Supplemental Digital Content is available in the text.
Keywords: children, endotheliopathy, multisystem inflammatory syndrome, severe acute respiratory syndrome coronavirus 2, shock
TRIAL REGISTRATION:
OBJECTIVES:
Severe acute respiratory syndrome coronavirus 2–related multisystem inflammatory syndrome in children is frequently associated with shock; endothelial involvement may be one of the underlying mechanisms. We sought to describe endothelial dysfunction during multisystem inflammatory syndrome in children with shock and then assess the relationship between the degree of endothelial involvement and the severity of shock.
DESIGN:
Observational study.
SETTING:
A PICU in a tertiary hospital.
PATIENTS:
Patients aged under 18 (n = 28) with severe acute respiratory syndrome coronavirus 2–related multisystem inflammatory syndrome in children and shock, according to the Centers for Disease Control and Prevention criteria.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
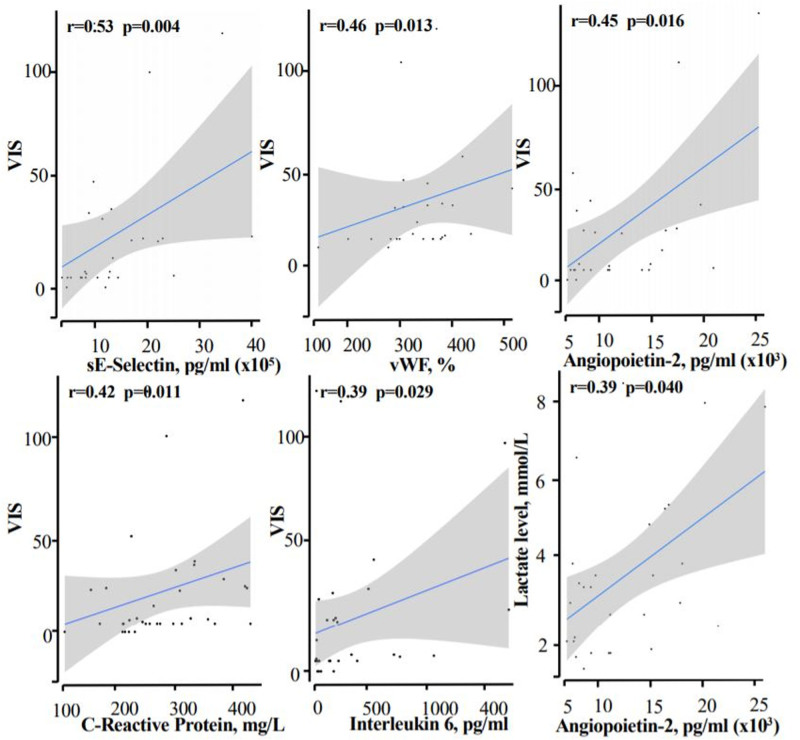
Correlations between endothelial marker levels and shock severity were assessed using Spearman coefficient. The median (interquartile range) age was 9 years (7.5–11.2 yr). Sixteen children presented with cardiogenic and distributive shock, 10 presented with cardiogenic shock only, and two presented with distributive shock only. The median left ventricular ejection fraction, troponin level, and lactate level were, respectively, 40% (35–45%), 261 ng/mL (131–390 ng/mL), and 3.2 mmol/L (2–4.2 mmol/L). Twenty-five children received inotropes and/or vasopressors; the median Vasoactive and Inotropic Score was 8 (5–28). Plasma levels of angiopoietin-2 (6,426 pg/mL [2,814–11,836 pg/mL]), sE-selectin (130,405 pg/mL [92,987–192,499 pg/mL]), von Willebrand factor antigen (344% [288–378%]), and the angiopoietin-2/angiopoietin-1 ratio (1.111 [0.472–1.524]) were elevated and significantly correlated with the Vasoactive and Inotropic Score (r = 0.45, p = 0.016; r = 0.53, p = 0.04; r = 0.46, p = 0.013; and r = 0.46, p = 0.012, respectively).
CONCLUSIONS:
Endothelial dysfunction is associated with severe acute respiratory syndrome coronavirus 2–related multisystem inflammatory syndrome in children with shock and may constitute one of the underlying mechanisms.
Multisystem inflammatory syndrome in children (MIS-C) is an emerging disease associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Although the etiopathogenesis of MIS-C remains unclear, the disease’s central features are systemic hyperinflammation and a cytokine storm after an immune response (1). In a French nationwide epidemiological study of MIS-C, severe cardiovascular manifestations (including cardiogenic and distributive shock) were observed in 67% of cases (2). In mechanistic terms, endothelitis and microvascular dysfunction might lead to distributive shock (through systemic capillary leakage and in a decrease in systemic vascular resistance) and/or cardiogenic shock (through myocardial edema) (3–6). The objectives of the present study were to: 1) describe endothelial dysfunction during MIS-C with shock and 2) assess the putative link between the severity of the acute cardiovascular manifestations and the degree of endothelial involvement.
MATERIALS AND METHODS
We conducted a retrospective study of a cohort of patients under 18 years old and having been admitted for MIS-C with shock to the PICU at Necker Hospital (Paris, France) between April 1, 2020, and May 31, 2020. MIS-C was diagnosed according to the Centers of Disease Control and Prevention criteria (7). All children with fever and shock were screened for SARS-CoV-2 infection and other viral/bacterial/fungal infections (Supplemental Methods, http://links.lww.com/CCM/G484), and included or excluded using a standardized case report.
Distributive, cardiogenic shock and acute heart failure were assessed according to the international definitions in children (8–10). Disease severity was rated by measuring the echocardiographic left ventricular ejection fraction (LVEF) and the troponin level (for acute heart failure) or the Vasoactive and Inotropic Score (VIS) (11) and the lactate level (for shock). The study was approved by an institutional review board.
Clinical assessments and blood sampling for biological evaluation were performed within the first 48 hours of admission to the PICU. Von Willebrand factor antigen (vWF:Ag) was measured on citrated plasma using an ACL Top coagulation analyzer (Instrumentation Laboratory, Le Pré Saint Gervais, France). Concentrations of soluble (s)E-selectin, P-selectin, endoglin, vascular endothelial growth factors, angiopoietins-1 and -2, and CD40L were measured on citrated with Human Magnetic Luminex Assays. Data were expressed as the median (interquartile range [IQR]) or the frequency (percentage). We evaluated correlations among biological data, endothelial markers, and the VIS by calculating Spearman coefficient. To assess the ability of the endothelial markers to predict a high VIS (>7), we analyzed the area under the receiver operating characteristic (ROC) curve and defined the corresponding optimal predictive cutoffs. All statistical analyses were performed using R studio software.
RESULTS
Thirty-two children with shock and fever were enrolled. We excluded children with typical Kawasaki disease shock syndrome (n = 1), documented septic shock (n = 2), and dilated cardiomyopathy (n = 1); hence, 28 children with MIS-C and shock were included. All 28 patients underwent nasopharyngeal swab polymerase chain reaction testing for SARS-CoV-2, and nine were found to be positive. All 28 were seropositive (immunoglobulins G) for SARS-CoV-2. No other viral, bacterial, or fungal agents were isolated. The study population’s demographic, clinical, and laboratory data are summarized in Table 1. The children were admitted to the PICU for shock a median (IQR) of 5 days (5–6 d) after symptom onset. Patients were started on IV immunoglobulins (n = 17) or IV immunoglobulins plus corticosteroids (n = 11) as soon as MIS-C was diagnosed. The children weaned off the inotropes and vasopressors within 3 days (1–4 d). Eleven children needed mechanical ventilation for a median duration of 5 days (3–6 d). All the children survived and were discharged from the PICU after a median of 5 days (4–7 d). In all cases, the left ventricular (LV) function had recovered fully. The plasma levels of angiopoietin-2, sE-selectin, vWF:Ag, and angiopoietin-2/angiopoietin-1 ratio were found to be elevated. VIS was significantly correlated with plasma angiopoietin-2, sE-selectin, and vWF:Ag levels and angiopoietin-2/angiopoietin-1 ratio (Fig. 1; and Supplemental Table 1, http://links.lww.com/CCM/G484). Similarly, VIS was significantly correlated with plasma C-reactive protein (CRP) and interleukin (IL)-6 levels (Fig. 1). The plasma angiopoietin-2 level was also correlated with the LVEF (r = −0.46; p = 0.01) and the lactate level (r = 0.39; p = 0.04). Based on the ROC curve, the optimal cutoffs for discriminating between the cases of severe (VIS >7) and moderate shocks (VIS <7) were 97,259 pg/mL for sE-selectin (Supplemental Fig. 1, http://links.lww.com/CCM/G484). To probe the putative effect of vasopressors on endothelial activation, we compared angiopoietin-2 and vWF:Ag levels in patients who received epinephrine and/or norepinephrine (n = 16) versus patients treated with inotropic drugs that lack a vasopressor effect (dobutamine and/or milrinone, n = 11); no significant intergroup differences were observed (angiopoietin-2, 9,545 pg/mL [4,044–12,989 pg/mL] in those receiving epinephrine and/or norepinephrine vs 4,293 pg/mL [2,381–6,544 pg/mL] in those receiving dobutamine and/or milrinone, p = 0.157; vWF:Ag, 353 pg/mL [313–390 pg/mL] vs 340 pg/mL [284–361 pg/mL], respectively, p = 0.202).
TABLE 1.
Clinical and Laboratory Data
| Study Population (n = 28) | Value | Normal Range |
|---|---|---|
| Demographic and clinical features | ||
| Age, yra | 9 (7.5–11.2) | |
| Male sex, n (%) | 14 (50) | |
| Epidemiologic link to a person with COVID-19, n (%) | 9 (32) | |
| Underlying conditions, n (%) | 2 (7) | |
| Pediatric Logistic Organ Dysfunction 2 scorea/Pediatric Risk of Mortality IV scorea | 3 (2–4)/3 (2–3) | |
| Acute heart failure, n (%) | 26 (93) | |
| Cardiogenic shock, n (%) | 10 (36) | |
| Distributive shock, n (%) | 2 (7) | |
| Cardiogenic and distributive shock, n (%) | 16 (57) | |
| Acute kidney injury, n (%) | 23 (82) | |
| Cardiac and laboratory variablesa | ||
| Left ventricular ejection fraction, % | 40 (34–45) | >55 |
| Troponin, ng/mL | 261 (131–390) | <26 |
| Brain natriuretic peptide, pmol/L | 2,374 (504–8,297) | <100 |
| N-terminal probrain natriuretic peptide, pmol/L | 21,708 (4,028–26,000) | <300 |
| Lactate, mmol/L | 3.2 (2.0–4.2) | <2 |
| d-dimer, ng/mL | 2,709 (1,939–5,177) | <500 |
| Fibrinogen, g/L | 6.2 (5.6–7.8) | 2–4 |
| Antithrombin, % | 64 (54–76) | 70–140 |
| C-reactive protein, mg/L | 279 (223–346) | <6 |
| Procalcitonin, ng/mL | 28.5 (9.1–65.2) | <0.5 |
| Interleukin-6, pg/mL | 131 (25–273) | <8.5 |
| Hemodynamic support | ||
| Inotropes and vasoactive drugs, n (%) | 25 (89) | |
| Dobutamine, n (%) | 18 (64) | |
| Dosing, µg/kg/mina | 5.00 (5.00–5.00) | |
| Epinephrine, n (%) | 15 (54) | |
| Dosing, µg/kg/mina | 0.10 (0.05–0.18) | |
| Milrinone, n (%) | 11 (39) | |
| Dosing, µg/kg/mina | 0.50 (0.30–0.55) | |
| Norepinephrine, n (%) | 7 (25) | |
| Dosing, µg/kg/mina | 0.37 (0.30–0.95) | |
| Vasoactive Inotropic Scorea | 8 (5–28) | |
| Endothelial marker levelsa | ||
| Angiopoietin-1, pg/mL | 6,854 (5,575–9,318) | |
| Angiopoietin-2, pg/mLb | 6,426 (2,814–11,836) | |
| Angiopoietin-2/angiopoietin-1 ratiob | 1.111 (0.472–1.524) | |
| CD40L, pg/mL | 3,368 (2,886–4,025) | |
| sEndoglin, pg/mL | 2,382 (2,256–2,964) | |
| sE-selectin, pg/mLb | 130,405 (92,987–192,499) | |
| sP-selectin, pg/mLb | 35,979 (27,647–41,818) | |
| Vascular endothelial growth factor-A, pg/mL | 64 (16–100) | |
| Soluble vascular endothelial growth factor receptor-2, pg/mL | 8,710 (7,621–10,612) | |
| von Willebrand factor antigen, %b | 344 (288–378) | 50%–150% |
aExpressed as the median (interquartile range).
bElevated values.
Figure 1.
Correlations between endothelial markers (angiopoietin-2, sE-selectin, and von Willebrand factor antigen [vWF:Ag]) or inflammatory markers (C-reactive protein and interleukin-6) and the vasoactive inotropic score (VIS) or the lactate level.
DISCUSSION
Although intense inflammation and hemostasis activation are well-known consequences of severe SARS-CoV-2 infections (5), the present study is the first to report evidence of endothelial dysfunction in MIS-C with shock. Plasma levels of markers of systemic inflammation (CRP, procalcitonin, and IL-6), endothelial dysfunction, and coagulation (particularly sE-selectin, angiopoietin-2, vWF:Ag, D-dimers, and fibrinogen) were notably higher than the reference values reported for controls (12) or for children without infections or organ dysfunctions admitted to the ICU (13).
Because of the observed significant correlations between the severity of shock and the plasma concentrations of both endothelial and inflammation markers, we hypothesize that hyperinflammation may play a role in the occurrence of shock in MIS-C via endothelial dysfunction. Remarkably and despite differences in the clinical manifestations of MIS-C, adults and children show a common endothelial pattern, with elevated angiopoietin-2 and sE-selectin levels in the more severe forms (5, 14).
The vascular growth factor angiopoietins-1 and 2 exert opposing effects on endothelial activation and dysfunction. Angiopoietin-1 promotes vascular stability and suppresses inflammation, whereas angiopoietin-2 is associated with vascular injury and microvascular leakage. Interestingly, the angiopoietin-2/angiopoietin-1 ratio was abnormally high in our study and was correlated with the severity of shock—as also observed in a study of pediatric septic shock (15). Furthermore, the level of angiopoietin-2 was correlated with heart failure. Together with the rapid recovery of LV systolic function, these findings argue in favor of myocardial stunning (without necrosis) as a result of endothelial leakage and edema (6).
Similar endothelial injury has already been described in patients with sepsis and in children with Kawasaki disease (15–17). Endothelial dysfunction and disruption may cause both myocardial dysfunction and distributive shock in these disease settings (4, 6, 16). Our findings are, therefore, not unexpected, since MIS-C shares some clinical features with Kawasaki disease shock syndrome and septic shock—even though some researchers have emphasized the specific clinical and mechanistic features of MIS-C (1).
We cannot rule out an accentuation of endothelial dysfunction by the vasoactive treatment administered for shock (4). However, many patients presented distributive shock (suggestive of endothelial dysfunction) prior to the initiation of vasopressor treatment. Moreover, the lactate level (a biomarker of the severity of shock) was correlated with angiopoietin-2. Furthermore, patients who received vasopressors and patients who received inotropic drugs (which are known to have a minor impact on the endothelium) did not differ significantly with regard to levels of angiopoietin-2 and vWF:Ag, both of which are released from Weibel-Palade bodies upon endothelial activation (18–20). Taken as a whole, these observations suggest that vasoactive treatment does not contribute greatly to endothelial dysfunction. Finally, all the samples were collected during early-stage disease, and some patients had already received IV immunoglobulins and corticosteroids; the endothelial dysfunction might have been less intense in these cases.
Our study suggests potential mechanisms of MIS-C but had some limitations. First, the study lacked a control group. Second, only one time point was evaluated, and we did not assess changes over time in endothelial marker levels during the patients’ recovery. Finally, the study’s observational design prevented us from proving that endothelial activation and dysfunction causes shock.
CONCLUSIONS
In conclusion, our data highlighted significant relationships among endothelial dysfunction, systemic hyperinflammation, and acute severe cardiovascular manifestations. Endothelial dysfunction may be one of the mechanisms underlying SARS-CoV-2–related MIS-C with shock.
ACKNOWLEDGMENTS
We thank Dr. Joffre for his crucial help and input in improving this article, the nurse team for their great job during the COVID-19 outbreak, the cardiologists (Dr. Belhadger, Dr. Bajolle, Pr. Houyel, and Dr. Legendre), the virologists (Drs. Leruez and Burgard), and all the pediatric intensivists for their significant contribution on the critical management of the patients.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Borgel, Renolleau, Smadja, and Oualha conceived and supervised the study. Drs. Grimaud, Philippe, Chareyre, Brakta, Lasne, Toubiana, Angoulvant, and Desvages analyzed the data. Dr. Chocron analyzed the data and supervised statistical analysis. Drs. Borgel, Chocron, Smadja, and Oualha drafted the article. All authors’ interpreted data, drafted and revised the article, and approved the final version.
This work has been funded, in part, with grants from the French National Research Agency’s Evaluation de la COagulopathie et de la dysfonction enDOthéliale comme facteur prédictif de la gravité de l'infection par SARSCoV2/COVID-19 (Assessment of coagualtion and entothelial dysfunction as a predictive severity factor of SARS-CoV-2/COVID-19 infection (SARCODO) program, the Fondation de France, and the Groupe Hospitalo-Universitaire APHP Centre-Université de Paris’s AP-HP Mécénat Crise COVID-19 program.
Dr. Borgel’s institution received funding from Leo PHARMA and ROCHE Financial. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rowley AH, Shulman ST, Arditi M. Immune pathogenesis of COVID-19-related multisystem inflammatory syndrome in children. J Clin Invest. 2020; 130:5619–5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belot A, Antona D, Renolleau S, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020; 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI of children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020; 297:E283–E288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson PI, Stensballe J, Ostrowski SR. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017; 21:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020; 69:1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox SE, Lameira FS, Rinker EB, et al. Cardiac endotheliitis and multisystem inflammatory syndrome after COVID-19. Ann Intern Med. 2020; 173:1025–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) [Internet]. Centers for Disease Control and Prevention, 2020. Accessed July 14, 2020. Available at: https://www.cdc.gov/mis-c/
- 8.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 9.Brissaud O, Botte A, Cambonie G, et al. Experts’ recommendations for the management of cardiogenic shock in children. Ann Intensive Care. 2016; 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker SB, Conlon TW, Zhang B, et al. Clinical signs to categorize shock and target vasoactive medications in warm versus cold pediatric septic shock. Pediatr Crit Care Med. 2020; 21:1051–1058 [DOI] [PubMed] [Google Scholar]
- 11.McIntosh AM, Tong S, Deakyne SJ, et al. Validation of the vasoactive-inotropic score in pediatric sepsis*. Pediatr Crit Care Med. 2017; 18:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter RP, Russell RT, Hu PJ, et al. Plasma angiopoietin-2/-1 ratio is elevated and angiopoietin-2 levels correlate with plasma syndecan-1 following pediatric trauma. Shock. 2019; 52:340–346 [DOI] [PubMed] [Google Scholar]
- 13.Whitney JE, Zhang B, Koterba N, et al. Systemic endothelial activation is associated with early acute respiratory distress syndrome in children with extrapulmonary sepsis. Crit Care Med. 2020; 48:344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smadja DM, Guerin CL, Chocron R, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020; 23:611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melendez E, Whitney JE, Norton JS, et al. Systemic angiopoietin-1/2 dysregulation in pediatric sepsis and septic shock. Int J Med Sci. 2019; 16:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breunis WB, Davila S, Shimizu C, et al. ; International Kawasaki Disease Genetics Consortium. Disruption of vascular homeostasis in patients with Kawasaki disease: Involvement of vascular endothelial growth factor and angiopoietins. Arthritis Rheum. 2012; 64:306–315 [DOI] [PubMed] [Google Scholar]
- 17.Joffre J, Hellman J, Ince C, et al. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020; 202:361–370 [DOI] [PubMed] [Google Scholar]
- 18.Ostrowski SR, Gaïni S, Pedersen C, et al. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: An observational study. J Crit Care. 2015; 30:90–96 [DOI] [PubMed] [Google Scholar]
- 19.Lemaire LC, de Kruif MD, Giebelen IA, et al. Dobutamine does not influence inflammatory pathways during human endotoxemia. Crit Care Med. 2006; 34:1365–1371 [DOI] [PubMed] [Google Scholar]
- 20.White M, Ducharme A, Ibrahim R, et al. Increased systemic inflammation and oxidative stress in patients with worsening congestive heart failure: Improvement after short-term inotropic support. Clin Sci (Lond). 2006; 110:483–489 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.