Abstract
Background
Wenzhou has achieved great progress in the prevention and control of the growing coronavirus disease 2019 (COVID-19) pandemic, and traditional Chinese medicine (TCM) has played an indispensable role in this fight. This study aimed to investigate the efficacy of Maxingshigan-Weijing decoction (MWD) in treating infected patients.
Methods
This study was an open-label randomized controlled trial. Inpatients with mild or moderate symptoms caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were randomly treated with routine supportive care alone or a combination of routine supportive care and MWD. The primary outcome was the rate of symptom (fever, fatigue, cough and difficulty breathing) recovery.
Results
Fifty-nine inpatients were enrolled, of whom 29 received routine supportive care alone (control group) and 30 received combination therapy (treatment group). The rate of symptom recovery was significantly higher in the treatment group than in the control group. The time to recovery of fever (3 vs. 7 days), fatigue (9 vs. 12 days), coughing (9 vs. 14 days) and difficulty breathing (4.5 vs. 9.5 days) was also significantly shorter in the treatment group (all p < 0.001). The syndrome score was lower after MWD treatment. However, neither group differed in the viral assay findings, hospitalization days, medication time or the rate of conversion to severe cases.
Conclusions
MWD increased the rate of symptom recovery and shortened the time to recovery of clinical symptoms without deterioration to death or critical care. These findings may provide opportunities for the use of complementary medicine in treating this infection.
Clinical trial registration
Chinese Clinical Trial Registry, ChiCTR2000030759.
Keywords: COVID-19, Maxingshigan-Weijing decoction, Symptom recovery, Traditional Chinese medicine
1. Introduction
In December 2019, an unexplained pneumonia outbreak occurred in Wuhan, one of the most populous cities in central China. The outbreak was identified and named COVID-19 (Coronavirus Disease-2019) by the World Health Organization (WHO) on January 30, 2020.1 This virus has spread with astonishing speed throughout China2 and the world and has been identified as a pandemic and a health emergency of global concern.3 According to WHO data (https://www.who.int/), more than 100,000,000 diagnosed cases in more than 210 different countries have been identified, causing more than 2,200,000 related deaths as of January 2021.
During these challenging and unprecedented times, many countries have implemented extensive precautionary measures for better control of disease spread, and scientists have expended substantial effort to identify options that will help these infected patients and prevent potential death. At the same time, a vaccine against COVID-19 is being or has been developed in approximately 90 institutions worldwide. Unfortunately, the spread of the virus remains uncontrolled, and the number of deaths continues to increase.
Wenzhou, the city with the highest incidence of COVID-19 in Hubei Province from January to March 2020, effectively and efficiently prevented and controlled this growing epidemic situation with a 99.8% cure rate and only 1 death.4 These impressive outcomes were attributed not only to the defensive strategies rolled out overnight in cutting off routes of transmission by the Wenzhou municipal government but also to the developed medical level.
In China, both Western medicine and traditional Chinese medicine (TCM) are recognized as important components of the existing health system. TCM has been used to treat infectious diseases for thousands of years. As one of the origins of TCM, people in Wenzhou commonly choose TCM as a form of complementary medicine. Accordingly, TCM is widely used to treat patients with COVID-19, and it merits special attention and even commendation.
During the early days of the 2009 H1N1 influenza A pandemic, the popular herbal formula Maxingshigan–Yinqiaosan was used widely by TCM practitioners to reduce symptoms.5 Moreover, research on the mechanism of Maxingshigan decoction against influenza virus is also ongoing.6 Weijing decoction, established by Sun Simiao, has been widely used to treat respiratory conditions for thousands of years. The curative effect of Weijing decoction on the treatment of community-acquired pneumonia was significant7; it also shortens the recovery time and reduces the recurrence of AECOPD,8 as well as fighting other lung diseases, such as lung cancer and pulmonary interstitial fibrosis.9
Hence, the aim of this study was to describe the effects of Maxingshigan-Weijing decoction (MWD) on the treatment of COVID-19 in Wenzhou, China.
2. Methods
2.1. Study design and setting
We conducted an open-label randomized controlled trial during the COVID-19 epidemic between February and May 2020 at the First Affiliated Hospital of Wenzhou Medical University. A structured summary of the study protocol has been published,10 and the trial has been reported according to CONSORT 2010 guidelines (Supplement). The results were evaluated by a blinded end-point committee, which was an independent unit.
2.2. Participants
The following inclusion criteria and exclusion criteria were used according to our previously published protocol10:
Inclusion criteria
-
1.
Age of 18-85 years, either male or female;
-
2.
Diagnosed as positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2);
-
3.
Symptomatic. Mild (mild clinical symptoms without signs of pneumonia on chest X-ray) and moderate (fever or respiratory symptoms with signs of pneumonia on chest X-ray);
-
4.
Signed the informed consent form before treatment;
-
5.
Agreed not to enroll in any other clinical trials; and
-
6.
Inpatients.
Exclusion criteria
-
1.
< 18 or > 85 years old;
-
2.
Pregnancy and lactation;
-
3.
Serious heart, liver, kidney and hematopoietic system diseases, abnormal liver or kidney function;
-
4.
Suffering from pneumonia caused by other known viruses;
-
5.
Allergy to Chinese herbal medicine or suffering from allergies; and
-
6.
Critically ill patients (respiratory failure treated by mechanical ventilation, shock or multiple organ failure).
2.3. Protocol Deviations
Deviations from the approved protocol were reflected in the sample size and definition of the primary outcome measure. When this clinical trial was conducted, people were unable to travel or gather due to lockdown during the COVID-19 outbreak. Therefore, we modified the trial and deviated from our protocol to some extent, completing the study in only 1 hospital instead of recruiting infected patients from 3 hospitals. This approach led to the reduced sample size, and the definition of the primary outcome measure also changed to ensure the integrity, accuracy and reliability of the research data. A revised protocol was submitted to the Ethics Committee, which classified the deviations to the relevant grades, analyzed their effects on subject safety and quality of data, and provided approval.
2.4. Randomization and sample size
The minimization method was used, balancing the two arms for pneumonia severity. Patients were randomized to each group. Clinical researchers obtained a random sequence number that was automatically generated by a random number generator (IBM Corp., Armonk, NY, USA) and sequentially numbered them in an opaque envelope. Researchers opened random allocation envelopes and assigned participants accordingly. Eligible patients were randomly divided into a control group and a treatment group.
A previous study reported that the efficacies of integrated traditional Chinese and Western medicine and simple Western medicine for COVID-19 were 94.1% and 61.1%, respectively.11 Assuming a statistical power of 80% (one-sided type-1 error of α = 5%, β = 20%) and a rate of withdrawal and loss to follow-up of 10%, 30 participants were required in both groups.
2.5. Intervention
The TCM formula that we used in our study was Maxingshigan-Weijing decoction (MWD), which consists of 10 g of Herba Ephedra (Mahuang), 10 g of Amygdalus Communis Vas (Xingren), 45 g of Gypsum Fibrosum (Shigao), 30 g of Rhizoma phragmitis (Lugen), 20 g of peach kernel (Taoren), 20 g of winter melon kernel (Dongguaren), 30 g of Trichosanthes Kirilowii Maxim (Gualou), 12 g of Pericarpium Citri Reticulatae (Chenpi), 12 g of Rhizoma Pinelliae (Jiangbanxia), 12 g of caulis bambusae in taeniis (Zhuru), 30 g of semen lepidii (Tingliz), 15 g of semen lepidii (Shichangpu), 10 g of curcuma zedoary (ezhu) and 5 g of Radix Glycyrrhizae (Gancao).
Patients in the control group were treated with routine supportive care alone, which included staying in bed, oxygen therapy provided by a nasal cannula, broad-spectrum antibiotics and antivirals. Inpatients in the treatment group were asked to take 200 mL of MWD orally 2 times daily for 14 consecutive days in addition to the routine supportive care mentioned above.
2.6. Outcome measures
2.6.1. Primary outcome
The primary outcome was the rate of symptom recovery after 14 days of treatment. Symptom recovery was defined as the complete disappearance of fever, fatigue, cough and difficulty breathing.
2.6.2. Secondary outcomes
Secondary outcomes consisted of the time to recovery of individual symptoms, TCM syndrome scores, the time of conversion of SARS-CoV-2 RNA assay, hospitalization days, medication time and the rate of conversion to severe cases. In addition to TCM syndrome scores, other outcomes were recorded daily in a case report form. TCM syndrome scores were evaluated at baseline and at the end of drug intervention.
2.6.3. Routine blood tests
Finally, routine blood tests and hepatic and renal function were also measured.
The TCM Syndrome Scoring System is a checklist covering 4 main, 7 secondary and 13 accompanying items. The 4 main items consist of fever, cough, malaise and shortness of breath use a four-point scale (0, 2, 4 and 6), depending on the severity; the 7 secondary items, including dysphoria, diarrhea, pharyngalgia, expectoration, muscular soreness, nasal obstruction and rhinorrhea, use a 0-3-point scale; the 13 accompanying items include chest pain, headache, aversion to cold, dizziness, nausea and vomiting, anorexia, abdominal distension, dry mouth, anxiety, spontaneous sweating, insomnia, wheezing and blood-tinged sputum, and each item is rated on 0-1 point scale (0 indicates asymptomatic and 1 indicates symptomatic). The total scores are summed and range from 0 to 58, with higher scores indicating more severe levels of disease.
2.7. Statistical analysis
Differences were considered statistically significant at a P value of < 0.05. Statistical analyses were performed with SAS 9.4 software. Normally distributed continuous variables were reported as the means ± standard deviations, and an independent t-test was used to generate statistical inferences about the differences between the groups. Continuous variables with a skewed distribution are presented as the median durations and were analyzed with the Mann–Whitney U test. Counts were adopted to summarize the categorical variables and were compared with the chi-square test. The primary endpoint was analyzed according to the intention-to-treat principle, with event curves for the time-to-first event based on the Kaplan–Meier analysis. Additionally, repeated-measures ANOVA was used for the longitudinal data analysis.
2.8. Ethical considerations
The study protocol was approved by the Ethical Research Committee of the First Affiliated Hospital of Wenzhou Medical University (KY2020-003). Written informed consent was obtained from all participants prior to enrollment. This study was conducted in accordance with the guidelines for Good Clinical Practice for trials.
3. Results
This study included 59 patients with mild or moderate symptoms caused by SARS-CoV-2 infection, of whom 29 and 30 received routine supportive care alone or a combination of routine supportive care and TCM treatment, respectively. The study flow chart is shown in Fig. 1.
Fig. 1.
Flow chart of the study design.
3.1. Demographics and baseline characteristics of the participants
The demographic characteristics, including age, sex, BMI and blood pressure, of the study population are shown in Table 1(a). These parameters did not differ significantly between the groups. However, men were more likely to suffer from SARS-CoV-2 infection.
Table 1.
Patients’ characteristics
| Characteristics | Control group (n = 29) | Treatment group (n = 30) | p value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 53.3±15.8 | 50.7±12.3 | 0.389 |
| Male, n (%) | 21 (72.4) | 19 (63.3) | 0.094 |
| BMI (kg/m2) | 24.9±3.0 | 23.6±3.6 | 0.087 |
| SBP (mmHg) | 125.0 (118.0, 135.0) | 125.0 (118.0, 135.0) | 0.690 |
| DBP (mmHg) | 77.0 (72.0, 81.0) | 80.0 (75.0, 85.0) | 0.087 |
| Main symptoms at baseline | |||
| Median symptom score (IQR)* | 12 (6.0, 15.0) | 12 (9.0, 15.0) | 0.354 |
| Symptom, n(%) | |||
| Fever | 9 (31.0) | 9 (30.0) | 0.946 |
| Fatigue | 23 (79.3) | 27 (90.0) | 0.252 |
| Cough | 21 (72.4) | 29 (96.7) | 0.002 |
| Difficulty breathing | 15 (51.7) | 19 (63.3) | 0.285 |
IQR, interquartile range.
The main symptoms experienced by the participants in the control and treatment groups were fever (31% vs. 31.7%), fatigue (79.3% vs. 90.5) and difficulty breathing (51.7% vs. 63.5%). Inpatients in the treatment group had only one significant symptom, cough (72.4 vs. 96.8, P = 0.002) compared with the control group (Table 1b).
3.2. Outcomes
3.2.1. Primary outcome
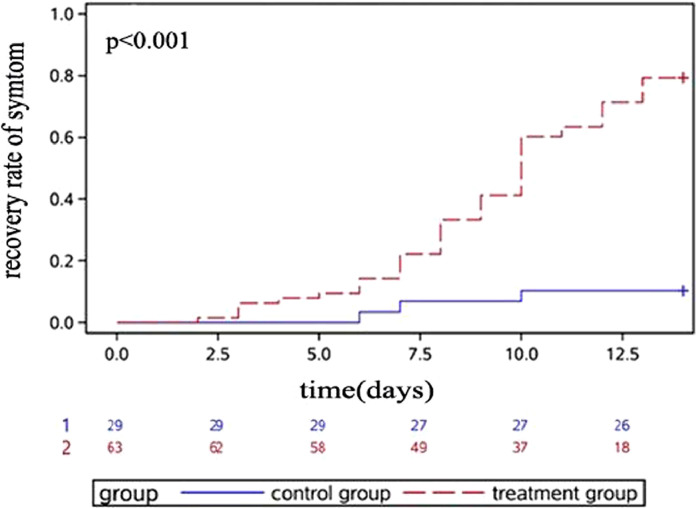
The rate of symptom recovery was significantly higher in the treatment group than in the control group based on the Kaplan–Meier curve (Fig. 2). Moreover, the difference became more significant over time.
Fig. 2.
Dynamic changes in the recovery rate. Kaplan–Meier curves for the symptom recovery rates (including fever, fatigue, coughing and difficulty breathing) are shown. The percentage of patients who achieved symptom recovery at individual time points is shown for the control group and treatment group.
3.2.2. Secondary outcomes
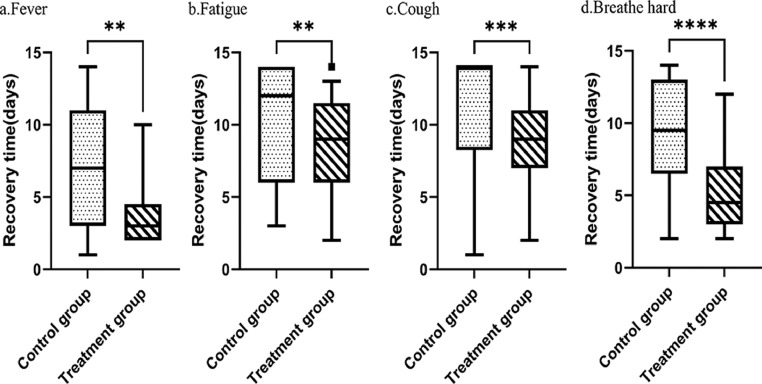
Figure 3 shows the comparison of times to individual symptom recovery. The treatment group exhibited a significantly shorter median time to the recovery of fever (3 days vs. 7 days, p = 0.009), fatigue (9 days vs. 12 days, p = 0.007), coughing (9 days vs. 14 days, p = 0.001) and difficulty breathing (4.5 days vs. 9.5 days, p < 0.001).
Fig. 3.
Comparison of the median times to symptom recovery. **p value <0.05, ***p value <0.01, and ***p value <0.005 compared with the control group.
As shown in Table 2, the TCM syndrome score was also significantly lower in the treatment group than in the control group based on repeated-measures ANOVA. In addition, the effect of MWD on the syndrome score was more significant over time.
Table 2.
Comparisons of the changes in TCM syndrome scores over time between the two groups
| Variables | df | Type III SS | F-value | P value |
|---|---|---|---|---|
| Treatment | 1 | 167.85 | 1.77 | 0.187 |
| Day | 4 | 479.93 | 67.87 | <0.001 |
| Treatment*Day | 4 | 76.006 | 10.75 | <0.001 |
df, degrees of freedom; SS, sum of squares of deviations
Treatment with MWD was not associated with a shorter conversion time of SARS-CoV-2 viral assay findings (14 days vs. 10 days) or a shorter hospitalization period (21 days vs. 18 days). However, the treatment group had a shorter median medication duration than the control group (10 days vs. 13 days), but the difference was not significant. No severe cases occurred in either group (Table 3).
Table 3.
Comparison of the secondary outcomes
| Variables | Control (n = 29) | Treatment (n = 30) | p value |
|---|---|---|---|
| Time to recovery of fever (Median, IQR), d | 7.0 (3.0, 11.0) | 3.0 (2.0, 4.0) | 0.009 |
| Time to recovery of fatigue (Median, IQR), d | 12.0 (6.0, 14.0) | 9.0 (6.0, 11.0) | 0.007 |
| Time to recovery of cough (Median, IQR), d | 14.0 (8.5, 14.0) | 9.0 (7.0, 11.0) | 0.001 |
| Time to recovery of difficulty breathing (Median, IQR), d | 9.5 (7.0, 13.0) | 4.5 (3.0, 7.0) | <0.001 |
| Time to viral assay conversion (Median, IQR), d | 10.0 (6.0, 16.0) | 14.0 (10.0, 18.0) | 0.021 |
| Days hospitalized (Median, IQR), d | 18.0 (12.0, 20.0) | 21.0 (17.0, 27.0) | 0.003 |
| Medication time (Median, IQR), d | 13.0 (9.0, 14.0) | 10.0 (9.0, 13.0) | 0.086 |
| Rate of conversion of severe cases, n (%) | 0 (0) | 0 (0) |
IQR, interquartile range.
3.2.3. Routine Blood Test
At the end of medication, hemoglobin levels improved significantly in the treatment group. However, no differences were observed in the changes in other blood parameters (e.g., leukocytes, neutrophil percentage, lymphocyte percentage, red blood cells, and platelets) (Table 4).
Table 4.
Main routine blood indices at the end of treatment
| Variables | Control (n = 29) | Treatment (n = 30) | p value |
|---|---|---|---|
| Leucocytes, × 109/L | 6.1 (5.5, 7.3) | 5.7 (4.9, 7.3) | 0.404 |
| Neutrophil percentage, % | 62.3±12.3 | 61.4±8.5 | 0.759 |
| Lymphocyte percentage, % | 26.4±10.7 | 26.6±8.8 | 0.952 |
| Hemoglobin, g/L | 115.5±21.1 | 127.3±18.8 | 0.041* |
| Red blood cells, × 1012/L | 3.9±0.6 | 4.2±0.6 | 0.071 |
| Platelets, × 109/L | 271.5 (228.0, 300.5) | 258.5 (216.0, 321.0) | 0.580 |
p < 0.05, compared to the control group.
3.2.4. Safety
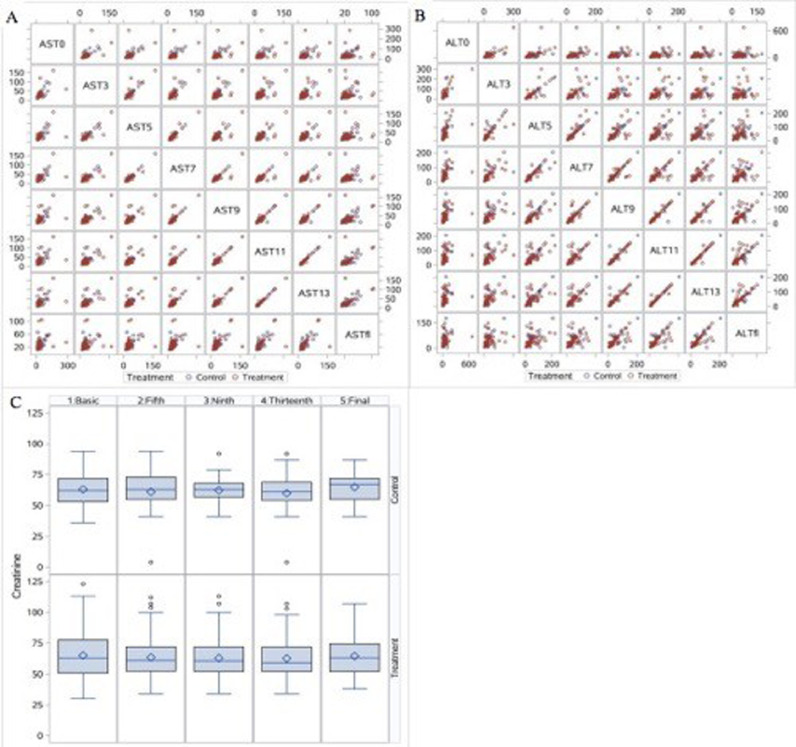
No obvious serious adverse events were reported, and no abnormal laboratory test findings were reported. Fig. 4 lists the changes in hepatic and renal function. The activities of alanine aminotransferase (ALT, Fig. 4A) and aspartate aminotransferase (AST, Fig. 4B) and serum creatinine levels (SCr, Fig. 4C) remained normal throughout the treatment period.
Fig. 4.
Changes in the values of biochemical parameters. A. Comparison of alanine aminotransferase (ALT) activity in the treatment and control groups shown in a matrix scatter plot. B. Comparison of aspartate aminotransferase (AST) activity in the treatment and control groups shown in a matrix scatter plot. C. Results from the mixed effects model showing changes in SCr levels over time.
4. Discussion
To our knowledge, this registered randomized, controlled trial is the first to investigate the efficacy and safety of Maxingshigan-Weijing decoction in patients with COVID-19. We found a higher rate of symptom recovery in patients treated with Maxingshigan-Weijing decoction than in patients receiving routine supportive care. In addition, the improvement in symptoms was significantly more rapid, the reduction in median time to fever recovery between the routine supportive care plus Maxingshigan-Weijing decoction and routine supportive care groups (3 days [CI, 2.0 to 4.0 days] vs. 7days [CI, 3.0 to 11.0]) was 57%, the reduction in the median time to fatigue recovery (9 days [CI, 6.0 to 11.0 days] vs. 12 days [CI, 6.0 to 14.0]) was 25%, the reduction in the median time to cough recovery (9 days [CI, 7.0 to 11.0 days] vs. 14 days [CI, 8.5 to 14.0]) was 35.7%, and the reduction in the median time to recovery of difficulty breathing (4.5 days [CI, 3.0 to 7.0 days] vs. 9.5 days [CI, 7.0 to 13.0]) was 52.6%. Therefore, regarding the clinical implications, we concluded that the combination of routine supportive care and Maxingshigan-Weijing decoction was superior to routine supportive care alone.
The mechanism of TCM in the treatment of COVID-19 is complex. Yang and colleagues12 established a rat model of LPS-induced pneumonia and found that Maxingshigan decoction mediated anti-inflammatory effects through the thrombin and Toll-like receptor (TLR) signaling pathways. Direct evidence obtained from a clinical study showed that Maxingshigan decoction not only improves fever by regulating the immune system but also improves patients' cough symptoms.11 Another classical Chinese medicine formula, Weijing decoction, is regarded as a folk medicine for clearing heat and toxic materials, removing blood stasis and expelling pus from the lung. Weijing decoction effectively reduces the levels of inflammatory factors (IL-6, IL-10, and TNF-α) and improves the quality of life of patients with senile pneumonia.13 Additionally, it rapidly downregulates PCT levels, reduces SOFA scores, and thus improves the prognosis of patients with severe pneumonia of phlegm-heat obstructing lungs.14 Moreover, Weijing decoction was used to shorten the mechanical ventilation time and hospital stay and improve the success rate of weaning in patients with severe pneumonia undergoing mechanical ventilation.15
According to the theory of TCM, scientists have found that the pathogenesis of COVID-19 is mainly characterized by heat, and it is located in the lung. Although Maxingshigan decoction exerts a reliable effect on attenuating fever, Weijing decoction mainly works on the lung, and thus a sensible approach is to combine Maxingshigan decoction with Weijing decoction to combat the mechanism of ‘heat’ and ‘phlegm’ syndrome and prevent the disease from progressing to a critical illness. In addition, Maxingshigan decoction and Weijing decoction are often used in combination in our clinical practice.16,17 Therefore, Maxingshigan-Weijing decoction (MWD) was adopted to treat COVID-19 in this study.
MWD used in this study is composed of the 14 herbal drugs mentioned above. Herba Ephedra (Mahuang), an important component of MWD, has been used to treat asthma, cold, flu, chills, fever, headache, nasal congestion, and cough in many countries.18 Amygdalus Communis Vas (Xingren) and semen lepidii (Tinglizi) decrease lung qi to relieve asthma, and Gypsum Fibrosum (Shigao) exerts an antipyretic effect by reducing the synthesis of inflammatory factors.19 Rhizoma phragmitis (Lugen), a key component of weijing-decoction, has been prescribed in traditional Korean and Chinese medicine to relieve fever, vomiting, dysuria, and constipation.20 Trichosanthes Kirilowii Maxim (Gualou), possesses a variety of pharmacological activities, such as anti-inflammatory, antioxidant, anticancer, neuroprotective activities.21 Pericarpium Citri Reticulatae, commonly referred to as “Chen-pi” in Chinese, exerts an antagonistic effect on the increase in ileal contraction induced by acetylcholine, BaCl2, and 5-HT.22 Caulis bambusae in taeniis (Zhuru), semen lepidii (Shichangpu) and winter melon kernel (Dongguaren) clear heat and transform phlegm, peach kernel (Taoren) and curcuma zedoary (ezhu) activate blood circulation to dissipate stasis, Rhizoma Pinelliae (Jiangbanxia) dries dampness and strengthen the spleens, and Radix Glycyrrhizae (Gancao) may reduce airway or pulmonary inflammation by modulating inflammation-related pathways to relieve cough.23 Therefore, this TCM formula clears fever, removes phlegm and relieves cough to improve the clinical symptoms of patients, provide additional benefits in terms of a decrease in symptom scores during treatment, blocks the transition of mild cases to severe cases, shortens the course of the disease and promotes self-recovery. Thus, it minimizes the incidence and mortality of severe illness, and enables the efficient use of tight and limited medical resources.
However, this study has limitations. First, the sample size was not large enough. The rapid outbreak of communicable disease affected the timely formation of a complete diagnosis and treatment plan. Second, research on the mechanisms of TCM is insufficient. Therefore, extended fundamental studies are needed to thoroughly explore the effects and theories of MWD on the resolution of all symptoms.
In conclusion, MWD exerted a therapeutic effect on COVID-19 by improving the recovery rate of symptoms, shortening the time to symptom recovery, and relieving TCM symptoms to decrease TCM scores, but with no obvious adverse outcomes. Thus, MWD could be considered a treatment for COVID-19.
Acknowledgments
Acknowledgments
We thank all patients and their families for their kind participation in the study. We would also like to acknowledge all the staff of the hospitals and Professor Mao Guangyun for evaluating the results.
Author contributions
Conceptualization: CJG and YR. Methodology: CJG and YR. Investigation: ZCC and YZZ. Formal Analysis: ZJH, WYT and XYY. Writing – Original Draft: ZCC and YZZ. Writing – Review and Editing: YZZ.
Conflict of interests
The authors have no competing interests to declare.
Funding
This study was supported by the Wenzhou Municipal Science and Technology Bureau (CN) (ZY202003). The funding body had no role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Ethical statement
This study received ethical approval from the institutional human ethics committee of the First Affiliated Hospital of Wenzhou Medical University (2020003), Wenzhou, China, on February 11, 2020.
Data availability
The data will be available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2021.100782.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang DY, Wang WL. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui DS, Azhar IE, Madani TA. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed February 19, 2020
- 4.Wenzhou News Network. Press Conference on COVID-19 Prevention and Control in Wenzhou. http://news.66wz.com/system/2020/03/16/105248853.shtml. Accessed on March 16,2020.
- 5.Wang C, Cao B, Liu QQ. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann Intern Med. 2011 Aug 16;155(4):217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Wu T, Lu GF. Effects of maxing Shigan decoction on the expression levels of CCL3 and CCL25 in lungs of mice infected with influenza virus. J Hunan Univ Chin Med. 2020;40(10):1216–1220. [Google Scholar]
- 7.Zhang YX, Li YX, Xu HR. Weijing Tang combined western medicine on pneumonia: a meta analysis of randomized controlled trials. Mod Chin Clin Med. 2019;26(04):47–51. [Google Scholar]
- 8.Liu S, Shergis J, Chen X. Chinese herbal medicine (weijing decoction) combined with pharmacotherapy for the treatment of acute exacerbations of chronic obstructive pulmonary disease. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/257012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong F, Jiang M, Chen M, Wang X, Zhang S, Zhou J. Study on inhibitory effect of MaiMenDong Decoction and WeiJing Decoction Combination with Cisplatin on NCI-A549 Xenograft in nude mice and its mechanism. J Cancer. 2017 Jul 23;8(13):2449–2455. doi: 10.7150/jca.17720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng C, Yuan Z, Pan X. Efficacy of Traditional Chinese Medicine, Maxingshigan-Weijing in the management of COVID-19 patients with severe acute respiratory syndrome: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):1029. doi: 10.1186/s13063-020-04970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia WG, An CQ, Zheng CJ. Clinical study of 34 cases of new coronavirus pneumonia treated by Chinese and western medicine. J Tradit Chin Med. 2020;61:375–382. (in Chinese) [Google Scholar]
- 12.Yang R, Liu H, Bai C. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren M, Wang FY. Curative effect of Qianjin Weizhi Decoction combined with moxifloxacin and ambroxol hydrochloride on senile pneumonia and its influence on IL-6, IL-10, TNF-α and quality of life. Hainan Med J. 2020;31:309–312. (in Chinese) [Google Scholar]
- 14.Huang DK, Cheng LM. Study on the effect of Qianjin Weijing Tang on PCT and SOFA scores in patients with severe pneumonia of phlegm-heat obstructing lungs type. J New Chin Med. 2019;51:70–72. (in Chinese) [Google Scholar]
- 15.Lin GY, Zhang Y. Effects of modified Weijing decoction combined with western medicine on respiratory mechanics, Th17 and Treg cytokine imbalance and arterial blood gas analysis in patients with severe pneumonia undergoing mechanical ventilation. Hebei Med J. 2018;40(4):520–526. (in Chinese) [Google Scholar]
- 16.He HL, Ye Y. Observation of integrated chinese and western medicine therapy for community - acquired pneumonia of phlegm-heat obstructing lung type. J New Chin Med. 2017;51:30–32. (in Chinese) [Google Scholar]
- 17.Wang SL, Zhang Y, Hou JH, Guo YR, Li F. Clinical efficacy of modified weijingtang and maxing shigan tang combined with half-dose hormones on refractory mycoplcasma pneumoniae pneumonia in children caused by toxic heat closing lung. Chin J Exp Trad Med Formul. 2020;26:69–74. (in Chinese) [Google Scholar]
- 18.González-Juárez DE, Escobedo-Moratilla A, Flores J. A review of the ephedra genus: distribution, ecology, ethnobotany, phytochemistry and pharmacological properties. Molecules. 2020 Jul 20;25(14):3283. doi: 10.3390/molecules25143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y.X., Li M., Tang Z.S., Wang B., Zhang B. An study on Gypsum compounds and their antipyretic function and anti-inflammatory mechanisms. J Shanxi College Tradit Chin Med. 2012;35(05):74–76. [Google Scholar]
- 20.Li H., Gao Y-M., Zhang J., Wang L., Wang X-X. Ultra-performance liquid chromatography fingerprinting for quality control of Phragmitis rhizoma (Lugen) produced in Baiyangdian. Pharmacogn Mag. 2013;9(36):285–289. doi: 10.4103/0973-1296.117810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang HQ, Liu P, Duan JA, Dong L, Shang EX, Qian DW. Hierarchical extraction and simultaneous determination of flavones and triterpenes in different parts of Trichosanthes kirilowii Maxim. by ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. J Pharm Biomed Anal. 2019;167:114–122. doi: 10.1016/j.jpba.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Guan F.L., Wang R.J., Wang J.H. Effect of chenpi and hesperidin on contractile activity of intestinal muscle strips in vitro. Lishizhen Med Mater Med Res. 2002;(02):65–67. [Google Scholar]
- 23.Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother Res. 2018;32(12):2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be available from the corresponding author upon reasonable request.