; 500 participants, 1 study; moderate‐certainty evidence) (Analysis 8.5).
8.5. Analysis.

Comparison 8: [Radial] Doppler assistance versus palpation and landmarks, Outcome 5: Time needed for a successful procedure
Major haematoma
No data are available for this outcome.
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
Ueda 2015 showed DUA may lead to no difference in haematoma or ischaemia when compared to palpation and landmarks up to 72 hours (RR 0.80, 95% CI 0.47 to 1.35; 500 participants, 1 study; low‐certainty evidence) (Analysis 8.6). We downgraded the certainty of evidence one level due to high risk of performance, reporting, and other bias, and one level due to imprecision: 95% CI is consistent with possible benefit and possible harm.
8.6. Analysis.

Comparison 8: [Radial] Doppler assistance versus palpation and landmarks, Outcome 6: Adverse events (haematoma or ischaemia)
No data are available for pain or other adverse events.
Quality of life
No data are available for this outcome.
3.7. Dynamic out‐of‐plane B‐mode ultrasound versus static out‐of‐plane B‐mode ultrasound
See Table 9. Only Bai 2020 had data for this comparison and was judged as having low overall risk of bias.
Primary outcomes
First‐attempt success rate
We are uncertain about the effect of dynamic out‐of‐plane B‐mode ultrasound on first‐attempt success rate compared to static out‐of‐plane B‐mode ultrasound up to one hour (RR 0.91, 95% CI 0.67 to 1.23; 131 participants; 1 study; very low‐certainty evidence) (Analysis 9.1).
9.1. Analysis.

Comparison 9: [Radial] Dynamic out‐of‐plane B‐mode ultrasound versus static out‐of‐plane B‐mode ultrasound, Outcome 1: First‐attempt success rate
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
We are uncertain about the effect of dynamic out‐of‐plane B‐mode ultrasound on overall success rate compared to static out‐of‐plane B‐mode ultrasound up to one hour (RR 1.07, 95% CI 0.92 to 1.25; 131 participants; 1 study; very low‐certainty evidence) (Analysis 9.2).
9.2. Analysis.

Comparison 9: [Radial] Dynamic out‐of‐plane B‐mode ultrasound versus static out‐of‐plane B‐mode ultrasound, Outcome 2: Overall success rate
Time (minutes) needed for a successful procedure
We are uncertain about the effect of dynamic out‐of‐plane B‐mode ultrasound on time needed for a successful procedure compared to static out‐of‐plane B‐mode ultrasound up to one hour (MD 0.37, 95% CI 0.07 to 0.66; 131 participants, 1 study; very low‐certainty evidence) (Analysis 9.3).
9.3. Analysis.

Comparison 9: [Radial] Dynamic out‐of‐plane B‐mode ultrasound versus static out‐of‐plane B‐mode ultrasound, Outcome 3: Time needed for a successful procedure
Major haematoma
No data are available for this outcome.
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
We are uncertain about the effect of dynamic out‐of‐plane B‐mode ultrasound on posterior wall puncture compared to static out‐of‐plane B‐mode ultrasound up to one hour (RR 0.52, 95% CI 0.34 to 0.81; 131 participants; 1 study; very low‐certainty evidence) (Analysis 9.3). We downgraded one level due to high risk of performance bias, one level due to indirectness (few participants are not representative of the overall relevant population), and one level due to imprecision (few participants).
No data are available for pain or other adverse events.
Quality of life
No data are available for this outcome.
3.8. Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound
See Table 10. We judged overall risk of bias as high for Cao 2020 and Zeng 2020, and as low for Abdalla 2017. We performed sensitivity analyses while excluding trials with high overall risk of bias and did not perform a sensitivity analysis for the study design because all studies had an individual parallel design.
Primary outcomes
First‐attempt success rate
Abdalla 2017, Cao 2020, and Zeng 2020 showed that the effect of oblique‐axis in‐plane B‐mode ultrasound on first‐attempt success rate is uncertain when compared to long‐axis in‐plane B‐mode ultrasound up to 72 hours (RR 1.11, 95% CI 0.44 to 2.79; 275 participants, 3 studies; I² = 87%; very low‐certainty evidence) (Analysis 10.1). The sensitivity analysis including only trials at low risk of bias (RR 2.36, 95% CI 1.35 to 4.14) (Analysis 10.2) changed the effect estimate.
10.1. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 1: First‐attempt success rate
10.2. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 2: First‐attempt success rate ‐ trials at low risk of bias
Pseudoaneurysm
No data are available for this outcome.
Secondary outcomes
Overall success rate
Abdalla 2017 and Cao 2020 showed oblique‐axis in‐plane B‐mode ultrasound may slightly improve the overall success rate compared to long‐axis in‐plane B‐mode ultrasound up to 72 hours (RR 1.27, 95% CI 1.05 to 1.53; 215 participants, 2 studies; I² = 0%; low‐certainty evidence) (Analysis 10.3). The sensitivity analysis including only trials at low risk of bias (RR 1.28, 95% CI 1.01 to 1.61) (Analysis 10.4) did not change the effect estimate.
10.3. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 3: Overall success rate
10.4. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 4: Overall success rate ‐ trials at low risk of bias
Time (minutes) needed for a successful procedure
Abdalla 2017, Cao 2020, and Zeng 2020 showed that the effect of oblique‐axis in‐plane B‐mode ultrasound on time needed for a successful procedure is uncertain when compared to long‐axis in‐plane B‐mode ultrasound up to 72 hours (MD ‐0.35 minutes, 95% CI ‐0.95 to 0.25; 275 participants; 3 studies; I² = 99%; very low‐certainty evidence) (Analysis 10.5). The sensitivity analysis including only trials at low risk of bias (MD ‐0.83 minutes, 95% CI ‐0.88 to ‐0.79) (Analysis 10.6) changed the effect estimate.
10.5. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 5: Time needed for a successful procedure
10.6. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 6: Time needed for a successful procedure ‐ trials at low risk of bias
Major haematoma
Abdalla 2017 and Cao 2020 showed that the effect of oblique‐axis in‐plane B‐mode ultrasound on overall success rate is uncertain when compared to long‐axis in‐plane B‐mode ultrasound up to 72 hours (RR 0.68, 95% CI 0.32 to 1.47; 215 participants, 2 studies; I² = 0%; very low‐certainty evidence) (Analysis 10.7). The sensitivity analysis including only trials at low risk of bias (RR 0.55, 95% CI 0.22 to 1.34) (Analysis 10.8) did not change the effect estimate.
10.7. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 7: Major haematoma
10.8. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 8: Major haematoma ‐ trials at low risk of bias
Adverse events (minor haematoma, pain, local infection, artery thrombosis, artery embolism, nerve injury, amputation, life‐threatening events; fatal events)
Zeng 2020 showed that the effect of oblique‐axis in‐plane B‐mode ultrasound on vasospasm or haematoma is uncertain when compared to long‐axis in‐plane B‐mode ultrasound up to 72 hours (RR 0.09, 95% CI 0.01 to 1.57; 60 participants; 1 study; very low‐certainty evidence) (Analysis 10.9).
10.9. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 9: Adverse events (vasospasm or haematoma)
Abdalla 2017 and Cao 2020 showed that the effect of oblique‐axis in‐plane B‐mode ultrasound on time needed for a successful procedure is uncertain when compared to long‐axis in‐plane B‐mode ultrasound up to 72 hours (RR 4.64, 95% CI 0.23 to 94.77; 215 participants, 2 studies; I² = 0%; very low‐certainty evidence) (Analysis 10.10). We did not perform the sensitivity analysis including only trials at low risk of bias because Abdalla 2017 reported no events in both groups.
10.10. Analysis.

Comparison 10: [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound, Outcome 10: Adverse events (ischaemia)
Abdalla 2017 also reported no events of local infection in both groups. Therefore, we could not estimate the effect of intervention on this outcome.
No data are available for pain or other adverse events.
Quality of life
No data are available for this outcome.
Discussion
Summary of main results
This review assessed the effects of ultrasound guidance for arterial (other than femoral) catheterisation in adults. We included 48 randomised controlled trials (RCTs) that used Doppler auditory ultrasound assistance (DUA) or direct ultrasound guidance (DUG) with B‐mode or any other modified ultrasound technique in 7997 participants. These studies compared ultrasound guidance with palpation and landmarks or with another ultrasound intervention for catheterisation in axillary, dorsalis pedis, or radial artery.
Two trials did not report any data that we could use in our analysis (Edanaga 2012; Fujita 2012). The other 46 included studies provided data for 10 different comparisons. We found few data related to pseudoaneurysm, adverse events, and quality of life (QoL), but all relevant outcomes had available data.
We found no data regarding indirect ultrasound guidance, nor data at more than 30 days after intervention.
Axillary artery
Real‐time B‐mode ultrasound versus palpation and landmarks
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on overall success rate, time needed for a successful procedure, and major haematoma when compared to palpation and landmarks up to one hour (Table 1). First‐attempt success rate, pseudoaneurysm, pain, and QoL were not reported.
Dorsalis pedis artery
Real‐time B‐mode ultrasound versus palpation and landmarks
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on first‐attempt success rate, overall success rate, and time needed for a successful procedure when compared to palpation and landmarks up to one hour (Table 2). Pseudoaneurysm, major haematoma, pain, and QoL were not reported.
Radial artery
Real‐time B‐mode ultrasound versus palpation and landmarks
Real‐time B‐mode ultrasound guidance may improve first‐attempt success rate or overall success rate, or may decrease time needed for a successful procedure up to one hour compared to palpation and landmarks. B‐mode ultrasound guidance probably decreases major haematoma up to one month compared to palpation and landmarks. We are uncertain about effects on pseudoaneurysm, pain, and QoL because the certainty of evidence is very low (Table 3).
Real‐time B‐mode ultrasound versus DUA
Real‐time B‐mode ultrasound guidance probably improves first‐attempt success rate and time needed for a successful procedure up to one hour compared to DUA. B‐mode ultrasound guidance may increase overall success up to one hour and adverse events up to 72 hours compared to DUA (Table 4).
Real‐time B‐mode ultrasound versus near‐infrared laser
We are uncertain about the effect of real‐time B‐mode ultrasound guidance on first‐attempt success rate, overall success rate, and time needed for a successful procedure up to the end of the procedure (less than one hour) compared to near‐infrared laser guidance (Table 5).
Real‐time B‐mode ultrasound versus modified real‐time B‐mode ultrasound
Real‐time B‐mode ultrasound guidance may decrease first‐attempt success rate, may reduce overall success rate, and may lead to no difference in time needed for a successful procedure up to one hour compared to modified real‐time B‐mode ultrasound guidance. We are uncertain about effects on major haematoma because the certainty of evidence is very low (Table 6).
In‐plane versus out‐of‐plane B‐mode ultrasound
In‐plane B‐mode ultrasound guidance may lead to no difference in overall success rate and time needed for a successful procedure up to one hour. It is uncertain if in‐plane B‐mode ultrasound guidance leads to no difference in first‐attempt success rate and haematoma up to one hour or leads to no difference in adverse events up to 72 hours compared to out‐of‐plane B‐mode ultrasound guidance because the certainty of evidence is very low (Table 7).
DUA versus palpation and landmarks
DUA may lead to no difference in first‐attempt success rate, overall success rate, or adverse events, and probably increases time needed for a successful procedure up to one hour compared to palpation and landmarks (Table 8).
Dynamic versus static out‐of‐plane B‐mode ultrasound
We are uncertain about the effect of dynamic out‐of‐plane B‐mode ultrasound guidance on first‐attempt success rate, overall success rate, time needed for a successful procedure, or adverse events up to one hour compared to static out‐of‐plane B‐mode ultrasound guidance (Table 9).
Oblique‐axis versus long‐axis in‐plane B‐mode ultrasound
Oblique‐axis in‐plane B‐mode ultrasound guidance may slightly improve overall success rate up to 72 hours compared to long‐axis in‐plane B‐mode ultrasound guidance. It is uncertain if oblique‐axis in‐plane B‐mode ultrasound guidance leads to no difference in first‐attempt success rate, time needed for a successful procedure, major haematoma, or adverse events up to 72 hours compared to long‐axis in‐plane B‐mode ultrasound guidance (Table 10). We assessed differences between analyses with studies judged at high overall risk of bias and those judged at low overall risk of bias in sensitivity analyses. The direction and size of effects changed substantially in favour of oblique‐axis intervention for first‐attempt success rate and time needed for a successful procedure in the sensitivity analysis.
Overall completeness and applicability of evidence
Most evidence was obtained from people hospitalised with a severe disease that required arterial sample for blood test or pressure monitoring (diagnosis purposes), undergoing major surgery, or at high risk of cardiovascular events; therefore, evidence regarding ultrasound guidance for arterial (other than femoral) catheterisation in adults at lower risk and for therapeutic purposes remains uncertain. Second, information on clinical endpoints for all included studies was based on data until 30 days after the intervention only (i.e. no included studies evaluated long‐term outcomes, mainly those as important as pseudoaneurysm, major haematoma, and other adverse events). Often follow‐up for these trials was less than one hour, hence information on long‐term efficacy and safety is absent. Cochrane Reviews focus on patient‐relevant outcomes, and pseudoaneurysm is the most relevant adverse event in this setting. All other adverse events, regardless of their prevalence, are already addressed in our other outcomes (major haematoma and adverse events), but related available evidence is sparse.
Although most studies reported our primary outcome of first‐attempt success rate, we identified very little evidence related to pseudoaneurysm and adverse events after arterial catheterisation. It is also noteworthy that no included studies clearly defined major haematoma, and only one study measured our secondary outcome quality of life.
We noted substantial heterogeneity in the methods of the included studies, some of which did not provide complete and clear information about their data. For instance, differentiation between ultrasound methods with or without needle tip control for a formal subgroup analysis is insufficient (fewer than 10 trials reported details). This is particularly relevant to allow the operator to sustain the target vessel and the needle, including the needle tip, under visual guidance during the entire dynamic procedure. This hindered quantitative analyses (e.g. increasing heterogeneity) and assessment of risk of bias in many studies.
The number of trials for nine of the ten possible comparisons was small, ranging from one to nine studies. Only one comparison involved 28 trials. Moreover, the included studies had small primary sample sizes (from 33 to 749 participants). Four studies randomised more than 400 participants, and all others analysed not more than 285 participants.
Another issue was the fact that we had two trials that fulfilled our selection criteria but could not be included in the analyses because their data were incompletely described, and we were unable to obtain full data, despite contacting the trialists.
Quality of the evidence
The certainty of evidence is very low to moderate. We downgraded the certainty of evidence due to risk of bias, particularly concerning lack of blinding of staff and participants, which could have an impact on the non‐pharmacological intervention. We downgraded the certainty of evidence due to heterogeneity not explained among studies. We also downgraded the certainty of evidence due to imprecision resulting from small numbers of participants and wide 95% confidence intervals that are consistent with possible benefit and possible harm.
Reporting bias was addressed in funnel plots with the aid of additional statistical tests; this approach shows a degree of asymmetry involving small sample size studies, with an effect favouring palpation and landmarks absent for some primary and secondary outcomes. Currently available trials have been conducted primarily for scientific purposes without industry financial support; even so, it seems likely that any such studies, especially those with a negative effect of the new technology (ultrasound), remain unpublished. Besides, it is likely that this seeming asymmetry is a result of smaller studies selecting a different (possibly higher‐risk) patient population to increase power.
Potential biases in the review process
We conducted a sensitive search of the literature, and we believe that we identified all relevant trials that met our inclusion criteria. However, we may have missed some trials, particularly in the grey literature.
We adhered to the inclusion and exclusion criteria prespecified in the protocol to limit subjectivity (Flumignan 2020). We made efforts to obtain additional relevant data from study authors but were unable to do so in some cases. If we can source supplementary data, we will consider them in future updates.
Selection, data extraction, and 'Risk of bias' assessment of included studies were performed in duplicate by two independent review authors to reduce potential bias in the review process. Additional analyses (subgroups and sensitivity analysis) were performed as planned in our protocol, but our conclusions were based on our primary analysis (Flumignan 2020).
Agreements and disagreements with other studies or reviews
A number of systematic reviews and meta‐analyses have examined ultrasound guidance for arterial access, but most of them have evaluated mixed populations with paediatric and adult participants. Ultrasound guidance seems to have a role for arterial access in children and neonates, who have significantly smaller arterial diameters, higher heart rates, and vasoreactivity compared to adults (Aouad‐Maroun 2016).
Shiloh 2011 searched randomised controlled trials (RCTs) in MEDLINE, Embase, Cochrane CENTRAL, and abstracts of societies of speciality without language or date limits. They searched only for RCTs comparing real‐time ultrasound guidance versus palpation and landmarks for radial artery access. They used the obsolete Jadad criteria for risk of bias assessment. Although they considered adults and children, Shiloh 2011 included less than 10% of our number of participants for the same comparison, maybe because they used a limited and not sensible search strategy, and they ran it 10 years ago. Nevertheless, Shiloh 2011 included 311 participants and found that real‐time B‐mode ultrasound guidance increased first‐attempt success rate in 71% compared to palpation. This group reported a reduction in haematoma from 50% in the palpation group to 7% in the ultrasound group in one of their included studies.
Gu 2014 used an even more limited search strategy in MEDLINE and Embase databases only. They included five trials with mixed adult and children populations ‐ 129 adults from two trials included ‐ that compared real‐time B‐mode ultrasound guidance versus palpation for radial artery catheterisation. They found 85% improvement in first‐attempt success rate and reduction of 21% in mean time to success, favouring ultrasound.
Tang 2014 searched PubMed, Embase, and Cochrane CENTRAL for RCTs that compared real‐time B‐mode ultrasound guidance versus palpation for radial artery catheterisation. They analysed seven trials with mixed children and adult populations (241 adults from four trials). Tang 2014 found that ultrasound guidance increased first‐attempt success rate in 51% and reduced haematoma rate in 83%.
Gao 2016 searched PubMed, Embase, and Cochrane CENTRAL for RCTs, published in English, that compared in‐plane versus out‐of‐plane ultrasound guidance for vascular access. They included five trials that analysed vein and artery (271 participants from two trials) access in adult populations. Gao 2016 found no difference between in‐plane and out‐of‐plane ultrasound guidance for first‐attempt success rate, mean time for success, and haematoma.
Gu 2016 searched PubMed, Embase, and Cochrane CENTRAL, and ClinicalTrials.gov for RCTs that compared real‐time B‐mode ultrasound guidance or Doppler auditory ultrasound assistance (DUA) versus palpation for radial artery access. They included 13 RCTs, which analysed B‐mode ultrasound guidance or DUA in paediatric or adult populations, used GRADE to assess the certainty of evidence, and used funnel plots to assess reporting bias. Gu 2016 analysed 2161 adults in 10 RCTs, which were also included in our review. They found that B‐mode ultrasound guidance increased first‐attempt success rate in 31% (moderate‐certainty evidence), mean time to success in 43 seconds (very low‐certainty evidence), and haematoma in 61% (low‐certainty evidence). They also found no difference between DUA and palpation for first‐attempt success rate (low‐certainty evidence). Gu 2016 declared that their evidence of effect was sufficient and conclusive, and they were not suspicious of reporting bias based on funnel plots.
White 2016 searched CINAHL, SCOPUS, PubMed, MEDLINE, and Web of Science for RCTs that compared B‐mode ultrasound guidance versus palpation for radial artery access in adult or paediatric populations. They included 11 trials, six of which analysed adult participants (n = 1080). They found that, compared with palpation, B‐mode ultrasound guidance increased by 40% the first‐attempt success rate, with no difference in haematoma.
Bhattacharjee 2018 searched PubMed and Cochrane CENTRAL for RCTs that compared B‐mode ultrasound guidance versus palpation for radial artery access in adults. They included only published trials that reported first‐attempt success rate or overall success rate, and they excluded trials that performed arterial puncture for blood sampling. They used a modified Cochrane tool for risk of bias (other bias was not assessed) and funnel plots for assessment of reporting bias. They found that, compared to palpation, B‐mode ultrasound guidance increased first‐attempt success rate (odds ratio (OR) 2.76, 95% confidence interval (CI) 1.86 to 4.1; n = 1835), led to no difference in overall success rate (OR 2.01, 95% CI 1.00 to 4.06; n = 1402), resulted in no difference in time for a successful procedure (standard mean difference (SMD) ‐0.31 minutes, 95% CI ‐0.65 to 0.04; n = 1855); and produced no difference in haematoma (OR 0.53, 95% CI 0.21 to 1.29; n = 790). Bhattacharjee 2018 declared that visual inspection of funnel plots revealed no publication bias.
Pacha 2018 searched MEDLINE, Embase, and Cochrane CENTRAL for RCTs that compared B‐mode ultrasound guidance versus palpation for radial artery access in adults. Although review authors applied no language restrictions, they included only published trials since 1996 to January 2018 ‐ those reporting first‐attempt success rate or overall success rate ‐ and excluded trials that performed arterial puncture for blood sampling. They found that, compared to palpation, B‐mode ultrasound guidance increased first‐attempt success rate in 35% but led to no difference in mean time for a successful procedure nor in haematoma.
Zhao 2020 searched PubMed, Embase, and Cochrane CENTRAL for RCTs that compared ultrasound‐guided versus palpation techniques for radial artery catheterisation in children and adults. They imposed no restrictions for publication status and language of trial reports, used a non‐validated scale to judge the quality of trials, examined funnel plots and additional statistical tests to investigate reporting bias, and did not use the GRADE approach for certainty of evidence. They found that, compared to palpation, B‐mode ultrasound guidance increased first‐attempt success rate (risk ratio (RR) 1.39, 95% CI 1.21 to 1.59), decreased mean time to success (SMD ‐41.18 seconds, 95% CI ‐75.43 to ‐6.93), and decreased haematoma (RR 0.40, 95% CI 0.22 to 0.72).
Our review seems to be more comprehensive than these identified previous reviews, which used limited search strategies, imposed language or date limits, searched overlapping databases (e.g. SCOPUS, PubMed, MEDLINE, Web of Science in the same review), or searched a limited number of databases (e.g. PubMed, Cochrane CENTRAL only). Besides, these previous reviews analysed only one or two of all our possible comparisons of ultrasound guidance for arterial (other than femoral) access. Although Bhattacharjee 2018, Gu 2016, and Zhao 2020 used funnel plots, they did not identify the suspicion of reporting bias with impact on the estimation of effect identified in our review. Only Gu 2016 used the GRADE approach to assess the certainty of evidence. Similar to our review, these review authors found an increased first‐attempt success rate in B‐mode ultrasound guidance compared to palpation for radial artery catheterisation. However, their results for time needed for a successful procedure and for haematoma are conflicting. Moreover, our conclusions are more circumspect.
Authors' conclusions
Implications for practice.
We are uncertain about effects for the following comparisons due to very low‐certainty evidence and unreported outcomes: real‐time B‐mode ultrasound versus palpation and landmarks for axillary and dorsalis pedis arteries, real‐time B‐mode ultrasound versus near‐infrared laser for radial artery, and dynamic versus static out‐of‐plane B‐mode ultrasound for radial artery. Besides, all low‐ to moderate‐certainty evidence is related to the radial artery.
Real‐time B‐mode ultrasound guidance probably has a relevant role for radial artery catheterisation guidance due to the body of evidence presented here. This systematic review and meta‐analysis found that B‐mode ultrasound guidance probably reduces major haematoma compared to palpation and probably improves first‐attempt success rate and time needed for a successful procedure compared to Doppler assistance. We also found that Doppler assistance increases time needed for a successful procedure compared to palpation for a radial artery procedure. Still, we were underpowered to find other moderate‐ and high‐certainty evidence for most other outcomes.
In addition, real‐time B‐mode ultrasound guidance may improve first‐attempt success rate and overall success rate and may decrease time needed for a successful procedure up to one hour compared to palpation and landmarks. Furthermore, real‐time B‐mode ultrasound guidance may improve overall success rate up to 72 hours compared to DUA. Finally, real‐time B‐mode ultrasound guidance may decrease first‐attempt success rate and overall success rate and may lead to no difference in time needed for a successful procedure up to one hour compared to modified real‐time B‐mode ultrasound guidance. However, data are lacking, or evidence is of very low certainty, for other relevant outcomes: it is uncertain whether real‐time B‐mode ultrasound guidance has any effect on pseudoaneurysm, pain, and quality of life (QoL) compared to palpation and landmarks; pseudoaneurysm, major haematoma, pain, and QoL were not reported for the comparison with DUA; and it is uncertain whether real‐time B‐mode ultrasound guidance has any effect on major haematoma compared to modified real‐time B‐mode ultrasound (pseudoaneurysm, pain, and QoL were not reported).
Evidence is lacking, is of very low certainty, or shows no significant differences related to all other relevant outcomes when in‐plane versus out‐of‐plane real‐time B‐mode ultrasound guidance are compared up to one hour; DUA is compared with palpation and landmarks up to 72 hours; and oblique‐axis is compared with long‐axis in‐plane B‐mode ultrasound guidance up to 72 hours. Data for indirect ultrasound guidance and for effects 30 days after the intervention are lacking; therefore, no conclusions for a long‐term time point can be drawn.
Implications for research.
Given that first‐attempt success rate and pseudoaneurysm are the most relevant outcomes for people who underwent arterial catheterisation and for their clinicians, it is important that future studies of ultrasound guidance for arterial catheterisation measure both as primary outcomes. Future trials need to be large enough to detect effects on clinical outcomes; they should not only include the main clinical outcomes (first‐attempt success rate and pseudoaneurysm), but should also measure overall success rate, time needed for a successful procedure, major haematoma, adverse events, and QoL, and they should use validated scales. All foreseen outcomes must be reported at the end of the trial. Finally, studies must be of at least six months' duration of long‐term effects of ultrasound guidance during the post‐intervention period are to be assessed. Six months may be long enough to provide additional data on rare adverse events following arterial catheterisation and to assess its effects during the post‐discharge period (e.g. pseudoaneurysm, thrombosis, major haematoma, nerve injury, amputation). Future trials should include participants with no or more previous arterial punctures, and should provide individual data by type of anaesthesia during the intervention. Continuous outcome data must be uniform, and similar scales should be used, especially for pain and for QoL.
Additional studies with the characteristics suggested above comparing ultrasound guidance with all other control interventions are needed to evaluate ultrasound guidance for wider clinical use in people who have undergone arterial catheterisation. The 20 ongoing studies that we identified, which aimed to recruit over 2737 participants altogether, will add to the evidence presented here related to real‐time B‐mode ultrasound guidance and DUA.
History
Protocol first published: Issue 4, 2020
Acknowledgements
The background and methods section of this review are based on a standard template provided by the Cochrane Heart Group.
We wish to thank Cochrane Heart, Cochrane Brazil, and the Division of Vascular and Endovascular Surgery of Universidade Federal de São Paulo, Brazil, for their methodological support. We are grateful to Nicole Martin, Andrea Takeda, Audrey Tan, Charlene Bridges, Eliano Navarese, Mahmood Ahmad, Jason Elliot‐Smith, and Rui Providencia from the Cochrane Heart editorial board; and to Andrew Miller, Erik Sloth, Fred Mihm, Leigh White, and Nicholas Harrison for their peer review contributions. We thank Patrícia Leika Hoshino, Universidade Estadual de Campinas, Brazil, for translating Edanaga 2012 from Japanese, and Jia Yean Thong, Fudan University Shanghai Medical College, China, for translating Cao 2018 from Chinese. We thank Vinicius Civile, Cochrane Brazil, for help with R software statistics. We also thank Erik Sloth (Gopalasingam 2017), Peter Juhl‐Olsen (Hansen 2014), Phong Nguyen (Nguyen 2019), and Eva Vaquerizo‐Carpizo (Vaquerizo 2014), for sharing raw data of interest for this review and for clarifying related doubts.
Appendices
Appendix 1. Glossary of terms
| Term | Definition |
| Ambulation | The act of walking |
| Angiography | A medical imaging technique used to visualise the inside, or lumen, of blood vessels and the heart chambers. This is traditionally performed by injecting a radio‐opaque contrast agent into the peripheral vein and imaging the body part using X‐ray‐based techniques such as fluoroscopy |
| Anticoagulants | Drugs that suppress, delay, or prevent blood clots |
| Antiplatelet agents | Drugs that prevent blood clots by inhibiting platelet function |
| Arterial | Relative to the artery |
| Atherosclerosis | A disease characterised by a buildup of abnormal fat, cholesterol, and platelet deposits on the inner wall of the arteries |
| B‐mode ultrasound | Brightness mode ultrasound is a 2‐dimensional image of a structure by ultrasound technology |
| Body mass index (BMI) | Body mass divided by the square of the body height, universally expressed in units of kg/m² |
| Brachial access | To access inside the blood vessels through the brachial artery, commonly using vascular devices |
| Catheterisation | A minimally invasive procedure to access inside of the blood vessels using a catheter |
| Coronary arteries | The arteries that carry blood to the cardiac muscle |
| Direct ultrasound guidance | Ultrasound scanning to verify the presence and position of a suitable target vessel at the time of needle insertion (i.e. real‐time ultrasound needle guidance) |
| Doppler auditory ultrasound assistance | Ultrasound scanning to verify the presence and position of a suitable target vessel. It commonly uses the Doppler effect transformed in auditory sound |
| Duplex ultrasound | Non‐invasive evaluation of blood flow through the arteries and veins by ultrasound devices |
| Dyslipidemia | Abnormal concentration of fats (lipids or lipoproteins) in the blood |
| Gasometry | A laboratory examination that evaluates gas dosages in a blood sample |
| Heparin | A drug that is used to prevent blood clotting (anticoagulant, blood thinner) |
| Indirect ultrasound guidance | Ultrasound scanning to verify the presence and position of a suitable target vessel by using B‐mode ultrasound before needle insertion without real‐time ultrasound needle guidance |
| Low‐molecular‐weight heparin | A drug that is used to prevent blood clotting (anticoagulant) |
| Obesity | Amount of body fat is beyond healthy conditions (BMI > 30 kg/m²) |
| Oedema | The excess watery fluid that collects in tissues of the body, causing swelling when fluid leaks out of the body's vessels |
| Overweight | Amount of body fat is over that of the average population but is less than in unhealthy conditions (BMI between 25 and 30 kg/m²) |
| Percutaneous | A procedure performed by a puncture in the skin without skin cutting and direct visualisation of the vessel or the interested structure |
| Peripheral artery disease (PAD) | An abnormal narrowing of arteries other than those that directly supply the heart or brain |
| Placebo | Substance or treatment with no active effect, like a sugar pill |
| Radioulnar arch | An artery structure that connects the ulnar and radial arteries in the hand |
| Randomised clinical trial (RCT) | A study in which participants are divided randomly into separate groups to compare different treatments |
| Sham | A placebo procedure that omits the step thought to be therapeutically necessary |
| Thrombosis | Local coagulation of blood (clot) in a part of the circulatory system |
| Ultrasound | Sound waves at a frequency higher than can be heard by a human being |
| Unfractionated heparin (UFH) | A mixture of heparins obtained from animals that is used to prevent blood coagulation. Used to avoid and treat clotting disorders |
| Vascular | Related to blood vessels (arteries and veins) |
| Virchow's triad | Three factors that contribute to thrombosis: (1) changes in the vessel wall; (2) changes in the pattern of blood flow; (3) changes in blood constituents (hypercoagulability) |
Appendix 2. Search strategies
CENTRAL
#1 MeSH descriptor: [Ultrasonography, Interventional] this term only
#2 MeSH descriptor: [Ultrasonography] this term only
#3 ultrasound*
#4 2D mode
#5 (two‐dimensional near/3 ultraso*)
#6 (in‐plane near/3 ultraso*)
#7 (out‐of‐plane near/3 ultraso*)
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 MeSH descriptor: [Catheterization] explode all trees
#10 MeSH descriptor: [Arteries] explode all trees
#11 ((artery or arteri*) near/3 puncture*)
#12 ((artery or arteri*) near/3 catheter*)
#13 (intra‐arterial near/3 catheter*)
#14 ((artery or arteri*) near/3 cannula*)
#15 arterial line*
#16 a‐line*
#17 art line*
#18 artery access
#19 vascular access
#20 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19
#21 #8 and #20
MEDLINE Ovid
1 Ultrasonography, Interventional/ or Ultrasonography/
2 ultrasound*.tw.
3 2D mode.tw.
4 (two‐dimensional adj3 ultraso*).tw.
5 (in‐plane adj3 ultraso*).tw.
6 (out‐of‐plane adj3 ultraso*).tw.
7 1 or 2 or 3 or 4 or 5 or 6
8 exp Catheterization/ and exp Arteries/
9 ((artery or arteri*) adj3 puncture*).tw.
10 ((artery or arteri*) adj3 catheter*).tw.
11 (intra‐arterial adj3 catheter*).tw.
12 ((artery or arteri*) adj3 cannula*).tw.
13 arterial line*.tw.
14 a‐line*.tw.
15 art line*.tw.
16 artery access.tw.
17 vascular access.tw.
18 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19 7 and 18
20 randomized controlled trial.pt.
21 controlled clinical trial.pt.
22 randomized.ab.
23 placebo.ab.
24 drug therapy.fs.
25 randomly.ab.
26 trial.ab.
27 groups.ab.
28 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
29 exp animals/ not humans.sh.
30 28 not 29
31 19 and 30
Embase Ovid
1 interventional ultrasonography/ or echography/
2 ultrasound*.tw.
3 2D mode.tw.
4 (two‐dimensional adj3 ultraso*).tw.
5 (in‐plane adj3 ultraso*).tw.
6 (out‐of‐plane adj3 ultraso*).tw.
7 1 or 2 or 3 or 4 or 5 or 6
8 exp catheterization/ or exp artery/
9 ((artery or arteri*) adj3 puncture*).tw.
10 ((artery or arteri*) adj3 catheter*).tw.
11 (intra‐arterial adj3 catheter*).tw.
12 ((artery or arteri*) adj3 cannula*).tw.
13 arterial line*.tw.
14 a‐line*.tw.
15 art line*.tw.
16 artery access.tw.
17 vascular access.tw.
18 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19 7 and 18
20 random$.tw.
21 factorial$.tw.
22 crossover$.tw.
23 cross over$.tw.
24 cross‐over$.tw.
25 placebo$.tw.
26 (doubl$ adj blind$).tw.
27 (singl$ adj blind$).tw.
28 assign$.tw.
29 allocat$.tw.
30 volunteer$.tw.
31 crossover procedure/
32 double blind procedure/
33 randomized controlled trial/
34 single blind procedure/
35 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34
36 (animal/ or nonhuman/) not human/
37 35 not 36
38 19 and 37
39 limit 38 to embase
CINAHL
S44 S20 AND S43
S43 S42 NOT S41
S42 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35
S41 S39 NOT S40
S40 MH (human)
S39 S36 OR S37 OR S38
S38 TI (animal model*)
S37 MH (animal studies)
S36 MH animals+
S35 AB (cluster W3 RCT)
S34 MH (crossover design) OR MH (comparative studies)
S33 AB (control W5 group)
S32 PT (randomized controlled trial)
S31 MH (placebos)
S30 MH (sample size) AND AB (assigned OR allocated OR control)
S29 TI (trial)
S28 AB (random*)
S27 TI (randomised OR randomized)
S26 MH cluster sample
S25 MH pretest‐posttest design
S24 MH random assignment
S23 MH single‐blind studies
S22 MH double‐blind studies
S21 MH randomized controlled trials
S20 S7 AND S19
S19 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18
S18 TX vascular access
S17 TX artery access
S16 TX art line*
S15 TX a‐line*
S14 TX arterial line*
S13 TX ((artery or arteri*) n3 cannula*)
S12 TX (intra‐arterial n3 catheter*)
S11 TX ((artery or arteri*) n3 catheter*)
S10 TX ((artery or arteri*) n3 puncture*)
S9 (MH "Arteries+")
S8 (MH "Catheterization+")
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S6 TX (out‐of‐plane n3 ultraso*)
S5 TX (in‐plane n3 ultraso*)
S4 TX (two‐dimensional n3 ultraso*)
S3 TX 2D mode
S2 TX ultrasound*
S1 (MH "Ultrasonography")
LILACS
Ultrasound$ or “2D mode” [Words] and Artery or arteri$ or intra‐arterial or a‐line$ or art line$ or “vascular access” [Words] and Puncture$ or catheter$ or cannula$ or access or line$ [Words]
IBECS
(mh: Ultrasonography or Ultrasonografía or Ultrassonografia or (Computer Echotomography) or (Diagnos* Ultrasonic) or (Diagnostic Ultrasound*) or Echography or Echotomography or (Echotomography Computer) or (Imaging Ultrasonic) or (Imaging* Ultrasonographic) or (Imaging* Ultrasound) or (Medical Sonography) or (Tomography Ultrasonic) or (Ultrasonographic Imaging*) or (Diagnóstico por Ultrasonido) or Ecografía or (Ecografía Médica) or Ecotomografía or (Ecotomografía por Computador) or (Imagen Ultrasonográfica) or (Imagen Ultrasónica) or (Imagen de Ultrasonido) or (Imagen por Ultrasonido) or (Sonografía Médica) or (Tomografía Ultrasonica) or (Diagnóstico por Ultrassom) or Ecografia or (Ecografia Médica) or Ecotomografia or (Ecotomografia por Computador) or (Imageamento Ultrassonográfico) or (Imagem Ultrassonográfica) or (Imagem Ultrassônica) or (Imagem de Ultrassom) or (Imagem por Ultrassom) or (Sonografia Médica) or (Tomografia Ultrassônica) or (2D mode ultraso*) or (two‐dimensional ultraso*) or (in‐plane ultraso*) or (out‐of‐plane ultraso*)) and (mh: Catheterization or Cateterismo or Cateterismo or Cannulation* or Catheterizations or E02.148 or E05.157 or Canulación or Cateterización or Canulação or Cateterização or mh: Arteries or Arterias or Artérias or Artery or ((artery or arteri*) puncture*) or ((artery or arteri*) catheter*) or (intra‐arterial catheter*) or ((artery or arteri*) cannula*) or (arterial line*) or a‐line* or (art line*) or (artery access) or (vascular access))
ClinicalTrials.gov
condition and other terms
#1
catheterization and ultrasonography
#2
artery access and ultrasonography
#1 or #2
WHO ICTRP
condition and intervention
#1
catheterization and ultrasonography
#2
artery access and ultrasonography
#1 or #2
Data and analyses
Comparison 1. [Axillary] B‐mode ultrasound guidance versus palpation and landmarks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall success rate | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.86] |
| 1.2 Time needed for a successful procedure | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐2.27 [‐7.36, 2.82] |
| 1.3 Major haematoma | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.22] |
| 1.4 Adverse events (venous puncture) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.20, 3.54] |
Comparison 2. [Dorsalis pedis] B‐mode ultrasound guidance versus palpation and landmarks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 First‐attempt success rate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.90, 1.82] |
| 2.2 Overall success rate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
| 2.3 Time needed for a successful procedure | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.16, 0.08] |
Comparison 3. [Radial] B‐mode ultrasound guidance versus palpation and landmarks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 First‐attempt success rate | 27 | 4708 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.29, 1.61] |
| 3.1.1 Experienced operators | 19 | 3384 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.23, 1.58] |
| 3.1.2 Inexperienced operators | 9 | 1324 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.31, 1.83] |
| 3.2 First‐attempt success rate ‐ trials at low risk of bias | 19 | 2762 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.33, 1.60] |
| 3.2.1 Experienced operators | 14 | 2106 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.26, 1.53] |
| 3.2.2 Inexperienced operators | 6 | 656 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.39, 2.12] |
| 3.3 First‐attempt success rate ‐ trials with individual parallel design | 25 | 4625 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.27, 1.59] |
| 3.3.1 Experienced operators | 18 | 3344 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.21, 1.56] |
| 3.3.2 Inexperienced operators | 8 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.28, 1.83] |
| 3.4 Pseudomaneurysm | 1 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 70.63] |
| 3.5 Overall success rate | 28 | 4955 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.06, 1.16] |
| 3.5.1 Experienced operators | 18 | 3132 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.03, 1.14] |
| 3.5.2 Inexperienced operators | 11 | 1823 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.05, 1.29] |
| 3.6 Overall success rate ‐ trials at low risk of bias | 19 | 2784 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.07, 1.22] |
| 3.6.1 Experienced operators | 15 | 2178 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.06, 1.21] |
| 3.6.2 Inexperienced operators | 5 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.97, 1.72] |
| 3.7 Overall success rate ‐ trials with individual parallel design | 26 | 4875 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.05, 1.15] |
| 3.7.1 Experienced operators | 17 | 3092 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.03, 1.14] |
| 3.7.2 Inexperienced operators | 10 | 1783 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.04, 1.28] |
| 3.8 Time needed for a successful procedure | 26 | 4902 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.54, ‐0.13] |
| 3.8.1 Experienced operators | 18 | 3430 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.35, 0.08] |
| 3.8.2 Inexperienced operators | 8 | 1472 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.69, ‐0.55] |
| 3.9 Time needed for successful procedure ‐ trials at low risk of bias | 18 | 2671 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.38, ‐0.08] |
| 3.9.1 Experienced operators | 14 | 2276 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.36, ‐0.02] |
| 3.9.2 Inexperienced operators | 4 | 395 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.81, ‐0.03] |
| 3.10 Time needed for a successful procedure ‐ trials with individual parallel design | 24 | 4822 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.51, ‐0.09] |
| 3.10.1 Experienced operators | 17 | 3390 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.11] |
| 3.10.2 Inexperienced operators | 7 | 1432 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.79, ‐0.56] |
| 3.11 Major haematoma | 16 | 2504 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.56] |
| 3.11.1 Experienced operators | 10 | 1918 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.19, 0.46] |
| 3.11.2 Inexperienced operators | 6 | 586 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.18, 1.09] |
| 3.12 Major haematoma ‐ trials at low risk of bias | 12 | 2081 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.21, 0.43] |
| 3.12.1 Experienced operators | 9 | 1735 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.19, 0.46] |
| 3.12.2 Inexperienced operators | 3 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.63] |
| 3.13 Adverse events (pain) | 4 | 883 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.66, 2.28] |
| 3.14 Adverse events (pain) ‐ trials at low risk of bias | 2 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.95, 0.75] |
| 3.15 Adverse events (pain) ‐ trials with individual parallel design | 3 | 497 | Mean Difference (IV, Random, 95% CI) | 1.22 [‐1.19, 3.64] |
| 3.16 Adverse events (bleeding, haematoma, ischaemia, or spasm) | 3 | 1303 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.52] |
| 3.16.1 Bleeding or haematoma | 1 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.34, 4.67] |
| 3.16.2 Bleeding, haematoma, or spasm | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.06, 1.36] |
| 3.16.3 Haematoma or ischaemia | 1 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.58, 1.57] |
| 3.17 Adverse events (local infection) | 3 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.15] |
| 3.18 Adverse events (local infection) ‐ trials at low risk of bias | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.02] |
| 3.19 Adverse events (oedema) | 2 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.64] |
| 3.20 Adverse events (arterial thrombosis) | 5 | 1496 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.54] |
| 3.21 Adverse events (arterial thrombosis) ‐ trials at low risk of bias | 4 | 1416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.13] |
| 3.22 Adverse events (death) | 1 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.85] |
| 3.23 Adverse events (spasm) | 5 | 1525 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.62, 1.97] |
| 3.24 Adverse events (spasm) ‐ trials at low risk of bias | 4 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.30, 2.64] |
| 3.25 Adverse events (posterior wall puncture) | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.28, 0.61] |
| 3.26 Quality of life (satisfaction) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.07, 1.07] |
Comparison 4. [Radial] B‐mode ultrasound versus Doppler assistance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 First‐attempt success rate | 1 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.11, 1.64] |
| 4.2 Overall success rate | 1 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.99, 1.29] |
| 4.3 Time needed for a successful procedure | 1 | 493 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐1.78, ‐1.36] |
| 4.4 Adverse events (haematoma or ischaemia) | 1 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.70, 2.05] |
Comparison 5. [Radial] B‐mode ultrasound versus near‐infrared laser guidance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 First‐attempt success rate | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.82, 2.16] |
| 5.2 Overall success rate | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.27, 8.45] |
| 5.3 Time needed for a successful procedure | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.09, 0.31] |
Comparison 6. [Radial] B‐mode ultrasound versus modified B‐mode ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 First‐attempt success rate | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.55, 0.84] |
| 6.2 Overall success rate | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| 6.3 Time needed for a successful procedure | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.01, 0.09] |
| 6.4 Major haematoma | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [1.37, 7.60] |
| 6.5 Adverse events (spasm) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.89, 2.16] |
| 6.6 Adverse events (posterior wall puncture) | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.00 [1.05, 60.89] |
Comparison 7. [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 First‐attempt success rate | 8 | 1051 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.12] |
| 7.2 First‐attempt success rate ‐ trials at low risk of bias | 5 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.17] |
| 7.3 Overall success rate | 8 | 1051 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.96, 1.05] |
| 7.4 Overall success rate ‐ trials at low risk of bias | 5 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.16] |
| 7.5 Time needed for a successful procedure | 9 | 1134 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.16, 0.05] |
| 7.6 Time needed for a successful procedure ‐ trials at low risk of bias | 6 | 699 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.23, 0.12] |
| 7.7 Major haematoma | 9 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.08] |
| 7.8 Major haematoma ‐ trials at low risk of bias | 6 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.54] |
| 7.9 Adverse events (thrombosis) | 5 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [0.13, 76.69] |
| 7.10 Adverse events (thrombosis) ‐ trials at low risk of bias | 4 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [0.13, 76.69] |
| 7.11 Adverse events (oedema) | 3 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.14] |
| 7.12 Adverse events (oedema) ‐ trials at low risk of bias | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.14] |
| 7.13 Adverse events (vasospasm) | 6 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.24, 2.69] |
| 7.14 Adverse events (vasospasm) ‐ trials at low risk of bias | 5 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.25, 3.54] |
| 7.15 Adverse events (posterior wall damage) | 3 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.10, 1.97] |
| 7.16 Adverse events (ischaemia) | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.42] |
7.16. Analysis.

Comparison 7: [Radial] In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound, Outcome 16: Adverse events (ischaemia)
Comparison 8. [Radial] Doppler assistance versus palpation and landmarks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 First‐attempt success rate | 2 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 8.2 First‐attempt success rate ‐ trials at low risk of bias | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.17] |
| 8.3 Overall success rate | 2 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.07] |
| 8.4 Overall success rate ‐ trials at low risk of bias | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.96, 1.03] |
| 8.5 Time needed for a successful procedure | 1 | 500 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [0.20, 0.70] |
| 8.6 Adverse events (haematoma or ischaemia) | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.47, 1.35] |
Comparison 9. [Radial] Dynamic out‐of‐plane B‐mode ultrasound versus static out‐of‐plane B‐mode ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 First‐attempt success rate | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.67, 1.23] |
| 9.2 Overall success rate | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.25] |
| 9.3 Time needed for a successful procedure | 1 | 131 | Mean Difference (IV, Random, 95% CI) | 0.37 [0.07, 0.66] |
| 9.4 Adverse events (posterior wall puncture) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.34, 0.81] |
9.4. Analysis.

Comparison 9: [Radial] Dynamic out‐of‐plane B‐mode ultrasound versus static out‐of‐plane B‐mode ultrasound, Outcome 4: Adverse events (posterior wall puncture)
Comparison 10. [Radial] Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 First‐attempt success rate | 3 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.44, 2.79] |
| 10.2 First‐attempt success rate ‐ trials at low risk of bias | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [1.35, 4.14] |
| 10.3 Overall success rate | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.05, 1.53] |
| 10.4 Overall success rate ‐ trials at low risk of bias | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.01, 1.61] |
| 10.5 Time needed for a successful procedure | 3 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.95, 0.25] |
| 10.6 Time needed for a successful procedure ‐ trials at low risk of bias | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐0.88, ‐0.79] |
| 10.7 Major haematoma | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.32, 1.47] |
| 10.8 Major haematoma ‐ trials at low risk of bias | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.22, 1.34] |
| 10.9 Adverse events (vasospasm or haematoma) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.57] |
| 10.10 Adverse events (ischaemia) | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 4.64 [0.23, 94.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdalla 2017.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 3‐arm parallel‐assignment open‐label study Egypt Duration: February 2015 to August 2015 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (artery in oblique view, real‐time, in‐plane) Comparator (longitudinal): ultrasound‐guided RA puncture (artery in long axis, real‐time, in‐plane) Comparator (transverse): ultrasound‐guided RA puncture (artery in short axis, real‐time, out‐of‐plane) Level of experience of person carrying out the procedure: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until 72 hours after the procedure |
|
| Notes | Funding: study authors declared there was nil financial support and sponsorship Conflicts of interest: quote: "none" Protocol available (NCT02550223) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated into 3 equal groups using closed envelope technique in 7 blocks of 18 (6 patients for each group)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated into 3 equal groups using closed envelope technique in 7 blocks of 18 (6 patients for each group)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "a prospective randomized nonblinded study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "a prospective randomized nonblinded study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Ammar 2017.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment open‐label study Pakistan Duration: December 2015 to July 2016. |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (artery in short axis, real‐time, out‐of‐plane) Comparator: percutaneously RA puncture by anatomical landmarks and palpation technique Level of experience of person carrying out the procedure: not reported Concomitant medications: local anaesthesia (details not provided) Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of interventions, we assumed that blinding of personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | Although there is no registered protocol, all prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Anand 2019.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment study (no mention of masking) India Total duration and date of study not clear |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided DPA puncture (artery in long axis, real‐time, in‐plane) Comparator: percutaneously punctured DPA by palpation technique Level of experience of person carrying out the procedure: quote: "all the cannulations were performed by a single investigator (Rahul Kumar Anand) who had experience of >50 DPA cannulations using each technique to minimise inter‐individual variability in skills" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until the end of the procedure (24 to 48 seconds) |
|
| Notes | Funding: study authors declared there was nil financial support and sponsorship Conflicts of interest: quote: "there are no conflicts of interest" Register number informed in the publication (CTRI/2018/04/019691) was not localised. We found another registration number (CTRI/2018/08/015525) related to this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization sequence was generated by a web‐based randomization program (www.randomizer.org)" |
| Allocation concealment (selection bias) | Low risk | Quote: "it was kept inside serially numbered opaque‐sealed envelopes. Sealed envelopes were opened to reveal allocation just before the DPA cannulation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the participant underwent the intervention after general anaesthesia, personnel for the intervention group were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "primary and secondary outcome data were collected by an unblinded anaesthesiologist who was not a part of this study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. Cross‐over was done for 3 participants (1 participant in experimental group and 2 in comparator group), and successful cannulation was done in 1 patient of comparator group. The other 2 participant procedures were abandoned |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Arora 2021.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised study Oman Duration: not mentioned |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental (longitudinal): ultrasound‐guided RA puncture (artery in long axis, real‐time, in‐plane) Comparator (transversal): ultrasound‐guided RA puncture (artery in long axis, real‐time, out‐of‐plane) Level of experience of person carrying out the procedure: quote: "the procedure in both groups was performed by the same experienced anesthesiologist, who had previously performed more than 50 radial artery cannulations in adult patients using either the in‐plane or the out‐of‐plane ultrasound approach" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: during the surgery procedure |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "study participants were randomly assigned to the out‐of‐plane USG group (group I, n = 42) or the in‐plane USG group (group II, n = 42) by a computerized random number generation chart (https://stattrek.com/statistics/random‐number‐generator.aspx)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "study participants were randomly assigned to the out‐of‐plane USG group (group I, n = 42) or the in‐plane USG group (group II, n = 42) by a computerized random number generation chart (https://stattrek.com/statistics/random‐number‐generator.aspx)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the participant underwent the intervention after general anaesthesia, personnel for the intervention group were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | High risk | There is a difference between outcomes described in the methods and in the results. The outcome 'number of failed attempts in the 2 ultrasound imaging planes' was not reported in the results. The outcome 'time for completion of the procedure' was described only in the results ‐ not in the methods |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Bai 2020.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised intervention, parallel‐assignment double‐masked study China, Beijing Duration: September 2018 to February 2019 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental (ultrasound DNTP): quote: "for the DNTP technique, the probe was placed to view the out‐of‐plane radial artery and moved to place the artery in the center of the ultrasound screen. Then, the needle was inserted at the point at which the middle mark of the probe contacted the skin and advanced through the skin at an angle of approximately 30 degrees until the tip was seen on the screen. The probe was moved along the long axis of the target artery away from the insertion point until the tip just disappeared from the screen. Then, we advanced the needle and catheter until the tip was just seen again. These steps were repeated until the tip was observed in the artery lumen" Control (ultrasound AD): quote: "the probe was placed to view the short‐axis plane of the target artery and moved to place the artery in the center of the ultrasound screen. Then, the distance from the surface of the skin to the anterior wall of the artery was measured. The needle was inserted at the point at which the middle mark of the probe contacted the skin and advanced through the skin. Since an initial angle of 45 degrees between needle and skin was used for a puncture, the distance between point of insertion and central point of the probe was approximately equal to the distance from surface of the skin to anterior wall of the artery. Then, the needle was advanced until blood appeared in the hub. The needle angle was decreased slightly while the catheter was advanced slightly. The catheter was advanced into the target artery only if blood continued to flow into the hub" Level of experience of person carrying out the procedure: quote: "the operator was an experienced senior anesthesiology resident in our department, who had already conducted the DNTP and AD techniques in over 100 patients each and was equally skilled in the 2 methods" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Outcomes Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: during the surgery procedure |
|
| Notes | Funding: quote: "no funding was obtained for this study" Conflicts of interest: quote: "the authors declare that they have no competing interests" Protocol available (NCT03656978) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "enrolled patients were randomized by computer generated numbers provided in sealed opaque envelopes to either the DNTP or AD group. The seal of the envelope was broken just before the cannulation procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "enrolled patients were randomized by computer generated numbers provided in sealed opaque envelopes to either the DNTP or AD group. The seal of the envelope was broken just before the cannulation procedure" Quote: "the anesthesiologist who conducted the cannulation procedure knew the allocation of the patients. The patients were blinded to the allocation. The statistician did not know the allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the anesthesiologist who conducted the cannulation procedure knew the allocation of the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the statistician did not know the allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | Investigators collected 1 additional secondary outcome that was not planned in the protocol Quote: "the number of skin punctures" |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Berk 2013.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment open‐label study Turkey Duration: June 2012 until August 2012. |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (artery in short axis, real‐time, out‐of‐plane) Comparator: ultrasound‐guided RA puncture (artery in long axis, real‐time, in‐plane) Level of experience of person carrying out the procedure: 2 researchers who had placed more than 50 arterial lines by using in‐plane or out‐of‐plane approach Concomitant medications: GA using intravenous induction with thiopental 5 to 8 mg/kg, fentanyl 1 to 2 mcg/kg, and rocuronium 0.6 to 1 mg/kg. After endotracheal intubation, anaesthetic maintenance consisted of sevoflurane 2 and 50% oxygen in nitrous oxide Excluded medication: local anaesthetic was not used |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: not reported Conflicts of interest: quote: "the authors declare that they have no conflict of interest" Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not sufficiently described. Quote: "patients were randomized by using sealed envelope method" |
| Allocation concealment (selection bias) | Unclear risk | Not sufficiently described. Quote: "patients were randomized by using sealed envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the participant underwent the intervention after general anaesthesia, personnel for the intervention group were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Bobbia 2013.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment open‐label study France Duration: 15 August 2010 to 30 September 2010 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery axis and plane with needle were not reported) Comparator: RA puncture using palpation and landmarks Level of experience of person carrying out the procedure: 13 physicians were all graduates of accredited French Society of Emergency Medicine, in which theoretical and practical training of the gesture was taught. To avoid confusion, 3 hours of simulator training was given before the start of the study Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the computer simultaneously created a time stamp in the research database, which represented the time of enrollment and designated the group in which the patient was (US‐guided [group 1] or not [group 2])" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "this was a prospective, nonblinded, randomized trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "this was a prospective, nonblinded, randomized trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Burad 2017.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 3‐arm parallel‐assignment open‐label study Oman Duration: 15 August 2010 to 30 September 2010 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery axis and plane with needle not reported) Comparator: RA puncture using palpation and landmarks Level of experience of person carrying out the procedure: quote: "all cannulations were performed by physicians well experienced with both techniques" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: not reported Conflicts of interest: quote: "the authors declare that they have no conflicts of interest" Protocol (NCT02825615) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "we randomized 100 adult patients... to one of the techniques by blindly picking chits from unlabeled boxes" No clear description of whether randomisation allowed the same chance of allocation for both groups (experimental and comparator) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "is a prospective, randomized, single‐centre, non‐blinded, intention‐to‐treat study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "is a prospective, randomized, single‐centre, non‐blinded, intention‐to‐treat study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Cao 2018.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment single‐blinded (outcomes assessor) study China Duration: December 2016 to May 2017. |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, needle out‐of‐plane) Comparator: RA puncture using palpation and landmarks Level of experience of person carrying out the procedure: quote: "nursing staff who have obtained the certificate of completion for critical care ultrasound" Concomitant medications: quote: "arterial cannulation was performed either before or after induction of general anesthesia based on the preference of the faculty anesthesiologist" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 5 minutes |
|
| Notes | Funding: quote: "this work was supported by the Research Fund of Health and Family Planning, Commission of Hunan Province, China (B2017012)" Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of interventions, we assumed that blinding of personnel is not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Cao 2020.
| Study characteristics | ||
| Methods | [Preprint] Single‐centre prospective randomised controlled 3‐arm parallel‐assignment open‐label study China Duration: 1 April 2020 until 28 July 2020 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: LA, in‐plane, B‐mode, real‐time; quote: "probe was placed parallel to the course of the artery, and the needle was directed parallel to the longitudinal axis of the probe" Comparator: SA, out‐of‐plane, B‐mode, real‐time; quote: "the probe was placed perpendicular to the course of the artery, and the needle was directed perpendicular to the longitudinal axis of the probe" Comparator: OA, in‐plane, B‐mode, real‐time; quote: "the probe was positioned transversely perpendicular to the artery as in the SAX group and then rotated clockwise in situ by 60° according to maximum visualization" Level of experience of person carrying out the procedure: all participants were trained by a teacher who was familiar with the 3 approaches; they were "anaesthesia residents with no more than one year of experience in blind palpation for radial artery cannulation and who previously performed ultrasound‐guided radial artery cannulation fewer than five times in patients" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: not mentioned |
|
| Notes | Funding: quote: "none" Conflicts of interest: quote: "the authors declare that they have no competing interest" Protocol available (ChiCTR200030416) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were assigned by a randomized block design to three groups. We allocated patients at a 1:1:1 ratio with a computer‐generated list of random numbers in blocks of three, with the results accessible to only research nurses" |
| Allocation concealment (selection bias) | Low risk | Quote: "the sealed envelopes were opened immediately before the procedure" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "with the results accessible to only research nurses" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There are losses (15 participants; 6.9%) that were not described regarding motivation or proportion among groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Edanaga 2012.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 3‐arm parallel‐assignment study; blinding not reported Japan Duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis (N = 12) and long axis (N = 12), needle plane not reported) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: anaesthesiologist staff have performed all punctures Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not described) |
|
| Notes | Funding: not reported Conflicts of interest: study authors declared no conflicts of interest Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | High risk | Study did not evaluate any safety outcome |
Fujita 2012.
| Study characteristics | ||
| Methods | [Abstract of event] Single‐centre prospective randomised controlled 2‐arms parallel‐assignment open‐label study Japan Duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery axis and plane with needle not reported) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Gibbons 2020.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arms parallel‐assignment open‐label study USA Duration: 1 Janurary 2018 to 31 December 2019 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: DNTP, out‐of‐plane, B‐mode, real‐time ultrasound‐guided puncture (artery axis not described) Comparator: palpation‐guided puncture Level of experience of person carrying out the procedure: all participants were trained by a teacher who was familiar with the approaches, but all were emergency interns Concomitant medications: 2 to 3 mL 1% lidocaine without epinephrine was used for patients who were conscious Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 day |
|
| Notes | Funding: quote: "no funding was provided for this study" Conflicts of interest: not reported Protocol available (NCT03326739) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "blinded study investigators selected sealed envelopes containing study materials and prerandomized selection into USG vs. LMGP using Research Randomizer (v 4.0, available at http://www.randomizer.org/) (21)" |
| Allocation concealment (selection bias) | Low risk | Quote: "blinded study investigators selected sealed envelopes containing study materials and prerandomized selection into USG vs. LMGP using Research Randomizer (v 4.0, available at http://www.randomizer.org/) (21)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "only novice emergency medicine interns, defined as interns with < 15 previous placements, who were not blinded, performed the cannulation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "an independent department research assistant who was blinded to the study’s objectives assessed the operator with respect to first‐pass success, number of attempts (limited to 3), time to completion using a stopwatch (limited to 15 min), complications, and need to crossover to the alternative method" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Gopalasingam 2014.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm cross‐over participant‐blinded study Denmark Duration: November 2012 to July 2013 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, and DNTP) as first intervention Comparator: RA puncture with palpation and landmarks as first intervention Level of experience of person carrying out the procedure: anaesthesiology residents (operators) had performed at least 20 of each procedure previous to this study Concomitant medications: after injection of 0.5 to 1.0 mL of lidocaine (10 mg/mL) Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: quote: "this work was funded by the Edgar Schnohr and wife Gilberte Schnohr’s fund and Helga and Peter Korning’s foundation" Conflicts of interest: quote: "none of the authors has financial interest related to this study to disclose" Protocol (NCT01690416) available Study authors provided data for the first phase, before the intervention cross‐over |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer randomisation (www.randomization.com) was conducted" |
| Allocation concealment (selection bias) | Unclear risk | No details provided Quote: "the randomisation order was revealed to the observer just prior to catheterisation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant blinded, but personnel not blinded Quote: "the study was randomised, controlled, patient‐blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding only for participants Quote: "the study was randomised, controlled, patient‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses. Besides the cross‐over design, another cross‐over (not planned) occurred in 4 participants in the comparator group (to ultrasound aid) and in none in the experimental group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | High risk | There is no plausible reason to change the outcome from primary on the protocol to secondary on the final report |
Goswami 2020.
| Study characteristics | ||
| Methods | [Abstract of event] Single‐centre prospective randomised controlled 2‐arm parallel‐assignment open‐label study India Duration: April to November 2019 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided puncture (not detailed) Comparator: palpation‐guided puncture Level of experience of person carrying out the procedure: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: not mentioned |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "recruitment and randomization was done using a computer‐generated table" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | High risk | Two outcomes (first‐attempt success in catheterisation and time taken to cannulate) of interest for this review were planned into trial methods but were not reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Grandpierre 2019.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arms parallel‐assignment open‐label study France Duration: February 2014 to June 2016 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, needle out‐of‐plane) Comparator: RA puncture with palpation and landmarks Level of experience of person carrying out the procedure: quote: "all physicians had a university degree in point‐of‐care ultrasound and all had previously used ABGA ultrasound guidance in clinical practice" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (quote: "followed until the ABGA was obtained") |
|
| Notes | Funding: quote: "this work was supported by the University Hospital of Nimes. No author has received funding. The University Hospital of Nimes supported data collection, data management, and analysis" Conflicts of interest: quote: "XB declares a competing interest as an ultrasound teacher for GE (GE MEDICAL SYSTEMS ULTRASOUND) customers. This does not alter our adherence to PLOS ONE policies and sharing data and materials. The other authors state they have no competing interests" Protocol (NCT01789801) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an SAS (Carry, NC, USA) program was used to create random block sizes of 4 or 6 and to stratify the reason for inclusion as non‐palpable artery and two failures by the nurse, with a ratio of 1:1" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the trial was conducted at a single center and was not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the trial was conducted at a single center and was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | High risk | Two of the outcomes (complications and participant satisfaction) of interest for this review were planned in the trial protocol but were not reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Hansen 2014.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm cross‐over participant‐blinded study Denmark Duration: 12 April 2012 to 3 October 2012 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, DNTP) as first intervention Comparator: RA puncture with palpation and landmarks as first intervention Level of experience of person carrying out the procedure: quote: "the traditional palpation technique was performed by experienced specialists in anesthesiology" and "arterial catheterization with the ultrasonography dynamic needle tip positioning technique was performed by an experienced specialist in anesthesiology" Concomitant medications: quote: "no local anaesthesia was used for this procedure" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: quote: "this work was funded by the Edgar Schnohr and wife Gilberte Schnohr’s fund and Helga and Peter Korning’s Foundation" Conflicts of interest: quote: "none of the authors has financial interest related to this study to disclose" Protocol not available Trial authors provided data for the first phase before intervention cross‐over |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were computer randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant blinded, but personnel not blinded Quote: "patients were blinded to randomization order and physically blinded to the individual technique used during the procedures" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no losses. Besides the cross‐over design, another cross‐over (not planned) occurred in 1 participant of the comparator group (to ultrasound aid) and in no participant of the experimental group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Khan 2018.
| Study characteristics | ||
| Methods | [Abstract of event] Single‐centre prospective randomised controlled 2‐arm parallel‐assignment open‐label study India Duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery short axis; needle in out‐of‐plane) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol (CTRI/2017/03/008020) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomization" |
| Allocation concealment (selection bias) | High risk | Quote: "an open list of random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no losses. The account of cross‐over to either intervention was planned but was not reported |
| Selective reporting (reporting bias) | High risk | Some data (final success rate, time for successful catheterisation (cannulation time), posterior wall haemorrhage, haematoma, incidence of spasm and other complications, cross‐over to either technique) of interest for this review were planned in the trial protocol but were not reported or were reported incompletely (cannulation time) |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Kiberenge 2018.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment single‐blinded (outcomes assessor) study USA Duration: May 2015 to December 2015 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, needle out‐of‐plane, DNTP) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: quote: "arterial cannulation was performed by anaesthesia residents or faculty members" and "the operators (anesthesia residents, fellows, and faculty) placing the arterial catheters were required to have placed at least 10 radial arterial catheters using each technique prior to participation in the study" Outcome data were reported separately by experienced and inexperienced operators Concomitant medications: quote: "arterial cannulation was performed either before or after induction of general anesthesia based on the preference of the faculty anesthesiologist" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 5 minutes |
|
| Notes | Funding: University of Iowa Hospitals and Clinics Conflicts of interest: quote: "the authors declare no conflicts of interest" Protocol (NCT02557828) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the assignments were computer generated, with randomly selected block sizes using nQuery Advisor 7.0 (Statistical Solutions Ltd, Cork, Ireland) and then placed in sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "the technique to be used for cannulation was determined when the research team member opened an opaque randomization envelope containing a piece of paper with either ultrasound or palpation printed on it" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding for participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Protocol states that this was an 'outcomes assessor' blinding study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. Protocol violations were reported and were treated in an ITT analysis Quote: "there were 3 protocol violations: one was due to the use of a different catheter, one operator refused to use the palpation technique after randomization, and another used a wire to guide the catheter into the vessel lumen while using the dynamic needle tip positioning technique. These 3 patients were treated as failed attempts in the intention to treat analysis" |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Killu 2011.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment open‐label study USA Duration: from 2005 to 2007 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided AA puncture (real‐time). Artery assessment axis and plane between needle and ultrasound not reported Comparator: percutaneously AA puncture by anatomical landmarks and palpation technique Level of experience of person carrying out the procedure: experimental (7 residents and 11 fellows), comparator (6 residents and 9 fellows) Concomitant medications: not reported Excluded medications: not reported Choice of right or left AA cannulation at the discretion of the operators. Right AA was cannulated in 63.6% (n = 21) of cases and left AA in 36.4% (n = 12). The AA catheter was inserted over a guide wire via the Seldinger technique |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until the end of the procedure. The highest value was not reported, but the highest mean was 14.82 ± 12.14 minutes for residents in the comparator group |
|
| Notes | Funding: not reported Conflicts of interest: quote: "the authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article" We did not identify a register number for this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomized using concealed allocation into 2 groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study could not be blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the study could not be blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | We did not identify an available protocol, but all prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Kim 2021a.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment open‐label study Korea Duration: 3 March 2017 to 16 November 2017 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: short‐axis, out‐of‐plane, B‐mode, real‐time ultrasound‐guided puncture Comparator: short‐axis, out‐of‐plane, B‐mode, real‐time ultrasound‐guided puncture plus electromagnetic guidance Level of experience of person carrying out the procedure: quote: "arterial cannulation was performed by a single anaesthesiologist who had successfully performed arterial cannulation under electromagnetic ultrasound guidance more than 50 times" Concomitant medications: all under general anaesthesia Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 day |
|
| Notes | Funding: quote: "this study was carried out with our departmental funding source. There was no other source of funding except our departmental funding source" Conflicts of interest: quote: "the authors declare that they have no competing interests" Protocol available (KCT0002476) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the participants were randomly allocated into two groups using a computerized, randomized table" |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocations were concealed in sequentially numbered, sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the provider could not be blinded because the activation of the electromagnetic guidance system was displayed on the screen" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Kim 2021b.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment study, with participants and outcome assessor blinded Korea Duration: 6 March 2019 to 29 July 2019 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: short‐axis, out‐of‐plane, B‐mode, real‐time ultrasound‐guided puncture (DNTP) Comparator: short‐axis, out‐of‐plane, B‐mode, real‐time ultrasound‐guided puncture plus electromagnetic guidance Level of experience of person carrying out the procedure: quote: "residents in their second year of the four‐year training were chosen as cannulation practitioners" Concomitant medications: all under general anaesthesia (1% lidocaine, propofol, and rocuronium) Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not detailed) |
|
| Notes | Funding: quote: "the authors received no specific funding for this work" Conflicts of interest: quote: "interests: the authors have declared that no competing interests exist" Protocol available (KCT0003507) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by an independent person using a computer‐generated random number list" |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation results were sealed in envelopes that were opened just before artery cannulation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "it was not possible to blind cannulation practitioners to method used. However, enrolled participants were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "separate observer who was blinded to patient group measured the diameter and depth of the radial artery and recorded the outcomes. A barrier was placed between the practitioner and the outcome observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Laursen 2015.
| Study characteristics | ||
| Methods | [Abstract of event] Single‐centre prospective randomised controlled 2‐arm study, with blinding not reported Denmark Duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, DNTP) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 hour after puncture |
|
| Notes | Funding: quote: "the publication charges for this supplement were funded by TrygFonden" Conflicts of interest: not reported Protocol (NCT01660724) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trial authors reported data for 224 of 238 randomised participants. Trial authors did not report data for 14 of 238 (5.8%) participants |
| Selective reporting (reporting bias) | High risk | Trial authors apparently collected all planned outcomes but reported numerical data for only 2 of them, which showed a difference between intervention groups (first‐attempt success rate and median time used for the procedure) |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Levin 2003.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not reported Israel Duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: quote: "both attendings and residents inserted arterial catheters during the course of the study." Attendings/residents: 7/55 attempts in experimental group, 14/100 attempts in comparator group Concomitant medications: quote: "for both techniques, local anesthetic (1% lignocaine) was infiltrated subcutaneously before commencing timing at the discretion of the anesthesiologist" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "before insertion of the radial artery catheter, the technique to be used was selected by random envelope" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | We did not identify an available protocol, but all prespecified outcomes were reported |
| Other bias | High risk | Trialists did not clearly describe or define the management of the outcome of number of attempts needed Quote: "failure of either technique was determined by the inserting physician subjectively when he or she felt uncomfortable proceeding with the current technique. In this event, data were recorded regarding subsequent attempts using the alternative technique but not included in the main analysis" |
Li 2016.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not reported China Duration: May 2014 to December 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: quote: "1 specialized nurse and 3 nurse‐in‐charge leaders with more than 10 years of experience in artery blind cannulation" Concomitant medications: quote: "continuous intravenous injection of butorphanol tartrate for analgesia and an intravenous injection of propofol for sedation" for all participants Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 3 days after the puncture |
|
| Notes | Funding: quote: "Zhejiang Provincial Program for the Cultivation of High‐level Innovative Health Talents (2014‐108) and Zhejiang Provincial Medical and Health Science and Technology Plan (No. 2015111582)" Conflicts of interest: quote: "the authors declare that they have no actual or potential conflicts of interest" Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a random number table developed by the clinical assessment center" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only 1 outcome (time to achieve early goal‐directed therapy) was assessed by blinded staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses were reported; did not differ between groups Quote: "four withdrawal cases existed in the palpation group, of which 2 died within 3 days and 2 cases were discharged against medical advice within 3 days. One rejection case and 3 withdrawal cases existed in the ultrasound group, of which 1 case was excluded due to flexion and stenosis of bilateral radial arteries, 1 case died within 3 days, and 2 cases were discharged against medical advice" |
| Selective reporting (reporting bias) | Low risk | We did not identify an available protocol, but all prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Nam 2020.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding for participant and outcome assessment Korea Duration: January to June 2018 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane, DNTP) Comparator: ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Level of experience of person carrying out the procedure: quote: "radial artery cannulations were performed prior to general anaesthesia induction by a single operator who performed more than 100 cases of radial artery cannulation per year" Concomitant medications: quote: "local anaesthesia using <1 mL of 2% lidocaine was then administered in both groups" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 5 minutes, or until the end of the procedure |
|
| Notes | Funding: not reported Conflicts of interest: quote: "the authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript" Protocol (NCT03405623) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated into the DNTP or LAX‐IP group using a computer‐generated random number table with a block size of two or four" |
| Allocation concealment (selection bias) | Low risk | Quote: "group assignments were sealed in opaque envelopes by a research assistant who was not involved in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the operator could not be blinded to the imaging methods" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "we blinded the assessor of secondary endpoints" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion after randomisation was described, was similar in both groups, and accounted for less than 10% of group amount. Experimental (3/70): due to surgery cancelled (1) and catheterised before surgery (2). Comparator (7/66): due to surgery cancelled (1), catheterised before surgery (5), and consent withdrawal (1) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Nasreen 2016.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not reported Pakistan Duration: 1 January 2014 to 31 December 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: quote: "in both groups, radial artery cannulation was performed by a consultant anesthesiologist" Concomitant medications: quote: "lignocaine 1 ml was injected above the radial artery" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 5 minutes, or until the end of the procedure |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
NCT01663779.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label parallel‐assignment study USA Duration: August 2012 to 31 August 2015 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: real‐time ultrasound‐guided RA puncture (artery axis and needle/ultrasound plane not reported) Comparator: RA puncture using palpation and landmarks (artery axis not reported) Level of experience of person carrying out the procedure: quote: "arterial lines are placed by either postgraduate year 2 residents (surgery & anaesthesia) rotating through the ICU on a monthly basis, or by mid‐level providers who are in the unit for indeterminate periods of time" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol available (NCT01663779) | results described as raw data at ClinicalTrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "masking: none (open‐label)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "masking: none (open‐label)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Nguyen 2019.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised (radial vs femoral and standard vs ultrasound) 2 × 2 factorial single‐blinded trial Australia Duration: November 2012 to November 2017 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery axis and needle plane not described) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: quote: "all operators had performed at least 75 coronary interventions in the previous year, a minimum of 50 standard transradial access and transfemoral access, and 10 proctored ultrasound‐guided access for both the radial and the femoral artery. All who satisfied the required training numbers were certified before taking part in the trial" Concomitant medications: quote: "lignocaine 1 ml was injected above the radial artery" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: quote: "all patients were followed up at one week and one month" |
|
| Notes | Funding: not reported Conflicts of interest: quote: "the authors have no conflicts of interest to declare" Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised (1:1) to radial or femoral access, and (1:1) to either standard or ultrasound guidance" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes balanced in blocks of 50 were used for randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "patients and investigators were not masked to access allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomised, single‐blinded, 2x2 factorial trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was similar between experimental and comparator groups (3% and 2.9%). Cross‐over rates were similar between experimental and comparator groups (7.2% and 8.1%) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Osuda 2020.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not reported Japan Duration: 1 January 2014 to 31 December 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Comparator: RA puncture using near‐infrared laser light Level of experience of person carrying out the procedure: quote: "our experience with the Mill Suss and LA‐IP ultrasound‐guided methods was very limited before this study" Concomitant medications: all participants under general anaesthesia; no details Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not specified) |
|
| Notes | Funding: not reported Conflicts of interest: quote: "the authors have no conflicts of interest associated with this study" Protocol (JPRN‐UMIN000021546) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "using a block randomization method" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Peters 2015.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm single‐blinded (outcome assessor) study Canada Duration: September 2013 to January 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure; quote: "all cardiac anaesthesiologists involved in the study had performed a minimum of 300 palpation‐guided and 10 ultrasound‐guided arterial catheter insertions prior to trial commencement" Concomitant medications: all participants under general anaesthesia; quote: "local anaesthetic in the form of 1% lidocaine 0.2‐1.0 mL was infiltrated superficially over the target structure at the discretion of the attending anaesthesiologist" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 5 minutes |
|
| Notes | Funding: quote: "this study was funded by departmental sources (Department of Anesthesia, St. Paul’s Hospital, Vancouver, BC). Dr. S. K. W. Schwarz holds the Dr. Jean Templeton Hugill Chair in Anesthesia, supported by the Dr. Jean Templeton Hugill Endowment for Anesthesia Memorial Fund" Conflicts of interest: quote: "none of the authors have any competing financial interests relating to patents and/or shareholdings in corporations involved in the development and/or marketing of any medication or medical device used in this study" Protocol (NCT02118441) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we used consecutively numbered sealed opaque envelopes containing individual folded group assignment cards that were generated (C.P.) prior to commencement of enrolment via urn randomization in blocks of six to minimize selection bias and keep group sizes balanced" |
| Allocation concealment (selection bias) | Low risk | Quote: "after enrolment of an individual patient, one sealed envelope was drawn and opened by the patient’s attending cardiac anesthesiologist who performed the allocated study intervention" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "it was not possible to blind data collectors to group allocation because data collection required direct observation of all insertions" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "it was not possible to blind data collectors to group allocation because data collection required direct observation of all insertions" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Quan 2014.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label study China Duration: September 2013 to January 2014 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Comparator: ultrasound‐guided RA puncture (real‐time, short axis, out‐of‐plane) Level of experience of person carrying out the procedure: quote: "the procedure in both groups was performed by the same experienced anesthesiologist, who had previously cannulated 450 radial arteries and used the ultrasound‐guided technique for approximately 200 procedures" Concomitant medications: all participants under general anaesthesia; quote: "local anaesthesia (0.2 mL, 2% lidocaine)" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 5 minutes |
|
| Notes | Funding: quote: "none" Conflicts of interest: quote: "the authors declare no conflicts of interest" Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not sufficiently described Quote: "using a sealed envelope method, patients were randomly assigned (allocation ratio 1:1) into 2 groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "using a sealed envelope method, patients were randomly assigned (allocation ratio 1:1) into 2 groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One dropout after randomisation (1/83, experimental group) due to participant consent withdrawn |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Rajasekar 2021.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 3‐arm open‐label parallel‐assignment trial India Duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane) Comparator: RA puncture via palpation and landmarks Level of experience of person carrying out the procedure: quote: "all the data were collected by the same anesthesiologist in all the patients" Concomitant medications: quote: "skin was infiltrated with 1 ml of 2% lignocaine" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure |
|
| Notes | Funding: not reported. Conflicts of interest: quote: "nil" Protocol available (CTRI/2019/02/017749) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the enrolled 90 patients were blocked randomized into one of the three groups (30 in each group) using computer‐generated randomization numbers and concealed by sealed enveloped technique" |
| Allocation concealment (selection bias) | Low risk | Quote: "the enrolled 90 patients were blocked randomized into one of the three groups (30 in each group) using computer‐generated randomization numbers and concealed by sealed enveloped technique" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "blinding was not possible in our study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "blinding was not possible in our study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses and the need for cross‐over among groups was balanced (5 (16.7%) palpation, 1 (3.3%) in‐plane, 2 (8.9%) out‐of‐plane) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | High risk | Trial authors changed the primary outcome of interest from 'Time of insertion of cannula' (protocol) to 'First‐attempt success rate' (article) without a reasonable motivation |
Rose 2018.
| Study characteristics | ||
| Methods | [Abstract of event] Single‐centre prospective randomised controlled 2‐arm open‐label study Site and duration not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery axis and needle plane not described) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "performed by emergency medicine residents with standard ultrasound training" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not described) |
|
| Notes | Funding: not described Conflicts of interest: not described Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described Quote: "patients were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | High risk | Baseline characteristics between groups and safety outcomes were planned but were reported only by descriptions (i.e. without numerical values) for each group |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Sethi 2017.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label study India Duration: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Comparator: ultrasound‐guided RA puncture (real‐time, short axis, out‐of‐plane) Level of experience of person carrying out the procedure: quote: "each of the two anaesthetists had placed more than 100 arterial lines by using either in‐plane or out‐of‐plane approaches before commencing this study" Concomitant medications: all participants under general anaesthesia Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not described) |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol (CTRI/2015/02/005552) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized into two groups according to a computer‐generated random number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization sequences were kept on an opaque sealed envelope to maintain confidentiality and were handed over to the operator just before arterial by an anesthesiologist who was not a part of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | High risk | One of the planned safety outcomes (posterior arterial wall puncture) was not reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Seto 2015.
| Study characteristics | ||
| Methods | Multi‐centre (6 hospitals) prospective randomised controlled 2‐arm open‐label study USA Duration: 1 December 2011 to 29 March 2013 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane); quote: "single‐ or double‐wall technique was used per operator preference" Comparator: RA puncture by palpation and landmarks; quote: "single‐ or double‐wall technique was used per operator preference" and "palpation‐guided procedures were allowed to cross over to rescue US guidance after 5 min of attempts" Level of experience of person carrying out the procedure; quote: "this study included operators experienced in transradial catheterization to minimize potential confounders" Concomitant medications: conscious sedation, intra‐arterial and/or subcutaneous lidocaine as per local practice, minimum of 2000 IU of intravenous unfractionated heparin or bivalirudin for anticoagulation, and minimum of 2.5 mg of intra‐arterial verapamil or 100 mg nitroglycerin for spasm prophylaxis Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: range 30 minutes to 24 hours (bleeding) |
|
| Notes | Funding: quote: "the study was investigator initiated and unsponsored" Conflicts of interest: quote: "Dr. Abu‐Fadel serves on the Speakers Bureau of Abbott Vascular. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose" Protocol (NCT01605292) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomized in a 1:1 fashion to either palpation or US guidance using sealed envelopes balanced in blocks of 50 to 80 generated at each center" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was imbalance in cross‐over interventions between experimental (0/347) and comparator (10/351) groups Quote: "ten patients in the control group required crossover to US guidance after 5 min of failed palpation attempts with 8 of 10 (80%) having successful sheath insertion with US" |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Seyhan 2021.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label parallel‐assignment study Turkey Duration: 1 January 2020 to 1 April 2020 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, short‐axis approach, out‐of‐plane) Comparator: palpation‐guided RA puncture Level of experience of person carrying out the procedure; quote: "clinicians who have point‐of‐care US certificate perform this procedure" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time point reported: up to the end of the procedure |
|
| Notes | Funding: quote: "the author(s) received no financial support for the research, authorship, and/or publication of this article" Conflicts of interest: quote: "the author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article" Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "once enrolment was complete, the patients were then randomized". It was not clear how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "this prospective‐pilot study was nonblinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "this prospective‐pilot study was nonblinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Shiver 2006.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label study USA Duration: 6 months (not specified). |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "none of the four had previously placed US‐guided arterial catheters, but all had experience placing US‐guided peripheral and central venous lines" Concomitant medications: all participants under general anaesthesia Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not described) |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "each patient included in the study was randomly assigned to either the US‐guided or palpation‐technique group by using a random‐number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization results with a data sheet were kept in a sealed envelope in a locked and secured area" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "because this study could not be effectively blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "because this study could not be effectively blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was an imbalance in cross‐over interventions between experimental (0/30) and comparator (11/30) groups Quote: "eleven (37%) patients in the palpation group required rescue with US guidance. None of the patients in the US‐guided group required more than two attempts, and none were switched to the palpation technique for rescue" |
| Selective reporting (reporting bias) | High risk | Two of the planned safety outcomes were not reported (arterial laceration and thrombosis) |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Tada 2003.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not described Japan Duration: April 1999 to March 2002 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: Doppler assistance‐guided RA puncture (real‐time, artery axis not applicable) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "board‐certified anaesthesiologist who had been trained in the ultrasound technique in 20 patients prior to the current study" Concomitant medications: all participants under general anaesthesia Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to the end of the procedure (not described) |
|
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "166 patients were randomly assigned to two study groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | High risk | There were imbalances between experimental and comparator groups: participant number (72/94), male participants (43/59), and female participants (29/35). This imbalance is considered not to be possible by chance |
Tangwiwat 2016.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not described Thailand Duration: November 2009 to October 2010 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "ten third‐year residents, having performed USG vascular catheterization as yet less than 3 times" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 24 hours |
|
| Notes | Funding: quote: "this work was supported by Siriraj Research Development Fund, Faculty of Medicine Siriraj Hospital, Mahidol University" Conflicts of interest: quote: "none" Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using computer generated block randomization (mixed block size)" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was an imbalance in cross‐over interventions between experimental (6/50) and comparator (1/50) groups |
| Selective reporting (reporting bias) | High risk | Some of the planned safety outcomes were not reported (infection, retained catheter, radial nerve damage, arterial thrombosis/ischaemia, carpal tunnel syndrome) |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Ueda 2015.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 3‐arm parallel‐assignment study, with outcomes assessment blinded USA Duration: February 2010 to December 2011 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental (Doppler): ultrasound‐assisted RA puncture (real‐time, artery axis not relevant, needle plane not relevant) Experimental (B‐mode): ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "we used a simulated artery to teach anaesthetists how to use Doppler and ultrasound probes, which they had used less than five times before the study" (article). Quote: "performed by anaesthesia residents" (register site) Concomitant medications: quote: "less than 1 ml lidocaine 2% was injected subcutaneously over the radial artery" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 3 days (article) and up to 5 minutes (register site) |
|
| Notes | Funding: quote: "no external funding and no competing interests declared" Conflicts of interest: quote: "no external funding and no competing interests declared" Protocol available, but not linked in the article (NCT01276171) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we allocated participants in a 1:1:1 ratio with a computer‐generated list of pseudo‐random numbers in blocks of six, accessible only to research nurses" |
| Allocation concealment (selection bias) | Low risk | Quote: "the total allocation sequence was prepared by research nurses before the first participant was recruited, using sealed envelopes" and "the sealed envelopes were opened immediately before arterial cannulation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "masking: single (outcomes assessor)" (register site) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were no losses, but we found inconsistencies related to time points (up to 3 days in the article vs up to 5 minutes in the protocol) and to number of events (Doppler group had 96 first attempt success events at article vs 101 events at protocol) |
| Selective reporting (reporting bias) | High risk | Two planned safety outcomes were not reported (thrombosis and infection) |
| Other bias | High risk | Trial authors stated that "all subsequent procedures were performed in a sterile fashion", but figure 1 shows an example of Doppler‐assisted radial artery cannulation without any sterile cover. Infection was one of the planned safety outcomes |
Wang 2017.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not described China Duration: 1 June 2017 to 27 October 2017 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "three anaesthesiologists who performed radial artery cannulation were divided into 3 categories according to the years of clinical training in anaesthesia after graduation from medical college: 1 year (CA1), 3 years (CA3), and 5 years (CA5). At least 15 cases of radial artery catheterization guided by the M‐LAINUT were performed for each operator before starting the study" Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 day and up to 3 days after the procedure |
|
| Notes | Funding: quote: "this work was supported in part by the Fujian Province Science and Technology Innovation Joint Fund Project of China (2017Y9008) and the National Natural Science Foundation of China (Grant number: 81641038). They were not involved in research design, collection, data analysis, or approved to submit articles for publication" Conflicts of interest: quote: "the authors have no conflicts of interest to disclose" Protocol (ChiCTR‐IOR‐17011474) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "all the random numbers were generated by a computer and placed in sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "study assignment was concealed until after the decision had been made to radial artery cannulation and the patient was enrolled in the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was balanced dropout among groups: experimental 1/144 (1 reluctance to randomise), comparator 2/144 (1, the attending anaesthesiologist, decided not to insert the cannula into the radial artery, 1 loss of medical records) |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | High risk | There are 2 preprints and 1 published article with the same registration number (ChiCTR‐IOR‐17011474) but with differences in study design and period of carry‐out. Wang 2020 reported randomisation in 2 different groups (1:1) and period of carry‐out between 1 June 2017 and 27 October 2017. Wang 2019 reported randomisation in 3 different groups (1:1:1) and period of carry‐out between 1 July 2018 and 24 November 2018. We considered these data as 2 different studies due to method characteristics, distinct participants, and period of carry‐out |
Wang 2019.
| Study characteristics | ||
| Methods | [Preprint] Single‐centre prospective randomised controlled 3‐arm study, with blinding not described China Duration: 1 July 2018 to 24 November 2018 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental (in‐plane): ultrasound‐guided RA puncture (real‐time, artery long axis, in‐plane) Experimental (out‐of‐plane): ultrasound‐guided RA puncture (real‐time, artery short axis, out‐of‐plane) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: punctures were performed "by two anaesthesiologists who had previously performed more than 160 arterial cannulations each year (including 30 in‐plane, 30 out‐of‐plane, and 100 palpation)" Concomitant medications: quote: "2% lidocaine could be used for local anaesthesia in the puncture site" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 day and up to 3 days after the procedure |
|
| Notes | Funding: quote: "this work was supported in part by Fujian Province Science and Technology Innovation Joint Fund Project of China(2017Y9008) and the National Natural Science Foundation of China (Grant number: 81641038)" Conflicts of interest: quote: "the authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article" Protocol (ChiCTR‐IOR‐17011474) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes used to ensure allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was balanced dropout among groups: in‐plane 1/67 (1 cancelled procedure), out‐of‐plane 2/67 (1 loss of medical data set and 1 transducer faulty for invasive blood pressure), palpation 2/67 (1 cancelled procedure and 1 loss of medical data set) |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | High risk | There are 2 preprints and 1 published article with the same registration number (ChiCTR‐IOR‐17011474) but with differences in study design and period of carry‐out. Wang 2017 reported randomisation in 2 different groups (1:1) and period of carry‐out between 1 June 2017 and 27 October 2017. Wang 2019 reported randomisation in 3 different groups (1:1:1) and period of carry‐out between 1 July 2018 and 24 November 2018. We considered these data as 2 different studies due to method characteristics, distinct participants, and period of carry‐out |
Yeap 2019.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not described USA Duration: 2014 to 2016 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery axis and needle plane not described) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "all residents had done at least 5 TBP and 5 USG radial arterial catheterizations prior to the study" Concomitant medications: all participants were under "general anaesthesia and endotracheal intubation". Details are not provided Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 day and up to 3 days after the procedure |
|
| Notes | Funding: quote: "the authors report no external funding source for this study" Conflicts of interest: quote: "the authors declare they have no competing interests" Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the participants were randomised by a computer program (Research Randomizer, www.randomizer.org)" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was imbalance between experimental and comparator cross‐over interventions Quote: "ultrasound rescue was required in 12 out of 151 patients in the blind palpation group. In contrast, only 1 out of 147 patient cross over to the palpation technique in ultrasound technique group" |
| Selective reporting (reporting bias) | High risk | There was an imbalance between experimental (0/206) and comparator (9/215) exclusions. The reason for 1 of the exclusions was not described. No safety outcomes were planned or reported. One of the planned outcomes (first‐time success rate) was not reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Yu 2019.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not described China Duration: October 2018 to December 2018 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery short axis, out‐of‐plane) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "the 2 operators were resident anaesthesiologists (had finished 3 years of Chinese standard training for residents) who were trained in arterial cannulation using ultrasonography or palpation and had performed the procedure at least 30 or 200 times, respectively" Concomitant medications: quote: "local anesthesia was administered with lidocaine 2% (Zhaohui Company, Shanghai, China) at the puncture site" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 day and up to 2 days after the procedure |
|
| Notes | Funding: quote: "this work is partially supported by National Natural Science Foundation of China (No.81770295) and Key Project of Excellent Youth in Higher Education Institution of Anhui Province (gxyqZD2018028)" Conflicts of interest: not reported Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "the technique used in certain patients was chosen via a sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was balanced exclusion in both groups. Two participants were excluded because their catheter retention time was > 6 hours (1 experimental and 1 comparator) |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Zaremski 2013.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment study, with blinding not described Switzerland Duration: not described |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery short axis, out‐of‐plane) Comparator: RA puncture by palpation and landmarks Level of experience of person carrying out the procedure: quote: "operator performing >200 transradial procedures per year" Concomitant medications: quote: "local anesthesia was administered with lidocaine 2% (Zhaohui Company, Shanghai, China) at the puncture site" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 day and up to 2 days after the procedure |
|
| Notes | Funding: quote: "HU was supported by an unrestricted research grant by the University of Basel, Switzerland" Conflicts of interest: quote: "Dr. Quesada is a member of the Abbott Advisory Board; a consultant for the Medicines Company; and a consultant/speaker’s bureau member for Abbott, Boston Scientific, Cordis Corporation, St Jude Medical, WL Gore, NMT Medical, and Terumo; he also reports travel expenses from the above companies" Protocol not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a balanced but large amount of cross‐over interventions in both groups (12/92 experimental (6 switch to palpation and 6 switch to femoral) and 12/91 comparator (all switch to ultrasound)). Ten participants in experimental group and 9 in comparator group were switched to femoral access |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Zeng 2020.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment study, with blinding not described China Duration: not described |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental (OA‐IP ‐ oblique axis/in‐plane): real‐time, B‐mode ultrasound‐guided RA puncture (quote: "after a longitudinal axis view of the radial artery was obtained, the probe was rotated 10 to 15 degrees (clockwise on the right hand, or counter‐clockwise on the left hand) to orient the longitudinal axis of the probe obliquely to the artery") Comparator (LA‐IP long axis/in‐plane): real‐time, B‐mode ultrasound‐guided RA puncture (artery in long axis, real‐time, needle in‐plane) Level of experience of person carrying out the procedure: quote: "radial artery cannulation was performed by one of two experienced anaesthesiologists: both had placed more than 50 radial arterial lines with in‐plane approaches before commencing this study" Concomitant medications: quote: "we induced anaesthesia with sufentanil, propofol and cisatracurium. After tracheal intubation, a radial arterial catheter was inserted" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time point reported: during the surgery procedure |
|
| Notes | Funding: quote: "none" Conflicts of interest: quote: "none" Protocol available (ChiCTR‐IOR‐16007748) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned to either of the OA‐IP or LA‐IP groups, according to a computer‐generated randomization" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were assigned to either of the OA‐IP or LA‐IP groups, according to a computer‐generated randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the participant underwent the intervention after general anaesthesia, personnel for the intervention group were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | High risk | One outcome, relevant for this review, was planned but was not reported (total success rate) |
| Other bias | Low risk | We do not suspect any other bias related to this study |
Zhefeng 2019.
| Study characteristics | ||
| Methods | Single‐centre prospective randomised controlled 2‐arm study, with blinding not described China Duration: March 2018 to May 2018 |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: ultrasound‐guided RA puncture (real‐time; artery short axis, out‐of‐plane) Comparator: modified ultrasound‐guided RA puncture (real‐time; artery short axis, out‐of‐plane with add of a developing line) Level of experience of person carrying out the procedure: quote: "all radial artery punctures were performed by interns who were in the anesthesiology rotation" Concomitant medications: all participants under general anaesthesia: quote: "patients were placed in supine position and administrated 0.05 mg/kg midazolam and 0.1 μg/kg sufentanil for analgesia and sedation" and "2% lidocaine was used for local anaesthesia" Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: up to 1 day and up to 2 days after the procedure |
|
| Notes | Funding: quote: "supported by Beijing Municipal Science and Technology Commission (no. Z171100001017036)" Conflicts of interest: quote: "none" Protocol (ChiCTR1800015337) available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to traditional ultrasound or ultrasound with developing line groups using a sealed envelope" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the limitations of this study include the lack of a double‐blind study design" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the limitations of this study include the lack of a double‐blind study design" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "there were no drop‐outs during the trial" |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | We do not suspect any other bias related to this study |
AA = axillary artery; ABGA = arterial blood gas analysis; AD = angle‐distance; ASA = Amerian Society of Anesthesiologists physical status classification system; BMI = body mass index; CA = coronary angiography; DNTP = dynamic needle tip positioning; DPA = dorsalis pedis artery; ENT = ear, nose, and throat speciality; GA = general anaesthesia; ICU = intensive care unit; INR = international normalised ratio; IP = in‐plane; ITT = intention‐to‐treat; IU = international unit; LA = long axis; OA = oblique axis; PCI = percutaneous coronary intervention; PVD = peripheral vascular disease; RA = radial artery; SA = short axis; SD = standard deviation; US = ultrasound; VAS = visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anantasit 2017 | Inadequate population. RCT that included only children |
| Cronin 1986 | Inadequate comparator. RCT that did not compare ultrasound guidance |
| CTRI/2018/11/016257 | Inadequate comparator. RCT that used the same ultrasound guidance in both groups |
| Dahl 1992 | Inadequate comparator. RCT that did not compare ultrasound guidance |
| Elmahdy 2018 | Inadequate comparator. RCT that did not compare direct or indirect ultrasound guidance (ultrasound was used as a method of selection of a favourable site for puncture) |
| Kucuk 2014 | Inadequate comparator. RCT that did not compare ultrasound guidance (same ultrasound‐guided technique was used for the 5 arms) |
| Min 2016 | Inadequate comparator. RCT that did not compare ultrasound guidance (same ultrasound‐guided technique was used for both arms) |
| Mori 2020 | Inadequate study design. Non‐randomised comparative trial |
| NCT03537118 | Inadequate population. RCT conducted with participants submitted to femoral access |
| NCT04001764 | Inadequate comparator. RCT that considered the same ultrasound‐guided short‐axis out‐of‐plane intervention for the 3 parallel arms |
| NCT04077762 | Inadequate comparator. RCT that did not compare ultrasound guidance |
| Vaquerizo 2014 | Inadequate study design. Quasi‐RCT. Eligible patients were randomised according to availability of staff to carry out the intervention |
| Wilson 2020 | Inadequate study design. Quasi‐RCT. Eligible patients were randomised by the last digit of their medical record number |
| Yao 2018 | Inadequate comparator. RCT that did not compare ultrasound guidance |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Flores‐Arévalo 2016.
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment open‐label study Ecuador Duration: July to August 2015 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: B‐mode, real‐time ultrasound‐guided puncture (arterial axis and ultrasound plane not reported) Comparator: palpation‐guided puncture Level of experience of person carrying out the procedure: not reported Concomitant medications: not reported Excluded medications: not reported |
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until the end of the procedure |
| Notes | Funding: not reported Conflicts of interest: not reported Protocol not available |
BMI: body mass index; SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800016772.
| Study name | Application of modified ultrasonication guidance technique in radial artery puncture: a prospective randomized controlled trial |
| Methods | Single‐centre prospective randomised controlled 3‐arm parallel‐assignment study (masking not reported) |
| Participants | 201 participants, 18 to 85 years old, female and male, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: modified ultrasound guidance Comparator: conventional out‐of‐plane ultrasound guidance technique Compartaor: traditional touch positioning guidance technique |
| Outcomes | Primary
Secondary
|
| Starting date | 01 July 2018 |
| Contact information | Jiebo Wang Fujian Medical University Union Hospital, China +86 15959004325 / 258960368@qq.com |
| Notes | ChiCTR1800016772 / no data provided |
ChiCTR‐IOR‐16009966.
| Study name | Comparison of ultrasound‐guided and traditional palpation radial artery cannulation in aged patients |
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel‐assignment study (masking not reported) |
| Participants | 130 participants, 70 years of age and older, female and male, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: ultrasound‐guided puncture Comparator: traditional palpation puncture |
| Outcomes | Primary
Secondary
|
| Starting date | 1 November 2016 |
| Contact information | Jing Yu Second Affiliated Hospital of Zhejiang University School of Medicine, China +86 13758159796 / janeyu1129@163.com |
| Notes | ChiCTR‐IOR‐16009966 / no data provided |
CTRI/2020/01/022989.
| Study name | Comparison of ultrasound‐guided versus blind arterial cannulation in ICU patients: a prospective randomized study |
| Methods | Single‐centre prospective randomised controlled 2‐arm participant‐blinded parallel‐assignment study |
| Participants | 188 participants, age not reported, sex not reported; radial, femoral, dorsalis pedis arteries Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: ultrasound‐guided arterial cannulation in radial, femoral, dorsalis pedis artery Comparator: arterial cannulation by digital palpation method in radial, femoral, dorsalis pedis artery |
| Outcomes | Primary
Secondary
|
| Starting date | 30 January 2020 |
| Contact information | Dr. Afzal Azim Department of Critical Care Medicine, Sanjay Gandhi Postgraduate Institute of Medical Sciences 226014 Lucknow, Uttar Pradesh, India 8004904730 | draazim2002@gmail.com |
| Notes | CTRI/2020/01/022989 | no data provided |
CTRI/2020/06/025543.
| Study name | Radial artery cannulation |
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label parallel‐assignment study |
| Participants | 80 participants, age not reported, sex not reported, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: ultrasound‐guided arterial cannulation in radial artery Comparator: arterial cannulation by digital palpation method in radial artery |
| Outcomes | Primary
Secondary
Time point: at baseline |
| Starting date | 15 June 2020 |
| Contact information | CR Saravanan Room No. 201, SRM Medical College Hospital and Research Centre, SRMIST Potheri 603203 Kancheepuram, Tamil Nadu, India 9884001153 | drcrsaravanan@gmail.com |
| Notes | CTRI/2020/06/025543 | no data provided |
CTRI/2020/08/027199.
| Study name | Comparing ultrasound versus palpatory method for posterior tibial artery cannulation |
| Methods | Single‐centre prospective randomised controlled 2‐arm parallel assignment study, with participant and outcome assessment blinded |
| Participants | 240 participants, age not reported, sex not reported, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: ultrasound‐guided arterial cannulation in tibial artery Comparator: arterial cannulation by digital palpation method in tibial artery |
| Outcomes | Primary
Secondary
|
| Starting date | 20 August 2020 |
| Contact information | Dr. Priyanka Gupta Department of Anaesthesiology, Level 6, Medical College Building, AIIMS Rishikesh 249203 Dehradun, Uttaranchal, India 9811894899 | drpriyankagupta84@gmail.com |
| Notes | CTRI/2020/08/027199 | no data provided |
CTRI/2020/09/028136.
| Study name | Two methods of radial artery cannulation with sonography in low blood pressure patients |
| Methods | Single‐centre prospective randomised controlled parallel‐group interventional trial |
| Participants | 90 participants, 18 to 80 years old, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention 1: radial artery cannulation in patients’ non‐dominant hand using a traditional ultrasound‐guided technique Intervention 2: radial artery cannulation in patients’ non‐dominant hand using focused acoustic shadowing facilitated ultrasound‐guided technique |
| Outcomes | Primary
Secondary
|
| Starting date | 29 September 2020 |
| Contact information | Dr. Shikha Soni Department of Anaesthesiology and Critical Care Medical College Jodhpur Rajasthan Jodhpur Rajasthan 342001 Jodhpur, RAJASTHAN, India | telephone: 9828036002 email: doctorudsharma@gmail.com affiliation: Dr. S.N. Medical College Jodhpur Dr. U.D. Sharma Department of Anaesthesiology and Critical Care Medical College Jodhpur Rajasthan Jodhpur Rajasthan 342001 Jodhpur, RAJASTHAN, India | telephone: 9828036002 email: doctorudsharma@gmail.com affiliation: Dr. S.N. Medical College Jodhpur |
| Notes | CTRI/2020/09/028136 / no data provided |
CTRI/2020/12/029455.
| Study name | Comparison of ultrasound guided versus conventional palpatory method of posterior tibial artery cannulation |
| Methods | Single‐centre prospective randomised parallel‐group trial |
| Participants | 76 participants, 18 to 65 years old, female and male, posterior tibial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: ultrasound‐guided posterior tibial artery cannulation Control: conventional palpatory method of posterior tibial artery cannulation |
| Outcomes | Primary
Secondary
|
| Starting date | 01 December 2020 |
| Contact information | Dr. Ankur Sharma address: Room No. 3124 Medical College Building Department of Trauma & Emergency (Anesthesia) AIIMS JODHPUR BASNI, phase 2, JODHPUR 342005 Jodhpur, RAJASTHAN, India | telephone: 9654045653 | email: ankuranaesthesia@gmail.com affiliation: AIIMS JODHPUR |
| Notes | CTRI/2020/12/029455 / no data provided |
CTRI/2021/02/031051.
| Study name | Comparison of USG‐guided and blind techniques for radial artery cannulation by residents in a teaching institute |
| Methods | Randomised parallel‐group active controlled interventional trial |
| Participants | 124 participants, adults Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention 1: ultrasound‐guided radial artery cannulation Control Intervention 1: palpation technique for radial artery cannulation |
| Outcomes | Primary
Secondary
|
| Starting date | 15 February 2021 |
| Contact information | Afroz Khan address: 6th floor Department of Anaesthesia & Critical Care, Main Building, Grant Government Medical College & Hospital, Byculla, Mumbai‐08 Quarter No. 11, Panchasheel Building, JJ Hospital, Byculla, Mumbai‐08 400008 Mumbai, MAHARASHTRA, India telephone: 09167296754 | email: drafrozkhan2003@gmail.com affiliation: grant government medical college |
| Notes | CTRI/2021/02/031051 / no data provided |
KCT0004903.
| Study name | The efficacy of combined ultrasound‐guided radial artery cannulation in adult surgical patients |
| Methods | Interventional primary study; parallel randomised controlled trial |
| Participants | 120 participants, radial artery, minimum 18 years old, maximum without limit of age Inclusion criteria
Exclusion criteria
|
| Interventions | 1. SA group (short‐axis approach group): only short‐axis approach was used to confirm radial artery 2. SLA group (combined short‐axis and long‐axis approach group): confirm midline of the radial artery through a short‐axis approach, then conduct a catheter with a long‐axis approach, confirming the actual catheter approach in real time |
| Outcomes | Primary
Secondary
|
| Starting date | 4 August 2020 |
| Contact information | Dowon Lee address: not informed telephone: not informed email: not informed affiliation: Pusan National University Hospital |
| Notes | KCT0004903 / no data provided |
NCT01189188.
| Study name | Ultrasound guidance for radial arterial blood sampling |
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label parallel‐assignment study |
| Participants | 74 participants, 18 years of age and older, female and male, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: artery puncture with ultrasound guidance Comparator: artery puncture without ultrasound guidance |
| Outcomes | Primary
Secondary
|
| Starting date | August 2010 |
| Contact information | Romain Genre‐Grandpierre Centre Hospitalier Universitaire de Nîmes, France phone and email not provided |
| Notes | NCT01189188 | no data provided |
NCT01561196.
| Study name | Conventional versus ultrasound‐guided arterial cannulation, with and without local anaesthesia |
| Methods | Single‐centre prospective randomised controlled 2‐arm single‐masking cross‐over assignment study |
| Participants | 20 participants, 20 to 90 years old, female and male, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: ultrasound‐guided arterial cannulation Comparator: conventional cannulation. The arterial needle is placed using the traditional method and lidocaine |
| Outcomes | Primary
Secondary
|
| Starting date | February 2012 |
| Contact information | Marlene A Hansen Aarhus University Hospital, Skejby, Denmark Anæstesiologisk‐Intensiv afdeling IAarhus, Jylland, Denmark, 8200 phone and email not provided |
| Notes | NCT01561196 | no data provided |
NCT02584673.
| Study name | Computer‐assisted instrument guidance (CAIG) for arterial line placement |
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label parallel‐assignment study |
| Participants | 30 participants, 18 years of age and older, female and male, target artery not described (e.g. radial, tibial, axillary) Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: existing ultrasound equipment with supplemental computer‐assisted instrument guidance system Comparator: traditional ultrasound methods and equipment (not described) |
| Outcomes | Primary
Secondary
|
| Starting date | October 2015 |
| Contact information | Irwin Gratz The Cooper Health System, Camden, NJ, USA phone: 856‐968‐8527 email: gratz‐irwin@cooperhealth.edu |
| Notes | NCT02584673 | no data provided |
NCT03144895.
| Study name | Arterial catheterisation by ultrasound: impact on success rates and complications in patients hospitalised in resuscitation |
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label parallel‐assignment study |
| Participants | 380 participants, 18 years of age and older, female and male, radial or femoral artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: laying a radial or femoral arterial catheter by anatomical placement alone Comparator: laying a radial or femoral arterial catheter by ultrasound tracking |
| Outcomes | Primary
Secondary
|
| Starting date | 2 May 2017 |
| Contact information | Elie Zogheib Centre Hospitalier Universitaire, Amiens, France +33322087832 | zogheib.elie@chu‐amiens.fr |
| Notes | NCT03144895 | no data provided |
NCT03995264.
| Study name | Ultrasound vs palpation for radial artery cannulation in patients undergoing bariatric surgery |
| Methods | Single‐centre prospective randomised controlled 2‐arm quadruple‐masking (participant, care provider, investigator, outcomes assessor) parallel‐assignment study |
| Participants | 120 participants, 19 to 85 years old, female and male, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: ultrasound‐guided radial artery cannulation Comparator: radial artery cannulation using palpation |
| Outcomes | Primary
|
| Starting date | 1 August 2019 |
| Contact information | Ji Eun Kim Ajou University School of Medicine, Seoul, Republic of Korea 82‐31‐219‐5575 | beye98@aumc.ac.kr |
| Notes | NCT03995264 | no data provided |
NCT04318990.
| Study name | Distal vs proximal radial artery access for cardiac catheterisation and intervention |
| Methods | Single‐centre prospective randomised controlled 2‐arm open‐label parallel‐assignment study |
| Participants | 300 participants, 18 years of age and older, female and male, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: distal radial artery access under ultrasound guidance Comparator: proximal radial artery access (ultrasound guidance not reported in this group) |
| Outcomes | Primary
Secondary
|
| Starting date | 6 March 2020 |
| Contact information | Preethi Ravindranathan Baylor Scott & White The Heart Hospital, USA 469‐814‐4721 | preethi.ravindranathan@bswhealth.org |
| Notes | NCT04318990 | no data provided |
NCT04617106.
| Study name | Radial artery cannulation using two different methods |
| Methods | Single‐centre prospective randomised parallel‐assignment clinical trial |
| Participants | 52 participants, 18 years of age and older, female and male, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Control group
Intervention group
|
| Outcomes | Primary
Secondary
|
| Starting date | 5 November 2020 |
| Contact information | Sujan Dhakal, MBBS, MD resident +9779851178234 | szndkl44@gmail.com Dr. Gentle Shrestha MBBS, MD +9779841248584 | gentlesunder@hotmail.com location: Nepal Tribhuwan University Teaching Hospital Bagmati, Nepal, 44600 contact: Sujan Dhakal, MBBS 9851178234 | szndkl44@gmail.com |
| Notes | NCT04617106 / no data provided |
NCT04806932.
| Study name | Comparison of the modified and conventional approach of radial artery cannulation under short‐axis ultrasound guidance in ICU hypotensive patients |
| Methods | Single‐centre prospective randomised controlled parallel‐assignment trial |
| Participants | 102 participants, 18 years to 100 years old (adult, older adult), female and male, radial artery Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: modified approach
The first 3 attempts will be performed via the modified approach. If the first 3 attempts fail, location or operator of subsequent attempts at artery puncture will be changed Comparator: conventional approach The first 3 attempts will be performed via the conventional approach. If the first 3 attempts fail, location or operator of subsequent attempts at artery puncture will be changed |
| Outcomes | Primary
Secondary
|
| Starting date | 11 April 2021 |
| Contact information | Hongyu He, Ph D021‐64041990 ext 692958 | he.hongyu@zs‐hospital.sh.cn Shanghai Zhongshan Hospital Recruiting Shanghai, Shanghai, China, 200032 Contact: Guowei Tu, PhD +8613501996995 | tu.guowei@zs‐hospital.sh.cn Contact: Zhe Luo, PhD +8613916127028 | luo.zhe@zs‐hospital.sh.cn |
| Notes | NCT04806932 / no data provided |
NTR6107.
| Study name | Catheterisation of the radial artery with fixated ultrasound transducer |
| Methods | Single‐centre prospective randomised controlled 3‐arm single‐masking parallel‐assignment study |
| Participants | 200 participants, 18 years of age and older, female and male, radial artery. Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: ultrasound‐guided puncture Comparator: ultrasound with a fixated transducer‐guided puncture Comparator: digital palpation puncture |
| Outcomes | Primary
Secondary
|
| Starting date | 1 November 2016 |
| Contact information | Harm Scholten Catharina Hospital Eindhoven, The Netherlands harm.scholten@cze.nl |
| Notes | NTR6107 | no data provided |
TCTR20210202004.
| Study name | A comparison of success rate of radial artery cannulation between ultrasound‐guided and conventional palpation technique in elderly patients |
| Methods | Prospective randomised interventional trial (masking not reported) |
| Participants | 60 participants, both sexes, 65 years of age or older Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental
Comparator
|
| Outcomes | Primary
Secondary
|
| Starting date | 1 July 2020 |
| Contact information | Narumon Ngorsorn address: Department of Anesthesiology, 40002 Khonkaen, Thailand telephone: 0834051482 | email: miracle_oneview@msn.com affiliation: Khon Kaen University Thanaporn Suwongkrua address: Department of Anesthesiology, 40002 Khonkaen, Thailand telephone: 0644469666 | email: por4807062@gmail.com affiliation: Khon Kaen University |
| Notes | TCTR20210202004 / no data reported |
UMIN000020698.
| Study name | The disturbing factors for residents to insert arterial catheter |
| Methods | Single‐centre prospective randomised controlled 3‐arm open‐label parallel‐assignment study |
| Participants | 150 participants, 20 years of age and older, female and male, target artery not described. Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: insert arterial catheter with ultrasound scan Comparator
|
| Outcomes | Primary
Secondary
|
| Starting date | 25 January 2016 |
| Contact information | Irie Tomoya Yokohama City University, Japan 045‐787‐2918 | tomoya.irie0216@gmail.com |
| Notes | UMIN000020698 | no data provided |
AA = axillary artery; ABGA = arterial blood gas analysis; ASA = Amerian Society of Anesthesiologists physical status classification system; BMI = body mass index; CA = coronary angiography; DNTP = dynamic needle tip positioning; DPA = dorsalis pedis artery; ENT = ear, nose, and throat speciality; GA = general anaesthesia; ICU = intensive care unit; INR = international normalised ratio; ITT = intention‐to‐treat; IU = international unit; PCI = percutaneous coronary intervention; PVD = peripheral vascular disease; RA = radial artery; SD = standard deviation; VAS = visual analogue scale.
Differences between protocol and review
In our protocol (Flumignan 2020), we planned to describe skewed data reported as medians and interquartile ranges, but in our review, we estimated the MD using the method reported by Wan 2014 to convert median and IQR into MD and CI. When it was not possible, we narratively described skewed data reported as medians and interquartile ranges.
In our protocol (Flumignan 2020), we planned to create funnel plots only for primary outcomes, but we created funnel plots for all outcomes where we were able to pool more than 10 trials, to explore possible small‐study biases.
Although we did not specify this in our protocol, in our review, we performed subgroup analysis only if we identified at least 10 studies for that outcome, to follow a Cochrane Heart recommendation.
Although we established "two‐dimensional ultrasound guidance as our intervention of interest", and we planned to include trials comparing any type of ultrasound guidance "versus any other techniques for arterial puncture", we did not list all possible comparisons in our protocol. Therefore, we amended the 'Types of interventions' section to include the following comparisons.
B‐mode ultrasound versus near‐infrared laser guidance.
B‐mode ultrasound versus modified B‐mode ultrasound.
In‐plane B‐mode ultrasound versus out‐of‐plane B‐mode ultrasound.
Doppler auditory ultrasound assistance versus palpation and landmarks.
Dynamic out‐of‐plane B‐mode ultrasound versus static out‐of‐plane B‐mode ultrasound.
Oblique‐axis in‐plane B‐mode ultrasound versus long‐axis in‐plane B‐mode ultrasound.
We planned to assess publication bias in our protocol, but we detailed in the review the additional statistical tests used to analyse these data.
Because 'in‐plane or out‐of‐plane ultrasound image' and 'vessel accessed in a longitudinal or transverse way' were considered in different comparisons, we deleted them from the subgroup analysis. We detailed better how we would deal with no sufficient information for subgroup analysis (e.g. Goswami 2020 for the experience of operators).
Contributions of authors
RLGF: acquired trial reports; selected trials; extracted, analysed and, interpreted data; performed risk of bias assessments and GRADE assessments; drafted the review; and acted as a guarantor of the review.
VFMT: analysed and interpreted data; and drafted the review.
RDL: analysed and interpreted data; and drafted the review.
JCCBS: analysed and interpreted data; and drafted the review.
CDQF: selected trials; extracted, analysed, and interpreted data; performed risk of bias assessments and GRADE assessments; and drafted the review.
LCUN: arbitrated any disagreement in trial selection, risk of bias, and GRADE judgements; analysed and interpreted data; and drafted the review.
Sources of support
Internal sources
-
Division of Vascular and Endovascular Surgery, Universidade Federal de Sao Paulo, Brazil
This project received methodological support from a collaboration between Cochrane Brazil and the Division of Vascular and Endovascular Surgery.
External sources
-
NIHR, UK
This project was supported by the NIHR via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Declarations of interest
RLGF: none known.
VFMT: none known.
RDL: declares grants from Bristol‐Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi via institution and consulting fees from Bayer, Boehringer Ingleheim, Bristol‐Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
JCCBS: none known.
CDQF: none known.
LCUN: none known.
New
References
References to studies included in this review
Abdalla 2017 {published data only (unpublished sought but not used)}
- Abdalla UE, Elmaadawey A, Kandeel A. Oblique approach for ultrasound-guided radial artery catheterization vs transverse and longitudinal approaches, a randomized trial. Journal of Clinical Anesthesia 2017;36:98-101. [DOI: 10.1016/j.jclinane.2016.10.016] [DOI] [PubMed] [Google Scholar]
- NCT02550223. Ultrasound Guided Radial Artery Cathetrization, A Novel Technique. clinicaltrials.gov/ct2/show/NCT02550223 (first received 3 September 2015).
Ammar 2017 {published data only (unpublished sought but not used)}
- Ammar A, Ali L, Furqan A. A randomized comparison of ultrasound guided versus blindly placed radial arterial catheters. Journal of Postgraduate Medical Institute 2017;31(1):8-11. [Google Scholar]
Anand 2019 {published data only (unpublished sought but not used)}
- Anand RK, Maitra S, Ray BR, Baidhya DK, Khanna P, Chowdhury SR, et al. Comparison of ultrasound-guided versus conventional palpatory method of dorsalis pedis artery cannulation: a randomized controlled trial. Saudi Journal of Anesthesia 2019;13(4):295-8. [DOI: 10.4103/sja.SJA_766_18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- CTRI/2018/08/015525. Palpation method versus ultrasound guided dorsalis pedis artery (DPA) cannulation in adult patients: a randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/08/015525 (first received 29 August 2018).
Arora 2021 {published data only (unpublished sought but not used)}
- Al Sheheimi R, Maddali M. Ultrasound-guided out-of-plane versus in-plane for radial arterial line cannulation. Oman Medical Journal 2020;35(1):48. [EMBASE: 633377632] [Google Scholar]
- Arora NM, Maddali MM, Al-Sheheimi RAR, Al-Mughairi H, Panchatcharam SM. Ultrasound-guided out-of-plane versus in-plane radial artery cannulation in adult cardiac surgical patients. Journal of Cardiothoracic and Vascular Anesthesia 2021;35(1):84-8. [DOI: 10.1053/j.jvca.2020.08.025] [DOI] [PubMed] [Google Scholar]
Bai 2020 {published data only (unpublished sought but not used)}
- Bai B, Tian Y, Zhang Y, Yu C, Huang Y. Dynamic needle tip positioning versus the angle-distance technique for ultrasound-guided radial artery cannulation in adults: a randomized controlled trial. BMC Anesthesiology 2020;20(1):231. [DOI: 10.1186/s12871-020-01152-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03656978. Ultrasound-guided vascular puncture and catheterization. clinicaltrials.gov/ct2/show/NCT03656978 (first received 21 August 2018).
Berk 2013 {published data only (unpublished sought but not used)}
- Berk D, Gurkan Y, Kus A, Ulugol H, Solak M, Toker K. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? Journal of Clinical Monitoring and Computing 2013;27(3):319-24. [DOI: 10.1007/s10877-013-9437-6] [DOI] [PubMed] [Google Scholar]
Bobbia 2013 {published data only (unpublished sought but not used)}
- Bobbia X, Grandpierre RG, Claret P, Moreau A, Pommet S, Bonnec J, et al. Ultrasound guidance for radial arterial puncture: a randomized controlled trial. American Journal of Emergency Medicine 2013;31(5):810-5. [DOI: 10.1016/j.ajem.2013.01.029] [DOI] [PubMed] [Google Scholar]
Burad 2017 {published data only (unpublished sought but not used)}
- Burad J, Date R, Kodange S, Hashim AH, Nollain K. Comparison of conventional and ultrasound guided techniques of radial artery cannulation in different haemodynamic subsets: a randomised controlled study. Intensive Care Medicine 2017;43(1):140-1. [DOI: 10.1007/s00134-016-4569-z] [DOI] [PubMed] [Google Scholar]
- NCT02825615. Better Arterial Cannulation Technique With Different Hemodynamics. clinicaltrials.gov/ct2/show/NCT02825615 (first received 3 July 2016).
Cao 2018 {published data only (unpublished sought but not used)}
- Cao L, Zhang L, Ai M, Li L, Tian D, Sun Y, et al. Application of radial arterial puncture cannulation under ultrasonic guidance in patients with critical diseases. Journal of Central South University Medical Sciences 2018;43(4):447-51. [DOI: 10.11817/j.issn.1672-7347.2018.04.018] [DOI] [PubMed] [Google Scholar]
Cao 2020 {published data only (unpublished sought but not used)}ChiCTR2000030416
- Cao Y, Su J, Hang H, Kang K, Zhang J, Cui M. Comparison of three ultrasound-guided radial artery cannulation methods performed by anaesthesia residents: a prospective randomized controlled trial. Research Square. [DOI: 10.21203/rs.3.rs-152370/v1] [DOI]
- ChiCTR2000030416. Comparison of three ultrasound-guided radial artery cannulation methods in anaesthesia residents: a prospective randomized controlled trial. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ChiCTR2000030416 (first received 1 March 2020).
Edanaga 2012 {published data only (unpublished sought but not used)}
- Edanaga M, Mimura M, Azumaguchi T, Kimura M, Yamakage M. Comparison of ultrasound-guided and blindly placed radial artery catheterization. Japanese Journal of Anesthesiology 2012;61(2):221-4. [PMID: ] [PubMed] [Google Scholar]
Fujita 2012 {published data only (unpublished sought but not used)}
- Fujita Y, Nakata J, Nakajima M, Sano I, Teramoto Y. Comparison of the real-time ultrasound guided catheterization and the traditional palpitation technique for radial artery catheterization. Anesthesia and Analgesia 2012;114(5 Suppl 1):S193. [DOI: 10.1213/01.ane.0000431534.65496.1e] [DOI] [Google Scholar]
Gibbons 2020 {published data only (unpublished sought but not used)}
- Gibbons RC, Zanaboni A, Murrett J, Patterson J, Tyner N, Saravitz S, et al. Ultrasound-vs landmark-guided arterial line placement in the emergency department: a randomized control trial. Academic Emergency Medicine 2020;27:S103‐. [DOI: 10.1111/acem.13961] [DOI] [Google Scholar]
- Gibbons RC, Zanaboni A, Saravitz S M, Costantino TG. Ultrasound guidance versus landmark-guided palpation for radial arterial line placement by novice emergency medicine interns: a randomized controlled trial. Journal of Emergency Medicine 2020;59(6):911-7. [DOI: 10.1016/j.jemermed.2020.07.029] [DOI] [PubMed] [Google Scholar]
- NCT03326739. Ultrasound guided versus landmark guided arterial line placement by emergency medicine interns. clinicaltrials.gov/ct2/show/NCT03326739 (first received 17 October 2017).
Gopalasingam 2014 {published and unpublished data}
- Gopalasingam N, Hansen MA, Thorn S, Sloth E, Juhl-Olsen P. Ultrasound-guided radial artery catheterisation increases the success rate among anaesthesiology residents: a randomised study. Journal of Vascular Access 2017;18(6):546-51. [DOI: 10.5301/jva.5000702] [DOI] [PubMed] [Google Scholar]
- NCT01690416. Conventional vs ultrasound guided arteria cannulation. clinicaltrials.gov/ct2/show/NCT01690416 (first received 13 September 2012).
- Sloth E. Details about your trial data [Ultrasound-guided radial artery catheterisation increases the success rate among anaesthesiology residents: a randomised study] [personal communication]. Email to: RLG Flumignan 20 September 2020. [DOI] [PubMed]
Goswami 2020 {published data only (unpublished sought but not used)}
- Goswami D. Comparative study between conventional (palpation guided) and ultrasound guided radial artery cannulation. Indian Journal of Critical Care Medicine 2020;24(Suppl 2):S1-S60. [DOI: 10.5005/jp-journals-10071-23353.183] [DOI] [Google Scholar]
Grandpierre 2019 {published data only (unpublished sought but not used)}
- Grandpierre RG, Bobbia X, Muller L, Markarian T, Occean B, Pommet S, et al. Ultrasound guidance in difficult radial artery puncture for blood gas analysis: a prospective, randomized controlled trial. PLoS One 2019;14(3):e0213683. [DOI: 10.1371/journal.pone.0213683] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01789801. A Randomized Study Evaluating the Role of Ultra-sound Guidance When Drawing Radial Arterial Blood Samples. clinicaltrials.gov/ct2/show/NCT01789801 (first received 9 February 2013).
Hansen 2014 {published data only}
- Hansen MA, Juhl-Olsen P, Thorn S, Frederiksen CA, Sloth E. Ultrasonography-guided radial artery catheterization is superior compared with the traditional palpation technique: a prospective, randomized, blinded, crossover study. Acta Anaesthesiologica Scandinavica 2014;58(4):446-52. [DOI: 10.1111/aas.12299] [DOI] [PubMed] [Google Scholar]
- Juhl-Olsen P. Details about your trial data [Hansen MA, Juhl-Olsen P, Thorn S, Frederiksen CA, Sloth E. Ultrasonography-guided radial artery catheterization is superior compared with the traditional palpation technique: a prospective, randomized, blinded, crossover study] [personal communication]. Email to: RLG Flumignan 10 October 2020. [DOI] [PubMed]
Khan 2018 {published data only}
- CTRI/2017/03/008020. Comparison of two methods, one with feeling blood vessel pulsation and one using sonography machine, for inserting catheter (tube) in wrist blood vessel in patient with low blood pressure. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/03/008020 (first received 06 March 2017).
- Khan MS, Myatra S, Bhagat V, Siddiqui S, Narkhede A, Prabhu N, et al. Comparison of real time ultrasound guidance versus palpation technique in radial artery catheterization in critically ill patients presenting with hypotension: a randomized controlled trial. Critical Care 2018;22(Suppl 1):P267. [DOI: 10.1186/s13054-018-1973-5] [DOI] [Google Scholar]
Kiberenge 2018 {published data only (unpublished sought but not used)}
- Comstock GT. Ultrasound-guided dynamic needle tip positioning technique versus palpation technique for radial arterial cannulation in adult surgical patients: a randomized control trial: Kiberenge RK, Ueda K, Rosauer B. Anesth Analg 2018;126:120-6. Journal of Emergency Medicine 2018;54(3):392-3. [DOI: 10.1016/j.jemermed.2018.01.017] [DOI] [PubMed] [Google Scholar]
- Kiberenge RK, Ueda K, Rosauer B. Ultrasound-guided dynamic needle tip positioning technique versus palpation technique for radial arterial cannulation in adult surgical patients: a randomized controlled trial. Anesthesia & Analgesia 2018;126(1):120-6. [DOI: 10.1213/ANE.0000000000002261] [DOI] [PubMed] [Google Scholar]
- NCT02557828. Dynamic Need Tip Positioning With Ultrasound Versus Palpation Technique for Radial Artery Cannulation: A Prospective Randomized Controlled Trial. clinicaltrials.gov/ct2/show/NCT02557828 (first received 22 September 2015).
Killu 2011 {published data only}
- Killu K, Oropello JM, Manasia AR, Kohli-Seth R, Bassily-Marcus A, Leibowitz AB, et al. Utility of ultrasound versus landmark-guided axillary artery cannulation for hemodynamic monitoring in the intensive care unit. ICU Director 2011;2(3):54-9. [DOI: 10.1177/1944451611407634] [DOI] [Google Scholar]
Kim 2021a {published data only (unpublished sought but not used)}
- KCT0002476. A novel electromagnetic ultrasound-guided arterial line cannulation comparing with conventional ultrasound-guided technique. cris.nih.go.kr/cris/search/detailSearch.do/7587 (first received 12 July 2017).
- KCT0002476. A novel electromagnetic ultrasound-guided arterial line cannulation comparing with conventional ultrasound-guided technique. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=KCT0002476 (first received 22 September 2017).
- Kim N, Kim HI, Kim D, Park D, Song SH, Byon H. A novel electromagnetic guidance ultrasound system on radial artery cannulation: a prospective randomized controlled trial. BMC Anesthesiology 2021;21(1):21. [DOI: 10.1186/s12871-021-01244-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2021b {published data only (unpublished sought but not used)}
- KCT0003507. Comparison between Ultrasound-guided and Palpation Technique for Radial Artery Cannulation. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=KCT0003507 (first received 14 February 2019).
- KCT0003507. Ultrasound-guided Dynamic Needle Tip Positioning Technique versus Palpation Technique for Radial Artery Cannulation in Elderly Patients. cris.nih.go.kr/cris/search/detailSearch.do/13031 (first received 12 December 2018).
- Kim SY, Kim KN, Jeong MA, Lee BS, Lim HJ. Ultrasound-guided dynamic needle tip positioning technique for radial artery cannulation in elderly patients: a prospective randomized controlled study. PLoS One 2021;16(5):e0251712. [DOI: 10.1371/journal.pone.0251712] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laursen 2015 {published data only (unpublished sought but not used)}
- Laursen CB, Pedersen RL, Lassen AT. Ultrasonographically guided puncture of the radial artery for blood gas analysis: a prospective, randomized controlled trial. Annals of Emergency Medicine 2015;65(5):618-9. [DOI: 10.1016/j.annemergmed.2015.01.016] [DOI] [PubMed] [Google Scholar]
- Laursen CB, Pedersen RL, Lassen AT. Ultrasound guided puncture of the radial artery for blood gas analysis: a prospective, randomized controlled trial. Scandivian Journal of Trauma, Resuscitation and Emergency Medicine 2015;23(Suppl 1):A16. [DOI: 10.1186/1757-7241-23-S1-A16] [DOI] [PubMed] [Google Scholar]
- NCT01660724. Ultrasound Guided Arterial Puncture: a Prospective, Blinded, Randomised Controlled Trial. www.clinicaltrials.gov/ct2/show/NCT01660724 (first received 16 July 2012).
Levin 2003 {published data only}
- Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Critical Care Medicine 2003;31(2):481-4. [DOI: 10.1097/01.CCM.0000050452.17304.2F] [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li X, Fang G, Yang D, Wang L, Zheng C, Ruan L, et al. Ultrasonic technology improves radial artery puncture and cannulation in intensive care unit (ICU) shock patients. Medical Science Monitor 2016;22:2409-16. [DOI: 10.12659/MSM.896805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nam 2020 {published data only}
- Fujii S. Ultrasound-guided cannulation: from its inception to future use. Minerva Anestesiologica 2020;86(1):4-6. [PMID: ] [DOI] [PubMed]
- Nam K, Jeon Y, Yoon S, Kwon SM, Kang P, Cho YJ, et al. Ultrasound-guided radial artery cannulation using dynamic needle tip positioning versus conventional long-axis in-plane techniques in cardiac surgery patients: a randomized, controlled trial. Minerva Anestesiologica 2020;86(1):30-7. [DOI: 10.23736/S0375-9393.19.13646-2] [DOI] [PubMed] [Google Scholar]
- NCT03405623. Randomized trial of ultrasound-guided radial artery cannulation using dynamic short axis versus conventional long-axis in-plane view in cardiac surgery patients. clinicaltrials.gov/ct2/show/NCT03405623 (first received 15 January 2018).
Nasreen 2016 {published data only (unpublished sought but not used)}
- Nasreen A, Khuwaja A M, Akhtar P, Amjad N, Rao ZA. A randomized comparison of ultrasound guided versus direct palpation method of radial artery cannulation techniques in adult patients undergoing open heart surgery. Anaesthesia, Pain and Intensive Care 2016;20(1):38-42. [Google Scholar]
NCT01663779 {unpublished data only}
- NCT01663779. Comparison of ultrasound-guided versus blind insertion of radial artery catheters. clinicaltrials.gov/ct2/show/NCT01663779 (first received 8 August 2012).
Nguyen 2019 {published and unpublished data}
- Jayanti S, Juergens C, Makris A, Hennessy A, Nguyen P. 848 Learning curve in performing transradial and ultrasound guidance vascular access. Heart Lung and Circulation 2020;29:S418. [DOI: 10.1016/j.hlc.2021.02.006] [DOI] [PubMed] [Google Scholar]
- Jayanti S, Nguyen P, Makris A, Hennessy A, Wang A, Park K, et al. Ultrasound-guided femoral access in patients with large thigh circumference: analysis from the standard versus ultrasound-guided radial and femoral access (SURF) trial. Heart Lung and Circulation 2019;28:S435. [DOI: 10.1016/j.hlc.2019.06.708] [DOI] [Google Scholar]
- Nguyen P, Makris A, Hennessy A, Jayanti S, Wang A, Park K, et al. Procedural success rates from the standard versus ultrasound-guided radial and femoral access (SURF) trial. Heart Lung and Circulation 2019;28:S422. [10.1016/j.hlc.2019.06.676] [Google Scholar]
- Nguyen P, Makris A, Hennessy A, Jayanti S, Wang A, Park K, et al. Standard versus ultrasound-guided radial and femoral access (SURF) - a randomised controlled trial. Heart Lung and Circulation 2019;28:S428. [DOI: 10.1016/j.hlc.2019.06.690] [DOI] [PubMed] [Google Scholar]
- Nguyen P, Makris A, Hennessy A, Jayanti S, Wang A, Park K, et al. Standard versus ultrasound-guided radial and femoral access in coronary angiography and intervention (SURF): a randomised controlled trial. EuroIntervention 2019;15(6):e522-30. [DOI: 10.4244/EIJ-D-19-00336] [DOI] [PubMed] [Google Scholar]
- Nguyen P, Makris A, Hennessy A, Jayanti S, Xuan W, Juergens C. Comparison of standard versus ultrasound guidance in radial and femoral access: a subanalysis of the randomised SURF trial. European Heart Journal 2020;41(Suppl 2):2500. [PMID: 10.1093/ehjci/ehaa946.2500] [DOI] [Google Scholar]
- Nguyen P. Details about your trial data [Standard versus ultrasound-guided radial and femoral access in coronary angiography and intervention (SURF)] [personal communication]. Email to: RLG Flumignan 9 September 2020.
Osuda 2020 {published data only}
- Osuda M, Edanaga M, Matsumoto T, Yamamoto A, Ihara S, Tanaka S, et al. Comparison of Mill Suss TM-guided radial artery catheterization with the long-axis in-plane ultrasound-guided method under general anesthesia: a randomized controlled trial. Journal of Anesthesia 2020;34:464-7. [DOI: 10.1007/s00540-020-02749-z] [DOI] [PubMed] [Google Scholar]
- UMIN000021546. The usefulness of Mill Suss-guided radial artery catheterization compared with ultrasound-guided catheterization. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000021546 (first received 21 March 2016).
Peters 2015 {published data only}
- NCT02118441. A comparison of ultrasound-guided versus direct palpation for radial artery catheterization among cardiac anesthesiologists. clinicaltrials.gov/ct2/show/NCT02118441 (first received 15 April 2014).
- Peters C, Schwarz S, Yarnold C, Kojic K, Kojic S, Head S, et al. Ultrasound guidance versus direct palpation for radial artery catheterization by expert operators: a randomized trial among Canadian cardiac anesthesiologists. Canadian Journal of Anaesthesia 2015;62(11):1161-8. [DOI: 10.1007/s12630-015-0426-8] [DOI] [PubMed] [Google Scholar]
Quan 2014 {published data only}
- Quan Z, Tian M, Chi P, Cao Y, Li X, Peng K. Modified short-axis out-of-plane ultrasound versus conventional long-axis in-plane ultrasound to guide radial artery cannulation: a randomized controlled trial. Anesthesia and Analgesia 2014;119(1):163-9. [DOI: 10.1213/ANE.0000000000000242] [DOI] [PubMed] [Google Scholar]
Rajasekar 2021 {published data only (unpublished sought but not used)}
- CTRI/2019/02/017749. Comparison of first attempt success rates of ultrasound guided radial artery cannulation against traditional palpatory method. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/02/017749 (first received 20 February 2019).
- Rajasekar M, Sukumar S, Selvaraj V. Comparison of success rates of different methods of Ultrasound guided radial artery cannulation (short axis and long axis methods) against traditional palpatory method in adult patients - a prospective randomised study. Turkish Journal of Anaesthesiology & Reanimation 2021;ahead of print:online. [DOI: 10.5152/TJAR.2021.1364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2018 {published data only}
- Rose D, Wilson C, Billioux V, Bright L. 170 A prospective randomized controlled trial comparing ultrasound guidance versus standard technique for radial arterial catheter placement by emergency medicine residents. Annals of Emergency Medicine 2018;72:S70. [DOI: 10.1016/j.annemergmed.2018.08.175] [DOI] [Google Scholar]
Sethi 2017 {published data only}
- CTRI/2015/02/005552. Comparison of two techniques for putting cannula for measurement of blood pressure by ultrasound. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/02/005552 (first received 18 February 2015).
- Sethi S, Maitra S, Saini V, Samra T, Malhotra SK. Comparison of short-axis out-of-plane versus long-axis in-plane ultrasound-guided radial arterial cannulation in adult patients: a randomized controlled trial. Journal of Anesthesia 2017;31(1):89-94. [DOI: 10.1007/s00540-016-2270-6] [DOI] [PubMed] [Google Scholar]
Seto 2015 {published data only}
- NCT01605292. Radial artery access with ultrasound trial. clinicaltrials.gov/ct2/show/NCT01605292 (first received 26 January 2012).
- Seto A, Roberts J S, Abu-Fadel M, Czak S, Latif F, Jain S, et al. Radial arterial access with ultrasound trial. Journal of the American College of Cardiology 2013;62(18):B90-1. [DOI: 10.1016/j.jacc.2013.08.1014] [DOI] [Google Scholar]
- Seto AH, Roberts JS, Abu-Fadel MS, Czak SJ, Latif F, Jain SP, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery access with Ultrasound Trial). JACC Cardiovascular Interventions 2015;8(2):283-91. [DOI: 10.1016/j.jcin.2014.05.036] [DOI] [PubMed] [Google Scholar]
Seyhan 2021 {published and unpublished data}
- Ak R. Re: Ultrasound guidance versus conventional technique for radial artery puncture in septic shock patients: a pilot study [personal communication]. Email to: CDQ Flumignan 10 July 2021. [DOI] [PubMed]
- Seyhan AU, Ak R. Ultrasound guidance versus conventional technique for radial artery puncture in septic shock patients: a pilot study. Journal of Vascular Access 2021;ahead of print:online. [DOI: 10.1177/11297298211023299] [DOI] [PubMed] [Google Scholar]
- Seyhan AU, Ak R. Ultrasound guidance versus conventional technique for radial artery puncture in septic shock patients: a randomized controlled trial. Authorea [preprint]. [DOI: 10.22541/au.161316255.50724520/v1] [DOI] [PubMed]
Shiver 2006 {published data only}
- Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Academic Emergency Medicine 2006;13(12):1275-9. [DOI: 10.1197/j.aem.2006.07.015] [DOI] [PubMed] [Google Scholar]
Tada 2003 {published data only}
- Tada T, Amagasa S, Horikawa H. Absence of efficacy of ultrasonic two-way Doppler flow detector in routine percutaneous arterial cannulation. Journal of Anesthesia 2003;17(3):206-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tangwiwat 2016 {published data only}
- Tangwiwat S, Pankla W, Rushatamukayanunt P, Waitayawinyu P, Soontrakom T, Jirakulsawat A. Comparing the success rate of radial artery cannulation under ultrasound guidance and palpation technique in adults. Journal of the Medical Association of Thailand 2016;99(5):505-10. [PubMed] [Google Scholar]
- Tangwiwat S, Pankla W, Rushatamukayanunt P, Waitayawinyu P, Soontrakom T, Jirakulsawat A. Comparing the success rate of radial artery cannulation under ultrasound guidance and palpation technique in adults. www.thaiscience.info/Journals/Article/JMAT/10983045.pdf (accessed 26 August 2020). [PubMed]
Ueda 2015 {published and unpublished data}
- NCT01276171. Ultrasound-image guided versus Doppler guided versus palpation technique for arterial cannulation in adults. clinicaltrials.gov/ct2/show/NCT01276171 (first received 11 January 2011).
- Ueda K, Bayman EO, Johnson C, Odum NJ, Lee JJ. A randomised controlled trial of radial artery cannulation guided by Doppler vs. palpation vs. ultrasound. Anaesthesia 2015;70(9):1039-44. [DOI: 10.1111/anae.13062] [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- ChiCTR-IOR-17011474. Application of Visualized Ultrasoniction Technique in Radial Artery Catheteriza: a prospective, randomized controlled study. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IOR-17011474 (first received 23 May 2017).
- Wang J, Lai Z, Weng X, Lin Y, Wu G, Huang Q et al. Modified Long-Axis In-Plane Ultrasound Technique Versus Conventional Palpation Technique For Radial Arterial Cannulation: A Prospective Randomized Controlled Trial. medRxiv. [DOI: 10.1101/19001586] [DOI] [PMC free article] [PubMed]
- Wang J, Lai Z, Weng X, Lin Y, Wu G, Su J et al. Modified long-axis in-plane ultrasound technique versus conventional palpation technique for radial arterial cannulation: A prospective randomized controlled trial. Medicine 2020;99(2):e18747. [DOI: 10.1097/MD.0000000000018747] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2019 {published data only}
- Wang J, Zhang L, Lai Z, Huang Q, Wu G, Lin L, et al. Modified Long-Axis In-Plane Ultrasound Versus Short-Axis Out-of-Plane Ultrasound For Radial Arterial Cannulation:A Prospective Randomized Controlled Trial. medRxiv. [10.1101/19005496]
Yeap 2019 {published data only}
- Yeap Y. Blind palpation vs ultrasound guided arterial line placement. Anesthesia and Analgesia 2016;122(5):S124. [DOI: 10.1213/01.ane.0000499505.96779.a0] [DOI] [Google Scholar]
- Yeap YL, Wolfe JW, Stewart J, Backfish KM. Prospective comparison of ultrasound-guided versus palpation techniques for arterial line placement by residents in a teaching institution. Journal of Graduate Medical Education 2019;11(2):177-81. [DOI: 10.4300/JGME-D-18-00592.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2019 {published data only}
- Yu Y, Lu X, Fang W, Liu X, Lu Y. Ultrasound-guided artery cannulation technique versus palpation technique in adult patients in pre-anesthesia room: a randomized controlled trial. Medical Science Monitor 2019;25:7306-11. [DOI: 10.12659/MSM.916252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaremski 2013 {published data only}
- Zaremski L, Quesada R, Kovacs M, Schernthaner M, Uthoff H. Prospective comparison of palpation versus ultrasound-guided radial access for cardiac catheterization. Journal of Invasive Cardiology 2013;25(10):538-42. [PubMed] [Google Scholar]
Zeng 2020 {published data only (unpublished sought but not used)}ChiCTR‐IOR‐16007748
- ChiCTR-IOR-16007748. Ultrasound-guided radial arterial cannulation: oblique axis/in-plane versus longitudinal axis/in-plane approaches. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ChiCTR-IOR-16007748 (first received 13 January 2016).
- Zeng C, Zhao G, Deng J, Li D. Oblique versus longitudinal axis/in-plane approaches for ultrasound-guided radial arterial cannulation A randomised controlled trial. European Journal of Anaesthesiology 2020;37(7):618-21. [DOI: 10.1097/EJA.0000000000001186] [DOI] [PubMed] [Google Scholar]
Zhefeng 2019 {published data only (unpublished sought but not used)}
- ChiCTR1800015337. Clinical Observation on Developing Line Location Technology for Ultrasound Guided Radial Artery Puncture in adult patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800015337 (first received 24 March 2018).
- Zhefeng Q, Luo C, Zhang L, Li X, He H, Chi P. Application of optimized ultrasonic localization system for radial artery puncture by intern doctors: a randomized trial. Medical Science Monitor 2019;25:1566-71. [DOI: 10.12659/MSM.913044] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Anantasit 2017 {published data only}
- Anantasit N, Cheeptinnakorntaworn P, Khositseth A, Lertbunrian R, Chantra M. Ultrasound versus traditional palpation to guide radial artery cannulation in critically ill children: a randomized trial. Journal of Ultrasound in Medicine 2017;36(12):2495-501. [DOI: 10.1002/jum.14291] [DOI] [PubMed] [Google Scholar]
Cronin 1986 {published data only}
- Cronin KD, Davies MJ, Domaingue CM, Worner MJ, Koumoundouros E. Radial artery cannulation - the influence of method on blood flow after decannulation. Anaesthesia and Intensive Care 1986;14(4):400-3. [DOI: 10.1177/0310057X8601400412] [DOI] [PubMed] [Google Scholar]
CTRI/2018/11/016257 {published data only}
- CTRI/2018/11/016257. Comparison of ultrasound guided dorsal radial artery cannulation and conventional radial artery cannulation at the volar aspect of wrist: a randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/11/016257 (first received 2 November 2018).
Dahl 1992 {published data only}
- Dahl MR, Smead WL, McSweeney TD. Radial artery cannulation: a comparison of 15.2- and 4.45-cm catheters. Journal of Clinical Monitoring 1992;8(3):193-7. [DOI] [PubMed] [Google Scholar]
Elmahdy 2018 {published data only}
- Elmahdy MF, Hassan M, Elguindy A. Role of vascular ultrasound scanning in repeated trans-radial coronary artery intervention (prospective randomized study). Catheterization and Cardiovascular Interventions 2018;92(5):862-70. [DOI: 10.1002/ccd.27413] [DOI] [PubMed] [Google Scholar]
Kucuk 2014 {published data only}
- Kucuk A, Yuce HH, Yalcin F, Boyaci FN, Yildiz S, Yalcin S. Forty-five degree wrist angulation is optimal for ultrasound guided long axis radial artery cannulation in patients over 60 years old: a randomized study. Journal of Clinical Monitoring and Computing 2014;28(6):567-72. [DOI: 10.1007/s10877-014-9552-z] [DOI] [PubMed] [Google Scholar]
Min 2016 {published data only}
- Min SW, Cho HR, Jeon YT, Oh AY, Park HP, Yang CW, et al. Effect of bevel direction on the success rate of ultrasound-guided radial arterial catheterization. BMC Anesthesiology 2016;16(1):34. [DOI: 10.1186/s12871-016-0202-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mori 2020 {published data only}
- Mori S, Hirano K, Yamawaki M, Kobayashi N, Sakamoto Yi, Tsutsumi M, et al. A comparative analysis between ultrasound-guided and conventional distal transradial access for coronary angiography and intervention. Journal of Interventional Cardiology 2020;2020:7342732-7342740. [DOI: 10.1155/2020/7342732] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03537118 {published data only}
- NCT03537118. Routine Ultrasound Guidance for Vascular Access for Cardiac Procedures: A Randomized Trial. clinicaltrials.gov/ct2/show/NCT03537118 (first received 15 May 2018).
NCT04001764 {published data only}
- NCT04001764. Comparison of the Efficacy of Radial Artery Catheterization in Three Different Regions in Intensive Care Patients. clinicaltrials.gov/ct2/show/record/NCT04001764 (first received 15 June 2019).
NCT04077762 {published data only}
- NCT04077762. Radial vs. State-Of-The-Art Femoral Access for Bleeding and Access Site Complication Reduction in Cardiac Catheterization (REBIRTH). clinicaltrials.gov/ct2/show/NCT04077762 (first received 26 August 2019).
Vaquerizo 2014 {published data only}
- Vaquerizo-Carpizo E, Fadrique-Millán LN, Torres-Sancho R, Benito-Bernal S. Comparative study between ultrasound-guided arterial puncture vs. the traditional technique [Estudio comparativo de la punción arterial ecoguiada frente a la técnica clásica]. Metas de Enfermería 2014;17(10):51-5. [Google Scholar]
- Vaquerizo-Carpizo E. Eco study [personal communication] [Estudio eco]. Email to: RLG Flumignan 28 October 2020.
Wilson 2020 {published data only}
- Wilson C, Rose D, Kelen GD, Billioux V, Bright L. Comparison of ultrasound-guided vs traditional arterial cannulation by emergency medicine residents. Western Journal of Emergency Medicine 2020;21(2):353-8. [DOI: 10.5811/westjem.2019.12.44583] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yao 2018 {published data only}
- Yao J, Yan H, Zeng Z, Wang L, Jiang W, Zhou Q, et al. The effect of application of a distal tourniquet on ultrasound guided radial artery cannulation in adult patients. American Journal of Emergency Medicine 2018;36(4):669-72. [DOI: 10.1016/j.ajem.2017.12.034] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Flores‐Arévalo 2016 {published data only (unpublished sought but not used)}
- Flores-Arévalo C, Bonilla-Cerda I, Bayas Y. Puncture of the radial artery guided by ultrasound for the obtaining of arterial blood gases [Punción de la arteria radial guiada por ultrasonido para obtención de gases arteriales ]. Revista Medica-Científica CAMbios 2016;15(2):18-21. [DOI: 10.36015/cambios.v15.n2.2016.233] [DOI] [Google Scholar]
References to ongoing studies
ChiCTR1800016772 {published data only}
- ChiCTR1800016772. Application of modified Ultrasonication Guidance Technique in Radial Artery puncture: a prospective, randomized, controlled trial. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800016772 (first received 23 June 2018).
ChiCTR‐IOR‐16009966 {published data only}
- ChiCTR-IOR-16009966. The use of ultrasound-guided radial artery cannulation in aged patients. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IOR-16009966 (first received 22 November 2016).
CTRI/2020/01/022989 {published data only}
- CTRI/2020/01/022989. Comparison of ultrasound guided verses blind arterial cannulation in ICU patients: a prospective randomized study. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/01/022989 (first received 27 January 2020).
CTRI/2020/06/025543 {published data only (unpublished sought but not used)}
- CTRI/2020/06/025543. Radial Artery Cannulation. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/06/025543 (first received 2 June 2020).
CTRI/2020/08/027199 {published data only}
- CTRI/2020/08/027199. Comparing ultrasound versus palpatory method for posterior tibial artery cannulation. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/08/027199 (first received 18 August 2020).
CTRI/2020/09/028136 {published data only}
- CTRI/2020/09/028136. Two methods of radial artery cannulation with sonography in low sonography in low blood pressure patients. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/09/028136 (first received 29 September 2020).
CTRI/2020/12/029455 {published data only (unpublished sought but not used)}
- CTRI/2020/12/029455. Comparison of ultrasound guided vs traditional palpatory procedure of posterior tibial artery cannulation. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/029455 (first received 1 December 2020).
CTRI/2021/02/031051 {published data only (unpublished sought but not used)}
- CTRI/2021/02/031051. Comparison of USG-guided and blind techniques for radial artery cannulation by residents in a teaching institute. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2021/02/031051 (first received 5 February 2021).
KCT0004903 {published data only}
- KCT0004903. The efficacy of combined ultrasound-guided radial artery cannulation in adult surgical patients. who.int/trialsearch/Trial2.aspx?TrialID=KCT0004903 (first register 8 April 2020).
NCT01189188 {published data only}
- NCT01189188. Ultrasound guidance for radial arterial blood sampling. clinicaltrials.gov/ct2/show/NCT01189188 (first received 25 August 2010).
NCT01561196 {published data only}
- NCT01561196. Conventional verses ultrasound guided arteria cannulation, with and without local anesthesia. clinicaltrials.gov/ct2/show/NCT01561196 (first received 13 March 2012).
NCT02584673 {published data only}
- NCT02584673. Computer assisted instrument guidance (CAIG) for arterial line placement. clinicaltrials.gov/ct2/show/NCT02584673 (first received 21 October 2015).
NCT03144895 {published data only}
- NCT03144895. Arterial catheterization by ultrasound: impact on success rates and complications in patients hospitalized in resuscitation. clinicaltrials.gov/ct2/show/NCT03144895 (first received 2 May 2017).
NCT03995264 {published data only}
- NCT03995264. Ultrasound vs palpation for radial artery cannulation in patients undergoing bariatric surgery. clinicaltrials.gov/ct2/show/NCT03995264 (first received 20 June 2019).
NCT04318990 {published data only}
- NCT04318990. Distal vs. proximal radial artery access for cardiac catheterization and intervention. clinicaltrials.gov/ct2/show/NCT04318990 (first received 19 March 2020).
NCT04617106 {published data only (unpublished sought but not used)}
- NCT04617106. Radial artery cannulation using two different methods. clinicaltrials.gov/show/NCT04617106 (first register 5 November 2020).
NCT04806932 {published data only}
- NCT04806932. Comparison of the modified and conventional approach of radial artery cannulation under short-axis ultrasound guidance in ICU hypotensive patients. clinicaltrials.gov/ct2/show/NCT04806932 (first report 19 March 2021).
NTR6107 {published data only}
- NTR6107. Catheterization of the radial artery with fixated ultrasound transducer. who.int/trialsearch/Trial2.aspx?TrialID=NTR6107 (first received 10 October 2016).
TCTR20210202004 {published data only (unpublished sought but not used)}
- TCTR20210202004. A comparison of success rate of radial artery cannulation between ultrasound guided and conventional palpation technique in elderly patients. who.int/trialsearch/Trial2.aspx?TrialID=TCTR20210202004 (first register 2 February 2021).
UMIN000020698 {published data only}
- UMIN000020698. The disturbing factors for residents to insert arterial catheter. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000020698 (first received 22 January 2016). [UMIN000020698]
Additional references
Aboyans 2018
- Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. Editor's Choice - 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2018;55(3):305-68. [PMID: ] [DOI] [PubMed] [Google Scholar]
AIUM 2013
- American Institute of Ultrasound in Medicine. AIUM practice guideline for the use of ultrasound to guide vascular access procedures. Journal of Ultrasound in Medicine 2013;32(1):191-215. [PMID: ] [DOI] [PubMed] [Google Scholar]
Aouad‐Maroun 2016
- Aouad-Maroun M, Raphael CK, Sayyid SK, Farah F, Akl EA. Ultrasound-guided arterial cannulation for paediatrics. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD011364. [DOI: 10.1002/14651858.CD011364.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arthurs 2008
- Arthurs ZM, Starnes BW, Sohn VY, Singh N, Andersen CA. Ultrasound-guided access improves rate of access-related complications for totally percutaneous aortic aneurysm repair. Annals of Vascular Surgery 2008;22(6):736-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Attie 2019
- Attie GA, Flumignan CDQ, Silva MAM, Barros EM, Daolio RM, Guedes Neto HJ, et al. What do Cochrane systematic reviews say about ultrasound-guided vascular access? Sao Paulo Medical Journal 2019;137(3):284-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharjee 2018
- Bhattacharjee S, Maitra S, Baidya DK. Comparison between ultrasound guided technique and digital palpation technique for radial artery cannulation in adult patients: an updated meta-analysis of randomized controlled trials. Journal of Clinical Anesthesia 2018;47:54-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brueck 2009
- Brueck M, Bandorski D, Kramer W, Wieczorek M, Holtgen R, Tillmanns H. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovascular Interventions 2009;2(11):1047-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Conte 2019
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Journal of Vascular Surgery 2019;69(6S):3S-125S.e40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 10 January 2020. Available at covidence.org.
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10. Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Dudeck 2004
- Dudeck O, Teichgraeber U, Podrabsky P, Lopez Haenninen E, Soerensen R, Ricke J. A randomized trial assessing the value of ultrasound-guided puncture of the femoral artery for interventional investigations. International Journal of Cardiovascular Imaging 2004;20(5):363-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. A nonparametric "trim and fill" method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association 2000;95(449):89-98. [DOI: 10.2307/2669529] [DOI] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman 2013
- Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the national cardiovascular data registry (2007-2012). Circulation 2013;127(23):2295-306. [PMID: ] [DOI] [PubMed] [Google Scholar]
Flumignan 2018
Franz 2017
- Franz RW, Tanga CF, Herrmann JW. Treatment of peripheral arterial disease via percutaneous brachial artery access. Journal of Vascular Surgery 2017;66(2):461-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gao 2016
- Gao YB, Yan JH, Ma JM, Liu XN, Dong JY, Sun F, et al. Effects of long axis in-plane vs short axis out-of-plane techniques during ultrasound-guided vascular access. American Journal of Emergency Medicine 2016;34(5):778-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gopalasingam 2017
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gu 2014
Gu 2016
- Gu WJ, Wu XD, Wang F, Ma ZL, Gu XP. Ultrasound guidance facilitates radial artery catheterization: a meta-analysis with trial sequential analysis of randomized controlled trials. Chest 2016;149(1):166-79. [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2018
- Harris E, Warner CJ, Hnath JC, Sternbach Y, Darling RC 3rd. Percutaneous axillary artery access for endovascular interventions. Journal of Vascular Surgery 2018;68(2):555-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JP, Eldridge S, Li T (editors). Chapter 23. Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgs 2005
- Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet 2005;366(9494):1407-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ibanez 2018
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal 2018;39(2):119-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jaroenngarmsamer 2019
- Jaroenngarmsamer T, Bhatia KD, Kortman H, Orru E, Krings T. Procedural success with radial access for carotid artery stenting: systematic review and meta-analysis. Journal of Neurointerventional Surgery 2019;12(1):87-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jolly 2009
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. American Heart Journal 2009;157(1):132-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kendall 2014
Kolkailah 2018
- Kolkailah AA, Alreshq RS, Muhammed AM, Zahran ME, Anas El-Wegoud M, Nabhan AF. Transradial versus transfemoral approach for diagnostic coronary angiography and percutaneous coronary intervention in people with coronary artery disease. Cochrane Database of Systematic Reviews 2018;4:CD012318. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamperti 2012
- Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Medicine 2012;38(7):1105-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2015
- Lee KS, Sos TA. Brachial artery access. Techniques in Vascular and Interventional Radiology 2015;18(2):87-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4. Searching for and selecting studies. In: Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al. (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2018
- Mason PJ, Shah B, Tamis-Holland JE, Bittl JA, Cohen MG, Safirstein J, et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circulation. Cardiovascular Interventions 2018;11(9):e000035. [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2013
- NICE Appraisal Committee Members. CG167. Myocardial infarction with ST-segment elevation: acute management. Available from nice.org.uk/guidance/cg167.
NICE 2014
- NICE Appraisal Committee Members. QS52. Peripheral arterial disease. Available from nice.org.uk/guidance/qs52.
NICE 2018
- NICE Appraisal Committee Members. CG147. Peripheral arterial disease: diagnosis and management. Available from www.nice.org.uk/guidance/cg147.
Pacha 2018
- Pacha HM, Alahdab F, Al-Khadra Y, Idris A, Rabbat F, Darmoch F, et al. Ultrasound-guided versus palpation-guided radial artery catheterization in adult population: a systematic review and meta-analysis of randomized controlled trials. American Heart Journal 2018;204:1-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Paganin 2018
- Paganin AC, Beghetto MG, Feijo MK, Matte R, Sauer JM, Rabelo-Silva ER. Vascular complications in patients who underwent endovascular cardiac procedures: multicenter cohort study. Revista Latino-Americana de Enfermagem 2018;26:e3060. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JT, Sterne JAC. Chapter 13. Assessing risk of bias due to missing results in a synthesis. In: Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Parviz 2015
- Parviz Y, Rowe R, Vijayan S, Iqbal J, Morton AC, Grech ED, et al. Percutaneous brachial artery access for coronary artery procedures: feasible and safe in the current era. Cardiovascular Revascularization Medicine 2015;16(8):447-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pascual 2017
- Pascual I, Carro A, Avanzas P, Hernández-Vaquero D, Díaz R, Rozado J, et al. Vascular approaches for transcatheter aortic valve implantation. Journal of Thoracic Disease 2017;9(Suppl 6):S478-87. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2008
- Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfeld JS, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC. Cardiovascular Interventions 2008;1(4):379-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
R Studio [Computer program]
- R Studio. Version 1.4.1106. Boston: R Studio, 2020.
Sandoval 2017
- Sandoval Y, Burke MN, Lobo AS, Lips DL, Seto AH, Chavez I, et al. Contemporary arterial access in the cardiac catheterization laboratory. JACC. Cardiovascular Interventions 2017;10(22):2233-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15. Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Seldinger 1953
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiologica 1953;39(5):368-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Seto 2010
- Seto AH, Abu-Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC. Cardiovascular Interventions 2010;3(7):751-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shiloh 2011
- Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest 2011;139(3):524-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Song 2016
- Song IK, Choi JY, Lee JH, Kim EH, Kim HJ, Kim HS, et al. Short-axis/out-of-plane or long-axis/in-plane ultrasound-guided arterial cannulation in children: a randomised controlled trial. European Journal of Anaesthesiology 2016;33(7):522-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Song 2018
- Song IK, Kim EH, Lee JH, Jang YE, Kim HS, Kim JT. Seldinger vs modified Seldinger techniques for ultrasound-guided central venous catheterisation in neonates: a randomised controlled trial. British Journal of Anaesthesia 2018;121(6):1332-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Soverow 2016
- Soverow J, Oyama J, Lee MS. Adoption of routine ultrasound guidance for femoral arterial access for cardiac catheterization. Journal of Invasive Cardiology 2016;28(8):311-4. [PMID: ] [PubMed] [Google Scholar]
Strauss 2021
- Strauss S, Siracuse JJ, Madassery S, Truesdell AG, Pereira K, Korngold EC, et al. Ultrasound‐guided versus anatomic landmark‐guided percutaneous femoral artery access. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD014594. [DOI: 10.1002/14651858.CD014594] [DOI] [Google Scholar]
Tang 2014
- Tang L, Wang F, Li Y, Zhao L, Xi H, Guo Z, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLoS One 2014;9(11):e111527. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ueda 2013
- Ueda K, Puangsuvan S, Hove MA, Bayman EO. Ultrasound visual image-guided vs Doppler auditory-assisted radial artery cannulation in infants and small children by non-expert anaesthesiologists: a randomized prospective study. British Journal of Anaesthesia 2013;110(2):281-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Valgimigli 2014
- Valgimigli M, Campo G, Penzo C, Tebaldi M, Biscaglia S, Ferrari R. Transradial coronary catheterization and intervention across the whole spectrum of Allen test results. Journal of the American College of Cardiology 2014;63(18):1833-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
White 2016
- White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. British Journal of Anaesthesia 2016;116(5):610-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Obesity: preventing and managing the global epidemic (WHO Technical Report Series 894). Available at www.who.int/nutrition/publications/obesity/WHO_TRS_894/en. [ISBN: 92 4 120894 5] [PubMed]
Zhao 2020
- Zhao W, Peng H, Li H, Yi Y, Ma Y, He Y, et al. Effects of ultrasound-guided techniques for radial arterial catheterization: a meta-analysis of randomized controlled trials. American Journal of Emergency Medicine 2020;46:1-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Flumignan 2020
- Flumignan RL, Trevisani VF, Lopes RD, Baptista‐Silva JC, Flumignan CD, Nakano LC. Ultrasound guidance for arterial (other than femoral) catheterisation in adults. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013585. [DOI: 10.1002/14651858.CD013585] [DOI] [PMC free article] [PubMed] [Google Scholar]